Abstract
This study tested the hypothesis that postsynaptic cutaneous vascular responses to endothelial-dependent and -independent vasodilators, as well as sweat gland function, are impaired in split-thickness grafted skin 5 to 9 months after surgery. Intradermal microdialysis membranes were placed in grafted and adjacent control skin, thereby allowing local delivery of the endothelial-dependent vasodilator, acetylcholine (ACh; 1 × 10−7 to 1 × 10−1 M at 10-fold increments) and the endothelial-independent nitric oxide donor, sodium nitroprusside (SNP; 5 × 10−8 to 5 × 10−2 M at 10-fold increments). Skin blood flow and sweat rate were simultaneously assessed over the semipermeable portion of the membrane. Cutaneous vascular conductance (CVC) was calculated from the ratio of laser Doppler-derived skin blood flow to mean arterial blood pressure. ΔCVC responses from baseline to these drugs were modeled via nonlinear regression curve fitting to identify the dose of ACh and SNP causing 50% of the maximal vasodilator response (EC50). A rightward shift in the CVC dose response curve for ACh was observed in grafted (EC50 = −2.61 ± 0.44 log M) compared to adjacent control skin (EC50 = −3.34 ± 0.46 log M; P = .003), whereas the mean EC50 for SNP was similar between grafted (EC50 = −4.21 ± 0.94 log M) and adjacent control skin (EC50 = −3.87 ± 0.65 log M; P = 0.332). Only minimal sweating to exogenous ACh was observed in grafted skin whereas normal sweating was observed in control skin. Increased EC50 and decreased maximal CVC responses to the exogenous administration of ACh suggest impairment of endothelial-dependent cutaneous vasodilator responses in grafted skin 5 to 9 months after surgery. Greatly attenuated sweating responses to ACh suggests either abnormal or an absence of functional sweat glands in the grafted skin.
Increases in skin blood flow and sweating are critical responses for humans to appropriately regulate internal temperature during exercise and/or hyperthermic exposure. If these heat-dissipating mechanisms are absent, exercise can increase internal temperature to unsafe levels within 10 minutes.1 Wounds such as burns seriously damage the skin, requiring, in many cases, excising of the damaged tissue and subsequent skin grafting. The excising process leaves little or no dermal tissue at the injured site. It is this dermal layer that contains sweat glands and a rich vascular network, both of which are vital for thermoregulation. Although some degree of revascularization occurs in grafted skin, little is known regarding the consequences of skin grafting with respect to the control of skin blood flow and sweating in the grafted tissue.
Split-thickness skin grafts, 5 to 9 months after surgery, have impaired reflex cutaneous vasodilation and sweating in response to indirect whole-body heating.2 In addition, grafted skin had reduced ability to maximally vasodilate in response to a local heating stimulus. 2 Taken together, these impairments indicate that grafted skin has a greatly attenuated capability to contribute to thermoregulation. However, the specific mechanisms responsible for the observed impairments in cutaneous vasodilation and sweating in grafted skin are unknown, but may be related to altered postsynaptic function (ie, decreased sensitivity to vasodilator neurotransmitters and cofactors).
The primary aim of this investigation was to determine whether altered postsynaptic function contributes to attenuated cutaneous blood flow and sweating responses observed during indirect whole-body heating in grafted skin 5 to 9 months after surgery.2 To address this question, we tested the hypothesis that endothelial-dependent and -independent vasodilation, as well as sweat gland function, are impaired in grafted skin compared with adjacent control skin in response to exogenous administration of the endothelial-dependent vasodilator, acetylcholine, and the endothelial-independent nitric oxide donor, sodium nitroprusside.
METHODS
Human Subjects
Twelve individuals (six men, six women) who had undergone split-thickness autograft application after tangential excision to viable fat within the previous 5 to 9 months participated in this study. Patients with shallow and deep dermal excisions were not enrolled. The mean age, height, and weight of the subjects were 32.7 ± 2.8 years, 168.0 ± 2.0 cm, and 84.8 ± 4.6 kg, respectively (mean ± SEM). Protocols were approved by the Institutional Review Board at the University of Texas Southwestern Medical Center at Dallas and Presbyterian Hospital of Dallas and subjects provided their informed consent. Subjects were not taking any medications that would affect cutaneous vasodilatory or sweating responses. Subjects refrained from caffeine, alcohol, and exercise 24 hours before the study.
Protocol 1: Endothelial-Dependent Vasodilation and Sweating
We inserted two intradermal microdialysis probes, consisting of two reinforced sections of polyimide tubing connected by a 1-cm dialysis membrane (Bio-analytical Systems, West Lafayette, IN), into unanesthetized grafted and adjacent control skin (Table 1) by advancing a 25-gauge needle 15 to 20 mm through the dermal layer, followed by threading the microdialysis probe through the lumen of the needle and withdrawing the needle. Microdialysis probes were perfused with lactated Ringer solution (Baxter, Deer-field, IL) at a rate of 2 µl/min via a perfusion pump (Harvard Apparatus, Holliston, MA) while hyperemia associated with insertion trauma subsided (a minimum of 90 minutes). A specially designed humidity chamber, having a small window (10 × 5 mm, ie, surface area of 0.5 cm2), was placed over each microdialysis probe such that sweating could be assessed directly over the semipermeable portion of each microdialysis membrane.
Table 1.
Location of split-thickness skin graft and skin donor site
| Subject No. |
Sex | Graft Site | Donor Site |
|---|---|---|---|
| 1 | Female | Left forearm | Right thigh |
| 2 | Male | Right forearm | Right calf |
| 3 | Male | Left thigh | Left thigh |
| 4 | Male | Right forearm | Right thigh |
| 5 | Female | Right hand/wrist | Right thigh |
| 6 | Female | Right forearm | Right thigh |
| 7 | Female | Left calf | Right thigh |
| 8 | Female | Left calf | Right thigh |
| 9 | Male | Right forearm | Right thigh |
| 10 | Male | Left forearm | Right thigh |
| 11 | Female | Right arm | Right thigh |
| 12 | Male | Left hand/wrist | Left thigh |
Sweat rate was assessed by the ventilated-capsule method with compressed nitrogen as the perfusion gas delivered at a rate of 300 ml/min. Humidity of the effluent gas was measured via a humidity-temperature probe (model HMP 35E, Vaisala, Woburn, MA) positioned 1 m from the capsule on the skin. The humidity and temperature probe was connected to a humidity data processor (HMT38, Vaisala, Woburn, MA) that calculated absolute humidity from the measurement of relative humidity and temperature. An integrating laser-Doppler flowmetry probe (model PF413, Perimed, Sweden) housed within the sweat chamber permitted simultaneous assessment of skin blood flow and sweat rate from the same location directly over each microdialysis membrane. Throughout the protocol, heart rate was obtained from an electrocardiogram (Agilent, Palo Alto, CA) and arterial blood pressure was measured from the upper arm via electro-sphygmomanometry (SunTech, Raleigh, NC).
At each site, dose–response curves for both skin blood flow and sweating were assessed upon administration of increasing doses of acetylcholine (1 × 10−7 M to 1 × 10−1 M at 10-fold increments). Each dose was administered for 5 min at a perfusion rate of 2 µl/min. Arterial blood pressure was measured during the final minute of each dose.
Protocol 2: Endothelial-Independent Vasodilation
Two intradermal microdialysis probes were inserted into grafted skin and adjacent control skin in a similar manner as described previously. Subjects were instrumented as noted previously with the exception of humidity chambers. An integrating laser Doppler flowmetry probe (model PF413, Perimed, Sweden) was placed over each microdialysis probe such that skin blood flow (model PF4000, Perimed, Sweden) could be assessed directly over the semi-permeable portion of each microdialysis membrane.
At each site, dose–response curves were obtained upon the administration of increasing doses of the endothelial-independent vasodilator sodium nitroprusside (5 × 10−8 M to 5 × 10−2 M at 10-fold increments), with each dose being delivered for 5 min at a perfusion rate of 2 µl/min. Skin blood flow was continuously measured directly above each microdialysis membrane during drug administration. Arterial blood pressure was obtained during the final minute of each dose.
Data and Statistical Analyses
For both protocols, data were continuously acquired at a sampling rate of 50 Hz using a data collection system (Biopac System, Santa Barbara, CA). Skin blood flow is reported in arbitrary units (au), given that the area sampled by these probes is unknown. One-minute-averaged responses were calculated at the end of each dose. Cutaneous vascular conductance (CVC) was calculated from the ratio of laser Doppler-derived skin blood flow to mean arterial blood pressure. CVC data, expressed as a change from baseline (ΔCVC), was mathematically modeled via non-linear regression curve fitting (GraphPad, San Diego, CA). The minimum and maximum ΔCVC at both grafted and adjacent control skin were generated from individual dose response curves for both acetylcholine (Protocol 1) and sodium nitroprusside (Protocol 2). The effective concentration causing 50% of the maximal response (EC50) also was calculated from nonlinear regression modeling. This parameter was used as an index of the drug responsiveness. 3 Mathematical modeling was unable to generate sweating dose response curves because of minimal sweating responses at all doses of acetylcholine in grafted skin.
Student’s paired t-tests were used to compare minimum and maximum ΔCVC responses, as well as EC50, between grafted and adjacent control skin for both protocols. Student’s paired t-tests also were used to compare differences in sweat rate from nor-mothermic baseline between grafted and adjacent control skin at the highest dose of acetylcholine. Statistical significance was accepted at P < .05. All data are presented as mean ± SEM.
RESULTS
Endothelial-Dependent Vasodilation
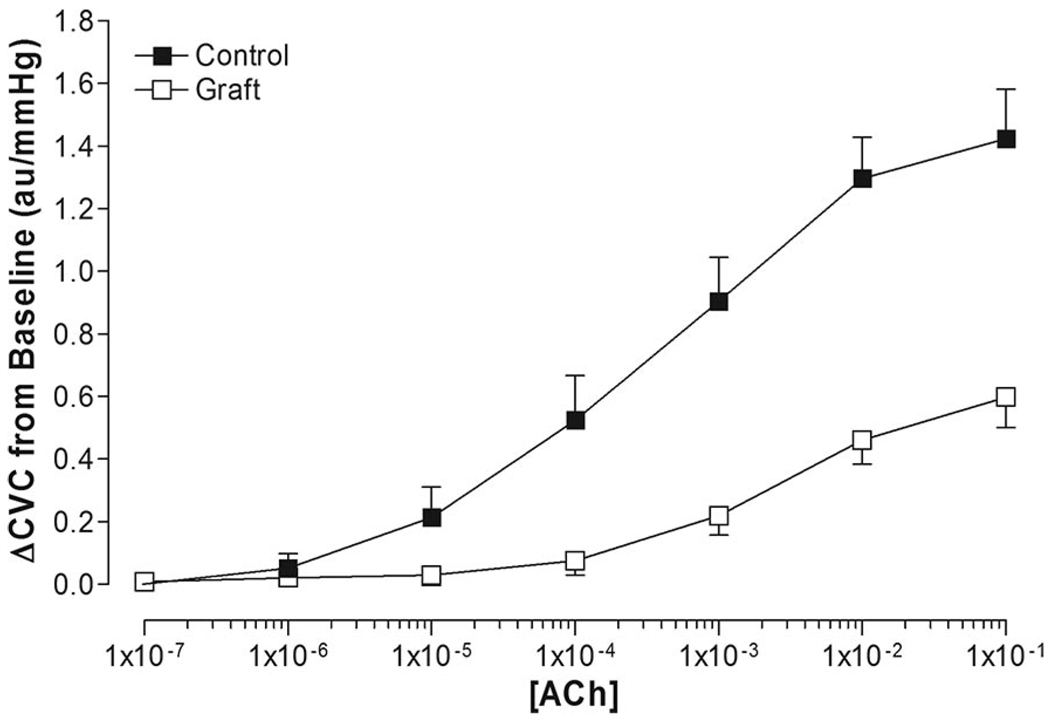
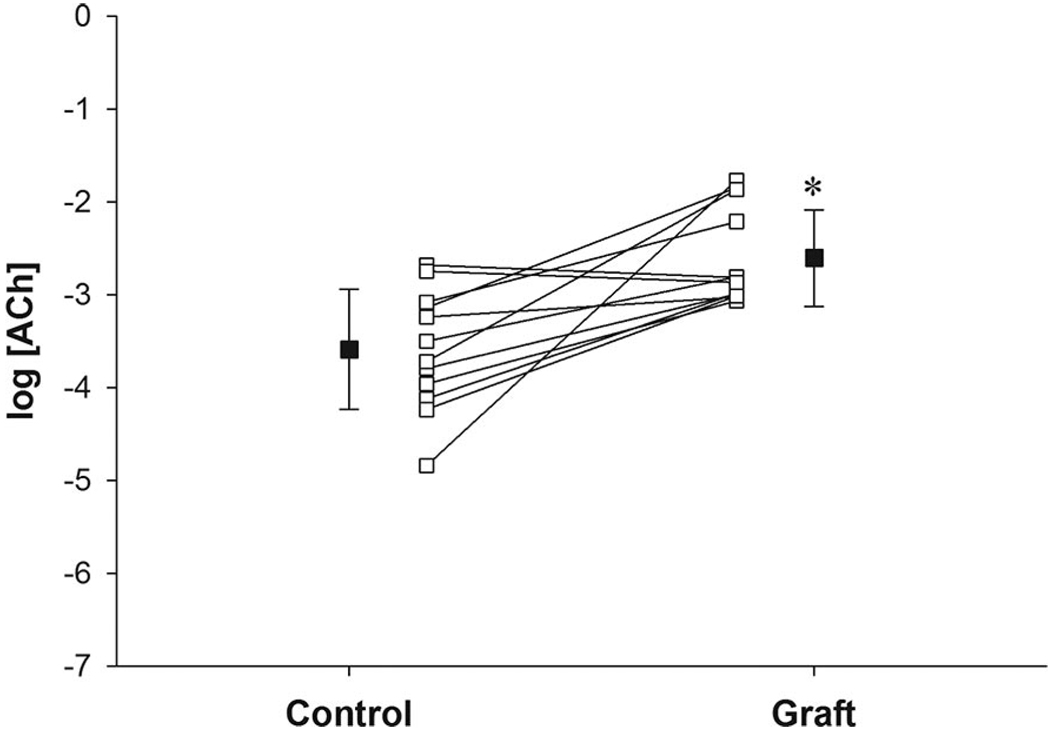
Baseline CVC was similar between the graft site (0.42 ± 0.09 au/mm Hg) and adjacent control skin (0.37 ± 0.09 au/mm Hg; P = .63). Dose–response curve modeling for acetylcholine had high goodness of fit in both control (mean R2 = 0.96 ± 0.01) and grafted skin (mean R2 = 0.88 ± 0.05; Figure 1). Minimum ΔCVC dose–response curve parameters were similar between grafted skin and adjacent control skin (Table 2). Maximum ΔCVC dose–response curve parameters were significantly lower in grafted skin (0.61 ± 0.09 au/mm Hg) compared with adjacent control skin (1.34 ± 0.15 au/mm Hg; P < .001; Table 2). The EC50 was significantly greater in grafted skin (−2.61 ± 0.15 log M) compared with adjacent control skin (−3.59 ± 0.19 log M; P = .003; Table 2 and Figure 2), indicating a rightward shift in the dose-response curve (ie, a higher dose of acetylcholine was needed to cause similar vasodilator responses) in grafted skin (Figure 1).
Figure 1.
Nonmodeled dose–response relationship of cutaneous vascular conductance (ΔCVC from baseline) to acetylcholine (ACh) administration in grafted skin (graft) and adjacent control skin (control).
Table 2.
Mean cutaneous vascular conductance (ΔCVC from baseline) dose–response curve parameters to the exogenous administration of acetylcholine
| Skin Type |
P Value (2-tailed paired t-test) |
||
|---|---|---|---|
| Acetylcholine Response |
Control | Graft | |
| Minimum ΔCVC (au/mm Hg) |
0.04 ± 0.03 | 0.02 ± 0.02 | .499 |
| Maximum ΔCVC (au/mm Hg) |
1.34 ± 0.15 | 0.61 ± 0.09 | <.001 |
| EC50 (log M) | −3.59 ± 0.19 | −2.61 ± 0.15 | .003 |
These values depict the minimum and maximum CVC response, as well as the effective concentration causing 50% of the maximum response (EC50) to increasing concentrations of acetylcholine.
Figure 2.
Mean responses ± SEM (■) and individual responses (□ with lines) for the effective concentration of acetylcholine (ACh) that yields 50% of the maximal response (EC50) in grafted skin (graft) and adjacent control skin (control). *Difference from control (P < .05).
Endothelial-Independent Vasodilation
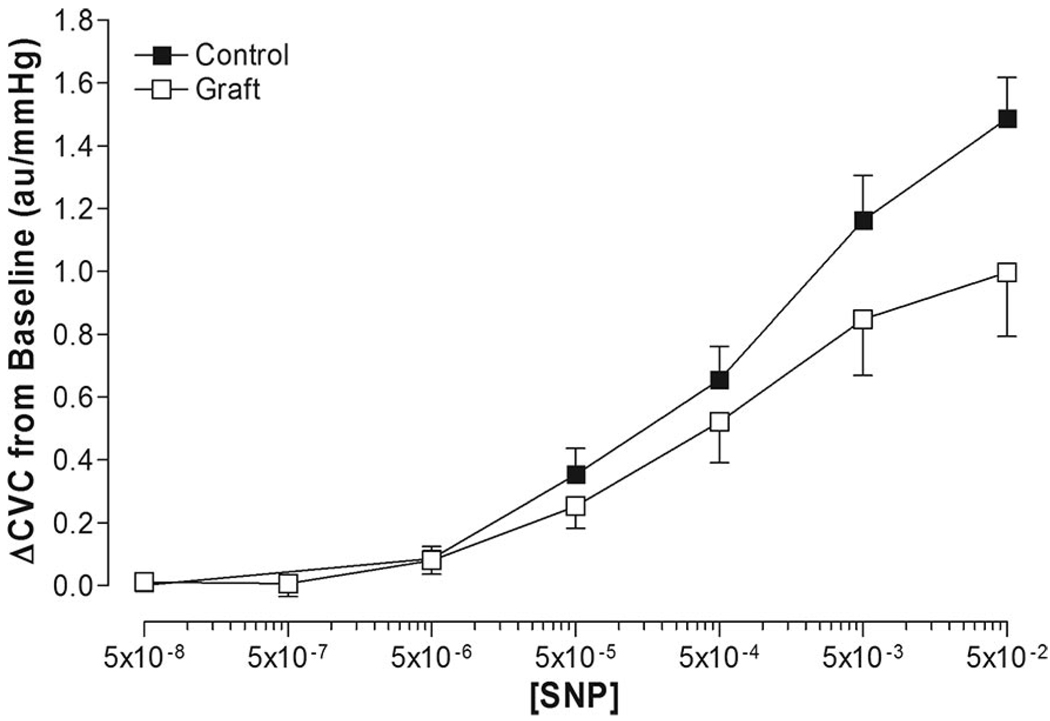
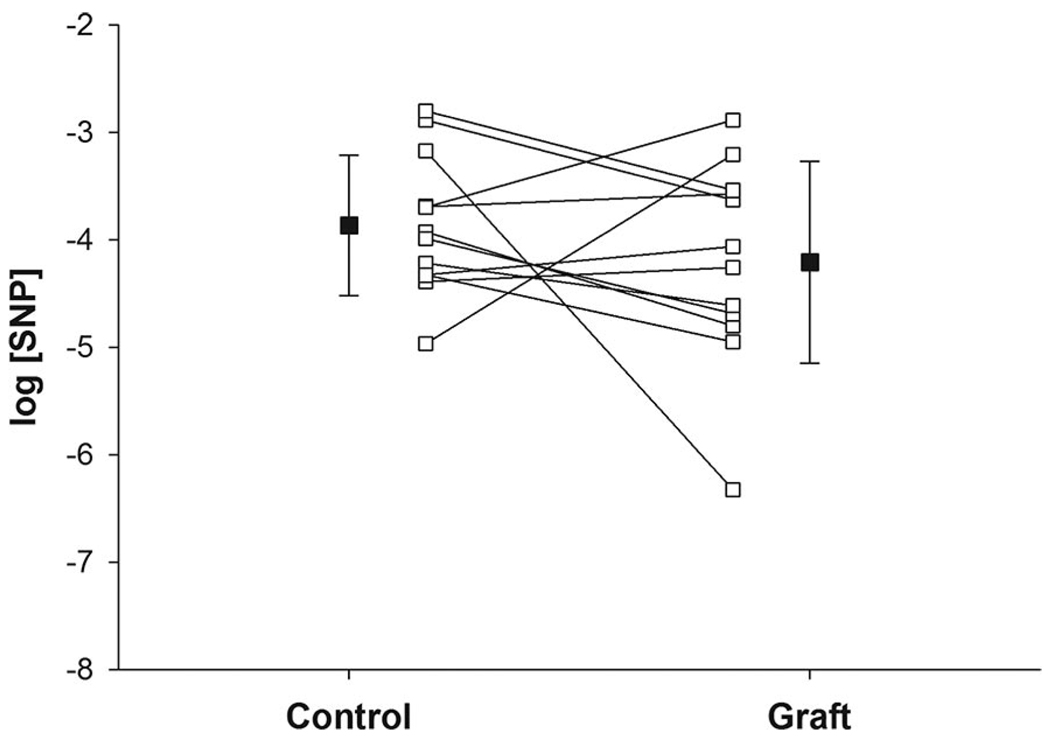
Baseline CVC was significantly greater in grafted skin (0.55 ± 0.09 au/mm Hg) compared with adjacent control skin (0.27 ± 0.04 au/mm Hg; P = .016). Dose–response curve modeling for sodium nitroprusside had high goodness of fit in both control skin (mean R2 = 0.97 ± 0.03) and grafted skin (mean R2 = 0.95 ± 0.06; Figure 3). Minimum ΔCVC dose–response parameters were similar between grafted skin and adjacent control skin (Table 3). Maximal ΔCVC tended to be lower in grafted skin (0.99 ± 0.20 au/mm Hg) compared to adjacent control skin (1.45 ± 0.12 au/mm Hg; P = .098; Table 3). No differences were observed in the EC50 of grafted skin compared to adjacent control skin (Table 3, Figure 4).
Figure 3.
Nonmodeled dose–response relationship of cutaneous vascular conductance (ΔCVC from baseline) to sodium nitroprusside (SNP) administration in grafted skin (graft) and adjacent control skin (control).
Table 3.
Mean cutaneous vascular conductance (ΔCVC from baseline) dose-response curve parameters to the exogenous administration of sodium nitroprusside
| Sodium Nitroprusside Response |
Skin Type |
P Value (2-tailed paired t-test) |
|
|---|---|---|---|
| Control | Graft | ||
| Minimum ΔCVC (au/mm Hg) |
0.05 ± 0.01 | 0.04 ± 0.04 | 0.772 |
| Maximum ΔCVC (au/mm Hg) |
1.45 ± 0.12 | 0.99 ± 0.20 | 0.098 |
| EC50 (log M) | −3.87 ± 0.19 | −4.21 ± 0.27 | 0.332 |
These values depict the minimum and maximum CVC response, as well as the effective concentration causing 50% of the maximum response (EC50) to increasing concentrations of sodium nitroprusside.
Figure 4.
Mean responses ± SEM (■) and individual responses (□ with lines) for the effective concentration of sodium nitroprusside (SNP) that yields 50% of the maximal response (EC50) in grafted skin (graft) and adjacent control skin (control).
Sweat Gland Function
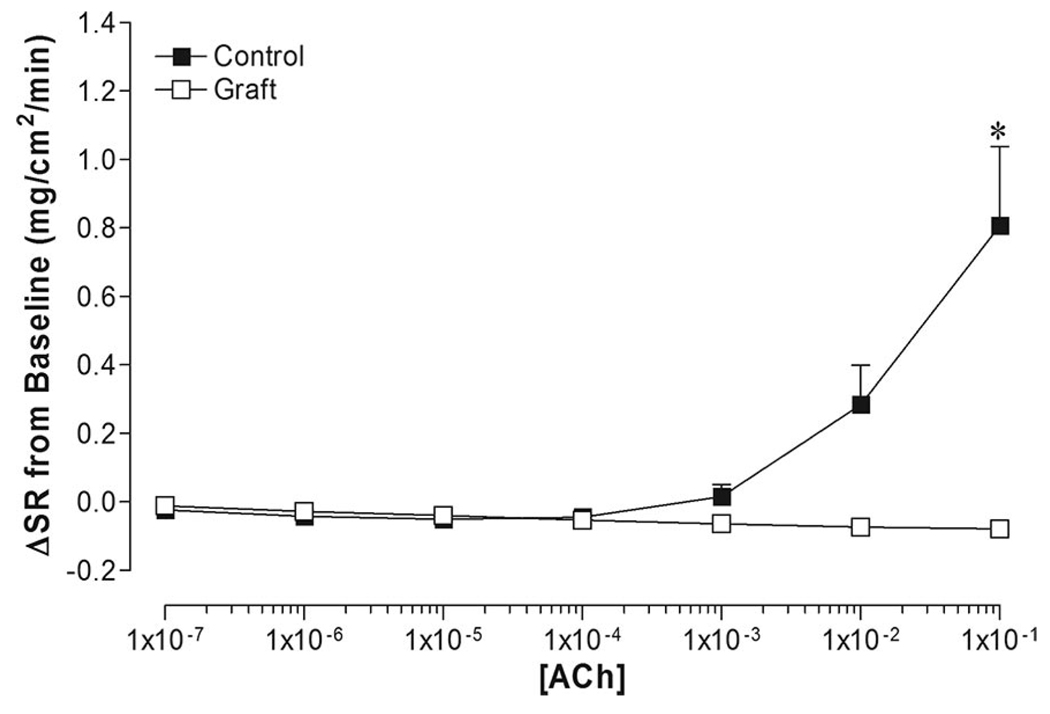
Nonlinear mathematical modeling was unable to generate dose–response curves for sweating due to minimal sweating responses at all doses of acetylcholine in grafted skin. The highest dose of acetylcholine (1 × 10−1 M) elicited a significantly large increase in ΔSR in control skin (0.81 ± 0.23 mg/cm2/min) compared to an absence of sweating in grafted skin (Figure 5).
Figure 5.
Dose–response relationship of sweat rate (ΔSR from baseline) to acetylcholine (ACh) administration in grafted skin (Graft) and adjacent control skin (control). *Difference from control (P < .05).
DISCUSSION
The primary finding of this investigation demonstrates that vasodilator responsiveness to exogenous agents is altered in grafted skin. Endothelial-dependent cutaneous vasodilation, as assessed via the administration of acetylcholine, is reduced in grafted skin 5 to 9 months after surgery compared with adjacent control skin. In addition, the absence of appreciable sweating to the administration of acetylcholine in grafted skin suggests either abnormal or an absence of functional sweat glands in grafted skin. Furthermore, maximal endothelial-independent cutaneous vasodilation to exogenous nitric oxide administration tends (P = .098) to be less in grafted compared with control skin at 5 to 9 months after surgery despite no observed differences in sensitivity (EC50) to nitric oxide. Given these findings, altered postsynaptic function is a possible mechanism for previously observed impairments in vasodilation and sweating in grafted skin during indirect whole-body heating and local heating.2
The active cutaneous vasodilator system mediates 85% to 95% of the increase in skin blood flow in nonglabrous skin during whole-body heating.4,5 Cutaneous active vasodilation is initiated by the release of neurotransmitters from sympathetic cholinergic nerves following increases in internal temperature. Acetylcholine is one of the neurotransmitters released from sympathetic cholinergic nerves and causes endothelial-dependent release of vasoactive factors, including nitric oxide, prostanoids, and endothelial-derived hyperpolarizing factor.6,7 Attenuated increases in CVC, accompanied with an elevated EC50 during acetylcholine administration, indicate an impairment of endothelial-dependent cutaneous vasodilator responses in grafted skin compared with adjacent control skin. The exact mechanism leading to this impaired response has yet to be identified.
Because nitric oxide is released from the endothelium during the administration of acetylcholine, altered nitric oxide-mediated vasodilation could account for impaired endothelial-dependent vasodilation. Nitric oxide is a potent vasodilator in many tissues, including skin. In the current study, the sensitivity to nitric oxide was similar (ie, no differences in EC50 for sodium nitroprusside) between grafted skin and adjacent control skin. However, maximum CVC responses tended to be different between sites (P = .098). These observations indicate that the vascular smooth muscle in grafted skin is responsive to exogenous nitric oxide, but maximal nitric oxide-mediated vasodilation may be altered in grafted skin 5 to 9 months after surgery. This tendency for attenuated maximal vasodilation could explain impaired vasodilation during local heating,2 as cutaneous vasodilation during sustained local heating is primarily nitric oxide dependent.8,9 Impairments in nitric oxide-mediated vasodilation could also partially explain attenuated cutaneous blood flow responses in grafted skin during indirect whole-body heating,2 as approximately 30% of cutaneous vasodilation during whole-body heating is nitric oxide dependent.10,11
The finding of similar vasodilator responses to sodium nitroprusside in grafted skin, coupled with attenuated vasodilator responses to acetylcholine, suggests that the impaired vasodilator responses to acetylcholine could be the result of either altered endothelial-dependent nitric oxide release and/or altered nonnitric oxide dependent vasodilation associated with the administration of acetylcholine. Regarding the latter, vasodilation caused by the exogenous administration of acetylcholine is not solely mediated by nitric oxide dependent pathways but may involve other pathways, including a prostanoid-dependent pathway, as well as a non-nitric oxide/nonprostanoid-dependent (ie, endothelial-derived hyperpolarizing factor) pathway.12–14 Recent findings provide evidence that prostanoids are involved in reflex cutaneous vasodilation during whole body heating.13 Thus, reduced endothelial-dependent vasodilation observed in this investigation and impaired reflex vasodilation during whole-body heat stress observed 5 to 9 months after surgery2 could be the result of disruptions in these non-nitric oxide pathways, including decreased endothelial production and release of vasoactive factors (eg, prostanoids and/or endothelial-derived hyperpolarizing factor) and/or decreased receptor sensitivity to these agents. The involvement of these pathways leading to impaired endothelial-dependent vasodilation in grafted skin warrants further investigation.
Impairments in endothelial-dependent vasodilation observed may only partially explain diminished reflex cutaneous vasodilation observed in grafted skin during whole-body and local heating.2 The primary neurotransmitter responsible for reflex cutaneous vasodilation during whole-body heating, remains unknown, although it is unlikely to be acetylcholine. 5,15–17 This unknown neurotransmitter responsible for active vasodilation has been proposed to be coreleased, presumably with acetylcholine, from sympathetic cholinergic nerves.16 Peptides, including vasoactive intestinal peptide and calcitonin gene-related peptide, also are co-released from sympathetic cholinergic nerves during and may be the neurotransmitters mediating heat stress induced cutaneous vasodilation.18–21 Alterations in the release of these vasoactive neurotransmitters and cofactors from sympathetic cholinergic nerves may play a role in impaired active vasodilation of grafted skin. The cutaneous vasculature also could be less responsive (ie, decreased sensitivity, decreased receptor density) to these neural factors after revascularization of the injured skin. Finally, impaired vasodilation observed in grafted skin during a whole-body heat stress2 may simply be explained by altered innervation of the cutaneous vasculature (ie, decreased number of nerves innervating cutaneous vessels). The relative role of these potential mechanisms in the impairment of vasodilation of grafted skin warrants further investigation.
Postsynaptic sweating responses to the administration of acetylcholine were absent in grafted skin. This finding is consistent with our findings of impaired sweating responses to indirect whole-body heating in grafted skin.2 This observation is also in agreement with previous reports documenting an absence of sweating from split thickness grafts.22–24 However, these previous studies were unable to identify whether the impaired sweating responses was due to nerve disruption or altered postsynaptic responses. The absence of sweating during the exogenous administration of acetylcholine suggests that impaired sweating in this skin is caused by a combination of the initial injury disrupting sweat glands at the recipient tissue, and/or the donor tissue in most split-thickness grafts not containing sweat glands.25,26 However, it is unknown whether sweat glands regenerate as the graft matures.
CONCLUSION
Attenuated endothelial-dependent cutaneous vasodilation and a tendency for attenuated endothelial-independent cutaneous vasodilation suggests altered postsynaptic function possibly contributing to impaired cutaneous vasodilator responses in grafted skin 5 to 9 months after surgery. Furthermore, the absence of appreciable sweating in grafted skin suggests either abnormal or an absence of functional sweat glands in grafted skin. These data suggest that split-thickness skin grafts at 5 to 9 months after surgery have reduced capability of contributing to thermoregulation through endothelial-dependent and perhaps nitric oxide-mediated vasodilator responses. Thus, individuals with split-thickness skin grafts may be at an increased risk of heat-related injury if the grafted area covers a large fraction of the skin surface. The long-term consequences of skin grafting with respect to the neural control of skin blood flow and sweating remains unknown.
ACKNOWLEDGMENTS
We thank Marilee Brown, RN, Obi Chukwumah, MBBS, and Kimberly Williams, RN, for their technical assistance. The considerable time and effort of the participants are greatly appreciated.
This project was supported by National Institute of General Medical Sciences (NIGMS) grant GM68865 (C. G. Crandall). S. L. Davis was supported by an individual National Research Service Award (GM71092).
REFERENCES
- 1.Rowell LB. Human Circulation: Regulation During Physical Stress. New York: Oxford University Press; 1986. [Google Scholar]
- 2.Davis SL, Shibasaki M, Low DA, et al. Impaired cutaneous vasodilation and sweating in grafted skin during whole-body heating. J Burn Care Res. 2007;28:427–434. doi: 10.1097/BCR.0B013E318053D312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson TE, Cui J, Crandall CG. Effect of whole-body and local heating on cutaneous vasoconstrictor responses in humans. Auton Neurosci. 2002;97:122–128. doi: 10.1016/s1566-0702(02)00046-2. [DOI] [PubMed] [Google Scholar]
- 4.Roddie IC. Circulation to skin and adipose tissue. In: Shepherd JT, Abboud FM, editors. Handbook of Physiology; Section 2: The Cardiovascular System. New York: Oxford University Press; 1983. pp. 285–317. [Google Scholar]
- 5.Roddie IC, Shepherd JT, Whelan RF. The contribution of constrictor and dilator nerves to the skin vasodilation during body heating. J Physiol (Lond) 1957;136:489–497. doi: 10.1113/jphysiol.1957.sp005775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 7.Rubanyi GM. Endothelium-derived relaxing and contracting factors. J Cell Biochem. 1991;46:27–36. doi: 10.1002/jcb.240460106. [DOI] [PubMed] [Google Scholar]
- 8.Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol. 2001;91:1619–1626. doi: 10.1152/jappl.2001.91.4.1619. [DOI] [PubMed] [Google Scholar]
- 9.Kellogg DL, Jr, Liu Y, Kosiba IF, et al. Role of nitric oxide in the vascular effects of local warming of the skin in humans. J Appl Physiol. 1999;86:1185–1190. doi: 10.1152/jappl.1999.86.4.1185. [DOI] [PubMed] [Google Scholar]
- 10.Kellogg DL, Jr, Crandall CG, Liu Y, et al. Nitric oxide and cutaneous active vasodilation during heat stress in humans. J Appl Physiol. 1998;85:824–829. doi: 10.1152/jappl.1998.85.3.824. [DOI] [PubMed] [Google Scholar]
- 11.Shastry S, Dietz NM, Halliwill JR, et al. Effects of nitric oxide synthase inhibition on cutaneous vasodilation during body heating in humans. J Appl Physiol. 1998;85:830–834. doi: 10.1152/jappl.1998.85.3.830. [DOI] [PubMed] [Google Scholar]
- 12.Boutsiouki P, Georgiou S, Clough GF. Recovery of nitric oxide from acetylcholine-mediated vasodilatation in human skin in vivo. Microcirculation. 2004;11:249–259. doi: 10.1080/10739680490425958. [DOI] [PubMed] [Google Scholar]
- 13.Holowatz LA, Thompson CS, Minson CT, et al. Mechanisms of acetylcholine-mediated vasodilatation in young and aged human skin. J Physiol (Lond) 2005;563:965–973. doi: 10.1113/jphysiol.2004.080952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kellogg DL, Jr, Zhao JL, Coey U, et al. Acetylcholine-induced vasodilation is mediated by nitric oxide and prostaglandins in human skin. J Appl Physiol. 2005;98:629–632. doi: 10.1152/japplphysiol.00728.2004. [DOI] [PubMed] [Google Scholar]
- 15.Kolka MA, Stephenson LA. Heat exchange through cutaneous vasodilation after atropine treatment in a cool environment. Aviat Space Environ Med. 1989;60:29–33. [PubMed] [Google Scholar]
- 16.Kellogg DL, Jr, Pergola PE, Piest KL, et al. Cutaneous active vasodilation in humans is mediated by cholinergic nerve co-transmission. Circ Res. 1995;77:1222–1228. doi: 10.1161/01.res.77.6.1222. [DOI] [PubMed] [Google Scholar]
- 17.Kolka MA, Stephenson LA. Cutaneous blood flow and local sweating after systemic atropine administration. Pflugers Arch. 1987;410:524–529. doi: 10.1007/BF00586536. [DOI] [PubMed] [Google Scholar]
- 18.Wilkins BW, Chung LH, Tublitz NJ, et al. Mechanisms of vasoactive intestinal peptide-mediated vasodilation in human skin. J Appl Physiol. 2004;97:1291–1298. doi: 10.1152/japplphysiol.00366.2004. [DOI] [PubMed] [Google Scholar]
- 19.Bennett LA, Johnson JM, Stephens DP, et al. Evidence for a role for vasoactive intestinal peptide in active vasodilatation in the cutaneous vasculature of humans. J Physiol (Lond) 2003;552:223–232. doi: 10.1113/jphysiol.2003.042135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Savage MV, Brengelmann GL, Buchan AM, et al. Cystic fibrosis, vasoactive intestinal polypeptide, and active cutaneous vasodilation. J Appl Physiol. 1990;69:2149–2154. doi: 10.1152/jappl.1990.69.6.2149. [DOI] [PubMed] [Google Scholar]
- 21.Wong BJ, Wilkins BW, Minson CT. H1 but not H2 histamine receptor activation contributes to the rise in skin blood flow during whole body heating in humans. J Physiol (Lond) 2004;560:941–948. doi: 10.1113/jphysiol.2004.071779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ponten B. Grafted skin. Acta Chir Scand Suppl. 1960;257:1–78. [PubMed] [Google Scholar]
- 23.Shapiro Y, Epstein Y, Ben-Simchon C, et al. Thermoregulatory responses of patients with extensive healed burns. J Appl Physiol. 1982;53:1019–1022. doi: 10.1152/jappl.1982.53.4.1019. [DOI] [PubMed] [Google Scholar]
- 24.McGibbon B, Beaumont WV, Strand J, et al. Thermal regulation in patients after the healing of large deep burns. Plast Reconstr Surg. 1973;52:164–170. doi: 10.1097/00006534-197308000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Ablove RH, Howell RM. The physiology and technique of skin grafting. Hand Clin. 1997;13:163–173. [PubMed] [Google Scholar]
- 26.Nedelec B, Hou Q, Sohbi I, et al. Sensory perception and neuroanatomical structures in normal and grafted skin of burn survivors. Burns. 2005;31:817–830. doi: 10.1016/j.burns.2005.06.007. [DOI] [PubMed] [Google Scholar]