Abstract
Eukaryotic translation initiation factor 4E (eIF4E) is a rate-limiting factor for cap-dependent protein synthesis and is regulated by PI3 kinase/mTOR and mitogen-activated protein kinase (MAPK)/Mnk signaling pathways. Recent studies have shown that Mnk-mediated eIF4E phosphorylation is absolutely required for eIF4E’s oncogenic function. Overexpression of eIF4E has been reported in many types of cancers; however, the expression of phosphorylated eIF4E (p-eIF4E) in human cancer tissues, particularly solid tumor tissues, has not been reported. The current study focused on evaluating p-eIF4E expression patterns in the tumor tissues obtained from patients with a variety of malignancies. Using three different tissue microarrays consisting of a total of 380 cases of human cancers and 146 cases of adjacent normal tissues, we detected p-eIF4E positive staining in 63.4% (241/380) of cancers, but only in 30.1% (44/146) of adjacent normal tissues. Thus, p-eIF4E expression is significantly higher in cancers than in adjacent normal tissues (P < 0.001). In general, there was no major difference in p-eIF4E staining between cancers with and without lymph node metastasis. In certain types of maligancies such as lung, gastric and colorectal cancers, p-eIF4E staining was significantly higher in the early stage (T1) than in the late stage (T3) disease (P < 0.05). Collectively, these findings suggest that p-eIF4E may play a critical role in cancer development, particularly early stages of tumorigenesis and support p-eIF4E as a good cancer therapeutic target.
Keywords: eIF4E, phosphorylation, cancer
Introduction
The eukaryotic translation initiation factor 4E (eIF4E) plays a pivotal role in the initiation of cap-dependent translation on cellular mRNAs. eIF4E is the cap-binding protein component of the eIF4F complex, which includes the RNA helicase eIF4A and the scaffolding protein eIF4G. Binding of eIF4E to the 7-methylguanosine (m7GppN) cap structure on the 5′ untranslated region (5′-UTR) of cellular mRNAs recruits the eIF4F complex to the mRNA. As a consequence, the eIF4F complex scans from the 5′ cap through the 5′-UTR, unwinds the secondary structure to reveal the translation initiation codon, enable ribosome loading and facilitates final protein translation 1, 2.
Because eIF4E is the least abundant among these initiation factors involved in eIF4F complex, eIF4E is the rate-limiting factor for cap-dependent translation initiation 2. Consequently, changes in eIF4E levels profoundly affect translation rates of oncogenic proteins such as c-Myc, VEGF, ODC, cyclin D1, HIF-1 and Mcl-1. These proteins are translationally repressed under physiological conditions, but are activated in the milieu of cancer. eIF4E expression is frequently elevated in many types of cancers and is associated with malignant progression. Inhibition of eIF4E effectively suppresses cellular transformation, tumor growth, invasiveness and metastasis 3, 4.
eIF4E is regulated by the PI3 kinase/mTOR and mitogen-activated protein kinase (MAPK)/Mnk signaling and may act as a convergence point of these pathways. The former enhances eIF4E activity via release from the 4E-BPs 1, 2, 5, whereas the latter can increase eIF4E phosphorylation (usually at Ser209) via Mnk1/2 5. In human cancers, the association between increased eIF4E expression and malignant transformation has been well documented in multiple cancer types 1, 3. In experimental cancer models, forced eIF4E overexpression in cultured fibroblasts or epithelial cells can induce cellular transformation and tumorigenesis, likely by selective increase in the synthesis of proteins necessary growth, angiogenesis, and survival factors 6. For example, ectopic expression of eIF4E in human mammary epithelial cells enables clonal expansion and anchorage-independent growth 7. In a mouse lymphoma model, it has been shown that eIF4E can recapitulate Akt action in oncogenesis and apoptotic resistance and is sufficient to confer resistance to a rapamycin-based therapy in vivo 8. Moreover, in transgenic mice, ectopic eIF4E expression increases the incidence of multiple cancers, including lymphomas, lung adenocarcinomas, hepatomas, and angiosarcomas, along with accelerated lymphomagenesis 9. eIF4E overexpression can also facilitatethe establishment of autocrine stimulatory loops, suppress apoptosis, and impart chemo- and radio-resistance, which are phenotypic alterations integral to malignant progression 1, 6.
The biological function of eIF4E phosphporylation in regulation of translation initiation is controversial. However, it has been suggested that phosphorylation of eIF4E increases its affinity for the cap of mRNA, and may also favor its entry into initiation complexes 5, 10, 11. It is possible that eIF4E phosphorylation vitally impacts cell transformation since p38 and ERK MAPKs, which regulate eIF4E phosphorylation through Mnks, are frequently activated in transformed cells or tissues. Indeed, it has been shown that overexpression of a mutant of eIF4E in which Ser209 has been altered to alanine is much less efficient than wild-type eIF4E in transforming NIH3T3 cells. In addition, the overexpression of wild-type, but not mutant eIF4E, increases cyclin D1 levels 12. Importantly, a recent study using a mouse lymphoma model has convincingly demonstrated that eIF4E phosphorylation at Ser209 by Mnks is absolutely required for eIF4E’s ability to inhibit apoptosis and promote tumorigenesis 13.
Although eIF4E overexpression in various types of cancers has been documented, the expression patterns of p-eIF4E in different human cancers, particularly solid tumors, have not been reported. Thus, the current study focused on detection of p-eIF4E expression patterns in different types of cancers including lung, head and neck, gastric and colorectal cancers as well other types of cancers. In addition, we analyzed the relationship between p-eIF4E and histology, disease stages, pathological grades and presence or absence of lymph node metastasis. We found that p-eIF4E expression was overall significantly higher in human cancers than their adjacent normal tissues, thus supporting the critical role of p-eIF4E in cancer development.
Materials and Methods
Tissue Microarrays (TMA)
In this study, we used three types of TMA. Human lung cancer TMA consisting of 40 cases of stage I-III lung cancer tissues, 10 cases of metastatic cancer tissues from the primary lung cancer, and 9 cases of adjacent normal human lung tissues was purchased from Imgenex (IMH-358; SanDiego, CA). Human head and neck TMA [AccuMax Array; A219 (II)) consisting of 28 cases of head and neck squamous cell carcinoma (HNSCC) tissues, 17 cases of other head and neck cancer tissues, and 8 cases of corresponding non-neoplastic tissues was purchased from Accurate Chemical & Scientific Corp (Westbury, NY). Multi-tumor TMA containing 289 cases of various malignant tumor and 129 cases of normal tissues (i.e., adjacent non-neoplastic tissues) was constructed by Cancer Research Institute, Xiangya School of Medicine, Central South University (Changsha, Hunan, China) and was a kind gift from Professor Guiyuan Li (Central South University, Hunan, China). The cancer types and tissue numbers are described in table 1.
Table 1.
Summary of the tissue types and sample number in the three tissue arrays
| Organ Site | Tumor (n) | Adjacent non-neoplastic tissue (n) | Note |
|---|---|---|---|
| Lung | 104 | 44 | SCC* (41), AC (37), LCC (6), AC-SCC (5), adenoid cystic carcinoma (3), mucoepidermoid cancer (3), bronchioloalveolar carcinoma (4), and SCLC (5). |
| Colorectal | 60 | 28 | |
| Head and neck | 49 | 22 | SCC |
| Breast | 29 | 3 | |
| Stomach | 28 | 12 | |
| Kidney and urethra | 21 | 7 | Kidney cancer (5), wilms tumor (2), urethral transitional cell carcinoma (14) |
| Liver | 19 | 14 | |
| Female genital organ | 18 | 4 | Ovary cancer (6), uterus endometrial cancer (4), cervical cancer (7), choriocarcinoma (1) |
| Soft tissue | 18 | 2 | Osteosarcoma (2), chondrosarcoma (3), malignant histological cell tumor (2), mesothelioma (1), leiomyosarcoma (2), lymphoma (3), fibrosarcoma (2), liposarcoma (1), undifferentiated sarcinoma (2). |
| Thyroid and parathyroid gland | 14 | 4 | Thyroid cancer (12), parathyroid cancer (2) |
| Skin | 7 | 3 | |
| Brain | 7 | 2 | Astrocytoma (5), meningioma (2) |
| Salivary gland | 6 | 1 | |
| Total | 380 | 146 |
SCC, squamous cell carcinoma; AC, adenocarcinoma; LCC, large cell carcinoma; AC-SCC, adeno-squamous cell carcinoma; SCLC, small cell lung cancer.
Imunohistochemistry (IHC) and Scores
The TMAs were stained with IHC using the EnVision™ + Dual Link System-HRP Kit (Dako; Carpinteria, CA). The rabbit monoclonal antibody against p-eIF4E (Ser209) was purchased from Epitomics, Inc. (Burlingame, CA) and used at 1:500 dilutions. The specificity of the antibody was determined with matched IgG isotype antibody as a negative control in IHC. Moreover, a single band with correct molecular weight in Western blotting was assured. p-eIF4E staining was scored as negative (< 10% staining) and positive staining (≥ 10% staining), respectively.
Statistical Analysis
Pearson’s chi-square test was used to compare the difference of p-eIF4E positive staining rates among the groups. Fisher’s exact test was used for small sample sizes which have less that five observations. The difference was considered statistically significant when P value was < 0.05. SAS 9.0 was used for the analysis.
Results
p-eIF4E Expression is Elevated in Tumors Compared to Adjacent Normal Tissues
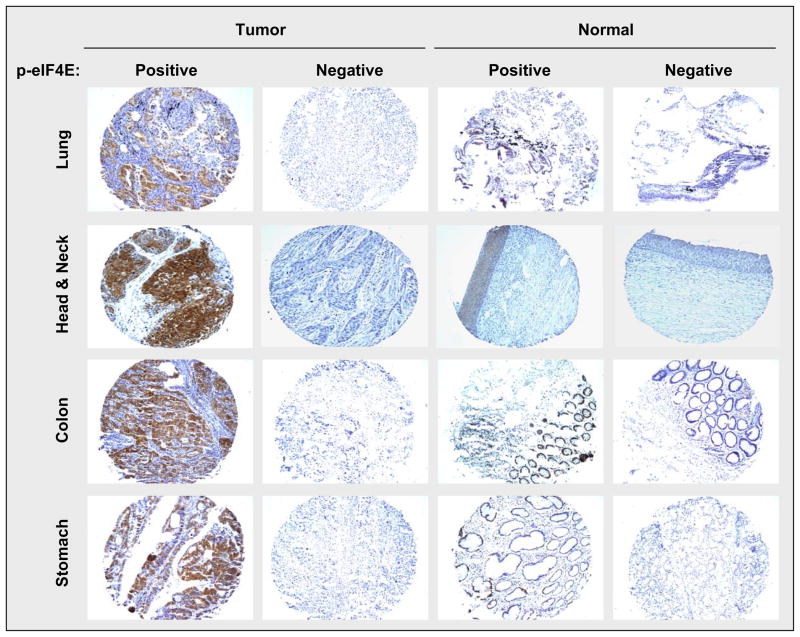
The three TMAs used in this study contained tissues from 17 types of human cancers (Table 1). Among them, lung, head and neck, colorectal, and gastric cancers had a relatively large number of cancer tissues and adjacent normal tissues. Accordingly, we detected significant higher expression of p-eIF4E in these cancer tissues than their corresponding adjacent normal tissues (Table 2) (Fig. 1). Specifically, the positive staining rates for p-eIF4E in lung, colorectal, gastric and head and neck cancers were 72.1% (75/104), 61.7% (37/60), 50% (14/28) and 81.6% (40/49), respectively; however, p-eIF4E positive staining rates in their corresponding normal tissues were only 22.7% (10/40), 28.6% (8/28), 16.7% (2/12) and 30.8% (4/13), respectively. Interestingly, p-eIF4E expression seemed lower in hepatocellular cancer (HCC) than in the above types of cancers. p-IF4E positivity was noted in 31.6% (6/19) of HCC compared to 21.4% (3/14) of adjacent normal tissues. Thus, the difference in p-eIF4E expression between HCC and adjacent normal tissues is not significant (P = 0.698). In the remainder of the solid tumors, the differences in expression of p-eIF4E between tumor and surrounding normal tissues was not conclusive due to smaller sample size of each individual tumor type. Nonetheless, the overall p-eIF4E positivity rate in all types of tumors (63.4%; 241/380) was significantly higher than that in the adjacent normal tissues (30.1%; 44/146) (P < 0.001).
Table 2.
Comparison of p-eIF4E expression between the tumors and adjacent normal tissues
| Tissue type | p-eIF4E positive %) (Positive/total case) |
P value# |
|---|---|---|
| Lung | ||
| Cancer | 72.1% (75/104) | < 0.001 |
| Normal | 22.7% (10/44) | |
| Colorectal | ||
| Cancer | 61.7% (37/60) | 0.004 |
| Normal | 28.6% (8/28) | |
| Stomach | ||
| Cancer | 50% (14/28) | 0.079 |
| Normal | 16.7% (2/12) | |
| Liver | ||
| Cancer | 31.6% (6/19) | 0.698 |
| Normal | 21.4% (3/14) | |
| Kidney | ||
| Cancer | 20% (1/5) | 0.416 |
| Normal | 50% (3/6) | |
| Wilms’ tumor | 0% (0/2) | |
| Urethra | ||
| Cancer | 85.8% (12/14) | 1.000 |
| Normal | 100 (1/1) | |
| Breast | ||
| Cancer | 75.9% (22/29) | 0.184 |
| Normal | 33.3% (1/3) | |
| Ovary | ||
| Cancer | 66.7% (5/6) | |
| Normal (N/A) | ||
| Cervical | ||
| Cancer | 85.7% (6/7) | 0.183 |
| Normal | 33.3% (1/3) | |
| Uterus endometrium | ||
| Endometrial Cancer | 75% (3/4) | 0.600 |
| Normal | 100% (1/1) | |
| Choriocarcinoma | 0% (0/1) | |
| Head and neck | ||
| HNSCC | 81.6% (40/49) | 0.002 |
| Normal SE* | 30.8 (4/13) | |
| AHSE | 77.8% (7/9) | |
| Salivary gland | ||
| Cancer | 33.3% (2/6) | 1.000 |
| Normal | 0% (0/1) | |
| Thyroid/parathyroid gland | ||
| Thyroid cancer | 41.7% (5/12) | 1.000 |
| Parathyroid cancer | 50% (1/2) | |
| Normal thyroid | 50% (2/4) | |
| Skin | ||
| Cancer | 42.9% (3/7) | 1.000 |
| Normal SE | 33.3% (1/3) | |
| Brain | ||
| Astrocytoma | 20% (1/5) | 1.000 |
| Meningioma | 0% (0/2) | |
| Normal brain tissue | 0% (0/2) | |
| Soft tissue | ||
| Soft tissue sarcoma | 44.4% (8/18) | 0.495 |
| Normal tissue | 0% (0/2) | |
| Total | ||
| All tumors | 63.4% (241/380) | < 0.001 |
| All normal tissues | 30.1% (44/146) | |
SE, squamous epithelium; AHSE: atypical hyperplasia squamous epithelium.
Comparison between tumors and normal tissues
Figure 1. Typical p-eIF4E expression in the representative cancer and normal tissues.
p-eIF4E was stained with IHC using a rabbit monoclonal p-eIF4E antibody at 1:500 dilutions.
In this study, human non-small cell lung carcinomas (NSCLC) was the most frequent tumor type that was studied (N = 99) (Table 1). Among the lung cancer cases, there were 41 cases of squamous cell carcinoma (SCC), 37 cases of adenocarcinoma (AC), 6 cases of larger cell carcinoma (LCC), 5 cases of adeno-squamous cancer (AC-SCC), and 10 cases of adenoid cystic carcinoma, mucoepidermoid cancer and bronchioloalveolar carcinoma (Table 3). p-eIF4E positivity in both SCC (75.6%; 31/41) and AC (81.1%; 30/37) were higher than in other types of NSCLC cancers (57.2%; 12/21) (P = 0.05 between AC and other cancers) (Table 3).
Table 3.
Correlation between p-eIF4E expression and clinical/pathologic features of NSCLC, HNSCC, colorectal cancer and gastric cancer
| p-eIF4E positive (%) (Positive/total cases) |
||
|---|---|---|
| NSCLC | ||
| Histology | SCC | 75.6% (31/41) |
| AC | 81.1% (30/37) a | |
| Others | 57.2% (12/21) | |
| Stage (SCC and AC only) | T1 | 86.5 %(32/37) |
| T2 | 62.4% (14/17) | |
| T3 | 62.5% (15/24)b | |
| Grade (SCC and AC only) | G1/G2 | 86% (43/50) |
| G3 | 64.3% (18/28)c | |
| LNM (SCC and AC only) | Yes | 74.4% (29/39) |
| No | 82.1% (32/39) | |
| Tumor site | Primary | 60% (6/10) |
| Metastatic | 80% (8/10) | |
| HNSCC | ||
| Stage | T1/T2 | 92.6% (25/27) |
| T3/T4 | 68.2% (15/22)d | |
| Grade | G1 | 80% (12/15) |
| G2 | 80.8% (21/26) | |
| G3 | 87.5% (7/8) | |
| LNM | Yes | 75% (15/20) |
| No | 86.2% (25/29) | |
| Colorectal cancer | ||
| Stage | T1 | 84.6% (11/13) |
| T2 | 59.3% (19/32) | |
| T3 | 46.7% (7/15)e | |
| Grade | G1/G2 | 63.6% (28/44) |
| G3 | 56.3% (9/16) | |
| LNM | Yes | 60.0% (18/30) |
| No | 63.3 % (19/30) | |
| Gastric cancer | ||
| Stage | T1/T2 | 76.9% (10/13) |
| T3 | 26.7% (4/15)f | |
| Grade | G1/G2 | 87.5% (7/8) |
| G3/G4 | 35% (7/20)g | |
| LNM | Yes | 56.5% (13/23) |
| No | 20% (1/5) | |
P = 0.05 compared with other NSCLC cancers;
P = 0.03 compared with T1 NSCLC;
P = 0.026 compared with G1/G2 NSCLC;
P = 0.028 compared with T1/T2 HNSCC;
P = 0.037 compared with T1 colorectal cancer;
P = 0.021 compared with T1/T2 Gastric cancer;
P = 0.033 compared with G1/G2 gastric cancer
The Correlation between p-eIF4E Expression and Cancer Stages or pathological Grades is Cancer Type-dependent
In certain types of cancers, we noted that p-eIF4E expression was reduced in the late clinical stages and/or higher pathological grades. In NSCLCs (AC and SCC only), p-eIF4E expression rate in the stage T3 disease (62.5%; 15/24) was significantly lower than that in the stage T1 (86.5%; 32/37) (P = 0.03). Similarly, the poorly differentiated grade 3 tumors also exhibited reduced p-eIF4E expression compared to G1 and G2 tumors (P = 0.026) (Table 3). In HNSCC, p-eIF4E expression in T3/T4 tumors (68.2%; 15/22) was significantly reduced compared with that in T1/T2 tissues (92.6%; 25/27) (P = 0.028). However, no significant differences were found among the pathological grades (Table 3). In colorectal cancer, p-eIF4E expression between T1 (84.6%; 11/13) and T3 (46.7%; 7/15) was statistically significant (P = 0.037) (Table 3). In gastric cancer, p-eIF4E staining was significantly reduced in T3 (76.9%; 10/13) compared to T1 and T2 (26.7%; 4/15) (P = 0.021). This was also true in the pathological grades [87.5% (7/8) in G1/G2 vs. 35% (7/20) in G3/G4] (P =0.033) (Table 3). In thyroid cancer, we noted a similar trend in p-eIF4E expression between T1 and T2 although the difference was not statistically significant, which is likely due to the limited number of cases (Table 4). In the rest types of cancers, p-eIF4E expression was not correlated with clinical stages or pathological grades (Table 4). Collectively, these results suggest that p-eIF4E may play an important role in the earlier stages of certain types of cancer.
Table 4.
Correlation between p-eIF4E expression and clinical/pathologic features of other cancers
| p-eIF4E positive (%) (Positive/total cases) |
P value | ||
|---|---|---|---|
| Hepatic cell carcinoma | |||
| Stage | T2 | 11.1% (1/9) | 0.141 |
| T3/T4 | 50% (5/10) | ||
| Grade | G2 | 20% (2/10) | 0.350 |
| G3 | 44.4% (4/9) | ||
| Breast cancer | |||
| Stage | T1 | 83.3% (5/6) | 1.000 |
| T2 | 70.6% (12/17) | ||
| T3 | 83.3% (5/6) | ||
| LNM | Yes | 70.6% (12/17) | 0.665 |
| No | 83.3% (10/12) | ||
| Urethral carcinoma | |||
| Stage | T2 | 100% (5/5) | 0.506 |
| T2/T3 | 77.8% (7/9) | ||
| Grade | G2 | 87.5% (7/8) | 1.000 |
| G3 | 83.3% (5/6) | ||
| Female genital organ | |||
| cancers | |||
| Stage | T1/T2 | 62.5% (5/8) | 0.608 |
| T3 | 80% (8/10) | ||
| Grade | G2 | 66.7% (8/12) | 0.615 |
| G3 | 83.3% (5/6) | ||
| LNM | Yes | 66.7% (4/6) | 1.000 |
| No | 75% (9/12) | ||
| Thyroid cancer | |||
| Stage | T1 | 66.7% (4/6) | 0.242 |
| T2 | 16.7% (1/6) | ||
| LNM | Yes | 66.7% (2/3) | 1.000 |
| No | 44.4% (4/9) | ||
p-eIF4E Expression Does Not Impact Lymph Node Involvement
We further compared p-eIF4E staining patterns between tumors from patients without and with lymph node metastasis (LNM). In NSCLC, HNSCC, colorectal cancer, gastric cancer, breast cancer, female genital organ cancers and thyroid cancer, there were no significant differences in p-eIF4E expression patterns between the two groups of tumors (Tables 3 and 4). In NSCLC, we had 10 cases of primary tumors and 10 cases of metastatic tumors. p-eIF4E staining was slightly higher in the metastatic tumors (80%; 8/10) than in primary tumors (60%; 6/10); however the difference was not statistically significant (P = 0.329) (Table 3).
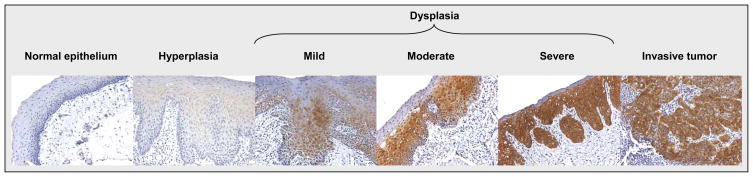
Increased eIF4E Phosphorylation Occurs in Premalignant Lesions of HNSCC
While we detected p-eIF4E in human HNSCC tissues, we noted that p-eIF4E was negative in normal head and neck squamous epithelium, but positive in some benign epithelial hyperplasia albeit with relatively weak staining. In some dysplastic epithelium, there was strong expression of p-eIF4E. Furthermore, in some cases of severe dysplasia, eIF4E phosphorylation was strongly positive comparable to that in the majority of invasive HNSCC tissues (Fig. 2). Overall, we detected 77.8% (7/9) positivity rate for p-eIF4E expression in the atypical hyperplasia squamous epitheliums, which was significantly higher than the 30.8% (4/13) cases positive for p-eIF4E in the normal squamous epitheliums (P = 0.03) (Table 2). These findings suggest that eIF4E phosphorylation may represent an early event and is involved in head and neck carcinogenesis.
Figure 2. Detection of p-eIF4E expression in early stages of head and neck carcinogenesis.
p-eIF4E was stained with IHC using a rabbit monoclonal p-eIF4E antibody at 1:500 dilutions. The pictures were taken at 200× magnitude.
Discussion
eIF4E has emerged as a therapeutic target for cancer, based on its expression in a variety of malignancies and the increasing knowledge of its oncogenic functions 6, 14. Recent preclinical studies have shown that eIF4E phosphorylation at Ser 209 is critical for its tumorigenic activity 12, 13. Therefore, evaluation of the expression of p-eIF4E could provide more accurate information regarding the role of this pathway in human cancer. The expression of phosphorylated eIF4E has not been studied in solid organ malignancies. In diffuse large B-cells lymphoma and Burkitt lymphoma, p-eIF4E was reported to be positive in 13/77 (16.9%) and in 6/8 (75%) cases, respectively 13. For the present study, we utilized TMAs consisting of total 380 tumors from 17 types of cancers and found an overall 63.4% (241/380) positivity rate for p-eIF4E. Moreover, we detected p-eIF4E in 146 cases of adjacent normal tissues and found an overall 30.1% (44/146) of p-eIF4E positive cases. p-eIF4E expression was significantly increased in cancers than in normal tissues (P < 0.001). To the best of our knowledge, this is the first study demonstrating p-eIF4E expression patterns in different types of cancers.
In experimental cancer models, enforced eIF4E overexpression in cultured fibroblasts or epithelial cells can induce cellular transformation 6. Moreover, ectopic eIF4E expression in transgenic mice increases the incidence of multiple cancers, including lymphomas, lung adenocarcinomas, hepatomas, and angiosarcomas, and accelerates lymphomagenesis 8, 9. In agreement, recent studies have shown that p-eIF4E is very critical for its transformation and tumorigenesis activity 12, 13. In our study, we found that p-eIF4E expression was significantly higher in the early stages of disease (e.g., T1) than in the advanced stages (e.g., T3) in certain types of cancers (e.g., colorectal and gastric cancers). Thus, it seems that p-eIF4E may play a more important role in the earlier stages of malignant transformation than in the late stage of these types of cancers. In HNSCC, we noted that p-eIF4E staining started to be positive in some benign epithelial hyperplasia albeit with relatively weak staining and became even stronger in some cases of severe dysplasia (Fig. 2). The positive rate of p-eIF4E in the atypical hyperplasia squamous epitheliums was as high as in HNSCC (Table 2). These results suggest that p-eIF4E may play a critical role in the early stage of head and neck carcinogenesis, supporting the role of p-eIF4E in promoting cell transformation. This finding suggests a role for inhibition of p-eIF4E for chemoprevention of patients at high risk for developing head and neck cancers.
In this study, we noted that p-eIF4E expression patterns were not significantly different between tumors from patients with and without LNM, suggesting that p-eIF4E expression does not predict LNM. In the NSCLC tumor tissues from primary and corresponding metastatic sites (e.g., lymph node and bone), we noted that p-eIF4E in the metastatic tissues had an increased trend compared with that in the primary tumors (80% vs. 60%). %). We intend to evaluate this further in a larger sample set to confirm the differential expression between primary and metastatic tumor specimens.
The limited number of tissues evaluated from other malignancies such as those of the kidney, uretha, breast, ovary, cervical, uterus endometrium, salivary, thyroid, parathyroid gland, skin, brain and soft tissue precludes making any definitive conclusions. Nonetheless, our current study demonstrates that p-eIF4E is significantly elevated in human cancers, particularly in earlier stages of cancers. This is the first step in our efforts to understand the precise biological role of p-eIF4E signaling in malignant transformation and tumor progression as we as to target eIF4E phosphorylation for cancer therapy.
It is known that the MAP-kinase signal-integrating kinases Mnk1 and Mnk2 are the only known kinases that phosphorylate eIF4E at Ser 209. Similar to p-eIF4E, constitutive activation of Mnk1 could mimic p-eIF4E to promote tumor formation 13. Both ERK and p38 MAPK directly activate Mnks 5. Two ERK and p38 MAPK phosphorylation sites have been identified in Mnk1, Thr197 and Thr202, which are essential for Mnk1 kinase activity 15. Since p38 and ERK MAPKs are frequently activated in transformed cells or tissues, we hypothesize that activated or phosphorylated Mnk1 is also elevated in human cancers as well. Therefore, our results on p-eIF4E in human cancers support further studies to evaluate the role of phosphorylated Mnk1 in human cancers and its correlation with p-eIF4E expression.
Acknowledgments
This study is supported by the Georgia Cancer Coalition Distinguished Cancer Scholar award (to S-Y. Sun) and the National Institute of Health RO1 CA118450 (to S-Y. Sun) and PO1 CA116676 (Project 1 to F.R. K and S-Y. S). S-Y. Sun, S. S. Ramalingam and F. R. Khuri are the Georgia Cancer Coalition Distinguished Cancer Scholars.
References
- 1.De Benedetti A, Graff JR. eIF-4E expression and its role in malignancies and metastases. Oncogene. 2004;23:3189–99. doi: 10.1038/sj.onc.1207545. [DOI] [PubMed] [Google Scholar]
- 2.Goodfellow IG, Roberts LO. Eukaryotic initiation factor 4E. Int J Biochem Cell Biol. 2007 doi: 10.1016/j.biocel.2007.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thumma SC, Kratzke RA. Translational control: a target for cancer therapy. Cancer Lett. 2007;258:1–8. doi: 10.1016/j.canlet.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 4.De Benedetti A, Harris AL. eIF4E expression in tumors: its possible role in progression of malignancies. Int J Biochem Cell Biol. 1999;31:59–72. doi: 10.1016/s1357-2725(98)00132-0. [DOI] [PubMed] [Google Scholar]
- 5.Raught B, Gingras AC. eIF4E activity is regulated at multiple levels. Int J Biochem Cell Biol. 1999;31:43–57. doi: 10.1016/s1357-2725(98)00131-9. [DOI] [PubMed] [Google Scholar]
- 6.Graff JR, Konicek BW, Carter JH, Marcusson EG. Targeting the eukaryotic translation initiation factor 4E for cancer therapy. Cancer Res. 2008;68:631–4. doi: 10.1158/0008-5472.CAN-07-5635. [DOI] [PubMed] [Google Scholar]
- 7.Avdulov S, Li S, Michalek V, Burrichter D, Peterson M, Perlman DM, Manivel JC, Sonenberg N, Yee D, Bitterman PB, Polunovsky VA. Activation of translation complex eIF4F is essential for the genesis and maintenance of the malignant phenotype in human mammary epithelial cells. Cancer Cell. 2004;5:553–63. doi: 10.1016/j.ccr.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 8.Wendel HG, De Stanchina E, Fridman JS, Malina A, Ray S, Kogan S, Cordon-Cardo C, Pelletier J, Lowe SW. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature. 2004;428:332–7. doi: 10.1038/nature02369. [DOI] [PubMed] [Google Scholar]
- 9.Ruggero D, Montanaro L, Ma L, Xu W, Londei P, Cordon-Cardo C, Pandolfi PP. The translation factor eIF-4E promotes tumor formation and cooperates with c-Myc in lymphomagenesis. Nat Med. 2004;10:484–6. doi: 10.1038/nm1042. [DOI] [PubMed] [Google Scholar]
- 10.Mahalingam M, Cooper JA. Phosphorylation of mammalian eIF4E by Mnk1 and Mnk2: tantalizing prospects for a role in translation. Prog Mol Subcell Biol. 2001;27:132–42. [PubMed] [Google Scholar]
- 11.Pyronnet S. Phosphorylation of the cap-binding protein eIF4E by the MAPK-activated protein kinase Mnk1. Biochem Pharmacol. 2000;60:1237–43. doi: 10.1016/s0006-2952(00)00429-9. [DOI] [PubMed] [Google Scholar]
- 12.Topisirovic I, Ruiz-Gutierrez M, Borden KL. Phosphorylation of the eukaryotic translation initiation factor eIF4E contributes to its transformation and mRNA transport activities. Cancer Res. 2004;64:8639–42. doi: 10.1158/0008-5472.CAN-04-2677. [DOI] [PubMed] [Google Scholar]
- 13.Wendel HG, Silva RL, Malina A, Mills JR, Zhu H, Ueda T, Watanabe-Fukunaga R, Fukunaga R, Teruya-Feldstein J, Pelletier J, Lowe SW. Dissecting eIF4E action in tumorigenesis. Genes Dev. 2007;21:3232–7. doi: 10.1101/gad.1604407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graff JR, Konicek BW, Vincent TM, Lynch RL, Monteith D, Weir SN, Schwier P, Capen A, Goode RL, Dowless MS, Chen Y, Zhang H, Sissons S, Cox K, McNulty AM, Parsons SH, Wang T, Sams L, Geeganage S, Douglass LE, Neubauer BL, Dean NM, Blanchard K, Shou J, Stancato LF, Carter JH, Marcusson EG. Therapeutic suppression of translation initiation factor eIF4E expression reduces tumor growth without toxicity. J Clin Invest. 2007;117:2638–48. doi: 10.1172/JCI32044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waskiewicz AJ, Flynn A, Proud CG, Cooper JA. Mitogen-activated protein kinases activate the serine/threonine kinases Mnk1 and Mnk2. Embo J. 1997;16:1909–20. doi: 10.1093/emboj/16.8.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]