Abstract
M. bovis BCG is an attractive vaccine vector against breast milk HIV transmission because it elicits Th1-type responses in newborns. However, BCG causes disease in HIV-infected infants. Genetically attenuated M. tuberculosis (Mtb) mutants represent a safer alternative for immunocompromised populations. In the current study, we compared the immunogenicity in mice of three different recombinant attenuated Mtb strains expressing an HIV envelope (Env) antigen construct. Two of these strains (ΔlysA ΔpanCD Mtb and ΔRD1 ΔpanCD Mtb) failed to induce significant levels of HIV Env-specific CD8+ T cell responses. In striking contrast, an HIV-1-Env-expressing attenuated ΔlysA Mtb containing a deletion in secA2, which encodes a virulence-related secretion system involved in evading adaptive immunity, generated consistently measurable Env-specific CD8+ T cell responses that were significantly greater than those observed after immunization with BCG expressing HIV Env. Similarly, another strain of ΔlysA ΔsecA2 Mtb expressing SIV Gag induced Gag- and Mtb-specific CD8+ T cells producing perforin or IFNγ, and Gag-specific CD4+ T cells producing IFNγ within 3 weeks after immunization in adult mice; in addition, IFNγ producing Gag-specific CD8+ T cells and Mtb-specific CD4+ T cells were observed in neonatal mice within 1 week of immunization. We conclude that ΔlysA ΔsecA2 Mtb is a promising vaccine platform to construct a safe combination HIV-TB vaccine for use in neonates.
1. Introduction
Tuberculosis is the major cause of morbidity and death among the estimated 33.2 million people with HIV-1 infection worldwide [1]. Infants in developing countries where HIV-1 and tuberculosis (TB) are highly endemic are at a high risk both for mother-to-infant HIV transmission (either through perinatal exposure to maternal secretions or subsequent exposure to breast milk) or Mycobacterium tuberculosis (Mtb) infection, as well as rapid progression to AIDS or disseminated tuberculosis after infection. Approximately 5–20% of babies born to HIV positive women in resource-limited countries are infected through breastfeeding [2] resulting in an estimated 200,000 cases of HIV-1 each year [3].
An effective pediatric vaccine against TB or HIV in Africa must be safe for infants at risk for HIV, will require a highly accelerated vaccine schedule and will likely need to elicit virus-specific neutralizing antibodies and cytotoxic T-lymphocyte (CTL) responses rapidly. CD8+ T-cell responses that may be critical to control intracellular pathogens including HIV and Mtb are inherently limited in human neonates. However, human and murine neonates generate functional CD8+ T cells after infections with viruses or immunization with live-attenuated immunogens that deliver antigen into the cytoplasm of APCs [4–7]. Thus far, only two candidate vaccines to protect against breast-milk HIV transmission (HIV-1 gp120 recombinant subunit and live-attenuated recombinant canarypox ALVAC vaccines) have been studied in human infants. These vaccines elicit HIV-1–specific T and B cell responses [8–10] that may contribute to control of HIV-1 infection [11–13]. However, antigen-specific immunity to these vaccines develops slowly, and only after repeated boosting. Therefore, there is an urgent need for a neonatal immunogen that generates HIV-specific immunity more rapidly.
Intradermal Mycobacterium bovis bacille Calmette-Guérin (BCG) vaccination in healthy newborns induces maturation of dendritic cells [14, 15] and rapidly primes strong adult-level Th1-type CD4+ and CD8+ T-cell responses against shared Mtb antigens [16–18] that may limit TB to pulmonary sites in infants [19]. Recombinant BCG (rBCG) has been developed as a candidate neonatal vaccine vector against pertussis [20], measles [21,22], RSV [23] and breastmilk HIV transmission [24]. Vaccines based on BCG vectors have the advantages that they are administered at birth, can be given orally, and rapidly generate long-lived T-cell responses against mycobacterial and co-expressed non-mycobacterial antigens when administered simultaneously to human infants [25]. Given the large geographical overlap between Mtb and HIV infection in Africa, BCG vaccination was until recently recommended at birth for all infants. However, it now appears that the annual risk for disseminated BCG disease in HIV-infected infants (~0.42%) clearly outweighs the potential benefits of BCG in children with HIV [26], [19]. Therefore, the WHO now advises against BCG vaccination in any HIV-infected child [1]. Alternative methods to control TB in infants with HIV are urgently needed.
We and others have observed that candidate attenuated M. tuberculosis (AMtb) strains ΔlysA ΔpanCD Mtb and ΔRD1 ΔpanCD Mtb that are avirulent in immunocompromised SCID and gamma-interferon knockout (GKO−/−) mice confer protection against a lethal aerosol challenge with Mtb H37Rv in immunocompetent mice equal to that observed after BCG immunization [27–29]. To determine the effectiveness of these AMtb vectors as HIV-1 immunogens for CD8+ T-cell responses, in the present study we measured the frequency of CD8+ T-cell responses against HIV-1 Env or SIV Gag after immunization with recombinant mutant Mtb strains expressing these HIV-1 or SIV antigens. Of three attenuated Mtb strains used for vaccine construction, we found that one induced significantly augmented CD8+ T cell responses to the expressed Env or Gag recombinant antigens. This strain combined a strongly attenuating auxotrophy mutation in the lysA gene with deletion of secA2, which encodes a virulence related secretion system that has been shown to participate in evasion of innate and adaptive immunity by M. tuberculosis.
Our results showed that single-dose immunization in neonatal mice with a ΔlysA ΔsecA2 AMtb strain expressing HIV Env rapidly generated high-frequency Env-specific CD8+ T cells among splenocytes, and significant Env-specific CD8+ T cell IFNγ and Mtb-specific CD4+ T cell IFNγ responses. In further studies using a ΔlysA ΔsecA2 AMtb strain expressing an SIV Gag antigen, we demonstrated enhanced responses of Gag-specific CD8+ T cells producing perforin and IFNγ, as well as Gag-specific CD4+ T cells producing IFNγ and Mtb-specific CD8+ T cells producing perforin These data suggest that ΔlysA ΔsecA2 Mtb could be developed further as a safe and effective neonatal combination HIV-TB vaccine platform.
2. Materials and methods
2.1. Bacterial strains and culture conditions
Table 1 lists the mycobacterial strains used in this work. The multicopy E.coli/mycobacterial shuttle episomal plasmid pJH222-gp120 (designated pEnv) that contains the Mtb-derived α antigen promoter that drives expression of a full-length human codon-optimized HIV-1 IIIB gp120 envelope gene (HXBc2) fused to an N-terminus 19-kDa signal sequence of Mtb and a carboxy-terminus HA tag, an aph gene encoding kanamycin resistance, a mycobacterial origin of replication (oriM) and the E. coli origin of replication (oriE), has been previously described [30] (Table 1). E. coli DH5α was used for amplification of plasmid DNA, which was purified using QIAGEN midiprep columns (QIAGEN, Inc., Valencia, CA). E. coli transformants were grown at 37°C in LB media supplemented with kanamycin (40 µg/ml). To generate pJH222 encoding SIV Gag, first, an insert that encodes the full-length SIVmac239 Gag sequence using the favored codon usage of M. tuberculosis (http://www.jcat.de/, with the exception of the following base pair modifications, 645 - G to A, 805 - C to T, 861- C to G and 1308 - C to A that were introduced to ablate two Pst1 and two Apa1 sites, respectively in order to facilitate cloning), the RGPGRAFVTI sequence (an H2Dd-restricted epitope of the V3 loop of HXBc2 Env, designated P18), SIINFEKL and the V5 epitope of paramyxovirus, SV5 [31] the target of V5 antibody-HRP (to permit immunoblotting), and that contained a 5' ApaI and a 3' PstI restriction site, plus an additional TA base pairs (to permit in-frame expression of the peptides) fused at the 3’ end of the insert (designated mgagp18) was synthesized (Bio Basic Inc, Canada). Next, mgagp18 was ligated into unique ApaI and PstI sites downstream of the alpha antigen promoter and the 19kDa signal sequence in the integrative mycobacterial shuttle vector pBRL34. NheI enzyme digestion at two sites in this intermediate construct, released a fragment containing the alpha antigen promoter and 19kDa signal sequence upstream of mgagp18 that was ligated in to pJH222 at the corresponding Nhe1 sites to generate pJH222-mgagp18 (designated pGAG).
TABLE 1.
Mycobacterial strains a
| Strain | Abbreviation | Relevant Characteristics | Source or reference |
|---|---|---|---|
|
M. bovis BCG strains | |||
| Pasteur | BCG | Vaccine strain | |
| mc25151 | BCG(pJH222) | BCG Pasteur with pJH222 | This study |
| mc25152 | BCG(pENV) | BCG Pasteur with pJH222::gp120 | This study |
|
M. tuberculosis H37Rv strains | |||
| mc26020 | 6020 | Δlys ΔpanCD, lysine and pantothenate auxotroph, Hyr; persists for < 3 weeks in vivo |
A |
| mc25153 | 6020(pJH222) | mc26020 with pJH222 | This study |
| mc25154 | 6020(pENV) | mc26020 with pJH222::gp120 | This study |
| mc26030 | 6030 | ΔRD1 ΔpanCD, RD1 knockout and pantothenate auxotroph, Hyr; persists for 28 weeks in vivo |
B |
| mc25155 | 6030(pJH222) | mc26030 with pJH222 | This study |
| mc25156 | 6030(pENV) | mc26030 with pYUB1404 | This study |
| mc25226 | 5226 | Δlys ΔsecA2, lysine auxotroph(96 base pair deletion) and pro-apoptotic |
This study |
| mc25127 | 5226(pJH222) | mc25226 with pJH222 | This study |
| mc25128 | 5226(pENV) | mc25226 with pENV | This study |
| mc23026 | 3026 | Δlys, lysine auxotroph(331base pair deletion) | C |
| mc25132 | 3026(pJH222) | mc23026 with pJH222 | This study |
| mc25133 | 3026(pENV) | mc23026 with pENV | This study |
| mc25222 | 5222 | Δlys ΔsecA2, lysine auxotroph (96 base pair deletion) and pro-apoptotic with hygromycin cassette |
This study |
| mc25129 | 5222(pGAG) | mc25222 with pGAG | This study |
Abbreviations: Hyr, Hygromycin and superscript r Hygromycin resistance
A. Sambandamurthy et al., 2005
B. Sambandamurthy et al., 2006
C. Pavelka et al., 1999
Individual clones of mycobacteria were grown in Middlebrook 7H9 broth (Becton Dickinson, Franklin Lakes, NJ) at 37°C. All broth and agar media contained 10% oleic acid-albumin dextrose-catalase (OADC) (Becton Dickinson Microbiological Systems, Sparks MD), 0.05% Tween 80 (Fisher Scientific, Fair Lawn, NJ). The auxotrophic strain 5226 was supplemented with lysine (200 µg/ml), 5222 with hygromycin (50µg/ml) and lysine, 6020 with both lysine and pantothenate (48 µg/ml) (Sigma Chemical Co., St. Louis, MO), and 6030 with pantothenate. pENV or pGAG was electroporated into competent mycobacterial cells as previously described [32, 33]. Strains 5222 and 5226 both contain an identical full-length secA2 deletion plus a lysA deletion, that contains an inserted hygromycin resistance gene cassette, and is larger in the former strain (Table 1) [34]. Control recombinant mycobacterial strains were transformed with pJH222 without a gene insert. AMtb and BCG transformants were selected on Middlebrook 7H10 (Becton Dickinson, Sparks, MD) or Middlebrook 7H11 agar containing 20 µg/ml of kanamycin (Sigma Chemical Co., St. Louis, MO). To prepare strains for mouse immunization, single colonies were grown in 7H9 broth containing kanamycin (20µg/ml) to mid log phase [OD600 0.6–0.8, that corresponds to an approximate concentration of 108 CFU (colony forming units)/ml], washed twice with PBS containing 0.05% Tween 80, and then resuspended to the desired concentration in PBS. Cell counts were verified by plating serial dilutions of the inocula on Middlebrook 7H10 agar plates.
To confirm expression of recombinant HIV-1 Env or SIV Gag in mycobacteria, strains were grown in 7H9 media with shaking at 75 rpm to mid log phase. A 5 ml aliquot of each culture was harvested, washed twice with phosphate buffered saline (PBS) plus 0.05% Tween 80, resuspended in 200µl of 2x protease inhibitor buffer (Roche Diagnostics, Germany) along with 200 µl of 0.1 mm silicon beads (BioSpec Products, Inc), subjected to FAStprep (Thermo Electron Corporation, MA) until grossly clear, and then incubated at 95° C for 45 min to kill viable mycobacteria. A 15 µl aliquot of each cell lysate was run on 12% (for BCG, 6020 and 6030 transformants) or 10–20% (for 5226 and 5222 transformants) gradient SDS-PAGE (Bio-Rad Laboratories, CA), electroblotted onto a nitrocellulose membrane, immunolabeled with an anti-HA monoclonal antibody (MAb) (clone 3F10) or V5 antibody-HRP, and then developed with a chemiluminescence detection kit according to the manufacturer’s protocol (Perkin Elmer, MA). The CFU was estimated based on the optical density where an OD of 1 corresponds to 3 × 108 CFU. Relative levels of recombinant gp120 expression in mycobacterial lysates were estimated using ImageJ software, version 1.34n (Wayne Rasband; http://rsb.info.nih.gov/ij/).
2.2. Animals and Immunization
Four to six-week-old (adult) female BALB/c mice (National Cancer Institute) were housed and cared for according to the Institutional Animal Care and Use Committee of the Albert Einstein College of Medicine (AECOM). Four-to-five-day old (neonatal) male and female mice were bred at AECOM from BALB/c breeding pairs obtained from the National Cancer Institute and were housed under specific pathogen-free conditions.
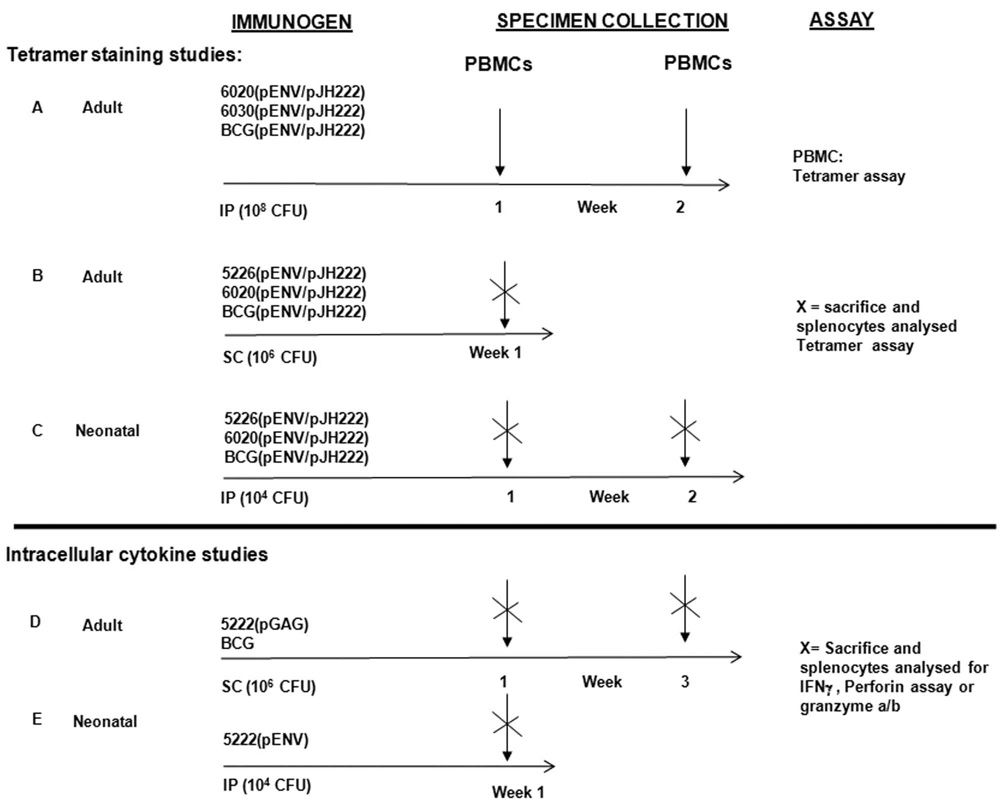
Adult BALB/c mice were inoculated with 106 or 108 CFU of each strain (in 0.2ml PBS) via either the intraperitoneal or the subcutaneous route. Neonatal mice were inoculated with 104 CFU of each strain via the intraperitoneal route (102 in 50µl PBS on either side at the peritoneum) using a 30 gauge needle. The various immunization schedules are represented in Fig 2.
FIG. 2. Figure summarizing the various vaccination schedules carried out in the study.
2.3. Tetramer staining
To determine the frequency of gp120-specific tetrameric responses in peripheral blood mononuclear cells (PBMCs) or splenocytes, blood was obtained from the retro-orbital plexus and spleens were harvested at weekly intervals starting 1 week after inoculation for up to six weeks (Fig. 2, Groups A–C). H-2Dd tetrameric complexes folded with the P18 peptide (RGPGRAFVTI) [35], a sequence found in the V3 loop of HIV-1 HXBc2 envelope protein, and which was encoded in the native env sequence in plasmid pENV and synthetically fused downstream of the gag sequence in pGAG, were prepared as described previously [36]. Fresh blood samples (200 µl from each mouse) or splenocyte suspensions (recovered after passage through 70-µm nylon cell strainer) were recovered from individual mice, resuspended in RPMI-1640 (Gibco BRL; Invitrogen), treated with RBC lysis buffer, diluted in 3 ml RPMI medium with 40 U/ml heparin, and then layered over Ficoll-Hypaque (lympholyte-M) before centrifugation at 800rpm for 20 min at 20°C. The mononuclear cell layer was carefully transferred to a fresh tube, diluted with 10 ml of PBS (GIBCO), pelleted, washed in 1 ml PBS with 2% fetal bovine serum (FBS), (Invitrogen, CA), and then resuspensed in 200 µl PBS with 2% FBS. The cells were stained with P18-tetramer phycoerythrin, vortexed briefly, incubated at 20°C for 20 min, and then incubated with allophycocyanin conjugated with anti-CD8α MAb (Ly-2) (BD Pharmingen, CA) for 20 min at 20°C. To control for nonspecific fluorescence, an aliquot of each PBMC or splenocyte sample was incubated without monoclonal antibody, with APC anti-CD8 alone, and with phycoerythrin anti-CD4 alone. The cells were washed with 5 ml PBS plus 2% FBS, resuspended in 2% formaldehyde in PBS, incubated at room temperature for 1 hour, vortexed, sorted by BD with a FACSCalibur cytometer and then analyzed using cell quest software (Becton Dickinson, CA). A minimum of 106 cells were analyzed for each sample.
2.4. Intracellular cytokine staining
To determine the frequency of CD3+ CD4+ or CD3+ CD8+ T cells producing IFNγ, perforin and/or granzyme a/b against Gag, Env, BCG or Mtb antigens, splenocytes were harvested from individual mice 1 and 3 weeks after immunization (Fig. 2, Groups D and E) and were resuspended in compete RPMI 1640 media supplemented with 10% FBS, 25mM HEPES, penicillin and streptomycin (1%), essential (1%) and non-essential amino acids. Lymphocyte suspensions were stimulated with anti-CD28 mAb (2µg/ml) plus either a pool of 15-mer SIVmac239 Gag peptides (4 µg/ml of each peptide; AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH, Germantown, MD; cat. #6204), P18 peptide (4 ug/ml), TB10.3/4:20–28 peptide (an Mtb-derived H-2Kd restricted sequence, GYAGTLQSL, that is present in both Mtb and BCG), [37] (Sigma Aldrich) (4µg/ml), mc25226 lysates (20 ug/ml), BCG lysates (20 ug/ml), granzyme a/b or media alone. Peptides were dissolved in dimethyl sulfoxide at 5mg/ml. IFNγ production, perforin content and or granzyme a/b content in CD4+ and CD8+ T cells were detected by combined surface and intracellular staining. In brief, the cells were incubated at 37° C with 10% CO2 for 2 hours and then treated with brefeldin-A (BFA) (Sigma Chemical Co., St. Louis, MO) at a final concentration of 10µg/ml and monensin A (2µM) (eBioscience). After another 4 hr incubation at 37° C, the cells were washed with PBS and treated with Live/Dead staining with blue fluorescent reactive dye (Invitrogen molecular probes, Oregon) incubated at 4° C, washed with PBS followed by cell surface staining with FITC anti-CD3, pacific blue anti-CD8, APC-Cy7 anti-CD4 (eBioscience, San Diego), for 15 min on ice protected from light. The cells were then washed with PBS buffer, resuspended and treated with freshly prepared Fixation/Permeabilization solution (eBioscience) and incubated overnight at 4° C in dark followed by washing with permeabilization buffer (eBioscience). The cells were then blocked with anti-CD16/32 monoclonal antibody (eBioscience) in permeabilization buffer for 15min at 4° C and stained with PE-Cy 7 anti-IFNγ (eBioscience) or APC anti-perforin (eBioscience) for 30 min at 4° C and washed with PBS followed by fixation with 2% paraformadehyde for 1 hour. Multicolor flow analysis was performed using the BD LSR II and the Flowjo software (Tree Star).
2.5 Statistical analysis
Statistical tests were performed using Student’s t test or Mann-Whitney analysis (Minitab, PA). P values of < 0.05 were considered significant.
3. Results
3.1. Expressing full-length HIV-1 Env or SIV Gag in recombinant AMtb
It has been shown previously that full-length heterologous viral proteins can be expressed in recombinant BCG and M. smegmatis, and that immunization with these constructs in animal models can elicit T-cell responses against a variety of viral pathogens including measles, [21, 22] HIV-1 [24, 30, 38] and simian immunodeficiency virus (SIV) [39, 40]. However expression of recombinant viral proteins has not to our knowledge been demonstrated previously in attenuated derivatives of Mtb. Following transformation with pENV, we detected expression of HIV gp120 protein in lysates of attenuated Mtb strains 6020, 6030 and 5226 and in BCG by Western immunoblotting (Fig. 1). Of note, the level of expression of HIV-1 Env in rBCG was estimated to be approximately eightfold higher than in the rAMtb strains by ImageJ version 1.40g analysis. Also, a higher proportion of break-down products was detected in rAMtb compared to BCG. Gp120 or SIVmac239 Gag expression in strain 5222 were also confirmed by Western immunoblotting (data not shown).
FIG. 1. Expression of recombinant HIV-1 HXBc2 gp120 in rBCG and rAMtb strains.
Each lane contains lysates of approximately 108 CFU of BCG, 6020, 6030 or 5226 harboring pEnv or pJH222, the corresponding control plasmid without the gp120 gene insert. Lysates were immunoblotted with HRP conjugated anti-HA antibodies and developed using chemiluminescence. The principal recombinant gp120 band migrates at approximately 55 kDa. Several lower molecular weight breakdown products, that are more prevalent in lysates of rAMtb than lystes of BCG, are also present.
3.2. Recombinant AMtb strain 6020(pEnv) engenders low-level Env-specific CD8+ T-cell responses in adult mice
To determine the feasibility of immunization with a live-attenuated Mtb, we compared the frequencies of gp120-specific tetrameric responses after immunization with either AMtb strains 6020(pENV) or 6030(pENV) or BCG(pENV) (Fig. 3). In a preliminary study, no gp120-specific responses above background levels were detected after immunization with 106 CFU of these strains. In a separate experiment, after a higher dose (108 CFU) inoculum, the frequency of P18 tetramer staining among CD8+ T cells in PBMCs recovered 1 week after immunization from groups of mice immunized with 6020(pENV) and BCG(pENV) (P=0.0003 and P=0.01 respectively), but not with 6030(pENV), (Fig. 3) was significantly higher than background frequencies detected among PBMCs from control-immunized groups.
FIG. 3. BCG-pEnv and rAMtb strain 6020-pEnv elicit Env-specific CD8+ T cells.
Tetramer staining and flow cytometric analysis of splenocytes after intraperitoneal immunization of 4-to-6-week old BALB/C mice with approximately 108 of either rBCG, 6020 or 6030 harboring pEnv. As a negative control, groups of mice were inoculated with the same dose of rBCG or rAMtb harboring the control pJH222 plasmid. The mean (± SEM) percent gp120 P18 tetramer-positive CD8+ T cells among splenocytes collected at the indicated time points is shown for each group of mice (n = 6 per group). The frequencies of CD8 and P18 tetramer staining responses were significantly higher after rBCG-pENV (at 1 and 2 weeks) and 6020-pEnv strain immunization (at 1 week) than after control rBCG and r6020 strain immunization (P < 0.05 [one asterisk]; P < 0.005 [two asterisks]).
3.3. Recombinant AMtb strain 5226(pENV) elicits high-frequency MHC class 1 restricted CD8+ T cell responses in adult and neonatal mice
The ability of BCG and other attenuated mycobacterial strains to persist within endosomes of infected macrophages and dendritic cells may restrict their ability to present antigens via the MHC class I pathway. Apoptotic macrophages infected with phagosome-enclosed pathogens appear to provide antigen to dendritic cells for cross-priming of CD8+ T cells via a detour pathway by which antigens derived from apoptotic vesicles act as cross-priming vehicles [41, 42]. In order to engage this detour pathway, we used the AMtb strain 5226 in which the lysine auxotrophy was combined with a deletion of the secA2 gene that is required for inhibition of host cell apoptosis by M. tuberculosis [43]. We compared the frequencies of gp120-specific tetrameric responses after immunization in adult and neonatal mice with rAMtb strain 5226 to the frequencies observed following immunization with either BCG or 6020 expressing HIV-1 Env in which the mechanisms for blocking apoptosis are intact. Remarkably, in adult mice inoculated with 5226(pEnv) the magnitude of the P18 tetrameric responses among splenocytes 1 week after immunization were sixfold higher than in mice inoculated with recombinant BCG(pEnv) (P<0.005) and twenty fourfold higher than in mice after 6020(pENV) inoculation (Fig. 4). In neonatal mice inoculated with 5226(pENV) at a 100-fold lower inoculum (104), the magnitude of the P18 tetrameric responses among splenocytes 1, 2 (Fig. 5) and 3 (data not shown) weeks after immunization were significantly higher than in mice inoculated with BCG(pEnv) (P<0.05) No responses above background were observed in mice immunized with strain 3026 (ΔlysA H37Rv) expressing Env (data not shown).
FIG. 4. rAMtb 5226(pEnv) elicits Env-specific CD8+ T cells markedly more effectively than either rAMtb 6020-pEnv or rBCG-pEnv.
Tetramer staining and flow cytometric analysis of splenocytes recovered 1 week after subcutaneous immunization of 4-to-6-week old BALB/C mice with approximately 106 of either 5226, 6020 or BCG harboring pEnv. As negative controls, groups of mice were inoculated with the same dose of rBCG or rAMtb harboring the control pJH222 plasmid. (A) Example of flow cytometric analysis from individual mice revealed that P18 tetramer-positive CD8+ T-cell responses were detected after immunization with 5226(pJH222), that were significantly higher than background frequencies detected from a corresponding control mouse, and that were dramatically higher than responses detected in a mouse after BCG(pENV) immunization. Gating was exclusively on the total CD8+ T cell population. (B) The mean (± SEM) percent gp120 P18 tetramer-positive CD8+ T cells among splenocytes collected 1 week after immunization is shown for each group of mice (n = 5 per group). The frequencies of CD8 and P18 tetramer staining responses were significantly higher after 5226-pENV than after either 6020-pEnv or BCG-pEnv immunization (P < 0.05 [one asterisk]; P < 0.005 [two asterisks]).
FIG. 5. rAMtb 5226(pEnv) elicits Env-specific CD8+ T cells markedly more effectively than rBCG-pEnv in neonatal mice.
Tetramer staining and flow cytometric analysis of splenocytes after intraperitoneal immunization of 4-to 5 day old BALB/C mice with approximately 104 of either rAMtb strain 5226 or rBCG harboring pEnv. As negative controls, groups of mice were inoculated with the same dose of rBCG or rAMtb harboring the control plasmid. pJH222. The mean (± SEM) percent gp120 P18 tetramer-positive CD8 T cells among splenocytes collected at the indicated time points is shown for each group of mice (n = 5 per group). The frequencies of P18 tetramer staining responses were significantly higher at 1 and 2 weeks after 5226(pENV) than after BCG(pENV) strain immunization. (P < 0.05 [one asterisks]; P < 0.005 [two asterisks])
3.4. Recombinant AMtb strain 5222(pENV) and 5222(pGAG) elicit CD4+ and CD8+ T-cell IFNγ and perforin responses against HIV, SIV and/or mycobacterial antigens in neonatal and adult mice
To determine whether live-attenuated Mtb generates mycobacteria-, HIV ENV- and SIV Gag-specific functional CD4+ and CD8+ T cell responses we evaluated antigen-specific CD4+ and CD8+ T cells for their functional maturation by assessing their expression of IFNγ, perforin and/or granzyme a/b using surface and/or intracellular staining with monoclonal antibodies and flow cytometric analysis. In adult mice, there were no antigen-specific responses detected 1 week after 5222(pGAG) immunization. However, 3 weeks after immunization, IFNγ and perforin producing CD8+ T cells specific for mycobacterial proteins (BCG lysate or TB10.3/4) were significantly elevated. In addition, Gag-specific CD8+ T cells producing perforin but not IFNγ were detected (Fig. 6A & 6B). Among CD4+ T cells significant levels of Gag-specific, but not mycobacteria-specific cells producing IFNγ were detected in splenocytes 3 weeks after immunization with 5222(pGAG) (Fig. 7A). In contrast, no mycobacterial-specific CD8+ (Fig. 6C & 6D) or CD4+ T cells producing IFNγ or perforin were detected after BCG immunization (Fig. 7B).
FIG. 6. rAMtb 5222(pGAG) elicits functional mycobacteria-specific CD8+ T cells in adult mice.
Surface and intracellular staining of splenocytes after subcutaneous immunization of 4-to-6-week old BALB/C mice with approximately 106 of either rAMtb strain 5222 harboring pGAG (Fig. 6A & B) or BCG (Fig. 6C&D). The mean (± SEM) percent of CD8 T+ cells among splenocytes collected at the indicated time points is shown for each group of mice (n = 4–5 per group). The frequencies of CD8+ T cells expressing IFNγ or perforin were significantly higher after stimulation with BCG lysates than after media only incubation (P < 0.05 [one asterisk]), as were the the frequencies of CD8+ T cells expressing perforin after stimulation with BCG lysate, TB10.3/4- and Gag peptide 3 weeks after 5222(pGAG) immunization. (P < 0.05 [one asterisks]; P < 0.005 [two asterisks]). There was no significant differences in the frequency of CD8+ T cells producing IFNγ or perforin after BCG lysate or TB10.3/4 stimulation compared to incubation with media alone after either BCG immunization.
FIG. 7. rAMtb 5222(pGAG) elicits a Gag-specific CD4+ T cell IFNγ response in adult mice.
Surface and intracellular staining of splenocytes was carried out after subcutaneous immunization of 4-to-6-week old BALB/C mice with approximately 106 of either rAMtb strain 5222 harboring pGAG (Fig. 7A) or BCG (Fig. 7B). The frequencies of CD4+ T cells expressing IFNγ were significantly higher after stimulation with a Gag peptide pool than after media only incubation (P < 0.05 [asterisk]). There was no significant difference in the frequency of CD4+ T cells secreting IFNγ after BCG stimulation compared to incubation with media alone after either 5222(pGAG) or BCG immunization.
In neonatal mice immunized with 5222(pENV), significant levels of ENV P18 peptide-specific (but not mycobacterial specific) CD8+ T cells producing IFNγ were detected in the spleen one week after immunization (Fig. 8A). In contract, no increase in P18-specific CD4+ T cells were detected, although IFNγ producing CD4+ T cells specific for mycobacterial antigens were augmented (Fig. 8B). No Mtb lysate-, TB 10.3/4- or P18-specific CD8+ T cells producing perforin were detected (Fig. 8C). For all experiments, P18 tetrameric responses among splenocyte CD8+ T cells 1 week after 5222(pGAG) or 5222(pENV) immunization were detected at frequencies of > 0.5% above levels of non-specific tetramer staining of splenocytes from groups of control immunized mice (data not shown).
FIG. 8. rAMtb 5222(pEnv) elicits Env- and Mtb-specific CD4+ and CD8+ T cell IFNγ responses in neonatal mice.
Surface and intracellular staining of splenocytes for antigen-specific CD8+ T cells producing IFNγ (Fig 8A), granzyme a/b (Fig 8B) or CD4+ T cells producing IFNγ (Fig 8C) were carried out after intraperitoneal immunization of 4-to-5-day old BALB/C mice with approximately 104 of either rAMtb strain 5222 harboring pENV. The frequency of CD8+ and T cells producing IFNγ was significantly higher after stimulation with a Env P18 peptide than after media only incubation (P < 0.05 [one asterisk]) and the frequency of CD4+ T cells producing IFNγ was significantly higher after stimulation with Mtb lysates than after media only incubation (P < 0.005 [two asterisks]) There was no significant difference in the frequency of CD8+ T cells producing granzyme a/b IFNγ after Mtb lysate or TB10.3/4 stimulation compared to incubation with media alone.
4. Discussion
Safe and effective strategies to vaccinate against HIV and TB at the earliest possible time after birth to prevent breast milk HIV transmission and childhood tuberculosis are urgently needed. We studied the immunogenicity of attenuated rAMtb vectors for HIV, SIV and Mtb-specific T cell responses. We observed that with the highly attenuated 6020 (ΔlysA ΔpanCD double auxotroph) or 6030 (ΔRD1 ΔpanCD) strains expressing HIV-1 Env antigen, Env-specific responses were either undetectable or observed at low-frequencies and only after a high-dose inoculum. We postulated that the poor immunogenicity of these attenuated Mtb strains was due to the fact that Mtb complex bacteria including the natural mammalian pathogens Mtb and M. bovis (and its derivated, BCG) have evolved strategies to evade cell-mediated immune responses. These organisms persist in phagosomes of infected macrophages, prevent phagosomal maturation [44, 45], and trigger apoptosis only weakly. Thus, they present antigen to the MHC class I pathway inefficiently. Taken together, these findings suggest that attenuated Mtb vectors could be made more effective by modifications that cause them to induce stronger apoptosis of infected cells.
In the present study, we observed that immunization with rAMtb strains 5226(pENV), 5222(pENV) or 5222(pGAG) which contain a deletion in secA2 along with the strongly attenuating ΔlysA mutation generate antigen-specific tetrameric responses by day seven after immunization in both adult and neonatal mice at frequencies that were dramatically higher than in mice vaccinated with either BCG or ΔlysA ΔpanCD H37Rv expressing the same antigens, as well as functional CD4+ and CD8+ T cells producing IFNγ or perforin against both HIV or SIV and Mtb antigens. This is the first reported observation of heterologous vaccination with a rAMtb strain that is highly attenuated for virulence and persistence. The deletion of lysA markedly attenuates for virulence in the majority of SCID mice [34] and the deletion of secA2 attenuates Mtb for virulence in immunocompetent and SCID mice only moderately relative to the wild type parental strain [46]. However, in order to minimize the potential that a ΔlysA ΔsecA2 rAMtb strain would revert to virulence, a second attenuated deletion (for example ΔpanCD [27]), would be required.
There are several advantages of a ΔlysA ΔsecA2 H37Rv-based immunogen against HIV-1 and Mtb over candidate BCG-based immunogens including superior safety, ease of manipulation to obtain a pro-apoptotic phenotype, immunogenicity and antigenic relevance to Mtb. Safety in SCID mice is considered a reliable first-screen for safety in humans with immunosuppression. BCG has been observed by other workers and us to be consistently lethal for 100% of SCID mice. In contrast, a high proportion (85%) of SCID mice infected ΔlysA ΔsecA2 Mtb survived for up to 642 days. (W.R. Jacobs, Jr. and M.H. Larsen, unpublished data). Although an auxotrophic ΔlysA BCG-based vaccine vector has been observed to be avirulent in SCID mice [34], single-dose recombinant ΔlysA BCG vaccination fails to elicit T-cell responses against heterologous antigens in immunocompetent mice [24]. Of note, the deletion of secA2 in BCG does not enhance its ability to induce apoptosis or elicit antigen-specific CD8+ T-cell responses (S.A. Porcelli and W.R. Jacobs, Jr., unpublished data). Also, 5222, 5226 and other Mtb-based strains contain the RD1 region (deleted during attenuation of BCG) that encodes several antigens (including ESAT-6 and CFP-10) that appear to be targets for protective T cells.
Breast milk HIV exposure accounts for approximately 15–20% all cases of mother-to-child HIV transmission, resulting in an estimated 200,000 new infections in infants each year [3]. Neonatal immunization may be the best approach to prevent infection or reduce the severity of HIV-related disease in these infants. Moreover, infants with HIV infection are at high risk for exposure, infection and rapidly progressive disease from tuberculosis. Because of the high rate of disseminated BCG infection after neonatal BCG immunization in infants with HIV-1, a safer alternative is urgently needed. We demonstrate here that a recombinant live attenuated Mtb vaccine rapidly elicited high-frequency HIV-1 specific CD8+ T-cell responses after only a single dose in 4 or 5-day-old neonatal mice as well as HIV-specific functional CD4+ and Mtb-specific CD8+ T cells producing IFNγ. These responses may be relevant to protection against HIV and Mtb in humans. IFNγ plays a critical role in T-cell immunity against both HIV and Mtb via potent activation of macrophages [47,48] and CD8+ T cells producing perforin are important to control of both pathogens [49, 50]. Others have observed that T cell responses against Gag alone may be sufficient to protect non-human primates against SIV infection [51] and to prevent progression to AIDS in humans after HIV-1 infection [52]. It is noteworthy that increases in the frequencies of CD4+ and CD8+ T cells producing IFNγ in response to Mtb antigens were observed three weeks after AMtb, but not after BCG immunization in adult mice. In BCG immunized animals higher frequencies of CD8+ and CD4+ T cells producing IFNγ spontaneously were detected among splenocytes, but these were not significantly enhanced by restimulation with mycobacterial antigens. This may reflect a non-specific immunostimulatory effect of BCG that, unlike 5222, continues to replicate after inoculation into mice, or may reflect a failure to detect specific responses with our assay. As suggested by data from human infants wherein BCG-specific T cells that produce IFNγ can be observed 10 weeks after neonatal BCG vaccination, [53] these responses may appear later.
The biological relevance of the rapidly induced HIV, and Mtb-specific CD4+ and CD8+ T cell responses that we observed following recombinant AMtb immunization in adult and neonatal mice will require further evaluation to determine the ability of these responses to protect against infection. Given the urgent need for safe and effective HIV and TB vaccines for infants in Africa, and the demonstrated ability to produce and administer live mycobacterial vaccines on a large scale, rAMtb-based HIV vaccines are an appealing platform for a human neonatal vaccine in lieu of BCG to prevent mother-to-child breast milk transmission of HIV. Vaccines designed on this platform could potentially establish immune memory for both HIV and TB that could be boosted with heterologous immunogens consisting of HIV or TB subunits [59, 60]. Ideally, such a rAMtb will need to be fully attenuated for virulence, while providing increased levels of immunogenicity compared to BCG.
Acknowledgements
We thank Ms. Karoline Biermann for providing the mc25226 and mc25222 knockout strains, Dr. Lydia Tesfa for assistance with FACS analysis, Dr. Jorge Sansarry for guidance on statistical analysis and Dr Norman Letvin for providing the p18 tetramer. This work was supported by National Institutes of Health grants PO1 AI052816 (P01 to GJF and WRJ), NDK 1R01DE019064-01 (R01 to GJF and MHL), AI 063537 (P01 to SAP and WRJ), the Center for AIDS Research at the Albert Einstein College of Medicine (AI 51519) and the Einstein Cancer Center Flow Cytometry Core Facility (CA13330).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.UNAIDS, W.H.O. 2007 AIDS epidemic update. 2007
- 2.De Cock KM, Fowler MG, Mercier E, de Vincenzi I, Saba J, Hoff E, et al. Prevention of mother-to-child HIV transmission in resource-poor countries: translating research into policy and practice. JAMA. 2000 Mar 1;283(9):1175–1182. doi: 10.1001/jama.283.9.1175. [DOI] [PubMed] [Google Scholar]
- 3.UNAIDS. Switzerland: AIDS epidemic update-special report on HIV/AIDS Geneva. 2006
- 4.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004 Jul;4(7):553–564. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 5.Siegrist CA, Lambert PH. Maternal immunity and infant responses to immunization: factors influencing infant responses. Dev Biol Stand. 1998;95:133–139. [PubMed] [Google Scholar]
- 6.Siegrist CA. Neonatal and early life vaccinology. Vaccine. 2001 May 14;19(25–26):3331–3346. doi: 10.1016/s0264-410x(01)00028-7. [DOI] [PubMed] [Google Scholar]
- 7.Franchini M, Abril C, Schwerdel C, Ruedl C, Ackermann M, Suter M. Protective T-cell-based immunity induced in neonatal mice by a single replicative cycle of herpes simplex virus. J Virol. 2001 Jan;75(1):83–89. doi: 10.1128/JVI.75.1.83-89.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borkowsky W, Wara D, Fenton T, McNamara J, Kang M, Mofenson L, et al. Lymphoproliferative responses to recombinant HIV-1 envelope antigens in neonates and infants receiving gp120 vaccines. AIDS Clinical Trial Group 230 Collaborators. J Infect Dis. 2000 Mar;181(3):890–896. doi: 10.1086/315298. [DOI] [PubMed] [Google Scholar]
- 9.Johnson DC, McFarland EJ, Muresan P, Fenton T, McNamara J, Read JS, et al. Safety and immunogenicity of an HIV-1 recombinant canarypox vaccine in newborns and infants of HIV-1-infected women. J Infect Dis. 2005 Dec 15;192(12):2129–2133. doi: 10.1086/498163. [DOI] [PubMed] [Google Scholar]
- 10.McFarland EJ, Johnson DC, Muresan P, Fenton T, Tomaras GD, McNamara J, et al. HIV-1 vaccine induced immune responses in newborns of HIV-1 infected mothers. AIDS. 2006 Jul 13;20(11):1481–1489. doi: 10.1097/01.aids.0000237363.33994.45. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham CK, Wara DW, Kang M, Fenton T, Hawkins E, McNamara J, et al. Safety of 2 recombinant human immunodeficiency virus type 1 (HIV-1) envelope vaccines in neonates born to HIV-1-infected women. Clin Infect Dis. 2001 Mar 1;32(5):801–807. doi: 10.1086/319215. [DOI] [PubMed] [Google Scholar]
- 12.McFarland EJ, Borkowsky W, Fenton T, Wara D, McNamara J, Samson P, et al. Human immunodeficiency virus type 1 (HIV-1) gp120-specific antibodies in neonates receiving an HIV-1 recombinant gp120 vaccine. J Infect Dis. 2001 Nov 15;184(10):1331–1335. doi: 10.1086/323994. [DOI] [PubMed] [Google Scholar]
- 13.Rowland-Jones S, Sutton J, Ariyoshi K, Dong T, Gotch F, McAdam S, et al. HIV-specific cytotoxic T-cells in HIV-exposed but uninfected Gambian women. Nat Med. 1995 Jan;1(1):59–64. doi: 10.1038/nm0195-59. [DOI] [PubMed] [Google Scholar]
- 14.Liu E, Law HK, Lau YL. BCG promotes cord blood monocyte-derived dendritic cell maturation with nuclear Rel-B up-regulation and cytosolic I kappa B alpha and beta degradation. Pediatr Res. 2003 Jul;54(1):105–112. doi: 10.1203/01.PDR.0000069703.58586.8B. [DOI] [PubMed] [Google Scholar]
- 15.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006 Jul 10;203(7):1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marchant A, Goetghebuer T, Ota MO, Wolfe I, Ceesay SJ, De Groote D, et al. Newborns develop a Th1-type immune response to Mycobacterium bovis bacillus Calmette-Guerin vaccination. J Immunol. 1999 Aug 15;163(4):2249–2255. [PubMed] [Google Scholar]
- 17.Murray RA, Mansoor N, Harbacheuski R, Soler J, Davids V, Soares A, et al. Bacillus Calmette Guerin vaccination of human newborns induces a specific, functional CD8+ T cell response. J Immunol. 2006 Oct 15;177(8):5647–5651. doi: 10.4049/jimmunol.177.8.5647. [DOI] [PubMed] [Google Scholar]
- 18.Vekemans J, Lienhardt C, Sillah JS, Wheeler JG, Lahai GP, Doherty MT, et al. Tuberculosis contacts but not patients have higher gamma interferon responses to ESAT-6 than do community controls in The Gambia. Infect Immun. 2001 Oct;69(10):6554–6557. doi: 10.1128/IAI.69.10.6554-6557.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet. 2006 Apr 8;367(9517):1173–1180. doi: 10.1016/S0140-6736(06)68507-3. [DOI] [PubMed] [Google Scholar]
- 20.Nascimento IP, Dias WO, Quintilio W, Christ AP, Moraes JF, Vancetto MD, et al. Neonatal immunization with a single dose of recombinant BCG expressing subunit S1 from pertussis toxin induces complete protection against Bordetella pertussis intracerebral challenge. Microbes Infect. 2008 Feb;10(2):198–202. doi: 10.1016/j.micinf.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Zhu YD, Fennelly G, Miller C, Tarara R, Saxe I, Bloom B, et al. Recombinant bacille Calmette-Guerin expressing the measles virus nucleoprotein protects infant rhesus macaques from measles virus pneumonia. J Infect Dis. 1997 Dec;176(6):1445–1453. doi: 10.1086/514140. [DOI] [PubMed] [Google Scholar]
- 22.Fennelly GJ, Flynn JL, ter Meulen V, Liebert UG, Bloom BR. Recombinant bacilli Calmette-Guerin priming against measles. J Infect Dis. 1995 Sep;172(3):698–705. doi: 10.1093/infdis/172.3.698. [DOI] [PubMed] [Google Scholar]
- 23.Bueno SM, Gonzalez PA, Cautivo KM, Mora JE, Leiva ED, Tobar HE, et al. Protective T cell immunity against respiratory syncytial virus is efficiently induced by recombinant BCG. Proc Natl Acad Sci U S A. 2008 Dec 30;105(52):20822–20827. doi: 10.1073/pnas.0806244105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Im EJ, Saubi N, Virgili G, Sander C, Teoh D, Gatell JM, et al. Vaccine platform for prevention of tuberculosis and mother-to-child transmission of human immunodeficiency virus type 1 through breastfeeding. J Virol. 2007 Sep;81(17):9408–9418. doi: 10.1128/JVI.00707-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ota MO, Vekemans J, Schlegel-Haueter SE, Fielding K, Sanneh M, Kidd M, et al. Influence of Mycobacterium bovis bacillus Calmette-Guerin on antibody and cytokine responses to human neonatal vaccination. J Immunol. 2002 Jan 15;168(2):919–925. doi: 10.4049/jimmunol.168.2.919. [DOI] [PubMed] [Google Scholar]
- 26.Hesseling AC, Marais BJ, Gie RP, Schaaf HS, Fine PE, Godfrey-Faussett P, et al. The risk of disseminated Bacille Calmette-Guerin (BCG) disease in HIV-infected children. Vaccine. 2007 Jan 2;25(1):14–18. doi: 10.1016/j.vaccine.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 27.Sambandamurthy VK, Derrick SC, Jalapathy KV, Chen B, Russell RG, Morris SL, et al. Long-term protection against tuberculosis following vaccination with a severely attenuated double lysine and pantothenate auxotroph of Mycobacterium tuberculosis. Infect Immun. 2005 Feb;73(2):1196–1203. doi: 10.1128/IAI.73.2.1196-1203.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sambandamurthy VK, Jacobs WR., Jr Live attenuated mutants of Mycobacterium tuberculosis as candidate vaccines against tuberculosis. Microbes Infect. 2005 May;7(5–6):955–961. doi: 10.1016/j.micinf.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Sampson SL, Dascher CC, Sambandamurthy VK, Russell RG, Jacobs WR, Jr, Bloom BR, et al. Protection elicited by a double leucine and pantothenate auxotroph of Mycobacterium tuberculosis in guinea pigs. Infect Immun. 2004 May;72(5):3031–3037. doi: 10.1128/IAI.72.5.3031-3037.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cayabyab MJ, Hovav AH, Hsu T, Krivulka GR, Lifton MA, Gorgone DA, et al. Generation of CD8+ T-cell responses by a recombinant nonpathogenic Mycobacterium smegmatis vaccine vector expressing human immunodeficiency virus type 1 Env. J Virol. 2006 Feb;80(4):1645–1652. doi: 10.1128/JVI.80.4.1645-1652.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Southern JA, Young DF, Heaney F, Baumgartner WK, Randall RE. Identification of an epitope on the P and V proteins of simian virus 5 that distinguishes between two isolates with different biological characteristics. J Gen Virol. 1991 Jul;72(Pt 7):1551–1557. doi: 10.1099/0022-1317-72-7-1551. [DOI] [PubMed] [Google Scholar]
- 32.Snapper SB, Melton RE, Mustafa S, Kieser T, Jacobs WR., Jr Isolation and characterization of efficient plasmid transformation mutants of Mycobacterium smegmatis. Mol Microbiol. 1990 Nov;4(11):1911–1919. doi: 10.1111/j.1365-2958.1990.tb02040.x. [DOI] [PubMed] [Google Scholar]
- 33.Wards BJ, Collins DM. Electroporation at elevated temperatures substantially improves transformation efficiency of slow-growing mycobacteria. FEMS Microbiol Lett. 1996 Nov 15;145(1):101–105. doi: 10.1111/j.1574-6968.1996.tb08563.x. [DOI] [PubMed] [Google Scholar]
- 34.Pavelka MS, Jr, Chen B, Kelley CL, Collins FM, Jacobs FM, Jacobs Jr WR., Jr Vaccine efficacy of a lysine auxotroph of Mycobacterium tuberculosis. Infect Immun. 2003 Jul;71(7):4190–4192. doi: 10.1128/IAI.71.7.4190-4192.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takahashi H, Nakagawa Y, Pendleton CD, Houghten RA, Yokomuro K, Germain RN, et al. Induction of broadly cross-reactive cytotoxic T cells recognizing an HIV-1 envelope determinant. Science. 1992 Jan 17;255(5042):333–336. doi: 10.1126/science.1372448. [DOI] [PubMed] [Google Scholar]
- 36.Staats HF, Bradney CP, Gwinn WM, Jackson SS, Sempowski GD, Liao HX, et al. Cytokine requirements for induction of systemic and mucosal CTL after nasal immunization. J Immunol. 2001 Nov 1;167(9):5386–5394. doi: 10.4049/jimmunol.167.9.5386. [DOI] [PubMed] [Google Scholar]
- 37.Majlessi L, Rojas MJ, Brodin P, Leclerc C. CD8+-T-cell responses of Mycobacterium-infected mice to a newly identified major histocompatibility complex class I-restricted epitope shared by proteins of the ESAT-6 family. Infect Immun. 2003 Dec;71(12):7173–7177. doi: 10.1128/IAI.71.12.7173-7177.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Honda M, Matsuo K, Nakasone T, Okamoto Y, Yoshizaki H, Kitamura K, et al. Protective immune responses induced by secretion of a chimeric soluble protein from a recombinant Mycobacterium bovis bacillus Calmette-Guerin vector candidate vaccine for human immunodeficiency virus type 1 in small animals. Proc Natl Acad Sci U S A. 1995 Nov 7;92(23):10693–10697. doi: 10.1073/pnas.92.23.10693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yasutomi Y, Reimann KA, Lord CI, Miller MD, Letvin NL. Simian immunodeficiency virus-specific CD8+ lymphocyte response in acutely infected rhesus monkeys. J Virol. 1993 Mar;67(3):1707–1711. doi: 10.1128/jvi.67.3.1707-1711.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawahara M, Matsuo K, Honda M. Intradermal and oral immunization with recombinant Mycobacterium bovis BCG expressing the simian immunodeficiency virus Gag protein induces long-lasting, antigen-specific immune responses in guinea pigs. Clin Immunol. 2006 Apr;119(1):67–78. doi: 10.1016/j.clim.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 41.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998 Mar 5;392(6671):86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 42.Winau F, Kaufmann SH, Schaible UE. Apoptosis paves the detour path for CD8 T cell activation against intracellular bacteria. Cell Microbiol. 2004 Jul;6(7):599–607. doi: 10.1111/j.1462-5822.2004.00408.x. [DOI] [PubMed] [Google Scholar]
- 43.Hinchey J, Lee S, Jeon BY, Basaraba RJ, Venkataswamy MM, Chen B, et al. Enhanced priming of adaptive immunity by a proapoptotic mutant of Mycobacterium tuberculosis. J Clin Invest. 2007 Aug;117(8):2279–2288. doi: 10.1172/JCI31947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Via LE, Deretic D, Ulmer RJ, Hibler NS, Huber LA, Deretic V. Arrest of mycobacterial phagosome maturation is caused by a block in vesicle fusion between stages controlled by rab5 and rab7. J Biol Chem. 1997 May 16;272(20):13326–13331. doi: 10.1074/jbc.272.20.13326. [DOI] [PubMed] [Google Scholar]
- 45.Via LE, Fratti RA, McFalone M, Pagan-Ramos E, Deretic D, Deretic V. Effects of cytokines on mycobacterial phagosome maturation. J Cell Sci. 1998 Apr;111(Pt 7):897–905. doi: 10.1242/jcs.111.7.897. [DOI] [PubMed] [Google Scholar]
- 46.Braunstein M, Espinosa BJ, Chan J, Belisle JT, Jacobs WR., Jr SecA2 functions in the secretion of superoxide dismutase A and in the virulence of Mycobacterium tuberculosis. Mol Microbiol. 2003 Apr;48(2):453–464. doi: 10.1046/j.1365-2958.2003.03438.x. [DOI] [PubMed] [Google Scholar]
- 47.Sarol LC, Imai K, Asamitsu K, Tetsuka T, Barzaga NG, Okamoto T. Inhibitory effects of IFN-gamma on HIV-1 replication in latently infected cells. Biochem Biophys Res Commun. 2002 Mar 8;291(4):890–896. doi: 10.1006/bbrc.2002.6532. [DOI] [PubMed] [Google Scholar]
- 48.Tascon RE, Stavropoulos E, Lukacs KV, Colston MJ. Protection against Mycobacterium tuberculosis infection by CD8+ T cells requires the production of gamma interferon. Infect Immun. 1998 Feb;66(2):830–834. doi: 10.1128/iai.66.2.830-834.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Migueles SA, Laborico AC, Shupert WL, Sabbaghian MS, Rabin R, Hallahan CW, et al. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat Immunol. 2002 Nov;3(11):1061–1068. doi: 10.1038/ni845. [DOI] [PubMed] [Google Scholar]
- 50.Stenger S, Hanson DA, Teitelbaum R, Dewan P, Niazi KR, Froelich CJ, et al. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science. 1998 Oct 2;282(5386):121–125. doi: 10.1126/science.282.5386.121. [DOI] [PubMed] [Google Scholar]
- 51.Liu J, O'Brien KL, Lynch DM, Simmons NL, La Porte A, Riggs AM, et al. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature. 2009 Jan 1;457(7225):87–91. doi: 10.1038/nature07469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kiepiela P, Ngumbela K, Thobakgale C, Ramduth D, Honeyborne I, Moodley E, et al. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat Med. 2007 Jan;13(1):46–53. doi: 10.1038/nm1520. [DOI] [PubMed] [Google Scholar]
- 53.Soares AP, Scriba TJ, Joseph S, Harbacheuski R, Murray RA, Gelderbloem SJ, et al. Bacillus Calmette-Guerin vaccination of human newborns induces T cells with complex cytokine and phenotypic profiles. J Immunol. 2008 Mar 1;180(5):3569–3577. doi: 10.4049/jimmunol.180.5.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Van Rompay KK, Abel K, Lawson JR, Singh RP, Schmidt KA, Evans T, et al. Attenuated poxvirus-based simian immunodeficiency virus (SIV) vaccines given in infancy partially protect infant and juvenile macaques against repeated oral challenge with virulent SIV. J Acquir Immune Defic Syndr. 2005 Feb 1;38(2):124–134. doi: 10.1097/00126334-200502010-00002. [DOI] [PubMed] [Google Scholar]
- 55.Hawkridge T, Scriba TJ, Gelderbloem S, Smit E, Tameris M, Moyo S, et al. Safety and immunogenicity of a new tuberculosis vaccine, MVA85A, in healthy adults in South Africa. J Infect Dis. 2008 Aug 15;198(4):544–552. doi: 10.1086/590185. [DOI] [PMC free article] [PubMed] [Google Scholar]