Abstract
This review discusses rational design of particles for use as therapeutic vectors and diagnostic imaging agent carriers. The emerging importance of both particle size and shape is considered, and the adaptation and modification of soft lithography methods to produce nanoparticles is highlighted. To this end, studies utilizing particles made via a process called Particle Replication In Non-wetting Templates (PRINT™) are discussed. In addition, insights gained into therapeutic cargo and imaging agent delivery from related types of polymer-based carriers are considered.
Keywords: PRINT, nanoparticles, diagnostic imaging, therapeutic drug delivery, shape
With rapid development of new pharmaceuticals and contrast agents, the need for the minimization of side effects in concert with simultaneous targeted delivery to specific tissues of interest continues to expand. Overcoming barriers for effective bioavailability of therapeutic agents has been especially challenging in the fields of gene therapy[1] and oncology.[2] As an illustration, despite their potential for wide application, only a few antisense oligonucleotides or small interfering RNA’s (siRNA) have entered clinical trials. The prevalence of hydrophobic drugs also necessitates the use of nanocarriers; for these systems, direct dissolution in the bloodstream is limited without the formation of a salt or use of a delivery vector.[3] One solution to this problem is the delivery of drugs, gene therapy agents, and imaging contrast agents via nano-scale vectors, and this has been an area of intense study for decades. Although multiple approaches have been explored and strides have been made in therapeutic drug delivery and diagnostic imaging agent carriers, a set of rules for the rational design of nanocarriers has not yet been fully established due to limited understanding of how all of the carrier properties (including size and shape as well as matrix functionality, porosity, flexibility, and surface chemistry) interact. Clearly, a strong motivation for the development of nanocarriers exists in cases in which the drug or imaging agent is too fragile, insoluble, or toxic for direct in vivo administration.
The term “nanoparticle” is broadly applied in the description of almost every pharmaceutical carrier or imaging agent system, so further classification is needed for clarity. One group of nanoparticles is comprised of organic macromolecules including single chain thermoplastic polymer-drug conjugates[4, 5] thermoplastic polymer colloids prepared via techniques such as emulsion polymerization,[6–9] crosslinked nanogel matrices,[10–12] dendrimers[13, 14], and carbon nanotubes.[15] For this group of materials, the carrier matrix is a single synthetic molecule with covalent bonds and a relatively large molar mass. Other types of drug delivery vectors often termed nanoparticles are comprised of self-assemblies of smaller molecules which are aggregated through intermolecular forces. Liposomes[16–18] and polyplexes[19–22] are the most studied of this class of particle, but this class of carriers also includes aggregates such as polymersomes[23, 24] and other assemblies of block copolymers,[25] colloidosomal aggregrates of latex particles,[26, 27] and protein or peptide assemblies such as Abraxane®,[28, 29] a protein-taxol assembly that has already moved through clinical trials. The dynamic nature of these types of systems depends upon the intermolecular forces in play and the biological conditions. Finally, as a testament to the variety of possible approaches to this problem, nanoparticle-based therapies and imaging agents in the pharmaceutical research pipeline include complexes based upon fullerenes, silica,[30] colloidal gold,[31–34] gold nanoshells, quantum dots, and superparamagnetic particles.
The potential delivery solutions of liposomal pharmaceutical carriers[17], polyplexes,[22, 35] and polymer-drug conjugates,[5, 36] which have shown promise on several fronts for decades, have been recently reviewed and will not be thoroughly elaborated upon herein. Indeed, the breadth and volume of therapeutic and imaging nanoparticle literature is considerable, and this review is not intended to be exhaustive. In this report, we focus on fabrication of well-defined, nonspherical, crosslinked or linear polymer particles as vectors for pharmaceutical and imaging agent delivery.
Rational Design of Drug and Contrast Agent Carriers
Several critical factors that must be considered in the design of contrast agent and/or polymeric drug carriers include the chemical functionality and mechanical flexibility of the matrix, the degree of cross-linking, if any, the dispersion or encapsulation of the drug within the matrix, the permeability of the cargo through the matrix of the particle, the number and the nature of phases that comprise the particle (one phase versus two or more phases e. g. drug rich phase and matrix rich phase,) the size and shape of the particle, and the surface chemistry. Many of these factors need to be studied and controlled in particle design for the delivery of imaging contrast agents and the delivery and release of cargos ranging from small molecules to proteins to nucleotides. A large body of in vivo studies has proven that particle size is a crucial factor in biodistribution, treatment of inflammation, and tumor bed penetration. The effect of particle shape, on the other hand, has received much less attention and is therefore not well understood.
Using advances at the interface between biology and the traditionally materials science based field of soft lithography,[37] researchers just recently have been able to access interesting shapes at the sub-micron size range on a sufficient volume scale to allow for extensive in vitro and in vivo biological studies. In this technique, a biological pattern or the pattern on a hard substrate master, typically a silicon wafer, is embossed onto to a more flexible, crosslinked polymer material, the mold. Soft lithography has been used extensively to mold naturally occurring objects and prepare patterns for microfluidics, and these topics have been the subject of recent reviews.[38] Herein, we review recent advances in soft lithography[37, 38] as a means for producing drug delivery and diagnostic imaging particles. A modification of these traditional lithographic methods, known as Particle Replication In Non-wetting Templates (PRINT®) (Figure 1),[39–48] has proven to be valuable for producing precisely-controlled polymeric vectors in the tens of nanometers to micron size range. The PRINT process constitutes a tunable particle preparation platform by allowing entrapment (transiently, if desired) of diagnostic imaging agents and a wide variety of therapeutic cargo types while simultaneously providing tremendous latitude in the chemical composition of the carrier matrix. In addition, the PRINT process is well suited for independent and precise variation of shape, size, and modulus (stiffness) of the particle.[49] Indeed, PRINT affords unprecedented control and flexibility in the engineering of rationally designed particles, and thus it offers advantages as a fabrication method when compared to techniques employed to prepare traditional carriers such as liposomes, dendrimers, and colloidal particles.
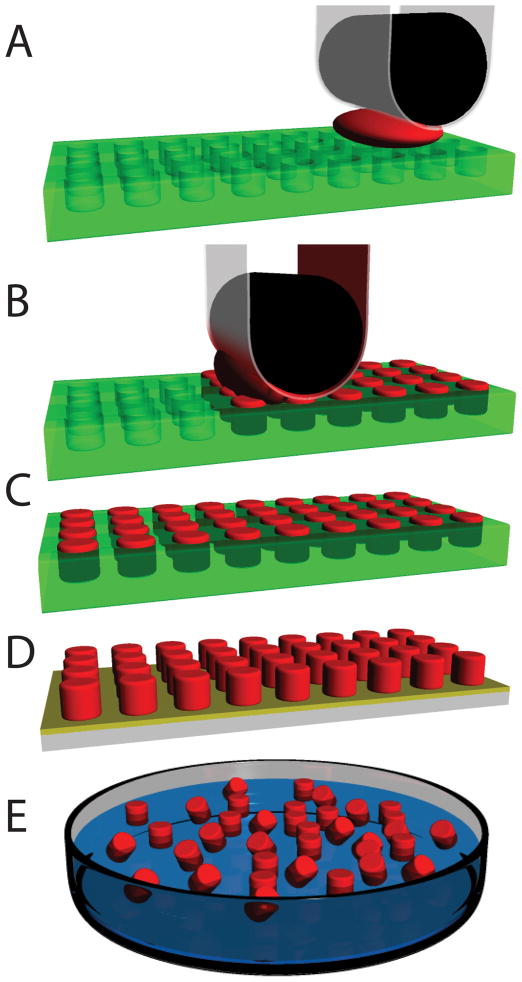
Figure 1.
Schematic representation of the PRINT process. a.) Empty mold (green), high surface energy polymer sheet (clear), roller (black) is brought into contact with the particle precursor solution (red) and the mold; b.) Roller evenly distributes particle precursor solution into the cavities of mold. Excess particle precursor solution is wicked away by the high surface energy polymer sheet; c.) Particles are cured in the mold; d.) Particles are removed from the mold; e.) Particles are collected or harvested using a number of different film based techniques and ultimately are dispersed in solution.
Particle Size and Shape Effects
Until recently, the general conclusions drawn about particle size effects on various biological processes relied on studies using primarily spherical particles. This is due to the fact that drug or contrast agent-loaded spherical particles with a narrow size distribution can be fabricated in a straight forward manner by a number of methods including emulsion, dispersion, and suspension polymerizations as well as precipitation and spraying techniques. The roles that particle size and composition play in biodistribution,[52] cellular binding,[53] cell entry pathways,[54] cell uptake,[55–57] and tumor bed penetration[58, 59] have been extensively studied for macromolecular carriers and spherical particles.
This work with spherical particles has resulted in valuable insights, but many naturally occurring objects are non-spherical, and biological processes typically occur under dynamic conditions in which the motion of spherical and non-spherical objects will differ. Indeed, Decuzzi and Ferrari’s theoretical work predicts that, under conditions of linear shear flow such as that in the bloodstream, oblate particles adhere more strongly to biological substrate than spherical particles; use of non-spherical particles for delivery is predicted to improve therapeutic and imaging efficacy.[60] Experimentally, the combined effects of particle size, shape, charge, and chemical composition on biodistribution and cell entry have not yet been fully elucidated,[61] but data suggest that seemingly small changes in chemical composition and modulus of the particle materials can have profound effects in vivo. Clearly the major roadblock to elucidating the interdependence of particle size, shape, surface chemistry, and modulus is the previous lack of a suitable particle fabrication technique.
Recently, techniques have been developed to prepare non-spherical particles by molding techniques such as the PRINT process, post-fabrication manipulation of spherical particles, or directly through microfluidics. Although particle size impact on bioavailability has been the subject of numerous studies, particle shape, on the other hand, has received less attention. Clearly shape is crucial to the particle’s mechanism of cell entry[44, 62, 63] and the release rate of the therapeutic cargo.[64, 65] Shape effects on biological processes are still not fully understood, particularly at the nanoscale, primarily due to past limitations in the control of particle fabrication. The work exploring shape effects in vitro and in vivo is detailed in the sections below and summarized in Table 1. Experimental explorations into the idea that unpredicted biological effects could result from non-spherical particle shapes are described in this section.
Table 1.
Recent Studies Elucidating Shape Effects on Polymer Nanoparticle-Mediated Drug Delivery and Therapeutic Imaging Applications
| Particle Matrix and Description | Size Range Studied | Shape | Reference |
|---|---|---|---|
| PEG-based Diblock copolymers | 22–66 nm × 0.5–8 μm | Spheres vs. flexible filaments | [66] |
| Polystyrene | 1–10 μm | Spheres, oblate or prolate ellipsoids, elliptical discs, rectangular discs, “UFO’s” | [62, 63] |
| Degradable and non-degradable PEG-based hydrogels | 100 nm – 5 μm | Cubes, cylinders, rods | [44–46, 67] |
| Degradable peptide diacrylates gels vs. Non-degradable PEG-based hydrogels | 50–400 nm | Squares, triangle, pentagons | [68] |
Top Down Methods for Shape-Specific Particle Fabrication
The PRINT Process
Crosslinked fluoropolymers have recently been employed as alternative materials to poly(dimethyl siloxane) (PDMS) for microfluidics[51], as molds for imprint lithography,[49, 60] and as a template for particle design and synthesis via the PRINT process.[40] PRINT is unique over the imprint lithography techniques promulgated by Whitesides et al. in that PRINT uses elastomeric fluoropolymers instead of silicones which results in three distinct features not possible with silicones. First of all, perfluoropolyether elastomers have a lower surface energy which enables the selective filling of nano-scale cavities in the mold using any organic liquid—without wetting the land area around the cavities—which enables distinct objects or particles to be formed at the micro- and nano-scales without the formation of the interconnecting “flash layer” noted as a major hurdle in traditional imprint lithography.[69] Secondly, organic liquids and sol-gel metal oxide precursors do not swell fluoropolymers like they do silicones, so one can make PRINT particles and particle arrays having a wide range of attributes (surface chemistries, degradation characteristics, deformability). Thirdly, the Teflon™-like characteristics of the fluoropolymer mold allows the resultant organic particles to be easily harvested or removed from the mold. Indeed, photocurable perfluoropolyethers (PFPEs) used in the PRINT process retain the advantageous flexibility of PDMS while imparting an even lower surface energy and exhibiting dramatically improved solvent resistance.[50, 51] The PFPE molding materials, which in the uncured state have low viscosities and positive coefficients of spreading on most substrates, have been shown to precisely mold even fragile or “soft” nanoscale objects such as block copolymer micelles and naturally occurring objects such as viruses.[41] Rogers and co-workers have recently published a side-by-side comparison of PDMS versus PFPE-based materials in soft-lithography.[70]
Perhaps the biggest potential drawback of fluorinated molding matrices when compared to silicone materials is cost. This disadvantage has been mitigated by the development of continuous mold manufacturing processes that employ an inexpensive backing material coated with a very thin film of the fluorinated molding material (Fig. 2).[71] This engineering advancement for rapid, economically feasible mold replication has taken the PRINT process from the realm of an academic method to reproduce small features to a scalable particle manufacturing process.
Figure 2.
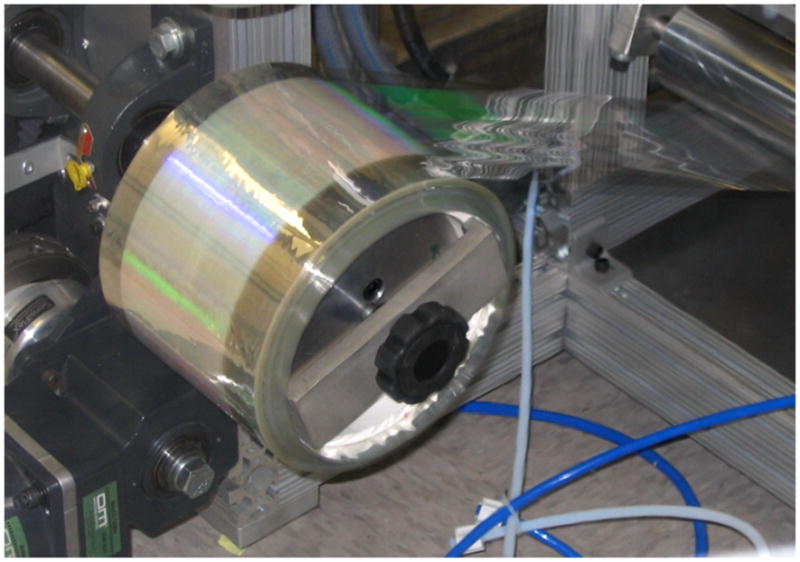
Continuous thin mold manufacturing for the PRINT process. Patterned surface can be seen in green.
Reprinted with permission from Liquidia Technologies.[71]
Using the PRINT process, particles have been constructed from matrices ranging from synthetic materials such as highly crosslinked hydrogels and linear polyesters[40, 42] to natural materials such as pure, biologically relevant proteins (Fig. 3).[48] The PRINT process can be adapted as needed to provide appropriate conditions for mold cavity filling and subsequent solidification of the particles using diverse processing techniques such as lyophilization, solvent evaporation, thermal curing/annealing, photocuring, vitrification, or crystallization. Particles made via the PRINT process have been shown to be effective delivery agents for both therapeutic cargos and imaging agents.[46, 47] For example, a reductively labile crosslinker was incorporated into hydrogel particles in the micron-size range fabricated via the PRINT process, and these particles effectively delivered doxorubicin to cervical cancer cells (HeLa cells.)[47] One of the key advantages of the PRINT process is the ability to precisely control particle size and shape in the nanometer-size regime independently of composition. The impact of this aspect of the technology is expanded in the next sections.
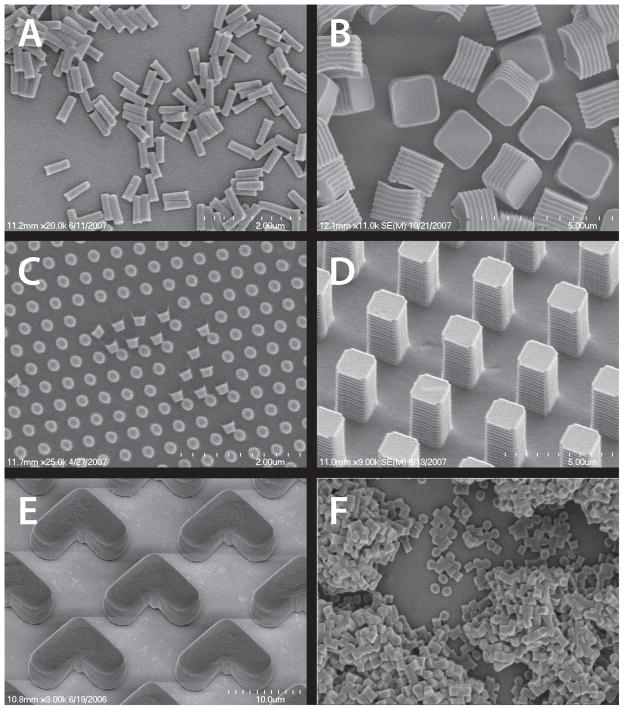
Figure 3.
SEM images of particles of various sizes, shapes, and compositions prepared via the PRINT process: (A) Hydrogel rods containing antisense oligonucleotide; (B) crosslinked degradable matrix cubes containing Doxorubicin HCl; (C) Abraxane™ harvested onto medical adhesive; (D) Insulin particles harvested onto a medical adhesive; (E) Hydrogel “boomerangs” containing 15 wt% iron oxide; (F) Hydrogel cylinders containing 10 wt% Omniscan™.
Reprinted with permission from references (B) [47];(C) [48]; (D) [48]; (E) [46].
Biodistribution and Cell Uptake of PRINT Nanoparticles
A preliminary study of the biodistribution and pharmacokinetic study of [125I]-labeled non-targeted, cylindrical particles prepared by the PRINT process in the 200 nm size range in healthy mice showed the expected uptake primarily in the liver and spleen.[43, 45] The conventional strategies to reduce the rapid clearance from the bloodstream and uptake by the liver and spleen have been to increase hydrophilicity of the particle surface and reduce particle size. In an alternative approach, however, Geng and coworkers have compared soft spherical assemblies with flexible filaments and found that the in vivo circulation time for the non-spherical filomicelles was about ten times longer than their analogous spherical counterparts.[66] They extended their study to the delivery of paclitaxel and showed significant tumor shrinkage in a xenograft mouse tumor model, and showed that an increase in the filomicelle length had the same relative therapeutic effect as a similar increase in the paclitaxel dosage. These results show that, in applications where a prolonged circulation time is desired, a long, worm-like structure can be more effective than a sphere.
In addition to dramatically affecting biodistribution, particle size and shape can play a key role in the mechanism of passage through the cell membrane into the cell. The biological details of cell internalization of macromolecules and particles have been reviewed.[72] Gratton et al. have completed experiments showing size and shape effects on the uptake of highly crosslinked acrylic particles fabricated via the PRINT process by HeLa cells.[44] A large library of crosslinked, PEG-based particles of various sizes and shapes were prepared via the PRINT process. Non-targeting particles fabricated from this formulation showed excellent uptake by HeLa cells (Fig. 4), and cellular uptake mechanisms have been elucidated. This work showed that, interestingly, it is not the particles of lowest volume that enter cells at the fastest rate; rod like particles showed kinetically preferential uptake.
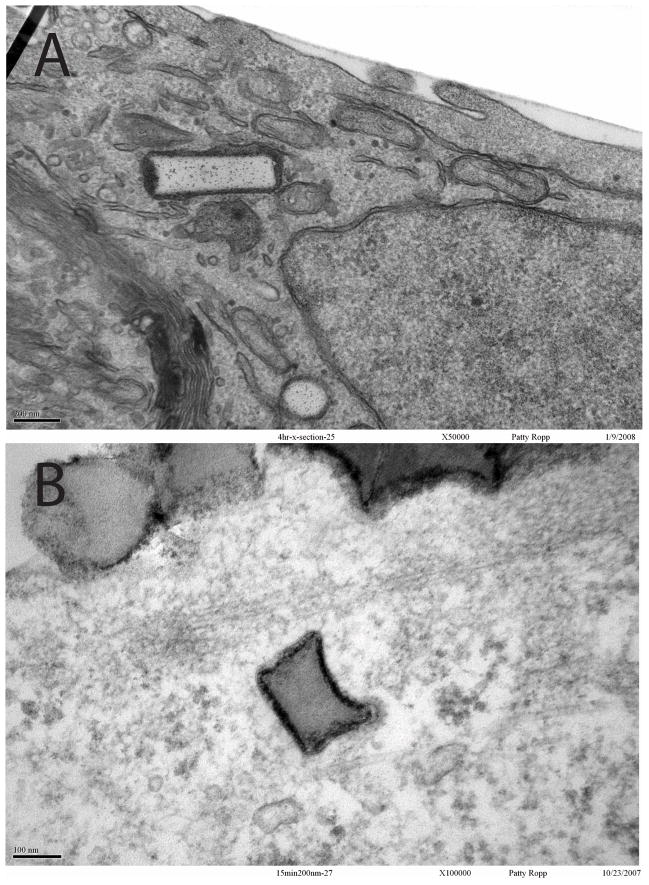
Figure 4.
TEM showing HeLa cell internalization of 150 nm × 450 nm (top) or 200 nm × 200 nm (bottom) cylindrical particles fabricated via the PRINT process.
Reprinted with permission from [44].
Step and Flash Imprint Lithography
In another study of carefully fabricated, non-spherical particles, Roy et al. have used step and flash imprint lithography (S-FIL) to prepare cubes as well as triangular and pentagonal cylinders (Figure 5). Drawing on a large body of literature for enzymatically degradable peptide sequences for drug delivery,[73–76] the authors were able to use cathepsins to trigger release of DNA plasmids from these particles in vitro. This work combined the superior shape control provided by lithographic techniques with clever cargo encapsulation and release strategies.
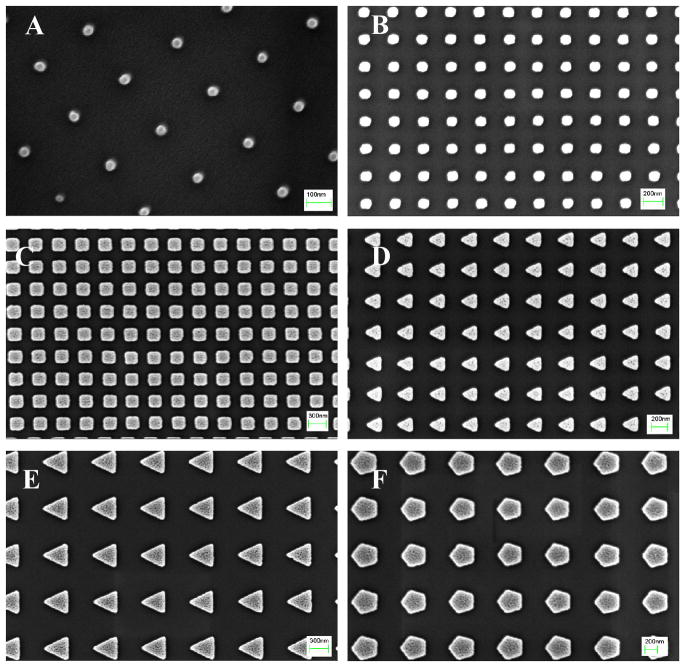
Figure 5.
SEM images of S-FIL imprinted (100% w/v, MW 3400) PEGDA nanoparticles: (A) 50 nm squares (scale bar = 100 nm), (B) 100 nm squares (scale bar = 200 nm), (C) 200 nm squares (scale bar = 300 nm), (D) 200 nm triangles (scale bar = 200 nm), (E) 400 nm triangles (scale bar = 300 nm), and (F) 400 nm pentagonal particles (scale bar = 200 nm).
Reprinted with permission from [68]
Recently Emerging PDMS-Molding Processes for Fabrication
The most widely studied molding material is lightly crosslinked PDMS, commonly referred to as silicone rubber. Advantages of using PDMS as a molding material are numerous and include low toxicity, low modulus (flexibility), low surface energy which allows facile release from master pattern templates as well as replication of molecular-scale objects,[77] and high gas permeability, which allows dead-end filling. For some applications, however, the swelling of PDMS molds in organic solvents and/or PDMS-fragment transfer to the sample constitute serious drawbacks. Alternative silicone-based materials and filled molding material formulations that minimize the mechanical distortion of the stamp have been developed.[78] In addition, various mold surface treatments,[79, 80] such as oxygen plasma and fluorosilane grafting, have been explored in efforts to make the mold surface more hydrophilic or hydrophobic, respectively, which has implications in both wetting during mold filling and release of the particles from the mold. Finally, cleaning procedures have been developed which virtually eliminate sample contamination by small fragments from the PDMS-stamp.[81]
With the target application of drug delivery, Hansford and coworkers have fabricated both thermoplastic and thermoset microparticles and medical devices in various shapes using PDMS stamps.[82–84] The design of unique shapes with “arms” that can self-fold to imbed into intestinal tissue[84] after oral delivery highlights the vast possibilities opened by the tools for precise shape control on the micron and nanometer size regimes. This group also prepared microcapsules for intravenous delivery containing sucrose as a model cargo with a thin poly(vinyl alcohol) (PVA) or poly(lactic-co-glycolic acid) (PLGA) based skin that swells in the presence of water due to osmotic pressure. In this case, release of the drug is dominated by diffusion out of the microcapsules through the thin membrane rather than dissolution of the thermoplastic matrix, so an entirely new drug transport profile is accessible. This offers some advantages over the use of conventionally prepared PGLA microspheres and nanoparticles,[85] such as a relatively high capacity for drug loading.
In another application of PDMS-based soft lithography to drug-carrier fabrication, Oudshoorn and co-workers have explored the use of various molding techniques including rigid micromolding, soft micromolding, and photolithography to prepare uniform microparticles comprised of methacrylated hyperbranched polyglycerols.[79] They obtained high yields of squares and hexagon shapes in the tens to hundreds of microns size regime, but, in the PDMS-based molding experiments, they noted that a number of particles had less well defined shape than they obtained using a rigid, epoxy-based micromold. Clearly, traditional soft lithography using PDMS-based molding materials offers wide latitude in the design of size and shape-specific particles, particularly on the micron size scale, and it constitutes an interesting research path toward delivery and imaging applications. However, for applications such as intracellular drug delivery and imaging where nanoscale vectors are often most efficacious, opportunities exist to improve on PDMS-based soft lithography using alternative molding techniques.
Other Methods for Shape-Specific Particle Fabrication
Because particles used for imaging and drug delivery are generally < 5 microns in their largest dimension, they are comparable in size to the pathogens combated during the evolution of the immune system.[86] Micron-sized range particles (0.5 μm and larger) have been described as invoking “bacteria-like responses,” primarily being removed from circulation via phagocytosis, while nanoparticles up to 200 nm in diameter are more likely to invoke “virus-like responses.”[86] Exploiting this trend, nanoparticles have been intentionally designed to either invoke, suppress, or avoid the immune response.[87] Pronounced invocation of the immune response has driven size-dependence research in particulate vaccine vectors (adjuvants),[88–90] suppression of an immune response could make particulate carriers useful during organ transplant therapy, while avoidance of the immune system and reduced systemic toxicity using targeted particulate carriers is most often desirable in the case of imaging and therapeutic agents.
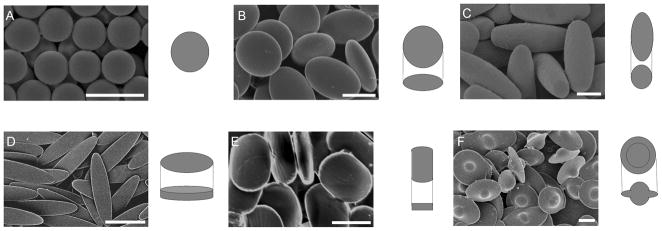
The emerging understanding of the importance of particle shape in phagocytosis has been described and carefully studied by Champion et al. (Figure 6). [62, 63] Recognition of the fact that naturally occurring immunological targets vary widely in both size and shape provided motivation for this work. By carefully varying shape at constant size, the authors concluded that it is indeed particle shape, rather than size, which plays a dominant role for determining the complexity of the local actin structure, and ultimately whether phagocytosis or simply spreading processes occur.
Figure 6.
a) Scanning electron micrographs and 3D illustrations of PS particles created for phagocytosis experiments. (A) Spheres. (B) Oblate ellipsoids (13%). (C) Prolate ellipsoids (7%). (D) Elliptical disks (9%). (E) Rectangular disks (5%). (F) UFOs (12%). Particles are monodispersed with average standard deviations of measured dimensions for each shape listed in parentheses. A portion of this variation is due to 2–5%standard deviation in the diameter of spheres used as starting materials. (Scale bars: 5 m.)
Reprinted with permission from [62]
In summary, numerous studies have shed light on the roles played by size and shape of particulate carriers in the complicated biological processes of biodistribution, cell uptake, and bioavailability. The ability to prepare particles in a manner than allows independent alteration of one variable at a time has proven fruitful for gaining insights into shape-dependent biological processes such as phagocytosis. Many techniques, such as stretching thermoplastic particles[62] or S-FIL preparation of crosslinked particles as described above,[68] can be employed to prepare small amounts of shape-specific particles. Practical, large-scale manufacturing techniques for fabrication of non-spherical, shape-specific particles constitute perhaps the biggest advantage gained by the application of the PRINT process to this area.
Imaging Modalities
While size and shape clearly play an important role in nanoparticle drug delivery, the field of medical imaging has seen very few applications or systematic studies exploring the effect of variation in particle size and shape. Therefore, the potential for important discoveries in the field of size and shape dependence on nanoparticle biodistribution and targeting is enormous. Without a doubt, studies directed toward successful targeted delivery of size and shape-specific nanoparticles as novel therapeutic agents will be contingent on the successful visualization of their whereabouts in vivo. Moreover, nanoparticle-mediated delivery provides an opportunity to reduce the toxicity effects associated with commonly employed contrast enhancement agents.
A number of different imaging modalities can be drawn upon for successful imaging of tissue via this new class of particles including, but not limited to, magnetic resonance (MR) imaging, positron emission tomography (PET), single photon emission computed tomography (SPECT), ultrasound imaging, and fluorescent imaging. Here we focus on recent advances for imaging agent carriers in MR and PET imaging.
MR Imaging
Magnetic Resonance (MR) imaging plays an ever increasing role in the development of targeted nanoparticle therapeutics and imaging agents because of its high spatial resolution and unparalleled imaging of soft tissue. Using conventional contrast agents such as iron oxide nanoparticles and small molecule gadolinium (Gd) chelates, MR imaging is capable of providing excellent spatial resolution and 3D anatomical information. Because of its low sensitivity, however, relatively large concentrations of contrast agent are required to observe a modest increase in contrast (especially for T1 weighted imaging). The potential side effects (such as nephrogenic systemic fibrosis or NSF) caused by these relatively large contrast agent doses have spurred interest in particle-mediated delivery of MR contrast agents. These contrast agents carry multiple MR beacons per particle and are capable of drastically improving the local contrast. Nanoparticle contrast agents are paving the way for a new generation of imaging in which smaller doses will be required both to monitor the in vivo distribution of nanoparticles and to perform targeted imaging of diseased tissue and tumors. Some of the most recent efforts to exploit polymer-based nanoparticles for delivery of iron oxide and Gd, respectively, are summarized below, and then initial efforts to apply the PRINT process to this problem are detailed.
In many respects, iron oxide nanocrystals are close to ideal nanoparticle contrast agents. They are nontoxic and are easily surface functionalized with stealthing and targeting agents.[91] Monodisperse samples of iron oxide nanocrystals have been shown to have different R1 and R2 relaxivities based solely on crystal size[92] as well as on the surface coating.[93] Spherical micelles of poly(styrene-b-acrylic acid) containing multiple iron oxide nanoparticles were recently reported by Taton and coworkers.[94] The micelles were surface crosslinked into a covalently bound assemblies in the 40 – 140 nm size range using a diamine and functionalized further with a fluorescein derivative. Using this method, larger polymer particles necessarily contained a larger number of iron oxide particles. A different method recently reported by Sailor et al. describes improved relaxivity and improved targeting of tumor cells over traditional iron oxide nanoparticles using nanoworms.[95] The worms were composed of iron oxide nanoparticles trapped in strands of dextran.[95] The authors believe that the unique shape of the elongated particles is the key to improving their efficacy.
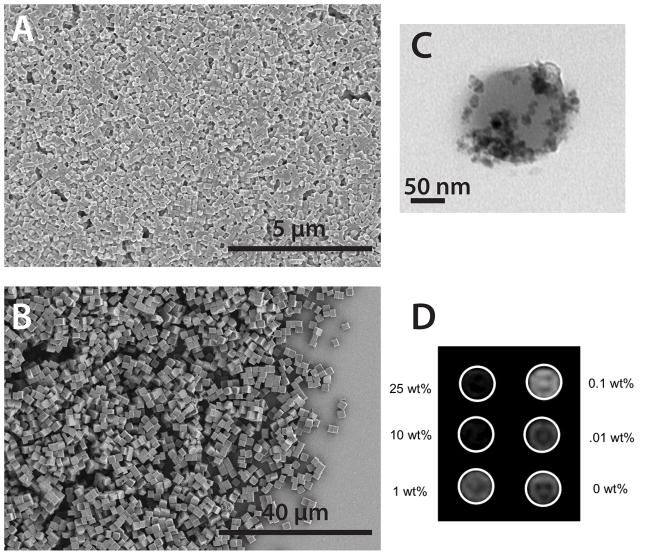
Herlihy et al. have fabricated 200 × 200 nm cylindrical and 2 × 2 × 2 μm cubic particles of a biocompatible hydrogel matrix containing iron oxide nanoparticles via the PRINT process (Figure 7).[46] This constitutes the first example of shape and size specific magnetic hydrogel particles produced by a lithographically-based process. They were able to systematically control the iron oxide content without altering the size or shape of the particles. Increasing the cargo content led to a decrease in signal intensity in T2 weighted phantom studies.[46] Since the cargo content for particles fabricated via the PRINT process can be controlled independently from the particle’s shape, size, and surface functionality, this provides a unique opportunity to tune the signal intensity without affecting other key factors such as biodistribution.
Figure 7.
Magneto-polymer composite polymer particles of well defined size and shape prepared via the PRINT process: (A) SEM image of 200 nm × 200 nm cylinders, (B) SEM image of 2×2×2 micron cubes (C) TEM of biocompatible 200 × 200 nm particle containing 15 wt% PEG-silane coated iron oxide nanocrystals, and (D) T2 phantom study of iron oxide containing particles in agarose gel. Equimolar concentrations of PRINT particles with increasing iron oxide loading.
As stated earlier, iron oxide based imaging agents are popular because of their low level of toxicity and the ease with which one can modify their surface with a variety of ligands, stealthing polymer coatings and fluorescent beacons for multimodal imaging. However, because the majority of iron oxide contrast agents create a decrease in signal intensity, causing tissue containing the contrast agent to “go dark,” researchers have pursued other compounds for contrast agents that increase signal intensity.
Small molecule chelates of Gd such as Gd-DTPA (diethylenetriaminepentaacetic acid) and Gd-DOTA (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid) and their derivatives create an increase in MR signal intensity. Caravan has described how the limited sensitivity of MR requires millimolar concentrations of these agents before any contrast is observed.[96] Coupling multiple Gd chelates to a single nanoparticle carrier can increase contrast enhancement. A number of synthetic approaches towards gadolinium-bound particles have been described including crosslinked micelles,[97] peptide containing liposomes,[98] dendrimers,[99] and hybrid systems.[100]
Very few examples are found of shape and size specific nanoparticles containing gadolinium. One approach involves the encapsulation of MR contrast agent within a nanocapsule. Landfester et al. demonstrate the use of inverse miniemulsions to encapsulate commercially available gadolinium-containing contrast agents inside crosslinked dextran nanocapsules.[101] The capsules are porous in nature allowing water exchange across the surface. While the nanocapsules show a small reduction in relaxivity from that of the small molecule encapsulated within, the crosslinked dextran coating provides potential for surface functionalization and altered biodistribution.
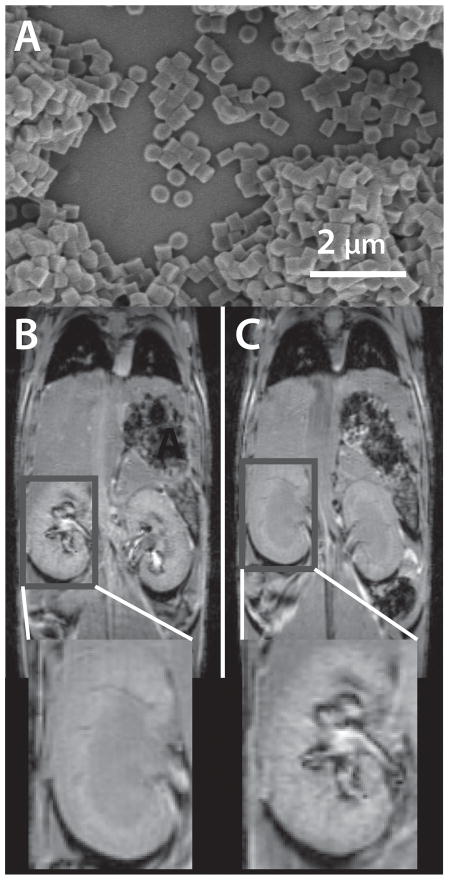
Recently Herlihy et al. demonstrated the first example of Gd-containing shape and size specific particles for MR imaging (Figure 8).[67] Two sets of biocompatible hydrogel particles, 200 × 200 nm cylinders and 2 × 2 × 2 μm cubes encapsulating a commercially available Gd contrast agent (Omniscan™), were administered to healthy mice. Particle biodistribution was different depending on the size of the particles. The small particles created an increase in contrast in the liver, kidney and blood vessels while the larger particles were observed mostly in the liver and blood vessels. Increased contrast was observed up to 3 hours after injection.
Figure 8.
T1 weighted MR images a bolus injection of 200 × 200 nm cylindrical particles (a) just before (b) and 60 minutes post (c) injection.
Position Emission Tomography (PET)
A number of groups have also adopted a nanoparticle platform for imaging using positron emission tomography (PET). PET relies on radioisotopes that can easily be bound to the surface, or interior, of a nanoparticle using simple chelation chemistry. Positron emitters such as 64Cu are detectable at concentrations as low as picograms per milliliter. The high sensitivity of PET makes it ideal for quantitative data analysis. Some limitations of PET include low spatial resolution, the inability to provide significant anatomical information, and need for contrast agents that are not widely available.
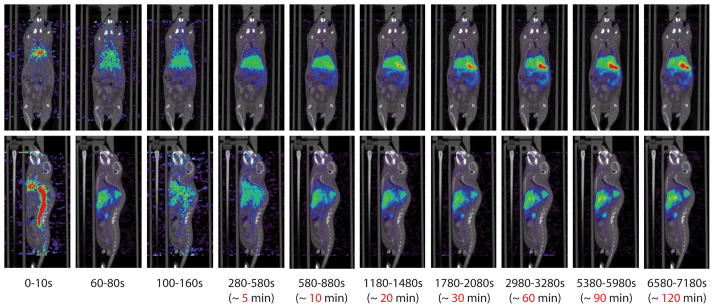
Work performed by Gratton et al. showed the first example of shape and size specific nanoparticles bound to 64Cu-DOTA for use in in vivo PET imaging (Figure 9).[45] To make these particles, DOTA-NHS-ester was bound to the surface of a 200 × 200 nm cylindrical, biocompatible hydrogel particle. DOTA is widely popular for binding radioisotopes such as 64Cu to polymers. The particles were sterically stabilized with high molecular weight PEG. Similar to the 200 nm particles used for MR, and supporting other biodistribution studies,[44] the particles containing 64Cu appeared to accumulate in the liver.
Figure 9.
MicroPET imaging with 64Cu-DOTA PRINT particles. Time resolved PET images consisting of a two hour dynamic scan. The PET/CT images are overlayed. Mouse was injected with 136.2 μCi of 64Cu-labeled DOTA-nanoparticle. Both the coronal view (top), and sagittal view (bottom) are presented.
Reprinted with permission from [45]
In 2007, Wooley et al. reported the optimization of radiolabeling crosslinked micellar assemblies polystyrene-b-poly(acrylic acid) with 64Cu-DOTA.[102] In this work, a DOTA-lysine derivative is covalently bound to the acrylic acid units of the copolymer that is subsequently labeled with 64Cu. The copolymers were then used to form micelles and were surface-crosslinked using 2,2′-(ethylenedioxy)-bis(ethylamine). By varying the relative amounts of DOTA-lysines per chain and the crosslinking ratio they were able to optimize signal enhancement as high as 400μCi/μg. Interestingly, the researchers found that the non-crosslinked micelles provided the most intense signal but pointed to the necessity of surface crosslinking for stable nanocontrast agents.
Using a surface crosslinked platform similar to the one described earlier, Wooley and coworkers[97] made 40 nm “knedel-like” particles with high relaxivities (39−1 mM−1). The high relaxivity was attributed to the slow rotation of the particle. In this work the particles were composed of assemblies of poly(acrylic acid-b-methyl acrylate) surface crosslinked with the same diamine. The particles were functionalized with a diethylenetriaminepentaacetic acid (DTPA) derivative that coordinated gadolinium. Nanoparticle platforms such as these may be useful in the future as molecular imaging agents.
Conclusion
In summary, nanoparticle mediated delivery of therapeutic and contrast agents constitutes a growing field of research. New techniques for independently controlling particle size, shape, composition, cargo loading, and surface functionality are beginning to pave the way for understanding the complicated interplay between these particle parameters and biological systems.
We have reviewed a number of studies in which polymer-based nanoparticles have been used as carriers for therapeutics and imaging contrast agents. Striking recent studies from a couple of different research groups have indicated that anisotropic, worm-like particles can enhance circulation time in comparison to previous studies with spherical particles. In addition, soft lithography methods, such as the PRINT process, are becoming valuable tools in the repertoire of techniques for imparting size, shape, and composition control. With this new capability, identical size and shape particles have been prepared for a number of different functions from delivery of small molecule chemotherapeutics to imaging kidneys. Moreover, independent variation of particle shape at constant composition has shown shape to be critical in areas such as cell entry kinetics.
Acknowledgments
We thank the STC Program of the NSF (CHE-9876674), NIH PO1-GM059299-07 (PPG), NIH U54-CA119343 (the Carolina Center of Cancer Nanotechnology Excellence), and Liquidia Technologies, Inc. for funding. We also thank Liz Enlow and Doug Betts for constructive comments during the editing of the manuscript.
Footnotes
Cross-References
MR relaxation properties of superparamagnetic iron oxide particles
Microlithographic delivery devices
Nano-encapsulation technology to deliver native protein drugs
Contributor Information
Dorian A. Canelas, Email: dcanelas@email.unc.edu, Department of Chemistry, University of North Carolina at Chapel Hill, Chapel Hill, NC, 27599
Kevin P. Herlihy, Email: herlihy@email.unc.edu, Department of Chemistry, University of North Carolina at Chapel Hill, Chapel Hill, NC, 27599
Joseph M. DeSimone, Email: desimone@unc.edu, Department of Chemistry, University of North Carolina at Chapel Hill, Chapel Hill, NC, 27599, Department of Pharmacology, University of North Carolina at Chapel Hill, Chapel Hill, NC, 27599, Lineberger Comprehensive Cancer Center, University of North Carolina, Chapel Hill, NC, 27599, and Department of Chemical Engineering, North Carolina State University, Raleigh, NC, 27695
References
- 1.Wong SY, Pelet JM, Putnam D. Polymer Systems for Gene Delivery - Past, Present, and Future. Prog Polym Sci. 2007;32:799–837. [Google Scholar]
- 2.Zamboni WC. Concept and Clinical Evaluation of Carrier-Mediated Anticancer Agents. The Oncologist. 2008;13:248–260. doi: 10.1634/theoncologist.2007-0180. [DOI] [PubMed] [Google Scholar]
- 3.Nie S, Xing Y, Kim GJ, Simons JW. Nanotechnology: Applications in Cancer. Annu Rev Biomed Eng. 2007;9:257–288. doi: 10.1146/annurev.bioeng.9.060906.152025. [DOI] [PubMed] [Google Scholar]
- 4.Cuchelkar V, Kopeček J. Polymer-Drug Conjugates. In: Uchegbu IF, Schaetzlain AG, editors. Polymers in Drug Delivery. Boca Raton, FL: CRC Press LLC; 2006. pp. 155–182. [Google Scholar]
- 5.Duncan R. The Dawning Era of Polymer Therapeutics. Nature Reviews Drug Discovery. 2003;2:347–360. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- 6.Kriwet B, Walter E, Kissel T. Synthesis of Bioadhesive Poly(acrylic acid) Nano- and Microparticles Using an Inverse Emulsion Polymerization Method for the Entrapment of Hydrophilic Drug Candidates. J Controlled Release. 1998;56(1–3):149–158. doi: 10.1016/s0168-3659(98)00078-9. [DOI] [PubMed] [Google Scholar]
- 7.Lambert G, Fattal E, Pinto-Alphandary H, Gulik A, Couvreur P. Polyisobutylcyanoacrylate Nanocapsules Containing an Aqueous Core as a Novel Colloidal Carrier for the Delivery of Oligonucleotides. Pharmaceutical Research. 2000;17(6):707–714. doi: 10.1023/a:1007582332491. [DOI] [PubMed] [Google Scholar]
- 8.Yan X, Gemeinhart RA. Cisplatin Delivery from Poly(acrylic acid-co-methyl methacrylate) Microparticles. J Controlled Release. 2005;106(1–2):198–208. doi: 10.1016/j.jconrel.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Gao K, Jiang X. Influence of Particle Size on Transport of Methotrexate Across Blood Brain Barrier by Polysorbate 80-coated Polybutylcyanoacrylate Nanoparticles. Int J Pharm. 2006;310(1–2):213–219. doi: 10.1016/j.ijpharm.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 10.Murthy N, Xu M, Schuck S, Kunisawa J, Shastri N, Fréchet JMJ. A Macromolecular Delivery Vehicle for Protein-based Vaccines: Acid-degradable Protein-loaded Microgels. Proc Natl Acad Sci USA. 2003;100(9):4995–5000. doi: 10.1073/pnas.0930644100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oh JK, Siegwart DJ, Matyjaszewski K. Synthesis and Biodegradation of Nanogels as Delivery Carriers for Carbohydrate Drugs. Biomacromolecules. 2007;8:3326–3331. doi: 10.1021/bm070381+. [DOI] [PubMed] [Google Scholar]
- 12.McAllister K, Sazani P, Adam M, Cho MJ, Rubinstein M, Samulski RJ, DeSimone JM. Polymeric Nanogels Produced via Inverse Microemulsion Polymerization as Potential Gene and Antisense Delivery Agents. J Am Chem Soc. 2002;124(51):15198–15207. doi: 10.1021/ja027759q. [DOI] [PubMed] [Google Scholar]
- 13.Lee CC, MacKay JA, Frechet JMJ, Szoka FC. Designing Dendrimers for Biological Applications. Nature Biotechnology. 2005;23(12):1517–1526. doi: 10.1038/nbt1171. [DOI] [PubMed] [Google Scholar]
- 14.Bai S, Thomas C, Rawat A, Ahsan F. Recent Progress in Dendrimer-Based Nanocarriers. Critical Reviews in Therapeutic. Drug Carrier Systems. 2006;23(6):437–495. doi: 10.1615/critrevtherdrugcarriersyst.v23.i6.10. [DOI] [PubMed] [Google Scholar]
- 15.Singh R, Pantarotto D, Lacerda L, Pastorin G, Klumpp C, Prato M, Bianco A, Kostarelos K. Tissue Biodistribution and Blood Clearance Rates of Intravenously Administered Carbon Nanotubes Radiotracers. Proc Natl Acad Sci USA. 2006;103(9):3357–3362. doi: 10.1073/pnas.0509009103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karmali PP, Chaudhuri A. Cationic Liposomes as Non-Viral Carriers of Gene Medicines: Resolved Issues, Open Questions, and Future Promises. Medicinal Research Reviews. 2007;27(5):696–722. doi: 10.1002/med.20090. [DOI] [PubMed] [Google Scholar]
- 17.Torchilin V. Recent Advances with Liposomes as Pharmaceutical Carriers. Nature Reviews Drug Discovery. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 18.Samad A, Sultana Y, Aqil M. Liposomal Drug Delivery Systems: An Update Review. Current Drug Delivery. 2007;4(4):297–305. doi: 10.2174/156720107782151269. [DOI] [PubMed] [Google Scholar]
- 19.Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr J-P. A Versatile Vector for Gene and Oligonucleotide Transfer Into Cells in Culture and in vivo: Polyethylenimine. Proc Natl Acad Sci USA. 1995;92(16):7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Felgner PL, Barenholz Y, Behr JP, Cheng SH, Cullis P, Huang L, Jessee JA, Seymour L, Szoka F, Thierry AR, Wagner E, Wu G. Nomenclature for Synthetic Gene Delivery Systems. Hum Gene Ther. 1997;8(5):511–513. doi: 10.1089/hum.1997.8.5-511. [DOI] [PubMed] [Google Scholar]
- 21.Pun SH, Davis ME. Development of a Nonviral Gene Delivery Vehicle for Systemic Application. Bioconjugate Chem. 2002;13(3):630–639. doi: 10.1021/bc0155768. [DOI] [PubMed] [Google Scholar]
- 22.Jeong JH, Kim SW, Park TG. Molecular Design of Functional Polymers for Gene Therapy. Prog Polym Sci. 2007;32(11):1239–1274. [Google Scholar]
- 23.Discher DE, Ahmed F. Polymersomes. Annu Rev Biomed Eng. 2006;8:323–341. doi: 10.1146/annurev.bioeng.8.061505.095838. [DOI] [PubMed] [Google Scholar]
- 24.Discher DE, Ortiz V, Srinivas G, Klein ML, Kim Y, Christian D, Cai S, Photos P, Ahmed F. Emerging Applications of Polymersomes in Delivery: From Molecular Dynamics to Shrinkage of Tumors. Prog Polym Sci. 2007;32(8–9):838–857. doi: 10.1016/j.progpolymsci.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forrest ML, Yanez JA, Remsberg CM, Ohgami Y, Kwon GS, Davies NM. Paclitaxel Prodrugs with Sustained Release and High Solubility in Poly(ethylene glycol)-b-poly(e-caprolactone) Micelle Nanocarriers: Pharmacokinetic Disposition, Tolerability, and Cytotoxicity. Pharmaceutical Research. 2007;25(1):194–206. doi: 10.1007/s11095-007-9451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dinsmore AD, Hsu MF, Nikolaides MG, Marquez M, Bausch AR, Weitz DA. Colloidosomes: Selectively Permeable Capsules Composed of Colloidal Particles. Science. 2002;298:1006–1009. doi: 10.1126/science.1074868. [DOI] [PubMed] [Google Scholar]
- 27.Shilpi S, Jain A, Gupta Y, Jain SK. Colloidosomes: An Emerging Vesicular System in Drug Delivery. Critical Reviews in Therapeutic. Drug Carrier Systems. 2007;24(4):361–391. doi: 10.1615/critrevtherdrugcarriersyst.v24.i4.20. [DOI] [PubMed] [Google Scholar]
- 28.www.abraxane.com.
- 29.Hawkins MJ, Soon-Shiong P, Desai N. Protein Nanoparticles as Drug Carriers in Clinical Medicine. Adv Drug Deliv Rev. 2008;60(8):876–885. doi: 10.1016/j.addr.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 30.Barbé C, Bartlett J, Kong L, Finnie K, Lin HQ, Larkin M, Calleja S, Bush A, Calleja G. Silica Particles: A Novel Drug-Delivery System. Adv Mater. 2004;16(21):1959–1966. [Google Scholar]
- 31.Kim J-H, Lee TR. Thermo- and pH-Responsive Hydrogel Coated Gold Nanoparticles. Chem Mater. 2004;16(19):3647–3651. [Google Scholar]
- 32.Rosi NL, Giljohann DA, Thaxton CS, Lytton-Jean AKR, Han MS, Mirkin CA. Oligonucleotide-Modified Gold Nanoparticles for Intracellular Gene Regulation. Science. 2006;312:1027–1030. doi: 10.1126/science.1125559. [DOI] [PubMed] [Google Scholar]
- 33.Chithrani BD, Ghazani AA, Chan WCW. Determining the Size and Shape Dependence of Gold Nanoparticle Uptake into Mammalian Cells. Nano Lett. 2006;6(4):662–668. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- 34.Chithrani BD, Chan WCW. Elucidating the Mechanism of Cellular Uptake and Removal of Protein-Coated Gold Nanoparticles of Different Sizes and Shapes. Nano Lett. 2007;7(6):1542–1550. doi: 10.1021/nl070363y. [DOI] [PubMed] [Google Scholar]
- 35.Heidel JD, Mishra S, Davis ME. Advances in Biochemical Engineering/Biotechnology. Vol. 99. Berlin: Springer GmbH; 2005. Molecular Conjugates, in Schaffer DV, Zhou W (eds): Gene Therapy and Gene Delivery Systems; pp. 7–39. [DOI] [PubMed] [Google Scholar]
- 36.Pasut G, Veronese FM. Polymer-Drug Conjugation, Recent Achievements and General Strategies. Prog Polym Sci. 2007;32(8–9):933–961. [Google Scholar]
- 37.Whitesides GM, Ostuni E, Takayama S, Jiang X, Ingber DE. Soft Lithography in Biology and Biochemistry. Annu Rev Biomed Eng. 2001;3:335–373. doi: 10.1146/annurev.bioeng.3.1.335. [DOI] [PubMed] [Google Scholar]
- 38.Gates BD, Xu Q, Stewart M, Ryan D, Willson CG, Whitesides GM. New Approaches to Nanofabrication: Molding, Printing, and Other Techniques. Chem Rev. 2005;105(4):1171–1196. doi: 10.1021/cr030076o. [DOI] [PubMed] [Google Scholar]
- 39.PRINT® is a registered trademark of Liquidia Technologies.
- 40.Rolland JP, Maynor BW, Euliss LE, Exner AE, Denison GM, DeSimone JM. Direct Fabrication and Harvesting of Monodisperse, Shape-Specific Nanobiomaterials. J Am Chem Soc. 2005;127(28):10096–10100. doi: 10.1021/ja051977c. [DOI] [PubMed] [Google Scholar]
- 41.Maynor BW, LaRue I, Hu Z, Rolland JP, Pandya A, Fu Q, Liu J, Spontak RJ, Sheiko S, Samulski RJ, Samulski ET, DeSimone JM. Supramolecular Nanomimetics: Replication of Micelles, Viruses, and Other Naturally Occurring Nanoscale Objects. Small. 2007;3(5):845–849. doi: 10.1002/smll.200600507. [DOI] [PubMed] [Google Scholar]
- 42.Olson DA, Gratton SEA, DeSimone JM, Sheares VV. Amorphous Linear Aliphatic Polyesters for the Facile Preparation of Tunable Rapidly Degrading Elastomeric Devices and Delivery Vectors. J Am Chem Soc. 2006;128(41):13625–13633. doi: 10.1021/ja063092m. [DOI] [PubMed] [Google Scholar]
- 43.Gratton SEA, Pohlhaus PD, Lee J, Guo J, Cho MJ, DeSimone JM. Nanofabricated Particles for Engineered Drug Therapies: A Preliminary Biodistribution Study of PRINT Nanoparticles. J Controlled Release. 2007;121(1–2):10–18. doi: 10.1016/j.jconrel.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gratton SEA, Ropp PA, Pohlhaus PD, Luft JC, Madden VJ, Napier M, DeSimone JM. Effect of Particle Design on Cellular Internalization Pathways. Proc Natl Acad Sci USA. 2008 doi: 10.1073/pnas.0801763105. accepted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gratton SEA, Williams SS, Napier M, Pohlhaus PD, Zhou Z, Wiles KB, Maynor BW, Shen C, Tseng H-R, Phelps ME, Samulski ET, DeSimone JM. The Pursuit of a Scalable Nano-Fabrication Platform for Use in Material and Life Science Applications. Accounts of Chemical Research 2008. 2008 doi: 10.1021/ar8000348. (accepted for publication) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herlihy KP, DeSimone JM. Magneto-polymer Composite Particles Fabricated Utilizing Patterned Perfluoropolyether Elastomer Molds. SPIE Preprint. 2007;6517(2) [Google Scholar]
- 47.Petros RA, Ropp PA, DeSimone JM. Reductively Labile PRINT Particles for the Delivery of Doxorubicin to HeLa Cells. J Am Chem Soc. 2008;130(15):5008–5009. doi: 10.1021/ja801436j. [DOI] [PubMed] [Google Scholar]
- 48.Kelly JY, DeSimone JM. Shape-Specific, Mono-Disperse Nano-Molding of Protein Particles. J Am Chem Soc. 2008;130(16):5438–39. doi: 10.1021/ja8014428. [DOI] [PubMed] [Google Scholar]
- 49.DeSimone JM, Gratton SEA, Galloway AL, Murphy AJ, Pohlhaus PD. Organic Delivery Vehicles for Probing and Treating Biological Systems: Adapting Fabrication Processes from the Electronics Industry for Use in Nano-Medicine. Polym Mater Sci Eng. 2007;96:268. [Google Scholar]
- 50.Rolland JP, Hagberg EC, Denison GM, Carter KR, DeSimone JM. High-Resolution Soft Lithography: Enabling Materials for Nanotechnologies. Angew Chem Int Ed. 2004;43(43):5796–5799. doi: 10.1002/anie.200461122. [DOI] [PubMed] [Google Scholar]
- 51.Rolland JP, Van Dam RM, Schorzman DA, Quake SR, DeSimone JM. Solvent-Resistant Photocurable Liquid Fluoropolymers for Microfluidic Device Fabrication. J Am Chem Soc. 2004;126(8):2322–2323. doi: 10.1021/ja031657y. [DOI] [PubMed] [Google Scholar]
- 52.Sun X, Rossin R, Turner JL, Becker ML, Joralemon MJ, Welch MJ, Wooley KL. An Assessment of the Effects of Shell Cross-linked Nanoparticle Size, Core Composition, and Surface PEGylation on in Vivo Biodistribution. Biomacromolecules. 2005;6(5):2541–2554. doi: 10.1021/bm050260e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cortez C, Tomaskovic-Crook E, Johnston APR, Scott AM, Nice EC, Heath JK, Caruso F. Influence of Size, Surface, Cell Line, and Kinetic Properties on the Specific Binding of A33 Antigen-Targeted Multilayered Particles and Capsules to Colorectal Cancer Cells. ACS Nano. 2007;1(2):93–102. doi: 10.1021/nn700060m. [DOI] [PubMed] [Google Scholar]
- 54.Rejman J, Oberle V, Zuhorn IS, Hoekstra D. Size-Dependent Internalization of Particles Via the Pathways of Clathrin- and Caveolae-Mediated Endocytosis. Biochem J. 2004;377:159–169. doi: 10.1042/BJ20031253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zauner W, Farrow NA, Haines AMR. In vitro Uptake of Polystyrene Microspheres: Effect of Particle Size, Cell Line, and Cell Density. J Controlled Release. 2001;71(1):39–51. doi: 10.1016/s0168-3659(00)00358-8. [DOI] [PubMed] [Google Scholar]
- 56.Win KY, Feng S-S. Effects of Particle Size and Surface Coating on Cellular Uptake of Polymeric Nanoparticles for Oral Delivery of Anticancer Drugs. Biomaterials. 2005;26:2713–2722. doi: 10.1016/j.biomaterials.2004.07.050. [DOI] [PubMed] [Google Scholar]
- 57.Hu Y, Xie J, Tong YW, Wang C-H. Effect of PEG Conformation and Particle Size on the Cellular Uptake Efficiency of nanoparticles with the HepG2 Cells. J Controlled Release. 2007;188:7–17. doi: 10.1016/j.jconrel.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 58.Goodman TT, Olive PL, Pun SH. Increased Nanoparticle Penetration in Collagenase-treated Multicellular Spheroids. Int J Nanomedicine. 2007;2(2):265–274. [PMC free article] [PubMed] [Google Scholar]
- 59.Dreher MR, Liu W, Michelich CR, Dewhirst MW, Yuan F, Chilkoti A. Tumor Vascular Permeability, Accumulation, and Penetration of Macromolecular Drug Carriers. J Nat Cancer Inst. 2006;98(5):335–344. doi: 10.1093/jnci/djj070. [DOI] [PubMed] [Google Scholar]
- 60.Decuzzi P, Ferrari M. The Adhesive Strength of Non-Spherical Particles Mediated by Specific Interactions. Biomaterials. 2006;27(30):5307–5314. doi: 10.1016/j.biomaterials.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 61.Minchin R. Sizing Up Targets with Nanoparticles. Nature Nanotechnology. 2008;3(1):12–13. doi: 10.1038/nnano.2007.433. [DOI] [PubMed] [Google Scholar]
- 62.Champion JA, Mitragotri S. Role of Target Geometry in Phagocytosis. Proc Natl Acad Sci USA. 2006;103(13):4930–4934. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Champion JA, Katare YK, Mitragotri S. Particle Shape: A New Design Parameter for Micro- and Nanoscale Drug Delivery Carriers. Journal of Controlled Release. 2007;121(1):3–9. doi: 10.1016/j.jconrel.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mader K, Bacic G, Domb A, Elmalak O, Langer R, Swartz HM. Noninvasive in vivo Monitoring of Drug Release and Polymer Erosion from Biodegradable Polymers by EPR Spectroscopy and NMR Imaging. J Pharmaceutical Sciences. 1997;86(1):126–134. doi: 10.1021/js9505105. [DOI] [PubMed] [Google Scholar]
- 65.Goldberg M, Langer R, Jia X. Nanostructured Materials for Applications in Drug Delivery and Tissue Engineering. J Biomater Sci Polymer Edn. 2007;18(3):241–268. doi: 10.1163/156856207779996931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Geng Y, Dalhaimer P, Cai S, Tsai R, Tewari M, Minko T, Discher DE. Shape Effects of Filaments Versus Spherical Drug Particles in Flow and Drug Delivery. Nature Nanotechnology. 2007;2(4):249–255. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Herlihy KP, Brannen CL, Lin W, An H, DeSimone JM. Size and Shape-specific Positive Contrast Agents for Magnetic Resonance Imaging: Synthesis, Characterization, and Preliminary In Vivo Results. Proceedings of the 236th ACS National Meeting; 2008. [Google Scholar]
- 68.Glangchai LC, Caldorera-Moore M, Shi L, Roy K. Nanoimprint Lithography Based Fabrication of Shape-Specific, Enzymatically-Triggered Smart Nanoparticles. J Controlled Release. 2008;125:263–272. doi: 10.1016/j.jconrel.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 69.Dumond J, Low HY. Residual Layer Self-Removal in Imprint Lithography. Adv Mater. 2008;20(7):1291–1297. [Google Scholar]
- 70.Truong TT, Lin R, Jeon S, Lee HH, Maria J, Gaur A, Hua F, Meinel I, Rogers JA. Soft Lithography Using Acryloxy Perfluoropolyether Composite Stamps. Langmuir. 2007;23(5):2898–2905. doi: 10.1021/la062981k. [DOI] [PubMed] [Google Scholar]
- 71.www.liquidia.com.
- 72.Conner SD, Schmid SL. Regulated Portals of Entry Into the Cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- 73.Kopecek J, Rejmanova P, Chytry V. Polymers Containing Enzymatically Degradable Bonds, 1. Chymotrypsin Catalyzed Hydrolysis of p-Nitroanilides of Phenylalanine and Tyrosine Attached to Side-Chains of Copolymers of N-(2-Hydroxypropyl)methacrylamide. Makromol Chem. 1981;182:799–809. [Google Scholar]
- 74.Binaschi M, Parlani M, Bellarosa D, Bigioni M, Salvatore C, Palma C, Crea A, Maggi CA, Manzini S, Goso C. Human and Murine Macrophages Mediate Activation of MEn 4901/t-01298: a New Promising Camptothecin Analogue-Polysaccharide Complex. Anti-Cancer Drugs. 2006;17(10):1119–1126. doi: 10.1097/01.cad.0000236307.20339.b4. [DOI] [PubMed] [Google Scholar]
- 75.Schmid B, Chung D-E, Warnecke A, Fichtner I, Kratz F. Albumin-Binding Prodrugs of Camptothecin and Doxorubicin with an Ala-Leu-Ala-Leu Linker That are Cleaved by Cathepsin B: Synthesis and Antitumor Efficacy. Bioconjugate Chem. 2007;18(3):702–716. doi: 10.1021/bc0602735. [DOI] [PubMed] [Google Scholar]
- 76.Ulbrich K, Strohalm J, Kope ek J. Polymers Containing Enzymatically Degradable Bonds. VI. Hydrophilic Gels Cleavable by Chymotrypsin. Biomaterials. 1982;3(3):150–154. doi: 10.1016/0142-9612(82)90004-7. [DOI] [PubMed] [Google Scholar]
- 77.Hua F, Sun Y, Gaur A, Meitl MA, Bilhaut L, Rotkina L, Wang J, Geil P, Shim M, Rogers JA, Shim A. Polymer Imprint Lithography with Molecular-Scale Resolution. Nano Lett. 2004;4(12):2467–2471. [Google Scholar]
- 78.Schmid H, Michel B. Siloxane Polymers for High-Resolution, High-Accuracy Soft Lithography. Macromolecules. 2000;33(8):3042–49. [Google Scholar]
- 79.Oudshoorn MHM, Penterman R, Rissmann R, Bouwstra JA, Broer DJ, Hennink WE. Preparation and Characterization of Structured Hydrogel Microparticles Based on Cross-Linked Hyperbranched Polyglycerol. Langmuir. 2007;23(23):11819–11825. doi: 10.1021/la701910d. [DOI] [PubMed] [Google Scholar]
- 80.Moran IW, Cheng Df, Jhaveri SB, Carter KR. High-Resolution Soft Lithography of Thin Film Resists Enabling Nanoscopic Pattern Transfer. Soft Matter. 2008;4:168–176. doi: 10.1039/b711506g. [DOI] [PubMed] [Google Scholar]
- 81.Thibault C, Severac C, Mingotaud A-F, Vieu C, Mauzac M. Poly(dimethylsiloxane) Contamination in Microcontract Printing and Its Influence on Patterning Oligonucleotides. Langmuir. 2007;23(21):10706–10714. doi: 10.1021/la701841j. [DOI] [PubMed] [Google Scholar]
- 82.Guan J, Chakrapani A, Hansford DJ. Polymer Microparticles Fabricated by Soft Lithography. Chem Mater. 2005;17(25):6227–6229. [Google Scholar]
- 83.Guan J, Ferrell N, Lee LJ, Hansford DJ. Fabrication of Polymeric Microparticles for Drug Delivery by Soft Lithography. Biomaterials. 2006;27(21):4034–4041. doi: 10.1016/j.biomaterials.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 84.Guan J, He H, Lee LJ, Hansford DJ. Fabrication of Particulate Reservoir-Containing, Capsulelike, and Self-folding Polymer Microstructures for Drug Delivery. Small. 2007;3(3):412–418. doi: 10.1002/smll.200600240. [DOI] [PubMed] [Google Scholar]
- 85.Astete CE, Sabliov CM. Synthesis and Characterization of PLGA Nanoparticles. J Biomater Sci Polymer Edn. 2006;17(3):247–289. doi: 10.1163/156856206775997322. [DOI] [PubMed] [Google Scholar]
- 86.Xiang SD, Scholzen A, Minigo G, David C, Apostolopoulos V, Mottram PL, Plebanski M. Pathogen Recognition and Development of Particulate Vaccines: Does Size Matter? Methods. 2006;40(1):1–9. doi: 10.1016/j.ymeth.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 87.Dobrovolskaia MA, McNeil SE. Immunological Properties of Engineered Nanomaterials. Nature Nanotechnology. 2007;2:469–478. doi: 10.1038/nnano.2007.223. [DOI] [PubMed] [Google Scholar]
- 88.Raychaudhuri S, Rock KL. Fully Mobilizing Host Defense: Building Better Vaccines. Nature Biotechnology. 1998;16(11):1025–1031. doi: 10.1038/3469. [DOI] [PubMed] [Google Scholar]
- 89.Fifis T, Gamvrellis A, Crimeen-Irwin B, Pietersz GA, Li J, Mottran PL, McKenzie IFC, Plebanski M. Size-Dependent Immunogenicity: Therapeutic and Protective Properties of Nano-Vaccines Against Tumors. J Immunology. 2004;173(5):3148–3154. doi: 10.4049/jimmunol.173.5.3148. [DOI] [PubMed] [Google Scholar]
- 90.O’Hagan DT, Singh M, Ulmer JB. Microparticle-Based Technologies for Vaccines. Methods. 2006;40(1):10–19. doi: 10.1016/j.ymeth.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 91.Gupta AK, Wells S. Surface-Modified Superparamagnetic Nanoparticles for Drug Delivery: Preparation, Characterization, and Cytotoxicity Studies. IEEE Trans Nanobioscience. 2004;3(1):66–73. doi: 10.1109/tnb.2003.820277. [DOI] [PubMed] [Google Scholar]
- 92.Thomassen T, Wiggen UN, Gundersen HG, Fahlvik AK, Aune O, Klaveness J. Structure Activity Relationship of Magnetic Particles as MR contrast Agents. Magnetic Resonance Imaging. 1991;9:255–258. doi: 10.1016/0730-725x(91)90018-h. [DOI] [PubMed] [Google Scholar]
- 93.LaConte LEW, Nitin N, Zurkiya O, Caruntu D, O’Connor CJ, Hu X, Bao G. Coating Thickness of Magnetic Iron Oxide nanoparticles Affects R2 Relaxivity. J Magnetic Resonance Imaging. 2007;26:1634–1641. doi: 10.1002/jmri.21194. [DOI] [PubMed] [Google Scholar]
- 94.Kim B-S, Qiu J-M, Wang J-P, Taton TA. Magnetomicelles: Composite Nanostructures from Magnetic Nanoparticles and Cross-Linked Amphiphilic Block Copolymers. Nano Lett. 2005;5(10):1987–1991. doi: 10.1021/nl0513939. [DOI] [PubMed] [Google Scholar]
- 95.Park J-H, von Maltzahn G, Zhang L, Schwartz MP, Ruoslahti E, Bhatia S, Sailor MJ. Magnetic Iron Oxide Nanoworms for Tumor Targeting and Imaging. Advanced Materials. 2008;20(9):1630–1635. doi: 10.1002/adma.200800004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Caravan P. Strategies for Increasing the Sensitivity of Gadolinium Based MRI Contrast Agents. Chem Soc Rev. 2006;35:512–523. doi: 10.1039/b510982p. [DOI] [PubMed] [Google Scholar]
- 97.Turner JL, Pan D, Plummer R, Chen Z, Whittaker AK, Wooley KL. Synthesis of Gadolinium-Labeled Shell-Crosslinked Nanoparticles form Magnetic Resonance Imaging Applications. Adv Funct Mater. 2005;15:1248–1254. [Google Scholar]
- 98.Accardo A, Tesauro D, Aloj L, Tarallo L, Arra C, Mangiapia G, Vaccaro M, Pedone C, Paduano L, Morelli G. Peptide-Containing Aggregates as Selective Nanocarriers for Therapeutics. Chem Med Chem. 2008;3:594–602. doi: 10.1002/cmdc.200700269. [DOI] [PubMed] [Google Scholar]
- 99.Rudovský J, Botta M, Hermann P, Hardcastle KI, Lukeš I, Aime S. PAMAM Dendrimeric Conjugates with a Gd-DOTA Phosphinate Derivative and Their Adducts with Polyaminoacids: The Interplay of Global Motion, Internal Rotation, and Fast Water Exchange. Bioconjugate Chem. 2006;17(4):975–987. doi: 10.1021/bc060149l. [DOI] [PubMed] [Google Scholar]
- 100.Kim JS, Rieter WJ, Taylor KML, An H, Lin W, Lin W. Self-Assembled Hybrid Nanoparticles for Cancer-Specific Multimodal Imaging. J Am Chem Soc. 2007;129(29):8962–8963. doi: 10.1021/ja073062z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jagielski N, Sharma S, Hombach V, Mailander V, Rasche V, Landfester K. Nanocapsules Synthesized by Miniemulsion Technique for Application as New Contrast Agent Materials. Macromol Chem Phys. 2007;208:2229–2241. [Google Scholar]
- 102.Sun G, Xu J, Hagooly A, Rossin R, Li Z, Moore DA, Hawker CJ, Welch MJ, Wooley KL. Strategies for Optimized Radiolabeling of Nanoparticles for in vivo PET Imaging. Adv Mater. 2007;19:3157–3162. [Google Scholar]