Abstract
A major challenge for inducing antitumor immune responses with native or modified tumor/self-Ags in tumor-bearing hosts relates to achieving efficient uptake and processing by dendritic cells (DCs) to activate immune effector cells and limit the generation of regulatory T cell activity. We analyzed the ability of therapeutic DC vaccines expressing a CD166 cross-reactive mimotope of the GD2 ganglioside, 47-LDA, to selectively expand adoptively transferred, tumor-specific T cells in NXS2 neuroblastoma tumor-bearing syngeneic mice. Before the adoptive cell transfer and DC vaccination, the tumor-bearing mice were lymphodepleted by nonmyeloablative total body irradiation or a myeloablative regimen that required bone marrow transplantation. The 47-LDA mimotope was presented to DCs either as a linear polypeptide in conjunction with universal Th epitopes or as a fusion protein with the murine IgG2a Fc fragment (47-LDA-Fcγ2a) to deliver the antigenic cassette to the activating Fcγ receptors. We demonstrate that immunization of adoptively transferred T cells in tumor-bearing mice with the 47-LDA mimotope expressed in the context of the activating Fc fusion protein induced higher levels of antitumor immune responses and protection than the 47-LDA polypeptide-DC vaccine. The antitumor efficacy of the therapeutic 47-LDA-Fcγ2a-DC vaccine was comparable to that achieved by a virotherapy-associated cancer vaccine using a recombinant oncolytic vaccinia virus expressing the 47-LDA-Fcγ2a fusion protein. The latter treatment, however, did not require total body irradiation or adoptive cell transfer and resulted in induction of antitumor immune responses in the setting of established tolerance, paving the way for testing novel anticancer treatment strategies.
It has become increasingly clear that enhancing the immunogenicity of tumor-associated Ags by the use of dendritic cells (DCs)3 and other potent vectors of cancer vaccines or by adoptive cell transfer (ACT) of autologous tumor-reactive T cells influences the kinetics and character of tumor growth. Ultimately, however, despite indications that some of the new cancer vaccines are capable of delaying tumor recurrence or extending the survival of cancer patients, their ability to induce cancer regression remains low (1–3). Recent reports that help to identify and prospectively remove the difficulties toward therapeutic vaccination of cancer patients indicate that the successful induction of antitumor immunity by cancer vaccine is not necessary associated with the induction of functional CTLs and that current vaccines may promote undesirable expansion of regulatory T cells (Tregs) (2, 4, 5). Treg cells are crucial mediators of peripheral tolerance and are elevated in cancers (6, 7). They possess a CD4+CD25+FoxP3+ phenotype and arise both in the thymus and through the conversion of FoxP3−CD4+ T cells in the periphery (7, 8). It is known that Treg cells limit primary responses to tumor/self-Ags in tumor-bearing host and prevent the generation of memory T cells (7–9). The interaction of Treg cells with DCs during the induction of an immune response to tumor-associated Ags and the regulation of its character remains under intensive study. The means to generate Ag-specific T effector cells (Teff) cells while limiting the generation of Ag-specific Tregs is crucial to optimal immunotherapy strategies. Several groups are now testing such strategies, including delivery of CD40 agonists plus TLR activation (10), a liposomal vaccine (11), CD137 single-chain Ab-expressing tumor cells (12), and DCs (2, 5).
The current cancer vaccines involving DCs have proven to be effective in inducing tumor-specific T cells (13–16), but their effectiveness in inducing clinical responses is still below expectations (3). A growing body of evidence indicates that DC behavior can be regulated by activating and inhibitory FcγR subtypes (17–19). FcγRs represent one of the best studied receptors for the uptake and targeting of Ags to DCs, either in the form of immune complexes or opsonized dying cells, such as those generated during Ab therapy of cancer (20). The FcγR system consists of a balance of activating and inhibitory FcRs that carry an ITAM or ITIM, respectively, in their cytoplasmic domain. (21). Human monocytes and DCs express an array of these receptors, including both activating and inhibitory FcRs (18, 22). Several studies have now shown that Ag uptake in the form of immune complexes or opsonized tumor cells is associated with enhanced Ag presentation by DCs and the generation of Ag-specific T cells (17, 23, 24). Selective blockade of inhibitory Fcγ receptors is associated with DC activation (18), and DCs from mice deficient in the inhibitory FcγRII have a more activated phenotype (25). These data suggest that the balance of activating vs inhibitory FcγRs may have a major impact on DC activation in vivo. Recently, it has been shown that selective targeting of activating FcγRs leads to the induction of a type I IFN response program in human monocytes and monocyte-derived DCs (26). Thus, the balance of FcR signaling may regulate the level of constitutive type I IFN signaling in myeloid cells, suggesting that the engagement of activating FcγRs on DCs by immune complexes may lead to major effects on the biology of DCs and the generation of adaptive immunity. Because individual Ab isotypes possess different affinities for Fcγ receptors (with activating Fcγ receptors having higher affinities for murine IgG2a and IgG2b isotypes or human IgG1 and IgG3 isotypes), differences in the ratios of activating-to-inhibitory receptor binding by the presented antigenic complex may predict the ability of DCs to induce immune responses (19). Harnessing this pathway may allow the recruitment of adaptive immunity and immunologic memory by Ab therapy or cancer vaccines.
In this study, we investigated whether triggering the activating FcγRs on APCs would generate tumor-specific Teff cells and offset the suppressive environment established by the tumor. Using a CD166 cross-reactive mimotope of GD2 ganglioside 47-LDA (27, 28), we investigated the efficacy of a DC vaccine expressing the 47-LDA-Fcγ2a fusion protein or 47-LDA linear polypeptide during ACT in syngeneic NXS2 tumor-bearing mice. Before the ACT and DC vaccination, the tumor-bearing mice were lymphodepleted by nonmyeloablative total body irradiation (TBI) or a myeloablative regimen that required bone marrow (BM) transplantation. To determine that the antitumor effect of immunization with the fusion protein is not limited to the DC vaccine and ACT in an irradiated tumor-bearing host, we examined the therapeutic efficacy of the 47-LDA-Fcγ2a construct expressed in the recombinant oncolytic vaccinia virus (rOVV) that exhibits tumor-selective replication (29, 30). The rOVV was selected as a vector for these studies for the following reasons: 1) vaccinia virus-based vaccines have been shown to break CD4+CD25+ Treg-mediated CD8 tolerance through TLR-dependent and -independent pathways (31, 32); 2) the thymidine kinase (TK)− and vaccinia growth factor (VGF)− mutant vaccinia virus is significantly attenuated and less pathogenic compared with the wild-type vaccinia vector (29); and 3) results from case series and clinical trials have shown systemic efficacy with rOVVs in patients with chronic lymphocytic leukemia, nonsmall cell lung carcinoma, melanoma, and liver tumors (33, 34). Furthermore, studies with murine tumor models confirmed the induction of cytotoxic CD8+ T cells against tumor Ags expressed by the oncolytic viruses (35).
We report that immunization of adoptively transferred T cells with the 47-LDA mimotope expressed in the context of the activating Fc fusion protein induced higher levels of antitumor immune responses and protection than the 47-LDA polypeptide-DC vaccine. Although delivery of 47-LDA-Fcγ2a fusion protein-expressing DCs has improved tumor-free survival in tumor-bearing mice that received ACT, the treatment required a myeloablative regimen and BM transplantation to elicit a significant protection level. In contrast, a comparable level of tumor regression associated with bypassing regulatory T cell-mediated tolerance was achieved by a virotherapy-associated cancer vaccine with rOVV expressing the 47-LDA-Fcγ2a fusion protein.
Materials and Methods
Animals and cell lines
Female A/J mice, 6 – 8 wk of age, were obtained from The Jackson Laboratory. The experimental procedures were performed in compliance with protocols approved by the Institutional Animal Care and Use Committee of the Roswell Park Cancer Institute. The murine NXS2 neuroblastoma cell line (provided by Dr. R. A. Reisfeld, Scripps Research Institute, La Jolla, CA) is a hybrid between the GD2-negative C1300 murine neuroblastoma (A/J background) and the GD2-positive murine dorsal root ganglioma cells. The hybrid cell line was shown to be MHC class I syngeneic to A/J mice by its H-2Kk-positive/H-2Kb-negative phenotype (36). The 14G2a hybridoma cell line secreting GD2-specific mAb (37) was provided by Dr. R. A. Reisfeld (Scripps Research Institute).
Generation of 47-LDA-Fcγ2a fusion protein
The construction of a 47-LDA expression vector consisting of the tissue plasminogen activator secretory signal sequence, two universal Th peptides, PADRE (AKFVAAWTLKAAA; Ref. 38) and the V3 loop of the HIV gp120 glycoprotein (CKRKIHIGPGQAFYT; Ref. 39), together with the 47-LDA mimetic peptide, was reported elsewhere (27). The murine 47-LDA-Fcγ2a fusion protein was generated by inserting the 47-LDA polypeptide coding sequence between the human EF1-HTLV promoter and the mouse IgG2a Fc region of the pFUSE-mIgG2Aa-Fc1 vector (Invivo-Gen) using the EcoRI and BglII restriction enzyme cleavage sites. Stable transfection of 293T cells was done with the 47-LDA-Fcγ2a fusion protein construct or sham vector using the Lipofectamine reagent (Invitrogen) followed by selection in zeocin-containing medium. The 47-LDA-Fcγ2a fusion protein was purified from culture supernatants of the transfected cells on the protein G column (Amersham Biosciences/GE Healthcare). The fusion protein was analyzed by electrophoresis with 15% SDS-polyacrylamide gels and immunoblotting with 14G2a mAb followed by ECL plus Western blotting detection system (Amersham Biosciences/GE Healthcare) according to the protocol of the manufacturer.
Immunization
DCs were generated in vitro from BM precursors as previously described (40). Briefly, BM cells were harvested from the tibias and femurs of 6- to 8-wk-old female A/J mice (n = 5) and then cultured in complete medium supplemented with 10 ng/ml GM-CSF at 37°C for 6 days. The medium was replenished every 2–3 days. On day 7, most of the nonadherent cells had acquired DC morphology and were CD11c-high and CD80-, CD86-, CD40-, and MHC class II-low, as determined by flow cytometric analyses. DCs were pulsed for 5 h with 10 μg/ml 47-LDA-Fcγ2a fusion protein or 47-LDA polypeptide (New England Peptide), incubated with LPS (1.0 μg/ml) for 1 h to induce maturation, washed, and injected i.v. (2 × 106) into mice (Fig. 1A). Twenty micrograms of DNA plasmid-encoded IL-15 and IL-21 cytokines were injected i.m. at the time of vaccination and 5 days later, respectively (41).
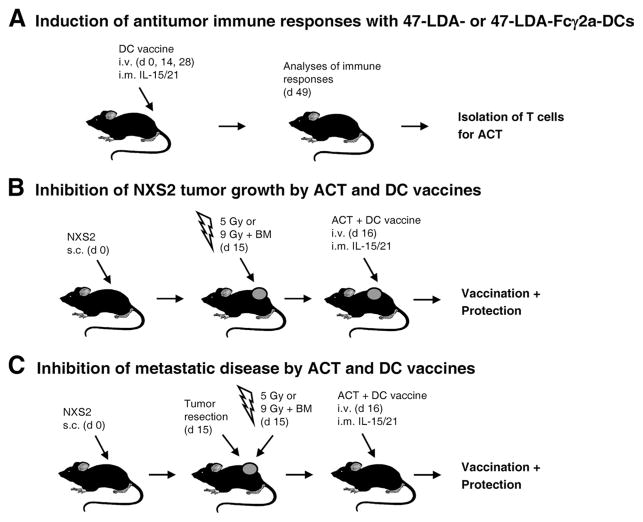
FIGURE 1.
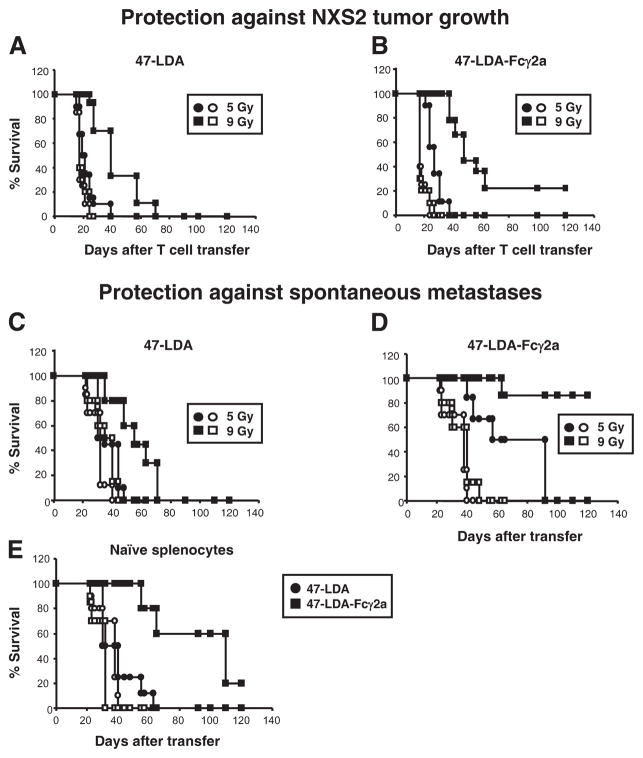
Schematic representation of vaccination with 47-LDA- or 47-LDA-Fcγ2a-pulsed DCs and ACT in tumor-bearing mice. A, Vaccination with 47-LDA- or 47-LDA-Fcγ2a-pulsed DCs. A/J mice (n = 5) were immunized in a 2-wk interval with 47-LDA- or 47-LDA-Fcγ2a-pulsed DCs in the presence of IL-15 and IL-21 vectors. Three weeks after the third immunization, CD8+ splenocytes were obtained by negative selection and used for the ACT in NXS2-bearing mice. B, Inhibition of primary tumor growth by ACT and DC vaccines. A/J mice were challenged with 2 × 106 NXS2 cells. Fifteen days later, lymphopenia in the tumor-bearing mice was induced by 5 Gy of TBI or 9 Gy of TBI plus BM transfer (107 cells) 1 day before the ACT and DC vaccination. Adoptive transfer of Ag-experienced CD8+ T cells (2 × 107) together with the DC vaccine was delivered on day 16. Tumor growth was monitored by measuring s.c. tumors once to thrice a week with a microcaliper and determining tumor volume (width × length × width/2 = mm3). C, Inhibition of metastatic disease by ACT and DC vaccine. NXS2-bearing mice underwent nonmyeloablative or myeloablative TBI at the time of tumor excision. One day later, the mice received ACT of CD8+ T cells (2 × 107) from the immunized mice or unseparated splenocytes from naive mice together with the DC vaccine. Survival was defined as the point at which mice were sacrificed due to extensive tumor growth or the development of metastases.
Flow cytometry
Immature DCs were incubated with a biotin-labeled 47-LDA-Fcγ2a fusion protein or a 47-LDA polypeptide followed by streptavidin-conjugated PE. For some experiments, DCs were stained with the following mAbs: anti-CD11c-FITC, anti-CD80-PE, anti-CD86-PE, anti-CD40-PE, or anti-MHC class II-PE (BD Biosciences). Splenocytes were labeled with anti-CD4-PE, anti-CD8-PE, anti-CD25-FITC (BD Biosciences) or the relevant isotype controls. Before specific Ab staining, cells were incubated with Fc blocker (anti-CD16/CD32 mAb) for 10 min. The cells were washed twice in HBSS containing 0.01% sodium azide and 1% FCS, fixed with 1% paraformaldehyde, and stored at 4°C in the dark before analyses. Background staining was assessed using isotype controls that included the appropriate fluorochrome-conjugated or unconjugated mouse IgG1, IgG2a, or IgG2b (BD Biosciences). The numbers of Treg cells in the axillary and brachial lymph nodes and spleen were determined by the intracellular staining of CD4+CD25+ lymphocytes with the anti-FoxP3-Alexa Fluor 647 mAb (eBioscience) according to the manufacturer’s protocol. The number of CD4+ and CD8+ splenocytes secreting IFN-γ or TNF-α was determined using anti-CD4-FITC and anti-CD8-FITC mAbs in conjunction with anti-IFN-γ-PE or anti-TNF-α-PE mAb (BD Biosciences). The intracellular expression of IL-12p70 in LPS-stimulated DCs was measured by staining with a rat anti-mouse IL-12p70 mAb that detects an epitope within the IL-12p35 subunit and does not cross-react with IL-12p40 (R&D Systems). The intracellular expression of CCL22 in LPS-stimulated DCs was determined by staining with the rat anti-mouse CCL22 mAb (R&D Systems). Sorting of 47-LDA-Fcγ2a+, 47-LDA polytope+ or CD86+ DCs was performed on a BD FACSAria flow cytometer (BD Biosciences). All flow cytometric evaluations were performed on a FACScan or FACSCalibur flow cytometer. After gating on forward and side scatter parameters, at least 10,000 gated events were routinely acquired and analyzed using CellQuest software (BD Biosciences).
Adoptive transfer of T cells and DC vaccine
A/J mice (n = 8 – 10 per group) were injected s.c. with 2 × 106 NXS2 neuroblastoma cells and treated 15 days later by i.v. injection with CD8+-enriched splenocytes isolated from 47-LDA-Fcγ2a- or 47-LDA polytope-DC-immunized mice, as depicted in Fig. 1A. The CD8+ splenocytes were negatively selected using paramagnetic microbeads conjugated to anti-mouse CD4 (L3T4) and anti-mouse CD45R (B220) mAbs (MACS; Miltenyi Biotec) according to the manufacturer’s instructions. The isolated CD8+ splenocytes (2 × 107) were incubated with LPS-matured 47-LDA polypeptide- or 47-LDA-Fc fusion protein-coated DCs. The mixtures of T cells and DCs (20:1 ratio) were injected i.v. into the lymphodepleted NXS2 tumor-bearing mice in the presence of recombinant mouse IL-2 (1 μg/dose) as described (42). Lymphopenia in the tumor-bearing mice was induced by 5 Gy of nonmyeloablative TBI or 9 Gy of myeloablative TBI plus BM transfer (107 cells) 1 day before the ACT and vaccination (Fig. 1B). Control mice bearing established s.c. NXS2 tumors were irradiated with 5 or 9 Gy plus BM transplantation. Mice were immunized in a 2-wk interval with the DC vaccine in the presence of IL-15 and IL-21 vectors as described (41). Tumor growth was monitored by measuring s.c. tumors once to thrice a week with a microcaliper and determining tumor volume (width × length × width/2 = mm3).
For the immunotherapy of disseminated disease, NXS2-bearing mice underwent nonmyeloablative (5 Gy) or myeloablative (9 Gy) TBI at the time of tumor resection (Fig. 1C). One day after tumor excision, the mice received ACT of splenocytes (2 × 107) from the immunized or naive mice together with the DC vaccine. Mice were immunized in a 2-wk interval with the DC vaccine in the presence of the IL-15 and IL-21 vectors as described (41). The control mice had the primary tumor excised with or without TBI and BM transplantation. Survival was defined as the point at which mice were sacrificed due to extensive tumor growth. Kaplan-Meier survival plots were prepared, and significance was determined using the log rank Mantel-Cox method.
In vitro analyses of vaccine-induced IFN-γ and TNF-α expression and T cell proliferation
Splenocytes from A/J mice immunized with the 47-LDA-Fcγ2a- or 47-LDA polypeptide-coated DCs were analyzed for IFN-γ and TNF-α expression after overnight stimulation with 47-LDA-expressing DCs at the 20:1 ratio. Cells isolated from sham vector-immunized mice served as controls. To investigate the induction of Treg cells in mice immunized with 47-LDA-DC and 47-LDA-Fcγ2a-DC vaccines, the spleen and the axillary and brachial lymph nodes were removed from the immunized mice 3 wk after the last immunization and analyzed for the number of Treg cells by staining with anti-CD4-PE, anti-CD25-FITC, and anti-FoxP3-Alexa Fluor 647 mAbs or the relevant isotype controls. To analyze the effect of Treg cells on Teff cell proliferation, CD8+ T cells from the 47-LDA-DC or 47-LDA-Fcγ2a-DC vaccine immunized mice were loaded with 25 μM CFSE for 10 min at 37°C and cultured with 47-LDA-expressing syngeneic DCs (ratio 20:1) for 72 h in the presence or absence of Treg cells (ratio 1:1). Cells were stained with PE-conjugated anti-CD8 mAbs and analyzed by flow cytometry. The Treg cell populations were isolated using the CD4+ CD25+ regulatory T cell isolation kit (Miltenyi Biotec) according to the manufacturer’s protocol.
To analyze proliferative CD4+ and CD8+ T cell responses in tumor-bearing and tumor-free mice after ACT and DC vaccination, splenocytes were labeled with 25 μM CFSE and incubated with 47-LDA-expressing DCs for 72 h. Cells were stained with PE-conjugated anti-CD4 or anti-CD8 mAbs and analyzed by flow cytometry.
CTL assay
Splenocytes were cultured with 47-LDA-expressing DCs at the 20:1 ratio in 15% T cell stimulatory factor (T-STIM culture supplement; Collaborative Biomedical Products) as a source of exogenous IL-2. After 3 days of stimulation, cells were split and cultured in medium supplemented with murine rIL-2 (0.3 ng/ml) (BD Biosciences). Before stimulation, CD8+ T cells were isolated by negative selection using T cell enrichment columns (Miltenyi Biotec) according to the manufacturer’s protocol. The cytolytic activity of CTLs against NXS2 tumor cells was analyzed 5 days later by a standard 4-h 51Cr-release assay. The percentage of specific lysis was calculated as follows: ([cpm experimental release − cpm spontaneous release]/[cpm maximum release − cpm spontaneous release]) × 100. Maximum release was determined from the supernatants of cells that were lysed by the addition of 5% Triton X-100. Spontaneous release was determined from target cells incubated with medium only.
Generation of rOVVs expressing enhanced GFP (EGFP) and the 47-LDA-Fcγ2a fusion protein for oncolytic virotherapy-based cancer vaccine
The rOVVs expressing EGFP and the 47-LDA-Fcγ2a fusion protein have been generated by homologous recombination in CV-1 cells (43) using the VSC20 vaccinia (44) and the vaccinia shuttle plasmids pSEL-EGFP and pCB023-47-LDA-Fcγ2a, respectively (29). The parental vaccinia virus VSC20 with lacZ gene cloned in place of the VGF gene was obtained from Dr. B. Moss (National Institutes of Health, Bethesda, MD). The pCB023 and pSEL-EGFP vaccinia shuttle plasmids were obtained from Dr. D. Bartlett (University of Pittsburgh Cancer Institute, Pittsburgh, PA). The pCB023-47-LDA-Fcγ2a shuttle plasmid was generated by cloning the 47-LDA-Fcγ2a fusion protein gene into the EcoRI and SmaI restriction enzyme sites of pCB023. After DNA sequence verification, the secretion of the 47-LDA-Fcγ2a fusion protein into the culture supernatant of 293T cell transfectants was confirmed by immunoblotting with the 14G2a mAb. Multiple plaques of the recombinant viruses were isolated in TK− cells by BrdU selection as described (45). After amplification on HeLa cells, the rOVV-EGFP and rOVV-47-LDA-Fcγ2a viruses were purified over the sucrose gradient, titered, and used for in vitro and in vivo studies in NXS2 cells. The expression of EGFP in rOVV-EGFP-infected NXS2 cells was confirmed by immunofluorescence microscopy, whereas secretion of the 47-LDA-Fcγ2a fusion protein from rOVV-47-LDA-Fcγ2a-infected NXS2 cells was determined by immunoblotting with 14G2a mAb.
For the oncolytic virotherapy-based vaccine, A/J mice (n = 10) were injected s.c. with 2 × 106 NXS2 cells and treated 15 days later with i.v. injection of 108 PFU of the rOVV-47-LDA-Fcγ2a or rOVV-EGFP vector. Tumor-bearing mice treated with PBS served as controls. Survival was defined as the point at which mice were sacrificed due to extensive tumor growth. Kaplan-Meier survival plots were prepared, and significance was determined using the log rank Mantel-Cox method.
Statistical analyses
The statistical significance of the difference between groups was performed using a two-tailed Student’s t test assuming equal variance. The p values for the pairwise group comparisons for average tumor growth were computed using the nonparametric Wilcoxon rank-sum test. Kaplan-Meier survival plots were prepared and median survival times were determined for NXS2-challenged groups of mice. Statistical differences in the survival across groups were assessed using the log rank Mantel-Cox method. Data were presented as arithmetic means ± SD and analyzed using the JMP program (SAS Institute) on a Windows-based platform.
Results
Characterization of murine 47-LDA-Fcγ2a fusion protein
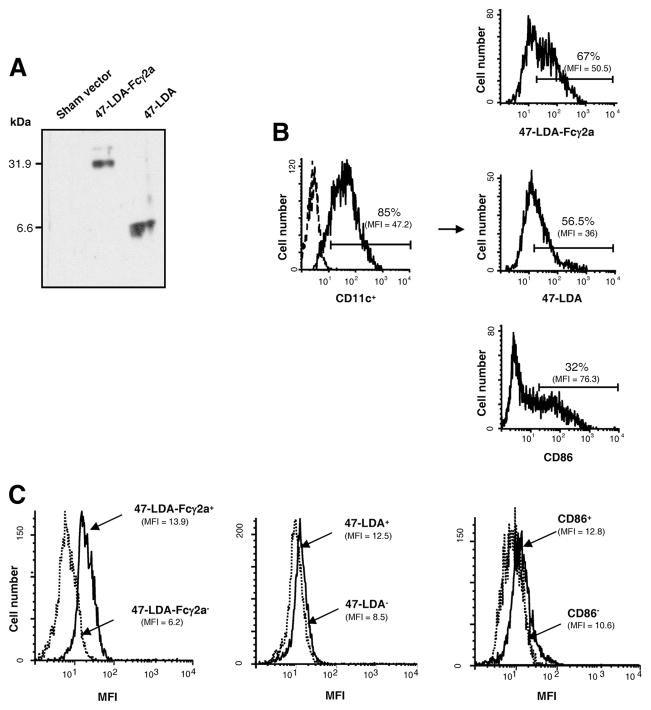
The murine 47-LDA-Fcγ2a fusion protein was generated by inserting the relevant coding sequence (including the tissue plasminogen activator secretory signal sequence, two universal Th peptides, and the 47-LDA mimotope) in-frame between the hEF1-HTLV promoter and the mouse IgG2a Fc region of the pFUSE-mIgG2Aa-Fc1 vector using the EcoRI and BglII restriction enzyme cleavage sites. After sequencing of the 47-LDA-Fcγ2a construct to confirm the presence of an uncorrupted open reading frame, immunofluorescence staining of 47-LDA-Fcγ2-transfected 293T cells with the 14G2a mAb followed by flow cytometry analysis was done 48 h after transfection to determine the expression of the fusion protein (not shown). The secretion of the fusion protein from 47-LDA-Fcγ2a stable transfectants, selected in zeocin-containing medium, was confirmed by immunoblotting of the culture supernatant with the 14G2a mAb. As shown in Fig. 2A, a prominent band of 31.9 kDa was detected with the 14G2a mAb in the culture supernatant harvested from 47-LDA-Fcγ2a transfectants. No band was present in the supernatant prepared from cell transfected with the sham plasmid. The 47-LDA polypeptide with a molecular mass of 6.6 kDa, which was included in the analysis as a positive control, was clearly recognized by the 14G2a mAb. Altogether, these results showed that the GD2 mimotope expressed in the context of 47-LDA-Fcγ2a fusion protein retained its native antigenic determinants of the synthetic peptide recognized by the 14G2a mAb.
FIGURE 2.
Characterization of the 47-LDA-Fcγ2a fusion protein and its interaction with DCs. A, Western blotting of 47-LDA-Fcγ2a fusion protein and 47-LDA polypeptide with biotinylated 14G2a mAb and streptavidin-HRP. B, Binding of 47-LDA-Fcγ2a fusion protein and 47-LDA polypeptide to immature CD11c+ DCs. BM-derived DCs were stained with FITC-conjugated CD11c-specific mAb in combination with biotinylated 47-LDA-Fcγ2a fusion protein or 47-LDA polypeptide followed by streptavidin-PE and analyzed by flow cytometry. DCs stained with anti-CD86-PE mAb were included as a specificity control. The percentage and mean fluorescent intensity (MFI) of 47-LDA-Fcγ2a, 47-LDA, and CD86 molecules on CD11c-positive DCs are given. Arrows indicate the source of cells in the histograms. Data are from one representative experiment of three performed. C, Intracellular expression of IL-12p70 × 47-LDA-Fcγ2a+, 47-LDA+, CD86+ DCs, and their negative counterparts after LPS stimulation. Immature DCs were stained with biotinylated 47-LDA-Fcγ2a fusion protein, 47-LDA polypeptide, or CD86-specific mAb followed by streptavidin-PE. The positive and negative populations were sorted on a BD FACSAria flow cytometer, incubated overnight with 1 μg/ml LPS, and analyzed for IL-12p70 expression by intracellular staining with a rat anti-mouse IL-12p70 mAb specific for the IL-12p35 subunit, followed by goat anti-rat secondary Ab. Background staining (MFI, <2.6) was assessed using an isotype control Ab. All flow cytometric evaluations were performed on a FACScan or FACSCalibur flow cytometer. After gating on forward and side scatter parameters, at least 10,000-gated events were routinely acquired and analyzed using CellQuest software. Data are from one representative experiment of three performed.
The binding of the 47-LDA-Fcγ2a fusion protein and the 47-LDA polypeptide to BM-derived immature DCs was determined by a flow cytometry analysis. As shown in Fig. 2B, >50% of CD11c+ DCs reacted with the biotinylated 47-LDA-Fcγ2a fusion protein or the biotin-labeled 47-LDA polypeptide. The binding of the 47-LDA-Fcγ2a fusion protein, but not that of the 47-LDA polypeptide, was inhibited by the Fc blocking Ab CD16/32, indicating a specific interaction between the 47-LDA-Fcγ2a fusion protein and its cellular ligands. It is noteworthy, however, that the binding of the 47-LDA-Fcγ2a fusion protein to the FcγR had no effect on DC maturation, because the addition of LPS was necessary to up-regulate the surface expression of CD86, CD40, and CD86 Ags (not shown).
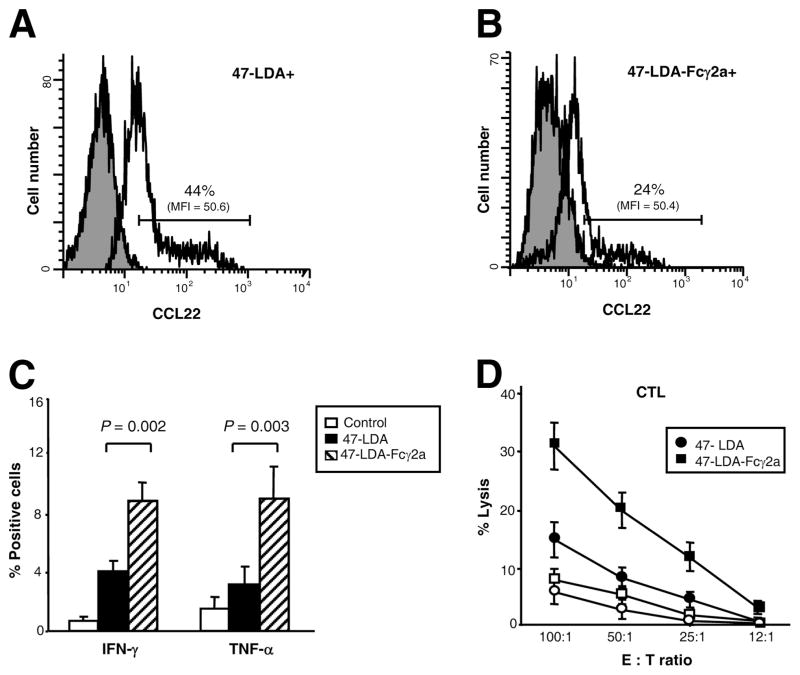
Because the activating and inhibitory FcγRs are critical for the modulation of effector immune responses during the administration of Ags to DCs in the form of immune complexes (25, 46) and IL-12 is a key cytokine involved in the generation of type 1 immunity (47), we investigated whether DCs that interact with the 47-LDA polypeptide or the 47-LDA-Fcγ2a fusion protein differ in their ability to express IL-12p70 upon stimulation with LPS. For these experiments, 47-LDA+ and 47-LDA-Fcγ2a+ DCs as well as their negative counterparts were obtained by cell sorting. DCs positive or negative for CD86 expression were included as a specificity control (Fig. 2, B and C). The sorted DC populations were stimulated overnight with 1 μg/ml LPS and analyzed for IL-12p70 expression by intracellular staining and flow cytometric analysis. Fig. 2C shows that 47-LDA-Fcγ2a+ DCs expressed significantly higher levels of IL-12p70 than their negative counterparts after stimulation with LPS (p = 0.008), whereas comparable levels of IL-12p70 were measured in 47-LDA+ and 47-LDA− DC populations or those sorted for the presence or absence of CD86 Ag expression. A parallel expression study of the Treg-attracting CCL22 chemokine (48, 49) in the sorted and LPS-stimulated 47-LDA+ and 47-LDA-Fcγ2a+ DCs revealed ~2-fold higher numbers of CCL22-positive cells in the former DC population (Fig. 3, A and B; p = 0.037).
FIGURE 3.
Differential expression of CCL22 in 47-LDA+ and 47-LDA-Fcγ2a+ DCs after LPS stimulation and induction of IFN-γ and TNF-α in CD4+ splenocytes by 47-LDA- and 47-LDA-Fcγ2a-DC vaccines. A and B, Immature DCs were stained with biotinylated 47-LDA polypeptide or 47-LDA-Fcγ2a fusion protein followed by streptavidin-PE. The 47-LDA+ (A) and 47-LDA-Fcγ2a+ (B) DCs were sorted on BD FAC-SAria flow cytometer, incubated with 1 μg/ml LPS for 24 h, and analyzed for CCL22 expression by intracellular staining with rat anti-mouse CCL22 mAb followed by goat anti-rat secondary Ab. The mean fluorescence intensity (MFI) and percentage of cells positive for CCL22 expression are indicated. The gray area denotes background staining assessed using an isotype control Ab. Data are from one representative experiment of three performed. C, Expression of IFN-γ and TNF-α in splenocytes of mice immunized with 47-LDA-DC and 47-LDA-Fcγ2a-DC vaccines (filled and hatched bars, respectively). A/J mice (n = 5) were immunized three times with DCs coated with 47-LDA polypeptide or 47-LDA-Fcγ2a fusion protein after LPS-induced maturation in the presence of IL-15 and IL-21 vectors. Cells isolated from mice immunized with LPS-treated DC served as controls (open bars). Three weeks after the last immunization, the expression of IFN-γ and TNF-α in CD4+ splenocytes was analyzed by intracellular staining after overnight stimulation with DCs expressing the 47-LDA mimotope. D, NXS2 neuroblastoma-specific CTL responses. CD8+ splenocytes from mice immunized with 47-LDA-DC (●) and 47-LDA-Fcγ2a-DC (■) vaccines were obtained by negative selection. Cells were cultured with 47-LDA-expressing DCs (filled symbols) or sham plasmid-transfected DCs (open symbols) at the 20:1 ratio as described in the Materials and Methods section. The CTL activities against NXS2 cells were analyzed in a standard 51Cr-release assay. All determinations were made in triplicate samples, and the SD was <10%. Results are presented as the means ± SD of four independent experiments.
Enhanced immunostimulatory activity of 47-LDA-Fcγ2a-pulsed DCs in vivo
The differences in the expression levels of IL-12 and CCL22 between LPS-stimulated 47-LDA-Fcγ2a+ DC and 47-LDA+ DCs suggest that these cells may have different abilities to interact with Teff and Treg cells in vivo. Therefore, we investigated the induction of tumor-specific Th1 and CTL responses after immunization of A/J mice with the 47-LDA-Fcγ2a- and 47-LDA-DC vaccines (Fig. 1A). Each DC vaccine was delivered by i.v. injection three times in a 2-wk interval together with DNA plasmid-encoded IL-15 and IL-21 delivered i.m. at the time of vaccination and 5 days later, respectively (41). Three weeks after the last immunization, CD4+ splenocytes from the immunized mice were analyzed for IFN-γ and TNF-α production by intracellular staining and flow cytometry after overnight stimulation with 47-LDA-expressing DCs. Cells isolated from mice immunized with DCs and IL-15 and IL-21 vectors were included as controls. As shown in Fig. 3C, the numbers of IFN-γ- and TNF-α-producing CD4+ cells were >4-fold higher in mice immunized with the 47-LDA-Fcγ2a fusion protein-coated DCs than in the control animals and >2-fold higher than the responses induced by the 47-LDA-DC vaccine (p = 0.002 and p = 0.003, respectively). The increases in IFN-γ-producing CD4+ T cell responses after immunization with 47-LDA-Fcγ2a fusion protein vaccine were associated with higher CD8+ T cell-mediated cytotoxic activities against the NXS2 neuroblastoma tumor compared with those induced by the 47-LDA polytope-coated DCs over a broad range of E: T ratios (Fig. 3D).
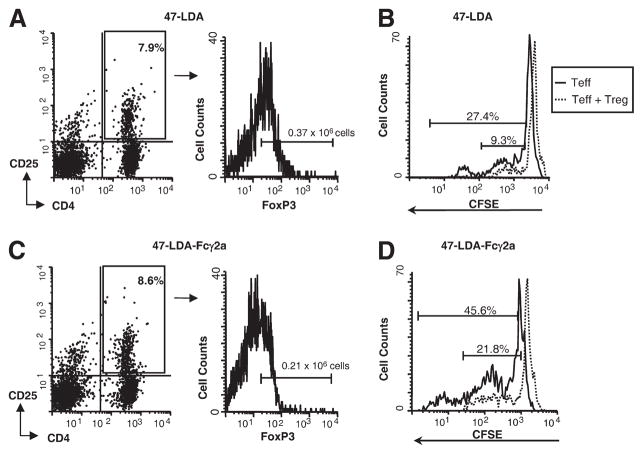
We also examined the induction of Treg cells after 47-LDA and 47-LDA-Fcγ2a-DC vaccines by a flow cytometric analysis of CD4+CD25+FoxP3+ lymphocytes in the axillary and brachial lymph nodes and spleen. In the axillary lymph nodes of 47-LDA-immunized mice, the absolute numbers of FoxP3+ cells within the CD4+CD25+ population ranged from 0.31 × 106 to 0.42 × 106 cells (Fig. 4A) and were ~2-fold higher compared with the animals immunized with the 47-LDA-Fcγ2a-DC vaccine (0.36 ± 0.06 vs 0.19 ± 0.04; p = 0.03). In the latter group of mice, the numbers of CD4+CD25+ lymphocytes positive for intracellular FoxP3 expression ranged from 0.17 × 106 to 0.24 × 106 cells (Fig. 4C). Similar differences in the frequencies of Treg cells were detected in the brachial lymph nodes and spleen of the immunized mice (data not shown). When CFSE-labeled CD8+ lymphocytes and Treg cells prepared from 47-LDA-DC- and 47-LDA-Fcγ2a-DC-immunized mice were mixed at a 1:1 ratio and cocultured with the respective LPS-matured 47-LDA+ DCs and 47-LDA-Fcγ2a+ DCs, both populations of Treg cells suppressed the expansion of Teff cells (Fig. 4, B and D, respectively). However, the proliferation rate of Teff cells in 47-LDA-Fcγ2a+ DC-stimulated cultures were significantly higher compared with stimulation elicited with 47-LDA+ DCs (p = 0.02).
FIGURE 4.
Analyses of Treg cell induction and function in mice immunized with 47-LDA-DC and 47-LDA-Fcγ2a-DC vaccines. Three weeks after the last immunization with 47-LDA-DCs (A) or 47-LDA-Fcγ2a-DCs (C), the axillary lymph nodes were removed and analyzed for Treg cell expression by staining with anti-CD4-PE, anti-CD25-FITC, and anti-FoxP3-Alexa Fluor 647 mAbs or the relevant isotype controls. The histograms illustrate the FoxP3 expression in the gated populations (CD4+CD25+ cells). To analyze the effect of Treg cells on Teff cell proliferation, CD8+ cells from 47-LDA-DC (B) or 47-LDA-Fcγ2a-DC (D) vaccine-immunized mice were loaded with CFSE and cultured with 47-LDA-expressing syngeneic DCs (ratio 20:1) for 72 h in the presence or absence of Treg cells (ratio 1:1). Cells were stained with PE-conjugated anti-CD8 mAbs and analyzed by flow cytometry. The percentage of cells that underwent more than one round of cell division is indicated. Data are from one representative experiment of three performed.
Myeloablative TBI in combination with the 47-LDA-Fcγ2a-DC vaccine enhances ACT therapy
To compare the therapeutic efficacy of the 47-LDA- and 47-LDA-Fcγ2a-DC vaccines, we performed ACT experiments with Ag-experienced CD8+ splenocytes in NXS2 tumor-bearing A/J mice (Fig. 1B). The CD8+ T cells were injected i.v. into the NXS2-bearing mice together with 47-LDA- or 47-LDA-Fcγ2a-DC vaccine to induce the proliferation of Teff cells and mount a secondary response upon adoptive transfer to the lymphodepleted host (50). Fig. 5A shows that lymphodepletion with a nonmyeloablative (5 Gy) regimen before ACT and 47-LDA polypeptide-coated DC vaccine immunization had a background effect on the inhibition of tumor growth (p = 0.28). The antitumor efficacy of the adoptively transferred CD8+ T cells was augmented after myeloablative (9 Gy) TBI, as determined by extension of the overall survival time of the treated mice from 25 to 70 days (Fig. 5A; p = 0.007). We found that the myeloablative regimen was particularly effective in combination with adoptively transferred CD8+ splenocytes delivered together with 47-LDA-Fcγ2a-DCs. As shown in Fig. 5B, the latter treatment resulted in complete remission of tumor growth in 22% of NXS2-bearing animals that received a myeloablative dose of TBI accompanied by a transplantation of syngeneic BM from naive mice (p = 0.0003). It is noteworthy that although the therapy with CD8+ T cells stimulated with 47-LDA-Fcγ2a-DC vaccine in the nonmyeloablative setting did not show complete tumor resolution, there was a significant prolongation of survival in the treated mice compared with the control group (Fig. 5B; p = 0.015). The antitumor efficacy of the ACT was specific because all of the control mice developed progressively growing tumors and had to be sacrificed by day 25.
FIGURE 5.
Inhibition of NXS2 primary and metastatic tumor growth by 47-LDA- and 47-LDA-Fcγ2a-DC vaccines and ACT. A and B, Protection against primary tumor growth by ACT and 47-LDA-DC vaccine (A) and 47-LDA-Fcγ2a-DC vaccine (B). A/J mice (n = 8 – 10) were injected s.c. with 2 × 106 NXS2 cells and treated 15 days later with i.v. adoptive transfer of CD8+-enriched splenocytes isolated from 47-LDA- or 47-LDA-Fcγ2a-vaccinated syngeneic mice. Lymphopenia of tumor-bearing mice was induced by TBI (5 Gy; ●) or (9 Gy; ■) plus BM (107 cells) transplantation 1 day before CD8+ T cell transfer as described in Materials and Methods. Mice were immunized in a 2-wk interval with the DC vaccines in the presence of the IL-15 and IL-21 vectors. NXS2 tumor-bearing mice that were irradiated with 5 Gy (○) or 9 Gy (□) plus BM transplant served as controls. C and D, Control of metastatic disease by adoptively transferred CD8+ splenocytes and 47-LDA-DC (C) and 47-LDA-Fcγ2a-DC (D) vaccines. For the immunotherapy of disseminated disease, NXS2-bearing A/J mice that underwent nonmyeloablative (5 Gy; ●) or myeloablative (9 Gy; ■) TBI at the time of tumor excision received ACT of CD8+ splenocytes (2 × 107 cells) from the immunized mice together with 47-LDA- or 47-LDA-Fcγ2a-DC vaccine. NXS2-challenged mice that were irradiated with 5 Gy (○) or 9 Gy (□) plus BM transplant served as controls. E, NXS2-challenged mice received ACT from naive A/J mice together with the DC vaccines. Mice were immunized in a 2-wk interval with the DC vaccine in the presence of the IL-15 and IL-21 vectors. The control mice had the primary tumor excised with (○) or without (□) TBI and BM transplantation. Survival was defined as the point at which mice were sacrificed due to extensive tumor growth. Kaplan-Meier survival plots were prepared, and significance was determined using the log rank Mantel-Cox method.
Control of metastatic disease by adoptively transferred CD8+ splenocytes and 47-LDA-Fcγ2a-DC vaccine
The ability of NXS2 neuroblastoma to develop spontaneous metastases after excision of the primary tumor (51) provided a model for investigating the efficacy of adoptively transferred CD8+ splenocytes to control disseminated disease (Fig. 1C). In view of the accumulating evidence that curative surgery in conjunction with the depletion of Treg cells enables the development of long-lived tumor protection and CD8+ T cell memory (9), NXS2 tumor-bearing mice underwent nonmyeloablative (5 Gy) or myeloablative (9 Gy) TBI at the time of tumor excision and ACT of Ag-experienced CD8+ splenocytes. As shown in Fig. 5C, transfer of CD8+ T cells expanded by 47-LDA polypeptide-coated DCs delayed progression of the metastatic disease that was observed only in the myeloablated mice (p = 0.024). Tumor-free survival was not observed in this group of animals.
The antitumor efficacy of the adoptively transferred CD8+ T cells was significantly enhanced by the ACT and the 47-LDA-Fcγ2a-DC vaccination, as only two of six NXS2-challenged mice that received 5 Gy of TBI before the ACT and the 47-LDA-Fcγ2a-DC vaccination developed spontaneous metastases within a 50-day period of the treatment (Fig. 5D; p = 0.003). The remaining mice in this group exhibited a delay in progression of the metastatic disease with the longest survival time of 90 days. As expected, the highest antitumor effectiveness of the adoptively transferred splenocytes, with >80% of tumor-free survival during a period of 120 days, was observed after a myeloablative regimen and ACT with 47-LDA-Fcγ2a-DC vaccine (Fig. 5D; p < 0.001). In contrast, the control mice that had the primary tumor excised and received TBI had to be sacrificed by day 40 due to disease progression. The metastatic lesions developed primarily in the brachial and axillary lymph nodes and were less frequent in liver, spleen, and BM as determined by staining with NXS2-reacting 14G2a mAb and RT-PCR analyses (data not shown).
Inhibition of metastatic disease by primary immune responses induced by the 47-LDA-Fcγ2a-DC vaccine
We next investigated the ability of 47-LDA- and 47-LDA-Fcγ2a-DC vaccines to induce antitumor immune responses capable of inhibiting metastatic disease after adoptive transfer of naive splenocytes to myeloablated mice that had the primary tumor resected before the treatment initiation (Fig. 5E). For these experiments the total population of naive splenocytes was used for ACT, because CD4+ T cells are required for providing CD8+ T cells with growth factors and also for eliciting antitumor responses (52). Fig. 5E shows that although the measurable therapeutic impact of the in vivo stimulated naive T cells in NXS2-bearing mice was lower compared with that mediated by the Ag-experienced CD8+ T cells, 20% of treated mice remained tumor-free 100 days after ACT with the 47-LDA-Fcγ2a-DC vaccine (p = 0.007). The 47-LDA+ DC vaccine was also capable of inducing antitumor T cells responses, albeit to a smaller degree. The control mice that had the primary tumor excised developed metastatic disease within the first 2 mo of treatment, indicating a significant efficacy of the tumor-specific T cells induced by active immunization with the 47-LDA-Fcγ2a-DC vaccine in NXS2-bearing mice.
Cellular responses in tumor-bearing and tumor-free mice after ACT and DC vaccination
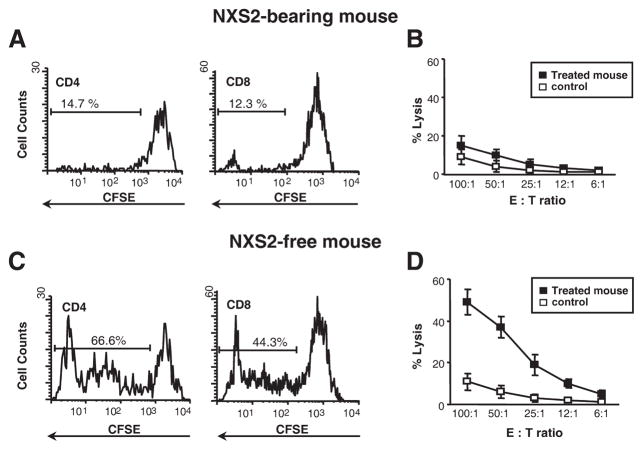
We next investigated cellular responses in tumor-bearing and tumor-free mice after ACT and immunization with DC vaccines. Both therapeutic 47-LDA- and 47-LDA-Fcγ2a-DC vaccines were delivered to myeloablated mice after excision of the primary NXS2 tumor together with adoptively transferred naive splenocytes and analyzed for the induction of Teff cells. Because no tumor-free survival was observed after ACT and immunization with 47-LDA+ DC vaccine, animals with visible metastatic lesions in the lymph nodes were examined for antitumor immune responses in the spleen. As shown in Fig. 6A, <15% of CD4+ or CD8+ splenocytes in the tumor-bearing mice underwent in vitro division after 72 h of stimulation with 47-LDA-expressing DCs, and the CTL responses to NXS2 cells were at a background level (Fig. 6B). In contrast, a robust proliferation of CD4+ and CD8+ splenocytes (ranges of 55 – 69% and 39 – 46%, respectively) was detected in tumor-free mice after receiving ACT and the 47-LDA-Fcγ2a-DC vaccine (Fig. 6C). The proliferative responses were also associated with antitumor CTL activities against NXS2 cells (Fig. 6D).
FIGURE 6.
Analyses of cellular responses in tumor-bearing and tumor-free mice after ACT and DC vaccination. A and B, Lack of proliferative responses (A) and CTL activity (B) in splenocytes of NXS2-bearing mice. A, Splenocytes isolated from mice that developed progressively growing tumors after ACT of naive splenocytes and 47-LDA polypeptide-DC vaccine were loaded with CFSE and cultured with 47-LDA-expressing syngeneic DCs (ratio 20:1) for 72 h. Cells were stained with PE-conjugated anti-CD4 or anti-CD8 mAbs and analyzed by flow cytometry. The percentage of cells that underwent more than one round of cell division is indicated. Data are from one representative experiment of four performed. B, CTL activities of stimulated or control CD8+ splenocytes from tumor-bearing mice against NXS2 cells were analyzed in a standard 51Cr-release assay. All determinations were made in triplicate samples, and results are presented as the means ± SD of three independent experiments. C and D, Analyses of proliferative responses (C) and CTL activities against NXS2 (D) in tumor-free mice after receiving ACT from naive mice and the 47-LDA-Fcγ2a-DC vaccine. Experiments were conducted as described in A and B.
Virotherapy-based cancer vaccine with rOVV expressing 47-LDA-Fcγ2a fusion protein
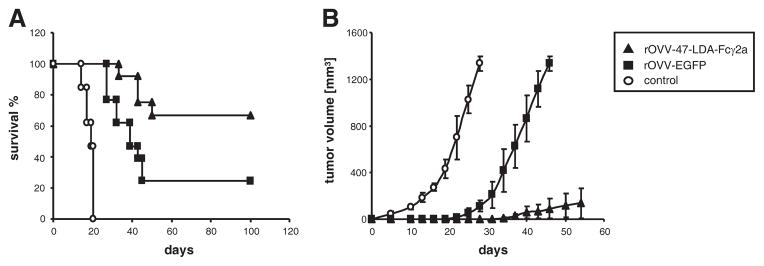
To determine that the antitumor effect of the 47-LDA-Fcγ2a-DC vaccine is not limited to the ACT in an irradiated tumor-bearing host, we next examined the therapeutic efficacy of the 47-LDA-Fcγ2a fusion protein expressed by the rOVV vaccinia virus. Because the oncolytic TK−VGF− mutant of vaccinia rOVV-47-LDA-Fcγ2a exhibits tumor-specific replication, it was anticipated that the fusion protein will be secreted from the infected cells and cross-presented by tumor-infiltrating DCs at the site of oncolysis (29, 30). In parallel studies, an additional group of NXS2 tumor-bearing mice was injected with the rOVV-EGFP vector as a specificity control to determine the extent of protection elicited by tumor-associated Ags released from virally infected NXS2 cells. Animals were examined for tumor growth by measuring s.c. tumors once to thrice a week. As shown in Fig. 7A, ~25% of NXS2-bearing mice that were treated with the control rOVV-EGFP vector became tumor free. In contrast, all tumor-bearing mice that were treated with PBS instead of the viral vector developed progressively growing tumors and had to be sacrificed by day 20. The highest antitumor effectiveness, characterized by >60% tumor-free survival for a period of 100 days, was observed in tumor-bearing mice after oncolytic immunovirotherapy with the rOVV-47-LDA-Fcγ2a vector (Fig. 7A; p = 0.0004).
FIGURE 7.
Therapeutic oncolytic virotherapy-based cancer vaccine with rOVV-EGFP or rOVV-47-LDA-Fcγ2a fusion protein. A, Inhibition of tumor growth by rOVV-47-LDA-Fcγ2a vector. A/J mice (n = 10) were injected s.c. with 2 × 106 NXS2 cells and treated 15 days later with i.v. injection of 108 PFU of rOVV-47-LDA-Fcγ2a (▲) or rOVV-EGFP(■) vector. Tumor-bearing mice treated with PBS served as controls (○). Survival was defined as the point at which mice were sacrificed due to extensive tumor growth. Kaplan-Meier survival plots were prepared, and significance was determined using the log rank Mantel-Cox method. B, Tumor-specific immune memory protected mice from NXS2 rechallenge. Tumor-free mice after treatment with rOVV-47-LDA-Fcγ2a (▲) or rOVV-EGFP (■) vector were rechallenged with NXS2 cells. Untreated mice challenged with the NXS2 tumor served as controls (○). Animals were examined daily until the tumor became palpable, after which tumor growth was monitored by measuring s.c. tumors once to thrice a week.
To obtain a more comprehensive understanding of the effect of oncolytic virotherapy treatment on the memory cell development in a tumor-bearing host, NXS2-challenged mice (n = 6) that remained free of tumors for at least 40 days after rOVV-EGFP or rOVV-47-LDA-Fcγ2a treatment were rechallenged s.c. with 106 NXS2 cells. Fig. 7B shows that all rOVV-EGFP-treated mice developed progressively growing tumors upon rechallenge with NXS2 tumor. There was, however, a 20-day delay in the initiation of tumor growth in the rOVV-EGFP-treated mice compared with the untreated animals. This finding is consistent with the ability of rOVV to induce antitumor immune responses due to the uptake of apoptotic cells by DCs and the cross-presentation of tumor Ags during oncolytic virotherapy (53). In contrast, only one of six rOVV-47-LDA-Fcγ2a-treated and NXS2 rechallenged mice developed slowly progressing tumor 35 days after tumor inoculation, reflecting a significant antitumor influence of the 47-LDA-Fcγ2a-induced immunity compared with rOVV-EGFP- or PBS-treated mice (Fig. 7B; p < 0.001).
Discussion
To develop a means of limiting the ability of cancer vaccines to interact with Treg cells in tumor-bearing mice, we first compared the generation of functional tumor-specific cellular responses by the 47-LDA peptide mimic presented to DCs either as a linear polypeptide in conjunction with universal Th epitopes or as a fusion protein with the murine IgG2a Fc fragment. We showed that the 47-LDA-Fcγ2a fusion protein-DC vaccine not only expanded tumor-specific CD8+ T cells but also induced primary immune responses after ACT of naive splenocytes to tumor-bearing mice that underwent myeloablative TBI and BM transplantation. The 47-LDA-Fcγ2a vaccine-induced CD8+ T cells inhibited growth of the primary tumors as well as protected the tumor-bearing mice from the development of minimal residual disease. In contrast, protection elicited by the 47-LDA polypeptide-DC vaccine resulted in a delay of tumor growth that was significant only after ACT in the myeloablative setting. The results of these studies are consistent with the findings that FcγRs are capable of modulating T cell-mediated immune responses during therapeutic vaccination in tumor-bearing mice (54 – 57).
The mechanisms responsible for the increased ability of 47-LDA-Fcγ2a+ DCs to induce higher levels of tumor-specific immune responses compared with those generated by the 47-LDA+ DCs remain to be elucidated. It is possible that the elevated number of IL-12-expressing cells within the 47-LDA-Fcγ2a+ DC population compared with the 47-LDA+ counterpart after LPS-induced maturation contributed to this effect, because APCs that express IL-12 have augmented capacity to prime type 1 rather than Treg cell responses (48, 58). This observation is further supported by the reduced expression of the CCL22 chemokine in 47-LDA-Fcγ2a+ DCs and the lower proportion of Treg cells in 47-LDA-Fcγ2a+ DC-immunized mice compared with animals that received the 47-LDA-DC vaccine. Our results are consistent with the previous findings that the character of the inflammatory environment can affect the balance between Teff and Treg cell activation by instructing the maturing DCs to adopt a stable propensity to interact with each of these T cell types (45, 48, 58 – 60). It is noteworthy, however, that although alteration of DC functions has been previously suggested in the design of a cancer vaccine (57, 61), the tuning of DC responses to counteract the immunosuppression associated with tumor progression (62 – 65) during therapeutic vaccination with a mimotope of tumor-associated Ags has not been extensively evaluated. Thus, these findings highlight a new application of peptide mimotopes for the immunotherapy of cancer.
It is well established that activating and inhibitory low-affinity FcγRs are critical for the modulation of effector immune responses (46). Their role in the induction of adaptive immunity showed that alteration in the function of activating/inhibitory receptors during the administration of Ags to DCs in a form of immune complexes can influence the activation of DCs in vivo and enhance antitumor T cell responses (55). This notion is supported by the observation that a selective blockade of the inhibitory FcγRIIb receptor enables human DC maturation and immunity to Ab-coated tumor cells (18, 25), and DCs from FcγRIIb-deficient mice show a higher expression of costimulatory molecules, which could account for an increased capacity to prime Ag-specific T cells (66). However, the use of large protein carriers creates difficulties in terms of reproducibility of the Ag binding that may endanger vaccine effectiveness and/or practical feasibility. Therefore, synthetic constructs encompassing the antigenic and helper epitopes as well as the Fc portion of the IgG Ab with increased binding affinity to activating FcγRs should offer distinct advantages over immune complexes in terms of manufacturing and characterization. This also suggests that the ability of the 47-LDA-Fcγ2a fusion protein to empower DCs to efficiently prime and expand specific CD8+ CTL responses in tumor-bearing mice might lead to the improved efficacy of tumor immunotherapy.
The study of antitumor immune responses after DC vaccines or surgery has shown that Treg cells are a fundamental obstacle to the development of T cell memory in hosts bearing poorly immunogenic tumors (9, 48). Although surgery currently remains the leading cure for solid tumors, memory T cell responses may be required for the durable prevention of tumor recurrence and metastasis following surgery. As seen in most patients with cancer, our results showed that surgery or DC vaccine alone does not induce protection against poorly immunogenic tumor-associated Ags. However, in our model, surgery in combination with lymphodepletion, ACT, and DC vaccination enables the development of long-lived tumor protection. Although we cannot exclude the possibility that the tumor itself served as the source of Ag, presumably priming T cell responses against a multitude of tumor Ags during the induction of postsurgical immunity in the absence of Tregs (24, 67, 68), the antitumor immunity generated in the absence of the 47-LDA mimotope DC vaccination only extended the survival of tumor-bearing mice. In contrast, active immunization with a 47-LDA-Fcγ2a fusion protein targeted to the activating FcγRs in a myeloablated tumor-bearing host led to a significant suppression of the primary tumor and protection from the development of spontaneous metastatic disease. The relative contribution of the tumor-induced vs vaccine-induced immunity in the setting of the 47-LDA-Fcγ2a-DC vaccine to the suppression of tumor growth remains unknown, and future work will be required to characterize the kinetics and diversity of antitumor T cell responses.
Although the use of ex vivo generated DCs provides a unique opportunity to avoid tumor-induced DC dysfunction and allows for precise manipulation of DC properties, the need for specialized cell culture facilities for the ex vivo manipulation of a patient’s cells prompted attempts to develop cell-free vaccines capable of targeting endogenous DCs within the bodies of tumor-bearing hosts. Such vaccines engineered to deliver tumor Ags or their mimotopes selectively to DCs can be coupled with strategies to induce DC polarization in vivo without any ex vivo treatment. Our results using the virotherapy approach with the rOVV-47-LDA-Fcγ2a vector, which delivers the transgene directly to tumor lesions (data not shown) for secretion and cross-presentation by DCs, demonstrated the induction of protective Teff cells as well as memory T cells in the absence of any exogenous cytokine treatment, ACT, TBI, or ex vivo manipulation. Further investigation is required to better define the effect of cross-presentation of a virally delivered 47-LDA-Fcγ2a fusion protein in the tumor microenvironment on the balance between Teffs and Tregs as well as the effectiveness and longevity of the virotherapy-induced antitumor immune responses. In this regard, several lines of evidence indicate that the vaccinia virus elicits innate immune responses through the TLR2/MyD88-dependent pathway, resulting in the production of proinflammatory cytokines and a TLR-independent pathway that leads to the activation of IFN-β in vitro and in vivo (32). Because sustained stimulation of TLRs of the innate immunity is required for breaking established Treg-mediated tolerance in vivo and the vaccinia virus can provide TLR signals, this unique potency of the vaccinia virus as a vaccine vehicle can lead to activation of host defense and protect the mice from tumor challenge (31, 69). Thus, the possibility of breaking CD8+ T cell tolerance by the virus-based vaccines in the presence of CD4+ CD25+ Tregs suggests that rOVV expressing the 47-LDA-Fcγ2a fusion protein or other tumor-associated Ags may prove an appealing alternative to DC vaccines for overcoming CD4+ CD25+ T cell-mediated CD8 tolerance in vivo. In summary, this work stresses the importance of exploring the uptake and processing of tumor/self-Ags by DCs to activate immune effector cells and to limit the ability of attracting anti-inflammatory Treg cells. Our findings illuminate a new paradigm for cancer immunotherapies aimed at the selective activation of the inflammatory vs the regulatory type of immune cell.
Acknowledgments
We are grateful to Dr. R. A. Reisfeld, B. Moss, and D. Bartlett for the reagents. We thank Earl Timm and Rosemary Furlage for help with a flow cytometric analysis.
Footnotes
Abbreviations used in this paper: DC, dendritic cell; ACT, adoptive cell transfer; BM, bone marrow; EGFP, enhanced GFP; rOVV, recombinant oncolytic vaccinia virus; TBI, total body irradiation; Teff, effector T cell; TK, thymidine kinase; Treg, regulatory T-cell; VGF, vaccinia growth factor.
Disclosures
The authors have no financial conflict of interest.
This work was supported by the National Institutes of Health Grants R21EB008071 (to D.K.), Association for Research of Childhood Cancer, Inc., (D.K. and B.B.), and Roswell Park Alliance Foundation (D. K. and B.B.).
References
- 1.Curiel TJ. Tregs and rethinking cancer immunotherapy. J Clin Invest. 2007;117:1167–1174. doi: 10.1172/JCI31202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalinski P, Urban J, Narang R, Berk E, Wieckowski E, Muthuswamy R. Dendritic cell-based therapeutic cancer vaccines: what we have and what we need. Future Oncol. 2009;5:379–390. doi: 10.2217/FON.09.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schadendorf D, Ugurel S, Schuler-Thurner B, Nestle FO, Enk A, Brocker EB, Grabbe S, Rittgen W, Edler L, Sucker A, et al. Dacarbazine (DTIC) versus vaccination with autologous peptide-pulsed dendritic cells (DC) in first-line treatment of patients with metastatic melanoma: a randomized phase III trial of the DC study group of the DeCOG. Ann Oncol. 2006;17:563–570. doi: 10.1093/annonc/mdj138. [DOI] [PubMed] [Google Scholar]
- 4.Zhou G, Drake CG, Levitsky HI. Amplification of tumor-specific regulatory T cells following therapeutic cancer vaccines. Blood. 2006;107:628–636. doi: 10.1182/blood-2005-07-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palucka AK, Ueno H, Fay JW, Banchereau J. Taming cancer by inducing immunity via dendritic cells. Immunol Rev. 2007;220:129–150. doi: 10.1111/j.1600-065X.2007.00575.x. [DOI] [PubMed] [Google Scholar]
- 6.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Yamaguchi T, Sakaguchi S. Regulatory T cells in immune surveillance and treatment of cancer. Semin Cancer Biol. 2006;16:115–123. doi: 10.1016/j.semcancer.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Wang HY, Wang RF. Regulatory T cells and cancer. Curr Opin Immunol. 2007;19:217–223. doi: 10.1016/j.coi.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Cote AL, Usherwood EJ, Turk MJ. Tumor-specific T-cell memory: clearing the regulatory T-cell hurdle. Cancer Res. 2008;68:1614–1617. doi: 10.1158/0008-5472.CAN-07-6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahonen CL, Wasiuk A, Fuse S, Turk MJ, Ernstoff MS, Suriawinata AA, Gorham JD, Kedl RM, Usherwood EJ, Noelle RJ. Enhanced efficacy and reduced toxicity of multifactorial adjuvants compared with unitary adjuvants as cancer vaccines. Blood. 2008;111:3116–3125. doi: 10.1182/blood-2007-09-114371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen W, Yan W, Huang L. A simple but effective cancer vaccine consisting of an antigen and a cationic lipid. Cancer Immunol Immunother. 2008;57:517–530. doi: 10.1007/s00262-007-0390-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Y, Yang S, Ye Z, Jaffar J, Zhou Y, Cutter E, Lieber A, Hellstrom I, Hellstrom KE. Tumor cells expressing anti-CD137 scFv induce a tumor-destructive environment. Cancer Res. 2007;67:2339–2344. doi: 10.1158/0008-5472.CAN-06-3593. [DOI] [PubMed] [Google Scholar]
- 13.Schuler T, Blankenstein T. Cutting edge: CD8+ effector T cells reject tumors by direct antigen recognition but indirect action on host cells. J Immunol. 2003;170:4427–4431. doi: 10.4049/jimmunol.170.9.4427. [DOI] [PubMed] [Google Scholar]
- 14.Schuler T, Kammertoens T, Preiss S, Debs P, Noben-Trauth N, Blankenstein T. Generation of tumor-associated cytotoxic T lymphocytes requires interleukin 4 from CD8+ T cells. J Exp Med. 2001;194:1767–1775. doi: 10.1084/jem.194.12.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schuler T, Qin Z, Ibe S, Noben-Trauth N, Blankenstein T. T helper cell type 1-associated and cytotoxic T lymphocyte-mediated tumor immunity is impaired in interleukin 4-deficient mice. J Exp Med. 1999;189:803–810. doi: 10.1084/jem.189.5.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dhodapkar MV, Steinman RM, Sapp M, Desai H, Fossella C, Krasovsky J, Donahoe SM, Dunbar PR, Cerundolo V, Nixon DF, Bhardwaj N. Rapid generation of broad T-cell immunity in humans after a single injection of mature dendritic cells. J Clin Invest. 1999;104:173–180. doi: 10.1172/JCI6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dhodapkar KM, Dhodapkar MV. Recruiting dendritic cells to improve antibody therapy of cancer. Proc Natl Acad Sci USA. 2005;102:6243–6244. doi: 10.1073/pnas.0502547102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dhodapkar KM, Kaufman JL, Ehlers M, Banerjee DK, Bonvini E, Koenig S, Steinman RM, Ravetch JV, Dhodapkar MV. Selective blockade of inhibitory Fcγ receptor enables human dendritic cell maturation with IL-12p70 production and immunity to Ab-coated tumor cells. Proc Natl Acad Sci USA. 2005;102:2910–2915. doi: 10.1073/pnas.0500014102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nimmerjahn F, Ravetch JV. Divergent immunoglobulin G subclass activity through selective Fc receptor binding. Science. 2005;310:49, 510–1512. doi: 10.1126/science.1118948. [DOI] [PubMed] [Google Scholar]
- 20.Ravetch JV, Bolland S. IgG Fc receptors. Annu Rev Immunol. 2001;19:275–290. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- 21.Dhodapkar MV, Dhodapkar KM, Palucka AK. Interactions of tumor cells with dendritic cells: balancing immunity and tolerance. Cell Death Differ. 2008;15:39–50. doi: 10.1038/sj.cdd.4402247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ravetch JV. A full complement of receptors in immune complex diseases. J Clin Invest. 2002;110:1759–1761. doi: 10.1172/JCI200217349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Regnault A, Lankar D, Lacabanne V, Rodriguez A, Thery C, Rescigno M, Saito T, Verbeek S, Bonnerot C, Ricciardi-Castagnoli P, Amigorena S. Fcγ receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J Exp Med. 1999;189:371–380. doi: 10.1084/jem.189.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Groh V, Li YQ, Cioca D, Hunder NN, Wang W, Riddell SR, Yee C, Spies T. Efficient cross-priming of tumor antigen-specific T cells by dendritic cells sensitized with diverse anti-MICA opsonized tumor cells. Proc Natl Acad Sci USA. 2005;102:6461–6466. doi: 10.1073/pnas.0501953102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalergis AM, Ravetch JV. Inducing tumor immunity through the selective engagement of activating Fcγ receptors on dendritic cells. J Exp Med. 2002;195:1653–1659. doi: 10.1084/jem.20020338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dhodapkar KM, Banerjee D, Connolly J, Kukreja A, Matayeva E, Veri MC, Ravetch JV, Steinman RM, Dhodapkar MV. Selective blockade of the inhibitory Fcγ receptor (FcγRIIB) in human dendritic cells and monocytes induces a type I interferon response program. J Exp Med. 2007;204:1359–1369. doi: 10.1084/jem.20062545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bolesta E, Kowalczyk A, Wierzbicki A, Rotkiewicz P, Bambach B, Tsao CY, Horwacik I, Kolinski A, Rokita H, Brecher M, et al. DNA vaccine expressing the mimotope of GD2 ganglioside induces protective GD2 cross-reactive antibody responses. Cancer Res. 2005;65:3410–3418. doi: 10.1158/0008-5472.CAN-04-2164. [DOI] [PubMed] [Google Scholar]
- 28.Wierzbicki A, Gil M, Ciesielski M, Fenstermaker RA, Kaneko Y, Rokita H, Lau JT, Kozbor D. Immunization with a mimotope of GD2 ganglioside induces CD8+ T cells that recognize cell adhesion molecules on tumor cells. J Immunol. 2008;181:6644–6653. doi: 10.4049/jimmunol.181.9.6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCart JA, Ward JM, Lee J, Hu Y, Alexander HR, Libutti SK, Moss B, Bartlett DL. Systemic cancer therapy with a tumor-selective vaccinia virus mutant lacking thymidine kinase and vaccinia growth factor genes. Cancer Res. 2001;61:8751–8757. [PubMed] [Google Scholar]
- 30.Gao P, Uekusa Y, Nakajima C, Iwasaki M, Nakahira M, Yang YF, Ono S, Tsujimura T, Fujiwara H, Hamaoka T. Tumor vaccination that enhances antitumor T-cell responses does not inhibit the growth of established tumors even in combination with interleukin-12 treatment: the importance of inducing intratumoral T-cell migration. J Immunother. 2000;23:643–653. doi: 10.1097/00002371-200011000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Yang Y, Huang CT, Huang X, Pardoll DM. Persistent Toll-like receptor signals are required for reversal of regulatory T cell-mediated CD8 tolerance. Nat Immunol. 2004;5:508–515. doi: 10.1038/ni1059. [DOI] [PubMed] [Google Scholar]
- 32.Zhu J, Martinez J, Huang X, Yang Y. Innate immunity against vaccinia virus is mediated by TLR2 and requires TLR-independent production of IFN-β. Blood. 2007;109:619–625. doi: 10.1182/blood-2006-06-027136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu TC, Kirn D. Systemic efficacy with oncolytic virus therapeutics: clinical proof-of-concept and future directions. Cancer Res. 2007;67:429–432. doi: 10.1158/0008-5472.CAN-06-2871. [DOI] [PubMed] [Google Scholar]
- 34.Liu TC, Kirn D. Gene therapy progress and prospects cancer: oncolytic viruses. Gene Ther. 2008;15:877–884. doi: 10.1038/gt.2008.72. [DOI] [PubMed] [Google Scholar]
- 35.Diaz RM, Galivo F, Kottke T, Wongthida P, Qiao J, Thompson J, Valdes M, Barber G, Vile RG. Oncolytic immunovirotherapy for melanoma using vesicular stomatitis virus. Cancer Res. 2007;67:2840–2848. doi: 10.1158/0008-5472.CAN-06-3974. [DOI] [PubMed] [Google Scholar]
- 36.Lode HN, Xiang R, Dreier T, Varki NM, Gillies SD, Reisfeld RA. Natural killer cell-mediated eradication of neuroblastoma metastases to bone marrow by targeted interleukin-2 therapy. Blood. 1998;91:1706–1715. [PubMed] [Google Scholar]
- 37.Mujoo K, Kipps TJ, Yang HM, Cheresh DA, Wargalla U, Sander DJ, Reisfeld RA. Functional properties and effect on growth suppression of human neuroblastoma tumors by isotype switch variants of monoclonal anti-ganglioside GD2 antibody 14.18. Cancer Res. 1989;49:2857–2861. [PubMed] [Google Scholar]
- 38.Alexander J, del Guercio MF, Maewal A, Qiao L, Fikes J, Chesnut RW, Paulson J, Bundle DR, DeFrees S, Sette A. Linear PADRE T helper epitope and carbohydrate B cell epitope conjugates induce specific high titer IgG antibody responses. J Immunol. 2000;164:1625–1633. doi: 10.4049/jimmunol.164.3.1625. [DOI] [PubMed] [Google Scholar]
- 39.Ahluwalia A, Gokulan K, Nath I, Rao DN. Modification of delivery system enhances MHC nonrestricted immunogenicity of V3 loop region of HIV-1 gp120. Microbiol Immunol. 1997;41:779–784. doi: 10.1111/j.1348-0421.1997.tb01926.x. [DOI] [PubMed] [Google Scholar]
- 40.Brinker KG, Garner H, Wright JR. Surfactant protein A modulates the differentiation of murine bone marrow-derived dendritic cells. Am J Physiol. 2003;284:L232–L241. doi: 10.1152/ajplung.00187.2002. [DOI] [PubMed] [Google Scholar]
- 41.Kowalczyk A, Wierzbicki A, Gil M, Bambach B, Kaneko Y, Rokita H, Repasky E, Fenstermaker R, Brecher M, Ciesielski M, Kozbor D. Induction of protective immune responses against NXS2 neuroblastoma challenge in mice by immunotherapy with GD2 mimotope vaccine and IL-15 and IL-21 gene delivery. Cancer Immunol Immunother. 2007;56:1443–1458. doi: 10.1007/s00262-007-0289-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeng R, Spolski R, Finkelstein SE, Oh S, Kovanen PE, Hinrichs CS, Pise-Masison CA, Radonovich MF, Brady JN, Restifo NP, et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med. 2005;201:139–148. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bolesta E, Kowalczyk A, Wierzbicki A, Eppolito C, Kaneko Y, Takiguchi M, Stamatatos L, Shrikant PA, Kozbor D. Increased level and longevity of protective immune responses induced by DNA vaccine expressing the HIV-1 Env glycoprotein when combined with IL-21 and IL-15 gene delivery. J Immunol. 2006;177:177–191. doi: 10.4049/jimmunol.177.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buller RM, Chakrabarti S, Cooper JA, Twardzik DR, Moss B. Deletion of the vaccinia virus growth factor gene reduces virus virulence. J Virol. 1988;62:866–874. doi: 10.1128/jvi.62.3.866-874.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kmieciak D, Wasik TJ, Teppler H, Pientka J, Hsu SH, Takahashi H, Okumura K, Kaneko Y, Kozbor D. The effect of deletion of the V3 loop of gp120 on cytotoxic T cell responses and HIV gp120-mediated pathogenesis. J Immunol. 1998;160:5676–5683. [PubMed] [Google Scholar]
- 46.Nimmerjahn F, Ravetch JV. Fcγ receptors: old friends and new family members. Immunity. 2006;24:19–28. doi: 10.1016/j.immuni.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 47.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 48.Muthuswamy R, Urban J, Lee JJ, Reinhart TA, Bartlett D, Kalinski P. Ability of mature dendritic cells to interact with regulatory T cells is imprinted during maturation. Cancer Res. 2008;68:5972–5978. doi: 10.1158/0008-5472.CAN-07-6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Banerjee DK, Dhodapkar MV, Matayeva E, Steinman RM, Dhodapkar KM. Expansion of FOXP3high regulatory T cells by human dendritic cells (DCs) in vitro and after injection of cytokine-matured DCs in myeloma patients. Blood. 2006;108:2655–2661. doi: 10.1182/blood-2006-03-011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parretta E, Cassese G, Barba P, Santoni A, Guardiola J, Di Rosa F. CD8 cell division maintaining cytotoxic memory occurs predominantly in the bone marrow. J Immunol. 2005;174:7654–7664. doi: 10.4049/jimmunol.174.12.7654. [DOI] [PubMed] [Google Scholar]
- 51.Neal ZC, Imboden M, Rakhmilevich AL, Kim KM, Hank JA, Surfus J, Dixon JR, Lode HN, Reisfeld RA, Gillies SD, Sondel PM. NXS2 murine neuroblastomas express increased levels of MHC class I antigens upon recurrence following NK-dependent immunotherapy. Cancer Immunol Immunother. 2004;53:41–52. doi: 10.1007/s00262-003-0435-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hunder NN, Wallen H, Cao J, Hendricks DW, Reilly JZ, Rodmyre R, Jungbluth A, Gnjatic S, Thompson JA, Yee C. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med. 2008;358:2698–2703. doi: 10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li QX, Liu G, Wong-Staal F. Oncolytic virotherapy as a personalized cancer vaccine. Int J Cancer. 2008;123:493–499. doi: 10.1002/ijc.23692. [DOI] [PubMed] [Google Scholar]
- 54.Amigorena S. Fcγ receptors and cross-presentation in dendritic cells. J Exp Med. 2002;195:F1–F3. doi: 10.1084/jem.20011925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boruchov AM, Heller G, Veri MC, Bonvini E, Ravetch JV, Young JW. Activating and inhibitory IgG Fc receptors on human DCs mediate opposing functions. J Clin Invest. 2005;115:2914–2923. doi: 10.1172/JCI24772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schuurhuis DH, Ioan-Facsinay A, Nagelkerken B, van Schip JJ, Sedlik C, Melief CJ, Verbeek JS, Ossendorp F. Antigen-antibody immune complexes empower dendritic cells to efficiently prime specific CD8+ CTL responses in vivo. J Immunol. 2002;168:2240–2246. doi: 10.4049/jimmunol.168.5.2240. [DOI] [PubMed] [Google Scholar]
- 57.Wenink MH, van den Berg WB, van Riel PL, Radstake TR. Fc γ receptor mediated modulation of dendritic cells as a potential strategy in the battle against rheumatoid arthritis. Neth J Med. 2006;64:103–108. [PubMed] [Google Scholar]
- 58.Kalinski P, Hilkens CM, Wierenga EA, Kapsenberg ML. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol Today. 1999;20:561–567. doi: 10.1016/s0167-5699(99)01547-9. [DOI] [PubMed] [Google Scholar]
- 59.Sporri R, Reis e Sousa C. Inflammatory mediators are insufficient for full dendritic cell activation and promote expansion of CD4+ T cell populations lacking helper function. Nat Immunol. 2005;6:163–170. doi: 10.1038/ni1162. [DOI] [PubMed] [Google Scholar]
- 60.Figdor CG, I, de Vries J, Lesterhuis WJ, Melief CJ. Dendritic cell immunotherapy: mapping the way. Nat Med. 2004;10:475–480. doi: 10.1038/nm1039. [DOI] [PubMed] [Google Scholar]
- 61.Hackstein H, Thomson AW. Dendritic cells: emerging pharmacological targets of immunosuppressive drugs. Nat Rev Immunol. 2004;4:24–34. doi: 10.1038/nri1256. [DOI] [PubMed] [Google Scholar]
- 62.Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, Divino CM, Chen SH. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–1131. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 63.Liu VC, Wong LY, Jang T, Shah AH, Park I, Yang X, Zhang Q, Lonning S, Teicher BA, Lee C. Tumor evasion of the immune system by converting CD4+CD25−T cells into CD4+CD25+ T regulatory cells: role of tumor-derived TGF-β. J Immunol. 2007;178:2883–2892. doi: 10.4049/jimmunol.178.5.2883. [DOI] [PubMed] [Google Scholar]
- 64.Sharma S, Yang SC, Hillinger S, Zhu LX, Huang M, Batra RK, Lin JF, Burdick MD, Strieter RM, Dubinett SM. SLC/CCL21-mediated anti-tumor responses require IFNγ MIG/CXCL9 and IP-10/CXCL10. Mol Cancer. 2003;2:22. doi: 10.1186/1476-4598-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sharma S, Yang SC, Zhu L, Reckamp K, Gardner B, Baratelli F, Huang M, Batra RK, Dubinett SM. Tumor cyclooxygenase-2/prostaglandin E2-dependent promotion of FOXP3 expression and CD4+ CD25+ T regulatory cell activities in lung cancer. Cancer Res. 2005;65:5211–5220. doi: 10.1158/0008-5472.CAN-05-0141. [DOI] [PubMed] [Google Scholar]
- 66.Iruretagoyena MI, Riedel CA, Leiva ED, Gutierrez MA, Jacobelli SH, Kalergis AM. Activating and inhibitory Fcγ receptors can differentially modulate T cell-mediated autoimmunity. Eur J Immunol. 2008;38:2241–2250. doi: 10.1002/eji.200838197. [DOI] [PubMed] [Google Scholar]
- 67.Dhodapkar MV, Krasovsky J, Olson K. T cells from the tumor microenvironment of patients with progressive myeloma can generate strong, tumor-specific cytolytic responses to autologous, tumor-loaded dendritic cells. Proc Natl Acad Sci USA. 2002;99:13009–13013. doi: 10.1073/pnas.202491499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Munz C, Steinman RM, Fujii S. Dendritic cell maturation by innate lymphocytes: coordinated stimulation of innate and adaptive immunity. J Exp Med. 2005;202:203–207. doi: 10.1084/jem.20050810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]