Abstract
Transforming growth factor β1 (TGFβ1), an important pleiotropic, immunoregulatory cytokine, uses distinct signaling mechanisms in lymphocytes to affect T-cell homeostasis, regulatory T (Treg)-cell and effector-cell function and tumorigenesis. Defects in TGFβ1 expression or its signaling in T cells correlate with the onset of several autoimmune diseases. TGFβ1 prevents abnormal T-cell activation through the modulation of Ca2+–calcineurin signaling in a Caenorhabditis elegans Sma and Drosophila Mad proteins (SMAD)3 and SMAD4-independent manner; however, in Treg cells, its effects are mediated, at least in part, through SMAD signaling. TGFβ1 also acts as a pro-inflammatory cytokine and induces interleukin (IL)-17-producing pathogenic T-helper cells (Th IL-17 cells) synergistically during an inflammatory response in which IL-6 is produced. Here, we will review TGFβ1 and its signaling in T cells with an emphasis on the regulatory arm of immune tolerance.
Introduction: TGFβ1 in T-cell tolerance
Self-tolerance is achieved primarily by negative selection of immature thymocytes and also through regulatory T (Treg)-cell activity in the periphery. Natural Treg (nTreg) cells develop in the thymus, are primarily self-antigen specific and express CD4, CD25 and forkhead box P3 (FOXP3). They home to peripheral tissues to maintain self-tolerance [1]. Treg cells are also generated in the periphery during an active immune response and are called adaptive or induced Treg cells. Treg-cell generation and function is regulated by several important factors. These include cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), glucocorticoid-induced tumour necrosis factor receptor (GITR), CD28 and CD25 on T cells and CD103, B7-1 and B7-2 on antigen-presenting cells (APCs), as well as transcription factor FOXP3 and the cytokines interleukin (IL)-10 and transforming growth factor β1 (TGFβ1) [1–6]. Treg cells are classified into various subsets depending on their surface markers, transcription factors and the cytokines they secrete [7]. The nTreg cells are the major Treg cells found under homeostatic conditions. These cells induce self-tolerance and prevent autoimmunity. They express FOXP3 and are thought to suppress naïve T cells through cell–cell contact and membrane-bound TGFβ1 [8] (Table 1). Treg cells are also induced in the periphery from CD4+CD25− T cells de novo, which are called inducible or adaptive Treg cells. These cells inhibit effector T cells primarily through the production of cytokines, such as IL-10 and TGFβ1. IL-10-producing Treg cells are called Tr1 cells, whereas TGFβ1-secreting Treg cells are called Th3 cells. A subset of CD8 T cells (CD8+CD122+) also suppresses the T-cell response through IL-10 production (reviewed in [7,9,10]). Tr1 cells are localized primarily in mucosal tissues, such as the colon, where they control inflammatory responses [11].
Table 1.
Regulatory T-cell subsetsa
| Treg subset | Regulatory mechanisms | FOXP3 expression |
Function |
|---|---|---|---|
| nTregs (CD4+CD25+) | Cell–cell contact dependent, cytokines (TGFβ1) |
Yes | Induces self-tolerance, inhibits allergy and allograft rejection |
| Tr1 cells | IL-10 | No | Inhibits mucosal inflammation |
| Th3 cells (iTreg cells) | TGFβ1 | FOXP3 | Inhibits autoimmunity |
| CD3+ CD4−CD8− (DN) T cells | Fas–FasL | No | Inhibits allograft rejection |
| CD4+CD25− Treg cells (anergic CD4+ cells) | Cell–cell contact dependent | ? | Inhibits allograft rejection |
| CD8+CD122+ | IL-10 | No | Inhibits T-cell responses and IFN-γ production |
| NK-Treg cells | IL-4, IL-10, TGFβ, cytotoxicity | ? | Suppression of autoimmunity, elimination of tumors |
TGFβ1 is an important immunoregulatory cytokine that is involved in the maintenance of self-tolerance and T-cell homeostasis [2]. It is produced by several immune and non-immune cell types and functions in a both autocrine and paracrine manner [2,12,13]; Treg cells are the primary source of TGFβ1 in the control of autoimmune disease. Last year, there were several important studies on the role of TGFβ1 and its signaling in T cells, both as a pro-and anti-inflammatory cytokine. Severe autoimmune diseases are induced in mice by blocking TGFβ signaling in T cells [14–16]. TGFβ signaling in T cells under inflammatory conditions is also important for the development of experimental autoimmune encephalomyelitis (EAE), a mouse model of human multiple sclerosis [17]. IL-6 inhibits Foxp3 expression and FOXP3+ Treg cells are increased in Il6 knockout (KO) mice, which are resistant to EAE induction [17,18]. Because, in T cells, TGFβ1 induces both FOXP3 and orphan nuclear receptor (RORγt) in the presence of IL-2 and IL-6, respectively, it is suggested that IL-6 counteracts the TGFβ1 effect on Treg-cell generation [19]. Here, we will review recent breakthroughs regarding the function of TGFβ1 in immune tolerance and we will discuss the implications for therapeutic intervention in auto-immune and inflammatory diseases.
TGFβ1 signaling in T cells
TGFβ1 signals primarily through membrane-bound serine/threonine kinase receptors and its signaling intermediates vary depending on cell type and response [19,20]. In T cells, TGFβ1 also signals through a Caenorhabditis elegans Sma and Drosophila Mad proteins (SMAD)-independent Ca2+–calcineurin–nuclear factor of activated T cells (NF-AT) cascade that inhibits naïve T-cell activation [21,22]. Lack of TGFβ ligand-binding might prevent translocation of FK506 binding protein 12 (FKBP12) from the TGFβ1-receptor complex to a ternary complex with the IP3 receptor and calcineurin, where it prevents Ca2+ channels from leaking [23]. AlthoughTGFβ1 inhibitsnaïve T-cell responses, it also induces Foxp3 expression in T cells and causes Th- to Treg-cell conversion and subsequent expansion through SMAD signaling[24].TGFβ1 also induces Ctla4 expression in naïve T cells, which, in turn, is responsible for the induction of Foxp3 in inducible Treg cells (Figure 1) [25]. Conditional deletion in T cells of the gene (Tgfbr2) for the TGFβ receptor type II (TGFβR2) confirms that TGFβ1 signaling is essential for T-cell homeostasis and Treg-cell suppressor function [14,15].
Figure 1.
Cross-talk between TCR and TGFβ signaling networks. TCR activation on recognition of MHC–Ag complexes on APCs leads to the phosphorylation of ZAP70, PLCγ and Lck. PLCγ hydrolyses PIP2 to generate DAG and IP3. DAG binds to and activates PKC, whereas IP3 binds to its receptor on the endoplasmic reticulum (ER) and releases Ca2+ into cytoplasm. Activated PKC activates NF-κB by degrading I-κB through phosphorylation. Increased cytosolic Ca2+ leads to the activation of calcineurin (CN), which, in turn, activates NF-AT through dephosphorylation. Active NF-κB and NF-AT translocate to the nucleus and induce the gene transcription that is required for the T-cell response. Co-stimulatory signals through CD28 activate the JNK/p38 MAPK pathway, which activates AP-1 (FOS/JUN). CD28 co-stimulatory signals inhibit Cblb, which is a negative regulator of TCR signals. Signaling through CD28 activates AKT, which, in turn, inhibits SMAD2/3 phosphorylation. Co-stimulatory signaling through CTLA-4, which is a negative regulator of TCR signaling, induces Cblb. TGFβ-receptor signaling activates SMAD2/3 through phosphorylation. Activated SMAD2/3 binds to SMAD4 and the complex translocates to the nucleus. FOXP3 inhibits SMAD7, which is an inhibitory SMAD that inhibits TGFβ signaling. CBL-B also mediates TGFβ signaling in Foxp3 induction. CD25 is the α subunit of the high-affinity receptor for the IL-2 complex and IL-2 is essential for TGFβ1-mediated induction of FOXP3. FKBP12, which is associated with the TGFβ receptor, is released on ligand binding. FKBP12 binds to the IP3 receptor and modulates Ca2+ release from ER.
TGFβ1 induces Th17-cell differentiation from naïve CD4+ cells on αCD3 and lipopolysaccharide (LPS) stimulation in the presence of dendritic cells (DCs). However, Th17 cells fail to develop from dominant negative (DN)-TGFβRII CD4+ T cells under the same culture conditions, suggesting that TGFβ signaling is required for Th17-cell differentiation [26]. The nuclear orphan receptor RORγt is required for the development of Th17 cells. TGFβ1 and IL-6 induce RORγt independently. FOXP3, which is induced by TGFβ1, inhibits RORγt-induced IL-17 expression [27]. Thus, a balance of IL-6 and TGFβ1 determines whether a T cell becomes a Treg or a Th17 cell.
TGFβ1 inhibits both Th1- and Th2-cell differentiation by inhibiting T-bet (Tbx21) and Gata3 expression, respectively. TGFβ1 inhibits Ifng expression through the inhibition of Tbx21 and Stat4, which are known to induce Th1-cell differentiation [12,28]. The inhibitory effect of TGFβ1 on Tbx21 expression is mediated through Src homology region 2 domain-containing protein tyrosine phosphatase-1 (SHP-1) [29], a negative regulator of T-cell receptor (TCR) signaling and Treg-cell generation. TGFβ1 also inhibits Th2-cell differentiation by inhibiting Gata3 expression in mouse but not human T cells [30,31]. GATA3 induces Th2-cell differentiation by inducing Il5, Il13 and Il4 expression and repressing Il12r expression [32]. It is unclear whether TGFβ1 uses different signaling pathways in inhibiting Th1- and Th2-cell differentiation. Our data indicate that TGFβ1 inhibits Th1-cell differentiation through a Ca2+–calcineurin pathway, although it is less clear whether this is the pathway used to inhibit Th2-cell differentiation [21]. Alternatively, it is possible that TGFβ1 induces Th3 or Th17 cells, depending on the cytokine environment, thereby causing an indirect suppression of Th1- and Th2-cell differentiation.
Phenotypes of TGFβ1-deficient mice
Tgfb1−/− mice die at weaning age from a multifocal autoimmune disease caused by self-reactive T cells [2,33]. Phenotypes of Tgfb1−/− mice on various gene-KO backgrounds are summarized in Table 2. Consistent with recent observations that TGFβ1 and IL-6 are essential for Th17-cell differentiation, Th17 cells are absent in Tgfb1−/− mice, suggesting that Tgfb1−/− T cells cause autoimmune disease without differentiating into Th17 cells [19,34]. Because Tgfb1−/− T cells produce interferon (IFN)-γ but not IL-17, it is possible that IFN-γ produced by Th1 cells inhibits Th17-cell development [22,34,35]. Tgfb1−/− T cells do differentiate into Th17 cells in vitro in the presence of added TGFβ1 and anti-CD3, suggesting that the lack of Th17-cell development in Tgfb1−/− mice is owing to the presence of abundant IFN-γ, the absence of TGFβ1 or both. In Tgfb1−/− Ifng−/− mice, inflammation is eliminated only in the liver but not in the heart and lungs [35]. This suggests that pathogenic Th17 cells might be induced by IL-6 in the absence of IFN-γ and TGFβ1, hence causing disease and early lethality in Tgfb1−/− Ifng−/− mice. EAE disease is exacerbated in IFN-γ-deficient mice. An interpretation of these data is that the deficiency of Th1 cells exacerbates autoimmune disease owing to a lack of inhibition of Th17-cell differentiation that results in Th17-cell expansion by TGFβ1 and IL-6. Because Tgfb1−/− mice produce increased amounts of IFN-γ, it is possible that Th1 cells cause disease in Tgfb1−/− mice, whereas Th17 cells cause disease in Tgfb1−/− Ifng−/− mice. We have observed that, in Tgfb1−/− mice, CD8 T cells also cause inflammation without CD4 helper T cells, although it is unclear whether the inflammation is caused by Th17 or CD8 T cells in Tgfb1−/− Ifng−/− mice [35,36] (Table 2). More recently, Korn et al. have shown that IL-21 is required for Th17-cell generation. Because FOXP3+ Treg cells are increased at the expense of effector T cells in Il6−/− mice, Th17 cells could be generated in Treg-cell-deleted IL-6-deficient mice, suggesting that IL-6 inhibits TGFβ1-induced Treg-cell generation, thereby enabling TGFβ1-induced Th17-cell generation [17,18]. However, IL-6 is more potent than IL-21 in inducing IL-17 and inhibiting FOXP3 production in the presence of TGFβ1.
Table 2.
Phenotypes of TGFβ1-deficient mice on various genetic backgrounds
| Genotype of KO mice | Survival age |
Phenotype | Comments | Refs |
|---|---|---|---|---|
| Tgfb1−/− | 3–4 wks | Severe multi-organ inflammation |
Lymphocytes, macrophages and neutrophils cause the lesions |
[86] |
|
Tgfb1−/− Rag*−/− (lymphocyte-deficient Tgfb1−/− mice) |
3–6 months |
Mild inflammation in the gut and lungs |
Macrophages and neutrophils cause the lesions. Inflammation in Tgfb1−/− mice is caused by lymphocytes primarily |
[87] |
|
Tgfb1−/− Cd4−/− (CD4+ T-cell-deficient Tgfb1−/− mice) |
3–4 wks | Severe multi-organ inflammation |
CD4+ T cells are not essential for inflammation in Tgfb1−/− mice |
[36] |
|
Tgfb1−/− Cd8−/− (CD8+ T-cell-deficient Tgfb1−/− mice) |
3–4 wks | Severe multi-organ inflammation |
CD8+ T cells are not essential for inflammation in Tgfb1−/− mice |
[36] |
|
Tgfb1−/− Igh6−/− (B-cell-deficient Tgfb1−/− mice) |
4–6 wks | Severe multi-organ inflammation |
T cells, macrophages and neutrophils are sufficient for inflammation in Tgfb1−/− mice |
[36] |
|
Tgfb1−/− nu/nu (Athymic nude Tgfb1−/− mice; T-cell deficient) |
2–3 months |
Mild inflammation in gut and lungs |
T cells are essential for inflammation in Tgfb1−/− mice |
[36] |
|
Tgfb1−/− Mhc2−/− (MHC class II-deficient Tgfb1−/− mice) |
3–4 wks | No CD4+ T cells, mild inflammation, no autoantibodies |
MHC class II antigen presentation exacerbates inflammation in Tgfb1−/− mice |
[88] (Doetschman, unpublished) |
|
Tgfb1−/− B2m−/− (MHC class I-deficient Tgfb1−/− mice) |
2–3 months |
No CD8+ T cells, mild inflammation, reduced autoantibodies |
MHC class I antigen presentation causes autoimmunity |
[89] |
| Tgfb1−/− Il6−/− | 1–3 months |
Mild inflammation | IL-6 mediates inflammation in Tgfb1−/− mice | (Doetschman, unpublished) |
| Tgfb1−/− Ifnγ−/− | 3–8 wks | Inflammation is eliminated only in the liver |
IFN-γ is required for hepatic inflammation in Tgfb1 −/− mice |
[35] |
| Tgfb1−/− Il4−/− | 3 wks | Inflammation severity is similar to that in Tgfb1−/− mice |
IL-4 is not essential for inflammation in Tgfb1−/− mice |
[35] |
|
Tgfb1−/− DO11.10 (TCR transgenic Tgfβ1−/− mice) |
2–3 months |
Mild inflammation | Self-antigen recognition by T cells causes inflammation and autoimmunity in Tgfb1−/− mice |
[33] |
| Tgfb1−/− Cd28−/− | 2–3 wks | Inflammation in DKO mice is more severe than in Tgfb1−/− mice |
T-cell co-stimulation is not essential for autoimmune disease in Tgfb1−/− mice |
[38] |
Rag* refers to Rag1 or Rag2 genes.
In contrast to the situation with Ctla4−/− mice, treatment of day-2 Tgfb1−/− mice with cytotoxic T-lymphocyte-associated protein 4-immunoglobulin fusion protein (CTLA-4-Ig), which binds to B7-1 with high affinity and blocks co-stimulation through both CD28 and CTLA-4 [37], does not reverse the severe autoimmune disease [38] (T. Doetschman et al., unpublished). These data suggest that TGFβ1-deficient T cells do not require co-stimulatory signals through CD28 for their activation and autoimmune response [2]. Recently, we demonstrated, in Tgfb1−/− mice, that elimination of self-antigen-reactive TCR-bearing T cells by genetic combination strongly inhibits activation of T cells and eliminates inflammation [33].We and others have also shown, using TCR-transgenic mice, that Treg-cell generation and maintenance in the periphery is not TGFβ1 dependent [39] (R. Bommireddy et al., unpublished). This suggests that TGFβ1 is important for the prevention of self-reactive T-cell activation, Treg-cell suppressor function or both. This is in contrast to the observations made in TGFβR2-deficient mice, which show a progressive loss of Treg cells in the periphery as they develop inflammation. The loss of Treg cells in these mice is owing to a cell-intrinsic defect in their survival mechanism [14,15], suggesting that TGFβ signaling through TGFβR2 is essential for Treg-cell survival in the periphery and that other TGFβ ligand(s) might provide survival signals for the maintenance of Treg cells in the periphery of Tgfb1−/− mice. Together, these studies indicate that TGFβ1 regulates the activation threshold levels of T cells in response to self-antigen recognition.
TGFβ function in Treg cells
Initial studies by Nakamura et al. and Piccirillo et al. have generated controversy regarding the role of TGFβ1 in Treg-cell function [40,41]. Although neutralizing TGFβ antibody blocked Treg-cell function in the first study, soluble TGFβR2 failed to block Treg-cell suppressor function in the other. The mechanism by which Treg cells suppress the T-cell response is now becoming clear with recent observations made in Tgfb1 and Tgfbr2 conditional-knockout (CKO) mice. Recently, Li et al. reported that T-cell-specific deletion of TGFβ1 causes T-cell activation and defective Treg-cell function, suggesting that TGFβ1 is required for suppressor function [42]. Consistently, T-cell-specific ablation of Tgfbr2 also causes T-cell activation and autoimmunity. These TGFβR2-deficient T cells are not suppressed by wild-type Treg-cells, which suggests that TGFβ signaling in effector T cells is essential for regulation [1,14,15].
TGFβ1-deficient Treg cells exhibit suppressor function both in vitro and in adoptive-transfer models of colitis and transgenic dn-Tgfbr2 T cells are also inhibited by Treg cells [43]. However, dn-Tgfbr2 mice live longer because trans-gene expression is leaky [41]. In mice with Lck/Cre-mediated CKO of Tgfbr2 in T cells, those cells with a TGFβR2 deficiency are resistant to Treg-cell suppression and have a similar T-cell phenotype as do Tgfb1−/− mice [14,15]. Also, neutralizing antibody to TGFβ1 abolishes suppressor function of TGFβ1-deficient Treg cells in vivo in an adoptive-transfer model of colitis, suggesting that paracrine TGFβ1 produced within recipient mice or other cell types in the case of in vitro studies might mediate the suppressor function of TGFβ1-deficient Treg cells [39,44]. More recently, Li et al. have shown, using Tgfb1 CKO mice (T-cell-specific deletion of TGFβ1), that FOXP3+ Treg cells are increased in Tgfb1 CKO mice but are defective in their suppressor function. They have also found that TGFβ1- deficient Treg cells fail to inhibit IFN production and T-cell activation in vivo and in vitro and also fail to protect mice from developing colitis in the adoptive-transfer model of colitis [42]. TGFβ1-independent Treg cells are unlikely to induce dominant tolerance because Tgfb1−/− mice die at approximately weaning age from multi-organ autoimmunity [2,33].
A decrease in functional Treg cells results in autoimmune diseases, such as diabetes and inflammatory bowel disease [45]. Over-expression of TGFβ1 in mouse T cells also increases the proportion of Th3 cells. These Th3 cells are protective in adoptive-transfer models of EAE and rescue IL-2-deficient mice from autoimmune disease [46]. This suggests that theTGFβ1 produced byTh3 cells might induce the conversion of Th cells to Treg cells in a cell–cell contact-dependent manner (infectious tolerance) [47]. TGFβ1 induces Foxp3 in T cells, which, in turn, inhibits Smad7 expression (inhibitory SMAD) in Treg cells in a positive autoregulatory manner. This is in contrast to the induction of Smad7 in naïve T cells in response to TGFβ signaling. Inhibition of Smad7 by Foxp3 also results in enhanced expression of SMAD3/4-response genes [24].
Our recent studies suggest that TGFβ1 does not regulate Foxp3 expression directly because the generation and maintenance of CD4+CD25+ Treg cells (FOXP3+) are actually increased in transgenic TCR-expressing Tgfb1−/− mice. CTLA-4 is required for Foxp3 expression in activated T cells in the presence of TGFβ1, although it is not required for Foxp3 expression in nTreg cells that develop in the thymus [48]. However, expression of FOXP3 alone in Treg cells is not sufficient for their function because FOXP3+ Treg cells in Tgfb1−/− mice are unable to prevent the activation of CD4+CD25− T cells, which suggests that TGFβ1 is required for their suppressor function (R. Bommireddy et al., unpublished).
Mouse models in which TGFβ signaling is disrupted develop cell-autonomous autoimmune disease (Table 3), suggesting an important physiological role for TGFβ1-dependent Treg cells. In nonobese diabetic (NOD) mice, which develop spontaneous autoimmune type 1 diabetes, CD4+CD25hi Treg cells, but not CD4+CD25+ cells, exhibit regulatory function and the absence of Treg-cell function in CD4+CD25+ cells is owing to a lack of TGFβ1 production [2,49]. In these mice, the proportion of TGFβ1-producing Treg cells decreases with age and correlates with disease onset and progression [50]. Consistent with this observation, Green et al. have shown that TGFβ signaling-deficient T cells are not inhibited by Treg cells in vivo, suggesting that TGFβ-dependent Treg cells are major factors in tolerance induction [51]. Treg-cell numbers are reduced in the periphery of mice either lacking TGFβR2 or expressing a dn-Tgfbr2 in T cells [14,15,52]. These data suggest that autocrine TGFβ1 in Treg cells is important for their maintenance, expansion and suppressor function. This is also supported by the finding that Tgfb1 KO mice, Tgfbr2 CKO in T cells and dn-Tgfbr2 transgenic mice all exhibit similar autoimmune phenotypes, with the delay of onset of autoimmunity in the dn-Tgfbr2 mice being a result of transgene leakiness (Table 3). Together, these in vivo and in vitro studies suggest that TGFβ1-deficient Treg cells are not effective in a TGFβ-deficient environment [39,44] (Figure 2). Further studies are required to understand the molecular mechanisms that control the expression of TGFβ1 in Treg cells during infection and inflammatory diseases.
Table 3.
TGFβ signaling is essential for the induction of self-tolerance in vivo
| Knockout or inactivation mice |
Phenotype | Comments | Refs |
|---|---|---|---|
| Tgfb1 | Multifocal inflammatory autoimmune disease mediated by IFN-γ and IL-6 and T-cell dependent. Mice die within 3 weeks after birth. |
TGFβ1 is essential to prevent T-cell activation. TCR down- modulation is an indication of self-antigen recognition in vivo. Disease is self-antigen specific. |
[22,86] |
|
Dn- Tgfbr2 (in T cells) |
Mice develop autoimmune inflammatory disease approximately 5 months after birth but are resistant to the induction of EAE. |
TGFβ signaling in T cells is essential for tolerance induction. Transgenic-receptor expression might be leaky, hence the delay. |
[90] [26,91] |
|
Tgfbr2 (deleted in T cells) |
Mice develop multi-organ autoimmune disease similar to Tgfβ1 KO mice. T-cell activation is not inhibited by wild-type Tregcells. Increased apoptosis of Treg cells and T cells with age and inflammation. |
TGFβ signaling is essential for prevention of activation of T cells and survival. TGFβ signaling is not required for generation of Treg cells but is essential for maintenance of Treg cells in the periphery. T-cell-intrinsic defect of TGFβ signaling causes spontaneous activation but no disease. |
[14,15] |
| Ctla4 | Massive lymphoproliferative inflammatory autoimmune disease mediated by Th2 cells. Mice die approximately 3 weeks after birth. |
CTLA-4 is essential for Treg-cell development and function. Over-expression of Foxp3 rescues these mice from lethal autoimmune disease. |
[58,92] |
| Foxp3 | Massive lymphoproliferative inflammatory autoimmune disease mediated by both Th1 and Th2 cells. Mice die approximately 3 weeks after birth. |
Foxp3 is essential for Treg-cell development and function. Retroviral transfection of CD4+ T cells programs them to become Treg cells. Up-regulates IL-10. |
See [58]and references in [1] |
| Smad3 | Multifocal formation of pyogenic abscesses within the wall of the stomach and the intestine. Involuted thymus, smaller spleen and enlarged lymph nodes. |
No apparent autoimmune diseases but impaired mucosal immunity. Increased number of activated T cells. Inflammation and cancer in the colon is due to activation of T cells against gut bacteria. |
[93] |
|
Smad4 (in T cells) |
Increased Th2 cytokines, gastrointestinal cancer. | Mice live longer (9–12 months). | [94] |
| Cblb | CD28-independent proliferation of T cells, mice are more susceptible for EAE, effector T cells are resistant to TGFβ1-inhibitory effect. |
Mice are normal and live longer. | [95] |
|
Ptpn6 (SHP-1) (motheaten mice) |
Thymocytes are hyper-responsive to TCR stimulation, increased Treg-cell generation, T cells are resistant to TGFβ1-inhibitory effect. |
Mice die early (2–3 wks) after birth. Effector T-cell resistance to Treg-cell suppression is the cause of T-cell activation. |
[29,96] |
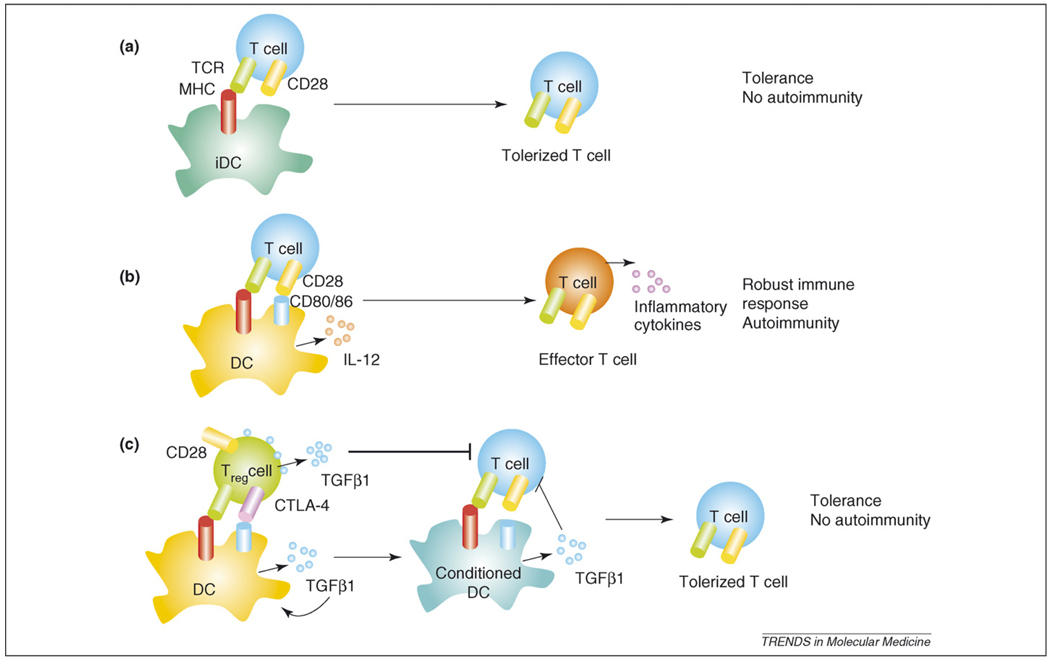
Figure 2.
Tolerance induction by Treg cells. Antigen presentation by iDCs to T cells induces tolerance because iDCs do not provide co-stimulation (a). However, Ag presentation by mature DCs activates T cells and the inflammatory environment can trigger the activation of autoreactive T cells (b). (c) Treg cells that express CTLA-4 and TGFβ1 in addition to CD28 interact with DCs and induce TGFβ1 production (conditioned DCs). Co-stimulatory molecules (CD80 and CD86) are down-modulated by TGFβ1 on conditioned DCs. Conditioned DCs induce tolerance in T cells by presenting Ag without co-stimulation and by TGFβ1 secretion.
Treg cells in tolerance and autoimmune diseases
Treg cells inhibit Th-cell proliferation in response to the TCR (αCD3) and CD4+CD25+-depleted CD4+ T cells proliferate more vigorously than undepleted CD4+ T cells in vitro [1]. Treg cells inhibit IL-2 and IFN-γ production by Th cells (Figure 2) and they also inhibit colitis, diabetes and graft-versus-host disease [11,53,54]. Treg-cell functions have been observed both in CD4+CD25+ and CD4+CD25− T cells. These data indicate that Treg cells, whether they express CD25 or not, act as suppressor cells that exhibit immunoregulatory function. Treg-cell development and function has been studied extensively in NOD mice ([2] and references therein).
Immune regulation is achieved by tightly controlled interactions among cytokines, surface receptors and transcription factors [55]. Deficiency in all or several of these, such as TGFβ1, CTLA-4 or FOXP3, leads to severe auto-immune disease and over-expression of either TGFβ1 or FOXP3 protects mice from autoimmune diseases [1,46,56]. Many autoimmune diseases have been attributed to the loss of Treg-cell function, which involves the production of TGFβ1 and IL-10 [1]. In IL-10-deficient mice, which develop colitis, TGFβ signaling is also deficient, suggesting that IL-10 regulates TGFβ signaling positively in intestinal epithelial cells [57].
FOXP3 in Treg-cell generation
The Foxp3 gene (Scurfin; forkhead/winged helix transcription factor) is essential for the development and maintenance of Treg cells in the periphery. A defect in the human FOXP3 gene leads to IPEX (immune deficiency, polyendocrinopathy, enteropathy, X-linked) syndrome, a systemic autoimmune disease, suggesting that FOXP3 is important for T-cell tolerance [58,59]. Recent studies from Rudensky’s lab suggest that targeted depletion of FOXP3-expressing Treg cells in mice results in severe autoimmune disease [60]. We propound the excellent reviews by Rudensky and Ziegler on the role of FOXP3 in immune tolerance [5,61]. FOXP3 complexed with NF-AT binds strongly to the Il2 promoter, thereby repressing its expression; the same complex induces Il2ra (CD25) and Ctla4 expression [62]. TGFβ1 inhibits NF-AT activation in T cells and also induces Foxp3 in vitro [24,63].
Recently, Ono et al. have shown that FOXP3 interacts physically with and inhibits the function of transcription factor acute myeloid leukemia-1 (AML-1)/Runt-related transcription factor 1 (Runx1), which is important for T-cell development and IL-2 and IFN-γ expression in CD4+ T cells [64]. These data suggest that FOXP3 induces Treg-cell generation through inhibition of IL-2 by interacting with other transcription factors that are involved in IL-2 induction. FOXP3 also induces Toll-like receptor (TLR)10 in Treg cells by binding to the promoter elements of TLR10 directly and by activating its expression [65]. TLR10 is expressed only in Treg cells but not in non-Treg cells and its expression is enhanced by TCR stimulation in a calcineurin-dependent manner. NF-AT cooperates with FOXP3 in inducing TLR10. Recently, it was shown that mere expression of FOXP3 by TGFβ1 in human CD4+ T cells does not confer suppressive properties to these Treg cells, suggesting that additional factors are required for FOXP3+ Treg-cell suppressor function [66]. All these data suggest that FOXP3 is essential for the development of Treg cells but is not sufficient to confer suppressor function. Although FOXP3 inhibits IL-2 within Treg cells, it must depend on some other factors, such as TGFβ1 or CTLA-4, in order to suppress effector T-cell responses.
CTLA-4 in Treg-cell generation and function
CTLA-4, a negative-regulatory molecule, is expressed constitutively on nTreg cells and is required for their suppressor function [1]. Ctla4−/− mice develop a lethal lymphoproliferative disorder, suggesting that CTLA-4 has an important role in preventing self-reactive T-cell activation. Over-expression of Foxp3 delays the development of auto-immune disease in Ctla4−/− mice, suggesting that FOXP3 compensates for the loss of CTLA-4 [58]. We propound a Sansom and Walker review on the role of CTLA-4 in T-cell regulation [67].
Ctla4−/− Treg cells cause suppression of T-cell proliferation in a TGFβ1-dependent manner in vitro [3]. Because cross-linking of CTLA-4 on activated T cells induces TGFβ1 production and because TGFβ1 accelerates CTLA-4 expression in vitro, it is plausible that CTLA-4-deficient T cells might not exhibit suppressor function owing to a deficiency in TGFβ1 production in vivo [68]. Activation of CTLA-4 by cross-linking enhances Treg-cell production of TGFβ1 and its surface accumulation at the site of cell–cell interactions and a loss of membrane-bound TGFβ1 is associated with the onset of diabetes in NOD mice [8,45,68]. Oida et al. also showed that blocking TGFβ signaling with a receptor-kinase inhibitor abrogated the suppressive function of Treg cells, suggesting that TGFβ is the primary mediator of Treg-cell suppressor function [8]. These observations suggest that TGFβ1 production is both regulated tightly and important for CD4+CD25+CTLA-4+ Treg-cell function in vivo (Figure 2).
The role of signal strength in T-cell activation and tolerance
For productive immune responses, T cells require at least two signals: a primary signal provided by the TCR or CD3 and a secondary co-stimulatory signal provided by CD28 interacting with CD80 and CD86 (B7-1 and B7-2) on APCs. Stimulation of T cells with ionomycin alone leads to anergy in the absence of protein kinase C (PKC) activation. Stimulation of T cells with phorbol 12-myristate 13-acetate (PMA) leads to the activation of nuclear factor-κB (NF-κB) and activator protein-1 (AP-1, a complex of two proteins, FOS and JUN), which prevents anergy induction by ionomycin [69]. FOXP3+ Treg cells are anergic to stimulation by αCD3 and αCD28. TGFβ1 induces the conversion of naïve T cells to Treg cells that express Foxp3 in the presence of αCD3 and αCD28 [24]. These data suggest that activation-signal strength instructs T cells to either become effector T cells or anergic (a state of unresponsiveness); TGFβ1 modulates TCR signal strength through its positive effects on casitas B-lineage lymphoma B (CBL-B) and CTLA-4 production and its negative effects on calcineurin [21,63,70]. Although CBL-B-deficient T cells express FOXP3, TGFβ1-induced FOXP3 expression is dependent on CBL-B to a large extent [70]. CBL-B-deficient effector T cells are also resistant to suppression by Treg cells and TGFβ, suggesting that TGFβ exerts its suppressor functions through CBL-B [71]. Blockade of co-stimulatory signals through CD4 or CD40L induces peripheral tolerance [72]. CD4+CD25+ Treg cells can be generated from CD4+CD25− T cells in vivo after treatment with non-mito-genic αCD3 F(ab’)2 fragments, suggesting that tolerance and the immune response to antigens are regulated by signal strength [47,49]. Similarly, another study by Ochi et al. using oral therapy of αCD3, which induces LAP+ [latency-associated peptide (positive)] CD4+ T cells, confirms that TGFβ1-dependent Treg-cell generation is important for preventing autoimmune disease [73].
DCs in antigen presentation and T-cell tolerance
DCs have important roles in antigen presentation and effective T-cell responses. If naïve T cells encounter antigen on immature DCs (iDCs), they are tolerized and become Treg cells rather than effector T cells. Co-stimulatory ligands (CD80 and CD86) are up-regulated on DC maturation. These mature DCs are more potent in activating naïve T cells to become effector T cells (Figure 2). Co-stimulatory molecules are also important for the generation of nTreg cells [74]. Elimination of these molecules by gene ablation results in decreased Treg cells. Bluestone et al. demonstrated that Treg cells interact directly with APCs, such as DCs, in vivo and prevent further arrest of effector T cells on DCs [53]. CTLA-4 down-modulates B7 molecules on DCs in vitro and these conditioned DCs (DCs that have encountered Treg cells) induce poor T-cell proliferation [75]. Conditioned DCs might produce the immunosuppressive cytokine TGFβ1, whereas those DCs that encounter naïve T cells might produce the pro-inflammatory cytokine IL-12 and induce a Th1 response. Consistently, CTLA-4 blockade results in decreased production of TGFβ1 and indoleamine 2,3-dioxigenase (IDO) and an increased T-cell response to viral infection [76]. Naïve T-cell interactions with conditioned DCs in a TGFβ1-rich environment could be tolerized rather than activated. Treg-cell-produced TGFβ1 can act on both DCs and naïve T cells and can down-regulate their responses. TGFβ1 prevents DC maturation by inhibiting co-stimulatory-molecule expression [77].
DCs express IDO on interaction with CTLA-4, which is expressed constitutively on Treg cells [78]. IDO catabolizes tryptophan and leads to immunosuppression by T-cell anergy [79]. Treg cells induce reverse signaling in DCs on their engagement with DCs through CTLA-4 and its ligand CD80 on DCs. Because Treg cells induce TGFβ1 in APCs, it is possible that there is signaling cross-talk in DCs that leads to TGFβ1 production. CTLA-4–Ig treatment causes T-cell anergy, which is dependent on IDO expression, because IDO-deficient DCs are not capable of inducing T-cell tolerance [80]. However, it is unclear whether TGFβ1 production is also impaired in IDO-deficient DCs. CTLA-4–Ig-treated Tgfb1−/− mice do not live longer than untreated Tgfb1−/− mice and they show no reduction in the severity of inflammation as compared with that in control Tgfb1−/− mice, therefore, we speculate that CTLA-4–Ig treatment induces tolerance through TGFβ1 production [38,81] (T. Doetschman, unpublished). More recently, Coombes et al. has shown that integrin CD103-expressing DCs are involved in peripheral Treg-cell generation, especially in the intestine, and are responsible for oral tolerance. These DCs capture food antigens and present them to T cells to induce oral tolerance. Blocking retinoic acid (RA) receptors prevented the FOXP3 induction by CD103+ DCs, suggesting that this process is dependent on the Vitamin A metabolite RA. CD103+ DCs express higher levels of Tgfb2, latent TGFβ-binding protein 3 (Ltbp3) and tissue-plasminogen activator compared with CD103− DCs [82]. Consistently, Mucida et al. have also found that RA inhibits TGFβ1-induced RORγt and Th17-cell generation in the presence of IL-6 from splenocytes in vitro, whereas it increased TGFβ1-induced FOXP3+ Treg-cell generation [83]. These data suggest an important role for TGFβ in the induction of oral tolerance by DCs in the gut.
Concluding remarks
The recent explosion in publications on Treg cells and the role of TGFβ1 in tolerance induction has led to the conclusion that TGFβ1 is central to self-tolerance. The primary role for TGFβ1 is to prevent T-cell activation by self-Ag presentation. A strong co-stimulatory environment over-rides the suppressor function of TGFβ1 and enables T cells to respond to invading pathogens. Treg cells have an important role in maintaining lymphocyte homeostasis and perturbations in Treg-cell generation or function lead to autoreactive T-cell expansion. The majority of Treg cells depend on TGFβ1 for their suppressor function, although they do not need to produce it. However, TGFβ signaling is essential for T cells to respond to Treg-cell function. CTLA-4 and FOXP3 are also important for Treg-cell generation, maintenance and function. Although FOXP3 is important for the generation of Treg cells, CTLA-4 is important for the maintenance of the Treg-cell pool in the periphery because it is involved in the induction of Foxp3 and the production of TGFβ1 [67]. It is noteworthy that, in humans, unlike mouse, TGFβ inhibits Th17 differentiation [84]. Current studies focusing on the role of TGFβ1 in T-cell subsets using CKO mice will help to clarify the complex roles for TGFβ1 in these processes (Box 1). Present approaches to restoring the Treg-cell pool using antibody therapy or gene-transfer therapy will lead to better treatment options for autoimmune diseases. However, either blocking Treg function or depleting Treg cells will boost the antitumor immune responses.
Outstanding questions
Unlike TGFβ1, FOXP3 is an intracellular transcription factor expressed only in Treg cells. How does it mediate the suppressor function of Treg cells?
Because FOXP3-deficient mice develop a similar autoimmune phenotype as Tgfb1−/− mice, does FOXP3 induce TGFβ1 expression in Treg cells?
Because TGFβ1 inhibits autoimmunity but does not inhibit the immune response to invading pathogens, is TGFβ1 expression regulated by the strength of the TCR signals?
Acknowledgements
We apologize to those investigators whose works have not been cited in this review owing to space restrictions. We thank Greg Boivin, University of Cincinnati, for his contributions to the IL-6 unpublished studies mentioned in the review and we thank George Babcock, University of Cincinnati, for consultation and use of his FACS equipment. We acknowledge the support of NIH grants AI067903 and CA084291 to TD.
References
- 1.Miyara M, Sakaguchi S. Natural regulatory T cells: mechanisms of suppression. Trends Mol. Med. 2007;13:108–116. doi: 10.1016/j.molmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Bommireddy R, Doetschman T. TGFβ, T-cell tolerance and anti-CD3 therapy. Trends Mol. Med. 2004;10:3–9. doi: 10.1016/j.molmed.2003.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang Q, et al. Distinct roles of CTLA-4 and TGFβ in CD4+CD25+ regulatory T cell function. Eur. J. Immunol. 2004;34:2996–3005. doi: 10.1002/eji.200425143. [DOI] [PubMed] [Google Scholar]
- 4.Shevach EM. Regulatory/suppressor T cells in health and disease. Arthritis Rheum. 2004;50:2721–2724. doi: 10.1002/art.20500. [DOI] [PubMed] [Google Scholar]
- 5.Rudensky A. Foxp3 and dominant tolerance. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005;360:1645–1646. doi: 10.1098/rstb.2005.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Annacker O, et al. Essential role for CD103 in the T cell-mediated regulation of experimental colitis. J. Exp. Med. 2005;202:1051–1061. doi: 10.1084/jem.20040662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beissert S, et al. Regulatory T cells. J. Invest. Dermatol. 2006;126:15–24. doi: 10.1038/sj.jid.5700004. [DOI] [PubMed] [Google Scholar]
- 8.Oida T, et al. TGFβ-mediated suppression by CD4+CD25+ T cells is facilitated by CTLA-4 signaling. J. Immunol. 2006;177:2331–2339. doi: 10.4049/jimmunol.177.4.2331. [DOI] [PubMed] [Google Scholar]
- 9.Izcue A, et al. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol. Rev. 2006;212:256–271. doi: 10.1111/j.0105-2896.2006.00423.x. [DOI] [PubMed] [Google Scholar]
- 10.Graca L, et al. Dominant transplantation tolerance. Curr. Opin. Immunol. 2003;15:499–506. doi: 10.1016/s0952-7915(03)00098-0. [DOI] [PubMed] [Google Scholar]
- 11.Uhlig HH, et al. Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J. Immunol. 2006;177:5852–5860. doi: 10.4049/jimmunol.177.9.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li MO, et al. Transforming growth factor-β regulation of immune responses. Annu. Rev. Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt-Weber CB, Blaser K. Regulation and role of transforming growth factor-β in immune tolerance induction and inflammation. Curr. Opin. Immunol. 2004;16:709–716. doi: 10.1016/j.coi.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Marie JC, et al. Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-β receptor. Immunity. 2006;25:441–454. doi: 10.1016/j.immuni.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 15.Li MO, et al. Transforming growth factor-β controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity. 2006;25:455–471. doi: 10.1016/j.immuni.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Rubtsov YP, Rudensky AY. TGFβ signalling in control of T-cell-mediated self-reactivity. Nat. Rev. Immunol. 2007;7:443–453. doi: 10.1038/nri2095. [DOI] [PubMed] [Google Scholar]
- 17.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 18.Korn T, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weaver CT, et al. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Chen W, Wahl SM. TGFβ: receptors, signaling pathways and autoimmunity. Curr. Dir. Autoimmun. 2002;5:62–91. doi: 10.1159/000060548. [DOI] [PubMed] [Google Scholar]
- 21.Bommireddy R, et al. TGFβ1 inhibits Ca2+-calcineurin-mediated activation in thymocytes. J. Immunol. 2003;170:3645–3652. doi: 10.4049/jimmunol.170.7.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bommireddy R, et al. TGFβ1 regulates lymphocyte homeostasis by preventing activation and subsequent apoptosis of peripheral lymphocytes. J. Immunol. 2003;170:4612–4622. doi: 10.4049/jimmunol.170.9.4612. [DOI] [PubMed] [Google Scholar]
- 23.Shou W, et al. Cardiac defects and altered ryanodine receptor function in mice lacking FKBP12. Nature. 1998;391:489–492. doi: 10.1038/35146. [DOI] [PubMed] [Google Scholar]
- 24.Fantini MC, et al. Cutting edge: TGFβ induces a regulatory phenotype in CD4+CD25− T cells through Foxp3 induction and down-regulation of Smad7. J. Immunol. 2004;172:5149–5153. doi: 10.4049/jimmunol.172.9.5149. [DOI] [PubMed] [Google Scholar]
- 25.Zheng SG, et al. TGFβ requires CTLA-4 early after T cell activation to induce FoxP3 and generate adaptive CD4+CD25+ regulatory cells. J. Immunol. 2006;176:3321–3329. doi: 10.4049/jimmunol.176.6.3321. [DOI] [PubMed] [Google Scholar]
- 26.Veldhoen M, et al. Signals mediated by transforming growth factor-β initiate autoimmune encephalomyelitis, but chronic inflammation is needed to sustain disease. Nat. Immunol. 2006;7:1151–1156. doi: 10.1038/ni1391. [DOI] [PubMed] [Google Scholar]
- 27.Ivanov II, et al. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17(+) T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 28.Lin JT, et al. TGFbeta 1 uses distinct mechanisms to inhibit IFN-γ expression in CD4+ T cells at priming and at recall: differential involvement of Stat4 and T-bet. J. Immunol. 2005;174:5950–5958. doi: 10.4049/jimmunol.174.10.5950. [DOI] [PubMed] [Google Scholar]
- 29.Park IK, et al. TGFβ1 inhibits T-bet induction by IFN-γ in murine CD4+ T cells through the protein tyrosine phosphatase Src homology region 2 domain-containing phosphatase-1. J. Immunol. 2005;175:5666–5674. doi: 10.4049/jimmunol.175.9.5666. [DOI] [PubMed] [Google Scholar]
- 30.Gorelik L, et al. Cutting edge: TGFβ inhibits Th type 2 development through inhibition of GATA-3 expression. J. Immunol. 2000;165:4773–4777. doi: 10.4049/jimmunol.165.9.4773. [DOI] [PubMed] [Google Scholar]
- 31.Lund R, et al. Identification of novel genes regulated by IL-12, IL-4, or TGFβ during the early polarization of CD4+ lymphocytes. J. Immunol. 2003;171:5328–5336. doi: 10.4049/jimmunol.171.10.5328. [DOI] [PubMed] [Google Scholar]
- 32.Zhou M, Ouyang W. The function role of GATA-3 in Th1 and Th2 differentiation. Immunol. Res. 2003;28:25–37. doi: 10.1385/IR:28:1:25. [DOI] [PubMed] [Google Scholar]
- 33.Bommireddy R, et al. Self-antigen recognition by TGFβ1-deficient T cells causes their activation and systemic inflammation. Lab. Invest. 2006;86:1008–1019. doi: 10.1038/labinvest.3700460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mangan PR, et al. Transforming growth factor-β induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 35.Gorham JD, et al. Genetic regulation of autoimmune disease: BALB/c background TGFβ1-deficient mice develop necroinflammatory IFN-γ-dependent hepatitis. J. Immunol. 2001;166:6413–6422. doi: 10.4049/jimmunol.166.10.6413. [DOI] [PubMed] [Google Scholar]
- 36.Bommireddy R, et al. Elimination of both CD4(+) and CD8(+) T cells but not B cells eliminates inflammation and prolongs the survival of TGFβ1-deficient mice. Cell. Immunol. 2004;232:96–104. doi: 10.1016/j.cellimm.2005.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manzotti CN, et al. Integration of CD28 and CTLA-4 function results in differential responses of T cells to CD80 and CD86. Eur. J. Immunol. 2006;36:1413–1422. doi: 10.1002/eji.200535170. [DOI] [PubMed] [Google Scholar]
- 38.Mamura M, et al. CD28 disruption exacerbates inflammation in Tgf-β1−/− mice: in vivo suppression by CD4+CD25+ regulatory T cells independent of autocrine TGFβ1. Blood. 2004;103:4594–4601. doi: 10.1182/blood-2003-08-2897. [DOI] [PubMed] [Google Scholar]
- 39.Fahlen L, et al. T cells that cannot respond to TGFβ escape control by CD4(+)CD25(+) regulatory T cells. J. Exp. Med. 2005;201:737–746. doi: 10.1084/jem.20040685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakamura K, et al. Cell contact-dependent immunosuppression by CD4 (+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor β. J. Exp. Med. 2001;194:629–644. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Piccirillo CA, et al. CD4(+)CD25(+) regulatory T cells can mediate suppressor function in the absence of transforming growth factor β1 production and responsiveness. J. Exp. Med. 2002;196:237–246. doi: 10.1084/jem.20020590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li MO, et al. T cell-produced transforming growth factor-β1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity. 2007;26:579–591. doi: 10.1016/j.immuni.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 43.Kullberg MC, et al. TGFβ1 production by CD4(+)CD25(+) regulatory T cells is not essential for suppression of intestinal inflammation. Eur. J. Immunol. 2005;35:2886–2895. doi: 10.1002/eji.200526106. [DOI] [PubMed] [Google Scholar]
- 44.Marie JC, et al. TGF{beta}1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J. Exp. Med. 2005;201:1061–1067. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gregg RK, et al. A sudden decline in active membrane-bound TGF{beta} impairs both T regulatory cell function and protection against autoimmune diabetes. J. Immunol. 2004;173:7308–7316. doi: 10.4049/jimmunol.173.12.7308. [DOI] [PubMed] [Google Scholar]
- 46.Carrier Y, et al. Th3 cells in peripheral tolerance. I. Induction of Foxp3-positive regulatory T cells by Th3 cells derived from TGFβ T cell-transgenic mice. J. Immunol. 2007;178:179–185. doi: 10.4049/jimmunol.178.1.179. [DOI] [PubMed] [Google Scholar]
- 47.Graca L, et al. Dominant tolerance: activation thresholds for peripheral generation of regulatory T cells. Trends Immunol. 2005;26:130–135. doi: 10.1016/j.it.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 48.Zheng SG, et al. Natural and induced CD4+CD25+ cells educate CD4+CD25− cells to develop suppressive activity: the role of IL-2, TGFβ, and IL-10. J. Immunol. 2004;172:5213–5221. doi: 10.4049/jimmunol.172.9.5213. [DOI] [PubMed] [Google Scholar]
- 49.Chatenoud L. CD3-specific antibodies restore self-tolerance: mechanisms and clinical applications. Curr. Opin. Immunol. 2005;17:632–637. doi: 10.1016/j.coi.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 50.Pop SM, et al. Single cell analysis shows decreasing FoxP3 and TGF{beta}1 coexpressing CD4+CD25+ regulatory T cells during autoimmune diabetes. J. Exp. Med. 2005;201:1333–1346. doi: 10.1084/jem.20042398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Green EA, et al. CD4+CD25+ T regulatory cells control anti-islet CD8+ T cells through TGF{beta}–TGF{beta} receptor interactions in Type 1 diabetes. Proc. Natl. Acad. Sci. U. S. A. 2003;100:10878–10883. doi: 10.1073/pnas.1834400100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schramm C, et al. TGFβ regulates the CD4+CD25+ T-cell pool and the expression of Foxp3 in vivo. Int. Immunol. 2004;16:1241–1249. doi: 10.1093/intimm/dxh126. [DOI] [PubMed] [Google Scholar]
- 53.Tang Q, et al. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat. Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Edinger M, et al. CD4(+)CD25(+) regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat. Med. 2003;9:1144–1150. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- 55.Kane LP, et al. Signal transduction by the TCR for antigen. Curr. Opin. Immunol. 2000;12:242–249. doi: 10.1016/s0952-7915(00)00083-2. [DOI] [PubMed] [Google Scholar]
- 56.Grewal IS, et al. Expression of transgene encoded TGFβ in islets prevents autoimmune diabetes in NOD mice by a local mechanism. J. Autoimmun. 2002;19:9–22. doi: 10.1006/jaut.2002.0599. [DOI] [PubMed] [Google Scholar]
- 57.Ruiz PA, et al. IL-10 gene-deficient mice lack TGFβ/Smad signaling and fail to inhibit proinflammatory gene expression in intestinal epithelial cells after the colonization with colitogenic Enterococcus faecalis. J. Immunol. 2005;174:2990–2999. doi: 10.4049/jimmunol.174.5.2990. [DOI] [PubMed] [Google Scholar]
- 58.Khattri R, et al. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 2003;4:337–342. [PubMed] [Google Scholar]
- 59.Gambinerix E, et al. Immune dysregulation, polyendocrinopathy, enteropathy, and X-linked inheritance (IPEX), a syndrome of systemic autoimmunity caused by mutations of FOXP3, a critical regulator of T-cell homeostasis. Curr. Opin. Rheumatol. 2003;15:430–435. doi: 10.1097/00002281-200307000-00010. [DOI] [PubMed] [Google Scholar]
- 60.Williams LM, Rudensky AY. Maintenance of the Foxp3-dependent developmental program in mature regulatory T cells requires continued expression of Foxp3. Nat. Immunol. 2007;8:277–284. doi: 10.1038/ni1437. [DOI] [PubMed] [Google Scholar]
- 61.Ziegler SF. FOXP3: of mice and men. Annu. Rev. Immunol. 2006;24:209–226. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]
- 62.Wu Y, et al. FOXP3 Controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126:375–387. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 63.Chen CH, et al. Transforming growth factor β blocks Tec kinase phosphorylation, Ca2+ influx, and NFATc translocation causing inhibition of T cell differentiation. J. Exp. Med. 2003;197:1689–1699. doi: 10.1084/jem.20021170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ono M, et al. Foxp3 controls regulatory T-cell function by interacting with AML1/Runx1. Nature. 2007;446:685–689. doi: 10.1038/nature05673. [DOI] [PubMed] [Google Scholar]
- 65.Bell MP, et al. Forkhead Box P3 regulates TLR10 expression in human T regulatory cells. J. Immunol. 2007;179:1893–1900. doi: 10.4049/jimmunol.179.3.1893. [DOI] [PubMed] [Google Scholar]
- 66.Tran DQ, et al. Induction of FOXP3 expression in naive human CD4+FOXP3− T cells by T cell receptor stimulation is TGF{beta}-dependent but does not confer a regulatory phenotype. Blood. 2007;110:2983–2990. doi: 10.1182/blood-2007-06-094656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sansom DM, Walker LS. The role of CD28 and cytotoxic T-lymphocyte antigen-4 (CTLA-4) in regulatory T-cell biology. Immunol. Rev. 2006;212:131–148. doi: 10.1111/j.0105-2896.2006.00419.x. [DOI] [PubMed] [Google Scholar]
- 68.Chen W, et al. Engagement of cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) induces transforming growth factor beta (TGFβ) production by murine CD4(+) T cells. J. Exp. Med. 1998;188:1849–1857. doi: 10.1084/jem.188.10.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Macian F, et al. Transcriptional mechanisms underlying lymphocyte tolerance. Cell. 2002;109:719–731. doi: 10.1016/s0092-8674(02)00767-5. [DOI] [PubMed] [Google Scholar]
- 70.Wohlfert EA, et al. Cutting edge: Deficiency in the E3 ubiquitin ligase Cbl-b results in a multifunctional defect in T cell TGFβ sensitivity in vitro and in vivo. J. Immunol. 2006;176:1316–1320. doi: 10.4049/jimmunol.176.3.1316. [DOI] [PubMed] [Google Scholar]
- 71.Wohlfert EA, et al. Resistance to CD4+CD25+ regulatory T cells and TGFβ in Cbl-b−/− mice. J. Immunol. 2004;173:1059–1065. doi: 10.4049/jimmunol.173.2.1059. [DOI] [PubMed] [Google Scholar]
- 72.Graca L, et al. Cutting edge: Anti-CD154 therapeutic antibodies induce infectious transplantation tolerance. J. Immunol. 2000;165:4783–4786. doi: 10.4049/jimmunol.165.9.4783. [DOI] [PubMed] [Google Scholar]
- 73.Ochi H, et al. Oral CD3-specific antibody suppresses autoimmune encephalomyelitis by inducing CD4(+)CD25(−)LAP(+) T cells. Nat. Med. 2006;12:627–635. doi: 10.1038/nm1408. [DOI] [PubMed] [Google Scholar]
- 74.Salomon B, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 75.Oderup C, et al. Cytotoxic T lymphocyte antigen-4-dependent down-modulation of costimulatory molecules on dendritic cells in CD4+ CD25+ regulatory T-cell-mediated suppression. Immunology. 2006;118:240–249. doi: 10.1111/j.1365-2567.2006.02362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hryniewicz A, et al. CTLA-4 blockade decreases TGFβ, IDO, and viral RNA expression in tissues of SIVmac251-infected macaques. Blood. 2006;108:3834–3842. doi: 10.1182/blood-2006-04-010637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lyakh LA, et al. TGFβ and vitamin D3 utilize distinct pathways to suppress IL-12 production and modulate rapid differentiation of human monocytes into CD83+ dendritic cells. J. Immunol. 2005;174:2061–2070. doi: 10.4049/jimmunol.174.4.2061. [DOI] [PubMed] [Google Scholar]
- 78.Grohmann U, et al. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat. Immunol. 2002;3:1097–1101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 79.Munn DH, et al. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22:633–642. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 80.Mellor AL, et al. Cutting edge: induced indoleamine 2,3 dioxygenase expression in dendritic cell subsets suppresses T cell clonal expansion. J. Immunol. 2003;171:1652–1655. doi: 10.4049/jimmunol.171.4.1652. [DOI] [PubMed] [Google Scholar]
- 81.Tivol EA, et al. CTLA4Ig prevents lymphoproliferation and fatal multiorgan tissue destruction in CTLA-4-deficient mice. J. Immunol. 1997;158:5091–5094. [PubMed] [Google Scholar]
- 82.Coombes JL, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF{beta}- and retinoic acid-dependent mechanism. J. Exp. Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mucida D, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 84.Acosta-Rodriguez EV, et al. Interleukins 1beta and 6 but not transforming growth factor-β are essential for the differentiation of interleukin 17-producing human T helper cells. Nat. Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 85.Shevach EM. From vanilla to 28 flavors: multiple varieties of T regulatory cells. Immunity. 2006;25:195–201. doi: 10.1016/j.immuni.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 86.Shull MM, et al. Targeted disruption of the mouse transforming growth factor-β1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Engle SJ, et al. Transforming growth factor β1 suppresses nonmetastatic colon cancer at an early stage of tumorigenesis. Cancer Res. 1999;59:3379–3386. [PubMed] [Google Scholar]
- 88.Letterio JJ, et al. Autoimmunity associated with TGFβ1-deficiency in mice is dependent on MHC class II antigen expression. J. Clin. Invest. 1996;98:2109–2119. doi: 10.1172/JCI119017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kobayashi S, et al. Beta 2-microglobulin-deficient background ameliorates lethal phenotype of the TGFβ1 null mouse. J. Immunol. 1999;163:4013–4019. [PubMed] [Google Scholar]
- 90.Gorelik L, Flavell RA. Abrogation of TGFβ signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12:171–181. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- 91.Lucas PJ, et al. Disruption of T cell homeostasis in mice expressing a T cell-specific dominant negative transforming growth factor β II receptor. J. Exp. Med. 2000;191:1187–1196. doi: 10.1084/jem.191.7.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tivol EA, et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 93.Yang X, et al. Targeted disruption of SMAD3 results in impaired mucosal immunity and diminished T cell responsiveness to TGFβ. EMBO J. 1999;18:1280–1291. doi: 10.1093/emboj/18.5.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim BG, et al. Smad4 signalling in T cells is required for suppression of gastrointestinal cancer. Nature. 2006;441:1015–1019. doi: 10.1038/nature04846. [DOI] [PubMed] [Google Scholar]
- 95.Chiang YJ, et al. Cbl-b regulates the CD28 dependence of T-cell activation. Nature. 2000;403:216–220. doi: 10.1038/35003235. [DOI] [PubMed] [Google Scholar]
- 96.Lorenz U, et al. Lack of SHPTP1 results in src-family kinase hyperactivation and thymocyte hyperresponsiveness. Proc. Natl. Acad. Sci. U. S. A. 1996;93:9624–9629. doi: 10.1073/pnas.93.18.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]