Abstract
Structural effect of polydisulfide Gd(III) chelates on their in vitro degradability, and cardiovascular and tumor imaging in mice were evaluated as biodegradable macromolecular MRI contrast agents. Polydisulfide Gd(III) chelates, Gd-DTPA cystamine copolymers (GDCC), Gd-DTPA L-cystine copolymers (GDCP), Gd-DTPA D-cystine copolymers (dGDCP) and Gd-DTPA glutathione (oxidized) copolymers (GDGP), with different sizes and narrow molecular weight distribution were prepared and evaluated both in vitro and in vivo in mice bearing MDA-MB-231 tumor xenografts. Large steric hindrance around the disulfide bonds in GDGP resulted in greater T1 and T2 relaxivities than GDCC, GDCP and dGDCP. The degradability of the polydisulfide by the endogenous thiols decreased with an increase in steric effects around the disulfide bonds in the order of GDCC > GDCP, dGDCP > GDGP. The size and degradability of the contrast agents had significant impact on vascular contrast enhancement kinetics. The agents with large size and low degradability resulted in more prolonged vascular enhancement than the agents with small size and high degradability. It seems that the size and degradability of the agents did not significantly affect tumor enhancement. All agents resulted in significant contrast enhancement in tumor tissue. This study has demonstrated that the vascular enhancement kinetics of the polydisulfide MRI contrast agents can be controlled by their sizes and structures. The polydisulfide Gd(III) chelates are promising biodegradable macromolecular MRI contrast agents for MR angiography and cancer imaging.
Keywords: Structure effect, polydisulfide contrast agents, magnetic resonance imaging, biodegradable, gadolinium
1. Introduction
Cancer and cardiovascular diseases are the main causes of the mortality. Earlier and more accurate detection of both diseases is essential for the patients to have positive prognoses [1]. Magnetic resonance imaging (MRI) is advantageous as compared to other clinical imaging modality because of the absence of radiation and its capacity of morphological and functional imaging with high spatial resolution. Contrast agents are frequently used in MRI to enhance the image contrast between the pathological and normal tissues [2]. Clinical MRI contrast agents, however, have small sizes and unfavorable pharmacokinetics, and show undesired background enhancement due to fast extravasation into the extracellular fluid space [3]. These limitations of small molecular contrast agents necessitate the development of macromolecular contrast agents. Recently, numerous macromolecular contrast agents have been prepared and evaluated for cancer and cardiovascular MR imaging. Preclinical studies have shown that macromolecular MRI contrast agents provide optimal pharmacokinetics and superior contrast enhancement for cancer and cardiovascular imaging. However, macromolecular contrast agents have not been approved for clinical applications because of the safety concerns related to their slow body excretion [4,5].
We have recently designed and developed a novel class of biodegradable macromolecular MRI contrast agents based on polydisulfide Gd(III) complexes to facilitate the excretion of the agents after contrast enhanced MRI [6–14]. Biodegradable disulfide bonds were incorporated into the polymer backbone and can be readily cleaved by the endogenous thiols, e.g. cysteine and glutathione, in the plasma. The reduced Gd(III) complexes and oligomers can then be readily eliminated via renal filtration. These biodegradable macromolecular MRI contrast agents can provide significant and prolonged contrast enhancement in the blood pool for effective contrast enhanced MR imaging, then degrade in vivo and excrete with minimal tissue accumulation [12,14]. In the previous studies, we have observed that the structure of the polydisulfide contrast agents may significantly affect their biodegradability and kinetics of in vivo contrast enhancement. For example, the introduction of steric hindrance around the disulfide bonds in the polydisulfide contrast agents decreases the degradation rate of the polymers, resulting in prolonged blood circulation as compared to the agents with less steric hindrance. These observations suggest that optimal in vivo contrast enhancement of the biodegradable macromolecular contrast agents can be fine tuned for various applications by structural modification. Better understanding of the structure-property correlation of polydisufide Gd(III) complexes will allow us to design and develop promising biodegradable macromolecular MRI contrast agents with optimal in vivo contrast enhancement and minimal tissue accumulation for further preclinical and clinical development.
In this study, we prepared polydisulfide Gd(III) complexes with different steric hindrance around the disulfide bonds and different molecular weights with narrow distribution to study the structural effects of the biodegradable macromolecular MRI contrast agents for tumor and cardiovascular MR imaging. These agents include Gd-DTPA cystamine copolymers (GDCC), Gd-DTPA L-cystine copolymers (GDCP), Gd-DTPA D-cystine copolymers (dGDCP) and Gd-DTPA glutathione (oxidized) copolymers (GDGP). The dGDCP was prepared to study the possible influence of polymer configuration on degradation and contrast enhancement. Three molecular weights, approximately 20, 45 and 110 kDa, were prepared and studied for each agent in nude mice bearing MDA-MB-231 xenography on a 3T MRI scanner.
2. Materials and Methods
2.1. Preparation and fractionation of polydisulfide contrast agents
DTPA cystamine copolymers (DCC), DTPA L-cystine copolymers (DCP), DTPA D-cystine copolymers (dDCP) and DTPA glutathione (oxidized) copolymers (DGP) were prepared by condensation polymerization according to the method described in the previous work [12]. The paramagnetic complexes GDCC, GDCP, dGDCP and GDGP were prepared by the complexation of DCC, DCP, dDCP and DGP with Gd(OAc)3 at pH 5.5~6.0, respectively, and then fractionated to prepare the agents with different molecular weights and narrow molecular weight distributions using size exclusion chromatography (SEC) equipped with a Superose® XK 50/100 column (Amersham Bioscience Corp., Piscataway, NJ). The loading amount of samples was 500 mg and the mobile phase was 20 mM Tris buffer with 0.1 M NaCl (pH 7.4). The molecular weights of the fractions were determined by SEC eluted with 20 mM Tris buffer with 0.1 M NaCl (pH 7.4). The fractionations were calibrated with poly-[N-(2-hydroxypropyl)methacrylamide] (PHPMA) standards. The Gd content for all agents was determined by inductively coupled plasma-optical emission spectroscopy (ICP-OES) (Perkin Elmer, Norwalk, CT, Optima 3100XL) at 342 nm.
2.2. Relaxivity
The T1 values of water protons in aqueous solutions of GDCC, GDCP, dGDCP, and GDGP with various concentrations were measured on a Siemens Trio 3T scanner at room temperature using an inversion recovery (IR)-prepared turbo spin echo (TSE) imaging pulse sequence and a birdcage human head coil [15]. Using a variety of inversion times (TIs) ranging from 22 ms to 2000 ms, T1 values were calculated by a nonlinear regression of MTI = M0 (1 − 2e(−TI/T1)), where M0 is the initial magnetization, and MTI is the magnetization after a particular TI. T1 relaxivity (r1) was determined based on the equation (1/T1)0bserved = (1/T1)background + r1 × [Gd3+]. To determine T2 relaxivity r2, T2 relaxation time was measured first using a turbo spin echo imaging sequence with turbo factor 3 T2 values and a serial of echo times TE = 12, 24, 36, 47, 59, 71, 83, 95 and 107 ms. T2 value for a given concentration was calculated from MTE = M0e(−TE/T2) after non-linear regression with various TE. Relaxivity r2 was then derived from the equation (1/T2)0bserved = (1/T2)background + r2 × [Gd3+].
2.3. Degradation of the polydisulfide complexes
Degradability of GDCC, GDCP, dGDCP and GDGP was evaluated in the presence of either one of four endogenous free thiols, i.e. cysteine, glutathione, cysteinylglycine and homocysteine. The agents with similar weight-averaged molecular weight of approximately 50 kDa and a similar polydispersity of approximately 1.1 were chosen in order to accurately compare their relative degradability. The experimental procedure is briefly described using GDCC as an example. GDCC (0.42 mM Gd) was incubated with 5 μM of the aforementioned free thiols in Tris pH 7.4 at 37 °C in triplicates. Samples were taken at 0, 5, 15, 60, 120 and 360 minute incubation and ultrafiltrated at 4,000 rpm and 4 °C for 10 minutes using YM-3 filter (Millipore, Bedford, MA) with molecular weight cutoff of 3,000 Da. The Gd contents in filtrates were determined using ICP-OES at 342 nm to calculate the amount of degradation products. The percentage of unfiltrated Gd were calculated to represent the relative stability of polydisulfide contrast agents to the corresponding free thiols. The degradation of GDCP, dGDCP and GDGP was similarly evaluated at a high free thiol concentration 50μM because of their relatively slow degradation rate.
The degradability of GDCC, GDCP, dGDCP and GDGP (0.42 mM Gd) in the presence of mixture of four thiols mimicking their composition in human and rat plasma was also evaluated with similar procedure as described above. The human plasma contains approximately 10μM cysteine, 5μM glutathione and 3μM cysteinylglycine, and rat plasma comprises of approximately 57μM cysteine and 26μM glutathione [16].
2.4. MR Imaging
Female athymic nude mice (5–6 weeks old, 20–25 g) were cared for according to an approved animal protocol and the guidelines of the University of Utah Institutional Animal Care and Use Committee. The mouse tumor model bearing MDA-MB-231 human breast carcinoma xenografts was developed as previously described [11]. The mice were anesthetized by the intramuscular administration of a mixture of ketamine (80 mg/kg) and xylazine (12 mg/kg) before administration of contrast agents. MR images of the mice were acquired on a Siemens Trio 3T scanner using a 3D fast low-angle shot (3D FLASH) pulse sequence with a human wrist coil before and at 2, 5, 10, 15, 30 and 60 min after injection of the contrast agents at a dose of 0.1 mmol-Gd/kg via a tail vein. Imaging parameters were 2.44 ms echo time (TE), 7.36 ms repitition time (TR), 25° RF tip angle, 120 mm field of view, 0.5 mm coronal slice thickness. A group of three mice were used for each polymeric contrast agent. T1-weighted axial images of tumors were also acquired using a 2D spin-echo sequence. The imaging parameters were 10 ms TE, 400 ms TR, 90° RF tip angle, 2 mm axial slice thickness.
2.5. Data analysis
The MR images were analyzed using a free software package OSIRIX (http://homepage.mac.com/rossetantoine/osirix/). Regions of interest (ROIs) were set on the right ventricle in the heart, tumor periphery and center of each mouse. Signal intensities in these ROIs were measured at different imaging time point. Each data point for the contrast enhancement pattern was the average from three different mice. Statistical analysis was performed to compare the polymeric contrast agents at each time point in each tissue using a one-way ANOVA with a Tukey post-test. A statistical significance was considered to be p<0.05.
3. RESULTS
3.1. Properties of polydisulfide contrast agents
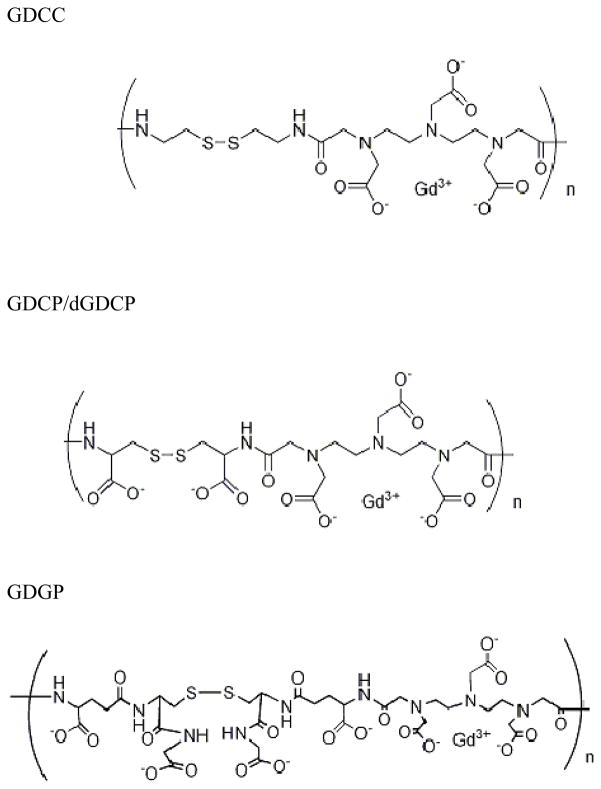
The chemical structures of GDCC, GDCP, dGDCP and GDGP are shown in Figure 1. GDCP, dGDCP and GDGP have negatively charged side chains at physiological pH 7.4, while GDCC is a neutral agent. The steric hindrance around the disulfide bonds increased in the order of GDCC, GDCP and GDGP. The polydisulfide contrast agents were prepared by condensation polymerization of DTPA dianhydride with cystamine, L-cystine, D-cystine and glutathione (oxidized form), respectively, followed by the complexation with Gd(OAC)3. The polymeric complexes had a broad molecular weight distribution and were fractionated with size exclusion chromatography. Three fractions with weight-averaged molecular weights of around 20, 45 and 110 kDa and a polydispersity index of approximately 1.1 were selected to represent low, medium and high molecular weights for this study. GDCC, GDCP and dGDCP had r1 and r2 relaxivities in the range of 5 - 8 mM−1s−1, while GDGP had higher r1 and r2. The physical parameters of the polydisulfide contrast agents are listed in Table 1.
Figure 1.
The chemical structures of GDCC, GDCP/dGDCP and GDGP.
Table 1.
Properties of polydisulfide contrast agents
| GDCC | GDCP | dGDCP | GDGP | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mw (kDa) | 23 | 43 | 113 | 22 | 45 | 105 | 23 | 46 | 112 | 20 | 43 | 114 |
| PDI | 1.07 | 1.13 | 1.11 | 1.10 | 1.08 | 1.05 | 1.12 | 1.10 | 1.06 | 1.04 | 1.13 | 1.06 |
| Diameter (nm) | 3.5 | 5.1 | 10.4 | 3.5 | 5.1 | 10.1 | 3.5 | 5.3 | 10.4 | 3.4 | 5.1 | 10.0 |
| Gd (mmol/g) | 1.21 | 1.14 | 1.09 | 1.03 | 1.11 | 1.02 | 0.97 | 0.97 | 0.95 | 0.75 | 0.72 | 0.72 |
| r1 (mM−1s−1) | 5.70 | 5.76 | 5.54 | 6.8 | 5.86 | 5.82 | 5.72 | 4.82 | 5.08 | 11.99 | 9.50 | 9.10 |
| r2 (mM−1s−1) | 7.70 | 7.07 | 6.63 | 8.20 | 8.17 | 7.40 | 5.87 | 6.77 | 6.97 | 10.6 | 12.73 | 13.07 |
3.2. Degradability of the polydisulfide complexes
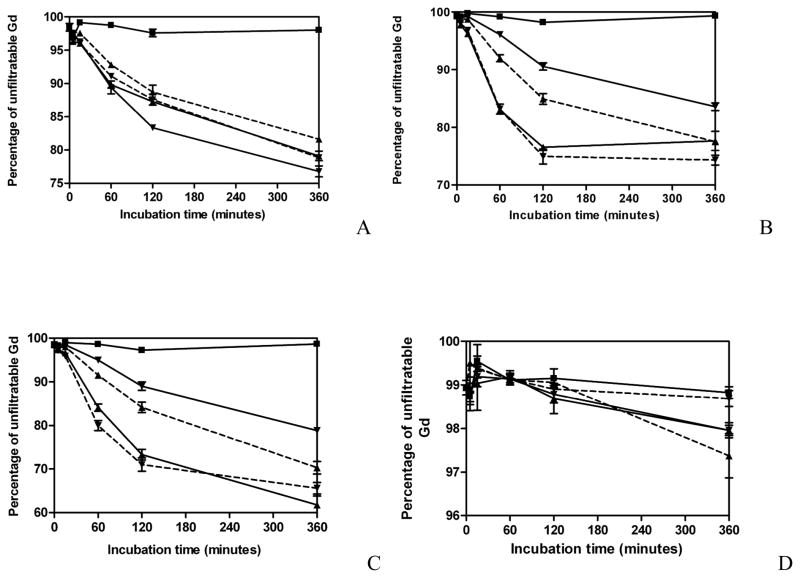
Degradability of GDCC, GDCP, dGDCP and GDGP in the presence of various endogenous thiols is shown in Figure 2. GDCC had similar degradability in the presence of 5μM of cysteine, cysteinylglycine, glutathione and homocysteine during 6 hour incubation, Figure 2A. GDCP, dGDCP and GDGP showed little degradation in the presence of 5μM thiols. Therefore, the degradation of these agents was evaluated with 50μM thiols. Both GDCP and dGDCP showed different degradability in different endogenous thiols, Figure 2B and 2C. GDCP and dGDCP degraded more rapidly in cysteine and cysteinylglycine than homocysteine and glutathione at physiological pH 7.4. Both agents had the lowest degradability in glutathione possibly due to the steric effect of its relatively large size. GDGP with large steric hindrance around the disulfide bonds had much lower degradability than GDCP and dGDCP and little degradation was observed during 6 hour incubation with 50μM of various endogenous thiols, Figure 2D.
Figure 2.
In vitro degradation of GDCC (A), GDCP (B), dGDCP (C) and GDGP (D) with Tris buffer pH7.4 (■) or various free thiols (5μM for GDCC, 50μM for GDCP, dGDCP and GDGP) (cysteine, ▲ and solid line; glutathione, ▼; and solid line; homocysteine ▲ and dashed line; cysteinylglycine ▼; and dashed line) (n=3).
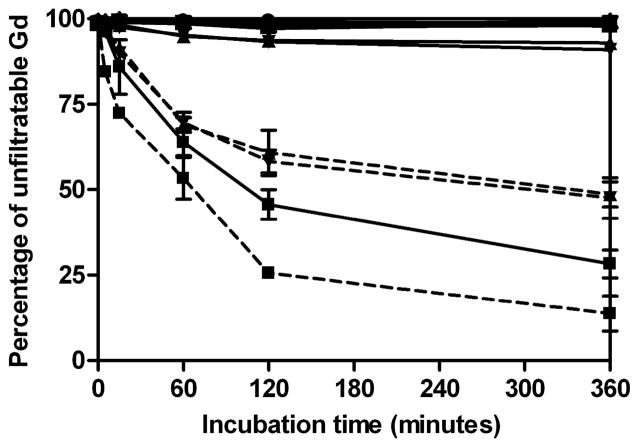
Figure 3 shows the degradability of GDCC, GDCP, dGDCP and GDGP in the thiol solutions mimicking the human and rat plasma compositions of major thiols. GDCC showed higher degradability in both human and rat plasma thiol compositions than the other agents. GDCC degraded more rapidly in rat thiol composition than human thiols composition due to the higher total thiol concentration in the former. GDCP and dGDCP also had much higher degradability in rat thiol composition than human thiol composition. GDGP had little degradation in both human and rat thiol compositions.
Figure 3.
In vitro degradation of polydisulfide contrast agents (0.42 mM Gd) with a mixture of major human plasma free thiols (cysteine (10μM), glutathione (5μM) and cysteinylglycine (3μM)) or rat plasma free thiols (cysteine (57μM) and glutathione (26μM). GDCC: Tris buffer pH7.4, □; human plasma free thiols, ■ and solid line; rat plasma free thiols, ■ and dashed line. dGDCP: Tris buffer pH7.4, ◻; human plasma free thiols, ▲ and solid line; rat plasma free thiols, ▲ and dashed line. GDCP: Tris buffer pH7.4, ▽; human plasma free thiols, ▼; and solid line; rat plasma free thiols, ▼; and dashed line. GDGP: Tris buffer pH7.4, ○; human plasma free thiols, ● and solid line; rat plasma free thiols, ● and dashed line.
3.3. Contrast enhanced cardiovascular MRI
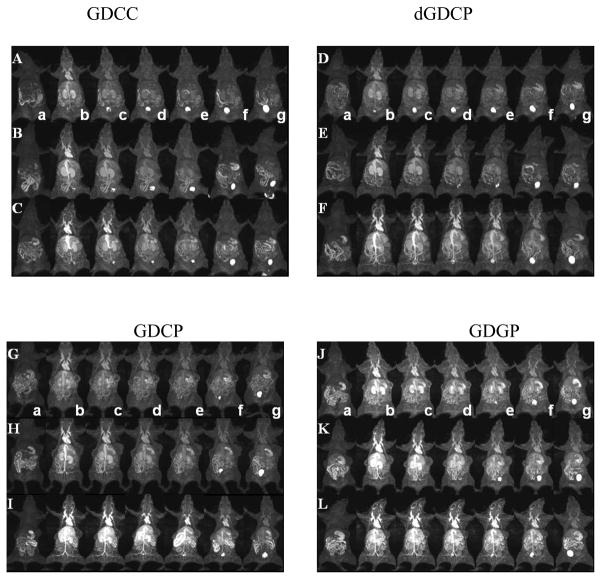
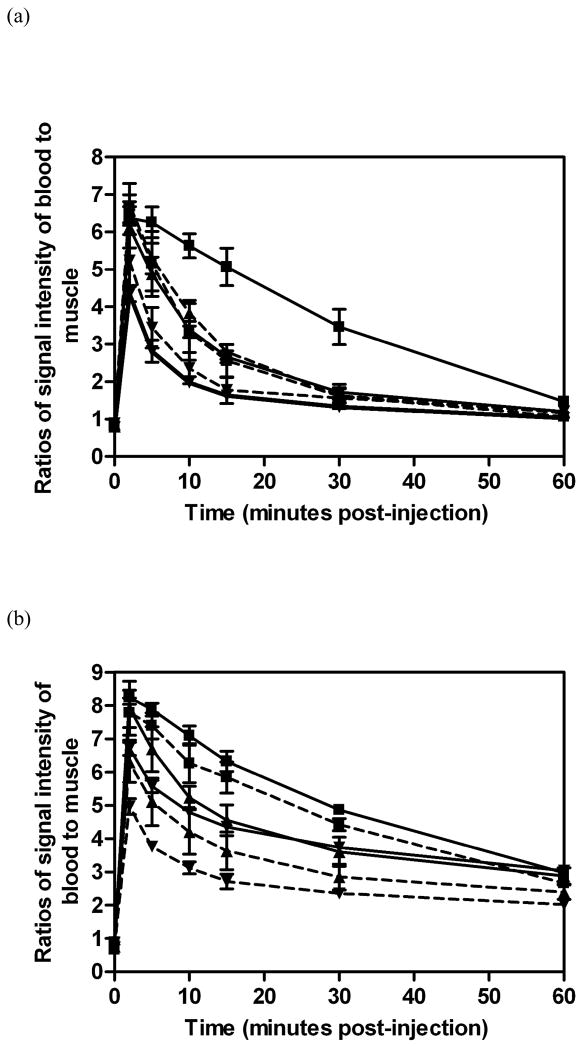
Figure 4 shows the representative three-dimensional maximum intensity projection (MIP) MR images of mice with MDA-MB-231 tumor xenografts before and at various time points after injection of GDCC, GDCP, dGDCP and GDGP with different molecular weights. Figure 5a shows the quantitative analysis of dynamic change of the signal intensity ratios of the blood in the heart to muscle, and figure 5b showed the contrast enhancement ratio in the vasculature to the surrounding tissues for all agents. Five to eight times contrast enhancement was observed in the heart, vasculature and kidneys for all agents at 2 min post-injection, and the signal intensity gradually decreased thereafter depending on their structures and sizes. For the four different agents with similar molecular weight, the duration of cardiovascular enhancement increased with the increasing steric hindrance around the disulfide bonds in the order of GDCC < dGDCP, GDCP < GDGP. The duration of the contrast enhancement in the heart and vasculature increased with the increasing molecular weight for the same agent. GDGP with high relaxivity and slow degradation rate resulted in more prominent contrast enhancement in the blood pool within the first 30 minutes post-injection even at the low molecular weight (21 kDa). Gradual enhancement in the urinary bladder was also observed for all polydisulfide contrast agents, indicating urinary clearance of Gd complexes after the degradation.
Figure 4.
Three dimensional maximum intensity projection MR images of mice before injection (a) and 2 (b), 5 (c), 10 (d), 15 (e), 30 (f) and 60 (g) minutes after injection of GDCC ( 23 kDa, A; 43 kDa, B; 113 kDa, C), dGDCP (23 kDa, D; 46 kDa, E; 112 kDa, F), GDCP ( 23 kDa, G; 43 kDa, H; 109 kDa, I) and GDGP( 21 kDa, J; 43 kDa, K; 108 kDa, L) at a dose of 0.1 mmol-Gd/kg via a tail vein.
Figure 5.
Relative MR signal intensities in the heart of mice before and at various time points after the intravenous injection of GDCC (a, dashed line) ( 23 kDa, ▼;; 43 kDa, ▲; 113 kDa, ■), dGDCP (a, solid line) (23 kDa, ▼;; 46 kDa, ▲; 112 kDa, ■), GDCP (b, dashed line) ( 23 kDa, ▼;; 43 kDa, ▲; 109 kDa, ■) and GDGP (b, solid line) ( 21 kDa, ▼;; 43 kDa, ▲; 108 kDa, ■) at a dose of 0.1 mmol-Gd/kg via a tail vein (n=3).
3.4. Contrast enhanced tumor imaging
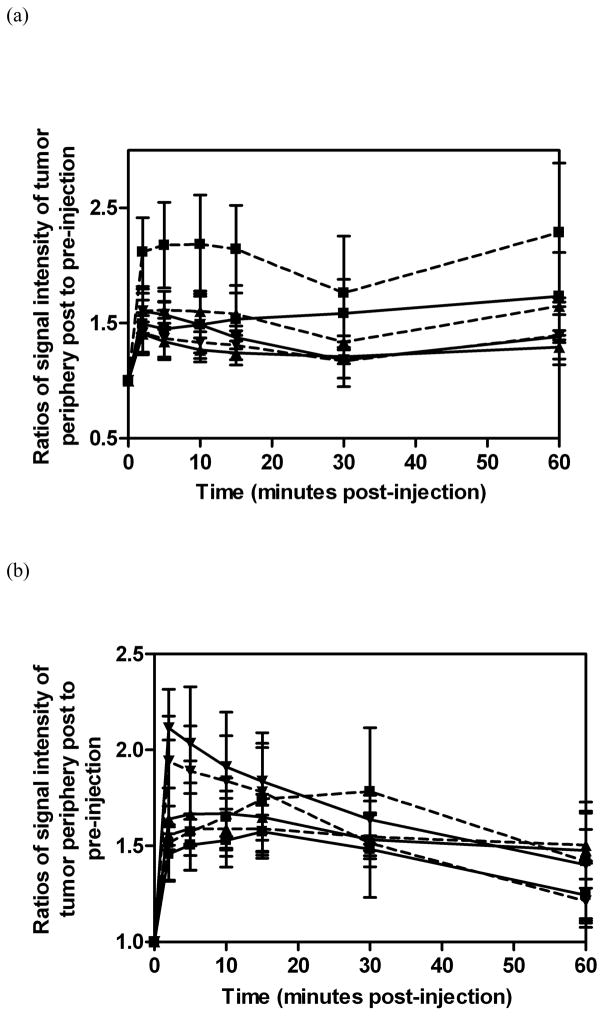
Figure 6 shows the two dimensional axial T1-weighted spin-echo images of tumor tissues before and after injection of the polydisulfide contrast agents. All agents resulted in substantial enhancement in the tumor periphery for at least 60 minutes. The quantitative analysis of the contrast enhancement in tumor periphery is shown in Figure 7. All agents resulted in 30 – 100% contrast enhancement in tumor periphery irrespective of their sizes and structures. However, GDCC showed a different size-dependent enhancement profile from GDCP, dGDCP and GDGP. The enhancement in the tumor periphery with GDCC increased with molecular weight, while GDCP, dGDCP and GDGP showed a reverse size-dependent enhancement pattern from GDCC within the first 10 to 15 minutes post-injection. Nevertheless, the difference among the agents in tumor enhancement is statistically insignificant.
Figure 6.
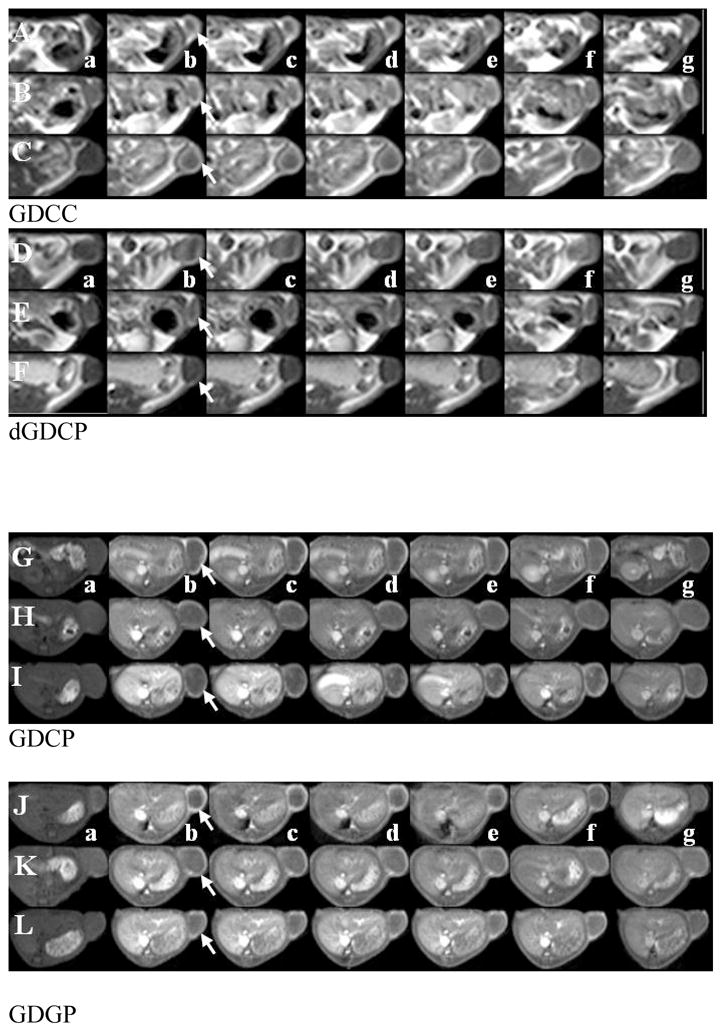
Two dimensional T1 weighted MR images of mice before injection (a) and 2 (b), 5 (c), 10 (d), 15 (e), 30 (f) and 60 (g) minutes after injection of GDCC ( 23 kDa, A; 43 kDa, B; 113 kDa, C), dGDCP (23 kDa, D; 46 kDa, E; 112 kDa, F), GDCP ( 23 kDa, G; 43 kDa, H; 109 kDa, I) and GDGP( 21 kDa, J; 43 kDa, K; 108 kDa, L) at a dose of 0.1 mmol-Gd/kg via a tail vein.
Figure 7.
Relative MR signal intensities in the tumor periphery in mice before and at various time points after the intravenous injection of GDCC (a, dashed line) ( 23 kDa, ▼;; 43 kDa, ▲; 113 kDa, ■), dGDCP (a, solid line) (23 kDa, ▼;; 46 kDa, ▲; 112 kDa, ■), GDCP (b, dashed line) ( 23 kDa, ▼;; 43 kDa, ▲; 109 kDa, ■) and GDGP (b, solid line) ( 21 kDa, ▼;; 43 kDa, ▲; 108 kDa, ■) at a dose of 0.1 mmol-Gd/kg via a tail vein(n=3).
4. Discussion
Macromolecular MRI contrast agents have advantages, such as high specificity for tumor characterization and prolonged enhancement for high-resolution vascular delineation as compared to small molecular contrast agents. Polydisulfide Gd(III) chelates have demonstrated superior contrast enhancement as macromolecular MRI contrast agents, and can be gradually degraded in the plasma and excreted with minimal tissue retention. In this study, we have shown that the degradability of the polydisulfide can be controlled by the structural modification. The introduction of steric hindrance around the disulfide bonds significantly decreased the degradation rate of the polydisulfides by the endogenous thiols.
GDGP has two larger substituents around the disulfide bonds than those in GDCC and GDCP. The steric hindrance inhibited the disulfide-thiol exchange reaction with the endogenous thiols, resulting in slow degradation in the presence of endogenous thiols [17–22]. The large steric hindrance also inhibited free rotation of the disulfide bonds in GDGP. Consequently, GDGP had higher T1 and T2 relaxvities than GDCC and GDGP.
The structure of the endogenous thiols did not significantly affected the degradation rate of GDCC, possibly due to the high reactivity of the disulfide bonds because of the lack of steric hindrance. For GDCP and dGDCP with intermediate degradability, their degradability was affected by the structure of the thiols. Both GDCP and dGDCP degraded more slowly in glutathione than other tested endogenous thiols because glutathione had a large steric effect around its thiol group. GDCP and dGDCP also had relatively low reactivity towards to homocysteine. The configuration of GDCP and dGDCP did not affect their degradation rate with the endogenous thiols, even though these thiols are amino acids or peptides with L-configuration.
The degradability of the polydisulfide Gd(III) chelates significantly affected the vascular enhancement within the vasculature. Vascular enhancement kinetics of the agents correlated well to their in vitro degradability in the presence of endogenous thiols. GDGP resulted in more prolonged vascular enhancement even at a molecular weight as low as 20 kDa, validating its low in vivo degradability as predicted by the in vitro study. The high relaxivity of GDGP might also contribute to its strong vascular enhancement. The GDCC with high in vitro degradability cleared more rapidly from the vascular circulation than the other agents. The agents with small sizes also cleared more rapidly than the same agents with large sizes. The agents with small sizes can be readily degraded into smaller oligomers that can be rapidly eliminated via renal filtration. The vascular enehancement significant decreased and significant enhancement was observed in the urinary bladder at 60 minutes post-injection for all agents, indicating that all agents can be degraded in vivo and excreted via renal filtration. However, the vascular enhancement kinetics of the biodegradable contrast agents observed in rodents may not be suitable for predicting the plasma kinetics in humans because the plasma thiol concentration in human is much lower than that in rodents. As we shown in this study, the degradability of GDCC and GDCP in human plasma thiol composition was slower than in rodents. It is reasonable to predict that the vascular enhancement duration by the polydisulfide agents in human might be longer than that in rodents.
It appears that the size and degradability of the polydisulfide Gd(III) chelates do not significantly affect tumor enhancement in mice. All agents resulted in 30–100% tumor enhancement, which should be sufficient to detect tumor tissue for diagnostic purpose. GDCC with high molecular weight (113 kDa) was more effective for tumor enhancement, possibly because it was a neutral agent and had relative longer blood retention. Neutral polymers may have a more flexible backbone than the charged polymers, and therefore have high permeability into extracellular matrix. High permeability and prolonged vascular circulation of high molecular weight GDCC may result in high tumor accumulation and strong tumor enhancement.
5. Conclusions
The structure of polydisulfide Gd(III) chelates had a significant impact on their degradability and in vivo contrast enhancement as biodegradable macromolecular MRI contrast agents. Degradability of the contrast agents decreased as the steric hindrance around the disulfide bonds increased. Strong steric effect also reduced free rotation of disulfide bonds and resulted in high relaxivities. The vascular enhancement of the contrast agents was improved with a decrease in degradability and an increase in polymer size. The size and degradability of polydisulfide contrast agents did not have significant effect on contrast enhanced tumor imaging. Optimal biodegradable macromolecular MRI contrast agents can be obtained by careful structural modification of the polydisulfide Gd(III) chelates for effective contrast enhanced MR angiography and cancer imaging.
Acknowledgments
We thank Dr. Yong-En Sun for technical assistant in animal experiments and Ms. Melody Johnson for the MR imaging. This work is supported in part by the NIH (R01EB000489).
References
- 1.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis—correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 2.Caravan P, Ellison J, McMurry T, Lauffer R. Gadolinium(III) chelates as MRI contrast agents: Structure, dynamics, and applications. Chem Rev. 1999;99:2293–352. doi: 10.1021/cr980440x. [DOI] [PubMed] [Google Scholar]
- 3.Brasch R, Turetschek K. MRI characterization of tumors and grading angiogenesis using macromolecular contrast media: status report. Eur J Radiol. 2000;34:148–55. doi: 10.1016/s0720-048x(00)00195-9. [DOI] [PubMed] [Google Scholar]
- 4.Duncan JR, Franano FN, Edwards WB, Welch MJ. Evidence of gadolinium dissociation from protein-DTPA-gadolinium complexes. Invest Radiol. 1994;29(SUPPL 2):S58–S61. doi: 10.1097/00004424-199406001-00020. [DOI] [PubMed] [Google Scholar]
- 5.Franano FN, Edwards WB, Welch MJ, Brechbiel MW, Gansow OA, Duncan JR. Biodistribution and metabolism of targeted and nontargeted protein-chelate-gadolinium complexes: Evidence for gadolinium dissociation in vitro and in vivo. Magn Reson Imaging. 1995;13:201–14. doi: 10.1016/0730-725x(94)00100-h. [DOI] [PubMed] [Google Scholar]
- 6.Lu ZR, Mohs AM, Zong Y, Feng Y. Polydisulfide Gd(III) chelates as biodegradable macromolecular magnetic resonance imaging contrast agents. Int J Nanomedicine. 2006;1:31–40. doi: 10.2147/nano.2006.1.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohs AM, Lu ZR. Gadolinium(III)-based blood-pool contrast agents for magnetic resonance imaging: status and clinical potential. Expert Opin Drug Deliv. 2007;4:149–64. doi: 10.1517/17425247.4.2.149. [DOI] [PubMed] [Google Scholar]
- 8.Lu ZR, Wang X, Parker DL, Goodrich KC, Buswell HR. Poly(L-glutamic acid) Gd(III)-DOTA conjugate with a degradable spacer for magnetic resonance imaging. Bioconjug Chem. 2003;14:715–19. doi: 10.1021/bc0340464. [DOI] [PubMed] [Google Scholar]
- 9.Lu ZR, Parker DL, Goodrich KC, Wang X, Dalle JG, Buswell HR. Extracellular biodegradable macromolecular gadolinium(III) complexes for MRI. Magn Reson Med. 2004;51:27–34. doi: 10.1002/mrm.10656. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Feng Y, Ke T, Schabel M, Lu ZR. Pharmacokinetics and tissue retention of (Gd-DTPA)-Cystamine copolymers, a biodegradable macromolecular magnetic resonance imaging contrast agent. Pharm Res. 2005;22:596–602. doi: 10.1007/s11095-005-2489-7. [DOI] [PubMed] [Google Scholar]
- 11.Zong Y, Wang X, Goodrich KC, Mohs AM, Parker DL, Lu ZR. Contrast-enhanced MRI with new biodegradable macromolecular Gd(III) complexes in tumor-bearing mice. Magn Reson Med. 2005;53:835–42. doi: 10.1002/mrm.20402. [DOI] [PubMed] [Google Scholar]
- 12.Zong Y, Guo J, Ke T, Mohs AM, Parker DL, Lu ZR. Effect of size and charge on pharmacokinetics and in vivo MRI contrast enhancement of biodegradable polydisulfide Gd(III) complexes. J Control Release. 2006;30:350–56. doi: 10.1016/j.jconrel.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Mohs AM, Zong Y, Guo J, Parker DL, Lu ZR. PEG-g-poly(GdDTPA-co-L-cystine): Effect of PEG chain length on in vivo contrast enhancement in MRI. Biomacromolecules. 2005;6:2305–11. doi: 10.1021/bm050194g. [DOI] [PubMed] [Google Scholar]
- 14.Mohs AM, Nguyen T, Jeong EK, Feng Y, Emerson L, Zong Y, Parker DL, Lu ZR. Modification of Gd-DTPA cystine copolymers with PEG-1000 optimizes pharmacokinetics and tissue retention for magnetic resonance angiography. Magn Reson Med. 2007;58:110–18. doi: 10.1002/mrm.21270. [DOI] [PubMed] [Google Scholar]
- 15.Fukushima E, Roeder SBW. Experimental pulse NMR: a nuts and bolts approach. Reading MA: Addison-Wesley; 1981. [Google Scholar]
- 16.Mansoor MA, Svardal AM, Ueland PM. Determination of the in vivo redox status of cysteine, cysteinylglycine, homocysteine and glutathione in human plasma. Anal Biochem. 1992;200:218–29. doi: 10.1016/0003-2697(92)90456-h. [DOI] [PubMed] [Google Scholar]
- 17.Mahieu JP, Gosselet NM, Sebille B, Garel MC, Beuzard Y. Reactivity of 42 disulfides with thiol group of human haemoglobin and human serum albumin. Int J Biol Macromol. 1993;15:233–40. doi: 10.1016/0141-8130(93)90043-l. [DOI] [PubMed] [Google Scholar]
- 18.Thorpe PE, Wallace PM, Knowles PP, Relf MG, Brown AN, Watson GJ, Knyba RE, Wawrzynczak EJ, Blakey DC. New coupling agents for the synthesis of immunotoxins containing a hindered disulfide bond with improved stability in vivo. Cancer Res. 1987;47:5924–31. [PubMed] [Google Scholar]
- 19.Greenfield L, Bloch W, Moreland M. Thiol-containing cross-linking agent with enhanced steric hindrance. Bioconjug Chem. 1990;1:400–10. doi: 10.1021/bc00006a006. [DOI] [PubMed] [Google Scholar]
- 20.Carroll SF, Bernhard SL, Goff DA, Bauer RJ, Leach W, Kung AH. Enhanced stability in vitro and in vivo of immunoconjugates prepared with 5-methyl-2-iminothiolane. Bioconjug Chem. 1994;5:248–56. doi: 10.1021/bc00027a010. [DOI] [PubMed] [Google Scholar]
- 21.Arpicco S, Dosio F, Brusa P, Crosasso P, Cattel L. New coupling reagents for the preparation of disulfide cross-linked conjugates with increased stability. Bioconjug Chem. 1997;8:327–37. doi: 10.1021/bc970025w. [DOI] [PubMed] [Google Scholar]
- 22.Hupe DJ, Wu D. Effect of charged substituents on rates of the thiol-disulfide interchange reaction. J Org Chem. 1980;45:3100–03. [Google Scholar]