Abstract
We previously showed that an elevated content of fibrinogen (Fg) increased formation of filamentous actin and enhanced endothelial layer permeability. In the present work we tested the hypothesis that Fg binding to endothelial cells (ECs) alters expression of actin-associated endothelial tight junction proteins (TJP). Rat cardiac microvascular ECs were grown in gold plated chambers of an electrical cell-substrate impedance system, 8-well chambered, or in 12-well plates. Confluent ECs were treated with Fg (2 or 4 mg/ml), Fg (4 mg/ml) with mitogen-activated protein kinase (MEK) kinase inhibitors (PD98059 or U0126), Fg (4 mg/ml) with anti-ICAM-1 antibody or BQ788 (endothelin type B receptor blocker), endothelin-1, endothelin-1 with BQ788, or medium alone for 24 h. Fg induced a dose-dependent decrease in EC junction integrity as determined by transendothelial electrical resistance (TEER). Western blot analysis and RT-PCR data showed that the higher dose of Fg decreased the contents of TJPs, occludin, zona occluden-1 (ZO-1), and zona occluden-2 (ZO-2) in ECs. Fg-induced decreases in contents of the TJPs were blocked by PD98059, U0126, or anti-ICAM-1 antibody. While BQ788 inhibited endothelin-1-induced decrease in TEER, it did not affect Fg-induced decrease in TEER. These data suggest that Fg increases EC layer permeability via the MEK kinase signaling pathway by affecting occludin, ZO-1, and ZO-2, TJPs, which are bound to actin filaments. Therefore, increased binding of Fg to its major EC receptor, ICAM-1, during cardiovascular diseases may increase microvascular permeability by altering the content and possibly subcellular localization of endothelial TJPs.
Fibrinogen (Fg) is a blood plasma adhesion glycoprotein that is normally synthesized and assembled in hepatocytes and fibroblasts. Its synthesis involves inflammatory cytokines such as interleukin-1 and interleukin-6 (Humphries, 1995; Vasse et al., 1996). An elevated blood content of Fg is considered to be a high risk factor for cardiovascular diseases (Chae et al., 2001; Danesh et al., 2005) and typically accompanies development of diseases such as hypertension (Letcher et al., 1981; Lominadze et al., 1998), diabetes (Lee et al., 2007), and stroke (D'Erasmo et al., 1993), which involve inflammatory processes. Hperfibrinogenemia may be an independent factor, or it may interact to modulate the severity and/or progression of vascular disorders (Kerlin et al., 2004). In addition, it is associated with increased formation of fibrin (Lord, 2007), which itself is a high vascular risk factor. We previously found that Fg can induce vasoconstriction through production of endothelin-1 (ET-1) (Lominadze et al., 2005). All these findings suggest a significant detrimental role of elevated blood Fg content in the cardiovascular system.
Increased microvascular permeability is a marker of inflammation. Impairment of endothelial cell (EC) integrity leading to significant tissue damage and inflammatory responses (Mehta and Malik, 2006), typically occurs during diseases such as hypertension (Letcher et al., 1981; Lominadze et al., 1998), diabetes (Lee et al., 2007), and stroke (D'Erasmo et al., 1993). Blood plasma components may pass through the endothelial barrier via two major transport mechanisms, transcellular and paracellular (Mehta and Malik, 2006). The present study focuses on the paracellular mechanism, which directly involves formation of filamentous actin (F-actin) in ECs (Mehta and Malik, 2006).
A pathologically high (4 mg/ml) content of Fg, which typically occurs during hypertension development (Lominadze et al., 1998), increases EC layer permeability to albumin (Tyagi et al., 2008). An increased Fg content leads to increased Fg binding to its endothelial receptors, intercellular adhesion molecule-1 (ICAM-1) (D'Souza et al., 1996; Plow et al., 2000) and α5β1 integrin (Luscinskas and Lawler, 1994; Plow et al., 2000), and increased formation of F-actin (Tyagi et al., 2008). Enhanced formation of F-actin may cause stiffening of the cells, actin filament retraction, and widening of inter-endothelial junctions (IEJs) (Qiao et al., 1995; Ehringer et al., 1999; Lominadze et al., 2004; Trepat et al., 2005; Mehta and Malik, 2006). Since most of the endothelial tight junction proteins (TJPs) are connected to actin filaments (Mehta and Malik, 2006), they could be involved in Fg-induced, increased albumin leakage through a paracellular transport mechanism (Tyagi et al., 2008).
ET-1 affects vascular integrity (Filep et al., 1991; Lopez-Belmonte and Whittle, 1994). Our previous studies showing that an increased Fg content enhances EC layer permeability (Tyagi et al., 2008), did not determine if permeability was altered by the higher content of Fg (Tyagi et al., 2008), or by ET-1 produced in response to Fg binding to ECs (Sen et al., 2009). The present study addresses the hypothesis that an increased content of Fg affects IEJs and alters TJPs connected to actin filaments. We show for the first time that Fg-induced impairment of EC integrity can be mediated by TJPs bound to actin filaments, and that this mechanism may involve extracellular signal-regulated kinase (ERK) signaling. Role of ET-1 in Fg-induced alterations of EC layer integrity and expression of TJPs has also being defined.
Materials and Methods
Reagents and antibodies
Human Fg (FIB-3, depleted of plasminogen, von-Willebrand factor, and fibronectin) was purchased from Enzyme Research Laboratories (South Bend, IN). Bovine serum albumin (BSA), 1-palmitoyl-sn-glycero-3-phosphocholine (LPC), fibronectin, and ET-1 were purchased from Sigma (St. Louis, MO). RIPA buffer was from Boston Bioproducts (Worcester, MA). Specific inhibitors of the mitogen-activated protein kinase (MEK) known as ERK kinase, PD98059 (2′-amino-3′-methoxyflavone) and U0126 (1,4-diamino-2,3-dicyano-1,4-bis[2-aminophenylthio] butadiene), were purchased from Calbiochem (La Jolla, CA). A function-blocking monoclonal antibody to rat ICAM-1 was purchased from Chemicon (Temecula, CA). BSA conjugated with Alexa Flour-488 dye (BSA-488), and secondary antibodies, conjugated with Alexa 555 dye or with Alexa 488 were purchased from Invitrogen (Carlsbad, CA). A specific blocker of endothelin type B (ETB) receptor, BQ-788, was purchased from Bachem Bioscience (King of Prussia, PA). Antibodies against rat zona occluden-1 (ZO-1) and zona occluden-2 (ZO-2) were purchased from Invitrogen (Carlsbad, CA) and an antibody against occludin was from BD Biosciences (Franklin Lakes, NJ).
Endothelial cell culture
Rat heart microvascular endothelial cells (RHMEC) were purchased from Vec Technologies (Rensselaer, NY). The endothelial nature of the cells was verified by uptake of acylated low-density lipoprotein and positive staining for CD-31 (Lincoln et al., 2003). The RHMECs were grown in MCDB-131 Complete medium (Vec Technologies, NY) at 37°C with 5% CO2 in air in a humidified environment and were used at the 5th or 6th passage for the experiments.
Endothelial cell integrity assays
Transendothelial electrical resistance (TEER) measurement was done according to the method described earlier (Tyagi et al., 2007). RHMECs were seeded onto evaporated gold microplates of an electrical cell-substrate impedance system (ECIS, Applied Biophysics, Troy, NY) and grown to a confluent monolayer that covered the microelectrodes of the system's 8-well chamber (Tiruppathi et al., 1992). Cells were treated with one of the following for 24 h: Fg (2, 3, or 4 mg/ml), Fg (4 mg/ml) with PD98059 (20 μM), U0126 (20 μM), or BQ788 (5 μM). To compare the effect of Fg with that of ET-1, cells were treated with ET-1 (10 nM) in the presence or absence of BQ788 (5 μM). To determine the effect of BQ788 or MEK inhibitors on TEER of the cells, the following substances were added to wells without Fg or ET-1: BQ788 (5 μM), PD98059 (20 μM), or U0126 (20 μM). Cells incubated with media alone, were used as a control group. In preliminary experiments we determined if the presence of albumin affected TEER results. Resistance baseline values for each microelectrode (i.e., each well) were taken as an average resistance for the last 30 min observation prior to the treatments. Resistance values after the treatments were plotted as changes from baseline vs. time. Differences between the experimental groups for TEER measurements were assessed at 6, 12, 18, and 24 h.
Fg-induced albumin leakage through the EC monolayer was assessed according to the method described earlier (Tyagi et al., 2007, 2008). Transwell permeable supports (Corning Inc., Corning, NJ) with polycarbonate membranes (Nuclepore Track-Etch, 6.5 mm in diameter, 0.4 μm pore size and pore density of 108/cm2) were coated with fibronectin for 1 h. The membranes were seeded with MHECs and the cells were grown to confluence. To confirm cell confluence and the presence of an intact monolayer on the membranes, cells in a separate test well (not membrane) were monitored by light microscopy. Cells in the test membranes were labeled with BCECF-AM (2′,7′-bis (2-carboxyethyl)-5-(and-6)-carboxyfluorescein, acetoxymethyl ester)(Molecular Probes, Eugene, OR) and observed under a fluorescence microscope (Olympus IX51, objective 20×) with 488 nm excitation and 516 nm emission for absence of visible gaps in the cell monolayer. The permeability assays were done after confirming that the cells in the test wells and Transwell membranes were fully confluent and formed an intact monolayer. Unlabeled BSA was added to each well to maintain its concentration similar to a normal plasma concentration of albumin (440 μM) and maintain the activity coefficient of Fg close to that in blood (Rivas et al., 1999). Although, in our preliminary experiments, we did not observe a difference in EC layer permeability to albumin between the groups with or without 440 μM of BSA, all the experiments in these series were done in the presence of 440 μM of BSA. The surface levels of solutions in the luminal (200 μl) and abluminal (700 μl) compartments of the Transwells were the same. The experimental set-up was similar to that used by Cooper et al. (1987), but the presence of albumin-mediated osmotic pressure in the present study makes it closer to in vivo conditions (Tyagi et al., 2007). In addition, we previously showed that albumin leakage through the membrane alone was about 9 times greater than that in the presence of the cells (Tyagi et al., 2007), demonstrating the validity of the experimental set-up.
For the permeability assay, cells were washed with PBS and incubated with medium alone (control) or various doses of Fg (2, 3, and 4 mg/ml) for 1 h. To test for a role of ET-1 produced as a result of Fg interaction with ECs (Sen et al., 2009), prior to adding 4 mg/ml of Fg, cells were treated with the ET-B receptor blocker BQ788 (5 μM) for 30 min. BQ788 was present in the wells when Fg was added. Another group of cells was treated with 10 nM ET-1 with or without BQ788 (5 μM) for 1 h. The dose of ET-1 was chosen based on results of a study demonstrating that 10 nM ET-1 caused constriction of third-order arterioles (Zhao et al., 2000). BSA-488 (3 mg/ml) was added to each of the wells described above and experiments were done three times in duplicate for each treatment. The cells were incubated in humidified conditions at 37°C. After incubation, media samples were collected from lower and upper wells. Fluorescence intensity of the samples was measured with a microplate reader (SpectraMax M2, Molecular Devices Corporation, Sunnyvale, CA) with excitation at 494 nm and emission at 518 nm. Results are expressed as fluorescence intensity units (FIU). To differentiate the effect of Fg on EC layer permeability from that of ET-1, the ratio of the florescence intensity of media samples from the group treated with Fg and BQ788 to that treated with Fg alone was compared to the ratio of the florescence intensity of media samples from the group treated with ET-1 and BQ788, to that treated with ET-1 alone.
Fibrinogen-induced alterations of occludin, ZO-1, and ZO-2
Expression of occludin, ZO-1, and ZO-2 messages was determined using the reverse transcription polymerase chain reaction assay (RT-PCR). Total RNA was isolated from confluent RHMECs using TRIzol® Reagent (Invitrogen, Carlsbad, CA). RNA was precipitated with ethanol overnight and then quantified at 260 nm. The expression of messenger RNAs (mRNAs) for occludin, ZO-1, and ZO-2 was examined using two-step RT-PCR. ImProm-III™ Reverse Transcription System obtained from Promega and used according to the manufacturer's specifications (Tyagi et al., 2009). cDNA samples were incubated for 2 min at 94°C, cycled 35 times (1 min at 94°C, 1 min at 54°C, and 1 min at 72°C), and extended for 5 min at 72°C. Products were visualized in a 1%TAE agarose gel, stained with ethidium bromide. Primers were: ZO-1 (forward) 5′-aaggcaattccgtatcgttg-3′ and (reverse) 5′-ccacagctgaaggactcaca-3′; ZO-2 (forward) 5′-aggcaacagacagctcgaat-3′ and (reverse) 5′-ctcccaagataatgccctga-3′; occludin (forward) 5′-gagggtacacagaccccaga-3′ and reverse 5′-caggattgcgctgactatga-3′ and GAPDH (forward) 5′-tatgtcgtggagtctactggcgtc-3′ and (reverse) 5′-gaatgggagttgctgttgaag-3′. Reactions were carried out in a MJ Research MiniCycler model PTC-150. Gels were photographed using the Kodak Bioimaging system (Gel Logic 200, Eastman Kodak, Rochester, NY).
Changes in protein content of occludin, ZO-1, and ZO-2 induced by Fg in RHMECs were assessed by western blot analysis. The cells were grown in 12-well plates (Corning, Corning, NY) until confluent. Cells were incubated with one of the following: Fg (2 or 4 mg/ml), Fg (4 mg/ml) with MEK inhibitors PD98059 or U0126 (50 μM each), or Fg (4 mg/ml) with anti-rat ICAM-1 (50 μg/ml) at 37°C for 24 h. Other cells were incubated with PD98059 or U0126 (50 μM each), or anti-ICAM-1 (50 μg/ml) at 37°C for 24 h. Cells incubated with serum-free media were used as a control. After incubation, cells were washed twice with ice-cold PBS and lysed with ice-cold RIPA buffer (containing 5 mM of EDTA), which was supplemented with phenylmethylsulfonyl fluoride (1 mM) and protease inhibitor cocktail (1 μl/ml of lysis buffer). Protein content of the lysate was determined using the Bicinchronic Acid (BCA) protein assay kit (Pierce, Rockford, IL). Equal amounts of protein (10 μg) were resolved on 10% SDS-PAGE and transferred onto a polyvinylidene difluoride membrane as described (Sen et al., 2009). The blots were incubated with monoclonal anti-rat occludin (1:400 dilution), anti-rat ZO-1 (1:500 dilution), or anti-rat ZO-2 (1:500 dilution) antibodies for 1 h at room temperature with gentle agitation. After incubation, the proteins on blots were detected as described (Sen et al., 2009). Membranes were stripped and reprobed for β-actin as a loading control. The blots were analyzed with Gel-Pro Analyzer software (Media Cybernetics, Silver Spring, MD) as described earlier (Lominadze et al., 2002). The protein expression intensity was assessed by the integrated optical density (IOD) of the area of the band in the lane profile. To account for possible differences in the protein load, the measurements presented are the IOD of each band under study (protein of interest) divided by the IOD of the respective β-actin band.
Immunohistochemistry and laser-scanning confocal microscopy were used to detect Fg-induced changes in occludin, ZO-1, and ZO-2 expression and subcellular distribution in ECs. RHMECs were grown until confluent in an 8-well chamber coated with fibronectin. The cells were washed and treated with low (2 mg/ml) or high (4 mg/ml) doses of Fg or Fg (4 mg/ml) with anti-ICAM-1 (50 μg/ml) antibody for 24 h. Since Fg binding affinity to ICAM-1 is higher than to its other endothelial receptor, α5β1 integrin (D'souza et al., 1996), we tested binding of Fg to endothelial ICAM-1. Cells incubated with media alone, were used as a control group. After incubation, cells were washed twice with PBS and incubated with LPC (100 μg/ml lysophosphatidylcholine, dissolved in 3.7% formaldehyde) for 30 min at 4°C in the dark (Lominadze et al., 2006). Then, cells were washed and incubated with anti-occludin (1:500), anti-ZO-1 (1:250), or anti-ZO-2 (1:250) antibodies for 24 h. After incubation, cells were washed twice with PBS and incubated with the appropriate fluorescence-conjugated secondary antibodies (dilution 1: 500) for 1 h at 4°C in dark. Cell nuclei were labeled with DAPI (1:1,000), which was added to the wells for 15 min. Cells were washed with PBS and digital images of the cells were taken with a confocal microscope (Olympus FV1000, objective 100×). Cell nuclei were visualized using a HeNe-G laser (596 nm) to excite the dye, while emission was observed above 620 nm. Occludin and ZO-2 (Alexa-488) were visualized using a HeNe-G laser (495 nm) to excite the dye, while emission was observed above 519 nm. ZO-1 (Alexa 555) was visualized using a HeNe-G laser (578 nm) to excite the dye, while emission was observed above 603 nm.
In three experiments that were done in duplicate (two wells per experimental group), Fg-induced changes in content (total fluorescence intensity) and location of occludin, ZO-1, or ZO-2 were assessed for each experimental group by analyzing the total fluorescence intensity in four random fields (in each well) with image analysis software (Image-Pro Plus, Media Cybernetics). Fluorescence intensity values for each experimental group were averaged and presented as a percent of control (cells treated with media alone).
Statistical analysis
All data are expressed as mean ±SEM. The experimental groups were compared by one-way ANOVA. If ANOVA indicated a significant difference (P < 0.05), Tukey's multiple comparison test was used to compare group means. Differences were considered significant if P < 0.05.
Results
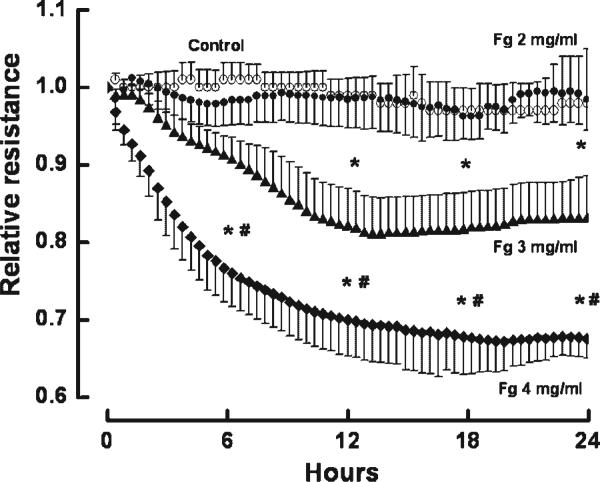
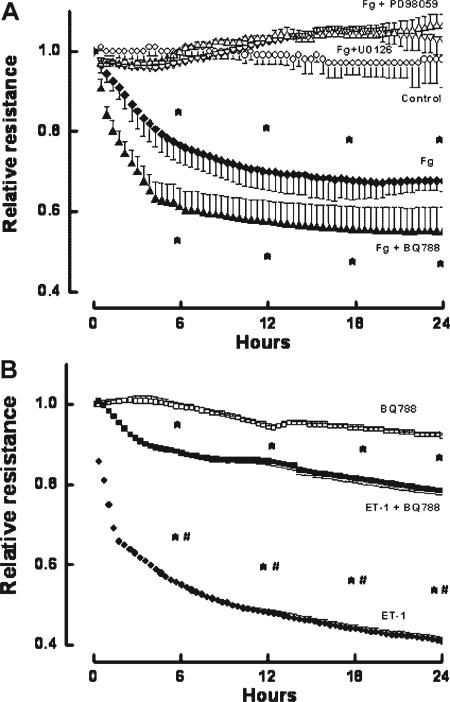
Fg induced a dose-dependent decrease in EC layer integrity as indicated by the decrease in TEER (Fig. 1). The decrease in EC integrity caused by the highest dose of Fg (4 mg/ml) was prevented by the presence of MEK inhibitors, PD98059 or U0126 (Fig. 2A). Since we showed that Fg may increase production of ET-1 (Lominadze et al., 2005), and that ET-1 decreases EC layer integrity (Filep et al., 1991; Lopez-Belmonte and Whittle, 1994), we tested whether endothelial layer integrity is altered by Fg, or if it is affected by ET-1 produced by Fg binding to ECs. Decreased EC integrity induced by Fg (4 mg/ml) was not altered by the ET-B receptor blocker BQ788 (Fig. 2A). In another series of experiments, EC integrity was drastically impaired by ET-1, but the ET-B receptor blocker, BQ788 prevented this drastic effect of ET-1 (Fig. 2B). However, BQ788, at the dose used in the present study, did not change EC layer integrity (Fig. 2B) or ameliorate ET-1-induced changes in EC integrity (Fig. 2B).
Fig. 1.
Changes in relative resistance of confluent rat heart microvascular endothelial cell monolayers after treatment with fibrinogen (Fg): 2 mg/ml (•), 3 mg/ml (▲), or 4 mg/ml (◆) for 24 h. Cells in the control group (○) were in media alone. Relative resistance was determined by comparing the resistance at the indicated times to the average resistance determined during 30 min prior to experiment. *P < 0.05 versus control. #P < 0.05 versus 3 mg/ml of Fg. Each point represents an average of three measurements in each group (n = 3).
Fig. 2.
Changes in relative resistance of confluent rat heart microvascular endothelial cell monolayers. A: Confluent cells were treated with 4 mg/ml of fibrinogen (Fg) (◆), 4 mg/ml Fg and 20 μM U0126 (Fg + U0126, △), 4 mg/ml Fg and 20 μM PD98059 (Fg + PD98059, ▽), or 4 mg/ml Fg and 5 μM BQ788 (Fg + BQ788, ▲). Cells in the control group (○) were in media alone. B: Confluent cells were treated with endothelin-1 (ET-1, 10 nM) (•), ET-1 and 5 μM of BQ788 (■), or 5 μM BQ788 alone (□). Relative resistance was determined by comparing the resistance at the indicated times to the average resistance determined during 30 min prior to experiment. *P < 0.05 versus control. #P < 0.05 versus ET-1 + BQ788. n = 3 for all groups.
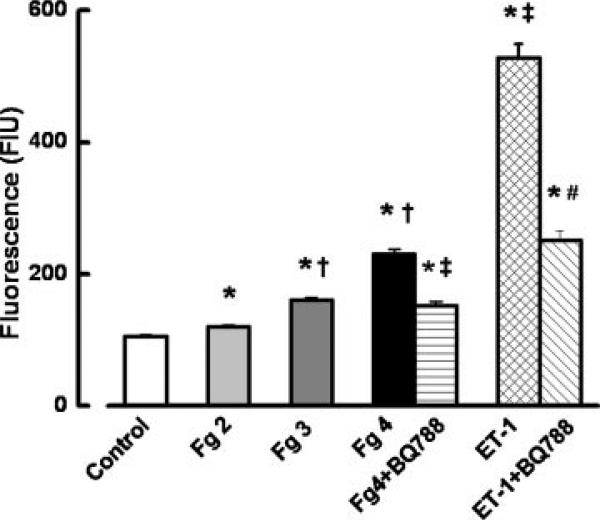
Fg also induced a dose-dependent increase in albumin leakage through the EC layer (Fig. 3). Treatment with BQ788 significantly inhibited the increased albumin leakage induced by 4 mg/ml of Fg (Fig. 3). ET-1 also increased albumin leakage (Fig. 3), which was significantly inhibited by BQ788 (Fig. 3). The ratio of the florescence intensity of media samples from the group treated with of Fg (4 mg/ml) and BQ788 to that treated with Fg (4 mg/ml) alone (0.67±0.03) was greater than the ratio of the florescence intensity of samples from the group treated with ET-1 and BQ788 to that treated with ET-1 alone (0.47±0.03). In the groups of ECs treated with 5 μM of BQ788 alone fluorescence intensity of BSA-488 measured in the lower chambers of Transwells was a 129±9 FIU.
Fig. 3.
Fg-induced albumin leakage through the endothelial cell monolayer. Fluorescence intensity of bovine serum albumin conjugated with FITC (BSA-488) in lower chambers of Transwells was measured by fluorimetry and presented as fluorescence intensity units (FIU). The following treatment groups were compared: media only (control), 2 mg/ml of Fg (Fg 2), 3 mg/ml of Fg (Fg 3), 4 mg/ml of Fg (Fg 4), 4 mg/ml of Fg and 5 μM BQ788 (Fg + BQ788), 10 nM ET-1, and 10 nM ET-1 with 5 μM BQ788 (ET-1 + BQ788). The cells were treated for 24 h. *P < 0.05 versus control. †P < 0.05 versus lower dose of Fg. ‡P < 0.05 versus 4 mg/ml of Fg. #P < 0.05 versus ET-1. n = 4 for all groups.
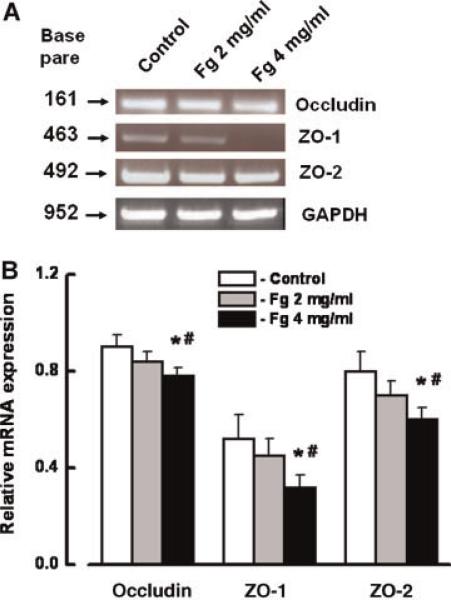
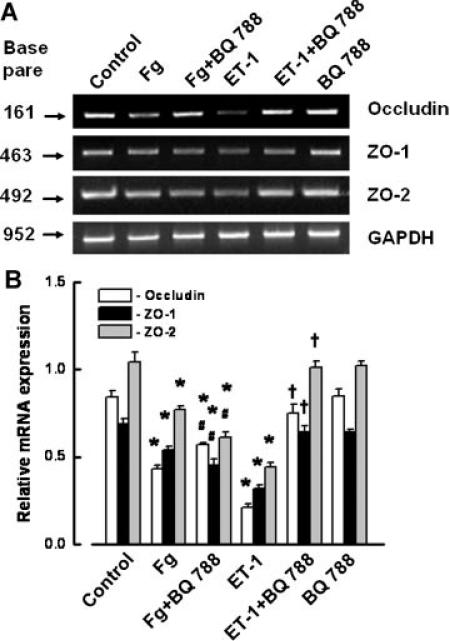
Expression of mRNAs for occludin, ZO-1, and ZO-2 was significantly less after treatment of the cells with a high concentration of Fg (4 mg/ml) for 24 h (Fig. 4). Although BQ788 slightly reversed the decreased expression of mRNAs for occludin, ZO-1, and ZO-2, they were still significantly less than in control (Fig. 5A,B). ET-1 also induced a significant decrease in expression of mRNAs for occludin, ZO-1, and ZO-2 (Fig. 5A,B). However, this ET-1-induced decreased expression of mRNAs of occludin, ZO-1, and ZO-2 was restored to control values by BQ788 (Fig. 5A,B). BQ788 did not affect expression of mRNAs of occludin, ZO-1, and ZO-2 (Fig. 5A,B).
Fig. 4.
Expression of occludin, zona occluden-1 (ZO-1), and zona occluden-2 (ZO-2) mRNA in endothelial cells (ECs) after tretament with 2 and 4 mg/ml of Fg for 24 h. A: RT-PCR shows changes in mRNA expression of occludin, ZO-1, and ZO-2 after tretament with 2 or 4 mg/ml of Fg. B: Relative mRNA expression of occludin, ZO-1, and ZO-2. Relative mRNA expression of each band is reported as a ratio of integrated optical density (IOD) of each band to the IOD of the respective GAPDH band. *P < 0.05 versus respective control. #P < 0.05 versus respective 2 mg/ml of Fg. n = 3 for all groups. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Fig. 5.
Effect of endothelin-1 (ET-1) in 4 mg/ml of Fg-induced changes in expression of mRNA of occludin, zona occluden-1 (ZO-1), and zona occluden-2 (ZO-2) in endothelial cells. A: RT-PCR shows changes in mRNA expression of occludin, ZO-1, and ZO-2 after tretament with 4 mg/ml of Fg (Fg), 4 mg/ml of Fg and 5 μM BQ788 (Fg + BQ788), 10 nM ET-1, 10 nM ET-1 with 5 μM BQ788 (ET-1 + BQ788), and 5 μM BQ788. B: Relative mRNA expression of occludin, ZO-1, and ZO-2. Relative mRNA expression of each band is reported as a ratio of integrated optical density (IOD) of each band to the IOD of the respective GAPDH band. *P < 0.05 versus respective control. #P < 0.05 versus respective Fg. †P < 0.05 versus respective ET-1. n = 3 for all groups.
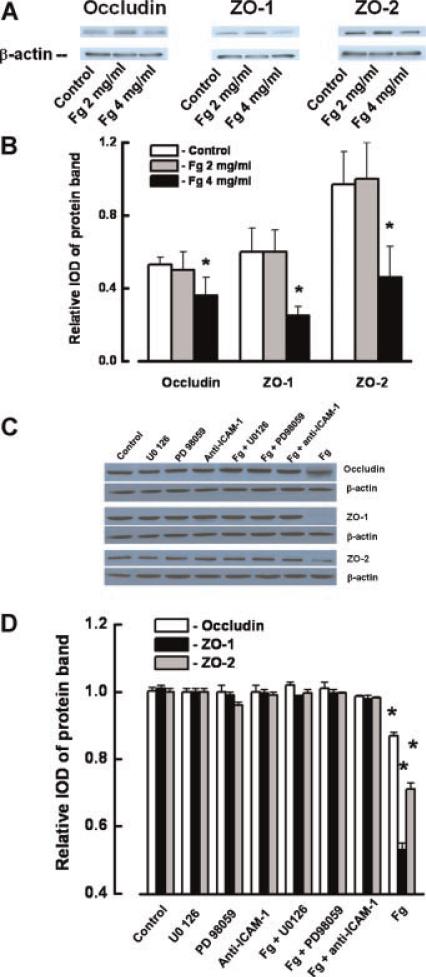
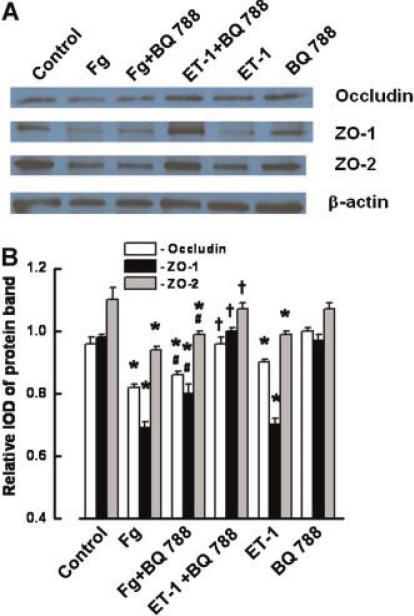
Treatment of ECs with Fg (4 mg/ml) also caused a significant decrease in the protein contents of occludin, ZO-1, and ZO-2 (Fig. 6A,B). This decrease was prevented by the presence of PD98059, U0126, or anti-ICAM-1 (Fig. 6C,D). Treatment of ECs with PD98059, U0126, or anti-ICAM-1antibody did not affect the content of occludin, ZO-1, and ZO-2 proteins in the ECs (Fig. 6C,D). Although BQ788 slightly reversed Fg-induced decreased expressions of occludin, ZO-1, and ZO-2, they were still significantly less than in control (Fig. 7A,B). However, BQ788 restored the expressions of occludin, ZO-1, and ZO-2, which were decreased by ET-1 (Fig. 7A,B). BQ788 had no effect on protein expression of occludin, ZO-1, and ZO-2 (Fig. 7A,B).
Fig. 6.
Changes in occludin, zona occluden-1 (ZO-1), and zona occluden-2 (ZO-2) protein contents in endothelial cells (EC) after tretament with fibrinogen (Fg). A: Changes in content of occludin, ZO-1, and ZO-2 in ECs after tretament with 2 or 4 mg/ml of Fg shown by Western blot analysis. B: Relative protein expression of occludin, ZO-1, and ZO-2. C: Changes in protein expression of occludin, ZO-1, and ZO-2 in ECs were determined by Western blot analysis after treatment with medium alone (control), U0126, PD98059 (20 μM each), anti-ICAM-1 antibody (50 μg/ml), 4 mg/ml of Fg (Fg), or 4 mg/ml of Fg in the presence of 20 μM of U0126 (Fg + U0126), 20 μM of PD98059 (Fg + PD98059), or 50 μg/ml of anti-ICAM-1 antibody (Fg + anti-ICAM-1) for 24 h. D: Relative protein expression of occludin, ZO-1, and ZO-2 for the tretament groups shown in C. Relative protrein expression is reported as a ratio of integrated optical density (IOD) of each band to the IOD of the respective β-actin band. *P < 0.05 versus respective control. n = 3 for all groups. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Fig. 7.
Effect of endothelin-1 (ET-1) in 4 mg/ml of Fg-induced changes in expression of occludin, zona occluden-1 (ZO-1), and zona occluden-2 (ZO-2) in endothelial cells. A: Expression of occludin, ZO-1, and ZO-2 after tretament with 4 mg/ml of Fg (Fg), 4 mg/ml of Fg and 50 μg/ml BQ788 (Fg + BQ788), 10 nM of ET-1, ET-1 (10 nM) with 50 μg/ml of BQ788 (ET-1 + BQ788), and 50 μg/ml BQ788 shown by Wetsrn blot analysis. B: Relative protein expression of occludin, ZO-1, and ZO-2. Relative protrein expression is reported as a ratio of integrated optical density (IOD) of each band to the IOD of the respective β-actin band. *P < 0.05 versus respective control. #P < 0.05 versus respective Fg. †P < 0.05 versus respective ET-1. n = 3 for all groups. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
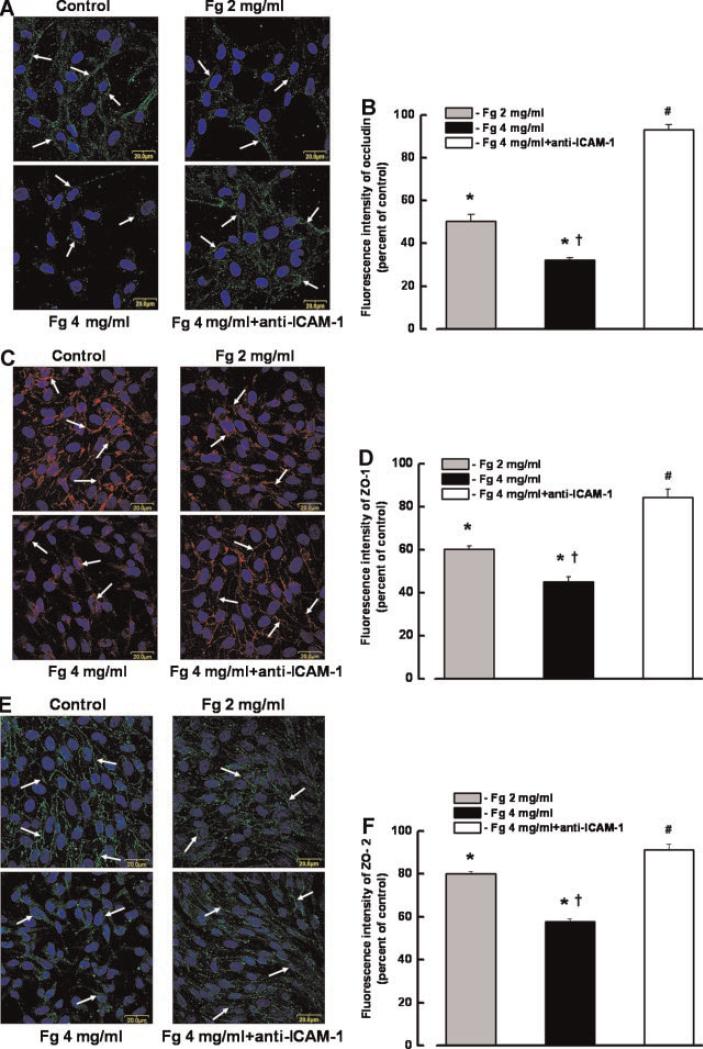
Confocal microscopy showed that Fg caused dose-dependent alterations of occludin, ZO-1, and ZO-2 expression and localization in ECs, which were prevented by the presence of anti-ICAM-1 antibody (Fig. 8A–F, respectively). Treatment of RHMECs with a high dose of Fg (4 mg/ml) for 24 h, induced a decrease in contents and translocation of ZO-1 and ZO-2 proteins into the cellular cytosol (Fig. 8C–F, respectively). Translocation of ZO-2 into the cytosol (Fig. 8E) was more pronounced than that of ZO-1 (Fig. 8C).
Fig. 8.
Fibrinogen (Fg)-induced changes in occludin, zona occluden-1 (ZO-1), and ZO-2 content and their locations in endothelial cells (ECs). A,C,E: Confocal images show a visible decrease of occludin (green, A), ZO-1 (red, C), and ZO-2 (green, E) (indicated by arrows) in ECs after treatment with 4 mg/ml of Fg, which were restored to normal in the presence of anti-ICAM-1 antibody (50 μg/ml). EC nuclei are indicated by blue color. B,D,F: Comparison of fluorescence (green for occludin, red for ZO-1, and green for ZO-2) intensity changes in ECs are presented by the histogram. *P < 0.05 versus control, †P < 0.05 versus 2 mg/ml Fg; #P < 0.05 versus 4 mg/ml Fg. n = 3 for all groups.
Discussion
A pathologically high concentration of Fg impaired EC layer integrity by affecting endothelial tight junction proteins. Inhibition of this Fg-induced impairment of IEJ integrity with MEK inhibitors (PD98059 and U0126) suggests that Fg binding to its endothelial receptors (e.g., ICAM-1) may activate ERK signaling. This signaling triggers alterations in a paracellular transport mechanism, such as that described in a previous study (Tyagi et al., 2008). Fg binding to its endothelial receptor, ICAM-1 (Altieri et al., 1995; D'souza et al., 1996), causes vasoconstriction (Lominadze et al., 2005) and leads to ICAM-1-induced activation of ERK (Lawson et al., 1999; Hubbard and Rothlein, 2000) and formation of F-actin (Tyagi et al., 2008). Formation of F-actin may cause stiffening of the cells and opening of IEJs, therefore facilitating an increase in EC layer permeability (Ehringer et al., 1999; Lominadze et al., 2004; Gordon et al., 2005; Lominadze et al., 2006).
Since ET-1 affects vascular integrity (Filep et al., 1991; Lopez-Belmonte and Whittle, 1994), we tested if endothelial monolayer permeability and tight junctional integrity were altered by a high content of Fg (Tyagi et al., 2008), or by ET-1 released in response to Fg binding to ECs (Sen et al., 2009). The role of Fg in alteration of rat cardiac IEJs was clearly shown by TEER measurements and suggest that Fg can be involved in impairment of paracellular transport seen previously (Tyagi et al., 2008). In the present study, Fg decreased TEER in the EC layer in a dose-dependent fashion and was not affected by the presence of an endothelial ET-1 receptor (ETB receptor) blocker, BQ788. In contrast, the decrease of EC integrity caused by ET-1 was inhibited by BQ788. Although Fg and ET-1 are capable of affecting EC layer integrity, this result suggests that Fg has an effect independent of ET-1. Therefore, it is plausible that an effect of Fg on EC integrity can be augmented by the effect of ET-1, when the concentration of Fg is elevated, and more ET-1 is produced (Sen et al., 2009).
The results of Fg-induced alterations of IEJs were confirmed by functional studies of the Fg effect on EC layer permeability to albumin. Albumin leakage induced by Fg (4 mg/ml) or by ET-1 (10 nM) was significantly decreased by the presence of BQ788. However, the effect of BQ788 was greater in the cells treated with ET-1 than in those treated with Fg. These results suggest that Fg itself may induce macromolecular leakage through the EC layer and that this leakage can be exacerbated by ET-1 produced in response to increased content of Fg (Sen et al., 2009).
We did not observe a difference in EC layer permeability to albumin between the groups with or without 440 μM of BSA (data not shown). This finding agrees with a study where exposure of EC to high shear stress (4 Pa) for 35 h resulted in increased albumin leakage through the EC layer (Kudo et al., 2005), suggesting that albumin may induce leakage through EC layer only after prolonged exposure of the cells to a high shear stress. Therefore, the use of albumin in our study is consistent with another in vitro model that has been used to study rheological properties of blood. In the present study we used cultured ECs and experiments were done with static conditions. Therefore, an effect of shear stress imposed by the blood flow in vasculature was not addressed. Separate studies using animals overexpressing occludin, ZO-1, or ZO-2 genes are needed to further define the correlation between the Fg-induced permeability and a role of these TJPs.
The present results support our previous finding that Fg increases paracellular transport (Tyagi et al., 2008). Others showed that ET-1 did not affect transendothelial [125I]-human serum albumin flux in EC monolayers (Horgan et al., 1991; Rodman et al., 1992). However, ET-1 enhances microvascular permeability (Filep et al., 1991; Lopez-Belmonte and Whittle, 1994). Since Fg may be involved in regulating production of ET-1 (Lominadze et al., 2005), the effect of Fg on EC paracellular transport may be enhanced by the presence of ET-1. The present experiments suggest a mechanism of increased EC layer permeability to albumin caused by a pathologically high content of Fg (Tyagi et al., 2008). However, the possible involvement of transendothelial transport is not ruled out.
It is well known that thrombin converts Fg to fibrin. We previously used a thrombin activity blocker, huridin, to eliminate a possible effect of fibrin formation, or of thrombin itself, during Fg-induced EC permeability experiments (Tyagi et al., 2008). Results from cells treated with Fg in the presence or absence of huridin (0.1 U/ml) were not different, suggesting an absence of functionally active thrombin, and therefore, fibrin formation in the Fg-treated groups (Tyagi et al., 2008). In addition, the purity of Fg before and after the experiments, was not compromised as demonstrated by Western blot analyses (Lominadze et al., 2005; Tyagi et al., 2008). Therefore, it is reasonable to conclude that the effects observed in the present study were due to undegraded Fg.
Our observation of decreased expression of occludin suggests that an elevated content of Fg may first affect an EC junction protein closest to the blood stream. Occludin, a tight junction protein located at the apical side of a cell (Mehta and Malik, 2006) is bound to the main cytoskeletal protein, actin, through proteins of the ZO family (Mehta and Malik, 2006). Decreased mRNA expression was accompanied by decreased protein content for occludin, ZO-1, and ZO-2 induced by elevated Fg. Association of a decreased content of occludin with formation of gaps between the ECs has clearly been shown by others (Reijerkerk et al., 2006). In the present study, the decreased expression of protein and mRNA for occludin, ZO-1, and ZO-2 were confirmed by confocal microscopy. Contents of occludin, ZO-1, and ZO-2 were significantly lower in the presence of 4 mg/ml of Fg. In addition, they (mainly ZO-1) were also translocated into the cell cytosol. These results suggest that Fg-induced formation of F-actin, which leads to stiffening of the cells and pulling of the cell edges inward, may be linked to decreased ZO protein expression and translocation of the ZO proteins from the actin cytoskeleton to the cytosol (Ehringer et al., 1999; Gordon et al., 2005; Lominadze et al., 2004, 2006). Decreased expression of ZO-1 and ZO-2 in response to increased Fg concentration helps to clarify the mechanism of Fg-induced enhanced formation of F-actin (Tyagi et al., 2008).
Although the endothelial ET-1 receptor (ETB receptor) blocker, BQ788, did not significantly affect Fg-induced decreases in protein and mRNA expression of occludin, ZO-1, or ZO-2, it inhibited their downregulation by ET-1. Enhanced production of ET-1 during an increase in Fg content (Sen et al., 2009) may exacerbate the effect of Fg. We were previously unable to show a role of the endothelin type A (ETA) receptor in Fg-induced production of ET-1 from ECs (Sen et al., 2009). Therefore, it appears that ETA receptor is not involved in Fg-mediated processes in microvascular ECs (Sen et al., 2009).
In the present study, a pathologically high content of Fg (4 mg/ml) compromised EC layer integrity, as indicated by changes in TEER. We also showed that the higher content of Fg led to downregulation of occludin, ZO-1, and ZO-2 proteins. These results clearly suggest that Fg-induced alterations in EC layer integrity can be a result of Fg-induced downregulation of occludin, ZO-1, and ZO-2 protein expression. Since these TJPs are associated with EC actin filaments, it is possible that their downregulation by a high content of Fg (4 mg/ml) could be involved in Fg-induced increased EC layer permeability, which has been reported (Tyagi et al., 2008). However, the results of the present study do not allow a direct conclusion that TJPs such as occludin, ZO-1, or ZO-2 can have a role in Fg-induced increased EC layer permeability. It is important to determine if an increased content of Fg can affect the integrity of the ECs that over-express occludin or ZO proteins. However, this goes beyond of the scope of the present study and requires separate investigation devoted to this specific question.
Mitogen-activated protein kinase kinase (MEK) is phosphorylated and activated by Raf. Once activated, MEK phosphorylates and activates ERK (Kolch, 2000). PD9805 and U0126 used in the present study are chemically unrelated MEK/ERK inhibitors and have different mechanisms of action (Alessi et al., 1995; Favata et al., 1998). While PD98059 binds to the inactive forms of MEK and prevents its activation by upstream activators such as Raf (Alessi et al., 1995), U0126 binds to the activated form of MEK (Favata et al., 1998). Formation of F-actin can occur through ERK signaling (Chandrasekar et al., 2003; Bourguignon et al., 2005). In addition, occludin binds to extracellular signal-regulated kinase (ERK) (Basuroy et al., 2006). Our data show that Fg-induced alterations in EC integrity and in the content of TJPs may be regulated by ERK signaling. These results, in combination with our previous data showing increased albumin leakage through the EC layer in the presence of 4 mg/ml of Fg (Tyagi et al., 2008), suggest that binding of Fg to its endothelial receptor, ICAM-1, activates MEK/ERK signaling. However, the possible effect of Fg interaction with another of its endothelial receptors, α5β1 integrin, should not be ruled out. The latter is involved in vasoconstriction (Mogford et al., 1997) and in Fg-induced increased EC layer permeability (Tyagi et al., 2008).
We found that MEK/ERK inhibitors, PD9805 or U0126, decreased Fg-induced downregulation of occludin, ZO-1 and ZO-2 expression. These results indicate that the mechanism for changes in expression of these TJPs, their subcellular localization, and decreased EC layer integrity induced by Fg binding to endothelial ICAM-1 involve ERK signaling. This agrees with our previous finding that an increased content of Fg activates ERK signaling, which is activated further by enhanced production of ET-1 as a result of the increased Fg concentration (Sen et al., 2009). Together, these findings indicate that Fg binding to its endothelial receptor, ICAM-1, activates MEK/ERK signaling and induces downregulation of occludin, ZO-1, and ZO-2 proteins. These events compromise IEJ integrity, and enhance production of ET-1. ET-1 further activates MEK/ERK signaling and exacerbates downregulation of the TJPs, causing an even greater impairment in EC layer integrity.
Increased blood Fg concentration is typical of many cardiovascular and cerebrovascular diseases (Ernst and Resch, 1993; Danesh et al., 1998; Chae et al., 2001; Danesh et al., 2005), which are accompanied by increased EC permeability (Parving et al., 1977; Laine, 1988). Increased EC layer permeability through formation of F-actin can be induced by an elevated level of Fg (Tyagi et al., 2008). The present investigation extends these observations by showing that increased Fg content compromises EC layer integrity by downregulating endothelial TJPs through MEK/ERK signaling.
Acknowledgments
Supported in part by NIH grants to D.L. (HL-80394) and to S.C.T. (HL-71010 and NS-051568).
Contract grant sponsor: NIH
Contract grant numbers: HL-80394, HL-71010, NS-051568.
Abbreviations
- Fg
fibrinogen
- EC
endothelial cell
- ET-1
endothelin-1
- ERK-1/2
extracellular signal-regulated kinase-1/2
- IEJ
inter-endothelial junctions
- ICAM-1
intercellular adhesion molecule-1
- TJP
tight junction proteins
- ZO-1
zona occluden-1
- ZO-2
zona occluden-2
Footnotes
Phani K. Patibandla and Neetu Tyagi contributed equally to this work.
Literature Cited
- Alessi D, Cuenda A, Cohen P, Dudley D, Saltiel A. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- Altieri D, Duperray A, Plescia J, Thornton G, Languino L. Structural recognition of a novel fibrinogen gamma chain sequence (117–133) by intercellular adhesion molecule-1 mediates leukocyte-endothelium interaction. J Biol Chem. 1995;270:696–699. doi: 10.1074/jbc.270.2.696. [DOI] [PubMed] [Google Scholar]
- Basuroy S, Seth A, Elias B, Naren A, Rao R. MAPK interacts with occludin and mediates EGF-induced prevention of tight junction disruption by hydrogen peroxide. Biochem J. 2006;393:69–77. doi: 10.1042/BJ20050959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon L, Gilad E, Rothman K, Peyrollier K. Hyaluronan-CD44 interaction with IQGAP1 promotes Cdc42 and ERK signaling, leading to actin binding, Elk-1/estrogen receptor transcriptional activation, and ovarian cancer progression. J Biol Chem. 2005;280:11961–11972. doi: 10.1074/jbc.M411985200. [DOI] [PubMed] [Google Scholar]
- Chae C, Lee R, Rifai N, Ridker P. Blood pressure and inflammation in apparently healthy men. Hypertension. 2001;38:399–403. doi: 10.1161/01.hyp.38.3.399. [DOI] [PubMed] [Google Scholar]
- Chandrasekar N, Mohanam S, Lakka SS, Dinh DH, Olivero WC, Gujrati M, Rao JS. Glial cell-induced endothelial morphogenesis is inhibited by interfering with extracellular signal-regulated kinase signaling. Clin Cancer Res. 2003;9:2342–2349. [PubMed] [Google Scholar]
- Cooper J, Del Vecchio P, Minnear F, Burhop K, Selig W, Garcia J, Malik A. Measurement of albumin permeability across endothelial monolayers in vitro. J Appl Physiol. 1987;62:1076–1083. doi: 10.1152/jappl.1987.62.3.1076. [DOI] [PubMed] [Google Scholar]
- Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: Meta-analyses of prospective studies. JAMA. 1998;279:1477–1482. doi: 10.1001/jama.279.18.1477. [DOI] [PubMed] [Google Scholar]
- Danesh J, Lewington S, Thompson SG, Lowe GD, Collins R, Kostis JB, Wilson AC, Folsom AR, Wu K, Benderly M, Goldbourt U, Willeit J, Kiechl S, Yarnell JW, Sweetnam PM, Elwood PC, Cushman M, Psaty BM, Tracy RP, Tybjaerg-Hansen A, Haverkate F, de Maat MP, Thompson SG, Fowkes FG, Lee AJ, Smith FB, Salomaa V, Harald K, Rasi R, Vahtera E, Jousilahti P, Pekkanen J, D'Agostino R, Kannel WB, Wilson PW, Tofler G, Arocha-Pinango CL, Rodriguez-Larralde A, Nagy E, Mijares M, Espinosa R, Rodriquez-Roa E, Ryder E, Diez-Ewald MP, Campos G, Fernandez V, Torres E, Coll E, Marchioli R, Valagussa F, Rosengren A, Wilhelmsen L, Lappas G, Eriksson H, Cremer P, Nagel D, Curb JD, Rodriguez B, Yano K, Salonen JT, Nyyssonen K, Tuomainen TP, Hedblad B, Lind P, Loewel H, Koenig W, Meade TW, Cooper JA, De Stavola B, Knottenbelt C, Miller GJ, Cooper JA, Bauer KA, Rosenberg RD, Sato S, Kitamura A, Naito Y, Iso H, Salomaa V, Harald K, Rasi V, Vahtera E, Jousilahti P, Palosuo T, Ducimetiere P, Amouyel P, Arveiler D, Evans AE, Ferrieres J, Juhan-Vague I, Bingham A, Schulte H, Assmann G, Cantin B, Lamarche B, Despres JP, Dagenais GR, Tunstall-Pedoe H, Woodward M, Ben Shlomo Y, Davey SG, Palmieri V, Yeh JL, Meade TW, Rudnicka A, Knottenbelt C, Ridker P, Rodeghiero F, Tosetto A, Shepherd J, Ford I, Robertson M, Brunner E, Shipley M, Feskens EJ, Kromhout D, Fibrinogen SC. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: An individual participant meta-analysis. JAMA. 2005;294:1799–1809. doi: 10.1001/jama.294.14.1799. [DOI] [PubMed] [Google Scholar]
- D'Erasmo E, Acca M, Celi F, Medici F, Palmerini T, Pisani D. Plasma fibrinogen and platelet count in stroke. J Med. 1993;24:185–191. [PubMed] [Google Scholar]
- D'Souza S, Byers-Ward V, Gardiner E, Wang H, Sung S-S. Identification of an active sequence within the first immunoglobulin domain of intercellular cell adhesion molecule-1 (ICAM-1) that interacts with fibrinogen. J Biol Chem. 1996;271:24270–24277. doi: 10.1074/jbc.271.39.24270. [DOI] [PubMed] [Google Scholar]
- Ehringer W, Yamany S, Steier K, Farag A, Roisen F, Dozier A, Miller F. Quantitative image analysis of F-actin in endothelial cells. Microcirculation. 1999;6:291–303. [PubMed] [Google Scholar]
- Ernst E, Resch K. Fibrinogen as a cardiovascular risk factor: A meta-analysis and review of the literature. Ann Intern Med. 1993;118:956–963. doi: 10.7326/0003-4819-118-12-199306150-00008. [DOI] [PubMed] [Google Scholar]
- Favata M, Horiuchi K, Manos E, Daulerio A, Stradley D, Feeser W, Van Dyk D, Pitts W, Earl R, Hobbs F, Copeland R, Magolda R, Scherle P, Trzaskos J. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- Filep J, Sirois M, Rousseau A, Sirois P. Effects of endothelin-1 on vascular permeability in the conscious rat: Interactions with platelet-activating factor. Br J Pharmacol. 1991;104:797–804. doi: 10.1111/j.1476-5381.1991.tb12509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S, Lominadze D, Saari J, Lentsch A, Schuschke D. Impaired deformability of copper-deficient neutrophils. Exp Biol Med. 2005;230:543–548. doi: 10.1177/153537020523000805. [DOI] [PubMed] [Google Scholar]
- Horgan M, Pinheiro J, Malik A. Mechanism of endothelin-1-induced pulmonary vasoconstriction. Circ Res. 1991;69:157–164. doi: 10.1161/01.res.69.1.157. [DOI] [PubMed] [Google Scholar]
- Hubbard A, Rothlein R. Intercellular adhesion molecule-1 (ICAM-1) expression and cell signaling cascades. Free Rad Biol Med. 2000;28:1379–1386. doi: 10.1016/s0891-5849(00)00223-9. [DOI] [PubMed] [Google Scholar]
- Humphries S. Genetic-regulation of fibrinogen. Eur Heart J. 1995;16:16–20. doi: 10.1093/eurheartj/16.suppl_a.16. [DOI] [PubMed] [Google Scholar]
- Kerlin B, Cooley BC, Isermann BH, Hernandez I, Sood R, Zogg M, Hendrickson SB, Mosesson MW, Lord S, Weiler H. Cause-effect relation between hyperfibrinogenemia and vascular disease. Curr Opin Hematol. 2004;103:1728–1734. doi: 10.1182/blood-2003-08-2886. [DOI] [PubMed] [Google Scholar]
- Kolch W. Meaningful relationships: The regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem J. 2000;351:289–305. [PMC free article] [PubMed] [Google Scholar]
- Kudo S, Tsuzaka M, Ikeda M, Tanishita K. Albumin permeability across endothelial monolayers under long-term shear stress. JSME Int J Ser C. 2005;48:419–442. [Google Scholar]
- Laine GA. Microvascular changes in the heart during chronic arterial hypertension. Circ Res. 1988;62:953–960. doi: 10.1161/01.res.62.5.953. [DOI] [PubMed] [Google Scholar]
- Lawson C, Ainsworth M, Yacoub M, Rose M. Ligation of ICAM-1 on endothelial cells leads to expression of VCAM-1 via a nuclear factor-{kappa}B-independent mechanism. J Immunol. 1999;162:2990–2996. [PubMed] [Google Scholar]
- Lee A, Lowe G, Woodward M, Tunstall-Pedoe H. Fibrinogen in relation to personal history of prevalent hypertension, diabetes, stroke, intermittent claudication, coronary heart disease, and family history: The Scottish Heart Health Study. Br Heart J. 2007;69:338–342. doi: 10.1136/hrt.69.4.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letcher R, Chien S, Pickering T, Sealey J, Laragh J. Direct relationship between blood pressure and blood viscosity in normal and hypertensive subjects. Role of fibrinogen and concentration. Am J Med. 1981;70:1195–1202. doi: 10.1016/0002-9343(81)90827-5. [DOI] [PubMed] [Google Scholar]
- Lincoln DW, Larsen AM, Phillips PG, Bove K. Isolation of murine aortic endothelial cells in culture and the effects of sex steroids on their growth. In Vitro Cell Dev Biol Anim. 2003;39:140–145. doi: 10.1007/s11626-003-0008-x. [DOI] [PubMed] [Google Scholar]
- Lominadze D, Joshua I, Schuschke D. Increased erythrocyte aggregation in spontaneously hypertensive rats. Am J Hypertens. 1998;11:784–789. doi: 10.1016/s0895-7061(98)00056-9. [DOI] [PubMed] [Google Scholar]
- Lominadze D, Schuschke D, Joshua I, Dean W. Increased ability of erythrocytes to aggregate in spontaneously hypertensive rats. Clin Exp Hypertens. 2002;24:397–406. doi: 10.1081/ceh-120005376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lominadze D, Saari J, Percival S, Schuschke D. Proinflammatory effects of copper deficiency on neutrophils and lung endothelial cells. Immunol Cell Biol. 2004;82:231–238. doi: 10.1046/j.1440-1711.2004.01231.x. [DOI] [PubMed] [Google Scholar]
- Lominadze D, Tsakadze N, Sen U, Falcone J, D'Souza S. Fibrinogen- and fragment D-induced vascular constriction. Am J Physiol. 2005;288:H1257–H1264. doi: 10.1152/ajpheart.00856.2004. [DOI] [PubMed] [Google Scholar]
- Lominadze D, Roberts A, Tyagi N, Tyagi S. Homocysteine causes cerebrovascular leakage in mice. Am J Physiol Heart Circ Physiol. 2006;290:H1206–H1213. doi: 10.1152/ajpheart.00376.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Belmonte J, Whittle B. The involvement of endothelial dysfunction, nitric oxide and prostanoids in the rat gastric microcirculatory responses to endothelin-1. Br J Pharmacol. 1994;112:267–271. doi: 10.1111/j.1476-5381.1994.tb13062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord S. Fibrinogen and fibrin: Scaffold proteins in hemostasis. Curr Opin Hematol. 2007;14:236–241. doi: 10.1097/MOH.0b013e3280dce58c. [DOI] [PubMed] [Google Scholar]
- Luscinskas F, Lawler J. Integrins as dynamic regulators of vascular function. FASEB J. 1994;8:929–938. doi: 10.1096/fasebj.8.12.7522194. [DOI] [PubMed] [Google Scholar]
- Mehta D, Malik A. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- Mogford J, Davis G, Meininger G. RGDN peptide interaction with endothelial alpha5beta1 integrin causes sustained endothelial-dependent vasoconstrictionto rat skeletal muscle arterioles. J Clin Invest. 1997;100:1647–1653. doi: 10.1172/JCI119689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parving H, Jensen H, Westrup M. Increased transcapillary escape rate of albumin and IgG in essential hypertension. Scand J Clin Lab Invest. 1977;37:223–227. doi: 10.3109/00365517709091486. [DOI] [PubMed] [Google Scholar]
- Plow E, Haas T, Zhang L, Loftus J, Smith J. Ligand binding to integrins. J Biol Chem. 2000;275:21785–21788. doi: 10.1074/jbc.R000003200. [DOI] [PubMed] [Google Scholar]
- Qiao R, Yan W, Lum H, Malik A. Arg-Gly-Asp peptide increases endothelial hydraulic conductivity: Comparison with thrombin response. AJP: Cell Physiol. 1995;269:C110–C117. doi: 10.1152/ajpcell.1995.269.1.C110. [DOI] [PubMed] [Google Scholar]
- Reijerkerk A, Kooij G, van der Pol SMA, Khazen S, Dijkstra CD, de Vries HE. Diapedesis of monocytes is associated with MMP-mediated occludin disappearance in brain endothelial cells. FASEB J. 2006;20:2550–2552. doi: 10.1096/fj.06-6099fje. [DOI] [PubMed] [Google Scholar]
- Rivas G, Fernandez J, Minton A. Direct observation of the self-association of dilute proteins in the presence of inert macromolecules at high concentration via tracer sedimentation equilibrium: Theory, experiment, and biological significance. Biochemistry. 1999;38:9379–9388. doi: 10.1021/bi990355z. [DOI] [PubMed] [Google Scholar]
- Rodman D, Stelzner T, Zamora M, Bonvallet S, Oka M, Sato K, O'Brien R, McMurtry I. Endothelin-1 increases the pulmonary microvascular pressure and causes pulmonary edema in salt solution but not blood-perfused rat lungs. J Cardiovasc Pharmacol. 1992;20:658–663. doi: 10.1097/00005344-199210000-00021. [DOI] [PubMed] [Google Scholar]
- Sen U, Tyagi N, Patibandla PK, Dean WL, Tyagi SC, Roberts AM, Lominadze D. Fibrinogen-induced endothelin-1 production from endothelial cells. AJP: Cell Physiol. 2009;296:C840–C847. doi: 10.1152/ajpcell.00515.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiruppathi C, Malik A, Del Vecchio P, Kees C, Giaever I. Electrical method for detection on endothelial cell shape change in real time: Assessment of endothelial barrier function. Proc Natl Acad Sci USA. 1992;89:7919–7923. doi: 10.1073/pnas.89.17.7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepat X, Grabulosa M, Buscemi L, Rico F, Farre R, Navajas D. Thrombin and histamine induce stiffening of alveolar epithelial cells. J Appl Physiol. 2005;98:1567–1574. doi: 10.1152/japplphysiol.00925.2004. [DOI] [PubMed] [Google Scholar]
- Tyagi N, Moshal KS, Tyagi SC, Lominadze D. γ-Aminbuturic acid a receptor mitigates homocysteine-induced endothelial cell permeability. Endothelium. 2007;14:315–323. doi: 10.1080/10623320701746164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi N, Roberts A, Dean W, Tyagi S, Lominadze D. Fibrinogen induces endothelial cell permeability. Mol Cell Biochem. 2008;307:13–22. doi: 10.1007/s11010-007-9579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi N, Gillespie W, Vacek J, Sen U, Tyagi S, Lominadze D. Activation of GABA-A receptor ameliorates homocysteine-induced MMP-9 activation by ERK pathway. J Cell Physiol. 2009;220:257–266. doi: 10.1002/jcp.21757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasse M, Paysant J, Soria J, Collet J, Vannier J, Soria C. Regulation of fibrinogen biosynthesis by cytokines, consequences on the vascular risk. Haemostasis. 1996;26:331–339. doi: 10.1159/000217313. [DOI] [PubMed] [Google Scholar]
- Zhao H, Joshua I, Porter J. Microvascular responses to endothelin in deoxycorticosterone acetate-salt hypertensive rats. Am J Hypertens. 2000;13:819–826. doi: 10.1016/s0895-7061(00)00260-0. [DOI] [PubMed] [Google Scholar]