Abstract
Background
Antihypertensive drugs with favorable metabolic effects on glucose and lipid levels are advocated for first-line therapy in hypertensive patients with metabolic/cardiometabolic syndrome (MetS). We compared outcomes by race in black and nonblack hypertensive individuals with and without MetS treated with a thiazide-type diuretic (chlorthalidone), a calcium channel blocker (amlodipine besylate), an α-blocker (doxazosin mesylate), or an angiotensin-converting enzyme inhibitor (lisinopril).
Methods
A post hoc subgroup analysis from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT), a randomized, double-blind, active-controlled hypertension treatment trial in 42 418 participants. We defined MetS as hypertension plus at least 2 of the following: fasting serum glucose level of at least 100 mg/dL, body mass index (calculated as weight in kilograms divided by height in meters squared) of at least 30 kg/m2, fasting triglyceride levels of at least 150 mg/dL, and high-density lipoprotein cholesterol levels of less than 40 mg/dL in men (or less than 50 mg/dL in women).
Results
Significantly higher rates of heart failure were consistent across all treatment comparisons in those with MetS. Relative risks (RRs) were 1.50 (95% confidence interval [CI], 1.18–1.90), 1.49 (95% CI, 1.17–1.90), and 1.88 (95% CI, 1.42–2.47) in black participants and 1.25 (95% CI, 1.06–1.47), 1.20 (95% CI, 1.01–1.41), and 1.82 (95% CI, 1.51–2.19) in nonblack participants for amlodipine, lisinopril, and doxazosin comparisons with chlorthalidone, respectively. Higher rates for combined cardiovascular disease were observed with lisinopril-chlorthalidone (RR, 1.24 [95% CI, 1.09–1.40] and 1.10 [95% CI, 1.02–1.19], respectively) and doxazosin-chlorthalidone comparisons (RR, 1.37 [95% CI, 1.19–1.58] and 1.18 [95% CI, 1.08– 1.30], respectively), in black and nonblack participants with MetS. Higher rates of stroke were seen in black participants only (RR, 1.37 [95% CI, 1.07–1.76] for the lisinopril-chlorthalidone comparison; RR, 1.49 [95% CI, 1.09–2.03] for the doxazosin-chlorthalidone comparison). Black patients with MetS also had higher rates of end-stage renal disease (RR, 1.70 1 [95% CI, 1.13– 2.55]) with lisinopril compared with chlorthalidone.
Conclusions
The ALLHAT findings fail to do not support the preference of for calcium channel blockers, α-blockers, or angiotensin-converting enzyme inhibitors compared with thiazide-type diuretics in patients with the MetS, despite their more favorable metabolic profiles. This was particularly true for black participants.
INTRODUCTION
Hypertensive patients with the Metabolic/Cardiometabolic Syndrome (MetS) are at especially high risk for complications of cardiovascular disease (CVD).(1–3) In addition, racial differences in the presentation of the MetS are well documented. For example, when compared to Caucasians, African-Americans with MetS have a higher prevalence of elevated blood pressure, type II diabetes, and obesity but lower triglyceride and higher HDL-cholesterol levels.(1) The primary management strategy for MetS includes lifestyle changes, optimizing blood pressure control, and reducing other cardiovascular risk factors. (1;2)
Despite the lack of supportive clinical outcome data, the use of antihypertensive drugs with a favorable metabolic profile [e.g., alpha-blockers, angiotensin converting enzyme (ACE)-inhibitors, and calcium channel blockers (CCBs)] has been advocated over classes of antihypertensive drugs with a less favorable profile (e.g., beta-blockers and thiazide-type diuretics).(4–7) Results from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) showed that neither an alpha-blocker, an ACE-inhibitor, nor a CCB was superior to a thiazide-type diuretic in preventing cardiovascular or renal events in the entire trial cohort or in subgroups stratified by race, diabetic status, or level of renal function.(8;9) Furthermore, ACE-inhibitors were significantly less effective in preventing several cardiovascular outcomes, particularly in Blacks.(9) However, it is unclear whether these agents might be more effective than diuretics in those with MetS. ALLHAT enrolled participants with hypertension and at least one additional coronary heart disease (CHD) risk factor, resulting in more than half meeting the definition for MetS. This report focuses on the effects by treatment group and race on cardiovascular and renal outcomes in ALLHAT participants with the MetS.
METHODS
The ALLHAT cohort consisted of men and women aged 55 years or older with stage 1 or stage 2 hypertension and at least 1 additional risk factor for CHD. ALLHAT participants (n=42,418), were randomly assigned to therapy with chlorthalidone (n=15,255), amlodipine (n=9,048), lisinopril (n=9,054), or doxazosin (n=9,061). Details of the ALLHAT study design have been previously published.(10) The study received appropriate review board approval, and all patients provided written informed consent.
For the purposes of this report, MetS at baseline was defined as hypertension, which all participants had at study entry, plus ≥2 of the following factors: glycemic disorder (fasting glucose ≥100 mg/dl, non fasting glucose ≥200 mg/dl, or history of diabetes), body mass index (BMI) ≥30, fasting triglycerides ≥150 mg/dl, or high density cholesterol (HDL) cholesterol <40 mg/dl in men or <50 mg/dl in women. This definition is consistent with that defined by the National Cholesterol Education Program except that BMI ≥30 was substituted for waist circumstance which was not collected during the trial – a validated substitution allowed by the WHO definition and previously used in a post-hoc analysis of a clinical trial or use and previously reported by others.(2;11)(12) Fasting glucose ≥100 rather than ≥110 mg/dl was used to reflect the changing definition of diabetes and impaired fasting glucose as reflected in the International Diabetes Federation definition of Mets.(13)
Blood pressure (BP) lowering was achieved by titrating the dose of the randomized (Step 1) study drug and by adding open-label step 2 (atenolol, clonidine, or reserpine) or step 3 (hydralazine) agents, as necessary, to obtain a SBP <140 mmHg.(10) All Step 1 (blinded) medications were identical in appearance. Follow-up visits were conducted at 1, 3, 6, 9, and 12 months, and then every 4 months thereafter for an average follow-up of 4.9 years for the diuretic versus ACE-inhibitor and CCB comparisons. However, upon recommendation of an independent review panel, the alpha-blocker arm of the trial was discontinued early resulting in a 3.2 yr average follow-up for the diuretic vs. alpha-blocker comparison.(14)
The primary outcome of the study was fatal CHD or nonfatal myocardial infarction (MI). Major secondary outcomes included (1) all-cause mortality, (2) fatal and nonfatal stroke, (3) combined CHD (primary outcome, coronary revascularization, or hospitalized angina), and (4) combined CVD (combined CHD, stroke, other treated angina, or heart failure [fatal, hospitalized, or treated non-hospitalized], or peripheral arterial disease). End-stage renal disease (ESRD) (dialysis, renal transplant or kidney disease death) and components of the major secondary outcomes were also pre-specified. Although not a pre-specified endpoint, we calculated changes in fasting glucose (FG) levels and the incidence of diabetes (FG>125 mg/dl) in the four treatment groups. Standardized procedures were employed for reporting and validating study outcomes.
Data were summarized as mean (SD) for continuous variables and number of subjects (percentage) for categorical variables. Baseline characteristics were compared in Black and non-Black participants with and without MetS using the Z-test for significance testing of continuous covariates and contingency table analyses for categorical data. Outcomes were analyzed using an intention-to-treat approach. The Cox proportional hazards model was used to determine time-to-event hazard ratios (HRs, 95% confidence intervals [CIs]). Cox test assumptions were examined using log-log plots and tests of treatment by time (time-dependent) interaction terms. When the assumptions were violated, a two-by-two table was used to estimate relative risk (RR).
The median follow-up for the doxazosin comparison with chlorthalidone was only 3.2 years because the doxazosin arm was terminated early in light of an increased cardiovascular risk compared with chlorthalidone (stroke 36% [p=0.001]; heart failure 80% [p<0.001]) and futility of achieving a statistically significant difference in the primary endpoint by the scheduled end of the trial. The shortened duration of follow-up for the diuretic vs. α-blocker comparison required a separate determination of the diuretic event rate for this comparison. Heterogeneity of treatment effects across MetS and race was examined by testing for treatment-covariate interaction with the proportional hazard model using a p<0.05. Analyses were unadjusted. Given the many subgroup and interaction analyses performed, statistical significance at the 0.05 level should be interpreted with caution. All statistical analyses were performed using STATA Version 9.0.
RESULTS
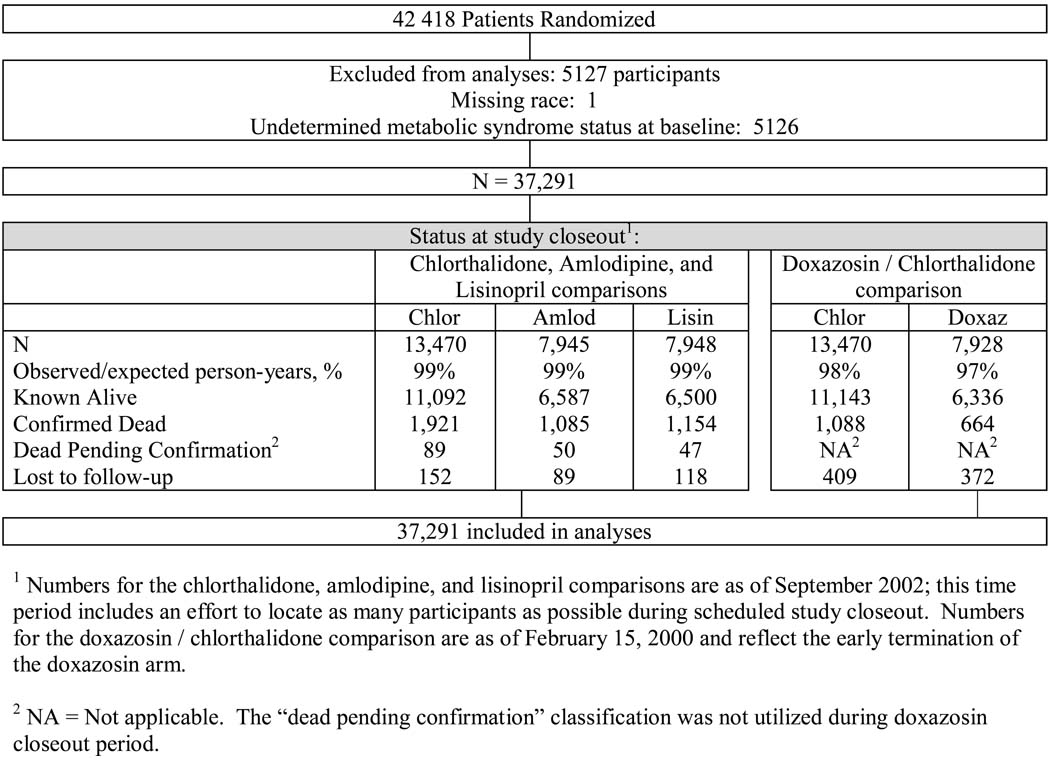
Baseline characteristics of the cohort group by MetS status, race and treatment group are outlined in Table 1, and follow-up is described in Figure 1. The criteria for MetS were met by 54% of ALLHAT participants (n=23,077). Although counterintuitive, ALLHAT participants without the MetS were more likely to qualify for the trial based upon the presence of CVD. This is because participants without diabetes at baseline (and less likely to be classified as having the MetS) had to have other CHD risk factors to qualify for ALLHAT. Participants with MetS from both race subgroups across randomized comparisons were more likely to be younger and female, and – as a result of the ALLHAT recruitment criteria – they were less likely to smoke, have LVH, or have a history of atherosclerotic cardiovascular disease (ASCVD). In Blacks with MetS, those randomized to amlodipine had significantly higher BMI and were more likely to be on aspirin than those in the chlorthalidone subgroup. Otherwise, baseline characteristics were similar across treatment groups. The ratio of observed to expected person-years in the trial was about 99% for comparisons of amlodipine and lisinopril with chlorthalidone, and 97–98% for comparisons of doxazosin with chlorthalidone.
Table 1.
Baseline Characteristics by Race and Metabolic Syndrome
| Black | Non-Black | ||||
|---|---|---|---|---|---|
| With MetS | Without MetS | With MetS | Without MetS | ||
| Number randomized[1] | 7,327 | 5,491 | 15,750 | 8,723 | |
| Age, Mean (SD) years | 65.4 (7.2) | 67.2 (8.1)** | 66.4 (7.3) | 68.4 (8.0)** | |
| 55–59, n (%) | 1,750 (23.9) | 1,032 (18.8)** | 3,021 (19.2) | 1,304 (15.0)** | |
| 60–69, n (%) | 3,575 (48.8) | 2,475 (45.1) | 7,617 (48.4) | 3,616 (41.5) | |
| 70–79, n (%) | 1,710 (23.3) | 1,530 (27.9) | 4,386 (27.9) | 2,975 (34.1) | |
| 80+, n (%) | 292 (23.3) | 454 (8.3) | 726 (4.6) | 828 (9.5) | |
| Women, n (%) | 4,456 (60.8) | 2,503 (45.6)** | 6,976 (44.3) | 3,459 (39.7)** | |
| Years of education, Mean (sd) | 10.2 (3.7) | 10.1 (4.0)* | 11.3 (4.1) | 11.6 (4.0)** | |
| Cigarette Smoking: | ** | ** | |||
| Current, n (%) | 1,428 (19.5) | 1,758 (32.0) | 2,684 (17.0) | 2,123 (24.3) | |
| Past, n (%) | 2,667 (36.4) | 1,818 (33.1) | 7,137 (45.3) | 3,609 (41.4) | |
| Never, n (%) | 3,232 (44.1) | 1,914 (34.9) | 5,929 (37.6) | 2,991 (34.3) | |
| BMI, mean (SD) mg/M2 | 33.3(6.5) | 27.0(5.0)** | 31.4(5.9) | 26.2(3.9)** | |
| Aspirin Use, n (%) | 1,832 (25.0) | 1,420 (25.9) | 6,431 (40.8) | 3,870 (44.4)** | |
| Antihypertensive treatment: | |||||
| Treated, n (%) | 6,757 (92.2) | 4,907 (89.4)** | 14,295 (90.8) | 7,724 (88.6)** | |
| Untreated, n (%) | 570 (7.8) | 584 (10.6) | 1,455 (9.2) | 999 (11.5) | |
| Atherosclerotic CVD, n (%) | 2,348 (32.1) | 2,306 (42.0)** | 7,238 (46.0) | 5,011 (57.5)** | |
| History MI or stroke, n (%) [2] | 1,287 (17.6) | 1,236 (22.5)** | 3,691 (23.4) | 2,425 (27.8)** | |
| History coronary revasc., n (%) [2] | 358 (4.9) | 307 (5.6) | 2,607 (16.6) | 1,569 (18.0)* | |
| Other Atherosclerotic CVD, n (%) [2,3] | 1,217 (16.6) | 1,206 (22.0)** | 3,695 (23.5) | 2,684 (30.8)** | |
| ST-T wave abnormality, n (%) [2] | 824 (11.5) | 725 (13.4)* | 1,272 (8.1) | 872 (10.0)** | |
| LVH by ECG or Echo, n (%) [2] | 1523(20.8) | 1886(34.4)** | 2049(13.0) | 1760(20.2)** | |
| Glycemic Status [4]: | ** | ** | |||
| Diabetes, n (%) | 4,860 (67.6) | 1,045 (19.1) | 8,040 (51.8) | 1,516 (17.5) | |
| Impaired fasting glucose, n (%) | 402 (5.6) | 122 (2.2) | 1,064 (6.9) | 173 (2.0) | |
| Normoglycemic, n (%) | 1,932 (26.9) | 4,308 (78.7) | 6,420 (41.4) | 7,000 (80.6) | |
| History of CHD at baseline, n (%) [2,5] | 1,180 (16.4) | 1,041 (19.2)** | 4,490 (28.6) | 2,849 (32.8)** | |
| Lipid trial participants, n (%) | 1,985 (27.1) | 1,474 (26.8) | 3,761 (23.9) | 2,249 (25.8)* | |
| Blood Pressure: | |||||
| SBP, mean (sd) mm Hg [1] | 146.2 (15.7) | 146.2 (16.0) | 146.0 (15.5) | 146.8 (15.5)** | |
| DBP, mean (sd) mm Hg | 84.4 (10.1) | 85.4 (10.1)** | 83.5 (10.0) | 83.7 (9.8) | |
| Serum potassium, mean (sd) mmol/L | 4.26 (0.76) | 4.29 (0.73) | 4.39 (0.64) | 4.41 (0.67) | |
| Fasting serum glucose, mean (sd) mg/dL | 144.2 (68.6) | 103.4 (45.5)** | 131.2 (57.1) | 101.8 (38.8)** | |
| Serum creatinine, mean (sd) mg/dl | 1.05 (0.35) | 1.08 (0.32)** | 0.99 (0.27) | 1.00 (0.26)* | |
| Total cholesterol, mean (sd) mg/dL | 219.7 (46.1) | 214.4 (41.3)** | 217.0 (43.6) | 212.4 (40.0)** | |
| LDL-cholesterol, mean (sd) mg/dL | 141.8 (40.5) | 136.2 (37.8)** | 134.6 (35.8) | 134.1 (34.1) | |
| HDL-cholesterol, mean (sd) mg/dL | 47.1 (13.4) | 57.2 (16.2)** | 40.1 (10.8) | 51.7 (14.6)** | |
| HDL-C <35 mg/dL, n (%) [2] | 591 (8.1) | 271 (4.9)** | 2,804 (17.8) | 745 (8.5)** | |
| Fasting triglycerides, mean (sd) mg/dL | 156.3 (109.2) | 102.3 (52.2)** | 224.3 (159.2) | 133.2 (94.5)** | |
SBP (p = 0.72) is the only variable in the table for which the Black versus Non-Black comparison is not statistically different. For each of the other variables the p-value for the Black vs. Non-Black comparison is ≤0.001.
Eligibility risk factors. For trial eligibility, participants had to have at least 1 other risk factor in addition to hypertension. Thus, the indicated risk factors are not mutually exclusive or exhaustive and may not represent prevalence.
Atherosclerotic CVD: history of angina pectoris; history of intermittent claudication, gangrene, or ischemic ulcers; history of transient ischemic attack; coronary, peripheral vascular, or carotid stenosis ≥50% documented by angiography or Doppler studies; ischemic heart disease documented by reversible or fixed ischemia on stress thalium or dipyridamole thalium, ST depression ≥1 mm for ≥1 minute on exercise testing or Holter monitoring; reversible wall motion abnormality on stress echocardiogram; ankle-arm index <0.9; abdominal aortic aneurysm detected by ultrasonography, computed tomography scan, or radiograph; carotid or femoral bruits.
Diabetes = History of diabetes at baseline or fasting glucose ≥126 mg/dL; Impaired fasting glucose = No history and baseline fasting glucose is 110 to 125 mg/dL inclusive; Normoglycemic = Not classified as impaired fasting glucose, no history and at least one of fasting glucose or non-fasting glucose is < 110 mg/dL.
CHD: known prior MI, angina, primary cardiac arrest, coronary stenosis >50%, reversible perfusion defect, prior coronary revascularization procedure.
p <0.05 , comparison of with/without MetS
p <0.001, comparison of with/without MetS
Figure 1.
Randomization and Follow-up of Participants in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT).
Blood Pressure Control (Table 2a, 2b)
Table 2.
| a. Blood Pressure and Biochemical Changes by Race and Treatment Group -- Participants With Metabolic Syndrome | ||||||||
|---|---|---|---|---|---|---|---|---|
| Black | Non-Black | |||||||
| Chlor (1) | Amlod | Lisin | Dox (2) | Chlor (1) | Amlod | Lisin | Dox (2) | |
| Fasting Glucose, mg/DL | ||||||||
| Mean (SD) | ||||||||
| Baseline | 145.5 (70.7) | 141.6 (66.0) | 145.5 (69.6) | 143.2 (66.7) | 131.5 (57.8) | 131.9 (58.1) | 130.7 (55.6) | 130.5 (56.2) |
| 2 years | 150.1 (73.1) | 147.1 (72.3) | 139.2 (68.0)* | 137.2 (66.9)* | 137.2 (60.1) | 130.2 (53.3)** | 130.1 (54.6)** | 126.9 (53.3)** |
| 4 years | 146.3 (72.7) | 141.8 (61.9) | 139.3 (72.0) | 130.1 (54.8) | 134.5 (56.8) | 132.3 (52.6) | 128.5 (50.5)* | 124.9 (52.2)* |
| 6 years | 133.0 (60.3) | 130.8 (55.1) | 135.9 (59.3) | - | 130.8 (48.5) | 130.9 (46.8) | 127.2 (46.7) | - |
| Potassium, mEq/L | ||||||||
| Mean (SD) | ||||||||
| Baseline | 4.27 (0.76) | 4.24 (0.77) | 4.28 (0.75) | 4.25 (0.74) | 4.37 (0.64) | 4.40 (0.66) | 4.40 (0.59)* | 4.41 (0.69)* |
| 2 years | 3.98 (0.67) | 4.26 (0.62)** | 4.38 (0.69)** | 4.28 (0.73)** | 4.07 (0.71) | 4.35 (0.63)** | 4.52 (0.71)** | 4.38 (0.59)** |
| 4 years | 4.10 (0.63) | 4.35 (0.73)** | 4.39 (0.59)** | 4.25 (0.57)** | 4.17 (0.65) | 4.45 (0.70)** | 4.61 (0.72)** | 4.46 (0.79)** |
| 6 years | 4.12 (0.61) | 4.40 (0.85)** | 4.44 (0.47)** | - | 4.24 (0.60) | 4.44 (0.49)** | 4.61 (0.52)** | - |
| Total Cholesterol, mg/dL | ||||||||
| Mean (SD) | ||||||||
| Baseline | 220.1 (46.5) | 221.6 (47.0) | 219.2 (47.1) | 218.1 (44.3) | 216.7 (43.8) | 218.4 (45.2) | 217.4 (42.5) | 216.0 (42.9) |
| 2 years | 209.6 (44.7) | 206.3 (45.9) | 205.9 (46.3) | 197.0 (42.7)** | 203.2 (41.3) | 201.7 (42.5) | 202.2 (43.2) | 195.2 (41.3)** |
| 4 years | 201.4 (44.0) | 200.2 (46.0) | 198.0 (42.3) | 189.3 (45.5)** | 194.3 (42.4) | 193.7 (40.7) | 193.4 (41.0) | 185.1 (36.9)** |
| 6 years | 191.1 (43.1) | 190.4 (43.3) | 190.3 (42.0) | - | 182.5 (41.4) | 184.3 (39.9) | 184.5 (40.8) | - |
| Total Cholesterol, Change from Baseline, mg/dL | ||||||||
| Mean (SD) | ||||||||
| 2 years | −10.1 (36.8) | −14.2 (40.3)* | −13.2 (41.0) | −21.0 (35.3)** | −12.8 (37.9) | −16.1 (39.7)* | −14.6 (39.2) | −19.8 (38.6)** |
| 4 years | −17.5 (40.5) | −19.7 (43.5) | −21.0 (42.1) | −28.0 (37.6)** | −20.5 (41.6) | −23.5 (41.9)* | −23.4 (42.5)* | −28.9 (37.7)** |
| 6 years | −28.3 (43.1) | −32.9 (45.7) | −29.8 (39.2) | - | −33.4 (45.1) | −33.2 (41.7) | −33.5 (43.6) | - |
| Systolic Blood Pressure, mm Hg | ||||||||
| Mean (SD) | ||||||||
| Baseline | 146.4(15.6) | 146.1(15.9) | 146.2(15.4) | 146.1(15.9) | 146.0(15.5) | 145.9(15.6) | 146.3(15.4) | 146.1(15.6) |
| 2 years | 137.9(17.1) | 139.1(15.5) | 143.4(19.1)** | 140.6(18.5)** | 135.4(15.3) | 136.4(14.3)* | 137.2(16.9)** | 137.2(16.2)** |
| 4 years | 135.5(16.5) | 136.9(15.5)* | 139.5(18.7)** | 138.9(17.7)* | 133.7(15.0) | 134.5(14.0)* | 134.1(15.9) | 136.3(16.5) |
| 6 years | 134.8(17.3) | 136.8(16.5) | 137.9(19.6)* | - | 133.5(15.3) | 134.1(14.7) | 131.8(16.6)* | - |
| Diastolic Blood Pressure, mm Hg | ||||||||
| Mean (SD) | ||||||||
| Baseline | 84.5(10.0) | 84.3(10.3) | 84.3(10.2) | 84.5(10.0) | 83.5(10.0) | 83.3(10.2) | 83.7(9.9) | 83.4(10.0) |
| 2 years | 79.6(10.1) | 79.3(10.0) | 80.9(10.7)** | 79.8(10.6) | 77.7(9.2) | 76.7(8.9)** | 77.5(9.8) | 77.3(9.5) |
| 4 years | 77.5(9.9) | 77.7(10.1) | 78.5(10.7)* | 78.0(11.0) | 75.7(9.5) | 74.8(9.0)** | 75.4(9.9) | 75.0(10.4) |
| 6 years | 75.1(10.8) | 75.2(9.6) | 75.2(10.2) | * | 73.3(9.7) | 72.3(9.6)* | 72.5(10.3) | - |
| At Goal BP (SBP < 140 mm Hg, DBP < 90 mm Hg) | ||||||||
| n, (%) | ||||||||
| Baseline | 729(27.7) | 454(29.2) | 421(26.7) | 435(27.8) | 1549(27.3) | 933(27.8) | 910(27.0) | 954(28.7) |
| 2 years | 1096(55.9) | 570(50.1)* | 464(42.3)** | 525(47.7)** | 2862(63.0) | 1578(60.8) | 1514(58.6)** | 1483(57.6)** |
| 4 years | 984(63.3) | 552(60.2) | 430(51.0)** | 240(54.4)* | 2525(68.7) | 1451(67.2) | 1429(68.5) | 530(60.4) |
| 6 years | 336(67.2) | 178(60.5) | 149(58.4)* | - | 756(70.3) | 441(68.4) | 444(72.9) | - |
| b. Blood Pressure and Biochemical cChanges by Race and Treatment -- Participants Without Metabolic Syndrome | ||||||||
|---|---|---|---|---|---|---|---|---|
| Black | Non-Black | |||||||
| Chlor (1) | Amlod | Lisin | Dox (2) | Chlor (1) | Amlod | Lisin | Dox (2) | |
| Fasting Glucose, mg/dL | ||||||||
| Mean (SD) | ||||||||
| Baseline | 103.0 (46.6) | 105.0 (46.7) | 102.5 (41.0) | 103.7 (46.9) | 102.7 (40.9) | 101.2 (36.9) | 101.8 (38.4) | 100.9 (37.3) |
| 2 years | 107.7 (43.8) | 107.1 (47.1) | 108.5 (50.2) | 107.9 (44.4) | 108.4 (46.3) | 103.3 (34.1)* | 102.5 (37.0)* | 103.5 (36.4)* |
| 4 years | 109.3 (39.2) | 105.0 (37.2) | 109.4 (40.0) | 105.2 (38.7) | 108.3 (36.6) | 107.4 (42.9) | 106.9 (37.1) | 104.8 (36.5) |
| 6 years | 106.0 (37.9) | 115.0 (47.7) | 111.3 (46.1) | - | 113.1 (36.5) | 103.4 (30.6)* | 107.2 (35.6) | - |
| Potassium, mEq/L | ||||||||
| Mean (SD) | ||||||||
| Baseline | 4.27 (0.70) | 4.28 (0.70) | 4.31 (0.79) | 4.30 (0.76) | 4.40 (0.68) | 4.39 (0.67) | 4.43 (0.68) | 4.41 (0.65) |
| 2 years | 3.99 (0.62) | 4.24 (0.73)** | 4.37 (0.65)** | 4.27 (0.77)** | 4.07 (0.70) | 4.34 (0.62)** | 4.55 (0.62)** | 4.36 (0.58)** |
| 4 years | 4.07 (0.67) | 4.37 (0.92)** | 4.41 (0.55)** | 4.41 (0.94)** | 4.16 (0.78) | 4.43 (0.61)** | 4.60 (0.63)** | 4.44 (0.90)** |
| 6 years | 4.10 (0.81) | 4.36 (0.69)** | 4.49 (0.76)** | - | 4.18 (0.78) | 4.44 (0.64)** | 4.61 (0.54)** | - |
| Total Cholesterol, mg/dL | ||||||||
| Mean (SD) | ||||||||
| Baseline | 214.8 (41.1) | 213.2 (41.6) | 214.3 (41.1) | 214.7 (41.4) | 213.6 (41.0) | 212.4 (40.6) | 211.8 (38.3) | 211.1 (39.4)* |
| 2 years | 207.6 (43.9) | 200.0 (39.3)** | 201.5 (44.4)* | 196.8 (38.4)** | 203.7 (39.5) | 201.1 (40.8) | 199.9 (38.8)* | 194.0 (36.3)** |
| 4 years | 201.8 (42.6) | 196.3 (41.0)* | 195.2 (39.6)* | 187.9 (37.2)** | 195.0 (39.0) | 193.7 (37.6) | 194.7 (38.6) | 188.3 (35.9)* |
| 6 years | 195.1 (42.4) | 188.1 (37.2)* | 190.6 (40.4) | - | 187.0 (37.6) | 188.5 (38.9) | 185.2 (35.8) | - |
| Total Cholesterol, Change from Baseline, mg/dL | ||||||||
| Mean (SD) | ||||||||
| 2 years | −8.2 (36.1) | −12.9 (33.5)* | −13.1 (35.9)* | −18.3 (34.3)** | −10.2 (36.7) | −10.9 (35.0) | −12.0 (35.1) | −17.8 (34.4)** |
| 4 years | −14.1 (36.5) | −15.9 (36.2) | −20.8 (36.7)** | −25.6 (34.1)** | −19.1 (38.6) | −18.7 (37.8) | −17.8 (38.2) | −24.3 (36.7) |
| 6 years | −22.9 (38.1) | −19.7 (36.2) | −24.2 (41.8) | - | −28.0 (39.2) | −24.5 (38.2) | −26.4 (36.7) | - |
| Systolic Blood Pressure, mm Hg | ||||||||
| Mean (SD) | ||||||||
| Baseline | 146.1(15.7) | 145.9(16.1) | 146.5(16.3) | 146.5(16.0) | 146.7(15.8) | 146.7(15.4) | 147.2(15.2) | 146.7(15.4) |
| 2 years | 135.8(16.2) | 138.0(16.2)* | 140.1(18.5)** | 140.4(18.0)** | 135.0(15.2) | 135.9(14.4) | 135.9(16.8) | 136.4(16.0)* |
| 4 years | 133.6(16.9) | 135.8(16.5)* | 136.6(18.3)** | 139.1(17.6)** | 132.8(15.4) | 132.7(14.1) | 134.5(17.2)* | 136.1(16.8)* |
| 6 years | 133.6(16.1) | 135.3(17.6) | 138.3(19.3)* | - | 132.8(15.6) | 131.3(15.0) | 133.2(16.7) | - |
| Diastolic Blood Pressure, mm Hg | ||||||||
| Mean (SD) | ||||||||
| Baseline | 85.3(10.1) | 84.9(10.2) | 85.8(9.9) | 85.7(10.0) | 83.7(9.7) | 83.7(9.9) | 83.8(9.8) | 83.6(9.8) |
| 2 years | 79.3(9.7) | 79.3(10.0) | 81.3(11.4)** | 81.0(10.9)** | 77.4(9.3) | 76.6(9.4)* | 77.1(9.6) | 77.4(9.5) |
| 4 years | 78.2(9.9) | 77.6(9.6) | 79.0(11.2) | 79.3(10.5) | 75.7(9.2) | 74.5(9.4)** | 75.6(10.0) | 75.6(10.5) |
| 6 years | 76.2(10.1) | 76.2(9.7) | 77.8(11.4) | - | 73.2(10.0) | 72.7(8.9) | 73.4(10.4) | - |
| At Goal BP (SBP < 140 mm Hg, DBP < 90 mm Hg) | ||||||||
| n, (%) | ||||||||
| Baseline | 520(26.5) | 327(28.0) | 285(24.9) | 318(26.2) | 864(27.1) | 508(27.3) | 455(24.6) | 465(25.6) |
| 2 years | 884(60.8) | 482(55.6)* | 397(48.0)** | 393(46.5)** | 1577(63.2) | 903(60.8) | 843(59.8)* | 860(61.0) |
| 4 years | 769(65.8) | 452(62.6) | 389(59.2)* | 175(51.3)* | 1418(70.7) | 875(70.8) | 746(65.6) | 310(62.6) |
| 6 years | 250(66.3) | 160(64.3) | 125(56.3)* | - | 379(72.1) | 244(73.9) | 208(67.5) | - |
Values for Chlorthalidone are as of September 30, 2002 (comparisons with amlodipine and lisinopril) and are comparable but may not be identical to those in the comparison with doxazosin, which was terminated early.
Comparisons are vs. Chlorthalidone values as of February 15, 2000.
p < 0.05 for comparison vs. chlorthalidone
p < 0.001 for comparison vs. chlorthalidone
Values for Chlorthalidone are as of September 30, 2002 (comparisons with amlodipine and lisinopril) and are comparable but may not be identical to those in the comparison with doxazosin, which was terminated early
Comparisons are vs. Chlorthalidone values as of February 15, 2000.
p < 0.05 for comparison vs. Chlorthalidone
p < 0.001 for comparison vs. Chlorthalidone
Mean baseline systolic and diastolic blood pressures and the percent of individuals with blood pressures controlled to <140/90 mmHg were similar across all treatment, race, and metabolic status subgroups. Among Black participants with MetS, systolic and diastolic blood pressures at 4 yrs were 3/1 mmHg higher in those randomized to lisinopril (p<0.001 and 0.03, respectively) and 2/1 mmHg higher at 4 yrs in those randomized to doxazosin (p=0.02 and 0.36, respectively). About 1 mmHg separated the blood pressures between the chlorthalidone and amlodipine treatment groups (p=0.05 SBP and 0.67 DBP). In Blacks without MetS, blood pressures at 4 yrs compared with chlorthalidone were 3/1 mmHg higher in those randomized to lisinopril (p<0.001 and 0.11, respectively) and 6/1 mmHg higher at 4 yrs in those randomized to doxazosin (p<0.001 and 0.06, respectively). In non-Blacks at 4 years, SBP differed by 0–3 mmHg between all treatment groups and 0–1 mmHg difference separated the DBP, regardless of MetS status.
Biochemical Changes (Table 2a, 2b)
The mean serum cholesterol levels in participants with and without MetS (~25% of whom were randomized in the ALLHAT lipid trial, half of whom received pravastatin) decreased more in participants randomized to receive amlodipine, lisinopril, and doxazosin treatments compared with chlorthalidone groups at 4 years. The difference in achieved levels was largest in those randomized to doxazosin by 8–14 mg/dl (Table 2a, 2b). Serum potassium levels for those with and without MetS were also slightly but significantly higher for amlodipine, lisinopril and doxazosin vs. chlorthalidone at year 4. Compared to those receiving chlorthalidone, fasting glucose levels at 4 years were lower for participants with MetS receiving amlodipine (by 2–5 mg/dl), lisinopril (by 6–7 mg/dl) and doxazosin (by 8–13 mg/dl). In those without the MetS, the differences in glucose levels were between 1–4 mg/dl higher on chlorthalidone than the other treatment groups. Notably, the differences in glucose levels were significant only for the lisinopril and doxazosin versus chlorthalidone comparisons in non-Blacks (p<.05).
Cardiovascular & Renal Endpoints
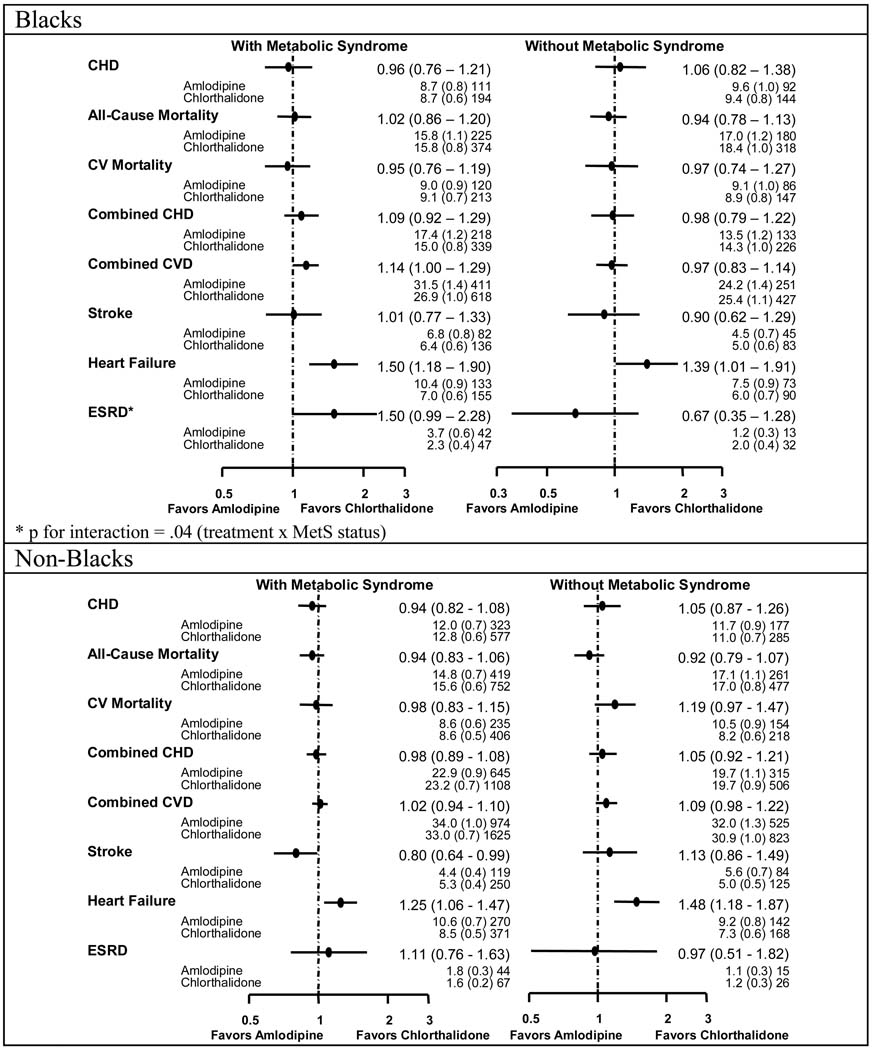
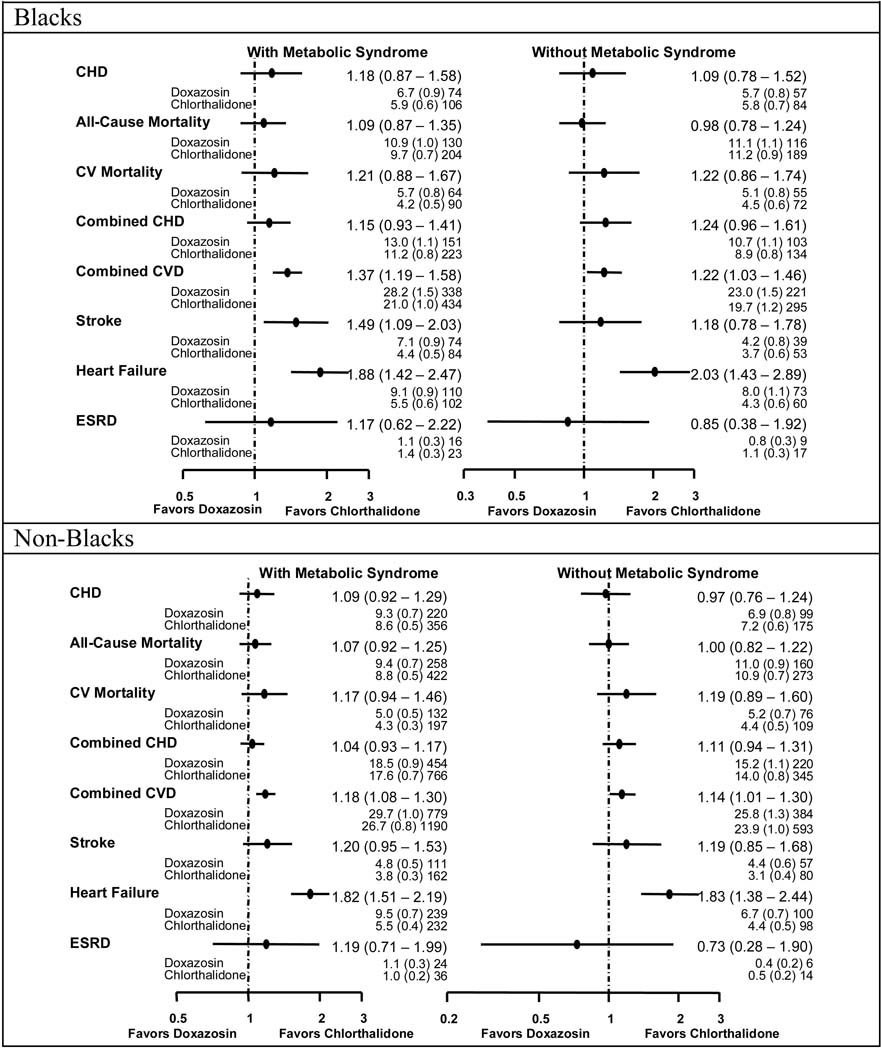
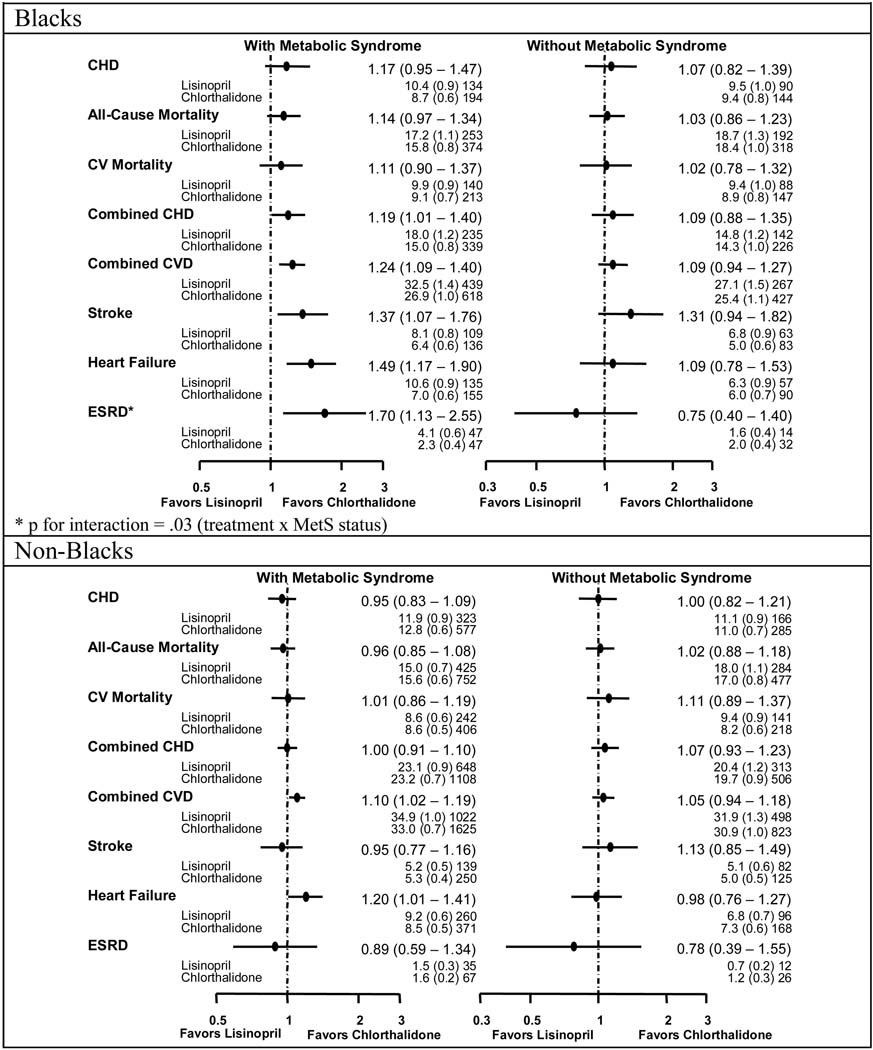
Figure 2–Figure 4 show the event rates and hazard ratios or relative risks (HR or RR, 95% CI) for the pre-specified outcomes by race, metabolic status, and treatment group. No differences were noted among the four treatment groups regardless of race or MetS status for the primary endpoint (nonfatal MI and fatal CHD).
Figure 2.
Comparisons of Amlodipine with Chlorthalidone - Hazard Ratios, Event Rates (Standard Errors), and Numbers of Events by Race and Metabolic Syndrome Status by Race
Figure 4.
Comparisons of Doxazosin with Chlorthalidone - Hazard Ratios, Event Rates (Standard Errors), and Numbers of Events by Race and Metabolic Syndrome Status by Race
Amlodipine / Chlorthalidone
In Blacks with MetS, participants randomized to amlodipine compared with those receiving chlorthalidone were more likely to have higher rates of combined CVD (HR=1.14, 1.00–1.29) and HF (HR=1.50, 1.18–1.90); ESRD also trended higher but was not statistically significant (HR=1.50, 0.99–2.28). Non-Blacks with MetS had higher rates of HF on amlodipine compared with chlorthalidone (RR=1.25, 1.06–1.47) but lower rates of stroke (HR=0.80, 0.64–.0.99). Participants without MetS randomized to amlodipine had similar rates of all outcomes vs. chlorthalidone but higher rates of HF in both Blacks (RR=1.39, 1.01–1.91) and non-Blacks (RR=1.48, 1.18–1.87). In Blacks, although the amlodipine/chlorthalidone comparisons for ESRD were not statistically significant for participants with or without MetS, there was a statistically significant difference in treatment effect by MetS for ESRD (HR=1.50, 0.99–2.28 for those with MetS and HR=0.67, 0.35–1.28 for those without MetS, p=.04 for interaction). In non-Blacks with the MetS, note is made of a statistically significant difference between amlodipine and chlorthalidone for stroke (HR=0.80, 0.64–0.99). However, this could be due to chance, since the p value for interaction comparing the results in participants with MetS with participants without MetS is not statistically significant (p=.051 for interaction).
Lisinopril / Chlorthalidone
Blacks with MetS on lisinopril compared with chlorthalidone were more likely to have higher rates of combined CHD (HR=1.19, 1.01–1.40), combined CVD (HR=1.24, 1.09–1.40), stroke (HR=1.37, 1.07–1.76), HF (RR=1.49, 1.17–1.90) and ESRD (HR=1.70, 1.13–2.55), while non-Blacks with MetS had higher rates of combined CVD (HR=1.10, 1.02–1.19) and HF (RR=1.20, 1.01–1.41). There were no significant differences in endpoints for lisinopril compared with chlorthalidone in either Blacks without MetS or in non-Blacks without MetS. In Blacks, there was a statistically significant difference in treatment effect for ESRD for participants with and without MetS (HR=1.70, 1.13–2.55 for those with MetS and HR=0.75, 0.40–1.40 for those without MetS, p=0.03 for interaction).
Doxazosin / chlorthalidone
Blacks with MetS randomized to doxazosin vs. chlorthalidone had higher rates of combined CVD (HR=1.37, 1.19–1.58), stroke (HR=1.49, 1.09–2.03), and HF (RR=1.88, 1.42–2.47), while non-Blacks with MetS had higher rates of combined CVD (HR=1.18, 1.08–1.30) and HF (RR=1.82, 1.51–2.19). Both racial groups without MetS treated with doxazosin had similarly higher rates of combined CVD and HF.
DISCUSSION
The major finding of this report is that despite a more favorable metabolic profile, neither the CCB, nor the ACE-inhibitor, or the alpha-blocker was superior to the thiazide-type diuretic in preventing hard clinical outcomes in hypertensive patients with the metabolic syndrome. The findings by race and MetS status also parallel the findings in the entire cohort and in all other subgroup analyses from the trial.(8;9;14–16)(17) In no subgroup analysis from ALLHAT has the CCB, ACEI or alpha-blocker been shown thus far to be more effective than the thiazide-type diuretic in preventing either the primary outcome (non-fatal MI or CHD death) or any other major cardiovascular or renal outcome. Note is made of a lower rate of stroke in non-Blacks with MetS assigned to amlodipine compared to those on chlorthalidone. However, this was not seen in Blacks with MetS, in either subgroup without MetS, and did not translate into lower rate of the composite CVD (presumably because of the excess heart failure). Given the number of comparisons and p=.05, the finding is likely due to chance.
The lack of benefit of the agents with the most favorable metabolic profile (i.e., ACE-inhibitors and alpha-blockers) was especially marked in the Black subgroup with MetS. The magnitude of the risk of ESRD (70%), HF (49%), and stroke (37%) and the increased risk of combined CVD and combined CHD, strongly argue against the preference of ACE-inhibitors over diuretics as initial therapy in Blacks with MetS. Similar higher risk was noted for those on the alpha-blocker vs those on the diuretic. While treatment-related differences in cardiovascular and renal outcomes in the lisinopril vs. chlorthalidone comparison may in part be attributed to BP differences in Black participants with MetS, no such attribution can explain the lack of superiority of amlodipine in either race subgroup or of doxazosin or lisinopril in non-Blacks with MetS, as BP differences were minimal.
ALLHAT is not only the first large clinical outcome trial to report on the comparative effects of different classes of antihypertensive drugs on cardiovascular and renal outcomes in patients with the MetS but, remarkably, it is also powered to do so by race, given the large number of participants in ALLHAT meeting the criteria for MetS. Its findings are consistent with published reports from other cohorts (including in diabetics) and with meta-analyses.(18–24) This report complements the findings in non-diabetic ALLHAT participants with and without MetS.(25) In addition, it extends these findings to include the alpha-blocker/diuretic comparison, analyses by race, and it includes diabetics in the definition to be consistent with most definitions of MetS.
The ALLHAT results seem to conflict with expectations. Some have suggested that the follow-up period was too short for the metabolic effects to manifest themselves as clinical outcomes and may not generalize to younger patients, especially those with MetS.(6;26;27) While longer-term treatment effects will be evaluated in an extended morbidity and mortality follow-up of the ALLHAT participants using national databases, until these data are available, little from this or other studies would predict a future reversal of our findings. First, differences in metabolic changes, while statistically significant, are relatively small. The largest difference in mean fasting glucose levels was between the diuretic and the alpha-blocker arms (up to 13 mg/dl). This would represent only a 0.16% lower HbA1c (28) and thus a small difference in diabetic outcomes.(29) Secondly, one would also have to assume that the drug-induced increases in glucose levels carry the same risk as a similar increase due to factors such as weight gain, sedentary lifestyle, etc. Although definitive data are not yet available, analyses of a 14+ year extended follow-up of the Systolic Hypertension in the Elderly Program (SHEP) participants showed a significant increase in both cardiovascular and total mortality in participants assigned placebo who developed diabetes during the double-blind phase of the trial.(19) However, no such increase was seen in those who developed diabetes in the chlorthalidone treatment arm (with atenolol added as needed for blood pressure control). An observational study by Verdecchia et al. reported an increase in CVD event rates associated with new-onset diabetes in hypertensives but the increase in CVD risk was not associated with diuretic therapy.(30) Furthermore, neither the ALLHAT data nor meta-analyses involving >2,800 CHD events, >5,000 CVD events, and >230,000 patient years (more if the alpha-blockers were to be included) suggest even the slightest signal for a lower rate of cardiovascular events with the ACE-inhibitors or the alpha-blockers, which represent the agents with the most favorable metabolic effects, compared with calcium antagonists and diuretics/beta-blockers. This was shown for individuals both with and without diabetes.(14;23;24) In fact, we have recently reported a similar lack of association between change in glucose and CVD outcomes from ALLHAT in those randomized to chlorthalidone, but interestingly a significantly higher CVD and CHD event rate associated with glucose elevations in those on lisinopril.(31) Finally, the recently published DREAM trial specifically designed to evaluate the effect ACEI treatment in patients with either impaired fasting glucose or impaired glucose tolerance reported no significant reduction in new-onset diabetes in participants randomized to ramipril compared to placebo.(32)
In conclusion, these findings fail to provide support for the selection of alpha-blockers, ACE-inhibitors, or CCBs over thiazide-type diuretics to prevent cardiovascular or renal outcomes in patients with the MetS, despite their more favorable metabolic profiles.
Figure 3.
Comparisons of Lisinopril with Chlorthalidone - Hazard Ratios, Event Rates (Standard Errors), and Numbers of Events by Race and Metabolic Syndrome Status by Race
Acknowledgements
Funding support: This study was supported by contract N01-HC-35130 from the National Heart, Lung, and Blood Institute. The Institute’s role included involvement in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript. The ALLHAT investigators acknowledge contributions of study medications supplied by Pfizer, Inc., (amlodipine and doxazosin), AstraZeneca (atenolol and lisinopril), and Bristol-Myers Squibb (pravastatin) and financial support provided by Pfizer, Inc.
Footnotes
Link to published article: http://archinte.ama-assn.org/cgi/reprint/168/2/207
Authors’ specific contributions:
Data access: Dr. Davis, Mr. Baimbridge, and Ms. Pressel had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Financial disclosures:
Previous presentations: International Society for Hypertension in Blacks (July 2005, June 2006), National Medical Association (July 2005).
Reference List
- 1.Hall WD, Clark LT, Wenger NK, Wright JT, Jr., Kumanyika SK, Watson K, et al. The Metabolic Syndrome in African Americans: a review. Ethn Dis. 2003;13(4):414–428. [PubMed] [Google Scholar]
- 2.Grundy SM, Brewer HB, Jr., Cleeman JI, Smith SC, Jr., Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109(3):433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 3.Eckel RH, Kahn R, Robertson RM, Rizza RA. Preventing cardiovascular disease and diabetes: a call to action from the American Diabetes Association and the American Heart Association. Circulation. 2006;113(25):2943–2946. doi: 10.1161/CIRCULATIONAHA.106.176583. [DOI] [PubMed] [Google Scholar]
- 4.Mykkanen L, Kuusisto J, Pyorala K, Laakso M, Haffner SM. Increased risk of non-insulin-dependent diabetes mellitus in elderly hypertensive subjects. J Hypertens. 1994;12(12):1425–1432. [PubMed] [Google Scholar]
- 5.Giles TD, Sander GE. Pathophysiologic, diagnostic, and therapeutic aspects of the metabolic syndrome. J Clin Hypertens (Greenwich ) 2005;7(11):669–678. doi: 10.1111/j.1524-6175.2005.04763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mancia G. The association of hypertension and diabetes: prevalence, cardiovascular risk and protection by blood pressure reduction. Acta Diabetol. 2005;42 Suppl 1:S17–S25. doi: 10.1007/s00592-005-0177-z. [DOI] [PubMed] [Google Scholar]
- 7.Wagh A, Stone NJ. Treatment of metabolic syndrome. Expert Rev Cardiovasc Ther. 2004;2(2):213–228. doi: 10.1586/14779072.2.2.213. [DOI] [PubMed] [Google Scholar]
- 8.The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHATThe ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group) JAMA. 2002;288(23):2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 9.Wright JT, Jr., Dunn JK, Cutler JA, Davis BR, Cushman WC, Ford CE, et al. Outcomes in hypertensive black and nonblack patients treated with chlorthalidone, amlodipine, and lisinopril. JAMA. 2005;293(13):1595–1608. doi: 10.1001/jama.293.13.1595. [DOI] [PubMed] [Google Scholar]
- 10.Davis BR, Cutler JA, Gordon DJ, Furberg CD, Wright JT, Jr., Cushman WC, et al. Rationale and design for the Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). ALLHAT Research Group. Am J Hypertens. 1996;9(4 Pt 1):342–360. doi: 10.1016/0895-7061(96)00037-4. [DOI] [PubMed] [Google Scholar]
- 11.Zhu S, Wang Z, Heshka S, Heo M, Faith MS, Heymsfield SB. Waist circumference and obesity-associated risk factors among whites in the third National Health and Nutrition Examination Survey: clinical action thresholds. Am J Clin Nutr. 2002;76(4):743–749. doi: 10.1093/ajcn/76.4.743. [DOI] [PubMed] [Google Scholar]
- 12.Pyorala K, Ballantyne CM, Gumbiner B, Lee MW, Shah A, Davies MJ, et al. Reduction of cardiovascular events by simvastatin in nondiabetic coronary heart disease patients with and without the metabolic syndrome: subgroup analyses of the Scandinavian Simvastatin Survival Study (4S) Diabetes Care. 2004;27(7):1735–1740. doi: 10.2337/diacare.27.7.1735. [DOI] [PubMed] [Google Scholar]
- 13.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23(5):469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 14.Diuretic versus alpha-blocker as first-step antihypertensive therapy: final results from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Hypertension. 2003;42(3):239–246. doi: 10.1161/01.HYP.0000086521.95630.5A. [DOI] [PubMed] [Google Scholar]
- 15.Rahman M, Pressel S, Davis BR, Nwachuku C, Wright JT, Jr., Whelton PK, et al. Renal outcomes in high-risk hypertensive patients treated with an angiotensin-converting enzyme inhibitor or a calcium channel blocker vs a diuretic: a report from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Arch Intern Med. 2005;165(8):936–946. doi: 10.1001/archinte.165.8.936. [DOI] [PubMed] [Google Scholar]
- 16.Rahman M, Pressel S, Davis BR, Nwachuku C, Wright JT, Jr., Whelton PK, et al. Cardiovascular outcomes in high-risk hypertensive patients stratified by baseline glomerular filtration rate. Ann Intern Med. 2006;144(3):172–180. doi: 10.7326/0003-4819-144-3-200602070-00005. [DOI] [PubMed] [Google Scholar]
- 17.Whelton PK, Barzilay J, Cushman WC, Davis BR, Iiamathi E, Kostis JB, et al. Clinical outcomes in antihypertensive treatment of type 2 diabetes, impaired fasting glucose concentration, and normoglycemia: Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Arch Intern Med. 2005;165(12):1401–1409. doi: 10.1001/archinte.165.12.1401. [DOI] [PubMed] [Google Scholar]
- 18.SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP) JAMA. 1991;265(24):3255–3264. [PubMed] [Google Scholar]
- 19.Kostis JB, Wilson AC, Freudenberger RS, Cosgrove NM, Pressel SL, Davis BR. Long-term effect of diuretic-based therapy on fatal outcomes in subjects with isolated systolic hypertension with and without diabetes. Am J Cardiol. 2005;95(1):29–35. doi: 10.1016/j.amjcard.2004.08.059. [DOI] [PubMed] [Google Scholar]
- 20.Black HR, Elliott WJ, Grandits G, Grambsch P, Lucente T, White WB, et al. Principal results of the Controlled Onset Verapamil Investigation of Cardiovascular End Points (CONVINCE) trial. JAMA. 2003;289(16):2073–2082. doi: 10.1001/jama.289.16.2073. [DOI] [PubMed] [Google Scholar]
- 21.Hansson L, Lindholm LH, Ekbom T, Dahlof B, Lanke J, Schersten B, et al. Randomised trial of old and new antihypertensive drugs in elderly patients: cardiovascular mortality and morbidity the Swedish Trial in Old Patients with Hypertension-2 study. Lancet. 1999;354(9192):1751–1756. doi: 10.1016/s0140-6736(99)10327-1. [DOI] [PubMed] [Google Scholar]
- 22.Brown MJ, Palmer CR, Castaigne A, de Leeuw PW, Mancia G, Rosenthal T, et al. Morbidity and mortality in patients randomised to double-blind treatment with a long-acting calcium-channel blocker or diuretic in the International Nifedipine GITS study: Intervention as a Goal in Hypertension Treatment (INSIGHT) Lancet. 2000;356(9227):366–372. doi: 10.1016/S0140-6736(00)02527-7. [DOI] [PubMed] [Google Scholar]
- 23.Turnbull F. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet. 2003;362(9395):1527–1535. doi: 10.1016/s0140-6736(03)14739-3. [DOI] [PubMed] [Google Scholar]
- 24.Turnbull F, Neal B, Algert C, Chalmers J, Chapman N, Cutler J, et al. Effects of different blood pressure-lowering regimens on major cardiovascular events in individuals with and without diabetes mellitus: results of prospectively designed overviews of randomized trials. Arch Intern Med. 2005;165(12):1410–1419. doi: 10.1001/archinte.165.12.1410. [DOI] [PubMed] [Google Scholar]
- 25.Black HR, Davis BR, Barzilay J, Nwachuku C, Baimbridge C, Duffy D, et al. Clinical outcomes in non-diabetic individuals with the metabolic syndrom assigned to chlorthalidone, amlodipine, or lisinopril as initial hypertension therapy: A report from the ALLHAT Study. Submitted. 2007 [Google Scholar]
- 26.Weber MA. Hypertension, the metabolic syndrome, and the risk of developing diabetes: is it time to change the guidelines? J Clin Hypertens (Greenwich ) 2004;6(8):425–427. doi: 10.1111/j.1524-6175.2004.03751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Messerli FH, Weber MA. Long-term cardiovascular consequences of diuretics vs calcium channel blockers vs angiotensin-converting enzyme inhibitors. JAMA. 2003;289(16):2067–2068. doi: 10.1001/jama.289.16.2067. [DOI] [PubMed] [Google Scholar]
- 28.Woerle HJ, Pimenta WP, Meyer C, Gosmanov NR, Szoke E, Szombathy T, et al. Diagnostic and therapeutic implications of relationships between fasting, 2-hour postchallenge plasma glucose and hemoglobin a1c values. Arch Intern Med. 2004;164(15):1627–1632. doi: 10.1001/archinte.164.15.1627. [DOI] [PubMed] [Google Scholar]
- 29.Stratton IM, Cull CA, Adler AI, Matthews DR, Neil HA, Holman RR. Additive effects of glycaemia and blood pressure exposure on risk of complications in type 2 diabetes: a prospective observational study (UKPDS 75) Diabetologia. 2006;49(8):1761–1769. doi: 10.1007/s00125-006-0297-1. [DOI] [PubMed] [Google Scholar]
- 30.Verdecchia P, Reboldi G, Angeli F, Borgioni C, Gattobigio R, Filippucci L, et al. Adverse prognostic significance of new diabetes in treated hypertensive subjects. Hypertension. 2004;43(5):963–969. doi: 10.1161/01.HYP.0000125726.92964.ab. [DOI] [PubMed] [Google Scholar]
- 31.Barzilay J, Davis BR, Cutler JA, Pressel SL, Whelton PK, Basil J, et al. Fasting glucose levels and incident diabetes mellitus in older non-diabetic adults randomized to three different classes of antihypertensive treatment. Arch Intern Med. 2006 doi: 10.1001/archinte.166.20.2191. [DOI] [PubMed] [Google Scholar]
- 32.Bosch J, Yusuf S, Gerstein HC, Pogue J, Sheridan P, Dagenais G, et al. Effect of ramipril on the incidence of diabetes. N Engl J Med. 2006;355(15):1551–1562. doi: 10.1056/NEJMoa065061. [DOI] [PubMed] [Google Scholar]