Abstract
Purpose
Numerous pre-clinical studies have demonstrated that local photodynamic therapy (PDT) of tumors enhances systemic anti-tumor immunity. However other than single case and anecdotal reports, this phenomenon has not been examined following clinical PDT. To determine whether PDT in a clinical setting enhances systemic recognition of tumor cells, we examined whether PDT of basal cell carcinoma (BCC) resulted in an increased systemic immune response to a BCC associated tumor antigen, Hip1.
Experimental Design
BCC lesions were treated with either PDT or surgically removed. Blood was collected from patients immediately before or 7–10 days following treatment. Peripheral blood leukocytes were isolated from HLA-A2 expressing patients and reactivity to an HLA-A2 restricted Hip1 peptide was measured by interferon-γ ELISpot assay.
Results
Immune recognition of Hip1 increased in patients whose BCC lesions were treated with PDT. This increase in reactivity was significantly greater than reactivity observed in patients whose lesions were surgically removed. Patients with superficial lesions exhibited greater enhancement of reactivity compared to patients with nodular lesions. Immune reactivity following PDT was inversely correlated with treatment area and light dose.
Conclusions
These findings represent the first demonstration that local tumor PDT can enhance systemic immune responses to tumors in patients and validate previous preclinical findings.
Introduction
Photodynamic therapy (PDT) is an anti-tumor modality that is approved for clinical use in a number of countries, including the US, for the elimination of early stage malignancies and the palliation of symptoms in patients with late stage tumors (1, 2). PDT has traditionally been considered to act locally, causing direct tumor destruction through generation of reactive oxygen species (ROS) and acting indirectly through vascular damage (3–5). However preclinical studies have shown that local PDT treatment of tumors can result in wide-spread systemic effects that include induction of systemic neutrophilia (6), induction of acute phase proteins (6, 7), increased circulating levels of complement proteins (8) and systemic release of pro-inflammatory cytokines (7, 9–13), all of which indicate the presence of a systemic inflammatory response. Subsequent studies showed that local PDT treatment of murine tumors results in resistance to subsequent tumor challenge (reviewed in (14, 15)) and in some cases an increased ability to control tumor growth outside the local treatment field (16, 17) that was dependent upon the presence of an intact immune response (17). The general conclusion from these pre-clinical studies was that PDT leads to the enhancement of anti-tumor immunity. However this hypothesis has never been tested in a clinical setting in part due to a lack of known tumor antigens associated with the tumors commonly treated with PDT.
Mutations in the gene for the receptor for sonic hedgehog protein (SHH), patched-1 (PTCH1) are causally involved in the development of basal cell carcinoma (BCC) (18, 19). Patched-1 negatively regulates the function of smoothen and mutations of PTCH1, which primarily result in truncated non-functional proteins, lead to unregulated activation of the hedgehog signaling pathway transcription factor family, GLI. mRNA levels for PTCH1 and the glioma-associated oncogene homolog (GLI) family members, GLI1, GLI2 and GLI3, have all been shown to be up regulated in BCC (20, 21). Recently a new member of the hedgehog signaling pathway gene family has been identified, HIP (hedgehog-interacting protein) (22). HIP encodes for type 1 transmembrane protein, Hip1, which binds to all members of the hedgehog family with an affinity similar to that of patched-1 and is thought to have a similar negative regulatory function (22). HIP is also over expressed in BCC (20, 21), but it appears to have lower expression in normal skin than PTCH1 and does not appear to be mutated in BCC. Thus it is possible that Hip1 can act as a tumor associated antigen (TAA) and that its overexpression can provide a target for the immune response. Vogt et al (23) demonstrated that immunization of mice prone to BCC (Ptch1+/− mice) with Hip1 resulted in increased immune reactivity to Hip1 and reduced incidence of BCC, further indicating the potential of this protein as a TAA. In the current study we examined the effect of PDT of BCC on immune reactivity to Hip1 in a cohort of HLA-A2 expressing patients in an attempt to determine whether PDT in a clinical setting could enhance immune reactivity to a TAA.
Patients and Methods
Identification of HLA-A2 binding Hip1 peptides
BIMAS (BioInformatics and Molecular Analysis Section, National Institutes of Health, Bethesda, MD1) was used to identify Hip1 peptides with HLA-A2 binding potential. BIMAS calculates a “theoretical” binding stability matrix based on the rate of dissociation from β2-microglobulin (β2M), which was developed by Parker et al. (24), to predict potential HLA-A2 binding peptides. Potential HLA-A2 binding Hip1 peptides were synthesized at the University of Georgia Molecular Genetics Instrumentation facility. Stock solutions of peptides were made in 100% DMSO and stored at −20°C until use. Working concentrations (500 µg/ml) of peptides were made in RPMI-1640. Peptide binding to HLA-A2 was confirmed using a modification of the T2 stabilization assay (25). Briefly 106 T2 cells were incubated in RPMI-1640 in the presence of peptide (25µg/ml) and 5nM β2M for 18h at room temperature, followed by 3h incubation at 37°C. The stabilized HLA-A2 molecules on the cell surface were detected using FITC-conjugated anti-HLA-A2 antibody (Pharmingen, San Diego, CA). Labeled cells were analyzed by flow cytometry using a FACScan (Becton Dickinson).
PDT treatment
The study was conducted in accordance with the Declaration of Helsinki. All patients were provided with written, informed consent. The Roswell Park Cancer Institute (RPCI) Investigational Review Board approved the study. Photosensitizers were applied prior to light application. Depending on the depth of the tumors, the physician determined whether patients were treated with 5-aminoleluvinic acid (ALA)-PDT or Porfimer sodium-PDT. ALA was applied as a 20% moisturel cream containing 240mg of crystalline ALA mixed with 960mg of liquid moisturel. The cream was applied in a thin layer over the lesion with about 0.5 – 1.0cm border of normal skin 4 – 24h before light application. The area was then covered with occlusive dressing to shield it from light and to keep the area moist until light application. Topically applied ALA was used primarily for thinner lesions as it has been shown to be less effective on thicker lesions due to lower permeability into the stratum corneum (26). Porfimer sodium (1mg/kg) in D5W was infused into patients intravenously, which allows for treatment of deeper lesions (27) but results in significant skin phototoxicity. Therefore Porfimer sodium treated patients were instructed to avoid direct sunlight as well as strong indoor lighting for up to 6 weeks post infusion. Light was applied directly on the lesions (both ALA and Porfimer sodium treatments) at a fluence of 100 – 260J at a fluence rate of 150mW/cm2 4h post ALA application or 48h post Porfimer sodium infusion. Lesions from 2- to 7-cm in diameter were exposed to 630-nm light derived from a Spectra Physics (Mountain View, CA) Model 171 argon laser pumping a Spectra Physics tunable dye laser (Model 375). Light was delivered to the sites using 400 mm or 600 mm diameter quartz fibers fitted with microlenses to give spots of light with uniform intensities. A filtered tungsten-halogen lamp (590–700 nm) that allows adjustment of the diameter of the illumination field (DUSA Pharmaceuticals, Inc, Wilmington, Mass) was used for larger fields with up to a 16-cm diameter. Clinical follow-up was performed at 3 and 6 months post-PDT and then on an average of every 6 months. Complete clinical responses were determined at ≥ 6 months.
Blood collection and lymphocyte isolation
Blood from BCC patients was drawn in the Dermatology clinic or Laboratory Medicine Department at RPCI 1–2 days before and 7–14 days post PDT treatment. Lymphocytes were isolated from patient peripheral blood mononuclear cells (PBMCs) by centrifugation over a Ficoll gradient (Ficoll-Paque™ PLUS; Amersham Biosciences). Isolated lymphocyte samples were frozen at −70°C in 80% human serum albumin (HSA), 10% Aim V medium and 10% DMSO until use. At the time of the assay, lymphocytes were thawed, washed two times in PBS, counted and resuspended in complete medium (RPMI 1640 containing 10% FBS).
Determination of HLA-A2 Expression
Patient lymphocytes (106 cells) were incubated with a FITC-conjugated anti-HLA-A2 monoclonal antibody (Pharmingen). Samples were then analyzed by flow cytometry on a Becton Dickinson FACScan. Mean fluorescence intensity (MFI) was compared to binding of cells to an isotype control antibody.
ELISpot Assay for IFN-γ
ELISpot plates (96 well) (Millipore, Cat. # MAHAS45 10) were coated with antihuman IFN-γ capture antibody (mAb1-D1K, Mabtech, USA) at a concentration of 0.5ug/ml in PBS O/N at 4°C. The wells were then washed with PBS and blocked with RPMI 1640 containing 10% FBS for at least 2 hours in a 37°C, 5% CO2 incubator. T2 target cells were pulsed with HIP peptide (25µg/ml) or an irrelevant peptide. The HLA-A2 binding peptide, gag77–85 from HIV p17 (28), was used as an irrelevant peptide control. The media was discarded off the blocked ELISpot plates and patient lymphocytes (1x105) were added to the wells in triplicate. The pulsed T2 (5x104) cells were added to appropriate wells containing lymphocytes. The plates were incubated in a 37°C, 5% CO2 incubator for 18–20 hours. Plates were then washed several times in PBS containing 0.5% tween-20 (PBS-Tween). Biotin conjugated anti-IFN-γ (mAb 7-B6-1-Biotin, Mabtech, USA) was added to each well and plates at 2 µg/ml and the plates were placed back in the incubator for 2hrs. Plates were washed several times with PBS-Tween. Streptavidin-HRP (SAv-HRP, BD Pharmingen) was added to each well at a 1:1000 dilution. Plates were incubated at room temperature for 1h. IFN-γ spots were developed using AEC staining kit (AEC-101, Sigma). 100ul of the AEC solution was added to each well and incubated for 5–15 minutes. The plates were washed and dried in the dark. The number of spot forming units in each well was enumerated by computer assisted image analysis using the Zeiss ELISPOT reader system equipped with v 4.1.56 software (ZEISS, Thornwood NJ) by the Roswell Park Cancer Center Immunomonitoring Core facility.
Statistical Analysis
To describe the observed variability in the data and test for variables related to immune response, a series of mixed linear models were fit to the data. The dependent variable for all considered models was immune response following treatment, repeated measures of which were taken for each patient. Independent variables included the corresponding pre-treatment value and random subject and day effects. The following variables were also included in the model one at a time: age, sex, diagnosis, drug, light dose, number of lesions treated, number of prior treatments, total area treated, and CCR. Standard diagnostic plots were used to assess model fit and all statistical tests was carried out using SAS version 9.1.3 statistical software (Cary, NC) and perform at the 0.05 level of significance.
Results
Identification of HLA-A2 Binding Peptides in Hip1
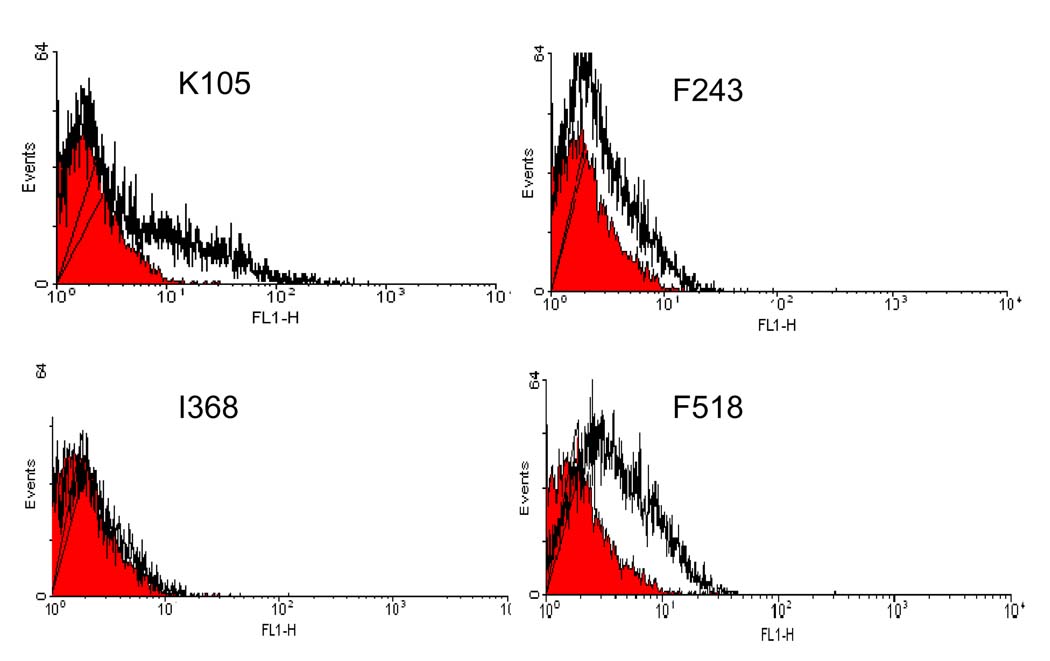
Several pre-clinical studies have shown that PDT enhanced anti-tumor immunity is dependent upon the presence of CD8+ T lymphocytes (reviewed in (14)). Tumor specific CD8+ T cells respond to peptide epitopes bound to major histocompatibility complex (MHC) I molecules. In order to determine whether PDT of BCC lesions enhanced immune recognition of the BCC TAA, Hip1, it was first necessary to identify MHC class I (MHC-I) binding peptides present in Hip1. HLA-A2 is the most common MHC-I allele in North America and its peptide binding motif is well characterized. Therefore analysis of the full-length amino acid sequence of Hip1 was performed using the BIMAS software program to predict Hip1 peptides capable of binding HLA-A2. BIMAS is a matrix-based algorithm that predicts peptide binding based on experimentally measured β2-microglobulin dissociation rates (24). Analysis revealed several peptides that could theoretically bind strongly to HLA-A2. The four peptides identified to have a high potential to bind HLA-A2 according to their dissociation constants were K105 (KMLSFKLLL), F243 (FILEKEGYV), I368 (ILGDGMITL), and F518 (FLTLQQSPV) (Table 1).
Table 1.
Predicted binding of Hip1 peptides to HLA-A2
| Peptide | Sequence | Position (a.a.) | Scorea |
|---|---|---|---|
| K105 | KMLSFKLLL | 105–113 | 607 |
| F243 | FILEKEGYV | 243–251 | 477 |
| I368 | ILGDGMITL | 368–376 | 342 |
| F518 | FLTLQQSPV | 518–526 | 320 |
Estimated Kd based on the BIMAS software package, which predicts MHC restricted peptides based on the amino acid sequence of the protein of interest.
The T2 stabilization assay was used to confirm peptide binding to HLA-A2 (25). T2 cells are TAP1/2 deficient; TAP1/2 molecules are needed for the loading of endogenous peptides onto nascent MHC-I molecules, which is required for protein stability and cell surface expression. T2 cells do not stably express cell surface HLA-A2 molecules; however, addition of exogenous peptides that bind to HLA-A2 results in stabilization of HLA-A2 and cell surface expression. Figure 1 shows that peptides K105, F243 and F518 stabilize cell surface expression of HLA-A2 on T2 cells, with K105 showing the best binding ability. Peptide I368 did not exhibit HLA-A2 binding, which was surprising. However the BIMAS algorithm depends on the assumption that each amino acid in the peptide contributes independently to binding and there are known cases where the combination of amino acids in the peptide do not behave as predicted, which is likely a result of the individual coefficients of binding not being determined accurately enough or a lack of knowledge of unfavorable amino acid preferences (24, 29).
Figure 1. Hip1 Peptide Stabilization of HLA-A2 Cell Surface Expression by T2 Cells.
T2 cells were incubated in RPMI-1640 in the presence of peptide (25µg/ml) and 5nM β2M for 18h at room temperature, followed by 3h incubation at 37°C. The stabilized HLA-A2 molecules on the cell surface were detected using FITC-conjugated anti-HLA-A2 antibody (Pharmingen, San Diego, CA). Representative histograms are shown depicting isotype binding (filled histogram) or anti-HLA-A2 binding (open histogram).
PDT enhances lymphocyte recognition of Hip1 peptide K105
To determine whether clinical PDT enhanced immune recognition of TAAs, blood was collected from patients with nodular or superficial BCC 1–2 days prior to and 7–14 days after PDT. Lymphocytes were isolated from HLA-A2+ patients tested for reactivity to Hip1. Reactivity was determined using ELISpot assays to measure IFN-γ production following incubation of patient lymphocytes with Hip1 peptide K105. A minimum of three independent tests were done on each pre- and post-treatment sample.
Samples were collected from 60 patients, 40% of which were HLA-A2+. Of the 24 patients that were HLA-A2+, pre- and post-PDT samples were collected from 21 patients and from 4 patients whose lesions were surgically removed. The patient profile (age, sex, and diagnosis), treatment parameters (photosensitizer and light dose used) and responses (clinical and immune reactivity) are shown in Table 2. The PDT treatment population consisted of 12 males and 9 females and includes 7 patients with nodular BCC and 14 patients with superficial BCC. Porfimer sodium-PDT was used to treat 3 patients, 1 with superficial BCC and 2 with nodular BCC. ALA-PDT was used to treat 13 patients with superficial BCC and 5 patients with nodular BCC. There was no significant difference in clinical response rates (CCR) between patients treated with Porfimer sodium-PDT and ALA-PDT (Evaluations made ≥ 6 months after PDT). The clinical response rate following treatment of superficial BCC with PDT was 92% and varied between 100% response and 50% response. The average clinical response rate for nodular BCC was less than that for superficial BCC with an average response rate of 60.2% that varied between a response of 90.9% and no response.
Table 2.
Patient Characteristics, Diagnosis, Treatment Conditions
| Patient | Sex | Age | Diagnosis | PSa | Light Dose |
# Prior Treatments |
# of Lesions Treated |
Total Area Treated (cm2) |
Clinical Responseb |
Change in Immune Responsec |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 50 | Superficial | Porfimer | 170 | 4 | 39 | 139 | 100 | I (>2 fold) |
| 2 | M | 56 | Nodular | Porfimer | 215 | 2 | 21 | 168 | 61.9 | I (>2 fold) |
| 3 | M | 43 | Nodular | Porfimer | 215 | 4 | 33 | 182 | 75.8 | I |
| 4 | F | 18 | Superficial | ALA | 195 | 8 | 16 | 446 | 100 | I (>2 fold) |
| 5 | F | 51 | Superficial | ALA | 200 | 6 | 2 | 6.3 | 100 | I (>2 fold) |
| 6 | M | 43 | Superficial | ALA | 200 | 5 | 39 | 74 | 100 | I (>2 fold) |
| 7 | M | 65 | Superficial | ALA | 200 | 0 | 4 | 53.6 | 50 | I (>2 fold) |
| 8 | F | 42 | Superficial | ALA | 193 | 0 | 2 | 10 | 100 | I (>2 fold) |
| 9 | F | 47 | Superficial | ALA | 200 | 0 | 10 | 24.2 | 80 | I (>2 fold) |
| 10 | M | 17 | Superficial | ALA | 260 | 0 | 64 | 139 | 100 | I (>2 fold) |
| 11 | F | 55 | Superficial | ALA | 200 | 0 | 32 | 57 | 81 | I (>2 fold) |
| 12 | F | 60 | Superficial | ALA | 200 | 0 | 32 | 57.8 | 84 | I (>2 fold) |
| 13 | M | 45 | Superficial | ALA | 200 | 5 | 71 | 262 | 96 | I (>2 fold) |
| 14 | M | 46 | Superficial | ALA | 200 | 7 | 50 | 86.7 | 100 | I (>2 fold) |
| 15 | M | 75 | Superficial | ALA | 189 | 0 | 2 | 10 | 100 | I (>2 fold) |
| 16 | F | 86 | Superficial | ALA | 186 | 5 | 2 | 10 | 100 | I (>2 fold) |
| 17 | F | 12 | Nodular | ALA | 200 | 0 | 11 | 676 | 0 | I |
| 18 | M | 21 | Nodular | ALA | 200 | 7 | 18 | 682 | 61.1 | D |
| 19 | F | 65 | Nodular | ALA | 260 | 0 | 11 | 1151 | 90.9 | D |
| 20 | M | 11 | Nodular | ALA | 100 | 1 | 42 | 64 | 64.9 | I |
| 21 | M | 50 | Nodular | ALA | 200 | 19 | 46 | 144 | 67.0 | D |
| 22 | F | 75 | Superficial | Surgery | I (>2 fold) | |||||
| 23 | M | 75 | Superficial | Surgery | D | |||||
| 24 | M | 55 | Superficial | Surgery | I | |||||
| 25 | M | 71 | Nodular | Surgery | D |
PS=photosensitizer;
% of treated lesion with complete clinical response;
I=Increased recognition of Hip peptide; D=decreased or no change in recognition of Hip peptide.
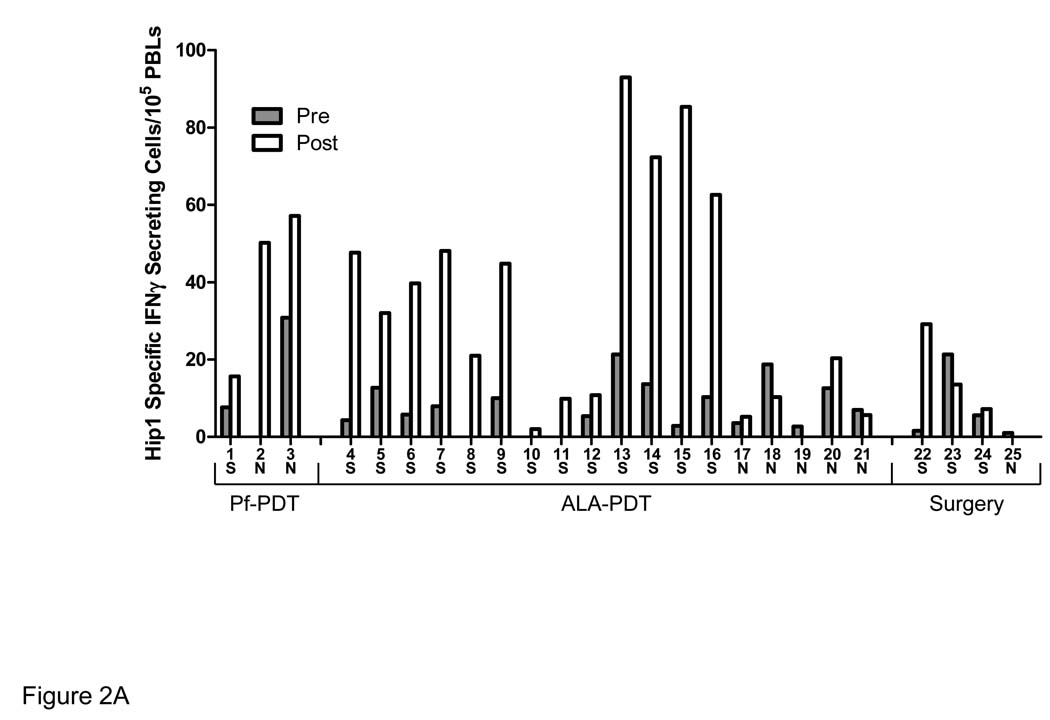
Seventeen of the 21 patients treated with PDT exhibited an increased response to Hip1 following treatment and 15 of the 17 responding patients exhibited a two-fold or greater increase in their response when the post-treatment mean was compared to the pre-treatment mean (Figure 2). Treatment with PDT resulted in significantly higher immune reactivity when compared to surgery (P≤0.03); only one of the four patients whose lesions were removed surgically had response was more than twice the pretreatment response. The reason for the increase in this patient is unknown as there was no apparent difference in tumor burden or removed lesion size between the four patients.
Figure 2. PDT Enhances Lymphocyte Recognition of Hip1.
Patient lymphocytes were tested for Hip1 reactivity by ELISpot analysis for IFN-γ secretion following incubation with the Hip1 derived peptide K105. The number of Hip1 specific IFN-γ secreting cells was determined by subtraction of the number of spots obtained following incubation with an irrelevant HLA-A2 binding peptide of the HIV protein gag from the number of spots obtained following incubation with peptide K105. Lymphocytes were obtained 1–2 days prior to PDT (Pre) and 7–14 days post-PDT (Post). Each sample was tested in a minimum two independent experiments each containing three replicates. (A) The average of the mean of the pre-treatment (filled bars) and post-treatment (open bars) values from the individual experiments is shown. Patients are grouped according to the treatment received; S=superficial lesion, N=nodular lesion.
The effect of PDT on reactivity to Hip1 was greater in patients following treatment of superficial lesions as compared to that of patients with nodular lesions. All of the patients who had superficial lesions treated with PDT demonstrated a greater than 2 fold increase in Hip1 reactivity. In contrast, only 57.1% (4 out of 7) patients treated for nodular BCC exhibited an increased reactivity to Hip1 following treatment and only 14.2% or 1 out of the 7 patients had a two fold or greater change in reactivity.
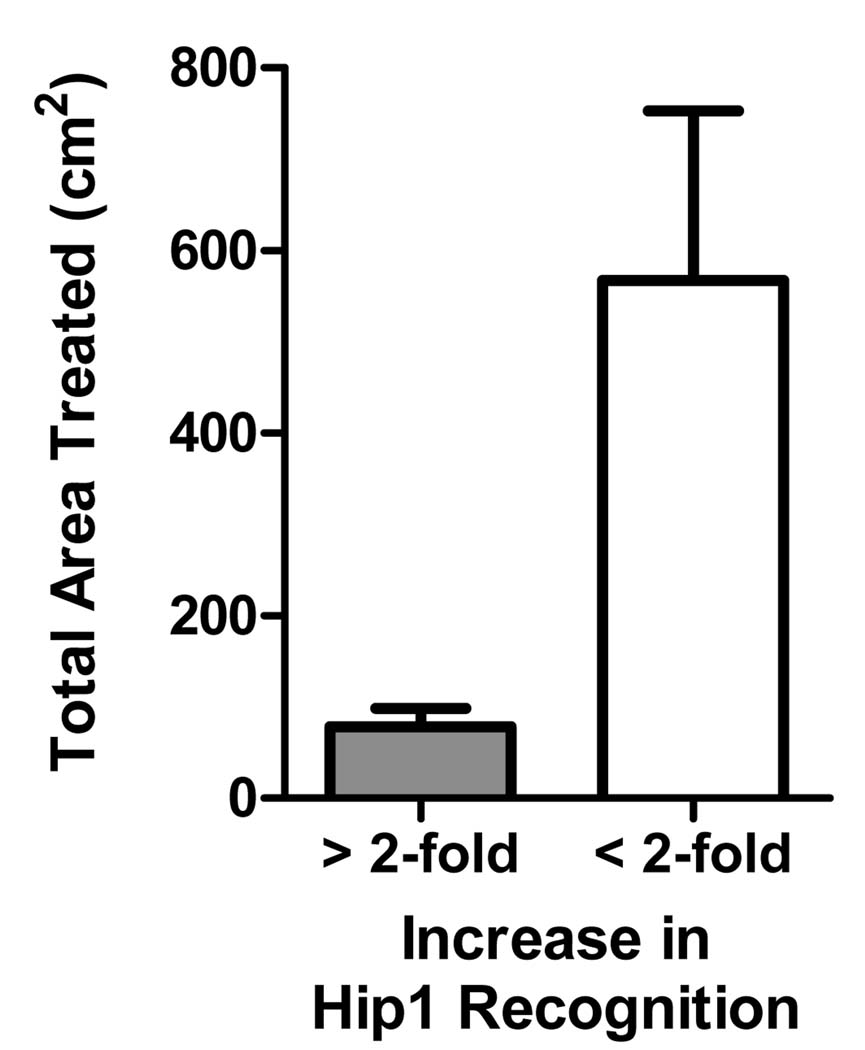
Patients treated with light doses above 200J/cm2 exhibited lower increases in immune reactivity to Hip1. Recognition of Hip1 following PDT was inversely affected by amount of area treated (P≤0.03), which is shown graphically in Figure 3.
Figure 3. Recognition of Hip1 Decreases with Increased Total Treatment Area.
Patient responses were segregated into categories representing responses greater than (n=15) or less than (n=6) the 2-fold pretreatment means and plotted against the total treatment area. Error bars represent standard error of the mean.
Interestingly, in two cases, Patients 4 and 13, we observed that lesions present at the time of treatment, but outside the treatment field, appeared to regress following PDT treatment. Unfortunately this study was not designed to follow regression of lesions outside the treatment field and thus these findings are limited and anecdotal.
Discussion
Although numerous pre-clinical studies have demonstrated that PDT of murine tumors can result in an increase in anti-tumor immunity (reviewed in (14)), to our knowledge the current study is the first to show that PDT in a clinical setting can enhance immune cell recognition of TAAs. Here we demonstrate that both ALA-PDT and Porfimer sodium-PDT enhance the immune response, as measured by IFN-γ secretion, against the BCC associated tumor antigen, Hip1. Importantly immune reactivity following treatment was significantly greater in patients treated with PDT as compared to those whose lesions were removed surgical.
Abdel-Hady et al. (30) first suggested the importance of anti-tumor immunity to clinical outcome. They showed that patients with vulval intraepithelial neoplasia (VIN) who did not respond to ALA-PDT were more likely to have MHC-I negative tumors than patients who responded to ALA-PDT. Responding patients also exhibited increased CD8+ T cell infiltration into their tumors after treatment as compared to non-responders. In addition a study by Dragieva et al. (31) on the use of ALA-PDT to treat actinic keratoses and Bowen’s disease in immuno-suppressed and immuno-competent patients showed that while both patient groups had similar initial response rates of greater than 80%, immuno-suppressed patients exhibited persistence of disease or appearance of new lesions that was greater than that of immuno-competent patients. Finally a recent case report shows that PDT of multifocal angiosarcoma of the head and neck resulted in increased immune cell infiltration into distant untreated tumors that was accompanied by tumor regression (32).
Both the Abdel-Hady et al. (30) study on VIN and the Thong et al. (32) study on multifocal angiosarcoma of the head and neck carcinoma show increased CD8+ T cell infiltration into the treated tumor following PDT. The current study demonstrates that PDT enhances recognition of MHC-I: antigen complexes by immune cells, suggesting that PDT of BCC enhances activation of tumor specific CD8+ T cells, which require MHC-I: antigen recognition for activation. The effects of PDT on immune recognition appeared to be greater in patients with superficial lesions as compared to those with nodular lesions. These patients also exhibited better clinical responses 6 months following treatment, suggesting that immune reactivity may contribute to outcome. Clinical response was also greater in patients with higher immune reactivity prior to treatment, which may be indicative of a more robust immune system and thus a better ability to respond to immune enhancement.
PDT enhancement of anti-tumor immunity appears to be inversely correlated with both fluence and fluence rate in preclinical and clinical studies (33, 34). Treatment with higher fluences and fluence rates leads to vascular shut down and limited immune cell infiltrate into the treated area (33, 35) and subsequent anti-tumor immunity (33). A recent case report showing that treatment of angiosarcoma with lower light dose and fluence rate resulted in remission of neighboring and distant untreated lesions (34), suggests induction of anti-tumor immunity occurs at lower light dose and fluence rates. These findings are corroborated by the current results showing that treatment of BCC lesions with lower light doses resulted in greater enhancement of immune recognition of Hip1. The current study also supports subsequent study into the effect of PDT on BCC lesions present outside the treatment field as two of the patients showed regression of untreated lesions following PDT.
Both ALA and Porfimer sodium photosensitizers were used in this study. These photosensitizers are similar in their mechanism of tumor eradication, which occurs directly via tumor cell death due to singlet oxygen release and indirectly via vasculature disruption and inflammation (3). ALA-PDT is thought to have less vascular effects than Porfimer sodium-PDT (36); however the extent to which that influences tumor response is unclear. Both treatments were able to stimulate increased recognition of Hip 1 and no significant difference was observed between the responses of patients treated with ALA-PDT vs. Porfimer sodium-PDT. In addition, there was no significant increase in either CCR or Hip 1 recognition in the nodular BCC patients treated with Porfimer sodium-PDT as compared to those treated with ALA-PDT; however the analysis is limited by the low patient number.
Certain PDT regimens have been shown to systemically suppress immune reactivity in pre-clinical models (reviewed in (37, 38)). The switch from immune enhancing to immune suppressing effects of PDT appears to be linked to the area of skin treated; whole body light irradiation in combination with photosensitizer resulted in immune suppression and reduction in autoimmunity in several model systems (37, 38). Our results show that enhancement of anti-tumor immunity is inversely related to the area treated, which may indicate that treatment of large surface areas leads to immune suppression rather than immune stimulation, although further study is required to confirm this finding.
The ability of PDT to enhance anti-tumor immunity suggest that this treatment modality may be used in an adjuvant setting with treatments that have either no or a negative effect on the patient immune response, such as surgery. Friedberg et al (39) reports increased survival for patients with non-small-cell lung cancer with pleural spread who receive surgery and PDT when compared to patients receiving surgery alone. Pleural PDT is accompanied by an increase in inflammation (13), which has been linked to enhanced anti-tumor immunity following PDT (14, 33).
A number of studies have suggested that the enhancement of anti-tumor immunity following PDT is due to the release of immunogenic peptides and danger signals from dead/dying tumor cells (14, 15, 32), which leads to the activation of dendritic cells and increased stimulation of tumor specific T cells. Dendritic cell activation and increased T cell stimulatory capacity can also be achieved by application of Toll-like receptor (TLR) agonists, such as imiquimod (40). Imiquimod has proven to be efficacious in the treatment of superficial BCC (41) and a recent study suggests that local application of imiquimod and photoimmunotherapy to advanced cutaneous melanoma lesions can result in systemic control of disease (42). Our study further supports the use of PDT as a means to enhance anti-tumor immunity in either a stand-alone or adjuvant setting.
In summary we have shown that treatment of BCC with either Porfimer sodium or ALA-PDT results in an enhancement of the ability of immune cells to recognize and respond to the tumor associated antigen, Hip1. This is the first study to directly examine the ability of PDT to enhance anti-tumor immunity in a clinical setting; the findings provide rationale for further mechanistic studies and for the development of PDT regimens to be used in concert with other cancer therapy modalities to enhance long-term survival and control of distant disease.
Statement of Translational Relevance
This study examines the ability of PDT to enhance immune recognition of tumor cells following treatment of BCC lesions. Numerous pre-clinical studies have suggested that enhancement of anti-tumor immunity plays a critical role in the long-term cure rate mediated by PDT. With the exception of single case and anecdotal reports this is the first study to examine the effect of PDT on the immune response in a clinical setting. The findings support the hypothesis that PDT is able to enhance anti-tumor immunity and suggest that enhancement of anti-tumor immunity is inversely correlated with the extent of the surface area treated and light dose. These results support recent findings that low fluence rates and lower light doses can lead to more effective PDT and suggest that modifications of the current clinical practice of high light dose and fluence rates may result in control of distant disease.
Acknowledgments
Grant Support: NIH Grants CA55791 and CA98156 and in part by the Roswell Park Cancer Center Support Grant CA16056
Footnotes
References
- 1.Brown SB, Brown EA, Walker I. The present and future role of photodynamic therapy in cancer treatment. Lancet Oncol. 2004;5:497–508. doi: 10.1016/S1470-2045(04)01529-3. [DOI] [PubMed] [Google Scholar]
- 2.Dougherty TJ. An update on photodynamic therapy applications. J.Clin.Laser Med.Surg. 2002;20:3–7. doi: 10.1089/104454702753474931. [DOI] [PubMed] [Google Scholar]
- 3.Henderson BW, Gollnick SO. In: Biomedical Photonics Handbook. Vo-Dinh T, editor. Boca Raton, FL: CRC Press; 2003. pp. 36-1–36-27. [Google Scholar]
- 4.Oleinick NL, He J, Xue LY, Separovic D. Stress-activated signalling responses leading to apoptosis following photodynamic therapy. SPIE. 1998;3247:82–88. [Google Scholar]
- 5.Oleinick NL, Evans HH. The photobiology of photodynamic therapy: cellular targets and mechanisms. Radiat Res. 1998;150(5 Suppl.):S146–S156. [PubMed] [Google Scholar]
- 6.Cecic I, Parkins CS, Korbelik M. Induction of systemic neutrophil response in mice by photodynamic therapy of solid tumors. Photochem Photobiol. 2001;74:712–720. doi: 10.1562/0031-8655(2001)074<0712:iosnri>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 7.Gollnick SO, Evans SE, Baumann H, et al. Role of cytokines in photodynamic therapy-induced local and systemic inflammation. Br J Cancer. 2003;88:1772–1779. doi: 10.1038/sj.bjc.6600864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cecic I, Stott B, Korbelik M. Acute phase response-associated systemic neutrophil mobilization in mice bearing tumors treated by photodynamic therapy. Int Immunopharmacol. 2006;6:1259–1266. doi: 10.1016/j.intimp.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Cecic I, Korbelik M. Mediators of peripheral blood neutrophilia induced by photodynamic therapy of solid tumors. Cancer Lett. 2002;183:43–51. doi: 10.1016/s0304-3835(02)00092-7. [DOI] [PubMed] [Google Scholar]
- 10.de Vree WJ, Essers MC, Koster JF, Sluiter W. Role of interleukin 1 and granulocyte colony-stimulating factor in photofrin-based phtodynamic therapy of rat rhabdomyosarcoma tumors. Cancer Res. 1997;57:2555–2558. [PubMed] [Google Scholar]
- 11.Ziolkowski P, Symonowicz K, Milach J, Szkudlarek T. In vivo tumor necrosis factor-alpha induction following chlorin e6-photodynamic therapy in Buffalo rats. Neoplasma. 1996;44:192–196. [PubMed] [Google Scholar]
- 12.Nseyo UO, Whalen RK, Duncan MR, Berman B, Lundahl SL. Urinary cytokines following photodynamic therapy for bladder cancer. Urology. 1990;36:167–171. doi: 10.1016/0090-4295(90)80220-h. [DOI] [PubMed] [Google Scholar]
- 13.Yom SS, Busch TM, Friedberg JS, et al. Elevated serum cytokine levels in mesothelioma patients who have undergone pleurectomy or extrapleural pneumonectomy and adjuvant intraoperative photodynamic therapy. Photochem.Photobiol. 2003;78:75–81. doi: 10.1562/0031-8655(2003)078<0075:esclim>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 14.Castano AP, Mroz P, Hamblin MR. Photodynamic therapy and anti-tumour immunity. Nat Rev Cancer. 2006;6:535–545. doi: 10.1038/nrc1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Canti G, De Simone A, Korbelik M. Photodynamic therapy and the immune system in experimental oncology. Photochem Photobiol Sci. 2002;1:79–80. doi: 10.1039/b109007k. [DOI] [PubMed] [Google Scholar]
- 16.Castano AP, Gad R, Zahra T, Hamblin MR. Specific anti-tumor immune response with photodynamic therapy mediated by benzoporphyrin derivative and chlorin(e6). In: Jacques SL, Duncan DD, Kirkpatrick SJ, Kriete A, editors. Laser Tissue Interaction XIII: Photochemical, Photothermal, and Photomechanical; The International Society for Optical Engineering, Proceedings of SPIE; Bellingham, WA. 2003. pp. 1–9. [Google Scholar]
- 17.Kabingu E, Vaughan L, Owczarczak B, Ramsey KD, Gollnick SO. CD8+ T cell-mediated control of distant tumours following local photodynamic therapy is independent of CD4+ T cells and dependent on natural killer cells. Br J Cancer. 2007;96:1839–1848. doi: 10.1038/sj.bjc.6603792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ingham PW. The patched gene in development and cancer. Curr Opin Genet Dev. 1998;8:88–94. doi: 10.1016/s0959-437x(98)80067-1. [DOI] [PubMed] [Google Scholar]
- 19.Wicking C, Smyth I, Bale A. The hedgehog signalling pathway in tumorigenesis and development. Oncogene. 1999;18:7844–7851. doi: 10.1038/sj.onc.1203282. [DOI] [PubMed] [Google Scholar]
- 20.Bonifas JM, Pennypacker S, Chuang P-T, et al. Activation of expression of hedgehog target genes in basal cell carcinomas. J Invest Dermatol. 2001;116:739–742. doi: 10.1046/j.1523-1747.2001.01315.x. [DOI] [PubMed] [Google Scholar]
- 21.Tojo M, Kiyosawa H, Iwatsuki K, Kaneko F. Expression of a sonic hedgehog signal transducer, hedgehog-interacting protein, by human basal cell carcinoma. Br J Derm. 2002;146:69–73. doi: 10.1046/j.1365-2133.2002.04583.x. [DOI] [PubMed] [Google Scholar]
- 22.Chuang P-T, McMahon AP. Vertebrate hedgehog signalling modulated by induction of a hedgehog-binding protein. Nature. 1999;397:617–621. doi: 10.1038/17611. [DOI] [PubMed] [Google Scholar]
- 23.Vogt A, Chuang PT, Hebert J, et al. Immunoprevention of basal cell carcinomas with recombinant hedgehog-interacting protein. J Exp Med. 2004;199:753–761. doi: 10.1084/jem.20031190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parker KC, Bednarek MA, Coligan JE. Scheme for ranking potential HLA-A2 binding peptides based on independent binding of individual peptide side-chains. J Immunol. 1994;152:163–175. [PubMed] [Google Scholar]
- 25.Elvin J, Cerundolo V, Elliott T, Townsend A. A quantitative assay of peptide-dependent class I assembly. Eur J Immunol. 1991;21:2025–2031. doi: 10.1002/eji.1830210909. [DOI] [PubMed] [Google Scholar]
- 26.Fien SM, Oseroff AR. Photodynamic therapy for non-melanoma skin cancer. J Natl Compr Canc Netw. 2007;5:531–540. doi: 10.6004/jnccn.2007.0046. [DOI] [PubMed] [Google Scholar]
- 27.Oseroff AR, Blumenson LR, Wilson BD, et al. A dose ranging study of photodynamic therapy with porfimer sodium (Photofrin) for treatment of basal cell carcinoma. Lasers Surg Med. 2006;38:417–426. doi: 10.1002/lsm.20363. [DOI] [PubMed] [Google Scholar]
- 28.McMichael AJ, Walker BD. Cytotoxic T lymphocyte epitopes: implications for HIV vaccines. AIDS. 1994;8 Suppl 1:S155–S157. [Google Scholar]
- 29.DiBrino M, Tsuchida T, Turner RV, Parker KC, Coligan JE, Biddison WE. HLA-A1 and HLA-A3 T cell epitopes derived from influenza virus proteins predicted from peptide binding motifs. J Immunol. 1993;151:5930–5935. [PubMed] [Google Scholar]
- 30.Abdel-Hady ES, Martin-Hirsch P, Duggan-Keen M, et al. Immunological and viral factors associated with the response of vulval intraepithelial neoplasia to photodynamic therapy. Cancer Res. 2001;61:192–196. [PubMed] [Google Scholar]
- 31.Dragieva G, Hafner J, Dummer R, et al. Topical photodynamic therapy in the treatment of actinic keratoses and Bowen's disease in transplant recipients. Transplantation. 2004;77:115–121. doi: 10.1097/01.TP.0000107284.04969.5C. [DOI] [PubMed] [Google Scholar]
- 32.Thong PS, Ong KW, Goh NS, et al. Photodynamic-therapy-activated immune response against distant untreated tumours in recurrent angiosarcoma. Lancet Oncol. 2007;8:950–952. doi: 10.1016/S1470-2045(07)70318-2. [DOI] [PubMed] [Google Scholar]
- 33.Kousis PC, Henderson BW, Maier PG, Gollnick SO. Photodynamic therapy (PDT) enhancement of anti-tumor immunity is regulated by neutrophils. Can Res. 2007;67:10501–10510. doi: 10.1158/0008-5472.CAN-07-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thong PS, Olivo M, Kho KW, et al. Immune response against angiosarcoma following lower fluence rate clinical photodynamic therapy. J Environ Pathol Toxicol Oncol. 2008;27:35–42. doi: 10.1615/jenvironpatholtoxicoloncol.v27.i1.40. [DOI] [PubMed] [Google Scholar]
- 35.Henderson BW, Gollnick SO, Snyder JW, et al. Choice of oxygen-conserving treatment regimen determines the inflammatory response and outcome of photodynamic therapy of tumors. Cancer Res. 2004;64:2120–2126. doi: 10.1158/0008-5472.can-03-3513. [DOI] [PubMed] [Google Scholar]
- 36.Henderson BW, Vaughan L, Bellnier DA, van LH, Johnson PG, Oseroff AR. Photosensitization of murine tumor, vasculature and skin by 5-aminolevulinic acid-induced porphyrin. Photochem Photobiol. 1995;62:780–789. doi: 10.1111/j.1751-1097.1995.tb08730.x. [DOI] [PubMed] [Google Scholar]
- 37.Granville DJ, Levy JG, Hunt DWC. Photodynamic treatment with benzoporphyrin derivative monoacid ring A produces protein tyrosine phosphorylation events and DNA fragmentation in murine P815 cells. Photochem Photobiol. 1998;67:358–362. [PubMed] [Google Scholar]
- 38.Hunt DW, Levy JG. Immunomodulatory aspects of photodynamic therapy. Expert Opin Investig Drugs. 1998;7:57–64. doi: 10.1517/13543784.7.1.57. [DOI] [PubMed] [Google Scholar]
- 39.Friedberg JS, Mick R, Stevenson JP, et al. Phase II trial of pleural photodynamic therapy and surgery for patients with non-small-cell lung cancer with pleural spread. J Clin Oncol. 2004;22:2192–2201. doi: 10.1200/JCO.2004.07.097. [DOI] [PubMed] [Google Scholar]
- 40.Bilu D, Sauder DN. Imiquimod: modes of action. Br J Dermatol. 2003;149 Suppl 66:5–8. doi: 10.1046/j.0366-077x.2003.05628.x. [DOI] [PubMed] [Google Scholar]
- 41.Lee S, Selva D, Huilgol SC, Goldberg RA, Leibovitch I. Pharmacological treatments for basal cell carcinoma. Drugs. 2007;67:915–934. doi: 10.2165/00003495-200767060-00007. [DOI] [PubMed] [Google Scholar]
- 42.Naylor MF, Chen WR, Teague TK, Perry LA, Nordquist RE. In situ photoimmunotherapy: a tumour-directed treatment for melanoma. Br J Dermatol. 2006;155:1287–1292. doi: 10.1111/j.1365-2133.2006.07514.x. [DOI] [PubMed] [Google Scholar]