Abstract
Objective
In 2004, after an 18-month investigation, the Food and Drug Administration (FDA) directed pharmaceutical manufacturers to add a black box warning to antidepressants regarding an increased risk of suicidality in children. It has been suggested that news media reporting played a critical role in the sharp declines in pediatric antidepressant use that occurred concurrent with this investigation. Our objective was to evaluate the quality, content and overall impression conveyed in news coverage of this issue.
Methods
We collected all news stories on pediatric antidepressant use and suicidality published in a convenience sample of 10 of the highest circulation print newspapers in the U.S., the three major television networks and a major cable news network in 2003 and 2004 (N=167). Two researchers coded news articles using a nine-item instrument. Item inter-rater reliability was .84 or greater.
Results
The quality of news reporting on key health messages included in FDA warnings was mixed. The overwhelming majority of news stories correctly described a risk of suicidality associated with pediatric antidepressant use as opposed to suicide itself. However, other key health messages highlighted in FDA warnings were often absent from news coverage. In terms of content, news stories, in particular television news, were more likely to include anecdotes of children harmed versus children helped by antidepressants while expert sources quoted were more likely to emphasize the benefits of antidepressants over their risks. However, the majority of news stories conveyed neither the overall impression that the risks of pediatric antidepressant use outweighed the benefits nor that the benefits outweighed the risks, and coverage became increasingly neutral over time.
Conclusions
Including key health messages in FDA safety warnings was not sufficient to ensure their communication to the public via the lay press, even though this information might have mitigated risks of pediatric antidepressant use.
Keywords: pediatric depression, FDA, antidepressants, news media
INTRODUCTION
The news media serves a vital function in conveying health care safety information to the public. Over half of the American public describes national, local, or cable news as their most important source of health information.1 In situations where there is uncertainty regarding the risks and benefits of medical care, the public looks to the media to flag potential safety problems, to interpret scientific data, and to identify qualified experts to comment on how to weigh risks and benefits. Health care providers may also rely on the news media for obtaining timely health safety information.2 Given the prominent role of the news media as an information source for the public and health professionals, assessing the quality and content of news reporting is essential. Disclosure of new risk information on pediatric antidepressant use and suicidality provides an opportunity to evaluate print and television news reporting on a health safety issue in the context of considerable scientific uncertainty.
Safety concerns regarding pediatric antidepressant use surfaced in 2003 when the manufacturer of the antidepressant Paxil identified evidence of increased risk of suicidality in clinical trial data. The FDA issued an advisory in June 2003 warning of possible risks associated with pediatric use of Paxil. In October 2003 and March 2004, the agency issued additional safety warnings regarding a broader set of antidepressants. In September 2004, an FDA advisory panel was presented with results from an FDA-sponsored meta-analysis that found that the rate of suicidality among children assigned to receive SSRIs was twice that of the placebo group.3 This evidence led to a 15 to 8 decision by the panel to recommend a black box warning related to the increased risk of suicidality in pediatric patients for all antidepressant drugs. This recommendation was adopted by the FDA and announced to the public in October 2004.
Supporters of the FDA labeling change argued that evidence of elevated risks of suicidality linked to use of antidepressants in youth was sufficiently serious to warrant warning providers and consumers. Critics countered that no children in the trials actually committed suicide and the FDA actions would reduce the use of an effective treatment for depression thereby producing poorer mental health outcomes in an under-treated population. Adding to the uncertainty, several studies using observational data found that pediatric SSRI use was negatively associated with suicide (i.e., protective) or that there was no significant association between antidepressant use and suicide.4,5,6,7,8,9
Concurrent with the FDA investigation and release of safety warnings, dramatic declines in pediatric antidepressant use occurred.10,11,12,13,14,15,16 It has been suggested that news media reporting on the issue played a critical role in the in severity of these declines,17,18 although to our knowledge no studies have examined the quality or content of media coverage. Prior research offers conflicting evidence on how well the news media functions as a conduit for communicating health risk information to the public. Moynihan and colleagues found that news coverage of medications often included inadequate or incomplete information about the benefits, risks, and costs.19 Schwartz and Woloshin compared news coverage of potential benefits and harms of tamoxifen, and found that the negative aspects of tamoxifen received greater emphasis.20 Another study provided evidence on the positive role of the news media in transmitting warnings about the association between pediatric aspirin use and Reyes Syndrome.21
We examined the quality, content and overall impression conveyed in news coverage of pediatric antidepressant use and suicidality risk in 2003 and 2004. We measure the quality of news reporting by determining whether key health messages emphasized in FDA warnings were mentioned in news coverage. We also examined news content related to the inclusion of anecdotes and expert source quotes and the overall impression of risks and benefits conveyed in news reports. In a recent commentary on health care journalism, Susan Dentzer characterized the news media’s role as “delivering to the public accurate, complete, and balanced messages about health” rather than solely reporting the news interpreted narrowly as “that which is new.”22 Our goal was to assess whether the news media achieved this standard in reporting on pediatric antidepressant use.
DATA AND METHODS
We collected all news stories on pediatric antidepressant use and suicidality published in a convenience sample of 10 of the highest circulation print newspapers in the U.S., the three major television networks (ABC, NBC, and CBS) and a major cable news network (CNN) in 2003 and 2004 (N=167). The news outlets included in our study are USA Today, Wall Street Journal, New York Times, Los Angeles Times, Washington Post, Chicago Tribune, New York Daily News, Philadelphia Inquirer, Denver Post/Rocky Mountain News, and Houston Chronicle. We used data from the Audit Bureau of Circulation to identify daily U.S. newspaper circulation rates23 and Nielsen Media Research to identify television viewership.24 We chose a convenience sample of news outlets with high circulation or viewership to analyze news coverage transmitted to a large subset of the American news-viewing public. In 2004, the daily circulation for the sampled newspapers exceeded 10 million, the three evening network news programs and the morning network news programs were watched by 17 million and 15 million viewers, and the average viewership for CNN daytime and primetime was 490,500 and 799,750, respectively.
News Coverage Selection
We used Lexis-Nexis, Factiva, and newspaper online archives to collect newspaper articles and Lexis-Nexis to collect television transcripts using the search terms suicide, antidepressant, and children. For a story to be included, all three terms or a close variant (e.g., suicidality, SSRI, pediatric) needed to appear in the story. We identified 327 newspaper articles and 191 television transcripts that met these search criteria. Our interest was in analyzing only hard news stories, feature stories, or health column stories. We excluded stories coded as editorials, corrections, book reviews, letters to the editor, business/stock information, Q&A format, duplicate wire stories, index, or obituaries. We also excluded stories where antidepressants and suicidality were not the primary focus and stories in which the primary focus was not children or adolescents. All exclusions were determined by the study authors (CLB, SHB) for a total of 167 stories analyzed.
Content Analysis
To analyze the quality, content and overall impression conveyed in each news story, we developed a nine-item coding instrument. The instrument was pilot tested by the authors, and minor adjustments were made to clarify question wording and item response coding. Once the instrument was finalized, each of the 167 articles was independently coded by each author. Inter-rater reliability for all items measured using kappa statistics was high. Raw agreement for a single, more subjective item assessing overall impression was 92% (κ=.84). Raw agreement was higher for the other items ranging from 93 to 99% (range for κ=.85 to κ=.99). To resolve coding disputes on specific items, the authors discussed and reached agreement on final coding. There were no items for which agreement could not be reached.
Measures
We included four measures of quality in news reporting. First, we examined whether news reports correctly described the association between pediatric antidepressant use and suicidality (versus suicide). Reporting an association between antidepressant use and suicide itself would constitute poor quality news reporting. We also examined inclusion of other key health messages emphasized in FDA safety warnings. These included: the importance of monitoring a child using antidepressants, the importance of tapering medication during antidepressant discontinuation, and that fluoxetine is the only FDA approved pharmaceutical treatment for pediatric depression. We chose to be expansive in our measure of monitoring and included references to medication monitoring as well as more general references to parent or physician monitoring. We also note that the warning stressed not only that fluoxetine was the only drug approved for pediatric depression, but it was also the only drug with proven efficacy in this population. Prior to FDA action, all three pieces of health information were available to the medical profession. However, since the FDA emphasized these health messages in each of its advisories beginning in October 2003, we viewed their inclusion in media coverage as an indicator of high quality reporting.
Next, we identified whether the news story referenced an individual child/adolescent who it was claimed was (a) harmed or (b) helped by antidepressant use. To be included, the child/adolescent had to be referenced specifically by name or as the child of a named parent. Conditional on a story referencing an individual child, we examined whether children harmed or helped were more often referenced. We also identified whether the news story included at least one quote from an expert source suggesting that (a) the risks of pediatric antidepressant use may outweigh the benefits or (b) the benefits of pediatric antidepressant may use outweigh the risks. Conditional on a news story including an expert quote on risks and benefits, we examined whether risk quotes or benefit quotes were more likely to be included.
Finally, we included one measure to assess whether the news story left the overall impression that the: (a) risks of pediatric antidepressant use outweigh the benefits; (b) benefits of pediatric antidepressant use outweigh the risks, or (c) neither. We chose a conservative approach and when only a weak overall impression of risk or benefit was conveyed, the story was coded as “neither.”
Data Analysis
We analyzed the quality, content and overall impression conveyed in news coverage in 2003 and 2004, and during the two week period following three FDA actions: the March 2004 advisory, the September 2004 panel vote, and the October 2004 decision. The news story was the unit of analysis. We tested differences between proportions of print and television news stories using logistic regression. We adjusted standard errors for lack of independence within news source. All analyses were conducted with STATA, version 9.
RESULTS
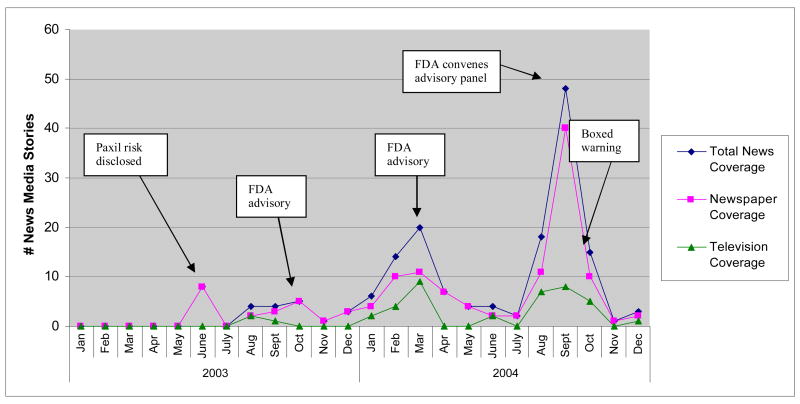
As indicated in Table 1, the study included 126 newspaper and 41 television stories reporting on pediatric antidepressant use and suicidality in 2003 and 2004. Figure 2 shows that the volume of news stories in our sample increased following FDA actions, and that peak coverage occurred with the FDA advisory panel’s vote to recommend issuance of a black box warning.
Table 1.
Descriptive Information on News Stories
| Characteristic | News Stories 2003–2004 no. (%) |
|---|---|
| Total news stories | 167 (100.0) |
| Print news stories | 126 (75.5) |
| Television news stories | 41 (24.5) |
| Year | |
| 2003 | 25 (15.0) |
| 2004 | 142 (85.0) |
| News stories in two week period following each specific FDA action | |
| 3/22/04 advisory1 | 17 (10.2) |
| 9/12–13/04 meeting2 | 41 (24.5) |
| 10/15/04 advisory3 | 12 (7.2) |
| Story length (mean words) | |
| Print news stories | 922 |
| Television news stories | 606 |
| Newspaper story type (N=126)4 | |
| Front page | 24 (19.0) |
| Byline | 87 (68.0) |
| News section (not front page) | 72 (57.1) |
| Business section | 8 (6.3) |
| Health/science section | 14 (11.1) |
| Other section | 6 (4.8) |
News stories during two week period from 3/22-4/05/2004.
News stories during two week period from 9/12-9/26/2004.
News stories during two week period from 10/15-10/29/2004.
Information is relevant to print news sources only.
Key Health Messages Emphasized in FDA Warnings
Table 2 indicates the quality of news reporting on key health messages emphasized in FDA warnings was mixed. The overwhelming majority of news stories (98%) correctly described a risk of suicidality associated with pediatric antidepressant use as opposed to suicide itself. However, other key health messages highlighted in FDA warnings were often absent from news coverage. Only 43% noted the importance of monitoring children using antidepressants, 13% mentioned that medications should be tapered when discontinued, and 40% mentioned fluoxetine as the only FDA approved pharmaceutical treatment for pediatric depression. Generally, news stories in the two weeks following FDA actions were no more likely to mention health information than in other time periods. The notable exception was the high level of coverage noting the importance of monitoring following the March 2004 advisory (88%).
Table 2.
News Media Coverage of Key Health Messages Emphasized in FDA Warnings
| News stories 2003–2004 |
News stories in any two week period following FDA action1 | News stories from other Time periods2 | News stories in two week period following specific FDA actions | |||
|---|---|---|---|---|---|---|
| 3/22/04 advisory3 | 9/12–13/04 panel meeting4 | 10/15/04 advisory5 | ||||
| Mention data indicates an increased risk of suicidality (versus suicide) (%) | 97.6 | 98.7 | 96.6 | 100.0 | 100.0 | 100.0 |
| Mention importance of monitoring (%) | 43.1 | 44.3 | 42.1 | 88.2 | 26.8 | 58.3 |
| Mention that when discontinuing, medication should be tapered (%) | 13.2 | 12.7 | 13.6 | 17.7 | 4.9 | 0.0 |
| Mention fluoxetine only FDA approved pharmaceutical treatment for pediatric depression (%) | 40.1 | 34.2 | 45.5 | 17.7 | 39.0 | 33.3 |
| N | 167 | 79 | 88 | 17 | 41 | 12 |
News stories during any of the two week periods following five FDA actions on pediatric antidepressant use and suicidality including release of the Paxil statement 6/19-7/03/2003, the first FDA advisory 10/27-11/10/2003, the second FDA advisory 3/22-4/05/2004, the FDA advisory meeting 9/12-9/26/2004, and the boxed warning decision 10/15-10/29/2004.
News stories during time periods other than the two weeks following the five FDA actions on antidepressant use and suicidality.
News stories during two week period from 3/22-4/05/2004.
News stories during two week period from 9/12-9/26/2004.
News stories during two week period from 10/15-10/29/2004.
Use of Anecdotes and Expert Quotes
Table 3 indicates that television news was significantly more likely than print news to include anecdotes involving individual children’s experiences with antidepressants, and both print and television new stories were more likely to include references to specific children harmed (35%) than helped (14%). Among news stories including an anecdote about a child (N=66), 89% mentioned a child who was harmed while only 36% mentioned a child who was helped.
Table 3.
Use of Anecdotes and Expert Source Quotes by Type of News Source
| News Stories 2003–2004 |
Print News Stories | Television News Stories | |
|---|---|---|---|
| Individual child referenced | |||
| Does the news story reference an individual child/adolescent who it is claimed was: | |||
| Harmed by antidepressant use? (%) | 35.3 | 27.8 | 58.5** |
| Helped by antidepressant use? (%) | 14.4 | 11.9 | 22.0* |
| Either harmed or helped by antidepressant use (%) | 39.5 | 31.0 | 65.9*** |
| Among news stories referencing an individual child/adolescent who was either harmed or helped by antidepressant use (N=66): | |||
| Story mentions a child/adolescent who was harmed (%) | 89.4 | 89.7 | 88.9 |
| Story mentions a child/adolescent who was helped (%) | 36.4 | 38.5 | 33.3 |
| Expert source quoted1 | |||
| Does new story include at least one quote from an expert source suggesting that: | |||
| Risks may outweigh benefits? (%) | 20.4 | 23.8 | 9.8 |
| Benefits may outweigh risks? (%) | 32.3 | 31.8 | 34.2 |
| Either risks may outweigh benefits or benefits may outweigh risks (%) | 43.7 | 44.4 | 41.5 |
| Among news stories including at least one quote from an expert source suggesting either the risks of antidepressant use outweigh the benefits or the benefits outweigh the risks (N=73): | |||
| Story mentions risks outweigh benefits (%) | 46.6 | 53.6 | 23.5 |
| Story mentions benefits outweigh risks (%) | 74.0 | 71.4 | 82.4 |
| N | 167 | 126 | 41 |
significant at 10%;
significant at 5%;
significant at 1%
Expert sources do not include family members or medical correspondents.
In contrast, fewer news stories included a quote from an expert source suggesting that the risks of pediatric antidepressant outweighed the benefits (20%), than suggested that the benefits outweighed the risks (32%). Among just the subset of news stories including an expert quote mentioning risks and benefits (N=73), a greater proportion mentioned that the benefits of antidepressants outweighed the risks (74%) than the risks of antidepressant use outweighed the benefits (47%). We did not detect statistically significant differences in expert source quotes related to risks and benefits by print versus television news.
Overall Impression of Risks and Benefits
The majority of stories (62%) conveyed neither the overall impression that the risks of pediatric antidepressant use outweighed the benefits nor that the benefits outweighed the risks (Table 4). The 38% of news stories that were not neutral were significantly more likely to convey the impression that the risks of pediatric antidepressant use outweighed the benefits (35%) than that the benefits outweighed the risks (4%). News stories became more neutral over time in their portrayal of risks and benefits. Following the March 2004 advisory, 53% of news stories emphasized the risks of antidepressants compared with only 8% of news stories following the October 2004 decision.
Table 4.
Overall Impression of Risks and Benefits of Pediatric Antidepressant Use
| News stories 2003–2004 |
News stories in two week period following specific FDA actions | |||
|---|---|---|---|---|
| 3/22/04 Advisory1 | 9/12–13/04 panel meeting2 | 10/15/04 Advisory3 | ||
| Does news story leave the overall impression that: | ||||
| Risks of pediatric antidepressant use outweigh benefits (%) | 34.7 | 53.0 | 27.0 | 8.3 |
| Benefits of pediatric antidepressant use outweigh risks (%) | 3.6 | 0.0 | 0.0 | 0.0 |
| Neither (%) | 61.7 | 47.0 | 73.0 | 91.7 |
| N | 167 | 17 | 41 | 12 |
News stories during two week period from 3/22-4/05/2004.
News stories during two week period from 9/12-9/26/2004.
News stories during two week period from 10/15-10/29/2004.
DISCUSSION
The purpose of this study was to assess the quality, content and overall impression conveyed in news reporting on FDA warnings pertaining to pediatric antidepressant use among national and regional news sources with high circulation or viewership. We found mixed evidence on the quality of news reporting. The overwhelming majority of news reports accurately described the association between pediatric antidepressant use and suicidality rather than suicide itself, an indicator of good quality reporting. However, other key health messages highlighted in FDA warnings pertaining to the importance of monitoring, the need to taper upon medication discontinuation and the FDA’s approval of fluoxetine to treat depression in children were often absent from news reports.
These findings suggest that including key health messages in FDA safety warnings was not sufficient to ensure its communication to the public via the lay press, even though this information might have mitigated the risks of pediatric antidepressant use. One research study found that monitoring did not increase following warnings despite the FDA’s emphasis on monitoring,25 and use of fluoxetine by incident child antidepressant users was still only 27% in September 2004 (up from 15% in October 2003).26 Beyond issuing safety warnings and contraindicating medications, the FDA relies on a number of other methods for informing consumers and providers about safety risks including the use of medication guides which include practical and reader-friendly product safety information. Nonetheless, the agency depends in part on the press to fulfill its mandate to communicate risk and benefit information to the public. Simply including key health messages in FDA press releases was not sufficient to ensure that journalists mention this information in news stories.
News stories, in particular television news, were more likely to include anecdotes of children harmed by antidepressants than children helped while expert source quotes were more likely to emphasis the benefits of antidepressants over their risks. That television news reporting relied heavily on anecdotes of children harmed by antidepressants is consistent with prior research indicating that television news tends to frame coverage in more personal terms by emphasizing individual experiences.27 Given evidence that patients may disproportionately weigh anecdotal versus statistical evidence,28 the use of anecdote of children harmed in news coverage could potentially have led to larger declines in pediatric antidepressant use than would have otherwise occurred.
While the greater emphasis on the benefits of antidepressants in expert source quotes may serve as a balance to use of anecdotes, increasing attention to conflicts of interest in medicine raise concern about journalists’ use of physicians or researchers as sources in the absence of disclosure policies. In this study, none of the news stories coded included specific information about whether the experts interviewed had specific conflicts of interest (e.g., receiving funding from the pharmaceutical industry). This is consistent with a recent study reporting that news articles reporting on medication studies often fail to report pharmaceutical company funding.29
Finally, the majority of news stories conveyed neither the overall impression that the risks of pediatric antidepressant use outweighed the benefits nor that the benefits outweighed the risks, and coverage became increasingly neutral over time.
A number of limitations are worth mentioning. Our results are limited to the extent the public and medical professionals learned about safety concerns related to pediatric antidepressant use via non-traditional news sources including blogs and other internet news sites. Given the current turmoil in the newspaper industry, the public is likely to rely increasingly heavily on these alternative sources of health information in the future. Likewise, it was not feasible to include local television news or smaller newspaper outlets since these news sources are not readily available to researchers for study. Finally, while families’ exposure to risk and benefit information via the news media may explain subsequent treatment patterns, assessing this causal relationship is beyond the scope of our study.
Because depression is an under-treated disease30 with the potential for long term negative consequences, the steep declines in pediatric antidepressant use that occurred in the aftermath of the FDA risk disclosure are troubling to the extent that they represent an increase in unmet need. Clinical trial data indicated that at least one antidepressant, fluoxetine, was clinically effective in treating children with major depressive disorder; 31 the efficacy of other antidepressants is treating children has not been proven. In the 1990s, increased antidepressant use following the introduction of SSRIs was hailed by many as an important advancement in public health. Steady declines in youth suicide rates suggested to some that antidepressants were saving lives. This trend reversed unexpectedly with the upswing in national youth suicide rates in 2004 and 2005, leading to the speculation that less antidepressant use following FDA safety warnings may have increased rather than decreased youth suicide, although other explanations are also possible (e.g., changes in youth access to firearms, the prevalence of alcohol use, web-based social networks). 32
In future years, the public may be expected to rely more on both traditional and non-traditional media to obtain health information given lowering consumer trust in the medical profession33 and the movement toward a more consumer-oriented health system. As medical decision making grows more complex, health care journalists are increasingly challenged to convey complex health information to the public. Further empirical research is needed to better understand how news reporting on medical risks and benefits may affect who does and does not get treated.
Figure 1.
News Stories on Pediatric Antidepressant Use and Suicidality, 2003–2004
List of Abbreviations
- FDA
Food and Drug Administration
Footnotes
Financial Disclosures:
The authors report no conflicts of interest. This study was supported by a grant from the National Institute of Mental Health (1 R01 MH 080883-01), of which Dr. Busch is principal investigator.
Contributor Information
Colleen L. Barry, Email: colleen.barry@yale.edu, Associate Professor of Public Health, Yale University School of Medicine, Department of Epidemiology and Public Health, 60 College Street, New Haven CT 06520, phone: (203) 785-4956, fax: (203) 785-6287
Susan H. Busch, Email: susan.busch@yale.edu, Associate Professor of Public Health, Yale University School of Medicine, Department of Epidemiology and Public Health, 60 College Street, New Haven CT 06520, phone: (203) 785-2927
References
- 1.Kaiser Family Foundation/Harvard School of Public Health. September/October 2001 Health News Index. Menlo Park, CA: Kaiser Family Foundation; 2001. [Google Scholar]
- 2.Dentzer S. Communicating Medical News: Pitfalls of Health Care Journalism. New England Journal of Medicine. 2009;360(1):1–3. doi: 10.1056/NEJMp0805753. [DOI] [PubMed] [Google Scholar]
- 3.Newman TB. A Black-Box Warning for Antidepressants in Children? New England Journal of Medicine. 2004;351(16):1595–1598. doi: 10.1056/NEJMp048279. [DOI] [PubMed] [Google Scholar]
- 4.Ludwig J, Marcotte DE. Anti-depressants, Suicide and Drug Regulation. Journal of Policy Analysis and Management. 2005;24(2):249–272. doi: 10.1002/pam.20089. [DOI] [PubMed] [Google Scholar]
- 5.Olfson M, Shaffer D, Marcus SC, et al. Relationship between antidepressant medication treatment and suicide in adolescents. Archives of General Psychiatry. 2003;60:978–82. doi: 10.1001/archpsyc.60.9.978. [DOI] [PubMed] [Google Scholar]
- 6.Rihmer Z, Belso N, Kalmar S. Antidepressants and Suicide Prevention in Hungary. Acta Psychiatrica Scandinavia. 2001;103:238–39. doi: 10.1034/j.1600-0447.2001.103003238.x. [DOI] [PubMed] [Google Scholar]
- 7.Ohberg A, Vuor E, Klaukka T, et al. Antidepressants and Suicide Mortality. Journal of Affective Disorders. 1998;50:225–33. doi: 10.1016/s0165-0327(98)00114-1. [DOI] [PubMed] [Google Scholar]
- 8.Hall WD, Mant A, Mitchell PB, et al. Association Between Antidepressant Prescribing and Suicide in Australia, 1991–2000: Trend Analysis. British Medical Journal. 2003;326:1008–13. doi: 10.1136/bmj.326.7397.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valuck RJ, Libby AM, Sills MR, Giese AA, Allen RR. Antidepressant Treatment and Risk of Suicide Attempt by Adolescents with Major Depressive Disorder. CNS Drugs. 2004;18(15):119–132. doi: 10.2165/00023210-200418150-00006. [DOI] [PubMed] [Google Scholar]
- 10.Rosack J. New Data Show Declines in Antidepressant Prescribing. Psychiatric News. 2005;40(17) [Google Scholar]
- 11.Harris G. Study Finds Less Youth Antidepressant Use. New York Times. 2004 September 21; [Google Scholar]
- 12.Gibbons RD, Hendricks Brown C, Hur K, Marcus SM, Bhaumik DK, Erkens JA, et al. Early Evidence on the Effects of Regulators’ Suicidality Warnings on SSRI Prescriptions and Suicide in Children and Adolescents. American Journal of Psychiatry. 2007;164:1356–1363. doi: 10.1176/appi.ajp.2007.07030454. [DOI] [PubMed] [Google Scholar]
- 13.Libby AM, Brent DA, Morrato EH, Orton HD, Allen R, Valuck RJ. Decline in Treatment of Pediatric Depression after FDA on Risk of Suicidality with SSRIs. American Journal of Psychiatry. 2007;164:884–891. doi: 10.1176/ajp.2007.164.6.884. [DOI] [PubMed] [Google Scholar]
- 14.Olfson M, Marcus SC, Druss BG. Effects of Food and Drug Administration Warnings on Antidepressant Drug Use in a National Sample. Archives of General Psychiatry. 2008;65(1):94–101. doi: 10.1001/archgenpsychiatry.2007.5. [DOI] [PubMed] [Google Scholar]
- 15.Nemeroff CB, Kalali A, Keller MB, Charney DS, Lenderts SE, Cascade EF, Stephenson H, Schatzberg AF. Impact of Publicity Concerning Pediatric Suicidality Data on Physician Practice Patterns in the United States. Archives General Psychiatry. 2007;64(4):466–72. doi: 10.1001/archpsyc.64.4.466. [DOI] [PubMed] [Google Scholar]
- 16.Busch SB, Frank RG, Barry CB. Declines in Pediatric Antidepressant Use After Safety Warnings: Who Stopped Using Medications? Working Paper. [Google Scholar]
- 17.Arehart-Treichel J. Suicide Attempts Decline with Psychotherapy or Antidepressants. Psychiatric News. 2007;45(15) [Google Scholar]
- 18.Satel S. Bad Medicine? National Review. 2004 September 12; [Google Scholar]
- 19.Moynihan R, Bero L, Ross-Degnan D, Henry D, Lee K, Watkins J, et al. Coverage by the News Media of the Benefits and Risks of Medication. New England Journal of Medicine. 2000;342(22):1645–1650. doi: 10.1056/NEJM200006013422206. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz LM, Woloshin S. News Media Coverage of Screening Mammography for Women in their 40s and Tamoxifen for Primary Prevention of Breast Cancer. JAMA. 2002;287:3136–3142. doi: 10.1001/jama.287.23.3136. [DOI] [PubMed] [Google Scholar]
- 21.Soumerai S, Ross-Degnan D, Kahn JS. Effects of Professional and Media Warnings about the Association Between Aspirin Use in Children and Reye’s Syndrome. Milbank Quarterly. 1992;70(1):155–182. [PubMed] [Google Scholar]
- 22.Dentzer S. Communicating Medical News Pitfalls of Health Care Journalism. New England Journal of Medicine. 2009;360(1):1–3. doi: 10.1056/NEJMp0805753. [DOI] [PubMed] [Google Scholar]
- 23.Newspaper circulation figures for the six months ending on March 31, 2004 filed with the Audit Bureau of Circulation and compiled by BurrellesLuce.
- 24.Television network and cable average viewership figures for 2004 collected by Nielsen Media Research and reported by the Pew Research Center’s Project for Excellence in Journalism: http://www.journalism.org/node/883
- 25.Morrato EH, Libby AM, Orton HD, Frank DeGruy, Brent DA, Allen R, Valuck RJ. Frequency of Provider Contact after FDA Advisory on Risk of Pediatric Suicidality with SSRIs. American Journal of Psychiatry. 2008;165:42–50. doi: 10.1176/appi.ajp.2007.07010205. [DOI] [PubMed] [Google Scholar]
- 26.Busch SH, Frank RG, Rosenheck R, Leslie D, Martin A, Barry CL. Antidepressants and Suicide Risk: How Did Risk Disclosure Affect Treatment Patterns in Youth? doi: 10.1176/appi.ps.61.1.11. Working Paper. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iyengar S. How Television Frames Political Issues. Chicago, IL: University of Chicago Press; 1991. Is Anyone Responsible? [Google Scholar]
- 28.Fagerlin A, Wang C, Ubel PA. Reducing the Influences of Anecdotal Reasoning on People’s Health Care Decisions: Is a Picture Worth a Thousand Statistics? Medical Decision Making. 2005:398–405. doi: 10.1177/0272989X05278931. [DOI] [PubMed] [Google Scholar]
- 29.Hochman M, Hochman S, Bor D, McCormick News Media Coverage of Medication Research: Reporting Pharmaceutical Company Funding and Use of Generic Medication Names. JAMA. 2008;300(13):1544–1550. doi: 10.1001/jama.300.13.1544. [DOI] [PubMed] [Google Scholar]
- 30.Kessler RC, Demler O, Frank RG, et al. Prevalence and Treatment of Mental Disorders, 1990–2003. New England Journal of Medicine. 2005;352(24):2515–2523. doi: 10.1056/NEJMsa043266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Treatment for Adolescents with Depression Study Team. Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for Adolescents With Depression Study (TADS) randomized controlled trial. JAMA. 2004;292:807–820. doi: 10.1001/jama.292.7.807. [DOI] [PubMed] [Google Scholar]
- 32.Bridge JA, Greenhouse JB, Weldon AH. Suicide Trends Among Youths Aged 10 to 19 Years in the United States, 1996–2005. JAMA. 2008;300(9):1025–1026. doi: 10.1001/jama.300.9.1025. [DOI] [PubMed] [Google Scholar]
- 33.Schlesinger M. A loss of faith: the sources of reduced political legitimacy for the American medical profession. Milbank Quarterly. 2002;80(2):185–235. doi: 10.1111/1468-0009.t01-1-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]