Abstract
Caveolin-1 (Cav-1), a member of the caveolin family, regulates caveolae-associated signaling proteins, which are involved in many biological processes, including cancer development. Cav-1 was found to exert a complex and ambiguous role as oncogene or tumor suppressor depending on the cellular microenvironment. Here we investigated Cav-1 expression and function in a panel of melanomas, finding its expression in all the cell lines. The exception was the primary vertical melanoma cell line, WM983A, characterized by the lack of Cav-1, and then utilized as a recipient for Cav-1 gene transduction to address a series of functional studies. The alleged yet controversial role of phospho-Cav-1 on cell regulation was also tested by transducing the non phosphorylatable Cav-1Y14A mutant. Wild type Cav-1, but not mutated Cav-1Y14A, increased tumorigenicity as indicated by enhanced proliferation, migration, invasion and capacity of forming foci in semisolid medium. Accordingly, Cav-1 silencing inhibited melanoma cell growth reducing some of the typical traits of malignancy. Finally, we detected a secreted fraction of Cav-1 associated with cell released microvesicular particles able to stimulate in vitro anchorage independence, migration and invasion in a paracrine/autocrine fashion and, more important, competent to convey metastatic asset from the donor melanoma to the less aggressive recipient cell line. A direct correlation between Cav-1 levels, the amount of MV released in the culture medium and MMP-9 expression was also observed.
Keywords: Melanoma, Caveolin-1, microvesicles, tumorigenicity
INTRODUCTION
Cav-1 is a 22–24 KDa protein originally identified as a structural component of caveolae, specialized invaginations of the plasma membrane. These caveolae represent compartments where key signaling transduction molecules are concentrated in order to provide an efficient system for cellular cross talk. Through its “scaffold domain” Cav-1 is able to self-interact and to regulate the activity of other caveolae-associated signaling proteins (1). When Cav-1 was evaluated for its expression in human cancer cell lines and tumor samples of different origin, the outcome was ambiguous. The observed expression profiles indicated that the role of Cav-1 varied according to tumor types (2, 3).
Although its growth inhibitory action has been clearly demonstrated in vivo in Cav-1−/− mice (4), more recent data supported the direct correlation between Cav-1 expression level and tumor aggressiveness (5) and, in some cases, with a poorer clinical outcome (6). Hence Cav-1 apparently possesses mutually exclusive functions, as tumor suppressor or tumor promoting gene, depending on tumor type/stage, cell context and the deriving availability of Cav-1 interacting partners. A specific example indicating the importance of Cav-1 partnership involves the formation of a Cav-1/E-cadherin complex at the plasma membrane. In primary cancer cells, where E-cadherin (E-cad) is generally expressed, Cav-1 exerts its anti-proliferative and pro-apoptotic properties, but in highly metastatic cells from colon or melanoma, where E-cad is usually silent, this ability is lost (7).
The two opposite roles of Cav-1 are also relatable to post-translational modifications of the different protein domains. One of them is the phosphorylation on tyrosine-14 by Src family kinases (8) induced by a number of stimuli. Cav-1 Y14-phosphorylation has been also associated with its translocation and clustering with integrins into focal adhesions (9, 10), suggestive of its role in cell adhesion, migration and invasion.
Finally, Cav-1 has been reported to be present in the secretory cellular components of pancreas and salivary glands (11), in differentiating osteoblasts (12) and in cancer (13). In this regard it is important to point out that in prostate carcinoma the secreted fraction of Cav-1 is associated with cell released microvescicles (MV) which are capable to confer tumorigenicity in vitro as well as in vivo (13–15). Differences in the protein composition of these so called “prostasomes”, recovered from healthy donors and prostate cancer cells, have been also shown, being Cav-1 undetectable in normal prostate epithelium and expressed in MV isolated from prostate cancer cell lines (16). Moreover in advanced prostate cancer the level of Cav-1 in the serum of patients appears as a significant diagnostic and prognostic marker (14, 15, 17).
Although Cav-1 expression and localization have been investigated in a number of human tumors (3), its possible function in human melanomas still remain elusive.
Here we analyzed Cav-1 expression level and role in a panel of differently staged melanoma cell lines in order to test whether Cav-1 might be causally involved in melanoma tumorigenicity. Moreover, we examined the possibility for Cav-1 to be exported by melanoma cells in secretory vesicles and their functional association with melanoma progression.
MATERIALS AND METHODS
Cell line culture and gene transduction
The human melanoma cell lines used in the current study were already described (18). The Cav-1 cDNA encompassing its complete coding sequence was cloned into the retroviral vector LXSN and infections performed as previously described (19). For transfection experiments the cDNA encoding Cav-1 was also subcloned into the pCB7vector, as described (20).
RNA analysis
The sequences of Cav-1 primers, utilized for RT-PCR analysis, were dir 5′-TCTTTGGCATCCCGATGG-3′ and rev 5′-GTTGATGCGGACATTGCT-3′. The sequences of the other primers are reported (19). The expression levels were evaluated, after exposure to a Typhoon Instrument, by the Image Quant software (Amersham Biosciences, UK). Real-time RT–PCR was performed by TaqMan technology, using the ABI PRISM 7700 DNA Sequence Detection System according to standard procedures. Commercial ready-to-use primers/probe mixes were also used (Assay on Demand #Hs00184697_m1, Applied Biosystems, Foster City, CA).
Cav-1 RNA silencing
Antisense experiments
Antisense inhibition studies with anti-Cav-1 oligomers were carried out as described (21). The oligo sequences used for Cav-1 experiments were: antisense 5′-TGGCCCGTGGCTGGATGA-3′ and scrambled 5′-CTGGTACGTATTCACTGT-3′.
Small interfering RNA
Cav-1 has been silenced by using a retroviral system based on the pSilencer 5.1-H1 vector (Ambion). Two different siRNA sequences targeting Cav-1 were selected and utilized in all the functional assays. Sequence so called “A” was designed with the aid of the Ambion sequence design software, whereas sequence “L” derives from Williams et al. 2005. The chosen sequences consisted of a 21–23 nt sense sequence linked to its reverse complementary antisense by a short spacer, being the resulting RNA transcript expected to fold back and form a stem loop. Stable cell lines were produced by retroviral infection and puromycin selection, according to standard techniques. As a control, a si-cyclophilin A infected cell line was also produced. Sense siCav-1#A 5′ AAAUACGUAGACUCGGAGGGAUU 3′ Sense siCav-1#L 5′ GCAAGTGTACGACGCGCACAC 3′.
Western blot in total cell lysates
Protein expression was evaluated in total cell lysates and in caveolae membrane fractions according to standard procedures. The antibodies used were: anti-Cav-1 polyclonal and anti.-MMP-2(H-76) polyclonal (Santa Cruz Biotechnology Inc., Santa Cruz, CA), anti-Ph-Cav-1(Y14) monoclonal (Transduction Laboratories, Lexington, UK), anti-MMP9 (Ab-8) monoclonal (Calbiochem, Darmstadt, Germany), anti-Lamp2 monoclonal (BD Pharmingen, S. Diego, CA). As a control, we used an anti-actin monoclonal antibody (Oncogene Research Products, Boston, MA).
Immunofluorescence analysis
Cells, fixed and permeabilized, according to standard procedures, were labeled with primary specific antibody 30 min at 37°C, followed by a goat Alexa Fluor 594 secondary antibody (Molecular Probes, Eugene, OR). Cells were then observed on a Leica TCS 4D apparatus. Image acquisition and processing were realized by using the Scanware Multicolor analysis (Leica Lasertechnik GmbH, Heidelberg, Germany).
Zymographic analysis
Zymography was performed using sodium dodecyl sulphate-polyacrylamide gels (7.5%) copolymerized with 1mg/ml gelatine type B. Samples were resuspended in cell lysis buffer (0.25% Triton X-100 and 10mM CaCl2), incubated 4 minutes at 60 °C and loaded. After electrophoresis, gels were washed twice for 15 minutes in 2.5% Triton X-100 at room temperature and incubated overnight at 37°C in Tris pH7.5, CaCl2 10mM, ZnCl2 1μM and Triton X-100 1%. Gels were then stained in Coomassie Blue R250 in methanol:acetic acid 4.5:1 for 1 hr and destained in ethanol:acetic acid 1:2.5. Bands were visualized and quantified by the Image Quant software in a Typhoon Instrument, (Amersham Biosciences, UK).
In vitro growth, migration and invasion
Functional studies were performed as previously described (18). The proliferative rate of melanoma cells was evaluated by a colorimetric assay XTT-based (Roche Molecular Biochemicals, Mannheim, Germany) and quantified using an ELISA plate reader (Wallac VICTOR2, Turku, Finland).
Migration was assayed, as previously described (22), using uncoated cell culture inserts (Corning Costar Corporation, Cambridge, MA) with 8μm pores. Five ×104 cells were placed in the upper compartment in 100 μl of DMEM serum-free, while 600 μl of DMEM supplemented with 10% FBS were placed into the lower compartment of the chamber. For invasion studies, membranes were coated with 100μg/cm2 of Matrigel growth factor reduced (Becton Dickinson, Bedford, MA) as a barrier, and 6×104−105 or cells were placed in the upper compartment. Assays were incubated at 37° C in 5% CO2. After 24–48 hr the cells attached to the upper side of the membrane were removed with a cotton swab; each membrane was fixed and stained with crystal violet solution (23). Chemotaxis and invasiveness, as relative number of cells on the undersurface of the membrane, were evaluated by a colorimetric assay at 595 nm in a microplate reader (Wallac VICTOR2, Turku, Finland). In some experiments microvesicles and/or a polyclonal Cav-1 antibody were added in the upper compartment.
The data were expressed as the mean absorbance ± SD for triplicate wells. The statistical analysis was performed by Student’s t test in all the reported experiments.
Growth in semisolid medium
Base layers of complete DME medium containing 0.5% agar were set in 60 mm plastic dishes. The bottom agar was overlaid with 1.5 ml of 0.3% (soft) or 0.9% (hard) agar containing 104 cells. Cultures were incubated for 4–6 weeks at 37°C and the colonies counted using an inverted microscope. Three experiments were performed for each cell line and results calculated as the average ± SD of three dishes for each condition.
Conditioned medium preparation
Conditioned media were prepared according to standard procedures (13). Briefly, 2×106cells/ml, were grown in 1% fetal bovine serum for 24/48 hr. After concentration of supernatants with Microcon YM-10 (Millipore), the secreted proteins were quantitated by spectrophotometer analysis and equal amounts (40 μg) of each sample were loaded for Western blot.
In migration and invasion studies the concentrated CM recovered from Cav-1-transduced WM983A cells was applied in the upper well of the chamber together with WM983A/LXSN cells in serum-free conditions. In some experiments a polyclonal Cav-1 antibody was added to the CM.
Microvesicles (MV) isolation
Melanoma cells were cultured with DME medium supplemented with 10% fetal bovine serum (FBS) previously deprived of bovine microvesicles (MVs) by ultracentrifugation (60 min at 100,000 × g). MVs, isolated by sequential centrifugations from supernatant of exponentially growing cells (cultured from 48 up to 72 hours), were purified on sucrose gradient (24,25). Gradient fractions were collected and analyzed by western blotting. Lamp-2 and Cav-1 positive fractions were pooled and used in all the experiments.
Eighty μg of Me665/1 MVs were layered on 60% to 10% continuous sucrose gradient and 35 μl from fraction 1–11 analyzed by Western blotting for Cav-1 and Lamp-2. Results indicated the presence of Lamp-2 positive MVs at a density ranging between 1.11 and 1.17 g/ml (fraction 3–6), corresponding to MVs flotation.
The presence of Cav-1 in association with MVs was analyzed in the conditioned medium (CM) untreated as well as depleted of MVs. Samples were concentrated in microcon tubes and same volumes compared for Cav-1 and Lamp-2 expression. In other experiments MVs released from the indicated melanomas were recovered at 2 days, quantified by protein assay and the amount normalized on the number of viable cells. Microvesicles (20 μg) secreted from WM983A, WM983A/Cav-1 and Me665/1 were analyzed by western blot and/or cytofluorimetry for Cav-1, Ph-Cav-1, Lamp-2, CD81, CD63 and Rab5B markers and for MMP-9 expression. For citofluorimetric analysis, microvesicles (20 μg) from melanoma cells were adsorbed onto aldehyde-sulphate latex beads (10 μl) (Invitrogen) and saturated overnight at 4°C with BSA. MVs/beads complexes (20 μl) were incubated in a final volume of 100 μl containing 0.5% BSA in PBS and 5 μl of antiCD63-FITC- and antiCD81-PE-conjugated antibodies (BD Pharmingen) for 30 min at 4°C. Beads were then washed and analysed on a FACS Canto-1 flow cytometer (Becton Dickinson, San Josè, CA). Data were analyzed by using the Flow Jo software (TreeStar Inc., USA).
Immunoprecipitation studies, were performed on MV-depleted conditioned medium (CM) (500 μg) and MVs (50 μg) purified from Me665/1 CM were lysed in a buffer containing Tris 50 mM pH 7.4, EDTA 1 mM, NaCl 150 mM with or without TX-100 1% and octylglucoside 60 mM (TX/OG) for 30 min at 4°C. AntiCav-1 antibody (S Cruz) and pre-washed beads were added to the samples and kept O/N a 4°C. Beads were then spun down, washed, loaded on 15% SDS-PAGE and subjected to western blotting analysis.
Microvesicles (MV) transfer assays
Microvesicles (MV) transfer in target cells was evaluated through a lipid mixing assay by using octadecyl rhodamine B chloride (R18) probe (Molecular Probes, Eugene, OR). MVs (10 μg protein/ml) were labelled in MES buffer (10 mM MES pH 7.0, 145 mM NaCl, 5 mM KCl) with 1 mM R18 for 30 min at room temperature. The unincorporated R18 was removed by using a Sephadex G-75 column. Five μg of labelled MVs were added to MES fusion buffer in a thermostated spectrofluorometer FluoroMax-2 (Spex), and fluorescence measured continuously at 560 nm excitation and 590 nm emission wavelengths (slits 1.5 nm). After an equilibration time of 200 seconds, unlabelled cells (106) were added and fluorescence monitored for further 400 seconds. The fusion reaction was stopped by addition of Triton X-100 plus octylglucoside (final concentrations 0.3% and 60 mM, respectively) which, representing the higher probe dilution, indicated the maximal emission (26).
In order to evaluate the incorporation of these microvescicles into cell membrane compartments, membranes from 106 WM983A cells, treated or not with Me665/1-derived MVs (65 μg for 4 hours at 37°C followed by two PBS washes), were compared. To purify membrane proteins from WM983A, cells were lysated (10 mM Tris-HCl pH 7.4, 1 mM EDTA) for 30 min at 4°C and centrifuged at 1.000 × g to remove nuclei. Supernatant was then centrifuged at 30.000 × g for 60 min in order to isolate membrane portions (pellet) from cytosolic proteins (supernatant). The whole amount of membranes recovered from untreated or MV-treated WM983A cells was analyzed by western blot for Cav-1 and Lamp-2 expression.
RESULTS
Cav-1 expression and function on melanoma cell proliferation
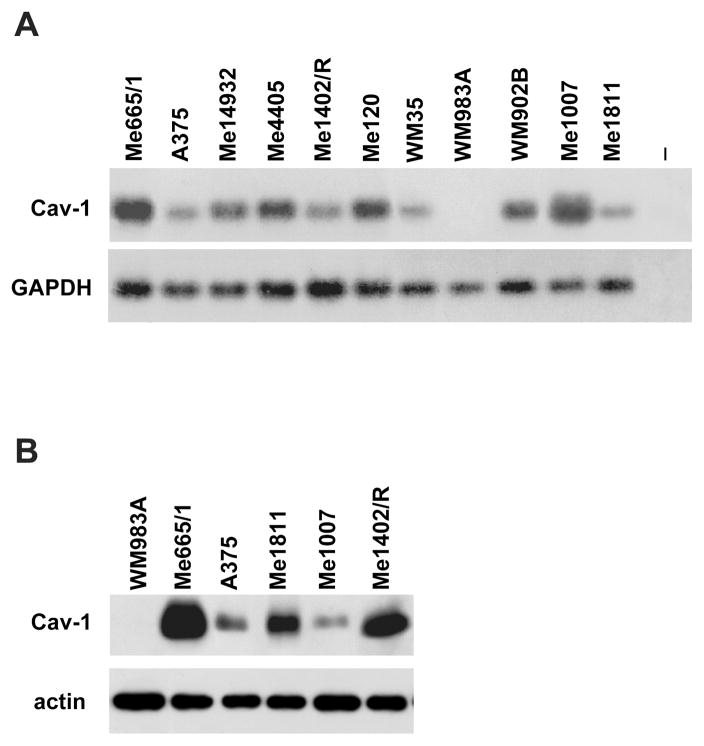
We evaluated Cav-1 expression in a panel of melanoma cell lines at different stages of progression, including radial (RGP) and vertical growth phase primary (VGP) melanomas, as well as subcutaneous and lymph-node metastases (19). All these melanoma cell lines expressed Cav-1, with the exception of the low-invasive WM983A, staged as a primary tumor VGP, where Cav-1 was not detectable at mRNA as well as protein levels (Fig. 1A, B). A barely detectable signal was visible only at very long time of exposure (data not shown).
Figure 1. Cav-1 expression analysis.
(A) RT-PCR analyses of Cav-1 (evaluated at 35 cycles), and control GAPDH (at 18 cycles). - is the negative control, including all the reaction reagents except cDNA; (B)Western blot analyses in total cell lysates.
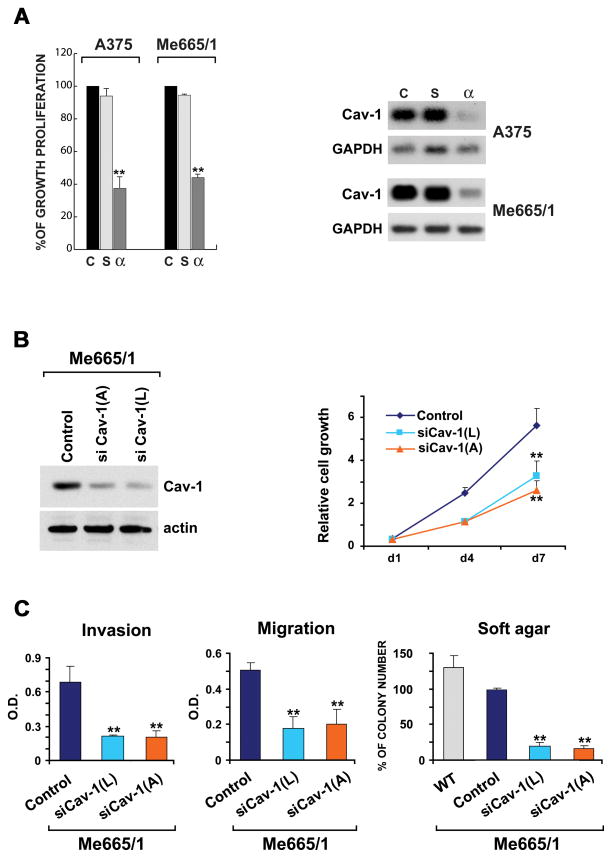
To analyze the possible functional roles of Cav-1, we abrogated its endogenous expression in two metastatic melanomas, whereas we restored Cav-1 expression in the sole Cav-1 negative melanoma cell line. The A375 and Me665/1 cell lines, respectively expressing low and high levels of Cav-1, were then treated with phosphorotioate antisense oligomers specifically targeting Cav-1. Interestingly, both melanoma cell lines showed 50–70% of cell growth inhibition as compared with scrambled oligomers treatment (Fig. 2A, left). Traditional and Real Time RT-PCR analyses confirmed the specific downregulation of Cav-1 mRNA in the antisense-treated cells (Fig. 2A right and not shown). These results were confirmed by a second group of experiments obtained by a siRNA-mediated specific silencing of Cav-1 in Me665/1 metastatic melanoma. Control and si-Cav-1-infected melanoma cell lines were analyzed for effective Cav-1 reduction and compared through some functional assays, including proliferation, migration, invasion and growth in semisolid medium (Fig. 2). Although we always observed a small residual amount of Cav-1, an important reduction of cell growth was observed in Cav-1 down-regulated Me665/1 cells (Fig. 2B); moreover, a decrease of the invasive and chemotactic properties, ranging between 50–75% (Fig. 2C, left) as well as of the foci size and number, were obtained (Fig. 2C, right and not shown).
Figure 2. Cav-1 functional role.
(A) (left) Evaluation of cell growth proliferation in A375 and Me665/1 melanoma cell lines treated or not with antisense oligos; control (c), cells treated with 15 μM scrambled (s) or antisense (α) phosphorothioate oligomers are shown; (right) RT-PCR analysis of Cav-1 and GAPDH in the same control (c), (s)- and (α)-Cav-1 treated cells (Cav-1 was evaluated at 35 cycles for Me665/1 and 40 for A375 cell lines). siRNA-mediated silencing of Cav-1. Control and si-Cav-1-infected melanoma cell lines were analyzed for Cav-1 expression level by WB (B, left panel), proliferation (B, right panel), invasion, migration and growth in semisolid medium (C). Values, reported as mean±SD of three separate experiments, are expressed as arbitrary units. **p<0.01.
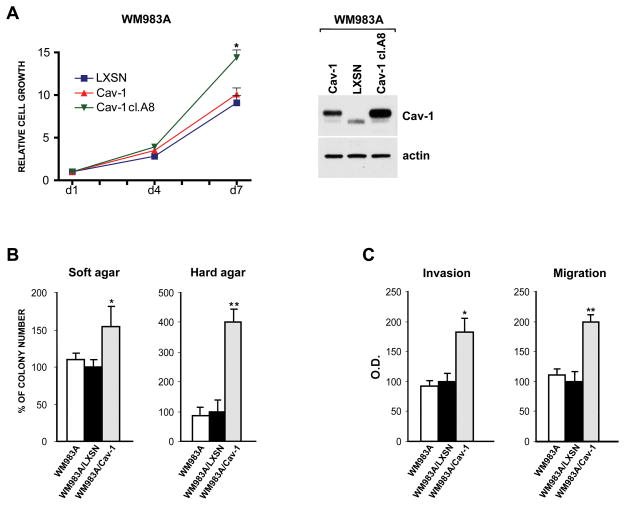
In parallel experiments the WM983A cell line was selected as a recipient for retroviral Cav-1 gene transduction; RT-PCR, Western-blot and Immunofluorescence analyses confirmed the correct transcription and translation of Cav-1 mRNA and protein in the retrovirally infected WM983A target cells (see Supplementary Fig. 1). Moreover analogous cellular distribution patterns were observed for Cav-1 protein endogenously produced by melanomas and for the ectopically expressed Cav-1 in WM983A melanoma cells (see Supplementary Fig. 1) and not shown). LXSN- and Cav-1-transduced WM983A cell lines were analyzed for cell proliferation at different times. Melanoma cells, expressing Cav-1, showed only a small increase of the proliferative rate when compared with wild type or empty vector-transduced WM983A cells, but proliferation appeared up-regulated in the WM983A/Cav-1 clone A8, selected among many others for its high level of Cav-1 expression (Fig. 3A).
Figure 3. Biological effects of Cav-1 expression.
(A) Growth kinetics (left) evaluated in LXSN-and Cav-1-transduced WM983A bulk populations. Proliferation was also compared with the WM983A/Cav-1 high expressing clone A8. Western blot analysis (right) comparing Cav-1 expression level in the same cells.(B) Evaluation of the number of colonies grown in soft- (0.3%) and hard-agar (0.9%) in WM983A cells. (C) Invasion and chemotaxis analyses upon Cav-1 overexpression. Values, expressed as percentage of WM983A/LXSN cells, are reported as mean±SD of three separate experiments. *p≤0.05; ** p≤0.001.
Role of Cav-1 on melanoma tumorigenicity
A series of functional studies were then performed to evaluate whether Cav-1 gene transduction could affect melanoma tumorigenicity.
We compared the anchorage-independent growth of the parental, the LXSN- and the Cav-1-transduced WM983A melanoma cells and a 1.5-fold increase of the number of Cav-1 expressing colonies was observed (Fig. 3B, left panel). To confirm the statistical significance of the small enhancement observed in the Cav-1-transduced cells, we performed the same experiment in the 0.9% less permissive concentration of agar obtaining a more significant 4-fold increase in the foci number (Fig. 3B, right panel).
We then analyzed whether the over-expression of Cav-1 could increase the malignant phenotype of the WM983A by using an in vitro matrigel invasion assay: a statistically significant induction of approximately 2-fold of WM983A/Cav-1 melanoma cell line compared to control cells was found (Fig. 3C, left). In the chemotaxis assays we observed essentially similar results: the WM983A migration towards the lower chamber was doubled in Cav-1 expressing cells (Fig. 3C, right).
Ph-Cav-1 functional role in melanoma migration
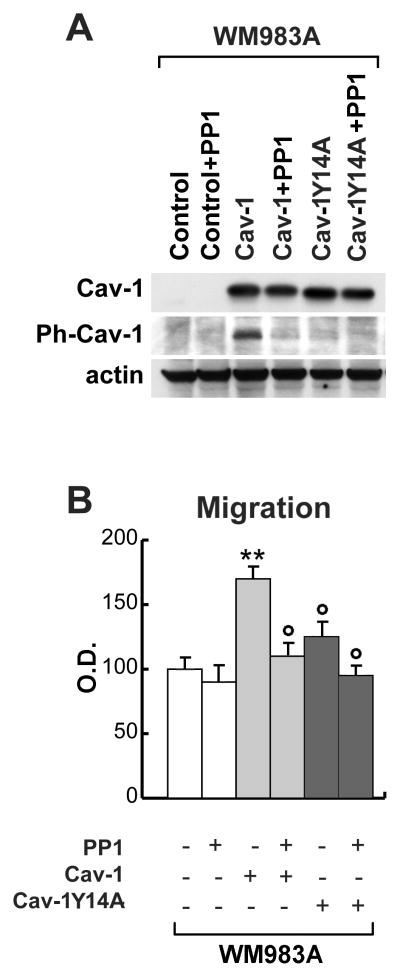
Since phophorylation of Cav-1 has been reported to induce profound effects on its localization and activity by some authors (10), while questioned by others (27), we decided to transfect the Cav-1-negative WM983A melanoma cell line either with the wild type Cav-1 or with a nonphosphorylatable Cav-1Y14A mutant in order to specifically analyze the role of the phosphorylated fraction of Cav-1 as a mediator of tumorigenesis in melanomas (Fig. 4). As a control, the cells were cultured in the presence of the Src-family kinase inhibitor PP1, which totally abrogated the phosphorylated fraction of Cav-1. Western blot analyses correctly confirmed the abrogation or the enforced expression of Cav-1 and Ph-Cav-1 (Fig. 4A).
Figure 4. Functional role of Ph-Cav-1 on melanoma migration ability.
(A) Western blot analyses in control, Cav-1- and Cav-1Y14A-transduced WM983A melanoma cells treated or not with PP1. (B) Migration assays in the same cell populations. All the results are reported as mean± SD. Each experiment was performed in triplicate. **p<0.01 vs untreated cells; ° p<0.01 vs. Cav-1-transduced cells
To evaluate the different levels of malignancy, we performed an in vitro migration assay: indeed Cav-1-transfected WM983A confirmed the statistically significant increase of cell migration (approximately 1.6-fold) and the abrogation of this effect in Cav-1Y14A mutant expressing cells. As expected, PP1-treatment did not affect the migration capability of the wild type, Cav-1-negative, WM983A melanoma (Fig. 4B), whereas it down-regulated the Cav-1-dependent increase obtained in WM983A/Cav-1 cells.
Tumorigenicity of the secreted fraction of Cav-1
The mechanisms of Cav-1 secretion, extensively analyzed in prostate cancer in vitro as well in vivo, have been linked to microvesicular particles. More recently melanoma-derived MV/exosomes have been identified and reported to induce tumor cell growth and immunosuppression (28) and to transfer genetic information in a murine tumor model (29).
In this view we investigated whether human melanoma cell lines expressing Cav-1 were also able to secrete its biologically active form. Western blot analysis showed the presence of Cav-1 in the concentrated conditioned media (CM) collected from the analyzed samples (Fig. 5A). Cav-1 from cell lysates co-migrated with the secreted fraction suggesting that no differences occurred as post-transcriptional modifications (not shown).
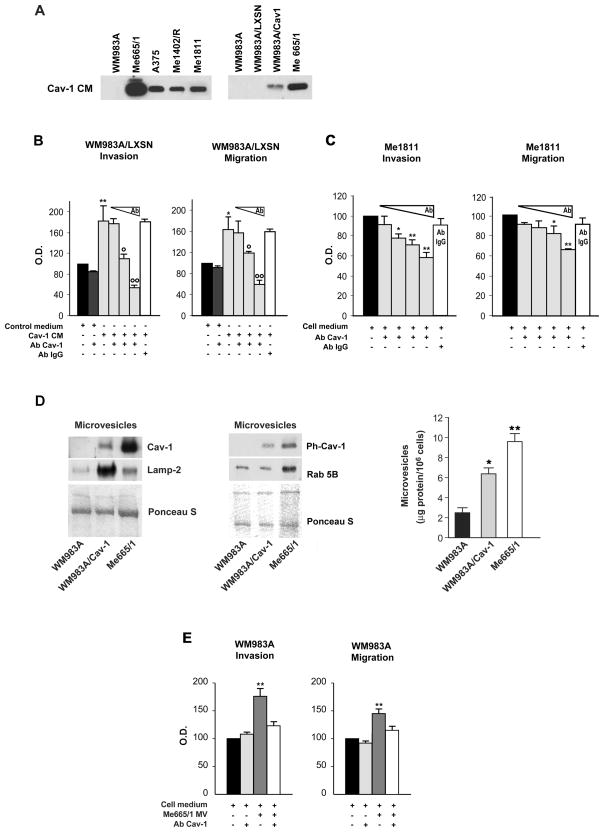
Figure 5. Biological effects of secreted Cav-1.
(A) Western blot analysis of secreted Cav-1 in the concentrated conditioned medium from some representative melanoma cell lines (left) and Cav-1-transduced WM983A (right). (B) Effects of the conditioned medium collected from the WM983A/LXSN (Control medium) or from the WM983A/Cav-1 (Cav-1CM) on the invasion and migration abilities of WM983A/LXSN cells. The polyclonal anti-Cav-1 antibody was added to the conditioned media, where indicated, at increasing doses from 2 to 10 μg/ml, *p≤0.05; ** p≤0.001 vs control - °p≤0.05; °°p≤0.001 vs Cav-1 CM; (C) Invasion and migration assays in Me1811 melanoma cell line performed with increasing doses of Cav-1 antibody added in the cell medium (concentrations of the anti-Cav-1 Ab were 4, 10, 20 and 40 μg/ml). All the results are reported as mean±SD. Each experiment was performed in triplicate. An anti-IgG Ab was included as a control. (D) (left and middle) Western blot analyses on microvesicles purified from the indicated melanomas; Ponceau S indicates the equal protein loading. (right) Normalized amounts of microvesicles released from the same samples. (E) Invasion and migration assays in control- or Me665/1 MV-fused WM983A cells; when indicated 10 μg/ml of an anti-Cav-1 Ab was included. (mean±SD derives from at least 3 independent experiments), ** p≤0.001 vs WM983A.
The activity of the secreted Cav-1 on cell migration and invasion was then analyzed by testing the effects of the CM collected from WM983A/Cav-1 on LXSN-transduced WM983A cells. As shown through an in vitro matrigel assay (Fig. 5B, left panel), we found an increase of the invasion cell ability of approximately 80% in WM983A/LXSN grown in the presence of Cav-1 CM, when compared to the control. To test whether such increase was specifically dependent on Cav-1 protein, a dose-response experiment was performed by adding a polyclonal Cav-1 antibody to the CM obtained from WM983A/Cav-1. Specific reductions, with a 25% residual activity at the higher Ab dose, were observed. Comparable results were obtained in the migration assay (Fig. 5B, right panel). An irrelevant Ab was included as a control. These data indicated that secreted Cav-1 was capable of promoting the invasive and chemotactic abilities of the WM983A melanoma cell line, according to an autocrine/paracrine fashion.
In parallel experiments the role of the secreted fraction of the endogenously produced Cav-1 was studied in Me1811, as a representative metastatic melanoma cell line producing intermediate amounts of Cav-1 (see Fig. 1). In the migration/invasion assays we observed that the addition of a Cav-1 antibody into the medium reduced the invasive and chemotactic capacities of this melanoma up to 40–50%. A dose-response curve with Cav-1 Ab confirmed the specificity of these decreases (Fig. 5C).
Secreted Cav-1 was found to be associated with microvesicles characterized and functionally involved in many biological processes (30, 31), thus we wondered whether we might find the same function/distribution in melanoma cells. At first, we performed a comparative Western blot analysis to show Cav-1 and Lamp-2 co-localization after a sucrose density gradient (Suppl. Fig. 2A). Beside that, for a deeper characterization, purified microvesicles were analyzed for other previously reported markers, such as CD81, CD63 and Rab5B (31, 32) (Fig. 5D, left and middle panels, and Supplementary Fig. 2B). After microvesicles depletion we found a virtual absence of Cav-1 and Lamp-2 in Me665/1 melanoma conditioned medium (Supplementary Fig. 2C). Immunoprecipitation studies were performed on CM as well as purified microvesicles, treated or not with Triton X-100/octylglucoside (TX/OG). Only TX/OG disrupted MVs can be immunoprecipitated confirming that most of Cav-1 protein, if not all, was contained in the MVs (see Supplementary Fig. 2D). Accordingly MVs analyzed by flow cytometry did not show any signal on their external surface. Finally, a MV-associated fraction of phosphorylated Cav-1 was also detected (Fig. 5D middle). Taken together these data support the hypothesis that secreted Cav-1 is MV-associated in melanoma cells.
We next explored the possible function of melanoma released Cav-1 microvesicles in promoting tumor progression. By comparing the amount of microvesicles in control or Cav-1-transduced WM983A and Me665/1 melanoma cell lines, we observed an increased recovery of microvesicles as a function of Cav-1 expression (Fig. 5D, right panel), thus suggesting the involvement of Cav-1 also in the MV releasing process. As microvesicles can readily fuse with cellular membranes through a phosphatidylserine-dependent mechanism (33), we looked for a possible horizontal transfer of the Me665/1 metastatic phenotype to the primary WM983A melanoma. To this purpose a lipid mixing assay, based on R18 lipophilic probe dequenching, was set up (Supplementary Fig. 2E, left panel; see also Materials and Methods). In order to test whether the proteins expressed by the metastatic Me665/1 cells were actually transferred to the recipient less aggressive WM983A cell line, Me665/1-derived microvesicles were incubated with WM983A cells and cell membranes analyzed for the level of Cav-1 and of the lysosomal membrane protein Lamp-2, as a representative marker associated with these organelles. Results indicated a consistent increase of these proteins (Supplementary Fig. 2E, right panel), suggestive of Me665/1 microvesicles incorporation into the WM983A recipient cell membranes and, possibly, of the consequent transfer of a more aggressive phenotype.
We then compared the invasive and chemotactic capabilities of wild type vs Me665/1/MV-fused WM983A cells. By using a Boyden chamber assay, increased levels ranging between 40 and 75% were observed in the latter, thus confirming the increased tumorigenicity of WM983A primary melanoma cell line after the up-take of Me665/1 secreted vesicles (Fig. 5E). Finally Cav-1-negative WM983A cells were incubated with MVs purified from WM983A cells transiently transfected either with wild type Cav-1 or its Y14A-non phosphorylatable mutant (Supplementary Fig. 3). After MV transfer, the recovered cells were compared by standard Boyden chamber assays. Interestingly, whereas Cav-1 containing MVs doubled the invasive capability of WM983A cells, the presence of Y14A mutated Cav-1 did not (see Supplementary Fig. 3).
Cav-1 regulation of metalloproteases
Based on all the above results, suggesting Cav-1 involvement in tumor progression, we studied the expression level of metalloproteases, as a function of Cav-1. Metalloproteases are a family of proteins capable of degrading the extracellular matrix, frequently overexpressed in various human cancers and recently associated with caveolae (34).
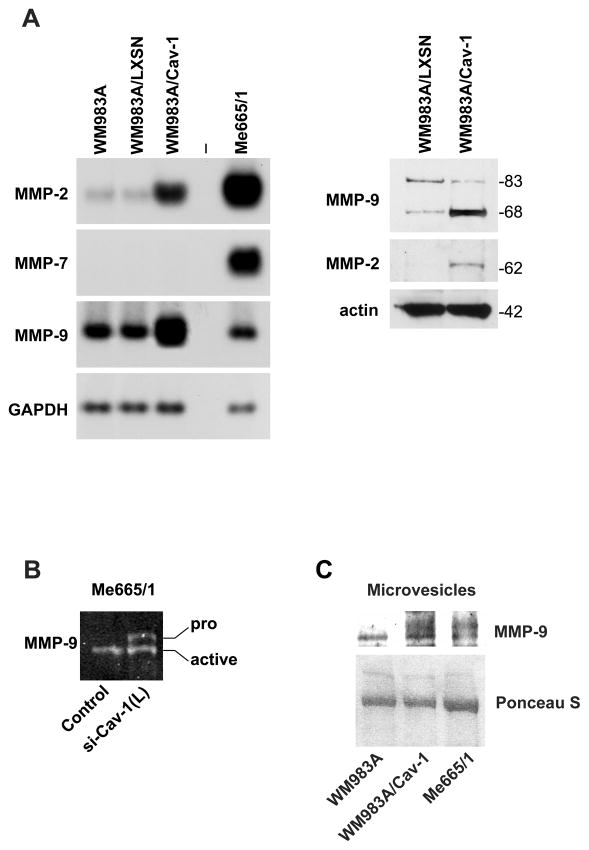
RT-PCR analysis of MMP-2, MMP-7 and MMP-9 performed in wild type, LXSN- and Cav-1-transduced WM983A cells showed an increase in the expression level of both MMP-2 and –9 (of 12- and 6-fold, respectively) in the WM983A/Cav-1 cell line compared with the untransduced and the LXSN-transduced WM983A cells, whereas MMP-7 remained constantly silent (Fig. 6A, left panel). This upregulation was confirmed at protein level by Western blot analysis (Fig. 6A, right panel). MMP-2 protein was barely or not detectable in the WM983A/LXSN cells, whereas in the Cav-1-transduced cells we observed a clear induction of the mature enzyme; a 3–5-fold increase was detected also for the active enzymatic form of MMP-9. The presence of increased levels of matrix degrading protease MMP-9 was confirmed by a zymographic analysis performed on Me665/1 vs Me665/1/siCav-1(L). In particular, Cav-1 silencing appeared to interfere with MMP-9 activation as indicated by the presence of the latent pro-MMP-9 form (Fig. 6B).
Figure 6. Cav-1 upregulates MMPs.
(A) (left) RT-PCR of MMP-2, -7 and -9 in parental, LXSN-and LCav-1SN-transduced WM983A melanoma cells. GAPDH was the internal control; (right) WB analysis showing the active enzymatic forms: 62KDa for MMP-2 and 83 and 68 KDa for MMP-9; the last one is attributable to autocatalytic cleavage of the COOH terminus. (B) MMP-9 zymographic analysis performed in control- and si-Cav-1(L)-infected Me665/1 cells. Pro- and active MMP-9 forms are indicated. (C) Western blot analyses on MV purified from the indicated samples. MVs from Me665/1 were loaded as a positive control; Ponceau S indicates the equal protein loading.
Finally, an enhancement of the MMP-9 mature enzymatic form was observed in MV purified from the WM983A/Cav-1 cells, confirming a direct correlation between MMP-9, Cav-1 as well as MV amounts (Fig. 6C).
DISCUSSION
The overall data on Cav-1 activity in tumor cells show a very complex picture. On one side Cav-1 down-regulation has been associated with loss of apoptosis and, consequently, with tumorigenesis, on the other Cav-1 over-expression has been demonstrated to stimulate cell survival and contributes to the metastatic process (1, 2, 5).
Here we originally reported the presence of Cav-1 in a panel of melanoma cell lines (Fig. 1) and its functional role as tumor promoting protein, as confirmed by a series of biological assays (Fig. 2 and 3). Thus far these data allow us to include melanomas in the group of neoplasias displaying Cav-1 higher expression in primary tumors and/or distant metastases compared to the corresponding normal tissues (2). This conclusion was supported by the finding of Cav-1 expression in all melanomas analyzed with higher levels in lymph nodal metastatic cell lines (Fig. 1). The sole exception to this apparent rule was represented by the WM983A primary VGP cell line lacking Cav-1. Accordingly, the retrovirally driven re-expression of Cav-1 in this melanoma cell line effectively induced increased cell growth, anchorage-independent, migration and invasion capabilities (Fig. 3 and Supplementary Fig. 1). Moreover the down-regulation of Cav-1 in metastatic Cav-1-expressing melanomas clearly reduces their proliferative rates as well as their tumorigenicity (Fig 2).
The phosphorylation status of Cav-1 was also demonstrated to play a role in melanoma (see Fig. 4). The functional involvement of Ph-Cav-1 was confirmed by PP1 treatment or Cav-1Y14A mutant over-expression which strongly reduced the chemotactic ability respect to the control cells. Accordingly, migration was improved by wild type Cav-1 overexpression (Fig. 4B). These results, although recently alleged controversial in view of the anti-PhTyr14Cav1 antibody cross-reactivity with the focal adhesion (FA) marker Ph-paxillin, are still suggestive for new intense investigation on Ph-Cav/Cav 1 role in cell adhesion, migration and invasion (27). Anyway the validity of western blot results was never debated (Fig. 4A). According to our data, phosphorylated Cav-1 has been reported as an effector of Rho/Rock signaling to promote tumor progression and metastasis in various human tumors (35).
The dual functional behaviour of Cav-1 might also find an explanation in the occurrence of different interacting partners that determine the pro- or anti-neoplastic functions of the complexes (1). An example supporting this hypothesis demonstrated Cav-1 requirement of E-cad for displaying its suppressive properties, including the downregulation of survivin (7). E-cad is silenced in melanomas and its downregulation is one of the possible causes of melanoma progression to metastasis (36, 37). Accordingly, E-cad re-expression in B16 murine melanoma cell line restores Cav-1 ability to control cell proliferation by repressing survivin (7).
Moreover, as first reported and accurately demonstrated for prostate cancer (13, 14), where serum levels of Cav-1 were proposed as diagnostic and prognostic markers (17), we detected a secreted fraction of Cav-1 in melanomas. In vitro experimental studies initially performed with concentrated conditioned medium and successively with purified microvesicles, showed Cav-1 capability of stimulating in vitro anchorage independence, migration and invasion in a paracrine/autocrine fashion (Fig. 1, 5 and Supplementary Fig. 3). These effects were abolished when an anti-Cav-1 specific Ab was included. Since Cav-1 molecules do not appear to protrude from MV surfaces, we should presume that the observed functional effects (see Fig. 5 and Supplementary Fig. 2) might derive from Cav-1-containing MVs and the anti-Cav-1 Ab interaction inside the cells. It is important to point out that we observed the genetic transfer of some metastatic properties into the less aggressive primary melanoma WM983A as a consequence of the uptake of metastatic Me665/1 melanoma-released vesicles (Supplementary Fig. 2E).
Cav-1 is known to be involved in the organization and remodelling of the extracellular matrix (38). Moreover key molecules, as matrix metalloproteases, have been reported to be positively (34) or negatively (2, 39) regulated by Cav-1. It is then important to consider the Cav-1-dependent upregulation of metalloproteases, MMP-2 and MMP-9, observed in melanomas (Fig. 6) and known to directly correlate with a more invasive phenotype in tumor cells (40). As reported for MMP-14, which is internalized, recycled to the plasma membrane and secreted in microvesicular exosomes (41), MMP-9 was detected in MV purified from the metastatic Me665/1 cell line and its increased expression observed in the WM983A cells after fusion with MV purified from the Me665/1 metastatic melanoma. These data confirmed the transferability of molecules functional to the increase of tumor aggressiveness and progression, reported for the murine B16 cell line (29), also in human melanoma cells. A direct correlation between Cav-1 levels, the amount of MV released in the culture medium and MMP-9 expression and activity was demonstrated in Cav-1-transduced vs. control-transduced WM983A cells and/or in control-compared with si-Cav-1-Me665/1 cell lines (Fig. 5, 6).
In conclusion, we have added some new insights on Cav-1 role in melanomas in that: i) the level of Ph-Cav-1 positively correlates with increased anchorage independence, invasion and migration (Fig. 4, Supplementary Fig. 3 and not shown); ii) Cav-1 can act in an autocrine/paracrine fashion in that the melanoma secreted MVs appear able to transfer a tumor-promoting ability, specifically associated with Cav-1 (Fig. 5). In support to this second finding, we showed that the amount of microvesicles released from melanoma cells appears as a function of Cav-1 cells expression (Fig. 5D, right panel), thus confirming the involvement of Cav-1 in this process.
Overall Cav-1 seems to exert a prominent role in melanoma progression, thus suggesting the future opportunity of utilizing it as a new diagnostic and/or prognostic molecular marker, as already hypothesized in metastatic prostate cancer (30). Moreover Cav-1 might eventually represent a new target to aim at in an innovative clinical setting, particularly relevant in those types of cancer, as melanoma, still lacking effective traditional therapies.
Supplementary Material
Supplementary Fig. 1. Cav-1 ectopic expression. (A) RT-PCR (left), Western blot (right) and (B) I.F. expression studies in parental, LXSN- and LCav-1SN-transduced WM983A melanoma cells.
Supplementary Fig. 2. Cav-1 association with MVs. (A) Western blot analysis of Cav-1 and Lamp-2 in Me665/1-microvesicles fractionated on a continuous sucrose gradient. Same volume amount for each fraction was loaded. (B) Microvesicles from melanoma cells were analyzed by citoflorimetry with the indicated antibodies. (C) The presence of Cav-1 in association with MVs was analyzed in the conditioned medium (CM) untreated or depleted from microvesicles (CM w/out MV). MVs purified from 10 fold higher volume of Me665/1 supernatant were loaded as a positive control. (D) Immunoprecipitation studies, performed on MV-depleted CM compared with purified MVs, treated or not with Triton X-100/octylglucoside (TX/OG), confirmed Cav-1 association with microvesicles. As a control, proteins extracted from purified MVs were loaded. (E) (left) MV transfer activity to recipient cells was evaluated through the increase in the fluorescence level resulting from mixing R18-labelled Me665/1 MV and WM983A parental cells; (right) MV transfer was confirmed by Cav-1 and Lamp-2 Western blot in membrane-lysates recovered from untreated (lane 2) or MV-treated WM983A cells (lane 3); MV from Me665/1 (lane 1) were loaded as a positive control.
Supplementary Fig. 3. Functional role of Ph-Cav-1-containing MVs. (A) Invasion assay in WM983A cells transiently transfected either with wild type Cav-1 or its Y14A-non phosphorylatable mutant. Values, expressed as arbitrary units, are reported as mean±SD of two separate experiments. ** p≤0.001 vs control. (B) WB analysis of the WM983A-transfected cells. LCav-1SN stably infected cells are included as a positive control.
Acknowledgments
We wish to thank G. Loreto for preparation of the figures. This work was partially supported by the Italian Ministry of Health (to AC) and by a FIRB grant (Grant RBNE03FMCJ_002) from the Italian Ministry for University and Research (to MS). K.F. is recipient of a postdoctoral fellowship from FIRB.
Abbreviations
- MV
Microvesicle
- Cav-1
Caveolin-1
- E-cad
E-cadherin
- CM
Conditioned Medium
References
- 1.Quest A, Gutierrez-Pajares JL, Torres V. Caveolin-1, an ambiguous partner in cell signalling and cancer. J Cell Mol Med. 2008;12:1130–50. doi: 10.1111/j.1582-4934.2008.00331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burgermeister E, Liscovitch M, Röcken C, Schmid RM, Ebert MP. Caveats of caveolin-1 in cancer progression. Cancer Lett. 2008;268:187–201. doi: 10.1016/j.canlet.2008.03.055. [DOI] [PubMed] [Google Scholar]
- 3.Williams TM, Lisanti MP. Caveolin-1 in oncogenic transformation, cancer and metastasis. Am J Physiol Cell Physiol. 2005;288:494–506. doi: 10.1152/ajpcell.00458.2004. [DOI] [PubMed] [Google Scholar]
- 4.Williams TM, Sotgia F, Lee H, Hassan G, Di Vizio D, Bonuccelli G, Capozza F, Mercier I, Rui H, Pestell RG, Lisanti MP. Stromal and epithelial caveolin-1 both confer a protective effect against mammary hyperplasia and tumorigenesis: Caveolin-1 antagonizes cyclin D1 function in mammary epithelial cells. Am J Pathol. 2006;169:1784–801. doi: 10.2353/ajpath.2006.060590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shatz M, Liscovitch M. Caveolin-1: a tumor-promoting role in human cancer. Int J Radiat Biol. 2008;84:177–89. doi: 10.1080/09553000701745293. [DOI] [PubMed] [Google Scholar]
- 6.Karam JA, Lotan Y, Roehrborn CG, Ashfaq R, Karakiewicz PI, Shariat SF. Caveolin-1 overexpression is associated with aggressive prostate cancer recurrence. Prostate. 2007;67:614–22. doi: 10.1002/pros.20557. [DOI] [PubMed] [Google Scholar]
- 7.Torres VA, Tapia JC, Rodriguez DA, Lladser A, Arredondo C, Leyton L, Quest AF. E-cadherin is required for caveolin-1-mediated down-regulation of the inhibitor of apoptosis protein survivin via reduced beta-catenin-Tcf/Lef-dependent transcription. Mol Cell Biol. 2007;27:7703–17. doi: 10.1128/MCB.01991-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glenney JR. Tyrosine phosphorylation of a 22-kDa protein is correlated with transformation by Rous sarcoma virus. J Biol Chem. 1989;264:20163–6. [PubMed] [Google Scholar]
- 9.Salanueva IJ, Cerezo A, Guadamillas MC, Del Pozo MA. Integrin regulation of caveolin function. J Cell Mol Med. 2007;11:969–80. doi: 10.1111/j.1582-4934.2007.00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.del Pozo MA, Balasubramanian N, Alderson NB, Kiosses WB, Grande-Garcia A, Anderson RG, Schwartz MA. Phospho-caveolin-1 mediates integrin-regulated membrane domain internalization. Nat Cell Biol. 2005;7:901–8. doi: 10.1038/ncb1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu P, Li WP, Machleidt T, Anderson RG. Identification of caveolin-1 in lipoprotein particles secreted by esocrine cells. Nat Cell Biol. 1999;1:369–75. doi: 10.1038/14067. [DOI] [PubMed] [Google Scholar]
- 12.Sawada N, Taketani Y, Amizuka N, Ichikawa M, Ogawa C, Nomoto K, Nashiki K, Sato T, Arai H, Isshiki M, Segawa H, Yamamoto H, et al. Caveolin-1 in extracellular matrix vesicles secreted from osteoblasts. Bone. 2007;41:52–8. doi: 10.1016/j.bone.2007.02.030. [DOI] [PubMed] [Google Scholar]
- 13.Tahir SA, Yang G, Ebara S, Timme TL, Satoh T, Li L, Goltsov A, Ittmann M, Morrisett JD, Thompson TC. Secreted caveolin-1 stimulates cell survival/clonal growth and contributes to metastasis in androgen-insensitive prostate cancer. Cancer Res. 2001;61:3882–5. [PubMed] [Google Scholar]
- 14.Ayala GE, Dai H, Tahir SA, Li R, Timme T, Ittmann M, Frolov A, Wheeler TM, Rowley D, Thompson TC. Stromal antiapoptotic paracrine loop in perineural invasion of prostatic carcinoma. Cancer Res. 2006;66:5159–64. doi: 10.1158/0008-5472.CAN-05-1847. [DOI] [PubMed] [Google Scholar]
- 15.Bartz R, Zhou J, Hsieh JT, Ying Y, Li W, Liu P. Caveolin-1 secreting LNCaP cells induce tumor growth of caveolin-1 negative LNCaP cells in vivo. Int J Cancer. 2008;122:520–5. doi: 10.1002/ijc.23142. [DOI] [PubMed] [Google Scholar]
- 16.Llorente A, de Marco MC, Alonso MA. Caveolin-1 and MAL are located on prostasomes secreted by the prostate cancer PC-3 cell line. J Cell Sci. 2004;117:5343–51. doi: 10.1242/jcs.01420. [DOI] [PubMed] [Google Scholar]
- 17.Tahir SA, Frolov A, Hayes TG, Mims MP, Miles BJ, Lerner SP, Wheeler TM, Ayala G, Thompsdn TC, Kadmon D. Preoperative serum caveolin-1 as a prognostic marker for recurrence in a radical prostatectomy cohort. Clin Cancer Res. 2006;12:4872–5. doi: 10.1158/1078-0432.CCR-06-0417. [DOI] [PubMed] [Google Scholar]
- 18.Felicetti F, Errico MC, Bottero L, Segnalini P, Stoppacciaro A, Biffoni M, Felli N, Mattia G, Petrini M, Colombo MP, Peschle C, Carè A. The promyelocitic leukemia zinc finger-microRNA-221/-222 pathway controls melanoma progression through multiple oncogenic mechanisms. Cancer Res. 2008;68:2745–54. doi: 10.1158/0008-5472.CAN-07-2538. [DOI] [PubMed] [Google Scholar]
- 19.Felicetti F, Bottero L, Felli N, Mattia G, Labbaye C, Alvino E, Peschle C, Colombo MP, Carè A. Role of PLZF in melanoma progression. Oncogene. 2004;23:4567–76. doi: 10.1038/sj.onc.1207597. [DOI] [PubMed] [Google Scholar]
- 20.Engelman JA, Wykoff CC, Yasuhara S, Song KS, Okamoto T, Lisanti MP. Recombinant expression of caveolin-1 in oncogenically transformed cells abrogates anchorage-independent growth. J Biol Chem. 1997;272:16374–81. doi: 10.1074/jbc.272.26.16374. [DOI] [PubMed] [Google Scholar]
- 21.Carè A, Silvani A, Meccia E, Mattia G, Stoppacciaro A, Permiani G, Peschle C, Colombo MP. HOXB7 constitutively activates basic fibroblast growth factor in melanomas. Mol Cell Biol. 1996;16:4842–51. doi: 10.1128/mcb.16.9.4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albini A, Iwamoto Y, Kleinman HK, Martin GR, Aaronson SA, Kozlowski JM, McEwan RN. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res. 1987;47:3239–45. [PubMed] [Google Scholar]
- 23.Niu J, Dorahy DJ, Gu X, Scott RJ, Draganic B, Ahmed N, Agrez MV. Integrin expression in colon cancer cells is regulated by the cytoplasmic domain of the beta6 integrin subunit. Int J Cancer. 2002;99:529–37. doi: 10.1002/ijc.10397. [DOI] [PubMed] [Google Scholar]
- 24.Andreola G, Rivoltini L, Castelli C, Huber V, Perego P, Deho P, Squarcina P, Accornero P, Lozupone F, Lugini L, Stringaro A, Molinari A, et al. Induction of lymphocyte apoptosis by tumor cell secretion of FasL-bearing microvesicles. J Exp Med. 2002;195:1303–16. doi: 10.1084/jem.20011624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161–72. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoekstra D, de Boer T, Klappe K, Wilschut J. Fluorescence method for measuring the kinetics of fusion between biological membranes. Biochemistry. 1984;23:5675–81. doi: 10.1021/bi00319a002. [DOI] [PubMed] [Google Scholar]
- 27.Hill MM, Scherbakov N, Schiefermeier N, Baran J, Hancock JF, Huber LA, Parton RG, Parat MO. Reassessing the Role of phosphocaveolin- 1 in cell adhesion and migration. Traffic. 2007;8:1695–1705. doi: 10.1111/j.1600-0854.2007.00653.x. [DOI] [PubMed] [Google Scholar]
- 28.Valenti R, Huber V, Iero M, Filipazzi P, Permiani G, Rivoltini L. Tumor-released microvesicles as vehicles of immunosuppression. Cancer Res. 2007;67:2912–5. doi: 10.1158/0008-5472.CAN-07-0520. [DOI] [PubMed] [Google Scholar]
- 29.Hao S, Ye Z, Li F, Meng Q, Qureshi M, Yang J, Xiang J. Epigenetic transfer of metastatic activity by uptake of highly metastatic B16 melanoma cell-released exosomes. Exp Oncol. 2006;28:126–31. [PubMed] [Google Scholar]
- 30.Tahir SA, Yang G, Goltsov AA, Watanabe M, Tabata K, Addai J, Fattah el MA, Kadmon D, Thompson TC. Tumor cell-secreted caveolin-1 has proangiogenic activities in prostate cancer. Cancer Res. 2008;68:731–9. doi: 10.1158/0008-5472.CAN-07-2668. [DOI] [PubMed] [Google Scholar]
- 31.Calzolari A, Raggi C, Deaglio S, Sposi NM, Stafsnes M, Fecchi K, Parolini I, Malavasi F, Peschle C, Sargiacomo M, Testa U. TfR2 localizes in lipid raft domains and is released in exosomes to activate signal transduction along the MAPK pathway. J Cell Sci. 2006;119:4486–98. doi: 10.1242/jcs.03228. [DOI] [PubMed] [Google Scholar]
- 32.Keller S, Sanderson MP, Stoeck A, Altevogt P. Exosomes: from biogenesis and secretion to biological function. Immunol Lett. 2006;107:102–8. doi: 10.1016/j.imlet.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 33.Del Conde I, Shrimpton CN, Thiagarajan P, López JA. Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood. 2005;106:1604–11. doi: 10.1182/blood-2004-03-1095. [DOI] [PubMed] [Google Scholar]
- 34.Williams TM, Medina F, Badano I, Hazan RB, Hutchinson J, Muller WJ, Chopra NG, Scherer PE, Pestell RG, Lisanti MP. Caveolin-1 gene disruption promotes mammary tumorigenesis and dramatically enhances lung metastasis in vivo. Role of Cav-1 in cell invasiveness and matrix metalloproteinase (MMP-2/9) secretion. J Biol Chem. 2004;279:51630–46. doi: 10.1074/jbc.M409214200. [DOI] [PubMed] [Google Scholar]
- 35.Joshi B, Strugnell SS, Goetz JG, Kojic LD, Cox ME, Griffith OL, Chan SK, Jones SJ, Leung SP, Masoudi H, Leung S, Wiseman SM, et al. Phosphorylated caveolin-1 regulates Rho/ROCK-dependent focal adhesion dynamics and tumor cell migration and invasion. Cancer Res. 2008;68:8210–20. doi: 10.1158/0008-5472.CAN-08-0343. [DOI] [PubMed] [Google Scholar]
- 36.Baldi A, Santini D, Battista T, Dragonetti E, Ferranti G, Petitti T, Groeger AM, Angelini A, Rossiello R, Baldi F, Natali PG, Paggi MG. Expression of AP-2 transcription factor and of its downstream target genes c-kit, E-cadherin and p21 in human cutaneous melanoma. J Cell Biochem. 2001;83:364–72. doi: 10.1002/jcb.1235. [DOI] [PubMed] [Google Scholar]
- 37.Nyormoi O, Bar-Eli M. Transcriptional regulation of metastasis-related genes in human melanoma. Clin Exp Metastasis. 2003;20:251–63. doi: 10.1023/a:1022991302172. [DOI] [PubMed] [Google Scholar]
- 38.Navarro A, Anand-Apte B, Parat MO. A role for caveolae in cell migration. FASEB J. 2004;18:1801–11. doi: 10.1096/fj.04-2516rev. [DOI] [PubMed] [Google Scholar]
- 39.Fiucci G, Ravid D, Reich R, Liscovitch M. Caveolin-1 inhibits anchorage-independent growth, anoikis and invasiveness in MCF-7 human breast cancer cells. Oncogene. 2002;21:2365–75. doi: 10.1038/sj.onc.1205300. [DOI] [PubMed] [Google Scholar]
- 40.Bergers G, Coussens LM. Extrinsic regulators of epithelial tumor progression: metalloproteinases. Curr Opin Genet Dev. 2000;10:120–7. doi: 10.1016/s0959-437x(99)00043-x. [DOI] [PubMed] [Google Scholar]
- 41.Hakulinen J, Sankkila L, Sugiyama N, Lehti K, Keski-Oja J. Secretion of active membrane type 1 matrix metalloproteinase (MMP-14) into extracellular space in microvesicular exosomes. J Cell Biochem. 2008;105:1211–8. doi: 10.1002/jcb.21923. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1. Cav-1 ectopic expression. (A) RT-PCR (left), Western blot (right) and (B) I.F. expression studies in parental, LXSN- and LCav-1SN-transduced WM983A melanoma cells.
Supplementary Fig. 2. Cav-1 association with MVs. (A) Western blot analysis of Cav-1 and Lamp-2 in Me665/1-microvesicles fractionated on a continuous sucrose gradient. Same volume amount for each fraction was loaded. (B) Microvesicles from melanoma cells were analyzed by citoflorimetry with the indicated antibodies. (C) The presence of Cav-1 in association with MVs was analyzed in the conditioned medium (CM) untreated or depleted from microvesicles (CM w/out MV). MVs purified from 10 fold higher volume of Me665/1 supernatant were loaded as a positive control. (D) Immunoprecipitation studies, performed on MV-depleted CM compared with purified MVs, treated or not with Triton X-100/octylglucoside (TX/OG), confirmed Cav-1 association with microvesicles. As a control, proteins extracted from purified MVs were loaded. (E) (left) MV transfer activity to recipient cells was evaluated through the increase in the fluorescence level resulting from mixing R18-labelled Me665/1 MV and WM983A parental cells; (right) MV transfer was confirmed by Cav-1 and Lamp-2 Western blot in membrane-lysates recovered from untreated (lane 2) or MV-treated WM983A cells (lane 3); MV from Me665/1 (lane 1) were loaded as a positive control.
Supplementary Fig. 3. Functional role of Ph-Cav-1-containing MVs. (A) Invasion assay in WM983A cells transiently transfected either with wild type Cav-1 or its Y14A-non phosphorylatable mutant. Values, expressed as arbitrary units, are reported as mean±SD of two separate experiments. ** p≤0.001 vs control. (B) WB analysis of the WM983A-transfected cells. LCav-1SN stably infected cells are included as a positive control.