Abstract
Development of an effective, safe, and convenient method for gene delivery to muscle is a critical step toward gene therapy for muscle-associated diseases. Toward this end, we have explored the possibility of combining the image-guided catheter insertion technique with the principle of hydrodynamic delivery to achieve muscle-specific gene transfer in pigs. We demonstrate that gene transfer efficiency of the procedure is directly related to flow rate, injection pressure, and injection volume. The optimal gene delivery was achieved at a flow rate of 15 ml/second with injection pressure of 300 psi and injection volume equal to 1.5% of body weight. Under such a condition, hydrodynamic injection of saline containing pCMV-Luc (100 µg/ml) resulted in luciferase activity of 106 to 107 relative light units (RLU)/mg of proteins extracted from the targeted muscle 5 days after hydrodynamic gene delivery. Result from immunohistochemical analysis revealed 70–90% transfection efficiency of muscle groups in the hindlimb and persistent reporter gene expression for 2 months in transfected cells. With an exception of transient edema and elevation of creatine phosphokinase, no permanent tissue damage was observed. These results suggest that the image-guided, intravenous hydrodynamic delivery is an effective and safe method for gene delivery to skeletal muscle.
Introduction
It has been a decade since we and Zhang et al. published the method of hydrodynamic gene delivery as an effective, simple, and convenient means to transfect mouse hepatocytes in vivo.1,2 Since then, this method has been extensively studied for delivery of naked DNA, RNA, proteins, and even viral vectors (for recent reviews, see refs. 3,4). Long-lasting gene expression at therapeutic level has been shown in animal studies. More recent work in large animals5,6,7,8,9 and in humans10 has provided direct evidence in support of hydrodynamic gene delivery as a viable approach for clinical gene therapy.
Application of hydrodynamic gene delivery to muscle was also studied.11,12,13,14,15 Using intra-arterial injection into the arms of nonhuman primates with blood flow obstruction, Zhang et al. reported successful gene transfer into muscle cells.12 Using a similar injection procedure to the femoral artery, Danialou et al. reported high level of reporter gene expression in muscle cells of the distal hindlimb in pigs.16 A drawback of the intra-arterial injection is that it requires surgical procedures to open the fascia, isolate the artery, and clamp the vein for injection. To simplify the procedure, Hagstrom et al. developed an anterograde intravenous injection procedure.17 By injecting plasmid DNA solution from a peripheral branch of the femoral vein into distal hindlimb with tourniquet occlusion, they obtained a high level of reporter gene expression in rats and rhesus macaques.17 Compared to intra-arterial injection, intravenous injection is more clinically favorable for treatment of muscular diseases such as muscular dystrophy because it is less invasive.
To extend these earlier studies, we investigated the possibility of intravenous gene transfer to large proximal hindlimb in pigs using a newly established procedure for liver gene transfer.5 The method employs an image-guided insertion technique to place the catheter into a specific site of the target organ and a computer-controlled injection device18 to drive intracellular gene transfer hydrodynamically. In this study, we demonstrate that the image-guided, intravenous hydrodynamic gene delivery to skeletal muscle in pigs is effective, safe, and site specific. Evaluation on persistency of reporter gene expression in the muscle showed long-term gene expression for at least 2 months (length of experiment). These results provide direct evidence in support of safety and effectiveness of hydrodynamic gene delivery to skeletal muscle.
Results
Image-guided catheter insertion for intravenous gene delivery to skeletal muscle
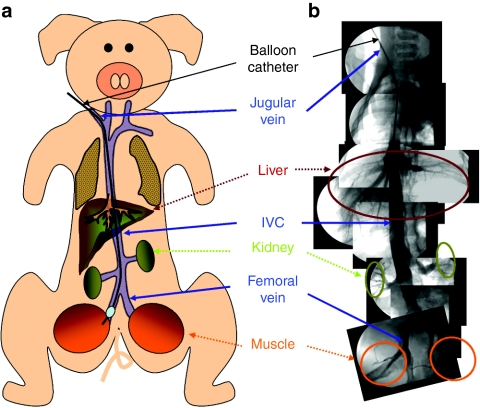
A number of previous studies have targeted the distal hindlimb for gene delivery by the peripheral vein.12,15,17 We aimed at the proximal hindlimb because of its larger size in muscle groups compared to that of the distal hindlimb. Figure 1a illustrates the experimental design for catheter insertion from the jugular vein. Catheter insertion from jugular vein at the neck was utilized instead of a direct insertion into the femoral vein to avoid surgical opening of the fasciae and connective tissue at the hindlimb. Traveling through the inferior vena cava, the inserted balloon catheter was placed at one of the femoral veins connecting to the hindlimb. Figure 1b shows the venography of the entire path of the balloon catheter from jugular vein to the femoral vein with images exhibiting the vascular connection to the inferior vena cava and the major organs such as liver and kidney. The balloon inserted to the femoral vein was inflated by infusion of 5 ml of contrast medium for fluoroscopic visualization. Before DNA injection, a small amount of contrast medium was injected to confirm the complete blockade of the femoral vein. A tourniquet was placed at the joint of the hindlimb to minimize the leakage of injected solution to the peripheral veins.
Figure 1.
Schematic presentation of image-guided catheter insertion and venography. (a) Schema of experimental design for catheter insertion from jugular to femoral vein through inferior vena cava (IVC). (b) Venography of vasculature. Images were assembled from a series photographs taken at different venous sections immediately after infusion of small volume of contrast medium into the selected parts of the vasculature.
Morphological change of the hindlimb upon hydrodynamic injection
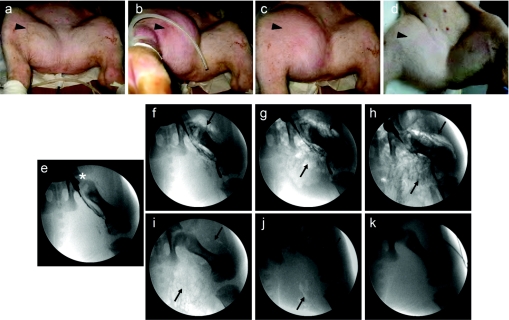
Pigs with body weight of 18–20 kg were used for the study. Figure 2a–d shows the exterior appearance of the hindlimb upon hydrodynamic injection of 300 ml (1.5% body weight) of DNA solution in 20 seconds. The injected hindlimb swells as soon as the injection starts, reaching the maximal (Figure 2b) at the end of injection and remaining swollen for at least 2 hours (Figure 2c). No swelling was seen in the injected limb 24 hours after the injection (Figure 2d) compared to noninjected limb (Figure 2a). No swelling was visible in other parts of the body, suggesting that the impact of the image-guided, intravenous hydrodynamic delivery is regional.
Figure 2.
Morphological impact of hydrodynamic injection on hindlimb. Hydrodynamic injection was made to the hindlimb at injection pressure of 300 psi with a volume equal to 1.5% body weight in 20 seconds. External appearance of the targeted muscle (black arrowhead) were photographed (a) before, (b) 20 seconds, (c) 2 hours, and (d) 24 hours after injection initiation. Distribution of injected solution in the targeted hindlimb was tape-recorded (e) before, (f) 5 seconds, (g) 10 seconds, (h) 20 seconds, (i) 30 minutes, (j) 2 hours, and (k) 24 hours after initiation of injection of CO2 containing solution (black arrow) through the balloon catheter (*).
We then examined the interior appearance of the hindlimb upon hydrodynamic injection (Figure 2e–k). To take advantage of the property of CO2 for high solubility in aqueous solution and its phase-contrast property for fluoroscopic imaging, we mixed CO2 with injection solution and followed its distribution in the muscle according to a procedure clinically used for angiography.19,20 Figure 2e shows the position of the inserted balloon catheter. Five seconds into the injection, extravasation of the injected solution was evident (Figure 2f). The solution spread in the entire field between 10 seconds (Figure 2g) and 20 seconds (Figure 2h). CO2 presence in the injected areas was still visible after 30 minutes (Figure 2i), barely seen in 2 hours (Figure 2j) and not seen 24 hours after (Figure 2k) the injection. These results suggest that the injected solution was able to reach the entire hindlimb.
Effect of hydrodynamic parameters on gene delivery efficiency
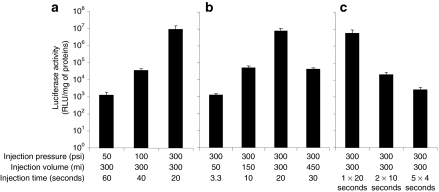
The effect of different hydrodynamic parameters on gene transfer was evaluated using pCMV-Luc reporter plasmid. Figure 3a shows that injection pressure is critical for gene delivery efficiency. Using injection volume at 300 ml (1.5% body weight), the injection pressure of 300 psi resulted in the highest level of luciferase gene expression at 107 relative light units (RLU)/mg of extracted proteins 5 days after the injection, which was ~200- and 1,000-fold higher than that obtained at injection pressures of 100 and 50 psi, respectively (P < 0.05). Injection pressure >300 psi was not tested because this is the upper limit of our injection device. These results suggest that the best injection pressure among the three pressures examined for intravascular gene delivery to the hindlimb is 300 psi.
Figure 3.
Effect of hydrodynamic parameters on gene delivery efficiency. (a) Effect of injection pressure, (b) effect of injection volume, and (c) effect of single injection with extended time or multiple injections with short duration. Luciferase activity was determined 5 days after hydrodynamic injection. The values represent mean ± SD (five samples for each of six muscle groups from two hindlimbs). 1 × 20 seconds, a single 20-second injection; 2 × 10 seconds, two 10-second injections with 5-second pause in between; and 5 × 4 seconds, five 4-second injections with 1-second pause in between (P < 0.05 between the peak level and any other level within each group).
The injection pressure at 300 psi was used to examine the effect of injection volume on gene delivery efficiency. Figure 3b shows that the highest level of luciferase gene expression (107 RLU/mg of extracted proteins) was achieved with an injection volume of 300 ml (1.5% body weight, injection time: 20 seconds). Interestingly, an injection volume of 450 ml (2.25% body weight) in 30 seconds resulted in 100-fold lower luciferase gene expression than that of 300 ml (P < 0.05). An injection at 150 ml (0.75% body weight) generated similar level of luciferase gene expression to that of 450 ml. Luciferase activity resulted from injection volume of 50 ml (injection time: 3.3 seconds) was 1,000-fold lower than that of 300 ml (P < 0.05). These results suggest that the optimal injection volume for intravascular gene delivery to the hindlimb is 300 ml.
Keeping the injection pressure at 300 psi and the injection volume at 300 ml, we then compared gene delivery efficiency between a single extended injection and multiple injections with short duration. Figure 3c shows that two consecutive 10-second injections with a 5-second pause in between and five 4-second injections with 1-second pause in between resulted in luciferase activity at 103 to 105 RLU/mg of extracted proteins, 100- to 1,000-fold lower than one single 20-second injection (P < 0.05). There was no impact of injection on physiological parameters such as electrocardiogram, heart rate, respiratory rate, blood pressure, and oxygen saturation level (data not shown). These results suggest that the optimal condition for intravascular gene delivery to proximal limb is a single injection of 300 ml (1.5% body weight) plasmid DNA in 20 seconds using 300 psi as the injection pressure.
Comparison of image-guided hydrodynamic gene delivery with conventional method of anterograde gene delivery
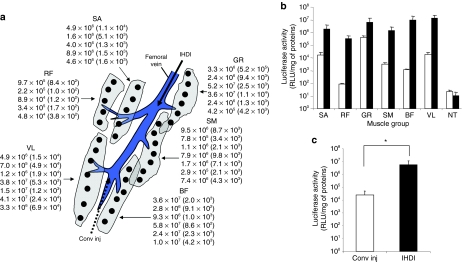
We then compared gene delivery efficiency between our image-guided hydrodynamic gene delivery and the conventional anterograde injection from peripheral vein. For conventional injection, an injection catheter was inserted from the peripheral branch of the femoral vein and plasmid-containing solution was injected anterograde at the flow rate of 2 ml/second according to the published procedure.17 Occlusion of blood flow was made at the proximal side of the femoral vein. Image-guided hydrodynamic injection in retrograde was performed as illustrated in Figure 1. Figure 4a shows the site and level of luciferase gene expression in different muscle groups (values in parenthesis represent luciferase activity that resulted from conventional injection). Luciferase gene expression was seen in all muscle groups including the sartorius, rectus femoris, gracilis, semimembranous, biceps femoris, and vastus lateralis. The muscle groups near the femoral vein such as vastus lateralis and gracilis showed higher luciferase activity compared to rectus femoris muscle located at the far side of the femoral vein. For conventional injection, the highest level of luciferase gene expression was seen in gracilis with luciferase activity at 105 RLU/mg of extracted proteins. The lowest gene expression was seen in rectus femoris (102 RLU/mg of extracted proteins, P < 0.05 to gracilis muscle). Among the muscle groups hydrodynamically transfected, the vastus lateralis, biceps femoris, and gracilis muscle showed the highest level of luciferase activity at 107 RLU/mg of extracted proteins, followed by the sartorius and semimembranous muscle at 106 RLU/mg of extracted proteins level. The lowest gene expression was seen in rectus femoris muscle at 105 RLU/mg of extracted proteins. Figure 4b shows the average luciferase activity from each muscle group resulted from conventional or image-guided hydrodynamic gene delivery. The average luciferase activity for the entire hindlimb (Figure 4c) that resulted from the image-guided hydrodynamic procedure is at least 100-fold higher than that of conventional method (Figure 4c) (P < 0.05).
Figure 4.
Comparison between image-guided hydrodynamic injection in retrograde and conventional injection in anterograde. Image-guided hydrodynamic gene delivery into the femoral vein was performed to right proximal hindlimb with an injection flow rate of 15 ml/second (volume of 300 ml in 20 seconds). Conventional injection into the peripheral vein was performed at injection rate of 2 ml/second (volume of 300 ml in 150 seconds) in anterograde. Luciferase gene expression was determined 5 days after the injection. (a) Site and level of luciferase expression (RLU/mg of extracted proteins) in various muscle groups in targeted hindlimb. Values in parentheses are luciferase activity resulted from conventional injection. Different shapes represent individual muscle group and their relevant position toward to femoral vein. The dots represent the approximate sites where muscle samples were collected. (b) Average of luciferase gene expression in various muscle groups transfected by image-guided hydrodynamic procedure (black bars) or by conventional injection in anterograde (open bars). (c) Average luciferase activity for the entire hindlimb (n = 2). Black solid and dotted arrow represents direction of the injection of IHDI and Conv inj, respectively (*P < 0.05). BF, biceps femoris; GR, gracilis; NT, nontransfected; RF, rectus femoris; SA, sartorius; SM, semimembranous; VL, vastus lateralis; Conv inj, conventional injection in anterograde direction; IHDI, image-guided hydrodynamic injection in retrograde.
Persistency of reporter gene expression
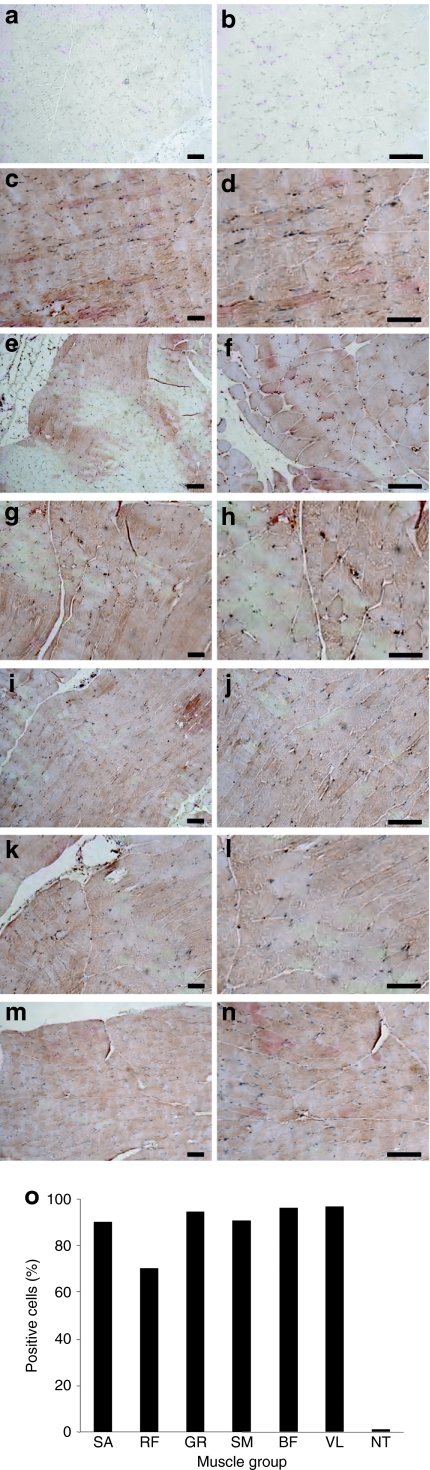
Experiments to examine persistency of transgene expression after image-guided hydrodnamic gene delivery were performed on two pigs using human α1 antitrypsin (hAAT) gene as a reporter and the best condition obtained (injection pressure: 300 psi, injection volume: 300 ml, injection time: 20 seconds). In animals with both proximal hindlimbs hydrodynamically transfected, hAAT protein level in serum was below detection limit of enzyme-linked immunosorbent assay (<1 ng/ml), but readily detectable in all muscle groups by immunohistochemistry. Figure 5 shows the result of antibody staining of different muscle groups 2 months after gene transfer. Compared to the control groups without gene transfer (Figure 5a,b), strong hAAT-positive cells were seen in all targeted muscle groups including the sartorius (Figure 5c,d), rectus femoris (Figure 5e,f), gracilis (Figure 5g,h), semimembranous (Figure 5i,j), biceps femoris (Figure 5k,l), and vastus lateralis (Figure 5m,n) muscle cells. Approximately 70–90% muscle cells stained positive for hAAT protein in all muscle groups (Figure 5o). The relatively heterogeneous distribution and smaller number of positively stained cells in rectus femoris muscle may be caused by uneven impact of the injection because of its distant location from the injection site. These results suggest that transgene expression is long lasting after the image-guided intravascular hydrodynamic gene delivery to skeletal muscle of the hindlimb.
Figure 5.
Detection of human α1 antitrypsin (hAAT) positive cells in muscle sections. Image-guided hydrodynamic injection of saline containing pAAT-hAAT plasmid (100 µg/ml) was performed at injection pressure of 300 psi and volume of 300 ml (1.5% body weight) in 20 seconds. Animals were euthanized 60 days after the hydrodynamic gene delivery and the muscle groups were collected for hAAT expression analysis. Tissue sections were immunostained with anti-α1 antitrypsin antibody. (a,b) NT muscle, (c,d) transfected SA, (e,f) transfected RF, (g,h) transfected GR muscle; (i,j) transfected SM muscle, (k,l) transfected BF muscle, (m,n) transfected VL muscle. Bar = 100 µm (a,c,e,g,i,k,m: ×100; b,d,f,h,j,l,n: ×200), (o) Percentage of the hAAT-positive cells (n = 600 muscle cells from sections of two hindlimbs). BF, biceps femoris; GR, gracilis; NT, nontransfected; RF, rectus femoris; SA, sartorius; SM, semimembranous; VL, vastus lateralis.
Assessment of tissue damage
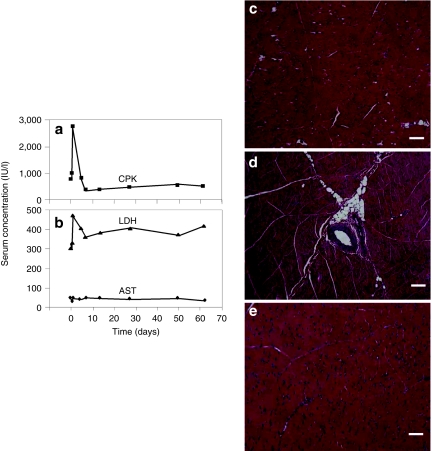
Blood biochemistry and histology of the targeted muscle were used to examine potential tissue damage of the procedure. Blood samples were collected before, 2 hours and 1, 5, 7, 14, 28, 50, and 60 days after hydrodynamic injection and full panel of serum biochemistry tests were performed. Figure 6a,b show blood concentration of three muscle related enzymes with time. A threefold increase of creatine phosphokinase was observed 24 hours after the injection (Figure 6a). Similarly, a spike increase of lactate dehydrogenase was also seen in the same time period. No increase was seen for serum concentration of aspartate aminotransferase (Figure 6b). Levels of creatine phosphokinase and lactate dehydrogenase returned to normal range 3 days after the injection. For the entire experimental period, the values for other serum components including total protein, albumin, globulin, alanine aminotransferase, γ-glutamic aminotransferase, alkaline phosphatase, total bilirubin, blood urea nitrogen, creatinine, glucose, sodium ion, potassium ion, and chloride ion remain unchanged (data not shown).
Figure 6.
Assessment of tissue damage by hydrodynamic injection. Blood samples were collected from the ear vein or peripheral vein in the limb before (time = 0), 2 hours, 1 day, and 5 days (n = 7), and 7, 14, 28, 50, and 60 days (n = 2). (a) Mean concentration of creatine phosphokinase (CPK), (b) lactate dehydrogenase (LDH), and aspartate aminotransferase (AST) in serum; hematoxylin and eosin staining of muscle from (c) nontransfected, (d) right after, and (e) 5 days after hydrodynamic gene delivery. Bar = 100 µm (×100).
Hematoxylin and eosin staining was utilized to evaluate the impact of the hydrodynamic injection on muscle structure. Compared to muscle structure of a noninjected hindlimb (Figure 5c), edema in injected muscle of animals right after the injection was identified (Figure 5d), which was resolved in 5 days (Figure 5e). The muscular edema is expected based on the significant swelling shown in Figure 2. These results suggest that the image-guided, intravascular hydrodynamic procedure is safe for gene delivery to skeletal muscle.
Discussion
Our results demonstrate that image-guided hydrodynamic gene delivery effectively transfects skeletal muscle cells (Figures 3–5) and results in long-term transgene expression (Figure 5). The pressure, volume, and time of injection are primary factors affecting gene transfer efficiency (Figure 3). The optimal parameters for hydrodynamic gene delivery to proximal hindlimb of 18–20 kg pigs are 300 psi as injection pressure, a volume equal to 1.5% body weight, and 20-second injection time (injection rate: 15 ml/second). No significant tissue damage is evidenced under the optimal condition (Figure 6).
The injected muscle was the predominant site of gene transfer and expression after hydrodynamic gene delivery to the femoral vein. Luciferase gene expression was detected only in the targeted proximal hindlimb, not in nontreated hindlimb, or other organs such as the liver, spleen, and kidney (data not shown). Images in Figure 2 demonstrating extravasation of injected solution and distribution to the entire muscle suggest that the procedure is capable of delivering gene into large number of cells. Immunohistochemistry study (Figure 5) suggests that the type of cells transfected are predominately skeletal muscle cells.
The image-guided hydrodynamic gene delivery was more effective than the conventional method of anterograde injection (Figure 4). This is likely due to the fact that hydrodynamics-based procedure is more effective in enhancing the permeability of the endothelium and the membrane of parenchyma cells. It is possible that the high flow rate generated by the pressurized injection into the vasculature induces transient change in endothelial and parenchymal structure leading to a more efficient gene transfer into the muscle cells. The impact of higher injection pressure and injection volume on gene expression level may be explained by the same mechanism. However, the observation that transfection efficiency with 450 ml was <300 ml (Figure 3) may reflect the presence of limit on the hydrodynamic impact on cells. Excessive hydrodynamic pressure can cause unfavorable structural changes and a lower level of transgene expression. Similar results had been reported by Danialou et al. using swine distal hindlimb injecting through its artery.16 Currently, it is unclear why multiple injections with shorter duration are not as effective as a single extended injection.
Sustained expression of hAAT was observed (Figure 5). Similar results were previously reported in small animals.14,17 Zhang et al. reported gene expression persisted for 6 months (ref. 14). The long-term expression may be in part explained by gene delivery to nondividing muscle cells. However, it is not currently clear whether the plasmid used in this study, especially the hAAT promoter, plays an important role in determining the persistence of transgene expression. Recent studies in mice showed that transgene expression in mouse liver after hydrodynamic delivery is significantly influenced by the number of CpG sequence in the plasmid.21,22 In addition, the promoter sequence23 and inclusion of intron sequence into the coding region of the transgene24,25,26 are also found important in determining persistence of transgene expression.
Skeletal muscle gene delivery can result in sustained level of therapeutic proteins that may be effective for treatment of muscular diseases. Alternatively, the image-guide hydrodynamic procedure may be used for delivery of therapeutic proteins, oligonucleotides or drug molecules directly to the muscle. As the image-guided catheter insertion is routinely used in clinic, the fact that image-guided, intravascular hydrodynamic delivery is capable of efficient gene transfer to proximal hindlimb in pigs provides the strong evidence of clinical applicability of this procedure. It is also conceivable that a modified procedure for image-guided delivery can be developed for delivery of DNA, RNA, proteins, or synthetic compounds to coronary vasculature or diseased cardiac muscle for basic or clinical studies.
Materials and Methods
Materials. DNA plasmids (pCMV-Luc and pAAT-hAAT) were purified by CsCl–ethidium bromide density-gradient ultracentrifugation and kept in Tris–EDTA buffer. Purity of the plasmids was verified by absorbency at 260 and 280 nm and 1% agarose gel electrophoresis. The luciferase assay kit was from Promega (Madison, WI). The introducer and the 12 Fr sheath for image-guided catheter insertion were from COOK (Bloomington, IN) and guide wire (ZIP wire) was from Boston Scientific (Natick, MA). Injection balloon catheters (8 Fr) were purchased from Medtronic (Minneapolis, MN). Contrast medium (OXILAN) was from Guerbet (Bloomington, IN). WavelineVet Vital Signs Monitor for monitoring physiological parameters on animals was from DRE Veterinary (Louisville, KY). Pigs (female, 18–20 kg) were from Wally Whippo (Enon Valley, PA).
Animal catheterization. All procedures performed on pigs were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh, Pittsburgh, Pennsylvania. Under general anesthesia, the animal was placed on the table of fluoroscopy machine YSF-100 from Shimadzu (Kyoto, Japan). A skin incision was made at the neck to expose the jugular vein and an 18 G peripheral catheter from TERUMO (Tokyo, Japan) was inserted. A 0.035-inch hydrophilic guide wire was inserted through the peripheral catheter and replaced later by a short sheath (12 Fr). Following an insertion of guide wire, the injection balloon catheter (8 Fr) was inserted to the targeted area of the femoral vein through the sheath. The venography was performed by injecting a small amount of contrast medium from the inserted catheter into appropriate venous sections. Inflation of the balloon was achieved by injecting 5 ml of contrast medium into the balloon. Obstruction of blood flow was verified by injecting a small volume of contrast medium into the femoral vein through the injection balloon catheter. To isolate the proximal hindlimb, peripheral occlusion was made at the join of proximal and distal hindlimb using a rubber band.
Intravascular hydrodynamic injection to hindlimb. Injection of saline containing either pCMV-Luc, pAAT-hAAT plasmid DNA (100 µg/ml), or contrast medium from the catheter was made via a computer-controlled injection device under the control of HydroJector600 software.18 Injection was driven by the pressure provided by a gas (CO2) cylinder with its pressure regulator set at the desirable pressure. Volume of injected DNA solution was set by the injection period and confirmed by subtraction the remaining volume in solution reservoir after the injection from the total volume before the injection. Multiple injections were controlled by the HydroJector600 injection system (single 20-second injection, two 10-second injections with an interval of 5 seconds, and five 4-second injections with 1-second interval time). Anterograde injection to the blood flow was performed through an 18 G peripheral catheter inserted to the superficial peripheral branch of the femoral vein at the flow rate of 2 ml/second as previously described.17 The change of internal and external appearance in targeted area before, during, and after hydrodynamic injection was recorded and visualized using a fluoroscope connected to a video cassette recorder and an SD1100 digital camera (Canon, Lake Success, NY), respectively. Physiological parameters (electrocardiogram, heart rate, respiratory rate, blood pressure, and oxygen saturation level) were continuously monitored and recorded before, during, and after the injection. At the end of injection, the balloon remained inflated for 20 minutes and then slowly deflated. The incision made at the animals' neck was sutured. Animals received a postoperative analgesia for 48 hours with ketoprofen and an antibiotic for 72 hours with amoxicillin.
Analysis of luciferase gene expression. Hydrodynamic gene delivery of pCMV-Luc plasmid DNA was performed with different settings of injection parameters. Five days after the hydrodynamic gene delivery, animals were euthanized and >5 samples from each targeted and nontargeted muscle group were collected. Tissue samples were immediately frozen on powdered dry ice and kept at −80 °C until use. For luciferase assay, 1 ml of lysis buffer was added to each sample (~100 mg) and the sample was thawed on ice and homogenized using a tissue Tearor (2 minutes, max speed). The tissue homogenate was centrifuged for 10 minutes in a microcentrifuge and the supernatant was collected. Supernatant (10 µl) was taken for luciferase and protein assay according to the previously established procedure.1 The level of reporter gene expression was presented as RLU per mg of total proteins in tissue homogenate. The amount of luciferase protein in tissue extract can be calculated using the equation derived from the standard curve in which luciferase protein (pg) = 7.98 × 10−5 RLU + 0.093 (R2 = 0.9999) (ref. 1).
Long-term expression study. Animals were hydrodynamically injected with the plasmid containing complementary DNA of hAAT gene driven by its natural promoter and euthanized 60 days after hydrodynamic gene delivery. Muscle samples were collected, fixed in 10% formalin, and embedded in paraffin. Sections (5 µm) were made and standard immunohistochemistry staining was performed with rabbit antihuman α1 antitrypsin antibody (ab9373, 1:20 dilution; Abcam, Cambridge, MA) and biotinylated goat anti-rabbit antibody as the 2nd antibody (BA-1000, Goat, 1:200 dilution; Vector Laboratories, Burlingame, CA). The distribution of α1 antitrypsin in muscle sections was visualized through avidin–biotin complex amplification using Vecstatin Elite ABC kit (PK-6100; Vector Laboratories) and AEC Chromogen Kit (ACG500; ScyTek Laboratories, Logan, UT). Microscopic examination was performed and photo images were recorded using a computer-controlled imaging system (Leitz DMRB; Leica Wetzlar, Germany). To quantify the percentage of hAAT-positive cells in the muscle, 200 cells in each of three different sections for each muscle group were counted and the percentage of positive cells calculated.
Assessment of tissue damage. Under general anesthesia, blood sample was collected at desirable time points from the ear vein or peripheral vein in the limb. Blood samples were collected before (time = 0), 2 hours, 1 day, and 5 days after the hydrodynamic injection of pCMV-Luc from five animals. Additional data points before the injection and at 2 hours, and 1, 5, 7, 14, 28, 50, and 60 days after the injection were collected from two pigs hydrodynamically injected with pAAT-hAAT. Automated concentration determination was performed using an IDEXX VetTest Chemistry Analyzer (Westbrook, ME). For microscopic examinations, muscle samples were collected from injected pigs described above right after the injection or 5 days after the injection and fixed in 10% formalin. Tissue embedding, sectioning (5 µm), and hematoxylin and eosin staining were done in the pathology lab of the Department of Pathology, University of Pittsburgh. Microscopic examination was performed and photographed under a regular light microscope.
Acknowledgments
We thank David Edwards for proof reading the manuscript. We acknowledge Michael Maranowski, Christopher Janssen, and all staff members in Division of Laboratory Animal Resources at the University of Pittsburgh for the excellent assistance for animal care. This work was supported in part by the National Institute of Health grant RO1EB007357. The authors declare that they have no conflict of interest.
REFERENCES
- Liu F, Song Y., and , Liu D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 1999;6:1258–1266. doi: 10.1038/sj.gt.3300947. [DOI] [PubMed] [Google Scholar]
- Zhang G, Budker V., and , Wolff JA. High levels of foreign gene expression in hepatocytes after tail vein injections of naked plasmid DNA. Hum Gene Ther. 1999;10:1735–1737. doi: 10.1089/10430349950017734. [DOI] [PubMed] [Google Scholar]
- Suda T., and , Liu D. Hydrodynamic gene delivery: its principles and applications. Mol Ther. 2007;15:2063–2069. doi: 10.1038/sj.mt.6300314. [DOI] [PubMed] [Google Scholar]
- Herweijer H., and , Wolff JA. Gene therapy progress and prospects: hydrodynamic gene delivery. Gene Ther. 2007;14:99–107. doi: 10.1038/sj.gt.3302891. [DOI] [PubMed] [Google Scholar]
- Kamimura K, Suda T, Xu W, Zhang G., and , Liu D. Image-guided, lobe-specific hydrodynamic gene delivery to swine liver. Mol Ther. 2009;17:491–499. doi: 10.1038/mt.2008.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman SJ, Baskin KM, Hodges BL, Chu Q, Gates A, Dreusicke R, et al. Development of catheter-based procedures for transducing the isolated rabbit liver with plasmid DNA. Hum Gene Ther. 2002;13:2065–2077. doi: 10.1089/10430340260395910. [DOI] [PubMed] [Google Scholar]
- Yoshino H, Hashizume K., and , Kobayashi E. Naked plasmid DNA transfer to the porcine liver using rapid injection with large volume. Gene Ther. 2006;13:1696–1702. doi: 10.1038/sj.gt.3302833. [DOI] [PubMed] [Google Scholar]
- Aliño SF, Herrero MJ, Noguera I, Dasí F., and , Sánchez M. Pig liver gene therapy by noninvasive interventionist catheterism. Gene Ther. 2007;14:334–343. doi: 10.1038/sj.gt.3302873. [DOI] [PubMed] [Google Scholar]
- Fabre JW, Grehan A, Whitehorne M, Sawyer GJ, Dong X, Salehi S, et al. Hydrodynamic gene delivery to the pig liver via an isolated segment of the inferior vena cava. Gene Ther. 2008;15:452–462. doi: 10.1038/sj.gt.3303079. [DOI] [PubMed] [Google Scholar]
- Khorsandi SE, Bachellier P, Weber JC, Greget M, Jaeck D, Zacharoulis D, et al. Minimally invasive and selective hydrodynamic gene therapy of liver segments in the pig and human. Cancer Gene Ther. 2008;15:225–230. doi: 10.1038/sj.cgt.7701119. [DOI] [PubMed] [Google Scholar]
- Budker V, Zhang G, Danko I, Williams P., and , Wolff J. The efficient expression of intravascularly delivered DNA in rat muscle. Gene Ther. 1998;5:272–276. doi: 10.1038/sj.gt.3300572. [DOI] [PubMed] [Google Scholar]
- Zhang G, Budker V, Williams P, Subbotin V., and , Wolff JA. Efficient expression of naked DNA delivered intraarterially to limb muscles of nonhuman primates. Hum Gene Ther. 2001;12:427–438. doi: 10.1089/10430340150504046. [DOI] [PubMed] [Google Scholar]
- Liang KW, Nishikawa M, Liu F, Sun B, Ye Q., and , Huang L. Restoration of dystrophin expression in mdx mice by intravascular injection of naked DNA containing full-length dystrophin cDNA. Gene Ther. 2004;11:901–908. doi: 10.1038/sj.gt.3302239. [DOI] [PubMed] [Google Scholar]
- Zhang G, Ludtke JJ, Thioudellet C, Kleinpeter P, Antoniou M, Herweijer H, et al. Intraarterial delivery of naked plasmid DNA expressing full-length mouse dystrophin in the mdx mouse model of duchenne muscular dystrophy. Hum Gene Ther. 2004;15:770–782. doi: 10.1089/1043034041648408. [DOI] [PubMed] [Google Scholar]
- Toumi H, Hegge J, Subbotin V, Noble M, Herweijer H, Best TM, et al. Rapid intravascular injection into limb skeletal muscle: a damage assessment study. Mol Ther. 2006;13:229–236. doi: 10.1016/j.ymthe.2005.07.699. [DOI] [PubMed] [Google Scholar]
- Danialou G, Comtois AS, Matecki S, Nalbantoglu J, Karpati G, Gilbert R, et al. Optimization of regional intraarterial naked DNA-mediated transgene delivery to skeletal muscles in a large animal model. Mol Ther. 2005;11:257–266. doi: 10.1016/j.ymthe.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Hagstrom JE, Hegge J, Zhang G, Noble M, Budker V, Lewis DL, et al. A facile nonviral method for delivering genes and siRNAs to skeletal muscle of mammalian limbs. Mol Ther. 2004;10:386–398. doi: 10.1016/j.ymthe.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Suda T, Suda K., and , Liu D. Computer-assisted hydrodynamic gene delivery. Mol Ther. 2008;16:1098–1104. doi: 10.1038/mt.2008.66. [DOI] [PubMed] [Google Scholar]
- Hawkins IF. Carbon dioxide digital subtraction arteriography. AJR Am J Roentgenol. 1982;139:19–24. doi: 10.2214/ajr.139.1.19. [DOI] [PubMed] [Google Scholar]
- Hawkins IF., and , Caridi JG. Carbon dioxide (CO2) digital subtraction angiography: 26-year experience at the University of Florida. Eur Radiol. 1998;8:391–402. doi: 10.1007/s003300050400. [DOI] [PubMed] [Google Scholar]
- Hodges BL, Taylor KM, Joseph MF, Bourgeois SA., and , Scheule RK. Long-term transgene expression from plasmid DNA gene therapy vectors is negatively affected by CpG dinucleotides. Mol Ther. 2004;10:269–278. doi: 10.1016/j.ymthe.2004.04.018. [DOI] [PubMed] [Google Scholar]
- Yew NS, Zhao H, Przybylska M, Wu IH, Tousignant JD, Scheule RK, et al. CpG-depleted plasmid DNA vectors with enhanced safety and long-term gene expression in vivo. Mol Ther. 2002;5:731–738. doi: 10.1006/mthe.2002.0598. [DOI] [PubMed] [Google Scholar]
- Xu ZL, Mizuguchi H, Ishii-Watabe A, Uchida E, Mayumi T., and , Hayakawa T. Optimization of transcriptional regulatory elements for constructing plasmid vectors. Gene. 2001;272:149–156. doi: 10.1016/s0378-1119(01)00550-9. [DOI] [PubMed] [Google Scholar]
- Notley C, Killoran A, Cameron C, Wynd K, Hough C., and , Lillicrap D. The canine factor VIII 3′-untranslated region and a concatemeric hepatocyte nuclear factor 1 regulatory element enhance factor VIII transgene expression in vivo. Hum Gene Ther. 2002;13:1583–1593. doi: 10.1089/10430340260201671. [DOI] [PubMed] [Google Scholar]
- Miao CH, Ye X., and , Thompson AR. High-level factor VIII gene expression in vivo achieved by nonviral liver-specific gene therapy vectors. Hum Gene Ther. 2003;14:1297–1305. doi: 10.1089/104303403322319381. [DOI] [PubMed] [Google Scholar]
- Wolff LJ, Wolff JA., and , Sebestyén MG. Effect of tissue-specific promoters and microRNA recognition elements on stability of transgene expression after hydrodynamic naked plasmid DNA delivery. Hum Gene Ther. 2009;20:374–388. doi: 10.1089/hum.2008.088. [DOI] [PubMed] [Google Scholar]