Abstract
Lung cancer is the leading cause of cancer-related death in the United States, and 85–90% of lung cancer cases are associated with tobacco use. Tobacco components promote lung tumorigenesis through genotoxic effects, as well as through biochemical modulation of signaling pathways such as the Akt/mTOR pathway that regulate cell proliferation and survival. This review will describe cell surface receptors and other upstream components required for tobacco-carcinogen induced activation of Akt and mTOR. Preclinical studies demonstrate that inhibitors of the Akt/mTOR pathway inhibit tumor formation in mouse models of carcinogen-induced lung tumorigenesis. Some of these inhibitors will be highlighted, and their clinical potential for the treatment and prevention of lung cancer will be discussed.
Keywords: Akt, mTOR, tobacco carcinogens, lung cancer
Background
The complex biological effects of tobacco smoke could be attributed to the large number of chemical agents it contains. At least 60 carcinogens are present in tobacco smoke that can be categorized into three groups: polycyclic aromatic hydrocarbons (PAH), nitrosamines, and aromatic amines. Additionally, nicotine, a precursor of many tobacco carcinogens, also promotes lung tumorigenesis (reviewed in (1)). Metabolic activation of tobacco components, such as the nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), generates metabolites that bind DNA and promote adduct formation. The persistence of DNA adducts results in somatic mutations in oncogenes and tumor suppressor genes such as K-Ras and TP53, which are critical to the formation of lung cancer. Although the genotoxic effects of tobacco carcinogens are well established, tobacco components can also promote lung tumorigenesis through modulation of signal transduction pathways that regulate cell proliferation, transformation, and survival. For example, nicotine and NNK are potent agonists of nicotinic acetylcholine receptors (nAChR), which are expressed in lung epithelial cells and can activate multiple signaling pathways such as Akt/mTOR, PKC (2), MEK/ERK (3, 4), and JAK/STAT pathways (5). Overexpression or activation of components of these pathways is prevalent in lung cancer. Although tobacco components have pleiotropic effects on signaling pathways in cells, arguably the most important mediator of tobacco-carcinogen induced lung tumorigenesis is the Akt/mTOR pathway.
Tobacco components stimulate multiple upstream activators of the Akt pathway
Activation of the Akt pathway by tobacco components increases lung epithelial cell proliferation and survival, and inhibits apoptosis in response to DNA damage. Tobacco components activate the Akt pathway by modulating the expression or activity of upstream regulators of Akt (Figure 1). Immediately upstream of Akt is the lipid kinase, PI3K. PI3K phosphorylates phosphoinositides at the D3 position, generating the biologically active lipids phsophatidylinositol-3,4-bisphosphate (PI(3,4)P2) and phosphatidylinositol-3,4,5-triphosphate (PI(3,4,5,)P3). Generation of these lipids, and subsequent binding to the pleckstrin homology (PH) domain of Akt, is critical to the activation of Akt. Activating mutations or amplification of PIK3CA, the gene that encodes the catalytic subunit of PI3K, p110α, occur in a subset of smoking-related lung cancers (6). The tumor suppressor PTEN is a lipid phosphatase that opposes this activity of PI3K. Loss of PTEN occurs in ~70 % of NSCLC through inactivating mutations, or more frequently, through promoter methylation (7, 8). Studies performed using an inhibitor of PI3K, LY294002, demonstrated that nicotine and NNK-induced activation of Akt in lung epithelial cells is dependent on PI3K (9, 10). Moreover, pretreatment of lung epithelial cells with LY294002 decreases tobacco-component induced proliferation and increases apoptosis.
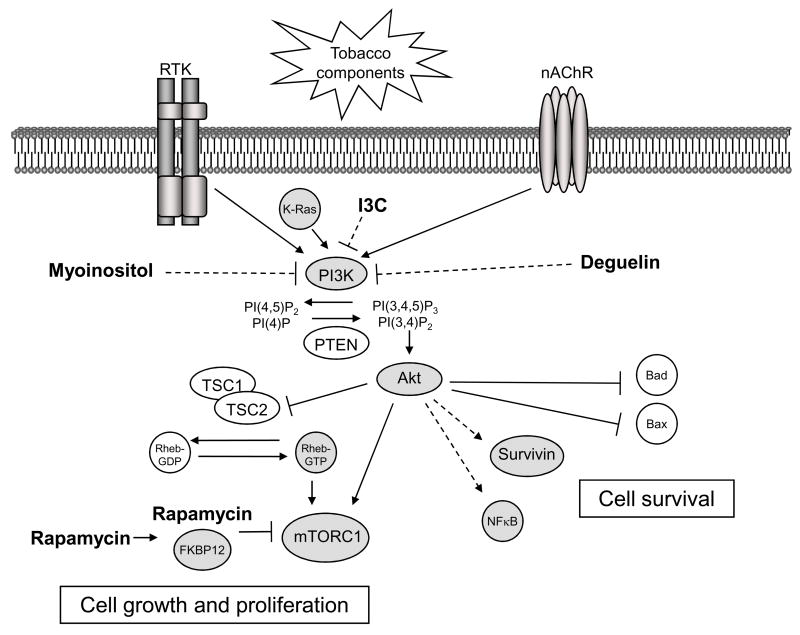
Figure 1. Upstream activators and downstream mediators of the Akt pathway in tobacco-carcinogen induced lung tumorigenesis.
Tobacco components stimulate cell surface receptors, such as members of the erbB family of receptor tyrosine kinases (RTK) and nicotinic acetycholine receptors (nAChR), which activate PI3K. PI3K can also be activated through direct interaction with K-Ras, which is commonly mutated in smoking-related lung cancers. The lipid kinase PI3K is required for tobacco-component induced activation of Akt. PI3K phosphorylates the phosphoinositides PI(4)P and PI(4,5)P2 at their D3 position, generating PI(3,4)P2 and PI(3,4,5)P3, respectively. The tumor suppressor PTEN opposes this activity of PI3K. PI(3,4)P2 and PI(3,4,5)P3 bind to the pleckstrin homology (PH) domain of Akt promoting its translocation to the cell membrane and subsequent activation. Akt activation in response to tobacco components increases cell survival by direct phosphorylation and inactivation of the proapoptotic proteins Bad and Bax. Additionally, Akt increases cell survival by indirectly inducing the anti-apoptotic protein survivin and the transcription factor NFkB. Tobacco-component induced activation of Akt can also increase cell growth and proliferation through activation of the mTOR pathway. Akt activates mTORC1 by at least two mechanisms. Akt can activate mTORC1 indirectly by phosphorylation and inactivation of TSC2, which suppresses the activity of the Rheb GTPase, an activator of mTORC1. Akt also directly activates mTORC1 through phosphorylation of PRAS40, a component of mTORC1. Drugs that inhibit the Akt/mTOR pathway could be effective in the treatment of prevention of tobacco-carcinogen induced lung tumorigenesis. The best-characterized inhibitors of this pathway (shown in bold) are rapamycin and its analogues, myoinositol, indole-3-carbinol (I3C), and deguelin. Rapamycin inhibits mTOR by binding to the immunophilin FKBP12. This complex, in turn, inhibits the activity of mTORC1. Conversely, myoinositol, indole-3-carbinol, and deguelin inhibit this pathway upstream of Akt, but whether PI3K is the direct target of these drugs is unclear (this is indicated by the dotted lines).
PI3K itself is activated by tobacco-components through multiple, upstream mechanisms. PI3K is composed of a catalytic subunit (p110) and a regulatory subunit (p85) that contains Src-homology 2 (SH2) domains. Interaction of these domains with phosphotyrosine residues located on growth factor tyrosine kinase receptors, such as erbB family members, activates PI3K. In vitro studies demonstrated that NNK-induced transformation of bronchial epithelial cells increases the expression of EGFR (11). Additionally, overexpression of EGFR and erb-B3 occur in the bronchial epithelium of smokers (12). PI3K activation can also occur by direct interaction of p110 with activated Ras. Activating mutations in K-Ras occur in approximately 25% of smoking-associated human lung adenocarcinomas (13). Also, exposure of A/J mice to the tobacco-specific carcinogen NNK induces K-Ras mutations, which promote lung tumorigenesis in this model (14). Immunohistochemical analysis of the lung adenomas and adenocarcinomas that develop in these mice demonstrated that Akt was activated in these lesions, which correlated with tumor progression (10). These studies demonstrate the importance of erbB family members and K-Ras in mediating tobacco-carcinogen induced activation of the PI3K/Akt pathway.
Another mechanism by which tobacco components can activate the PI3K/Akt pathway is via stimulation of nAChR. These receptors are prototypic ligand-gated ion channels that consist of either homo-pentamers derived from α7–α10 subunits or hetero-pentamers derived from a combination of α1–α6 and β2–β4 subunits. nAChR are important mediators of fast synaptic transmission in neurons, but they are also expressed in many non-neuronal cell types such as immune cells, keratinocytes, and epithelial and endothelial cells (reviewed in (15)). RT-PCR and microarray analyses demonstrated that lung epithelial cells vary in their expression of nAChR (9, 16). Epithelial cells of the small airways selectively express α2 and α4 subunits, whereas large airway epithelial cells express α3 and α5 subunits. Both cell types express α7– α10, β2, and β4 subunits. Quantitative PCR and microarray analysis demonstrated that the α4 and β4 subunits are preferentially expressed by NSCLC cells compared to normal lung epithelium (17). Additionally, analysis of tumors from patients with NSCLC showed differential expression of nAChR between smokers and non-smokers, with higher expression of the α6β3 receptor in the tumors from non-smokers. Genome wide association studies have suggested that individual nAChR confer an increased risk for tobacco-related lung cancer. Specifically, the gene locus 15q24 was associated with increased lung cancer risk and nicotine dependence (18–20). This locus contains genes that encode for the α3, α5 and β4 subunits of nAChR. Collectively, these studies support the role of nAChR in mediating tobacco-induced lung carcinogenesis.
Subunit composition of nAChR determines agonist-specific responsiveness. For example, nicotine and NNK are potent agonists of hetero-pentameric and α7 homo-pentameric nAChR, respectively. Studies performed using nAChR isoform-specific antagonists demonstrated that inhibitors of α3- and α4-containing nAChR decreased nicotine-induced activation of Akt, whereas inhibitors of α7-containing nAChR blocked NNK-induced activation of Akt (9). These receptors are also required for tobacco-component induced Akt activation in NSCLC cells, which promotes resistance to chemotherapy and radiation (21). Although the ability of nAChR to activate Akt is dependent on PI3K, the mechanism by which these receptors activate PI3K is still unclear.
mTOR is a critical mediator of tobacco-carcinogen-induced, Akt-driven lung tumorigenesis
Akt can promote tobacco-carcinogen induced lung tumorigenesis by regulation of multiple signaling pathways (Figure 1). For example, Akt increases lung epithelial cell survival in response to NNK and nicotine by phosphorylation and inactivation of the pro-apoptotic proteins Bad and Bax (16, 22–24), as well as through induction of the anti-apoptotic protein survivin (16, 25). Additionally, Akt activates the transcription factor NFκB, which increases NSCLC cell survival in vitro (21), and promotes tumor growth and angiogenesis via VEGF in vivo (26, 27). Another important mechanism by which Akt promotes tobacco-carcinogen induced lung tumorigenesis is through activation of the mTOR pathway.
Similar to Akt, mTOR regulates cellular processes critical to tumorigenesis such as cell growth, proliferation, and metabolism, and many cancers are characterized by aberrant activation of mTOR, including lung cancer (reviewed in (28)). mTOR functions in two distinct complexes in cells, mTORC1 and mTORC2. mTORC1 increases protein synthesis and cell growth through activation of S6K1 (p70 ribosomal protein S6 kinase) and inactivation of 4E-BP1 (eIF4E binding protein 1). Although the role of mTORC2 in regulating cellular processes is not well understood, mTORC2 directly phosphorylates members of the AGC family of kinases, such as PKCα and Akt itself, which could promote tumorigenesis (29–31). Akt activates mTORC1 by at least two mechanisms. Akt activates mTORC1 indirectly by phosphorylating and inhibiting the tumor suppressor TSC2 (32, 33). Because TSC2 suppresses the activity of the Ras-related GTPase Rheb, a selective activator of mTORC1, inhibition of TSC2 by Akt results in activation of mTORC1 (34). Additionally, Akt-mediated phosphorylation of PRAS40, a component of mTORC1, attenuates its inhibitory effect on mTORC1 (35, 36).
Preclinical studies have shown that mTOR promotes tobacco-component induced lung tumorigenesis. In vitro studies performed in lung epithelial or NSCLC cells showed that nicotine and NNK activate mTORC1, as assessed by increased phosphorylation of S6K and 4E-BP1 (9, 21). Nicotine can also activate mTORC1 by increasing the expression of the matrix glycoprotein fibronectin (37). In these studies, the ability of nicotine or NNK to activate mTORC1 was dependent on the Akt/mTOR pathway because treatment of NSCLC cells with Akt siRNA or the mTOR inhibitor rapamycin inhibited fibronectin-induced cell proliferation (37). A preferential role for mTOR in tobacco-carcinogen induced lung tumorigenesis was also suggested by analysis of NNK-induced lung lesions in A/J mice. Phenotypic progression of NNK-induced lung lesions correlated with increased activation of Akt and mTOR, but not other substrates of Akt such as GSK-3β (10).
Clinical data also validate the importance of the Akt/mTOR pathway in tobacco-carcinogen induced lung tumorigenesis. In hyperplastic and dysplastic lung lesions from heavy smokers, Akt and mTOR are progressively activated (38–41). Additionally, activation of Akt is associated with poor prognosis in patients with early-stage NSCLC (42). These studies suggest that activation of mTOR downstream of the PI3K/Akt pathway might be an important and early event in tobacco-carcinogen induced lung tumorigenesis.
Clinical-Translational Advances
Inhibitors of the Akt/mTOR pathway for the treatment or prevention of tobacco-carcinogen induced lung tumorigenesis
Because there is strong rationale to target the Akt/mTOR pathway in the treatment or prevention of tobacco-carcinogen induced lung tumorigenesis, inhibitors of this pathway have been evaluated in mouse models of lung cancer and in clinical trials. The inhibitors of the Akt/mTOR pathway that are best characterized are rapamycin and its analogs, myoinositol, indole-3-carbinol, and deguelin (Figure 1).
mTOR inhibitors
The most clinically developed inhibitors of the Akt/mTOR pathway are the mTOR inhibitors rapamycin (sirolimus) and its analogues, CCI-779 (temsirolimus) and RAD001 (everolimus). Rapamycin is a macrolide antibiotic that is FDA approved as an immunosuppressant for the prevention of renal allograft rejection. Rapamycin inhibits the activity of mTORC1 by binding to the immunophilin FKBP12. This complex, in turn, binds mTORC1 and inhibits its phosphorylation of substrates, such as S6K and 4E-BP1. Although originally reported to be insensitive to rapamycin, long-term treatment of mammalian cells with rapamycin also inhibits mTORC2 (43). Preclinical studies have demonstrated that rapamycin and rapamycin analogues are effective in the treatment or prevention of tobacco-induced lung tumorigenesis in multiple mouse models.
Exposure of A/J mice to the tobacco-specific carcinogen NNK causes K-Ras-mediated lung tumorigenesis (44), which is characterized by inflammation of the lung and increased activation of the mTOR pathway in tumors. Administration of rapamycin to A/J mice 26 weeks after exposure to NNK led to a 50% decrease in tumor volume, which correlated with decreased proliferation and inhibition of the mTOR pathway in tumors (45). Additionally, treatment of A/J mice with rapamycin following exposure to NNK, but prior to the development of tumors, reduced lung tumorigenesis by 90%. Rapamycin similarly prevented lung tumorigenesis in A/J mice exposed to another tobacco carcinogen, benzo(a)pyrene (BaP) (46).
Rapamycin could also inhibit tobacco-carcinogen induced lung tumorigenesis by affecting the tumor microenvironment. For example, administration of NNK significantly increases the number of lung-associated Foxp3+ regulatory T cells (Treg) prior to the development of tumors in A/J mice (47). Treg are a subset of CD4+ T cells that express the transcription factor Foxp3 and suppress autoreactive T cells, thus preventing autoimmunity (48, 49). Preclinical studies suggest that Treg may play an important role in limiting the development of an effective immune response against cancer (reviewed in (50)). Interestingly, rapamycin rapidly reverses the induction of lung-associated Treg by NNK, and maintains depletion of Foxp3+ Treg throughout treatment. These results suggest that rapamycin inhibits NNK-induced lung tumorigenesis by inhibition of mTOR in tumor cells and by decreasing lung- and tumor-associated Treg.
Similar results were obtained in a genetically engineered mouse model that genocopies and phenocopies tobacco-carcinogen induced lung tumorigenesis. Studies performed using mice bearing a mutant K-Ras allele, K-RASLA1, also demonstrated that CCI-779 decreased tumor growth, in part, by modulating components of the immune system (40). Specifically, these studies showed that inhibition of alveolar macrophage function and modulation of chemokine signaling contributed to inhibition of lung-tumorigenesis by CCI-779. Collectively, these studies demonstrate that mTOR inhibitors are effective in the treatment or prevention of tobacco-carcinogen induced lung tumorigenesis by inhibiting the mTOR pathway in tumor cells, as well as by affecting the tumor microenvironment.
Because mTOR inhibitors could be effective in the treatment of tobacco-carcinogen induced lung tumorigenesis, rapamycin and its analogues are currently being evaluated in clinical trials for the treatment of NSCLC. However, the therapeutic response in these trials has been modest (reviewed in (51)), and prolonged administration of these drugs could be limited due to toxicities in patients. Preclinical and clinical studies also suggest that the efficacy of mTOR inhibitors as anticancer agents could be limited due to feedback activation of Akt (52). Improved clinical response might be achieved by combining mTOR inhibitors with inhibitors of upstream components of the Akt/mTOR pathway that could negate feedback activation. mTOR inhibitors might also be more effective in the setting of lung cancer chemoprevention.
Myoinositol
Myoinositol is an isomer of glucose that is structurally similar to the head group of phosphatidylinositol, the endogenous substrate of PI3K in cells. Studies performed using compounds related to myoinositol demonstrated that this class of compounds inhibits the PI3K/Akt pathway, which is required for their anticancer activity (53, 54). Additionally, treatment of human bronchial epithelial cells (HBEC) with myoinositol inhibits basal levels of Akt activity as well as nicotine-induced activation of Akt, which correlates with inhibition of cell proliferation (41). Interestingly, myoinositol does not inhibit Akt nor inhibit the proliferation of NSCLC cells in vitro, suggesting that myoinositol might be more effective in preventing the development of lung cancer. Preclinical studies performed using A/J mice exposed to a mixture of tobacco carcinogens, NNK and BaP, demonstrated that myoinositol significantly prevented lung tumorigenesis when administered concurrently or following exposure to these carcinogens (55, 56). Assessment of P-Akt (S473) in lung tissues from these mice showed only modest inhibition by myoinositol. However, this analysis was performed on whole lung lysates, which would not distinguish between the effects of myoinositol on Akt activity in tumor cells and normal lung epithelium. Additionally, phosphorylation of Akt at S473 alone may not be an accurate readout for the activity of Akt (57). Taken together, these studies suggest that myoinositol could prevent tobacco carcinogen-induced lung tumorigenesis.
A Phase I clinical prevention trial with myoinositol in heavy smokers showed that it was well-tolerated even at doses as high as 18 g/day, and based on historical comparisons, the investigators suggested that treatment with myoinositol caused regression of preexisting bronchial dysplastic lesions (58). To elucidate possible mechanisms by which myoinositol might prevent lung tumorigenesis in smokers, immunohistochemical analysis was performed on 206 paired premalignant lesions from these patients using an antibody specific for P-Akt (T308), a site important to the activation of Akt. Prior to treatment with myoinositol, active Akt was detected in 71% of hyperplastic/metaplastic lesions and 90% of dysplastic lesions, and correlated with phenotypic progression (41). Regression of dysplastic lesions by myoinositol correlated with decreased phosphorylation of Akt. In addition, myoinositol decreased ERK phosphorylation in these lesions, a component of the MEK/ERK pathway that is also activated during tobacco-component induced lung tumorigenesis. These studies suggest that inhibition of signaling pathways by myoinositol underlies its ability to prevent lung tumorigenesis in smokers. A Phase II trial is currently being conducted with myoinositol in current and former smokers to further evaluate its ability to reverse bronchial dysplasia.
Indole-3-carbinol
Indole-3-carbinol (I3C) is a metabolite of glucobrasscins that are prevalent in cruciferous vegetables. Epidemiological studies indicate that consumption of cruciferous vegetables inversely correlates with lung cancer risk (59). Preclinical studies performed using A/J mice exposed to NNK and BaP demonstrated that I3C inhibited lung tumorigenesis in a dose-dependent manner when it was administered either during or one week following exposure to these carcinogens (60). Immunohistochemical and immunoblotting analyses of lung tissues from these mice showed that I3C significantly decreased P-Akt (S473) in tumors, as well as phosphorylation of the proapoptotic protein Bad, a substrate of Akt. However, quantitative proteomic analyses demonstrated that I3C modulated the expression of multiple proteins altered by the carcinogens in this model (61), and the relative contribution of Akt inhibition to the anti-cancer activity of this compound is unclear. Also, the clinical utility of I3C may be limited because I3C can promote colon and hepatic carcinogenesis in other mouse models (reviewed in (62)).
Deguelin
Deguelin is a naturally occurring rotenoid that can be isolated from several plant species. Studies performed using premalignant and malignant human bronchial epithelial cells demonstrated that deguelin inhibits the PI3K/Akt pathway, which was required for its ability to inhibit cell proliferation and induce apoptosis (63). In A/J mice, deguelin significantly decreased tumor multiplicity, volume, and overall tumor burden when administered before or following exposure to BaP or a mixture of NNK and BaP, respectively (64, 65). Immunohistochemical analysis showed that inhibition of tumorigenesis correlated with decreased P-Akt (S473) in bronchial epithelium and lung adenomas. Although these studies suggest that deguelin could be effective in the prevention of tobacco-carcinogen induced lung tumorigenesis, the clinical potential of deguelin might be limited due to toxicities (66). Efforts are currently underway to synthesize derivatives of deguelin that are less toxic but retain its anti-cancer activity.
Conclusion
Modulation of signaling pathways by tobacco components is an important aspect of tumor promotion. The Akt/mTOR pathway is an especially intriguing target for tobacco related lung tumorigenesis, based on the preclinical efficacy of pathway inhibitors and the results of early phase clinical trials. Drugs such as rapamycin, myoinositol, I3C, and degeulin that inhibit the pathway at different steps have clinical potential. As the complexity of mTOR regulation increases, options for new drugs to intervene in tobacco related carcinogenesis will also increase. For example, activation of the LKB1/AMPK pathway inhibits mTOR in an Akt-independent manner. Activators of AMPK such as the anti-diabetic drug metformin might therefore also inhibit tobacco carcinogen-induced lung tumorigenesis through inhibition of mTOR. Future studies will determine the best method to counteract the effects of tobacco components on signaling pathways.
References
- 1.Catassi A, Servent D, Paleari L, Cesario A, Russo P. Multiple roles of nicotine on cell proliferation and inhibition of apoptosis: implications on lung carcinogenesis. Mutat Res. 2008;659:221–31. doi: 10.1016/j.mrrev.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Clark AS, West KA, Blumberg PM, Dennis PA. Altered protein kinase C (PKC) isoforms in non-small cell lung cancer cells: PKCdelta promotes cellular survival and chemotherapeutic resistance. Cancer Res. 2003;63:780–6. [PubMed] [Google Scholar]
- 3.Dasgupta P, Rastogi S, Pillai S, et al. Nicotine induces cell proliferation by beta-arrestin-mediated activation of Src and Rb-Raf-1 pathways. J Clin Invest. 2006;116:2208–17. doi: 10.1172/JCI28164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jull BA, Plummer HK, 3rd, Schuller HM. Nicotinic receptor-mediated activation by the tobacco-specific nitrosamine NNK of a Raf-1/MAP kinase pathway, resulting in phosphorylation of c-myc in human small cell lung carcinoma cells and pulmonary neuroendocrine cells. J Cancer Res Clin Oncol. 2001;127:707–17. doi: 10.1007/s004320100289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arredondo J, Chernyavsky AI, Jolkovsky DL, Pinkerton KE, Grando SA. Receptor-mediated tobacco toxicity: cooperation of the Ras/Raf-1/MEK1/ERK and JAK-2/STAT-3 pathways downstream of alpha7 nicotinic receptor in oral keratinocytes. FASEB J. 2006;20:2093–101. doi: 10.1096/fj.06-6191com. [DOI] [PubMed] [Google Scholar]
- 6.Samuels Y, Wang Z, Bardelli A, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 7.Soria JC, Lee HY, Lee JI, et al. Lack of PTEN expression in non-small cell lung cancer could be related to promoter methylation. Clin Cancer Res. 2002;8:1178–84. [PubMed] [Google Scholar]
- 8.Marsit CJ, Zheng S, Aldape K, et al. PTEN expression in non-small-cell lung cancer: evaluating its relation to tumor characteristics, allelic loss, and epigenetic alteration. Hum Pathol. 2005;36:768–76. doi: 10.1016/j.humpath.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 9.West KA, Brognard J, Clark AS, et al. Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. J Clin Invest. 2003;111:81–90. doi: 10.1172/JCI16147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.West KA, Linnoila IR, Belinsky SA, Harris CC, Dennis PA. Tobacco carcinogen-induced cellular transformation increases activation of the phosphatidylinositol 3′-kinase/Akt pathway in vitro and in vivo. Cancer Res. 2004;64:446–51. doi: 10.1158/0008-5472.can-03-3241. [DOI] [PubMed] [Google Scholar]
- 11.Lonardo F, Dragnev KH, Freemantle SJ, et al. Evidence for the epidermal growth factor receptor as a target for lung cancer prevention. Clin Cancer Res. 2002;8:54–60. [PubMed] [Google Scholar]
- 12.O’Donnell RA, Richter A, Ward J, et al. Expression of ErbB receptors and mucins in the airways of long term current smokers. Thorax. 2004;59:1032–40. doi: 10.1136/thx.2004.028043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Westra WH, Slebos RJ, Offerhaus GJ, et al. K-ras oncogene activation in lung adenocarcinomas from former smokers. Evidence that K-ras mutations are an early and irreversible event in the development of adenocarcinoma of the lung. Cancer. 1993;72:432–8. doi: 10.1002/1097-0142(19930715)72:2<432::aid-cncr2820720219>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 14.Matzinger SA, Crist KA, Stoner GD, et al. K-ras mutations in lung tumors from A/J and A/J x TSG-p53 F1 mice treated with 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and phenethyl isothiocyanate. Carcinogenesis. 1995;16:2487–92. doi: 10.1093/carcin/16.10.2487. [DOI] [PubMed] [Google Scholar]
- 15.Gotti C, Clementi F. Neuronal nicotinic receptors: from structure to pathology. Prog Neurobiol. 2004;74:363–96. doi: 10.1016/j.pneurobio.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Dasgupta P, Kinkade R, Joshi B, Decook C, Haura E, Chellappan S. Nicotine inhibits apoptosis induced by chemotherapeutic drugs by up-regulating XIAP and survivin. Proc Natl Acad Sci U S A. 2006;103:6332–7. doi: 10.1073/pnas.0509313103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lam DC, Girard L, Ramirez R, et al. Expression of nicotinic acetylcholine receptor subunit genes in non-small-cell lung cancer reveals differences between smokers and nonsmokers. Cancer Res. 2007;67:4638–47. doi: 10.1158/0008-5472.CAN-06-4628. [DOI] [PubMed] [Google Scholar]
- 18.Hung RJ, McKay JD, Gaborieau V, et al. A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature. 2008;452:633–7. doi: 10.1038/nature06885. [DOI] [PubMed] [Google Scholar]
- 19.Amos CI, Wu X, Broderick P, et al. Genome-wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. Nat Genet. 2008;40:616–22. doi: 10.1038/ng.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thorgeirsson TE, Geller F, Sulem P, et al. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008;452:638–42. doi: 10.1038/nature06846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsurutani J, Castillo SS, Brognard J, et al. Tobacco components stimulate Akt-dependent proliferation and NFkappaB-dependent survival in lung cancer cells. Carcinogenesis. 2005;26:1182–95. doi: 10.1093/carcin/bgi072. [DOI] [PubMed] [Google Scholar]
- 22.Jin Z, Gao F, Flagg T, Deng X. Nicotine induces multi-site phosphorylation of Bad in association with suppression of apoptosis. J Biol Chem. 2004;279:23837–44. doi: 10.1074/jbc.M402566200. [DOI] [PubMed] [Google Scholar]
- 23.Xin M, Deng X. Nicotine inactivation of the proapoptotic function of Bax through phosphorylation. J Biol Chem. 2005;280:10781–9. doi: 10.1074/jbc.M500084200. [DOI] [PubMed] [Google Scholar]
- 24.Jin Z, Gao F, Flagg T, Deng X. Tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone promotes functional cooperation of Bcl2 and c-Myc through phosphorylation in regulating cell survival and proliferation. J Biol Chem. 2004;279:40209–19. doi: 10.1074/jbc.M404056200. [DOI] [PubMed] [Google Scholar]
- 25.Jin Q, Menter DG, Mao L, Hong WK, Lee HY. Survivin expression in normal human bronchial epithelial cells: an early and critical step in tumorigenesis induced by tobacco exposure. Carcinogenesis. 2008;29:1614–22. doi: 10.1093/carcin/bgm234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heeschen C, Weis M, Aicher A, Dimmeler S, Cooke JP. A novel angiogenic pathway mediated by non-neuronal nicotinic acetylcholine receptors. J Clin Invest. 2002;110:527–36. doi: 10.1172/JCI14676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heeschen C, Jang JJ, Weis M, et al. Nicotine stimulates angiogenesis and promotes tumor growth and atherosclerosis. Nat Med. 2001;7:833–9. doi: 10.1038/89961. [DOI] [PubMed] [Google Scholar]
- 28.Guertin DA, Sabatini DM. An expanding role for mTOR in cancer. Trends Mol Med. 2005;11:353–61. doi: 10.1016/j.molmed.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 29.Jacinto E, Loewith R, Schmidt A, et al. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol. 2004;6:1122–8. doi: 10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- 30.Sarbassov DD, Ali SM, Kim DH, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 31.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 32.Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–57. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 33.Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol Cell. 2002;10:151–62. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- 34.Garami A, Zwartkruis FJ, Nobukuni T, et al. Insulin activation of Rheb, a mediator of mTOR/S6K/4E-BP signaling, is inhibited by TSC1 and 2. Mol Cell. 2003;11:1457–66. doi: 10.1016/s1097-2765(03)00220-x. [DOI] [PubMed] [Google Scholar]
- 35.Sancak Y, Thoreen CC, Peterson TR, et al. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell. 2007;25:903–15. doi: 10.1016/j.molcel.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Vander Haar E, Lee SI, Bandhakavi S, Griffin TJ, Kim DH. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol. 2007;9:316–23. doi: 10.1038/ncb1547. [DOI] [PubMed] [Google Scholar]
- 37.Han S, Khuri FR, Roman J. Fibronectin stimulates non-small cell lung carcinoma cell growth through activation of Akt/mammalian target of rapamycin/S6 kinase and inactivation of LKB1/AMP-activated protein kinase signal pathways. Cancer Res. 2006;66:315–23. doi: 10.1158/0008-5472.CAN-05-2367. [DOI] [PubMed] [Google Scholar]
- 38.Tsao AS, McDonnell T, Lam S, et al. Increased phospho-AKT (Ser(473)) expression in bronchial dysplasia: implications for lung cancer prevention studies. Cancer Epidemiol Biomarkers Prev. 2003;12:660–4. [PubMed] [Google Scholar]
- 39.Balsara BR, Pei J, Mitsuuchi Y, et al. Frequent activation of AKT in non-small cell lung carcinomas and preneoplastic bronchial lesions. Carcinogenesis. 2004;25:2053–9. doi: 10.1093/carcin/bgh226. [DOI] [PubMed] [Google Scholar]
- 40.Wislez M, Spencer ML, Izzo JG, et al. Inhibition of mammalian target of rapamycin reverses alveolar epithelial neoplasia induced by oncogenic K-ras. Cancer Res. 2005;65:3226–35. doi: 10.1158/0008-5472.CAN-04-4420. [DOI] [PubMed] [Google Scholar]
- 41.Han W, Gills JJ, Memmott RM, Lam S, Dennis PA. The chemopreventive agent myoinositol inhibits Akt and extracellular signal-regulated kinase in bronchial lesions from heavy smokers. Cancer Prev Res (Phila Pa) 2009;2:370–6. doi: 10.1158/1940-6207.CAPR-08-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsurutani J, Fukuoka J, Tsurutani H, et al. Evaluation of two phosphorylation sites improves the prognostic significance of Akt activation in non-small-cell lung cancer tumors. J Clin Oncol. 2006;24:306–14. doi: 10.1200/JCO.2005.02.4133. [DOI] [PubMed] [Google Scholar]
- 43.Sarbassov DD, Ali SM, Sengupta S, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–68. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 44.Belinsky SA, Devereux TR, Maronpot RR, Stoner GD, Anderson MW. Relationship between the formation of promutagenic adducts and the activation of the K-ras protooncogene in lung tumors from A/J mice treated with nitrosamines. Cancer Res. 1989;49:5305–11. [PubMed] [Google Scholar]
- 45.Granville CA, Warfel N, Tsurutani J, et al. Identification of a highly effective rapamycin schedule that markedly reduces the size, multiplicity, and phenotypic progression of tobacco carcinogen-induced murine lung tumors. Clin Cancer Res. 2007;13:2281–9. doi: 10.1158/1078-0432.CCR-06-2570. [DOI] [PubMed] [Google Scholar]
- 46.Yan Y, Wang Y, Tan Q, et al. Efficacy of polyphenon E, red ginseng, and rapamycin on benzo(a)pyrene-induced lung tumorigenesis in A/J mice. Neoplasia. 2006;8:52–8. doi: 10.1593/neo.05652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Granville CA, Memmott RM, Balogh A, et al. A central role for Foxp3+ regulatory T cells in K-Ras-driven lung tumorigenesis. PLoS One. 2009;4:e5061. doi: 10.1371/journal.pone.0005061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 49.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 50.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 51.Pal SK, Figlin RA, Reckamp KL. The role of targeting mammalian target of rapamycin in lung cancer. Clin Lung Cancer. 2008;9:340–5. doi: 10.3816/CLC.2008.n.049. [DOI] [PubMed] [Google Scholar]
- 52.O’Reilly KE, Rojo F, She QB, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–8. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang C, Ma WY, Hecht SS, Dong Z. Inositol hexaphosphate inhibits cell transformation and activator protein 1 activation by targeting phosphatidylinositol-3′ kinase. Cancer Res. 1997;57:2873–8. [PubMed] [Google Scholar]
- 54.Piccolo E, Vignati S, Maffucci T, et al. Inositol pentakisphosphate promotes apoptosis through the PI 3-K/Akt pathway. Oncogene. 2004;23:1754–65. doi: 10.1038/sj.onc.1207296. [DOI] [PubMed] [Google Scholar]
- 55.Hecht SS, Kenney PM, Wang M, Upadhyaya P. Dose-response study of myo-inositol as an inhibitor of lung tumorigenesis induced in A/J mice by benzo. Cancer Lett. 2001;167:1–6. doi: 10.1016/s0304-3835(01)00454-2. [DOI] [PubMed] [Google Scholar]
- 56.Kassie F, Matise I, Negia M, et al. Combinations of N-Acetyl-S-(N-2-Phenethylthiocarbamoyl)-L-Cysteine and myo-inositol inhibit tobacco carcinogen-induced lung adenocarcinoma in mice. Cancer Prev Res (Phila Pa) 2008;1:285–97. doi: 10.1158/1940-6207.CAPR-08-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Williams MR, Arthur JS, Balendran A, et al. The role of 3-phosphoinositide-dependent protein kinase 1 in activating AGC kinases defined in embryonic stem cells. Curr Biol. 2000;10:439–48. doi: 10.1016/s0960-9822(00)00441-3. [DOI] [PubMed] [Google Scholar]
- 58.Lam S, McWilliams A, LeRiche J, MacAulay C, Wattenberg L, Szabo E. A phase I study of myo-inositol for lung cancer chemoprevention. Cancer Epidemiol Biomarkers Prev. 2006;15:1526–31. doi: 10.1158/1055-9965.EPI-06-0128. [DOI] [PubMed] [Google Scholar]
- 59.Lam TK, Gallicchio L, Lindsley K, et al. Cruciferous vegetable consumption and lung cancer risk: a systematic review. Cancer Epidemiol Biomarkers Prev. 2009;18:184–95. doi: 10.1158/1055-9965.EPI-08-0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kassie F, Matise I, Negia M, Upadhyaya P, Hecht SS. Dose-dependent inhibition of tobacco smoke carcinogen-induced lung tumorigenesis in A/J mice by indole-3-carbinol. Cancer Prev Res (Phila Pa) 2008;1:568–76. doi: 10.1158/1940-6207.CAPR-08-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kassie F, Anderson LB, Scherber R, et al. Indole-3-carbinol inhibits 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone plus benzo(a)pyrene-induced lung tumorigenesis in A/J mice and modulates carcinogen-induced alterations in protein levels. Cancer Res. 2007;67:6502–11. doi: 10.1158/0008-5472.CAN-06-4438. [DOI] [PubMed] [Google Scholar]
- 62.Dashwood RH. Indole-3-carbinol: anticarcinogen or tumor promoter in brassica vegetables? Chem Biol Interact. 1998;110:1–5. doi: 10.1016/s0009-2797(97)00115-4. [DOI] [PubMed] [Google Scholar]
- 63.Chun KH, Kosmeder JW, 2nd, Sun S, et al. Effects of deguelin on the phosphatidylinositol 3-kinase/Akt pathway and apoptosis in premalignant human bronchial epithelial cells. J Natl Cancer Inst. 2003;95:291–302. doi: 10.1093/jnci/95.4.291. [DOI] [PubMed] [Google Scholar]
- 64.Yan Y, Wang Y, Tan Q, Lubet RA, You M. Efficacy of deguelin and silibinin on benzo(a)pyrene-induced lung tumorigenesis in A/J mice. Neoplasia. 2005;7:1053–7. doi: 10.1593/neo.05532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee HY, Oh SH, Woo JK, et al. Chemopreventive effects of deguelin, a novel Akt inhibitor, on tobacco-induced lung tumorigenesis. J Natl Cancer Inst. 2005;97:1695–9. doi: 10.1093/jnci/dji377. [DOI] [PubMed] [Google Scholar]
- 66.Caboni P, Sherer TB, Zhang N, et al. Rotenone, deguelin, their metabolites, and the rat model of Parkinson’s disease. Chem Res Toxicol. 2004;17:1540–8. doi: 10.1021/tx049867r. [DOI] [PubMed] [Google Scholar]