Abstract
OBJECTIVE
Despite overwhelming biological plausibility, evidence for a protective effect of estrogen on cognitive function in postmenopausal women is inconsistent. This study examines the association between endogenous estrogen levels and subsequent four-year decline in cognitive function test performance in community-dwelling older women.
DESIGN
Longitudinal cohort study
PARTICIPANTS
343 postmenopausal women (median age 70 yrs)
MEASUREMENTS
Between 1984 and 1987, serum for measurement of sex hormones was obtained along with relevant covariates. Cognitive function was assessed in 1988–91 and again in 1992–96 using the Category Fluency test, the Mini-Mental Status Exam (MMSE) and Trail Making Test B (Trails B).
RESULTS
Women in the highest tertile of estrone and bioavailable estradiol had, respectively, 1.75 (95% CI 1.02, 3.07) and 1.79 (95% CI 1.04, 3.10) higher odds of 4 year decline in Category Fluency, a test of frontal lobe function, compared to those in the lowest tertile, independent of age and education. The 20% of women with highest tertile levels of both estrone and bioavailable estradiol had a two-fold higher odds of verbal fluency loss (OR=2.17; 95% CI 1.21, 3.89) Adjustment for testosterone levels or for obesity-related factors associated with high endogenous estrogens (higher BMI, waist girth, and triglycerides, and lower HDL cholesterol) did not alter results. Neither estrogen was associated with change in MMSE or Trails B scores.
CONCLUSIONS
Higher endogenous estrogen levels were associated with greater decline in verbal fluency in postmenopausal women. This association was not explained by elevated androgens or by obesity or obesity-related factors.
Keywords: estrogen, cognitive function, aging, postmenopausal women
INTRODUCTION
Considerable biological and epidemiologic evidence suggest a beneficial effect of exogenous estrogens on cognitive function in postmenopausal women,1,2 however nearly all long term clinical trials failed to show benefit.3–6 Observational studies of endogenous estrogen and cognitive function are inconclusive. Some report harmful associations,7,8 some protective,9,10 and others identified no clinically meaningful association between serum estrogens and cognitive ability.11
Early cross-sectional studies of older community-dwelling women from the Study of Osteoporotic Fractures, found higher levels of estrone were associated with poorer performance on one of three measures of cognitive function,7 whereas total and bioavailable estradiol were not related to current cognitive ability.9 The Rancho Bernardo Study also failed to identify any beneficial cross-sectional association of estrone or total and bioavailable estradiol with cognitive function test performance in postmenopausal women11. In contrast, a longitudinal analysis from the Study of Osteoporotic Fractures showed women with high concentrations of free and bioavailable estradiol were less likely to develop cognitive impairment after 6 years than women with low concentrations,9 consistent with the thesis that high endogenous estrogen levels may help preserve normal cognitive function in older women.
The objectives of the present study were to investigate the prospective association of endogenous estradiol and estrone levels with cognitive decline over four years in community-dwelling postmenopausal women and to test the independence of any observed association from obesity and obesity-related factors which could impact vascular brain health.
METHODS
Study population
Participants were postmenopausal women enrolled in the Rancho Bernardo Study, a population-based study of healthy aging in a predominantly Caucasian Southern California community. Between 1984 and 1987, 82% of surviving community-dwelling participants attended a research clinic visit when serum was obtained for the measurement of sex hormones. Cognitive function testing was first performed in 1988 to 1991 and was repeated using identical protocols during a clinic visit in 1992 to 1996. The average time between the baseline hormone measurements and the first cognitive function assessment was 4.3 years and between the first and second cognitive assessments was 4.0 years. The study protocol was approved by the Institutional Review Board of the University of California, San Diego; all participants gave written informed consent prior to participation.
Eligibility criteria for the present analysis included 1) age 50 or older, postmenopausal and no estrogen or insulin use at the time blood was obtained for sex hormone assay (1984–87), 2) sufficient archived frozen sera for estrogen measurements, 3) no history of stroke at the time of the cognitive function tests and 4) assessment of cognitive function at two follow-up visits. Of the 862 non-estrogen using women who had estrogens measured in 1984–87, 13 were excluded for age less than 50, 8 for pre-menopausal status, 2 because of insulin use, 15 because of stroke history and 11 because they had estrogen levels above the normal physiologic range.
Cognitive function assessment
Three standardized tests, chosen to assess different aspects of cognitive function, were administered by a trained interviewer at both the 1988–91 and the 1992–96 clinic visits. The Mini-Mental State Examination12,13 assesses orientation, registration, attention, calculation, language, and recall and is used as a test of global cognitive function; scores range from 0 to 30. Trail Making Test B (Trails B, from the Halstead-Reitan Neuropsychological Test Battery) tests visuomotor scanning and executive function.14 Participants scan a page continuously to identify numbers and letters in a specified sequence while shifting from number to letter sets. A maximum of 300 seconds is allowed; performance is rated by the time required to finish the test; the greater the score, the poorer the test performance. The Animals Naming Category Fluency test15 assesses cognitive flexibility and executive function. Participants name as many animals as possible in 60 seconds (the score is the number of non-repetitive animals produced). Pre-specified scores of ≤24 for the MMSE, ≥132 for Trails B, and ≤12 for Category Fluency are used to indicate categorically defined poor performance.
Sex hormone measurement
In 1984–87, blood samples were obtained by venipuncture between 0730 h and 1100 h after a requested 12-h fast; serum and plasma were separated and frozen at −70° C. Steroid hormone levels were measured on first-thawed serum samples between 1992 and 1994 in the UCSD reproductive endocrinology research laboratory. Estrone, estradiol and testosterone were measured by RIA after organic solvent extraction and celite column chromatography. Bioavailable (non-SHBG-bound) estradiol and testosterone were measured by an adaptation of the ammonium-sulfate precipitation method.16 The sensitivity and intra- and interassay coefficients of variation, respectively, were 10 pmol/L, 6% and 7% for estradiol; 10 pmol/L pmol/L × percentage free, 6% and 8% for bioavailable estradiol; 11 pmol/L, 6% and 8% for estrone; 0.07 nmol/L, 4% and 5% for testosterone; 0.07 nmol/L × percentage free, 7%, and 11% for bioavailable testosterone. Ten percent (n=35) of women had estradiol levels, and 3% (n=11) had estrone levels, below the assay sensitivity; values equivalent to 3.67 pmol/L (1 pg/ml) below theassay sensitivity were assigned to these individuals. Hormone levels did not varyby years of frozen sample storage or by season of sampling.
Covariates
During the hormone measurement visit (1984–87), standardized questionnaires were used to obtain medical history, date of last menstrual period, type of menopause, medication use, physical activity (exercise 3+ times per week, yes/no), and alcohol consumption (1+ drinks/day versus less or none). Current estrogen therapy was validated by examination of pills and prescriptions brought to the clinic for that purpose. Education was defined as years of schooling completed. Depressed mood was assessed using the Beck Depression Inventory (BDI)17. Height, weight, and waist and hip girth were measured in the clinic with participants wearing light clothing and no shoes. Body mass index (BMI) (kg/m2) and waist to hip ratio were used as estimates of overall and central adiposity, respectively.
Fasting plasma total, high-density lipoprotein (HDL), and low-density lipoprotein (LDL) cholesterol and triglyceride levels were measured in a Center for Disease Control and Prevention Certified Lipid Research Clinic Laboratory. Total cholesterol and triglyceride levels were measured by enzymatic techniques using an ABA-200 biochromatic analyzer (Abbott Laboratories, Irving, TX). HDL was measured after precipitation of the other lipoproteins with heparin and manganese chloride. LDL was estimated using the Friedewald formula18. Plasma glucose levels were measured by the glucose oxidase method. Blood pressures were measured twice in seated resting subjects using the Hypertension Detection and Follow–Up Program protocol19; the mean of two readings was used in analyses. The metabolic syndrome was defined according to 2002 National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) criteria20.
Statistical analysis
Sex hormone levels were not normally distributed and were log-transformed for analyses; reported values are medians and 95% ranges. General linear models were performed to compare those with complete data to those without complete data. To assess the association between baseline hormone level and change in cognition, the difference in each test score (1992–1996 value minus 1988–1991 value) was divided by the number of years between assessments and used as the outcome measure. For Trails B, the difference was multiplied by −1 so that negative changes would indicate worsening scores for all tests. Annualized change in each cognitive test score was modeled as a function of hormone level in regression analyses adjusting for age, education and the mean of the baseline and follow-up test score (to correct for regression to the mean21). Linear and quadratic associations were tested. Categorical decline in annualized change scores was defined as the tertile of greatest decline versus less or no decline. Logistic regressions were used to test the association of estrogen tertiles with categorical decline in test performance adjusting for age, education and the mean of the baseline and follow-up test score; tertile cutpoints (in pmol/L) were: estrone (T1:≤48; T2:48–74; T3:>74); estradiol (T1:≤11; T2:11–22; T3:≥22); and bioavailable estradiol (T1:≤6.6; T2:6.6–14.7; T3:≥14.7).
Secondary models tested confounding or effect modification by selected CVD risk factors, prior estrogen use and type of menopause. There was no significant multicollinearity (variance inflation factor >2) between the independent variables. Analysis of variance was used to test differences among estrogen groups defined on the basis of tertiles of estrone and bioavailable estradiol; the significance of differences among individual estrogen groups was assessed by post-hoc testing using the Fisher’s Least Significant Difference (LSD) test. Following the recommendation of Rothman,22 no adjustment was made for multiple comparisons; rather, exact P-values for two-sided tests are shown; p ≤0.05 was considered statistically significant. Data were analyzed using SPSS v15.0 (SPSS Inc, Chicago, IL).
RESULTS
At the 1984–87 clinic visit, the average age of the 343 non-estrogen using women with hormone measurements and serial cognitive function testing was 69.0 years (SD, 8.8). Means and the prevalence of relevant characteristics are summarized in Table 1. More than a quarter of these women were college graduates, most reported exercising regularly, and only 12% were current smokers. The mean score for each cognitive function test at the first assessment (1988–91) was above traditional cut points for poor cognitive performance, reflecting the relatively high functioning community-dwelling cohort (Table 2). Using categorical definitions of poor performance, baseline (1988–91) test scores were in the poor performance range for 14% (n=48) of women for Category Fluency, 4% (n=13) for the MMSE, and 39% (n=132) for Trails B. Over the following 4 years, MMSE scores did not decline on average, whereas mean change scores were negative for Trails B and Category Fluency indicating worsened performance. At the 1992–96 follow-up, 22% (n=75) of women were poor performers for Category Fluency, 6% (n=19) for MMSE, and 48% (n=163) for Trails B.
Table 1.
Baseline (1984–87) Characteristics, Selected CVD Risk Factors, and Hormone Levels for 343 Postmenopausal, Non-estrogen Using Women.
| Characteristic | Mean (SD) |
|---|---|
| Age (yrs) | 69.0 (8.8) |
| BMI (kg/m2) | 24.2 (3.5) |
| Waist girth (cm) | 78.4 (9.1) |
| Waist to hip ratio | 0.79 (0.06) |
| Beck Depression Score | 6.0 (4.1) |
| Percent | |
| College Graduate | 26.4 |
| 3+ Alcohol drinks/wk | 47.1 |
| Ever smoker | 50.6 |
| Current smoker | 12.1 |
| Exercise, 3+ times/wk | 81.8 |
| Prior estrogen use | 59.8 |
| Surgical menopause | 16.6 |
| CVD risk factors | Median (95% interval) |
| Systolic blood pressure (mm Hg) | 136 (103, 179) |
| Diastolic blood pressure (mm Hg) | 75 (59, 94) |
| Total cholesterol (mg/dl) | 6.05 (4.24, 8.12) |
| LDL cholesterol (mg/dl) | 3.70 (2.02, 5.77) |
| HDL cholesterol (mg/dl) | 1.71 (1.03, 2.74) |
| Triglycerides (mg/dl) | 1.06 (0.41, 3.18) |
| Fasting plasma glucose (mg/dl) | 5.38 (4.22, 8.88) |
| Steroid hormones | Median (95% interval) |
| Estrone (pmol/L) | 62.9 (7.4, 151.7) |
| Estradiol (pmol/L) | 18.3 (7.3, 58.7) |
| Bioavailable estradiol (pmol/L) | 11.0 (2.6, 35.6) |
| Testosterone (nmol/L) | 0.14 (0.03, 0.41) |
| Bioavailable testosterone (nmol/L) | 0.04 (.008, 0.14) |
Table 2.
Cognitive Function Test (CFT) Score Characteristics and Multivariate Linear Associations (standardized β-coefficients) of Estrogens with Annualized Change in CFT Scores Over 4 Years.
| Cognitive Function Test | Mean Baseline Score (SD) | Mean Annual Change (SD) | Linear association with annualized change in CFT score |
|||||
|---|---|---|---|---|---|---|---|---|
| Estrone | Estradiol | Bioavailable Estradiol | ||||||
| β-coeff. | p-value | β-coeff. | p-value | β-coeff. | p-value | |||
| MMSE | 27.5 (1.6) | .09 (.48) | 0.03 | .55 | 0.03 | .61 | 0.05 | .37 |
| Trails B | 130.2 (59.2) | −4.6 (14.2) | 0.05 | .39 | −0.02 | .78 | 0.01 | .96 |
| Category fluency | 17.5 (4.6) | −.30 (1.03) | −0.12 | .04 | −0.04 | .50 | −0.10 | .09 |
Models are adjusted for age, education and mean CFT score.
Estrogen levels were log-transformed for analyses.
The association of endogenous estrogens and change in cognitive function test scores was first examined using continuous values for both variables. In linear regression analyses adjusting for age, education and the mean of baseline and 4 year follow-up scores (to account for regression to the mean), higher baseline estrone levels predicted worsened Category Fluency scores after 4 years (p=.04); higher bioavailable estradiol (BioE2) showed a similar trend (p=.09) (Table 2). Total estradiol levels were not significantly related to change in Category Fluency as a continuous variable. No measured estrogen was related to change in MMSE or Trails B scores. Addition of a quadratic estrogen term to each model revealed no evidence of nonlinear estrogen associations with change in CFT performance over time; exclusion of the mean of the baseline and 4 year scores from regression models did not materially change results (data not shown).
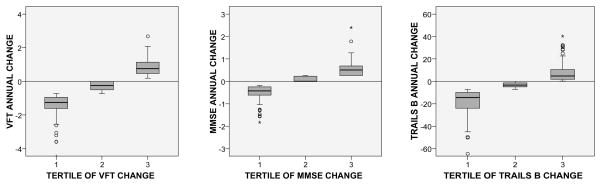
Next, threshold associations were tested using categorical variables. As shown in Figure 1, women in the lowest tertile of change (greatest reduction in score) showed a decline in test performance on average for each cognitive function test, whereas average change scores were close to zero for women in the second tertiles and positive for women in the third tertiles. Therefore, categorical decline in annualized score was defined as the tertile of greatest decline compared with less or no decline. In logistic regressions adjusted for age and education, there was no significant association of tertile of any measured estrogen with 4 year categorical decline in MMSE or Trails B scores (data not shown). However, women in the highest tertiles of estrone and BioE2 had 1.75 (95% CI 1.02, 3.07; p=.041) and 1.79 (95% CI 1.04, 3.10; p=.037) higher odds of decline in Category Fluency scores, respectively, compared to those in the lowest tertiles (Table 3). Baseline CFT scores did not differ by estrogen tertile and additional adjustment for the mean of baseline and 4 year scores did not alter risk estimates (data not shown), thus regression to the mean is unlikely to account for these results.
Figure 1.
Box plots of tertile of annual change in Category Fluency (VFT), MMSE and Trails B test scores. For Trails B, the annual change was multiplied by −1 so that negative changes would indicate worsening scores for all tests.
Table 3.
Odds Ratios for Decline in Category Fluency Test Score by Estrogen Tertile
| Odds ratio (95% CI)* |
||||
|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | p-trend | |
| Estrone | 1.0 (ref) | 1.17 (0.59, 1.92) | 1.75 (1.02, 3.07) | .015 |
| Estradiol | 1.0 (ref) | 1.13 (0.64, 1.99) | 1.14 (0.66, 1.97) | .42 |
| Bioavailable estradiol | 1.0 (ref) | 1.37 (0.75, 2.49) | 1.79 (1.04, 3.10) | .008 |
Adjusted for age and education
Although estrone and BioE2 levels were significantly correlated with each other (r=0.39, p<.001), only 59% of women in the highest tertile of estrone were also in the highest tertile of BioE2 and vice versa (p for chi-square <.001). The independence of estrone and BioE2 associations with decline in Category Fluency was investigated by categorizing participants into 4 groups based on “lower” (lowest two tertiles) and “high” (highest tertile) levels of each estrogen. Fifty-two percent (n=179) of the 343 women had lower levels of estrone and BioE2, 15% (n=50) had high estrone alone, 14% (n=47) high BioE2 alone, and 20% (n=67) had high levels of both estrone and BioE2. Women with high levels of both estrone and BioE2 had significantly higher odds of declining Category Fluency (OR=2.17; 95% CI 1.21, 3.89, p = .009) compared to those with lower levels of both estrogens (Table 4).
Table 4.
Odds Ratios for Decline in Category Fluency Test Score by Estrogen Category
| Normal E1 and BioE2 | Higher E1 only | Higher BioE2 only | Higher E1 and BioE2 | ||||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Model 1 | 1.0 (ref) | 1.26 (0.64, 2.48) | .50 | 1.20 (0.58, 2.47) | .62 | 2.17 (1.21, 3.89) | .009 |
| Model 2 | 1.0 (ref) | 1.39 (0.70, 2.77) | .35 | 1.20 (0.58, 2.50) | .62 | 2.18 (1.15, 4.12) | .016 |
| Model 3 | 1.0 (ref) | 1.27 (0.64, 2.50) | .49 | 1.19 (0.58, 2.45) | .64 | 2.07 (1.14, 3.76) | .017 |
| Model 4 | 1.0 (ref) | 1.08 (0.52, 2.22) | .84 | 0.88 (0.39, 1.98) | .75 | 2.57 (1.30, 5.09) | .007 |
E1=estrone, BioE2=bioavailable estradiol
Model 1 adjusted for age and education
Model 2 adjusted for age, education, BMI, waist to hip ratio, HDL cholesterol, and triglycerides
Model 3 adjusted for age, education and metabolic syndrome (yes/no)
Model 4 adjusted for age and education and excluding 57 women with the metabolic syndrome
Insight into potential mechanisms for the unfavorable estrogen effect was sought by comparing population characteristics among the estrogen groups (Table 5). Women with high levels of both estrone and BioE2 had significantly higher BMI, waist girth, and triglycerides and lower HDL cholesterol than women with lower estrogen levels or with elevated levels of only one estrogen; almost a third of these women met ATPIII criteria for the metabolic syndrome compared to 10–13% of women in the other estrogen groups (p <.001). Adjusting for the factors that distinguished women with high levels of both estrogens, including the metabolic syndrome (yes/no), did not alter the strength of the high estrogen association with declining Category Fluency (Table 4). Excluding the 57 women who met criteria for the metabolic syndrome increased the odds for declining Category Fluency in women with high levels of both estrogens to 2.57 (95% CI 1.30, 5.09; p = .007) (Table 4).
Table 5.
Baseline Characteristics, Selected CVD Risk Factors, and Hormone Levels by Estrogen Category.
| N (%) | Normal E1 & BioE2 179 (52) | High E1 only 50 (15) | High BioE2 only 47 (14) | High E1 & BioE2 67 (20) | |
|---|---|---|---|---|---|
| Characteristic | Mean | Mean | Mean | Mean | p-value |
| Age (yrs) | 69.3 | 71.8 | 65.2a,b | 68.9 | .002 |
| BMI (kg/m2) | 23.4 | 24.0 | 24.5 | 26.6a,b,c | <.001 |
| Waist girth (cm) | 76.1 | 78.0 | 79.3 | 83.8a,b,c | <.001 |
| Waist to hip ratio | .786 | .783 | .801 | .813a,b | .013 |
| Beck Depression Score | 5.8 | 6.4 | 5.3 | 6.5 | .37 |
| Percent | Percent | Percent | Percent | ||
| College Graduate | 29.2 | 28.0 | 19.1 | 20.9 | .38 |
| 3+ Alcohol drinks/wk | 46.9 | 44.0 | 59.6 | 43.3 | .32 |
| Ever Smoker | 52.0 | 46.0 | 59.6 | 44.8 | .40 |
| Current Smoker | 11.2 | 16.0 | 19.1 | 7.5 | .23 |
| Exercise, 3+ times/wk | 83.2 | 76.0 | 80.9 | 82.1 | .71 |
| Prior Estrogen Use | 60.9 | 62.0 | 55.3 | 58.2 | .88 |
| Metabolic syndrome | 13.5 | 8.0 | 12.8 | 32.8a,b,c | .001 |
| CVD risk factors | Mean | Mean | Mean | Mean | |
| Systolic blood pressure (mmHg) | 136.1 | 140.0 | 132.6 | 140.0 | .18 |
| Diastolic blood pressure (mmHg) | 75.1 | 75.3 | 75.6 | 77.0 | .53 |
| Total cholesterol (mg/dl) | 230.9 | 226.6 | 248.0a,b | 237.3 | .03 |
| LDL cholesterol (mg/dl) | 142.4 | 136.9 | 157.5b | 149.2 | .03 |
| HDL cholesterol (mg/dl) | 68.2 | 71.0 | 68.0 | 61.7a,b | .011 |
| Triglycerides (mg/dl) | 89.1 | 85.1 | 97.7 | 120.2a,b | <.001 |
| Fasting plasma glucose (mg/dl) | 100.1 | 96.8 | 99.2 | 117.0 | .11 |
| Steroid hormones | Mean | Mean | Mean | Mean | |
| Estrone (pmol/Ll) | 41.4 | 91.8 | 49.2 | 108.0a,c | <.001 |
| Estradiol (pmol/L) | 13.9 | 20.2 | 25.3 | 36.0a,b,c | <.001 |
| Bioavailable estradiol (pmol/L) | 6.6 | 7.7 | 17.3 | 22.0a,b,c | <.001 |
E1=estrone, BioE2=bioavailable estradiol
Values for estrogens are geometric means
p<.01 vs normal,
p<.01 vs high E1 only,
p<.01 vs high BioE2 only by LSD post-hoc tests
In secondary analyses, there was no evidence of effect modification by age, prior estrogen use, or central adiposity (waist to hip ratio) or for confounding by depressed mood (BDI) or surgical menopause (data not shown). Separate analyses adjusting for lifestyle (smoking, exercise, alcohol intake) did not influence results (data not shown). Total and bioavailable testosterone levels were not related to category fluency tests scores as continuous or categorical variables and adjustment for either total or bioavailable testosterone did not materially change estrogen associations (data not shown).
Although observations with missing data were excluded from the analysis, we compared those with complete information (n=343) to those without complete information (n=519) to assess the potential impact on study findings. Compared to those without cognitive function assessments at both follow-up visits, participants included in this study were younger (means 69.0 vs 73.5 yrs, p<.001), had lower Beck Depression Inventory scores (means 6.0 vs 7.2, p<.001) and were more likely to report exercising 3 or more times per week (82% vs 74%, p=.012). They did not differ in terms of weight, BMI, waist girth, waist to hip ratio, education, or alcohol and tobacco use. Age and education-adjusted cognitive function scores at the first assessment were higher in the women who returned than those who did not (means, Category Fluency: 17.2 vs 16.4, p=.035; MMSE: 27.4 vs 26.8, p=.002; Trails B: 134.5 vs 159.2, p<.001), whereas age-adjusted total and BioE2 levels were lower (means, estradiol: 18.4 vs 21.3 pmol/L, p=.002; BioE2: 9.5 vs 11.0 pmol/L, p=.009).
DISCUSSION
In this cohort of community-dwelling postmenopausal women, higher levels of endogenous estrone and bioavailable estradiol predicted a greater four year decline in performance on a category fluency test of cognitive flexibility and executive function. Women with high levels of both estrogens were twice as likely to experience verbal fluency loss as women with lower levels. Estrogen levels were not related to changing performance on another test involving executive function and visuomotor scanning (Trails B) or on a measure of global cognitive status (MMSE). Because the MMSE is designed to screen for possible dementia, and scores showed little decline in these relatively high functioning women, the absent association of endogenous estrogens with MMSE is not surprising. However, Trails B performance worsened to a significant degree in about a third of women and this decrease in function was not associated with estrone or estradiol. These inconsistent results may mean that the negative effect of high estrogens is specific to one component of cognitive function, or that the association with verbal fluency is spurious.
The performance of category fluency or category naming tests depends on sustained attention, verbal intelligence, and the efficiency of semantic processing23 and is a measure of executive function subserved predominantly by the frontal lobes.24 Although the hippocampus has long been presumed to be the primary site of action of estrogens on cognition,25 functional neuroimaging studies support estrogen effects on frontal lobe functioning.26,27 Keenan and colleagues25 propose that estrogen may temper age-related cognitive decline by helping to maintain frontal lobe-mediated processes involved in executive function. Our results suggest the opposite, higher levels of endogenous estrogen were associated with poorer retention of executive function as assessed by the Category Fluency test. Trails B is also a test of executive function that involves visuomotor scanning and activation of the prefrontal cortex,28,29 however the neural substrates for the multiple functions measured with Trails B involve systems distributed throughout the brain,30 which may account for the absent association of endogenous estrogens with declining Trails B performance in the present study.
A surprising number of the few large, population-based cross-sectional studies of endogenous estrogens and cognition in postmenopausal women suggest harm, but, as in the present study, not on all tests. An earlier analysis of the Rancho Bernardo cohort 11 failed to identify any association of total estradiol, bioavailable estradiol or estrone with current performance on twelve cognitive function tests, with a single exception: women with estradiol levels high enough to be measured had poorer performance on a delayed recall test than those with very low levels. (This test was not repeated at the second visit so change could not be evaluated.) The Rotterdam Scan Study also showed an inverse association of total estradiol levels with current memory performance based on a delayed recall test, the only cognitive function test reported.8 In another cross-sectional analysis, women with higher estrone levels had worse scores on the Digit Symbol test, but no difference in modified MMSE or Trails B scores.7 To our knowledge, only one large study has reported a protective cross-sectional association of serum estrogens and cognitive performance. In that study of 402 women recruited from a Dutch cohort,10 those in the highest quintile of either estradiol or estrone were 50% less likely to have mild cognitive impairment (MMSE<27) than women in the lowest quintile; the MMSE was the only test administered.
We are aware of only one other large cohort of older women in which the association of endogenous estrogens with change in cognitive function test scores over time was reported. Among more than 400 women in the Study of Osteoporotic Fractures, those with high concentrations of free and bioavailable estradiol were less likely to develop cognitive impairment (defined as a decrease of 3+ points on the modified MMSE) after 6 years than women with low concentrations.9 However high estrone was associated with greater decline in Trails B scores and estrogens were not related to change in Digit Symbol scores.7
There is no obvious reason for these divergent findings of null, harmful, and protective associations involving varying measures of estrogen and varying cognitive domains. Differences in postmenopausal estrogen use might play a role: one of these population-based reports selected women who had never used postmenopausal estrogen;10 three excluded women using estrogen at the time of the blood draw, but not prior users;8,11 and two included current estrogen users.7,9 However, the present study, and others,7–9,31 adjusted for estrogen use with no effect on results. Differences in populations, estrogen assays or cognitive assessment tools are also possible explanations, but none seem universal or satisfactory.
Our results are generally compatible with data from clinical trials showing a null or adverse effect of estrogen therapy on cognitive function and future dementia in postmenopausal women. In the four largest and longest trials (ULTRA, HERS, WHIMS, and WHISCA) women assigned to low dose estradiol or to estrogen alone or with medroxyprogesterone acetate (MPA) showed no difference in tests for most cognitive domains after 2 to 5.4 years of treatment compared to those assigned to placebo.3–5,32 Although visual memory improved, verbal memory declined in the estrogen plus progestin group in WHISCA,32 and estrogen/progestin treated women in the WHIMS trial were more likely to develop categorically defined mild cognitive impairment or dementia.5 Category fluency was not effected in WHISCA or ULTRA, was worse in women assigned to hormones in HERS and was not tested in WHIMS.
Estrone is abundant throughout the brain in higher concentrations than estradiol33, and has both ER-mediated and ER-independent actions.1 The non-SHBG bound (bioavailable) fraction of estradiol is believed to be more biologically active than protein-bound estradiol, and to cross the blood brain barrier more readily.34 Thus, dual or complementary actions of estrone and bioavailable estradiol in the frontal lobe and other brain areas may account for the strong association with verbal fluency loss observed in the present study for women with high levels of both estrogens.
In the past decade, interest has focused on the possibility of a causal link between increasing vascular disease and decreasing cognitive function in women after menopause. Adipose tissue is the main source of estrogens in postmenopausal women. The association of higher endogenous estrogens with memory loss observed in this and other studies could be secondary to the tendency for women with higher estrogens to be more obese and to have higher levels of obesity-related cardiovascular risk factors. We tested this possibility by adjusting for cardiovascular risk factors that distinguished the women with higher estrogen levels with negligible effects on results. Further, exclusion of women with the metabolic syndrome, who were three times more likely to be in the high estrogen group, actually strengthened the observed estrogen association. Nonetheless, it is still possible that the association reflects residual confounding, due to obesity-related vascular factors we did not consider, rather than direct harmful effects of estrogen on brain areas controlling cognition.
The association of estrogens with declining verbal fluency could also be due to androgen affects, since estrogens in postmenopausal women are derived from the peripheral conversion of androgen precursors, and serum testosterone has been linked to cognitive ability in women. Premenopausal women with polycystic ovarian syndrome and elevated free testosterone had poorer performance on tests of verbal fluency,35 which was improved by pharmacologically lowering free testosterone levels.36 In addition, a large population-based study of 1276 middle-aged and elderly women reported higher levels of free testosterone in women with lower verbal fluency.37 In the present study, total and bioavailable testosterone levels were not related to decline in verbal fluency and adjustmentfor either had minimal effect on estrogen associations.
This study has several strengths and limitations. One strength is the sensitivity of the estradiol assay compared to the low sensitivity of the estrogen assays used in most other early studies, in which 25% or more of postmenopausal women had undetectable levels. We used the gold standard methods available at the time of these estrogen assays and were able to detect estradiol levels in 90% of participants and estrone in 97%. Our results are based on single estimates of estrogen levels obtained approximately 4 years prior to the first cognitive assessment. Multiple and/or concurrent measures might characterize endogenous estrogen levels better. Although others have shown that a single measurement of estrone reliably characterizes postmenopausal women over a 2 to 3-year period, estradiol levels are less reproducible.38,39
The main limitation of this study is survival and participation bias, a characteristic of cohort studies of the elderly. This study is limited to participants who had the same cognitive function tests administered at two visits, 4 years apart. Women who did not return were older and less healthy (32% had died) than women who did, and had lower baseline cognitive function scores, which might have obscured a stronger harmful effect but would have been unlikely to conceal a protective association, or cause the adverse association. Age and education-adjusted levels of total and bioavailable estradiol were higher in those who did not return compared to those who did, thus selective attrition of women with low estrogens is unlikely to explain the observed associations.
In summary, higher levels of endogenous estrogens were associated with declining verbal fluency over four years in relatively high performing, community-dwelling postmenopausal women. This association was not explained by obesity (the primary determinant of endogenous estrogens after the menopause) or obesity-related cardiovascular risk factors, and was not related to depressed mood or lifestyle. These results do not support the hypothesis that estrogen preserves brain function in older women, and add to the evidence of possible harm.
Acknowledgments
The Rancho Bernardo Study was funded by research grants AG07181 and AG028507 from the National Institute on Aging and grant DK31801 from the National Institute of Diabetes and Digestive and Kidney Diseases.
References
- 1.Spencer JL, Waters EM, Romeo RD, Wood GE, Milner TA, McEwen BS. Uncovering the mechanisms of estrogen effects on hippocampal function. Front Neuroendocrinol. 2008;29:219–237. doi: 10.1016/j.yfrne.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr Rev. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- 3.Yaffe K, Vittinghoff E, Ensrud KE, Johnson KC, Diem S, Hanes V, Grady D. Effects of ultra-low-dose transdermal estradiol on cognition and health-related quality of life. Arch Neurol. 2006;63:945–950. doi: 10.1001/archneur.63.7.945. [DOI] [PubMed] [Google Scholar]
- 4.Grady D, Yaffe K, Kristof M, Lin F, Richards C, Barrett-Connor E. Effect of postmenopausal hormone therapy on cognitive function: the Heart and Estrogen/progestin Replacement Study. Am J Med. 2002;113:543–548. doi: 10.1016/s0002-9343(02)01270-6. [DOI] [PubMed] [Google Scholar]
- 5.Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, Hsia J, Margolis KL, Hogan PE, Wallace R, Dailey M, Freeman R, Hays J. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. Jama. 2004;291:2959–2968. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- 6.Resnick SM, Coker LH, Maki PM, Rapp SR, Espeland MA, Shumaker SA. The Women’s Health Initiative Study of Cognitive Aging (WHISCA): a randomized clinical trial of the effects of hormone therapy on age-associated cognitive decline. Clin Trials. 2004;1:440–450. doi: 10.1191/1740774504cn040oa. [DOI] [PubMed] [Google Scholar]
- 7.Yaffe K, Grady D, Pressman A, Cummings S. Serum estrogen levels, cognitive performance, and risk of cognitive decline in older community women. J Am Geriatr Soc. 1998;46:816–821. doi: 10.1111/j.1532-5415.1998.tb02713.x. [DOI] [PubMed] [Google Scholar]
- 8.den Heijer T, Geerlings MI, Hofman A, de Jong FH, Launer LJ, Pols HA, Breteler MM. Higher estrogen levels are not associated with larger hippocampi and better memory performance. Arch Neurol. 2003;60:213–220. doi: 10.1001/archneur.60.2.213. [DOI] [PubMed] [Google Scholar]
- 9.Yaffe K, Lui LY, Grady D, Cauley J, Kramer J, Cummings SR. Cognitive decline in women in relation to non-protein-bound oestradiol concentrations. Lancet. 2000;356:708–712. doi: 10.1016/S0140-6736(00)02628-3. [DOI] [PubMed] [Google Scholar]
- 10.Lebrun CE, van der Schouw YT, de Jong FH, Pols HA, Grobbee DE, Lamberts SW. Endogenous oestrogens are related to cognition in healthy elderly women. Clin Endocrinol (Oxf) 2005;63:50–55. doi: 10.1111/j.1365-2265.2005.02297.x. [DOI] [PubMed] [Google Scholar]
- 11.Barrett-Connor E, Goodman-Gruen D. Cognitive function and endogenous sex hormones in older women. J Am Geriatr Soc. 1999;47:1289–1293. doi: 10.1111/j.1532-5415.1999.tb07427.x. [DOI] [PubMed] [Google Scholar]
- 12.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 13.Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc. 1992;40:922–935. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- 14.Reitan R. Validity of the trail-making test as an indicator of organic brain disease. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- 15.Borkowski JG, Benton AL, Spreen O. Word fluency and brain damage. Neuropsychologia. 1967:5. [Google Scholar]
- 16.Tremblay RR, Dube JY. Plasma concentrations of free and non-TeBG bound testosterone in women on oral contraceptives. Contraception. 1974;10:599–605. doi: 10.1016/0010-7824(74)90099-7. [DOI] [PubMed] [Google Scholar]
- 17.Hebert LE, Scherr PA, McCann JJ, Beckett LA, Evans DA. Is the risk of developing Alzheimer’s disease greater for women than for men? Am J Epidemiol. 2001;153:132–136. doi: 10.1093/aje/153.2.132. [DOI] [PubMed] [Google Scholar]
- 18.Friedwald W, Levy R, Frederickson D. Estimation of the concentration of low-density lipoprotein cholesterol in plasma without use of preparative ultracentrifuge. Clin Chem. 1972:459–502. [PubMed] [Google Scholar]
- 19.The hypertension detection and follow-up program: Hypertension detection and follow-up program cooperative group. Prev Med. 1976;5:207–215. doi: 10.1016/0091-7435(76)90039-6. [DOI] [PubMed] [Google Scholar]
- 20.National Cholesterol Education Program (NCEP) Expert Panel on Detection, E., and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 21.Oldham PD. A note on the analysis of repeated measurements of the same subjects. J Chronic Dis. 1962;15:969–977. doi: 10.1016/0021-9681(62)90116-9. [DOI] [PubMed] [Google Scholar]
- 22.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
- 23.Joyce EM, Collinson SL, Crichton P. Verbal fluency in schizophrenia: relationship with executive function, semantic memory and clinical alogia. Psychol Med. 1996;26:39–49. doi: 10.1017/s0033291700033705. [DOI] [PubMed] [Google Scholar]
- 24.Anderson V, Jacobs R, Anderson P. Executive functions and the frontal lobes: a lifespan perspective. Psychology Press; 2008. [Google Scholar]
- 25.Keenan PA, Ezzat WH, Ginsburg K, Moore GJ. Prefrontal cortex as the site of estrogen’s effect on cognition. Psychoneuroendocrinology. 2001;26:577–590. doi: 10.1016/s0306-4530(01)00013-0. [DOI] [PubMed] [Google Scholar]
- 26.Berman KF, Schmidt PJ, Rubinow DR, Danaceau MA, Van Horn JD, Esposito G, Ostrem JL, Weinberger DR. Modulation of cognition-specific cortical activity by gonadal steroids: a positron-emission tomography study in women. Proc Natl Acad Sci U S A. 1997;94:8836–8841. doi: 10.1073/pnas.94.16.8836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaywitz SE, Shaywitz BA, Pugh KR, Fulbright RK, Skudlarski P, Mencl WE, Constable RT, Naftolin F, Palter SF, Marchione KE, Katz L, Shankweiler DP, Fletcher JM, Lacadie C, Keltz M, Gore JC. Effect of estrogen on brain activation patterns in postmenopausal women during working memory tasks. Jama. 1999;281:1197–1202. doi: 10.1001/jama.281.13.1197. [DOI] [PubMed] [Google Scholar]
- 28.Shibuya-Tayoshi S, Sumitani S, Kikuchi K, Tanaka T, Tayoshi S, Ueno S, Ohmori T. Activation of the prefrontal cortex during the Trail-Making Test detected with multichannel near-infrared spectroscopy. Psychiatry Clin Neurosci. 2007;61:616–621. doi: 10.1111/j.1440-1819.2007.01727.x. [DOI] [PubMed] [Google Scholar]
- 29.Zakzanis KK, Mraz R, Graham SJ. An fMRI study of the Trail Making Test. Neuropsychologia. 2005;43:1878–1886. doi: 10.1016/j.neuropsychologia.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 30.Coffey CE, Ratcliff G, Saxton JA, Bryan RN, Fried LP, Lucke JF. Cognitive correlates of human brain aging: a quantitative magnetic resonance imaging investigation. J Neuropsychiatry Clin Neurosci. 2001;13:471–485. doi: 10.1176/jnp.13.4.471. [DOI] [PubMed] [Google Scholar]
- 31.Geerlings MI, Launer LJ, de Jong FH, Ruitenberg A, Stijnen T, van Swieten JC, Hofman A, Witteman JC, Pols HA, Breteler MM. Endogenous estradiol and risk of dementia in women and men: the Rotterdam Study. Ann Neurol. 2003;53:607–615. doi: 10.1002/ana.10521. [DOI] [PubMed] [Google Scholar]
- 32.Resnick SM, Maki PM, Rapp SR, Espeland MA, Brunner R, Coker LH, Granek IA, Hogan P, Ockene JK, Shumaker SA. Effects of combination estrogen plus progestin hormone treatment on cognition and affect. J Clin Endocrinol Metab. 2006;91:1802–1810. doi: 10.1210/jc.2005-2097. [DOI] [PubMed] [Google Scholar]
- 33.Lanthier A, Patwardhan VV. Sex steroids and 5-en-3 beta-hydroxysteroids in specific regions of the human brain and cranial nerves. J Steroid Biochem. 1986;25:445–449. doi: 10.1016/0022-4731(86)90259-1. [DOI] [PubMed] [Google Scholar]
- 34.Pardridge WM, Mietus LJ. Transport of steroid hormones through the rat blood-brain barrier. Primary role of albumin-bound hormone. J Clin Invest. 1979;64:145–154. doi: 10.1172/JCI109433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schattmann L, Sherwin BB. Testosterone levels and cognitive functioning in women with polycystic ovary syndrome and in healthy young women. Horm Behav. 2007;51:587–596. doi: 10.1016/j.yhbeh.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 36.Schattmann L, Sherwin BB. Effects of the pharmacologic manipulation of testosterone on cognitive functioning in women with polycystic ovary syndrome: a randomized, placebo-controlled treatment study. Horm Behav. 2007;51:579–586. doi: 10.1016/j.yhbeh.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 37.Thilers PP, Macdonald SW, Herlitz A. The association between endogenous free testosterone and cognitive performance: a population-based study in 35 to 90 year-old men and women. Psychoneuroendocrinology. 2006;31:565–576. doi: 10.1016/j.psyneuen.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 38.Hankinson SE, Manson JE, Spiegelman D, Willett WC, Longcope C, Speizer FE. Reproducibility of plasma hormone levels in postmenopausal women over a 2–3-year period. Cancer Epidemiol Biomarkers Prev. 1995;4:649–654. [PubMed] [Google Scholar]
- 39.Cauley JA, Gutai JP, Kuller LH, Powell JG. Reliability and interrelations among serum sex hormones in postmenopausal women. Am J Epidemiol. 1991;133:50–57. doi: 10.1093/oxfordjournals.aje.a115801. [DOI] [PubMed] [Google Scholar]