Abstract
Myeloid-derived suppressor cells (MDSC) are present in most cancer patients and are potent inhibitors of T-cell-mediated anti-tumor immunity. Their inhibitory activity is attributed to production of arginase, reactive oxygen species, inducible nitric oxide synthase, and IL-10. We now report that MDSC also block T cell activation by sequestering cystine and limiting the availability of cysteine. Cysteine is an essential amino acid for T cell activation because T cells lack cystathionase, which converts methionine to cysteine, and because they do not have an intact xc− transporter and therefore cannot import cystine and reduce it intracellularly to cysteine. T cells depend on antigen presenting cells (APC) such as macrophages and dendritic cells to export cysteine which is imported by T cells via their ASC neutral amino acid transporter. MDSC express the xc− transporter and import cystine; however, they do not express the ASC transporter and do not export cysteine. MDSC compete with APC for extracellular cystine, and in the presence of MDSC, APC release of cysteine is reduced, thereby limiting the extracellular pool of cysteine. Therefore, MDSC consume cystine and do not return cysteine to their microenvironment, thereby depriving T cells of the cysteine they require for activation and function.
Introduction
Many patients and experimental animals with cancer are immune suppressed because they contain cell populations that inhibit anti-tumor immunity (1). Suppressive populations from both the lymphoid and myeloid compartments have been identified. Myeloid-derived suppressor cells (MDSC) are found in most patients with advanced cancers (2–5), and are potent inhibitors of innate and adaptive immunity. MDSC are a heterogenous population of cells that impair immunity by inhibiting the activation of CD4+ (6) and CD8+ (2, 7, 8) T cells, blocking NK cell cytotoxicity (9), blocking T cell expression of L-selectin (CD62L) which is needed for T cells to home to lymph nodes (10), and polarizing immunity towards a tumor-promoting type 2 phenotype through the down-regulation of IL-12 and production of IL-10 (11). Studies with inhibitors of arginase, inducible nitric oxide synthase (iNOS), and reactive oxygen species (ROS) demonstrated that both arginase and nitric oxide contribute to MDSC inhibition of T cell activation (6, 12–14). However, inhibitors of these molecules do not completely reverse suppression of all MDSC populations, suggesting that MDSC may use additional mechanisms to block T cell activation.
Mammalian cells require the amino acid cysteine for protein synthesis and proliferation. Cysteine can be generated by cells through two pathways. If cells express the plasma membrane cystine transporter xc−, which consists of the xCT and 4F2 light and heavy chains, respectively, they import disulfide-bonded cystine from the oxidizing extracellular environment (15). Within the reducing intracellular environment, imported cystine is reduced to cysteine (16). Alternatively, if cells synthesize cystathionase they can convert intracellular methionine to cysteine (17, 18). However, T cells do not contain cystathionase or the xCT chain of the xc− transporter (19–21), so they are dependent on other cells to produce cysteine which is then imported by T cells through the plasma membrane ASC neutral amino acid transporter. T cells require cysteine during antigen presentation and subsequent T cell activation, and typically obtain it from macrophages and/or dendritic cells (DC) which provide it through one of two mechanisms. These cells import cystine, convert it to cysteine, and then export the cysteine through their plasma membrane ASC transporter (22–24). Additionally, DC and macrophages secrete thioredoxin which converts extracellular cystine to cysteine which is then available for up-take by T cells (25, 26).
The dependence of T cells on exogenously generated cysteine led us to hypothesize that MDSC inhibit T cell activation by limiting extracellular cysteine. We now report that MDSC express the xCT and 4F2 heterodimeric cystine transporter xc-, so they can acquire cystine from their environment. However, MDSC do not express the ASC neutral amino acid transporter, so they do not export cysteine. Furthermore, MDSC do not express cystathionase so their requirement for cysteine must be fulfilled by their uptake and reduction of cystine. As a result, MDSC limit the amount of cysteine in their extracellular environment by consuming cystine and not exporting cysteine, and by sequestering cystine which would normally be imported, reduced, and exported as cysteine by macrophages and DC, or converted in the local environment to cysteine by thioredoxin. Therefore, in the presence of MDSC, DC and macrophages cannot support T cell proliferation so tumor-specific T cells are not activated and anti-tumor immunity is suppressed.
Materials and Methods
Mice and cells
BALB/c, BALB/c DO11.10 transgenic (specific for chicken ovalbumin (OVA) peptide323–339 restricted to I-Ad), and C57BL/6 OT-I transgenic (specific for OVA peptide257–264 restricted to H-2Kb) mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Mating pairs of transgenic BALB/c Clone 4 and TS1 (TCRs specific for influenza hemagglutinin (HA) peptide 518–526 restricted to H-2Kd and 110–119 restricted to I-Ed, respectively) were provided by Dr. E. Fuchs (Johns Hopkins). Mice were bred and maintained in the University of Maryland Baltimore County (UMBC) animal facility according to NIH guidelines. All animal procedures were approved by the UMBC Institutional Animal Care and Use Committee. 4T1 mouse mammary carcinoma cells were maintained as described (6).
RT-PCR
Total RNA was isolated from 5–10×106 cells and treated with RNAase-free DNAase I (Qiagen, Hilden, UK). First-strand cDNA synthesis was performed using an RNAeasy mini kit (Qiagen Sciences, MD) and an iScript cDNA synthesis kit (BioRad, CA). RT-PCR mixture contained: 1–1.5μg of cDNA combined with one pellet of puReTaq Ready-To-GoPCR beads (GE healthcare, Buckinghamshire, UK) containing stabilizers, BSA, dNTPs, 2.5U of puReTaq DNA polymerase, reaction buffer, and 1μl of a 10μM stock of each of the following upstream and downstream primers, respectively: GAPDH (421 bps, 5′ AGTATGATGACATCAAGAAGG 3′; 5′ ATGGTATTCAAGAGAGTAGGG 3′); xCT (213bps; 5′ TCTCCATCATCATCGGCACC 3′, 5′ AAGGACCAAAGACCTCCAGAATG 3′); cystathionase (342 bps, 5′ GCCTAGTTTCCAGCATTTCG 3′, 5′ GATGCCACCCTCCTGAAGTA 3′); 4F2 heavy chain (402 bps, 5′ CGACCTTCAGGCCTTTGTAG 3′, 5′ GCCAAGTACAAGGGTGCATT 3′); ASC transporter (385bps, 5′ AGGGGTAGTAGCGGTGGATT 3′, 5′ GGTGACCAGGAAAAAGAGCA 3′). cDNA was amplified in a PTC-200 Peltier Thermal Cycler (MJ Research) using the following conditions: denature at 94°C for 15 min followed by 35 cycles at 94° C for 1 min, 55° C for 1 min, and 72° C for 1 min, and a final incubation at 72° C. PCR products were analyzed on 1.2–1.4% agarose gels.
Reagents, peptides, antibodies, flow cytometry
OVA323–339 (ISQAVHAAHAEINEAGR), OVA257–264 (SIINFEKL), HA110–119 (SFERFEIFPK), and HA518–526 (IYSTVASSL) peptides were synthesized in the University of Maryland, Baltimore, Biopolymer Core facility. Gr1-FITC, CD11b-PE, F4/80-FITC, CD11c-PerCP, CD11c-PE, CD86-PE, MHC II-FITC, c-kit-PE, FITC-rat IgG2a, PE-rat IgG2a and goat anti-rabbit FITC were from BD Pharmingen (San Jose, CA); rabbit anti-mouse xCT from Abcam (Cambridge, MA); rabbit anti-mouse ASC from Transgenic Inc. (Japan); rabbit anti-mouse CD98-FITC (4F2hc) from MBL International (Woburn, MA); β-mercaptoethanol (β-ME) from Ultrapure Bioreagent (Phillipsburg, NJ). Sca-1-APC was from R&D Systems (Emeryville, CA). Cells were stained with mAbs and analyzed on an Epics XL or Cyan ADP flow cytometer (6) using FCS express or Summit v4.3 software.
Macrophages, MDSC, splenocytes, T cells
Peritoneal macrophages were prepared by injecting mice i.p. with 3% sodium thioglycolate. Peritoneal exudate cells were removed 4–5 days later using a 10 ml syringe with an 18g needle. RBC were lysed with Gey’s solution (11). The remaining macrophages were activated with lipopolysaccharide (LPS; 10ng/ml;) for 16–18 hrs and were > 87 % F4/80+ by flow cytometry. Macrophages were identified by gating on F4/80+ splenocytes. For Gr1+CD11b+ MDSC from tumor-free mice, 14–15 naive BALB/c mice were bled from the retro-orbital sinus and isolated by Miltenyi purification as described (6). Purified naive MDSC were 85–87% Gr1+CD11b+. For Gr1+CD11b+ MDSC from tumor-bearing mice, female BALB/c mice were inoculated in the abdominal mammary gland with 7×103 – 1×104 4T1 cells. Three to four weeks later when primary tumors were 11.57 ± 0.96 mm in diameter and metastatic disease was established, mice were bled from the retro-orbital sinus or by cardiac puncture, and RBCs were lysed. The resulting leukocytes were 85%–98% Gr1+CD11b+ as measured by flow cytometry. Splenocytes from DO11.10, Clone 4, TS1, and OT1 mice were purified using a CD4+ T cell isolation kit (Miltenyi Biotech).
Dendritic cell isolation
Bone marrow dendritic cells (DC) were prepared according to the procedure of (27). Briefly, bone marrow was flushed from the femurs of 2–3 4–6 month old male mice, depleted of RBCs and the remaining cells cultured at 37°C and 5% CO2 in DC medium (RPMI-1640 supplemented with 10% fetal calf serum, 2mM glutamine, 1% non-essential amino acids, 1% AA and 1% gentamycin). Ten to twenty × 106 cells/10ml were cultured in 25cm2 T flasks for 8–9 days with 20ng/ml recombinant mouse GM-CSF and 10ng/ml recombinant IL-4 (Fitzgerald, Concord, MA). Five ml of medium were removed on days 4 and 7 and replaced with 5ml DC medium containing 10ng/ml recombinant GM-CSF and 5ng/ml recombinant IL-4. On day 8, cells were harvested and positively selected with CD11c magnetic beads (Miltenyi Biotech). DC were matured by overnight incubation in DC medium supplemented with 1μg/ml LPS. Non-matured and LPS-matured DC were 80–90% CD11c+ and expressed MHC II and CD86 as analyzed by flow cytometry.
Glutamate uptake
Glutamate uptake was measured using a modified procedure of (28). All reagents were from Sigma unless stated otherwise. Macrophages, purified CD4+ T cells, and DC (all from tumor-free mice), and 4T1-induced blood MDSC were pre-incubated for 20 min at 37°C at 6×105 cells/200μl in 2 ml microfuge tubes or in 96 well flat bottom plates with 200–500μl of Buffer A (140mM –methyl-D-glucamine, 5.4mM KCL, 0.4mM KH2PO4, 10mM HEPES, 5mM D-glucose, 1.8mM CaCl2, 0.8mM MgSO4; pH 7.4). Cells were pelleted and resuspended in 200–300μl of Buffer A containing 170nM L-[3H]-glutamate (52Ci/mmol, GE Healthcare, Piscataway, NJ) with or without 2.5mM unlabeled amino acid competitor (L-cystine) or non-competitor (L-leucine) and incubated for 20 min at 37°C. Glutamate uptake was terminated by washing with excess cold Buffer A 3– 4 times. Washed cells were lysed with 200μl of 0.5% Triton X-100 in 0.1M potassium phosphate buffer, pH 7.0. Fifty μl of cell lysate were mixed with 500μl of Optiphase Supermix (PerkinElmer, Waltham, MA) and counted using a Wallac 1450 Microbeta liquid scintillation counter (PerkinElmer). Percent inhibition of glutamate transport = 100% × [(cpm without cystine − cpm with cystine)/(cpm without cystine)].
Cysteine release
Free thiols were measured according to (25) with the following modifications: Purified CD4+ T cells, DC, and peritoneal macrophages (all from tumor-free mice), and 4T1-induced blood MDSC were incubated for 6 hrs at 37°C in HL-1 or RPMI serum-free medium in 96 well plates (6×105 cells/200μl/well). One hundred μl of each supernatant was combined with 100μl of dilution buffer (30mM Tris HCL, 3mM EDTA pH 8.2) and 25μl of 5,5′-dithio-bis(2-nitrobenzoic acid; Ellman’s reagent; DTNB (Sigma, St. Louis, MO)) solution (29.7mg DTNB in 25ml methanol), incubated at room temperature for 10–15 min, and optical density was measured at 412nm. NAC was used as a standard. For experiments containing a mixture of MDSC and macrophages, 6×105–6×106 blood MDSC were plated in 200μl/well of Buffer A in 96 well flat bottom plates. After a 20 min incubation at 37°C, MDSC were washed twice with serum-free HL-1 medium, and 6×105 BALB/c macrophages were added per well in a total volume of 200μl/well of serum-free HL-1 medium. Co-cultures were incubated for 20 min at 37°C after which thiol release was measured using the DTNB cysteine release assay. Percent inhibition = 100% − {[(macrophages + MDSC) − HL-1 medium]/(macrophages - HL1 medium)}.
T cell activation, MDSC suppression, cystine levels
T cell proliferation was measured as described (6). For experiments including N acetyl-cysteine (NAC), transgenic splenocytes (1×105 cells/200 μl/well) were co-cultured with their cognate peptide (14μM OVA, 28μM HA peptide) in HL-1 medium (BioWhittaker, MD) containing 1% penicillin, 1% streptomycin, and 1% Glutamax (Invitrogen Life Technologies, Carlsbad, CA). T cell suppression assays were done as described (6) using transgenic splenocytes (D011.10, Clone 4, OT-1, TS1; 2×105/well) and their cognate peptides (14μM OVA, 28μM HA, 12μM OVA, 10μM HA peptide, respectively) in the presence of 25Gy-irradiated blood MDSC from 4T1 tumor-bearing mice in a total volume of 200μl serum-free HL-1 medium. β-ME was added to some wells. Percent suppression = 100% × [{(T cells + peptide) − (T cells + peptide + MDSC)}/(T cells + peptide)]. Serum cystine levels were measured using high-performance liquid chromatography as described (29).
Statistical analysis
Data were analyzed using Student’s two tailed t test for unequal variances or the Wilcoxon paired rank test.
Results
Exogenous beta-mercaptoethanol facilitates T cell proliferation
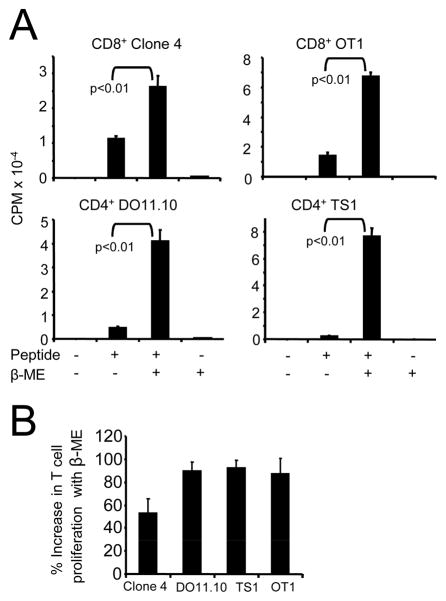
Because T cells cannot generate cysteine from cystine or methionine, they must import this essential amino acid. However, extracellular spaces are oxidizing environments, so cystine, the disulfide-bonded form, rather than the free amino acid, cysteine, is present extracellularly. Therefore, the reducing agent, β-ME, is included in T cell cultures to reduce cystine to cysteine (19–21). To confirm that a reducing environment facilitates activation of the transgenic T cells in the present study, splenocytes from TCR-transgenic CD4+ DO11.10 and TS1, and CD8+ Clone 4 and OT-I mice were co-cultured with cognate peptide in the presence or absence of β-ME. T cell proliferation in the presence of β-ME was greater than proliferation without β-ME for all transgenic populations (Figure 1).
Figure 1. β-mercaptoethanol facilitates antigen-driven T cell activation.
A, Transgenic CD4+ DO11.10 and TS1, and CD8+ Clone 4 and OT-I splenocytes were co-cultured with their respective peptides in the presence or absence of 5×10−5 M β-ME. Data are from one of four (TS1 and Clone 4) and one of five (DO11.10 and OT-I) independent experiments. Results of the pooled experiments for each transgenic population are significant at p <0.01 (Wilcoxon paired-sample test). B, Average percent increase ± SD in T cell proliferation for DO11.10, TS1, Clone 4, and OT-I splenocytes activated in the presence of β-ME shown in panel A.
MDSC express the cystine transporter, but lack cystathionase and the ASC neutral amino acid transporter
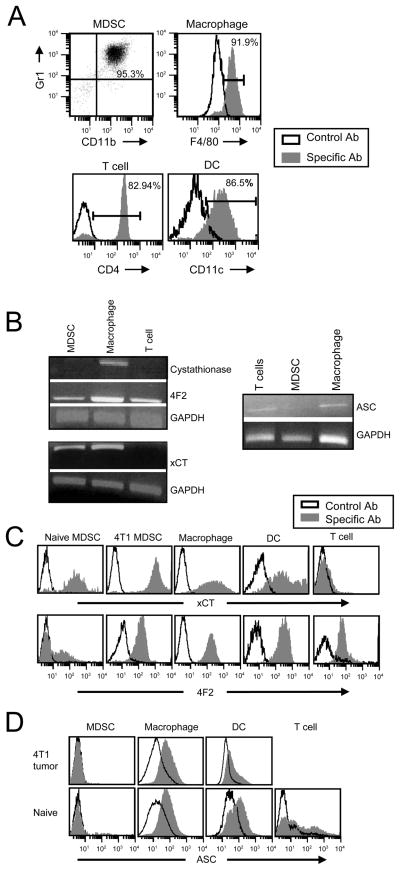
If MDSC limit T cell activation by sequestering cystine, then they may import cystine and not export cysteine. To determine if MDSC acquire, generate, and/or export cystine and/or cysteine, 4T1-induced blood MDSC (95.3% Gr1+CD11b+) (Figure 2A, top left) were assayed by RT-PCR for the cystine importer xc− (xCT and 4F2), the ASC neutral amino acid transporter, and cystathionase. Activated macrophages, which express xCT, 4F2, ASC, and cystathionase (22–24) (Figure 2A, top right), and T cells (Figure 2A, bottom left), which lack cystathionase and xCT, served as positive and negative controls, respectively. MDSC contain mRNA encoding the xc− transporter (xCT and 4F2), but do not contain mRNA encoding cystathionase or the ASC transporter (Figure 2B). Protein expression of xCT, 4F2 and ASC in macrophages, T cells, and MDSC from tumor-bearing mice was confirmed by flow cytometry. Bone marrow DC (Figure 2A, bottom right) were included in this experiment because they also produce cysteine. Consistent with the RT-PCR and published results (30), macrophages and DC contain a complete xc− cystine transporter, while T cells lack xCT. MDSC from tumor-free (naive) and from mice with 4T1 tumors also contain a complete xc− complex (Figure 2C). Macrophages (splenic and peritoneal), DC (splenic and bone marrow), and T cells express cell surface ASC; however, Gr1+CD11b+ cells from tumor-free or tumor-bearing mice do not express the ASC transporter (Figure 2D and supplemental figure 1). C-kit+Sca-1− myeloid progenitor cells express low levels of ASC (supplemental figure 2). Therefore, macrophages, DC, and MDSC can import cystine; however, unlike macrophages and DC, MDSC are unable to export cysteine. Because there are relatively few myeloid progenitor cells and because they do not localize to sites of T cell activation, they are unlikely to impact extracellular cystine levels.
Figure 2. MDSC express the heterodimeric xc− transporter (xCT + 4F2), and do not express cystathionase or the ASC neutral amino acid transporter.
A, Peritoneal macrophages, purified CD4+ splenic T cells, and DC (all from naive BALB/c mice), and 4T1-induced blood MDSC were stained with mAbs to Gr1, CD11b, F4/80, CD4, and/or CD11c and analyzed by flow cytometry. Purity of each population is shown. B, Total RNA was isolated from the MDSC, macrophages, and CD4+ T cells shown in panel A, reverse transcribed, and PCR amplified for the xc− (xCT and 4F2) and ASC transporters, cystathionase, and GAPDH. PCR for cystathionase, 4F2, xCT, and ASC was performed on 2, 3, 4, and 3 independent cell preparations, respectively, for each cell type, C, Peritoneal macropahges, bone marrow DC, purified splenic CD4+ T cells, and blood Gr1+CD11b+ cells (all from naive mice), and 4T1-induced blood MDSC were stained with antibodies to xCT, 4F2, Gr1, CD11b, F4/80, CD11c, and CD4. Gated populations (MDSC: Gr1+CD11b+; macrophages: F4/80+; DC: CD11c+; and T cells: CD4+) were analyzed for expression of xCT or 4F2. Data are from one of three independent cell preparations. D, Splenic macrophages, DC, purified T cells, and blood MDSC from tumor-free (naive) or 4T1-tumor-bearing mice were stained with antibodies to Gr1, CD11b, F4/80, CD11c, CD4, and ASC, and the gated Gr1+CD11b+, F4/80+, CD4+, or CD11c+CD11b+ populations analyzed by flow cytometry.
Extracellular cysteine partially reverses T cell suppression
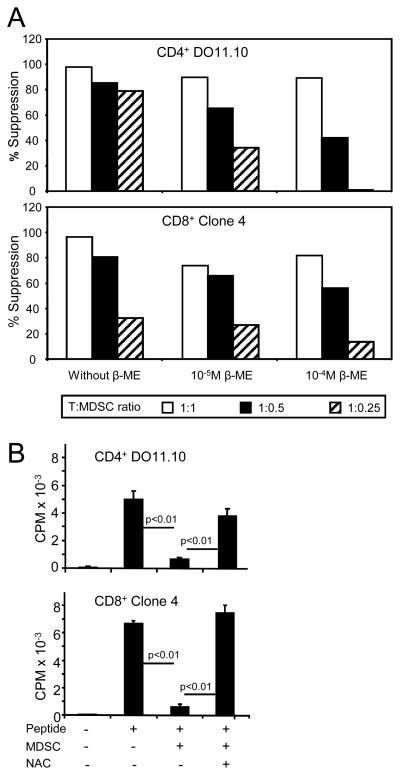
If cysteine deprivation contributes to the immunosuppressive effects of MDSC, then providing exogenous cysteine may reverse suppression. To test this hypothesis CD4+ DO11.10 and CD8+ Clone 4 transgenic splenocytes were co-cultured with their respective peptides and tumor-induced MDSC in the presence or absence of β-ME. T cell activation was measured by 3H-thymidine uptake (Figure 3A). Inclusion of MDSC suppressed T cell activation regardless of the presence of β-ME; however, β-ME partially restored activation. Therefore, β-ME reverses some, but not all, MDSC-mediated suppression, consistent with the concept that cysteine deprivation contributes to MDSC-mediated suppression.
Figure 3. MDSC suppression is reversed by β-ME or NAC).
A, Transgenic splenocytes and their respective peptides were co-cultured at varying ratios with 4T1-induced blood MDSC (90% Gr1+CD11b+ cells) with or without β-ME, and T cell activation measured by 3H-thymidine uptake. Data are plotted as percent suppression relative to T cells plus peptide without MDSC, and are from one of six independent experiments. Values for percent suppression in the presence of β-ME vs. no β-ME for the pooled experiments are significant at p <0.0005 (Wilcoxon paired-sample test). B, Transgenic splenocytes were co-cultured as in A in the presence of 0.5mM NAC and without β-ME. Ratio of splenocytes to MDSC for panel B was 1:0.5. Data are from one of three independent experiments. Pooled experiments are significant at p <0.005 (Wilcoxon paired-sample test).
β-ME is a general reducing agent and may affect T cell proliferation by acting on many molecules. To determine if extracellular cysteine is specifically involved, transgenic splenocytes were co-cultured with their respective peptides in the presence or absence of N–acetyl-cysteine (NAC), which is stable in the extracellular oxidizing environment (31). NAC enters cells via the ASC neutral amino acid transporter and is rapidly hydrolyzed intracellularly to the free amino acid cysteine. Inclusion of increasing amounts of NAC increases T cell proliferation similar to that seen for β-ME (supplemental Figure 3). To determine if NAC overcomes MDSC-mediated suppression, NAC was added to cultures of MDSC with transgenic T cells and their cognate peptides (Figure 3B). NAC restored proliferation of both CD4+ and CD8+ T cells. As shown by Nefedova and colleagues, five days of culture of MDSC with NAC, GM-CSF, and tumor cell-conditioned medium differentiates MDSC into non-suppressive macrophages and/or DC (31), raising the possibility that the results of figure 3B are due to MDSC differentation. However, one day old cultures of MDSC with NAC under the conditions of Figure 3B contained <0.5% macrophages or DC as measured by percent F4/80+Gr1− or CD11c+Gr1− cells (supplemental Table 1), and 97.5% of the cells were dead by day 3, indicating that the MDSC had not differentiated during the course of the experiment. Therefore, excess cysteine partially reverses MDSC suppressive activity, consistent with the hypothesis that MDSC inhibit T cell proliferation by depleting the environment of cysteine.
MDSC import cystine through the xc− transporter and do not export cysteine
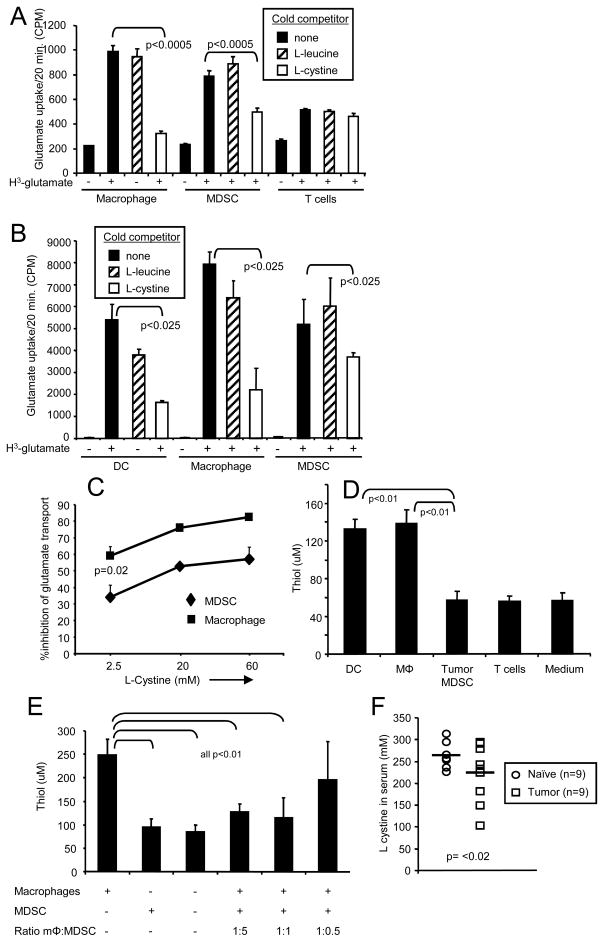
If MDSC suppress T cell activation by competing with macrophages and/or DC for the uptake of cystine, then their rate of cystine uptake should be similar to that of macrophages and DC. In macrophages and DC, the xc− transporter imports cystine and exports glutamate due to the higher concentration of cystine and glutamate outside and inside cells, respectively. Since radioactive cystine is not commercially available, and the cystine transporter is an antiporter, radioactive glutamate is used to measure cystine uptake (28, 32, 33). Equal numbers of blood MDSC, purified splenic T cells, peritoneal macrophages (Figure 4A), and DC (Figure 4B) were incubated with 3H-glutamate. To ascertain the specificity of the xc− transporter, cold competitor (L-cystine) or non-competitor (L-leucine) amino acids were included in some wells. MDSC, DC, and macrophages incorporated glutamate at approximately the same rate. Cold L-cystine, but not cold L-leucine, competed for uptake in MDSC, macrophages, and DC. The same concentration of L-cystine competed glutamate binding for macrophages and DC (Figure 4B), whereas, a higher concentration of L-cystine was required to block glutamate uptake by MDSC (Figure 4C), demonstrating that MDSC have a higher intracellular concentration of cystine than macrophages and DC. In agreement with published data, T cells had a minimally functional xc− transporter. Gr1+CD11b+ cells from tumor-free mice had similar uptake kinetics of glutamate as Gr1+CD11b+ MDSC from tumor-bearing mice (supplemental figure 4). Therefore, MDSC have a cystine transporter that imports cystine at a rate similar to that of macrophages and DC.
Figure 4. MDSC import cystine and do not export cysteine.
A and B, Peritoneal macrophages (92% F4/80+), T cells (85% CD4+) and bone marrow DC (85% CD11c+) (all from tumor-free BALB/c mice), and 4T1-induced blood MDSC (92% Gr1+CD11b+) were cultured in sodium-free buffer for 20 min, followed by incubation in buffer with 3H-glutamate in the presence or absence of cold competitors L-cystine or L-leucine. Data for panels A and B are from one of five and two independent experiments, respectively. Results of the pooled experiments are significant at p <0.0005 and p<0.025, respectively. C. Higher concentrations of cold competitor L-cystine are required to inhibit glutamate uptake by MDSC relative to peritoneal macrophages. Peritoneal macropahges from tumor-free mice and 4T1-induced MDSC were cultured with 3H-glutamate as in panels A and B in the presence of cold L-cystine. Data are from one of four independent experiments. D, Macrophages and DC, but not MDSC, export cysteine. Peritoneal macrophages (85% F4/80+), DC (82% CD11c+), and purified CD4+ T cells (95% CD4+) (all from tumor-free mice), and 4T1-induced blood MDSC (97% Gr1+CD11b+) were incubated in serum-free HL-1 medium and cysteine content of the culture supernatants measured by the DTNB colorimetric assay. Data are from one of five independent experiments. Results of the pooled experiments are significant at p<0.0005. E, MDSC prevent macrophages from releasing cysteine. Macrophages (86.7% F4/80+) and titered numbers of 4T1-induced MDSC (90.6% Gr1+CD11b+) were co-cultured in serum-free HL-1 medium, and cysteine release measured by the DTNB colorimetric assay. Data are from one of two independent experiments and are significant at p<0.005 for the pooled. F, Serum was obtained from tumor-free BALB/c mice or from BALB/c mice with 4T1 tumors (primary mammary tumors of >5mm in diameter and >60% Gr1+CD11b+ leukocytes). Serum levels of cystine were determined by HPLC. Each symbol represents an individual mouse; horizontal bars indicate the mean. The two groups are significantly different at p<0. Statistics for all pooled results used the Wilcoxon paired-sample test.
MDSC do not export cysteine
Since MDSC do not express the ASC neutral amino acid transporter, they are unlikely to export cysteine. To measure cysteine export, supernatants from MDSC, macrophages, DC, and purified T cells were tested using the DTNB colorimetric assay that detects free thiols (−SH group) cysteine and glutathione (GSH) (Figure 4D). Since GSH is retained intracellularly, it will not be present in supernatants and the only thiol detected in this assay will be cysteine (34). Supernatants from activated macrophages and DC, but not from MDSC or T cells contain thiols demonstrating that MDSC do not export cysteine.
If MDSC sequester cystine and limit the amount of extracellular cysteine, then co-cultures of MDSC with APC will contain less extracellular cysteine than cultures containing only APC. To test this hypothesis, macrophages were co-cultured with varying numbers of MDSC and the amount of extracellular cysteine measured (Figure 4E). At ratios of 1:5 and 1:1 macrophages to MDSC, macrophage release of cysteine was inhibited by >73%, and at a ratio of 1:0.5 cysteine release was inhibited 32%, consistent with the concept that MDSC sequester cystine so that APC are limited in the amount of cystine they import and cysteine they export.
Tumor-bearing mice have lower serum levels of cystine than tumor-free mice
If MDSC are scavenging cystine, then tumor-bearing mice with elevated levels of blood MDSC should have less cystine in their blood as compared to tumor-free mice. To test this possibility, tumor-free and 4T1-tumor-bearing BALB/c mice (primary tumors > 6mm in diameter and blood levels of >50% Gr1+CD11b+ MDSC) were bled and their serum tested for cystine by HPLC (figure 4F). Tumor-bearing mice have on average less cystine in their serum than tumor-free mice, consistent with the concept that MDSC sequester cystine which results in reduced extracellular cysteine.
Discussion
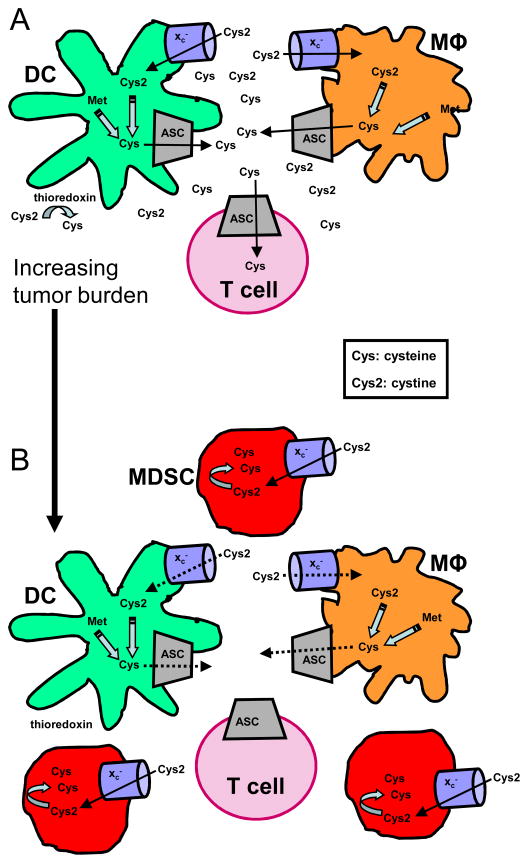
MDSC contribute to tumor progression by facilitating neo- angiogenesis (35) and by inhibiting innate (9, 11) and adaptive anti-tumor immunity (6). MDSC immune effects have been attributed to the production of arginase, inducible nitric oxide synthase (36) and reactive oxygen species (37), and to the nitration of TCRs (38). We now report that MDSC also block T cell activation by limiting the extracellular pool of cysteine which is required for T cell proliferation. Most cells synthesize cysteine from methionine using the enzyme cystathionase, or they use their xc− transporter to import cystine which is converted to cysteine in the intracellular reducing environment. However, T cells lack cystathionase and the xc− transporter and therefore must import extracellular cysteine through their ASC neutral amino acid transporter. Cysteine is essential for the protein synthesis that supports antigen-driven T cell activation, and the required extracellular cysteine is provided locally by macrophages and/or DC during antigen presentation (25, 30). If MDSC are present during T cell activation, they perturb these processes. MDSC do not synthesize cystathionase or the ASC neutral amino acid transporter and therefore must generate all of their cysteine from cystine that is imported through their xc− transporter. Since MDSC lack the ASC neutral amino acid transporter, they do not recycle cystine and return it to their surroundings as cysteine. As a result, while T cells are being activated by antigen, MDSC consume cystine that would otherwise be taken up by macrophages and DC and returned to the local environment as cysteine. Furthermore, increasing levels of MDSC that accumulate with progressive tumor growth limit the extracellular pool of cystine that could be converted to cysteine by thioredoxin produced by DC and macrophages (25, 26). As a result, the extracellular cysteine pool is reduced, and T cell activation is limited (Figure 5).
Figure 5.
MDSC suppress T cell activation by sequestering cystine and cysteine. A, DC and macrophages generate cysteine by reducing cystine imported through their xc− transporter, or by the conversion of methionine (Met) to cysteine through the action of cystathionase. Excess intracellular cysteine is exported by DC and macrophages through the ASC neutral amino acid transporter. Extracellular cysteine is also generated by the thioredoxin-mediated reduction of cystine to cysteine. During antigen presentation and T cell activation, T cells, which are unable to produce their own cysteine because they lack cystathionase and the xc− transporter, import cysteine through their ASC neutral amino acid transporter. B, MDSC must obtain all of their cysteine by importing cystine and reducing it to cysteine since they do not synthesize cystathionase and do not express the ASC neutral amino acid transporter. Therefore, as tumor burden and MDSC levels increase, MDSC consume increasing quantities of cystine. Since MDSC do not contain the ASC neutral amino acid transporter, they do not return cysteine to their surroundings. The competition between macrophages, DC, and MDSC for cystine leads to reduced uptake of cystine by macrophages and DC, and the concomitant decrease in cysteine released by these cells. Because of the lower levels of extracellular cystine, thioredoxin-mediated generation of extracellular cysteine is also reduced. Collectively, these effects result in the local depletion of cysteine and the inhibition of T cell activation.
The rate of uptake of cystine by MDSC is very similar to that of macrophages and DC, indicating that MDSC compete equally on a per cell basis with these APC. Since MDSC require higher concentrations of cold L-cystine to compete glutamate uptake, they have higher intracellular stores of cystine as compared to macrophages, consistent with the concept that MDSC sequester much of their imported cystine. Therefore, as tumor growth drives MDSC levels, increasing numbers of MDSC directly compete with APC for cystine, and act as a cystine “sink” to deprive macrophages and DC of cystine.
In addition to blocking T cell proliferation, limiting quantities of cysteine may also make T cells more susceptible to oxidative stress. GSH, a major intracellular redox molecule that protects cells from oxidative stress, is essential for optimal T cell proliferation and activation (39), and it’s synthesis is limited by cysteine (33, 40). Therefore, cysteine deprivation by MDSC is also likely to reduce intracellular T cell levels of GSH and thereby make T cells more susceptible to oxidative stress and its detrimental consequences.
Provision of cysteine in the form of NAC to MDSC cultured with GM-CSF and tumor cell-conditioned medium induces MDSC to differentiate into macrophages and DC, demonstrating that NAC also modulates MDSC function by converting MDSC to non-suppressive cells (31). However, the MDSC in our studies are not differentiating, indicating that the benefits of NAC are potentially two-fold: limiting the quantity of MDSC by promoting their differentiation, and providing cysteine for T cell activation. An epidemiological study showing that high levels of serum cysteine are associated with reduced risk of breast cancer (41) provides clinical support for the harmful effects of cysteine deprivation and the potential benefits of NAC.
As in vivo MDSC levels increase, free cystine/cysteine will decrease, and the extent of immune suppression by amino acid deprivation will vary depending on the quantity of MDSC at the site of antigen presentation and initial T cell activation. MDSC are present in the blood, lymph nodes, and at tumor sites of cancer patients (2) and additionally in the spleen of mice with tumors (6). Since antigen presentation and T cell priming occur in lymph nodes and spleen, but not in the blood, cystine/cysteine deprivation by MDSC is likely to be most pronounced in these secondary lymphoid organs. If antigen presentation and T cell activation occur within tumors, then cystine/cysteine deprivation by MDSC will also impair T cell activation in tumor.
NAC has been proposed as an anti-tumorigenic agent because it reduces the oxidative stress that promotes genetic instability. In vivo experiments using mice with progressively growing tumors have demonstrated therapeutic efficacy (42, 43). Our studies demonstrate that NAC may have the additional benefit of facilitating T cell activation by increasing extracellular cysteine levels. However, MDSC also suppress T cell activation through their production of arginase and nitric oxide (12, 44), so that supplemental cysteine alone may not significantly reduce the suppressive effects of MDSC. We have observed that peptide activation of transgenic T cells is increased in tumor-bearing mice maintained on NAC-supplemented water, but the increase was not statistically significant (unpublished data). However, administration of NAC, an already FDA-approved drug (45, 46), in combination with other agents that block MDSC, and combined with active immunotherapy may limit suppression by MDSC and thereby facilitate the treatment of established metastatic cancers.
Supplementary Material
Acknowledgments
We thank Drs. Jacobus Bosch and Erica Hanson for their helpful discussions, Ms. Sandy Mason for care of our mice, and Dr. Peter Gout for advice on the glutamate uptake protocol. These studies were supported by NIH R01CA115880 and R01CA84232, and Susan G. Komen for the Cure BCTR0503885.
References
- 1.Finn OJ. Cancer vaccines: between the idea and the reality. Nat Rev Immunol. 2003;3:630–41. doi: 10.1038/nri1150. [DOI] [PubMed] [Google Scholar]
- 2.Almand B, Clark JI, Nikitina E, et al. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166:678–89. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 3.Filipazzi P, Valenti R, Huber V, et al. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. 2007;25:2546–53. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 4.Young MR, Lathers DM. Myeloid progenitor cells mediate immune suppression in patients with head and neck cancers. Int J Immunopharmacol. 1999;21:241–52. doi: 10.1016/s0192-0561(99)00008-9. [DOI] [PubMed] [Google Scholar]
- 5.Young MR, Petruzzelli GJ, Kolesiak K, et al. Human squamous cell carcinomas of the head and neck chemoattract immune suppressive CD34(+) progenitor cells. Hum Immunol. 2001;62:332–41. doi: 10.1016/s0198-8859(01)00222-1. [DOI] [PubMed] [Google Scholar]
- 6.Sinha P, Clements VK, Ostrand-Rosenberg S. Reduction of myeloid-derived suppressor cells and induction of M1 macrophages facilitate the rejection of established metastatic disease. J Immunol. 2005;174:636–45. doi: 10.4049/jimmunol.174.2.636. [DOI] [PubMed] [Google Scholar]
- 7.Bronte V, Wang M, Overwijk WW, et al. Apoptotic death of CD8+ T lymphocytes after immunization: induction of a suppressive population of Mac-1+/Gr-1+ cells. J Immunol. 1998;161:5313–20. [PMC free article] [PubMed] [Google Scholar]
- 8.Kusmartsev SA, Li Y, Chen SH. Gr-1+ myeloid cells derived from tumor-bearing mice inhibit primary T cell activation induced through CD3/CD28 costimulation. J Immunol. 2000;165:779–85. doi: 10.4049/jimmunol.165.2.779. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki E, Kapoor V, Jassar AS, et al. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11:6713–21. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- 10.Hanson EM, Clements VK, Sinha P, et al. Myeloid-derived suppressor cells down-regulate L-selectin expression on CD4+ and CD8+ T cells. J Immunol. 2009;183:937–44. doi: 10.4049/jimmunol.0804253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sinha P, Clements VK, Bunt SK, et al. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol. 2007;179:977–83. doi: 10.4049/jimmunol.179.2.977. [DOI] [PubMed] [Google Scholar]
- 12.Bronte V, Serafini P, Mazzoni A, et al. L-arginine metabolism in myeloid cells controls T-lymphocyte functions. Trends Immunol. 2003;24:302–6. doi: 10.1016/s1471-4906(03)00132-7. [DOI] [PubMed] [Google Scholar]
- 13.Zea AH, Rodriguez PC, Atkins MB, et al. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 2005;65:3044–8. doi: 10.1158/0008-5472.CAN-04-4505. [DOI] [PubMed] [Google Scholar]
- 14.Kusmartsev S, Nefedova Y, Yoder D, et al. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J Immunol. 2004;172:989–99. doi: 10.4049/jimmunol.172.2.989. [DOI] [PubMed] [Google Scholar]
- 15.Mansoor MA, Svardal AM, Ueland PM. Determination of the in vivo redox status of cysteine, cysteinylglycine, homocysteine, and glutathione in human plasma. Anal Biochem. 1992;200:218–29. doi: 10.1016/0003-2697(92)90456-h. [DOI] [PubMed] [Google Scholar]
- 16.Arner ES, Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem. 2000;267:6102–9. doi: 10.1046/j.1432-1327.2000.01701.x. [DOI] [PubMed] [Google Scholar]
- 17.Ishii I, Akahoshi N, Yu XN, et al. Murine cystathionine gamma-lyase: complete cDNA and genomic sequences, promoter activity, tissue distribution and developmental expression. Biochem J. 2004;381:113–23. doi: 10.1042/BJ20040243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gout PW, Buckley AR, Simms CR, et al. Sulfasalazine, a potent suppressor of lymphoma growth by inhibition of the x(c)- cystine transporter: a new action for an old drug. Leukemia. 2001;15:1633–40. doi: 10.1038/sj.leu.2402238. [DOI] [PubMed] [Google Scholar]
- 19.Gmunder H, Eck HP, Droge W. Low membrane transport activity for cystine in resting and mitogenically stimulated human lymphocyte preparations and human T cell clones. Eur J Biochem. 1991;201:113–7. doi: 10.1111/j.1432-1033.1991.tb16263.x. [DOI] [PubMed] [Google Scholar]
- 20.Eagle H, Washington C, Friedman SM. The synthesis of homocystine, cystathionine, and cystine by cultured diploid and heteroploid human cells. Proc Natl Acad Sci U S A. 1966;56:156–63. doi: 10.1073/pnas.56.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bannai S. Transport of cystine and cysteine in mammalian cells. Biochim Biophys Acta. 1984;779:289–306. doi: 10.1016/0304-4157(84)90014-5. [DOI] [PubMed] [Google Scholar]
- 22.Gmunder H, Eck HP, Benninghoff B, et al. Macrophages regulate intracellular glutathione levels of lymphocytes. Evidence for an immunoregulatory role of cysteine. Cell Immunol. 1990;129:32–46. doi: 10.1016/0008-8749(90)90184-s. [DOI] [PubMed] [Google Scholar]
- 23.Sato H, Watanabe H, Ishii T, et al. Neutral amino acid transport in mouse peritoneal macrophages. J Biol Chem. 1987;262:13015–9. [PubMed] [Google Scholar]
- 24.Iwata S, Hori T, Sato N, et al. Thiol-mediated redox regulation of lymphocyte proliferation. Possible involvement of adult T cell leukemia-derived factor and glutathione in transferrin receptor expression. J Immunol. 1994;152:5633–42. [PubMed] [Google Scholar]
- 25.Angelini G, Gardella S, Ardy M, et al. Antigen-presenting dendritic cells provide the reducing extracellular microenvironment required for T lymphocyte activation. Proc Natl Acad Sci U S A. 2002;99:1491–6. doi: 10.1073/pnas.022630299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castellani P, Angelini G, Delfino L, et al. The thiol redox state of lymphoid organs is modified by immunization: role of different immune cell populations. Eur J Immunol. 2008;38:2419–25. doi: 10.1002/eji.200838439. [DOI] [PubMed] [Google Scholar]
- 27.Son YI, Egawa S, Tatsumi T, et al. A novel bulk-culture method for generating mature dendritic cells from mouse bone marrow cells. J Immunol Methods. 2002;262:145–57. doi: 10.1016/s0022-1759(02)00013-3. [DOI] [PubMed] [Google Scholar]
- 28.Shih AY, Murphy TH. xCt cystine transporter expression in HEK293 cells: pharmacology and localization. Biochem Biophys Res Commun. 2001;282:1132–7. doi: 10.1006/bbrc.2001.4703. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez PC, Ernstoff MS, Hernandez C, et al. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 2009;69:1553–60. doi: 10.1158/0008-5472.CAN-08-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edinger AL, Thompson CB. Antigen-presenting cells control T cell proliferation by regulating amino acid availability. Proc Natl Acad Sci U S A. 2002;99:1107–9. doi: 10.1073/pnas.042707999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nefedova Y, Fishman M, Sherman S, et al. Mechanism of all-trans retinoic acid effect on tumor-associated myeloid-derived suppressor cells. Cancer Res. 2007;67:11021–8. doi: 10.1158/0008-5472.CAN-07-2593. [DOI] [PubMed] [Google Scholar]
- 32.Lo M, Ling V, Wang YZ, et al. The x(c)(−) cystine/glutamate antiporter: a mediator of pancreatic cancer growth with a role in drug resistance. Br J Cancer. 2008 doi: 10.1038/sj.bjc.6604485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.La Bella V, Valentino F, Piccoli T, et al. Expression and developmental regulation of the cystine/glutamate exchanger (xc−) in the rat. Neurochem Res. 2007;32:1081–90. doi: 10.1007/s11064-006-9277-6. [DOI] [PubMed] [Google Scholar]
- 34.Hadzic T, Li L, Cheng N, et al. The role of low molecular weight thiols in T lymphocyte proliferation and IL-2 secretion. J Immunol. 2005;175:7965–72. doi: 10.4049/jimmunol.175.12.7965. [DOI] [PubMed] [Google Scholar]
- 35.Yang L, DeBusk LM, Fukuda K, et al. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6:409–21. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 36.Marigo I, Dolcetti L, Serafini P, et al. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol Rev. 2008;222:162–79. doi: 10.1111/j.1600-065X.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- 37.Nagaraj S, Gabrilovich DI. Tumor escape mechanism governed by myeloid-derived suppressor cells. Cancer Res. 2008;68:2561–3. doi: 10.1158/0008-5472.CAN-07-6229. [DOI] [PubMed] [Google Scholar]
- 38.Nagaraj S, Gupta K, Pisarev V, et al. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007;13:828–35. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smyth MJ. Glutathione modulates activation-dependent proliferation of human peripheral blood lymphocyte populations without regulating their activated function. J Immunol. 1991;146:1921–7. [PubMed] [Google Scholar]
- 40.Sakakura Y, Sato H, Shiiya A, et al. Expression and function of cystine/glutamate transporter in neutrophils. J Leukoc Biol. 2007;81:974–82. doi: 10.1189/jlb.0606385. [DOI] [PubMed] [Google Scholar]
- 41.Zhang SM, Willett WC, Selhub J, et al. A prospective study of plasma total cysteine and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2003;12:1188–93. [PubMed] [Google Scholar]
- 42.Gao P, Zhang H, Dinavahi R, et al. HIF-dependent antitumorigenic effect of antioxidants in vivo. Cancer Cell. 2007;12:230–8. doi: 10.1016/j.ccr.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sablina AA, Budanov AV, Ilyinskaya GV, et al. The antioxidant function of the p53 tumor suppressor. Nat Med. 2005;11:1306–13. doi: 10.1038/nm1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodriguez PC, Ochoa AC. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives. Immunol Rev. 2008;222:180–91. doi: 10.1111/j.1600-065X.2008.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsuyuki S, Yamauchi A, Nakamura H, et al. Possible availability of N-acetylcysteine as an adjunct to cytokine therapy for hepatocellular carcinoma. Clin Immunol Immunopathol. 1998;88:192–8. doi: 10.1006/clin.1998.4574. [DOI] [PubMed] [Google Scholar]
- 46.Onda T, Matsumoto K, Shibata T, et al. Phase III trial of upfront debulking surgery versus neoadjuvant chemotherapy for stage III/IV ovarian, tubal and peritoneal cancers: Japan Clinical Oncology Group Study JCOG0602. Jpn J Clin Oncol. 2008;38:74–7. doi: 10.1093/jjco/hym145. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.