Abstract
Background
Wide variation exists in the treatment of suspected gastroesophageal reflux disease (GERD) in premature infants; it is unknown to what degree diagnosis and treatment are impacted by the treating physician's medical specialty or interpretation of the medical literature.
Methods
This study involved an online survey of board-certified neonatologists, pediatric pulmonologists, and pediatric gastroenterologists about their beliefs regarding the symptoms, diagnosis, and treatment of GERD in premature infants in the neonatal intensive care unit (NICU), based on both clinical impression and interpretation of the literature.
Results
1021 neonatologists, 232 pediatric pulmonologists, and 222 pediatric gastroenterologists participated in the study (47.5% response). There was disagreement among specialties in nearly all aspects of the survey. Pulmonologists were most likely to report that respiratory symptoms are caused by GERD (p<0.001). Neonatologists were least likely to report that a therapeutic trial of pharmacologic agents would be useful for diagnosing GERD (p<0.001) or that lansoprazole, ranitidine, or cimetidine are safe or effective (p<0.001). No pharmacologic therapy had greater than 50% of respondents supporting its effectiveness. There was moderate correlation between physician belief based on the medical literature and belief based on clinical impression (Spearman Rank Correlation 0.47-0.75). For therapies supported by multiple meta-analyses in infants versus therapies with few infant trials, physicians rated the evidence for effectiveness similarly.
Conclusion
There is wide variation among pediatric specialties regarding beliefs about GERD in premature infants, as well as about the weight of evidence in the medical literature for this patient population. Physician beliefs do not seem to be driven by the degree of evidence in the neonatal literature. With no agreed-upon standard of care in the setting of widespread use of anti-reflux medications, greater understanding is needed about the ways physicians form clinical impressions and access, process, and apply medical evidence to patient care.
Keywords: premature infants, gastroesophageal reflux, clinical practice, evidence based medicine, specialty care
Introduction
While the collection of high-quality research data is of utmost importance, data from any study—even from large randomized controlled trials—in and of itself is not enough to change the landscape of clinical practice. It is also important to understand how evidence from clinical trials is disseminated, interpreted, and applied by practicing clinicians [1, 2]. The study of pediatric specialists' beliefs about gastroesophageal reflux disease (GERD) in premature infants provides an opportunity to explore the relationship between physicians' clinical impressions and interpretation of research data because the symptoms [3-9], diagnosis [10-13] and treatment [14-23] remain controversial. Despite this controversy in the literature, however, the use of anti-reflux medications in NICU patients is pervasive in the United States [24, 25].
There is variability in the self-reported knowledge, attitudes, and practice styles of pediatricians with regard to the management of GERD [26]; however, there is a paucity of information about the beliefs of specialists treating GERD in preterm infants. The present study, therefore, was carried out as a first step towards better understanding the link between a controversial body of medical literature and reported physician prescribing behaviors. This study first describes the range of physician beliefs, based both on clinical impression and medical research, about the symptoms, diagnosis, and treatment of GERD in premature infants in a NICU setting. Secondly, it assesses the correlation between physician beliefs based on clinical impression and based on medical literature. Finally, it evaluates the association between physician specialty and beliefs about GERD.
Methods
Study Design
This was a survey of physician beliefs about the symptoms, diagnosis, and treatment of GERD in premature infants in a NICU setting. Physicians rated their beliefs regarding their overall clinical impression, as well as their beliefs about the evidence in the medical literature. All questions included a clinical scenario involving the management of a 6-week old infant, former 28 weeks gestation, in the NICU with suspected GERD. Respondents used five-point ordinal scales to rate the strength of their beliefs and also provided demographic information.
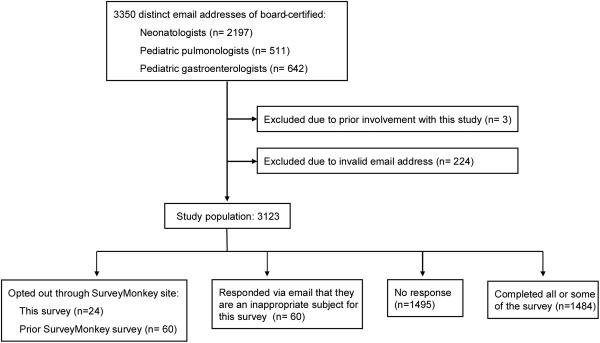
Neonatologists, pediatric pulmonologists, and pediatric gastroenterologists were identified as the physicians most likely to care for this patient population. Surveys were sent to all of the 3,123 physicians identified as board-certified in the United States in the fields of Neonatal-Perinatal Medicine, Pediatric Pulmonology, and Pediatric Gastroenterology who were not involved with the study and who had distinct, valid email addresses available to the research team (Figure 1). This amounted to 2016 neonatologists, 477 pulmonologists, and 570 gastroenterologists. This study was approved by the Cleveland Clinic Institutional Review Board.
Figure 1.
Inclusion/Exclusion and Responses
Questionnaire Development
A 20-item survey was developed based on the Tailored Design Method [27], as no appropriate instrument was identified in the medical literature. It was reviewed for content and clarity by 18 attending physicians and fellows in the fields of neonatology (n=14), pediatric pulmonology (n=1), pediatric gastroenterology (n=1), and general academic pediatrics (n=2) from four academic children's hospitals in the United States. Survey questions about physician beliefs are provided in Appendix 1.
Data Collection
The questionnaire was administered through the online survey company SurveyMonkey (www.surveymonkey.com). An introductory email was sent to describe the study and to invite participation. Reminder emails were sent to non-responders at 3 weeks, 6 weeks, 9 weeks, and 12 weeks after the initial email. The survey remained open for a total of 12 weeks from December 2007 to March 2008.
Statistical Methods
Demographic characteristics of participants and Likert scale responses were described using frequencies, percents, and their 95% confidence intervals; medians for the ordinal scales were also reported. Analyses of Likert scales used nonparametric methods suited to ordinal data, including the Kruskal-Wallis test and, when the Kruskal-Wallis yielded a significant result, the Steel-Dwass pairwise comparison for comparing two groups of ordinal variables. The Spearman rank correlation was used to assess association between two ordinal variables (such as personal beliefs and beliefs about the medical literature). All tests were two-tailed and performed at an overall significance level of 0.05. SAS 9.1 software (SAS Institute, Cary, NC) was used for all analyses.
Sample Size and Power
Based on three recent surveys of pediatricians [26, 28, 29], we expected a 25% response rate, yielding an expected sample size of 842. With this sample size, we would have had 90% power to detect correlations as small as 0.12, and 95% confidence intervals on percents would have been at most 6% wide (for example, the 95% confidence interval on 421/842 would be 47%-53%).
Results
Of the 3,123 physicians included in the study, 1,484 completed some or all of the survey (response rate= 47.5%). Of these, 1,021 were neonatologists (50.6% response), 232 were pediatric pulmonologists (48.6% response), 222 were pediatric gastroenterologists (38.9% response), and nine, who were excluded from analysis, reported a different specialty. Physicians from across the United States were represented in each of the three specialties. Additionally, 59 (1.9%) responded via email but declined to participate because they either had a conflict of interest, no longer practiced clinical medicine, or felt their practice fell outside of the scope of the research questions. 84 opted out directly through the SurveyMonkey site, either by opting out of this particular survey (n=24) or by opting out of a different SurveyMonkey survey (n=60), (Figure 1). Demographic data is reported in Table 1.
Table 1.
| Neonatology, N (%) | Ped. Pulmonology, N (%) | Ped. Gastroenterology, N (%) | |
|---|---|---|---|
| # Diplomates Certified | 4428 | 821 | 990 |
| # included in survey | 2016 | 477 | 570 |
| # Responded | 1021 (50.6) | 232 (48.6) | 222 (38.9) |
| Sex | |||
| Male | 536 (51.5) | 141 (60.8) | 140 (63.1) |
| Female | 370 (36.2) | 67 (28.9) | 60 (27.0) |
| Did not reply | 115 (11.3) | 24 (10.3) | 22 (9.9) |
| Yrs. since fellowship | |||
| 0-10 | 342 (33.5) | 83 (35.8) | 61 (27.4) |
| 11-20 | 436 (42.7) | 83 (35.8) | 75 (33.8) |
| 21-30 | 110 (10.8) | 38 (16.4) | 49 (22.1) |
| >30 | 27 (2.6) | 4 (1.7) | 13 (5.9) |
| Did not reply | 106 (10.4) | 23 (9.9) | 24 (10.8) |
| n/a | 1 (0.4) | ||
| NICU avg. daily census | |||
| No NICU | 3 (0.3) | 2 (0.9) | 2 (0.9) |
| <5 | 32 (3.1) | 0 (0.0) | 3 (1.4) |
| 5-14 | 137 (13.4) | 6 (2.6) | 14 (6.3) |
| 15-25 | 187 (18.3) | 36 (15.5) | 20 (9.0) |
| >25 | 572 (56.0) | 168 (72.4) | 163 (73.4) |
| Did not reply | 90 (8.8) | 20 (8.6) | 20 (9.0) |
Beliefs based on Overall Clinical Impression
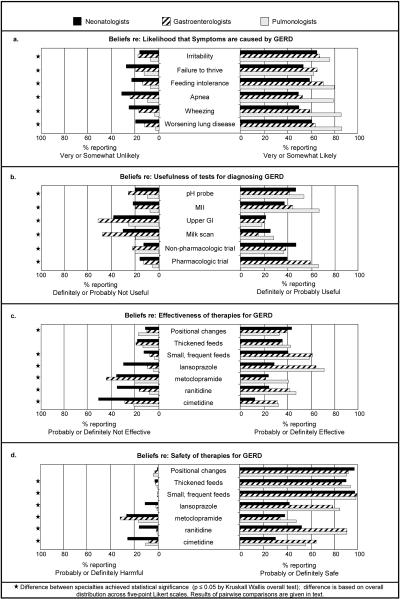
Significant variation in beliefs was seen among neonatologists, pediatric pulmonologists and pediatric gastroenterologists in nearly all questions about the symptoms, diagnosis and treatment of GERD in premature infants in the NICU. The variation reported here refers to differences in the entire distribution of responses, by specialty, across the five-point Likert scales. There were differences in belief among specialties about the likelihood that each of the six symptoms surveyed (irritability, failure to thrive, feeding intolerance, apnea, wheezing, and worsening lung disease) is caused by GERD (all p≤0.001) (Figure 2a). Pairwise comparisons revealed that pulmonologists were most likely to report that apnea, wheezing, and worsening lung disease are caused by GERD (all p≤0.001). Pulmonologists were also more likely than neonatologists to believe that irritability is caused by GERD in this population (p=0.001). Furthermore, neonatologists were least likely to believe that failure to thrive or feeding intolerance is caused by GERD (all p≤0.027).
Figure 2.
Physician beliefs by specialty, based on overall clinical impression
Similarly, significant differences were found regarding the usefulness of all six diagnostic strategies (pH probe, multichannel intraluminal impedance (MII), upper GI, scintigraphy (also known as a milk scan), and therapeutic trials of non-pharmacologic or pharmacologic treatments) (all p<0.001) (Figure 2b). Pulmonologists were most likely to believe that the pH probe or MII are useful for diagnosing GERD (all p≤0.001), and gastroenterologists were least likely to believe that an upper GI study or scintigraphy are useful in this population (all p≤0.002). With regard to therapeutic trials, neonatologists were most likely to believe that a non-pharmacologic trial is useful (both p≤0.018) and least likely to believe that a pharmacologic trial is useful as a diagnostic test (both p≤0.001).
The safety and effectiveness of several treatments for suspected GERD (positional changes; thickened feeds; smaller, more frequent feedings; lansoprazole; metoclopramide; ranitidine; and cimetidine) are also controversial (Figures 2c-d). In terms of non-pharmacologic treatments, neonatologists were more likely than pulmonologists to believe that positional changes offer effective treatment (p=0.008), and neonatologists were least likely of the three groups to believe that smaller, more frequent feedings are effective (both p≤0.001). Regarding the pharmacologic treatments, pulmonologists and gastroenterologists were more likely than neonatologists to believe that lansoprazole, ranitidine, or cimetidine are safe or effective (all p≤0.001). Pulmonologists were most likely to believe that metoclopramide is safe or effective (all p≤0.003).
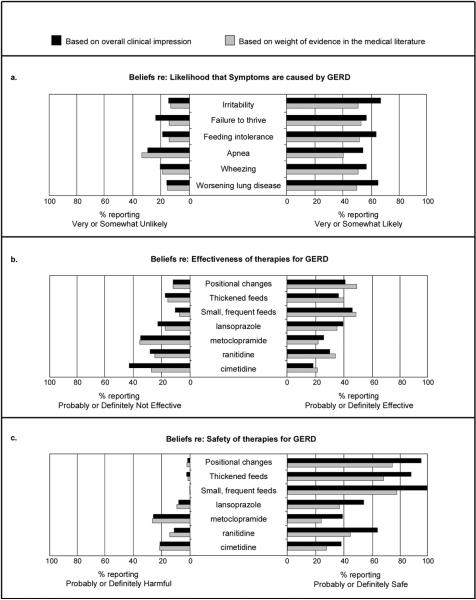
In addition to the differences seen among specialties, there was also a lack of consensus within specialties (Figure 2b). For example, 28.1% of neonatologists reported that lansoprazole is probably or definitely effective, while 29.3% of neonatologists believed lansoprazole to be probably or definitely not effective. Support for each of the pharmacologic therapies for treating GERD in this population ranged from between 18.1% and 38.7% of all respondents with regard to effectiveness (Figure 3b) and between 37.1% and 62.6% with regard to safety (Figure 3c). Only 184 respondents (12.4%) rated at least one of these therapies as definitely effective based on overall clinical impression.
Figure 3.
Physician beliefs (all respondents)
NICU average daily census was also associated with physician beliefs about the likelihood that symptoms are caused by GERD. Irritability, feeding intolerance, failure to thrive, and apnea were all rated significantly differently (all p≤0.003). Pairwise comparisons revealed that physicians who practice in the smallest NICUs, with average daily censuses less than five, were more likely to attribute all four of these symptoms to GERD than were physicians who practiced in larger NICUs with average daily censuses of 15-25 (all p≤0.03) or greater than 25 (all p≤0.009). Few differences were appreciated when the safety or effectiveness of different therapies were analyzed based on NICU average daily census.
Beliefs about Evidence from the Medical Literature
For each of the six symptoms included in the survey, between 39.3% and 51.6% of respondents reported that they are somewhat or very likely to be caused by GERD based on evidence from the medical literature (Figure 3a). None of the four pharmacologic therapies had more than 34.5% of respondents reporting that its effectiveness is supported by the medical literature (Figure 3b) or more than 43.7% reporting that its safety is supported by the literature (Figure 3c). For the non-pharmacologic therapies, each was rated, based on evidence from the medical literature, as probably or definitely effective by 39.3% to 48.2% of respondents (Figure 3b) and probably or definitely safe by between 66.4% and 75.7% (Figure 3c). Physician beliefs based on clinical impression were moderately correlated with beliefs based on medical literature (Spearman Rank Correlation ranges 0.47 – 0.75).
Finally, 168 respondents (11.3%) rated at least one of the pharmacologic therapies as definitely effective based on medical literature. Of note, lansoprazole was the most supported with 128 respondents (8.6%) rating it as definitely effective based on medical literature.
Qualitative Response
Several participants emailed the research team with comments about the survey and/or their approach to GERD. Representative comments are included:
…[Your survey] addressed some issues that have for years been pet peeves of mine and many peds pulmonology colleagues.. In the NICU, the neonatologists have an incredibly high threshold for even considering treating obvious GERD. We hear so many stories of kids who… have a chronic cough… and obvious GER -- parents almost always say the neonatologists knew about it and didn't treat. (-Pulmonologist)
… I feel that you could get a much better feedback on GERD in the NICU from a neonatologist or a gastroenterologist, rather than a pulmonologist like me. (-Pulmonologist)
Although I am a peds GI doc, I rarely participate in the care of the neonates in our hospital's NICU and really never for reflux concerns. (-Gastroenterologist)
This is a complex area, and most of us in GI tailor our approach to the specific patient presentation. One size does not fit all, unlike the approach of those who do pH probes on everyone! (-Gastroenterologist)
GERD is very vexing and overdiagnosed…we have no definitive protocol for dealing with it at our institution/NICU. (-Neonatologist)
Discussion
The results of this survey suggest several important points. First, the results demonstrate a wide range of beliefs, indicating that there is no apparent consensus about the management of GERD in premature infants in the NICU, based either on clinical impression or on assessment of the medical literature. There is clearly no agreed-upon standard of care. Second, significant differences in belief were appreciated among neonatologists, gastroenterologists, and pulmonologists, suggesting that patient care could be influenced by referral patterns and that specialists may be relying on different sources to shape their beliefs. Such differences in belief among specialists have the potential to impact patient care. Finally, a better understanding is needed of how medical literature and clinical experience are processed and implemented by physicians.
The range of beliefs about the symptoms, diagnosis and treatment of GERD in premature infants could be the result of many different factors. Gastroesophageal reflux, or the retrograde passage of stomach contents into the esophagus, is common in infants [8, 19, 30] and often considered physiologic [31-33]. Defining gastroesophageal reflux disease, however, or reflux with negative sequelae, has been fraught with difficulty, especially in premature infants. They are excluded from North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) guidelines on the management of GERD [31], and research on GERD in this population has been limited by the complexities of studying vulnerable populations, the heterogeneity of NICU populations, and the lack of agreed-upon diagnostic criteria [34]. While the lack of consistent guidelines and evidence in this population may explain the lack of agreement within specialty groups, it explains neither the pervasive use of anti-reflux drugs in this population, nor the variability in beliefs among specialist groups.
Differences among specialties suggest that premature infants may be treated differently based on the provider's clinical background. Neonatologists may be least likely to suspect GERD as a cause of symptoms, to test for GERD, or to treat, especially with pharmacologic therapies. Pulmonologists, who frequently treat a range of respiratory diseases associated with reflux [35, 36], were most likely to associate GERD with all three of the included respiratory symptoms. Distinct training pathways may also include different bedside teaching traditions and familiarity with different literature, which could also drive these differences. Neonatologists might be more familiar with the unique pharmacokinetics, pharmacodynamics, disease manifestations, and responses to therapies in the premature infant population, as well as with adverse events specific to this population such as the possibility of late-onset sepsis or necrotizing enterocolitis with ranitidine [37, 38]. Gastroenterologists and pulmonologists, however, treat older patients with GERD and may be more familiar with a wider range of GERD research. Institutional differences in consultation patterns may also play a role; these differences are suspected due to the range of responses to the survey, both from specialists who do not treat GERD in premature infants and from those who hold strong opinions (see Qualitative Response, above).
These results also raise questions about the role of medical literature in patient care and about physician evaluation of medical literature. Despite a minority of respondents believing any of the therapies to be definitely, or even probably, effective, the use of anti-reflux medications is widespread in NICUs and increasing in infants [24, 25, 39]. This indicates a potential disconnect between physicians' clinical impressions, interpretation of medical literature, and behavior. Indeed, this study demonstrated only moderate correlation between physician's clinical impressions and their assessment of the evidence in the literature. Additionally, physicians rated the effectiveness of thickened feeds and lansoprazole similarly based on the medical literature (Figure 3b) despite the fact that at the time of the survey, the effectiveness of thickened feeds for reducing reflux symptoms [19] and reducing the frequency of frank emesis [21] in infants was supported by two meta-analyses, while the effectiveness of lansoprazole and other PPIs in infants was supported by only a few small trials with some evidence for improvement in physiologic measures but not in normalization of symptoms [40-47]. Although it is possible that respondents rated these interventions similarly because of the lack of evidence specifically in preterm populations, in this case we would have expected lower effectiveness ratings for both. Finally, we note that 27% of respondents rated cimetidine as probably or definitely safe based on medical literature, despite a trial stopped by its data safety monitoring board in which cimetidine was associated with a higher risk of death and severe intraventricular hemorrhage in premature infants [17].
Although this study generated significant interest, with data from nearly 1,500 pediatric specialists across the United States, certain limitations are recognized. First, the survey captured self-reported beliefs about a single, limited case scenario rather than direct observation of physician behavior. Also, the literature that informed each physician's assessments is unknown. Although the 47.5% response rate was quite high for a survey of physician beliefs, certainly the possibility of non-response bias and coverage error exists. The Likert scales introduce additional limitations. For example, two physicians might rate the same therapy as “probably effective based on the medical literature,” where one bases this opinion on a positive study in full-term infants, and the other on the lack of negative studies in premature infants. Finally, we have no data on the frequency of individual respondent care of NICU patients; this variable may relate to the differences in beliefs seen within the different pediatric specialties. Nevertheless, the results of this study suggest important specialty differences in clinical impressions and in the uptake and evaluation of medical literature. Results clearly suggest the lack of agreed-upon standard of care and contribute to a dialogue about how clinical decisions are made in the face of objective uncertainty.
The management of premature infants in the NICU with suspected GERD is a controversial topic, with evidence-based management complicated by the lack of definitive studies, the heterogeneity of NICU populations, and the potential involvement of multiple pediatric specialties. Despite these challenges, and despite the lack of majority support for any therapy, anti-reflux medications are among the most frequently used drugs in NICUs. This study illustrates that there is no agreed-upon standard of care and demonstrates significant differences in belief among pediatric specialties. It also suggests that factors outside of the medical literature influence the development of clinical impressions and the assessment of published evidence. A better understanding of the way physicians review, interpret, and implement evidence from the literature is necessary to identify ways in which the practice of evidence-based medicine can be enhanced. There is a need for collaboration among specialties in both future research and in the diagnosis and management of GERD in preterm infants.
Supplementary Material
Acknowledgements
The authors wish to thank those physicians who participated in the survey.
Financial Disclosures and Conflicts of Interest Financial support was provided by National Institutes of Health grant K23-HD056299 and by the Cleveland Clinic Children's Hospital.
Abbreviations
- GER
Gastroesophageal reflux
- GERD
Gastroesophageal reflux disease
- MII
Multichannel intraluminal impedance
- NICU
Neonatal intensive care unit
- PPI
Proton pump inhibitor
Footnotes
E. Rome is on the speakers' bureau of Merck & Co.; the authors have no other conflicts of interest.
References
- 1.Freed GL. Society for Pediatric Research--2007 presidential address: expanding the research continuum--from bench to implementation. Pediatr Res. 2007;62(3):370–3. doi: 10.1203/PDR.0b013e318140b02a. [DOI] [PubMed] [Google Scholar]
- 2.Haines A, Donald A. Making better use of research findings. BMJ. 1998;317(7150):72–5. doi: 10.1136/bmj.317.7150.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marino AJ, et al. The incidence of gastroesophageal reflux in preterm infants. J Perinatol. 1995;15(5):369–71. [PubMed] [Google Scholar]
- 4.Di Fiore JM, et al. Apnea is not prolonged by acid gastroesophageal reflux in preterm infants. Pediatrics. 2005;116(5):1059–63. doi: 10.1542/peds.2004-2757. [DOI] [PubMed] [Google Scholar]
- 5.Heine RG, et al. Clinical predictors of pathological gastro-oesophageal reflux in infants with persistent distress. J Paediatr Child Health. 2006;42(3):134–9. doi: 10.1111/j.1440-1754.2006.00812.x. [DOI] [PubMed] [Google Scholar]
- 6.Finer NN, et al. Summary proceedings from the apnea-of-prematurity group. Pediatrics. 2006;117(3 Pt 2):S47–51. doi: 10.1542/peds.2005-0620H. [DOI] [PubMed] [Google Scholar]
- 7.Deal L, et al. Age-specific questionnaires distinguish GERD symptom frequency and severity in infants and young children: development and initial validation. J Pediatr Gastroenterol Nutr. 2005;41(2):178–85. doi: 10.1097/01.mpg.0000172885.77795.0f. [DOI] [PubMed] [Google Scholar]
- 8.Poets CF. Gastroesophageal reflux: a critical review of its role in preterm infants. Pediatrics. 2004;113(2):e128–32. doi: 10.1542/peds.113.2.e128. [DOI] [PubMed] [Google Scholar]
- 9.Fuloria M, et al. Gastroesophageal reflux in very low birth weight infants: association with chronic lung disease and outcomes through 1 year of age. J Perinatol. 2000;20(4):235–9. doi: 10.1038/sj.jp.7200352. [DOI] [PubMed] [Google Scholar]
- 10.Vandenplas Y, et al. Will esophageal impedance replace pH monitoring? Pediatrics. 2007;119(1):118–22. doi: 10.1542/peds.2006-1753. [DOI] [PubMed] [Google Scholar]
- 11.Magista AM, et al. Multichannel intraluminal impedance to detect relationship between gastroesophageal reflux and apnoea of prematurity. Dig Liver Dis. 2007;39(3):216–21. doi: 10.1016/j.dld.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 12.Condino AA, et al. Evaluation of infantile acid and nonacid gastroesophageal reflux using combined pH monitoring and impedance measurement. J Pediatr Gastroenterol Nutr. 2006;42(1):16–21. doi: 10.1097/01.mpg.0000188008.66752.72. [DOI] [PubMed] [Google Scholar]
- 13.Orenstein SR, Shalaby TM, Cohn JF. Reflux symptoms in 100 normal infants: diagnostic validity of the infant gastroesophageal reflux questionnaire. Clin Pediatr (Phila) 1996;35(12):607–14. doi: 10.1177/000992289603501201. [DOI] [PubMed] [Google Scholar]
- 14.Chao HC, Vandenplas Y. Effect of cereal-thickened formula and upright positioning on regurgitation, gastric emptying, and weight gain in infants with regurgitation. Nutrition. 2007;23(1):23–28. doi: 10.1016/j.nut.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Jordan B, et al. Effect of antireflux medication, placebo and infant mental health intervention on persistent crying: a randomized clinical trial. J Paediatr Child Health. 2006;42(12):49–58. doi: 10.1111/j.1440-1754.2006.00786.x. [DOI] [PubMed] [Google Scholar]
- 16.Hibbs AM, Lorch SA. Metoclopramide for the treatment of gastroesophageal reflux disease in infants: a systematic review. Pediatrics. 2006;118(2):746–52. doi: 10.1542/peds.2005-2664. [DOI] [PubMed] [Google Scholar]
- 17.Cotton RB, et al. Cimetidine does not prevent lung injury in newborn premature infants. Pediatr Res. 2006;59(6):795–800. doi: 10.1203/01.pdr.0000219397.35473.5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Canani RB, et al. Therapy with gastric acidity inhibitors increases the risk of acute gastroenteritis and community-acquired pneumonia in children. Pediatrics. 2006;117(5):e817–20. doi: 10.1542/peds.2005-1655. [DOI] [PubMed] [Google Scholar]
- 19.Craig WR, et al. Metoclopramide, thickened feedings, and positioning for gastrooesophageal reflux in children under two years. Cochrane Database Syst Rev. 2004;(4):CD003502. doi: 10.1002/14651858.CD003502.pub2. [DOI] [PubMed] [Google Scholar]
- 20.Huang RC, Forbes DA, Davies MW. Feed thickener for newborn infants with gastro-oesophageal reflux. Cochrane Database Syst Rev. 2002;(3):CD003211. doi: 10.1002/14651858.CD003211. [DOI] [PubMed] [Google Scholar]
- 21.Carroll AE, Garrison MM, Christakis DA. A systematic review of nonpharmacological and nonsurgical therapies for gastroesophageal reflux in infants. Arch Pediatr Adolesc Med. 2002;156(2):109–13. doi: 10.1001/archpedi.156.2.109. [DOI] [PubMed] [Google Scholar]
- 22.Khalaf MN, et al. Clinical correlations in infants in the neonatal intensive care unit with varying severity of gastroesophageal reflux. J Pediatr Gastroenterol Nutr. 2001;32(1):45–9. doi: 10.1097/00005176-200101000-00014. [DOI] [PubMed] [Google Scholar]
- 23.Orenstein SR, et al. Multicenter, Double-Blind, Randomized, Placebo-Controlled Trial Assessing the Efficacy and Safety of Proton Pump Inhibitor Lansoprazole in Infants with Symptoms of Gastroesophageal Reflux Disease. J Pediatr. 2008 doi: 10.1016/j.jpeds.2008.09.054. [DOI] [PubMed] [Google Scholar]
- 24.Clark RH, et al. Reported medication use in the neonatal intensive care unit: data from a large national data set. Pediatrics. 2006;117(6):1979–87. doi: 10.1542/peds.2005-1707. [DOI] [PubMed] [Google Scholar]
- 25.Malcolm W, et al. Annual Meeting of the Pediatric Academic Societies. San Francisco, CA: 2006. Anti-reflux medications (ARM) at NICU discharge for extremely low birthweight (ELBW) infants. [Google Scholar]
- 26.Diaz DM, et al. Knowledge, attitudes and practice styles of North American pediatricians regarding gastroesophageal reflux disease. J Pediatr Gastroenterol Nutr. 2007;45(1):56–64. doi: 10.1097/MPG.0b013e318054b0dd. [DOI] [PubMed] [Google Scholar]
- 27.Dillman DA. Mail and Internet Surveys: The Tailored Design Method. 2nd ed. John Wiley & Sons, Inc.; New York: 2000. [Google Scholar]
- 28.McMahon SR, et al. Comparison of e-mail, fax, and postal surveys of pediatricians. Pediatrics. 2003;111(4 Pt 1):e299–303. doi: 10.1542/peds.111.4.e299. [DOI] [PubMed] [Google Scholar]
- 29.Pujazon-Zazik M. In: Email re: medical student research project. Golski C, editor. Cleveland: 2007. [Google Scholar]
- 30.Dhillon AS, Ewer AK. Diagnosis and management of gastro-oesophageal reflux in preterm infants in neonatal intensive care units. Acta Paediatr. 2004;93(1):88–93. [PubMed] [Google Scholar]
- 31.Rudolph CD, et al. Guidelines for evaluation and treatment of gastroesophageal reflux in infants and children: recommendations of the North American Society for Pediatric Gastroenterology and Nutrition. J Pediatr Gastroenterol Nutr. 2001;32(Suppl 2):S1–31. doi: 10.1097/00005176-200100002-00001. [DOI] [PubMed] [Google Scholar]
- 32.Nelson SP, et al. One-year follow-up of symptoms of gastroesophageal reflux during infancy. Pediatric Practice Research Group. Pediatrics. 1998;102(6):E67. doi: 10.1542/peds.102.6.e67. [DOI] [PubMed] [Google Scholar]
- 33.Nelson SP, et al. Prevalence of symptoms of gastroesophageal reflux during infancy. A pediatric practice-based survey. Pediatric Practice Research Group. Arch Pediatr Adolesc Med. 1997;151(6):569–72. doi: 10.1001/archpedi.1997.02170430035007. [DOI] [PubMed] [Google Scholar]
- 34.Slocum C, et al. Infant apnea and gastroesophageal reflux: a critical review and framework for further investigation. Curr Gastroenterol Rep. 2007;9(3):219–24. doi: 10.1007/s11894-007-0022-3. [DOI] [PubMed] [Google Scholar]
- 35.Boesch RP, Acton JD. Outcomes of fundoplication in children with cystic fibrosis. J Pediatr Surg. 2007;42(8):1341–4. doi: 10.1016/j.jpedsurg.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 36.Hancox RJ, et al. Associations between respiratory symptoms, lung function and gastro-oesophageal reflux symptoms in a population-based birth cohort. Respir Res. 2006;7:142. doi: 10.1186/1465-9921-7-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bianconi S, et al. Ranitidine and late-onset sepsis in the neonatal intensive care unit. J Perinat Med. 2007;35(2):147–50. doi: 10.1515/JPM.2007.017. [DOI] [PubMed] [Google Scholar]
- 38.Terrin G, et al. Ranitidine treatment is associated with an increased risk of necrotizing enterocolitis in newborn. Digestive and Liver Disease. 2007;39(10):A62–A62. [Google Scholar]
- 39.Barron JJ, et al. Proton pump inhibitor utilization patterns in infants. J Pediatr Gastroenterol Nutr. 2007;45(4):421–7. doi: 10.1097/MPG.0b013e31812e0149. [DOI] [PubMed] [Google Scholar]
- 40.Khoshoo V, Dhume P. Clinical response to 2 dosing regimens of lansoprazole in infants with gastroesophageal reflux. J Pediatr Gastroenterol Nutr. 2008;46(3):352–4. doi: 10.1097/MPG.0b013e31815667d7. [DOI] [PubMed] [Google Scholar]
- 41.Moore DJ, et al. Double-blind placebo-controlled trial of omeprazole in irritable infants with gastroesophageal reflux. J Pediatr. 2003;143(2):219–23. doi: 10.1067/S0022-3476(03)00207-5. [DOI] [PubMed] [Google Scholar]
- 42.Omari T, et al. Pharmacokinetics and acid-suppressive effects of esomeprazole in infants 1-24 months old with symptoms of gastroesophageal reflux disease. J Pediatr Gastroenterol Nutr. 2007;45(5):530–7. doi: 10.1097/MPG.0b013e31812e012f. [DOI] [PubMed] [Google Scholar]
- 43.Omari TI, et al. Effect of omeprazole on acid gastroesophageal reflux and gastric acidity in preterm infants with pathological acid reflux. J Pediatr Gastroenterol Nutr. 2007;44(1):41–4. doi: 10.1097/01.mpg.0000252190.97545.07. [DOI] [PubMed] [Google Scholar]
- 44.Orenstein SR. Esomeprazole in infants 1 to 24 months old. Curr Gastroenterol Rep. 2008;10(3):292–3. [PubMed] [Google Scholar]
- 45.Orenstein SR. Omeprazole in preterm infants. Curr Gastroenterol Rep. 2008;10(3):291. [PubMed] [Google Scholar]
- 46.Orenstein SR. Omeprazole in infants 3 to 12 months old. Curr Gastroenterol Rep. 2008;10(3):291. [PubMed] [Google Scholar]
- 47.Orenstein SR, Hassall E. Infants and proton pump inhibitors: tribulations, no trials. J Pediatr Gastroenterol Nutr. 2007;45(4):395–8. doi: 10.1097/MPG.0b013e31812e011d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.