Abstract
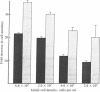
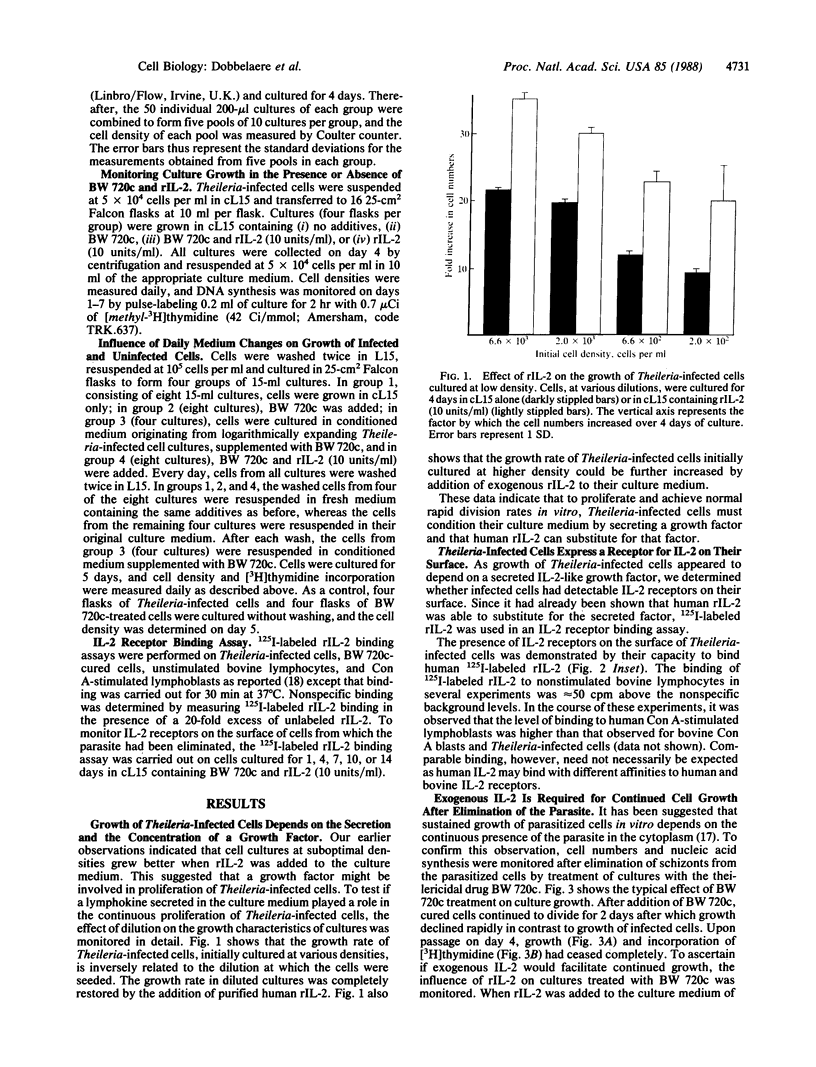
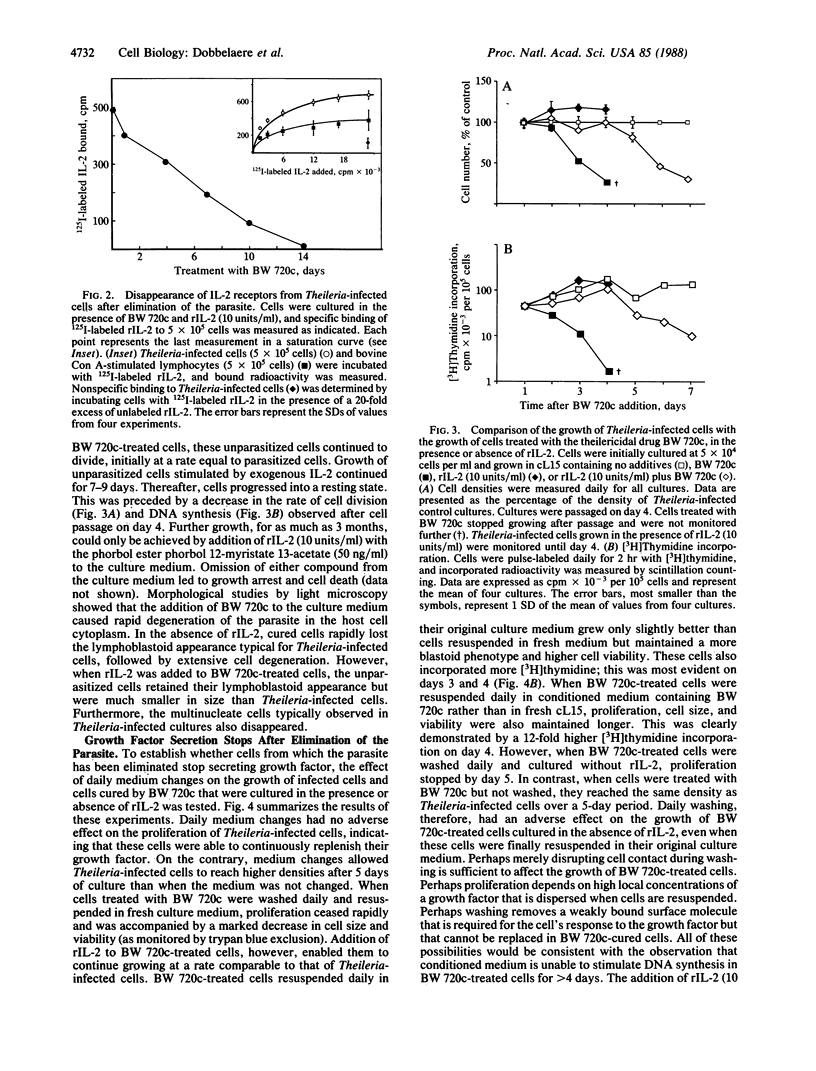
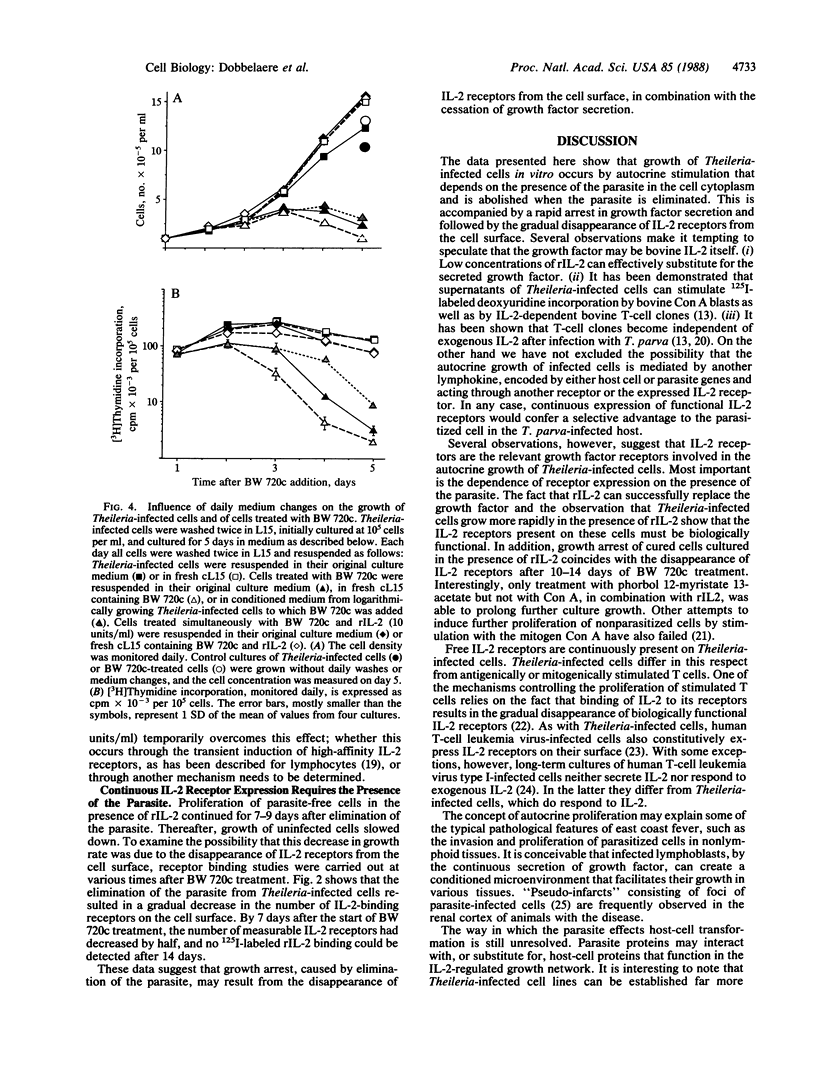
Bovine lymphocytes infected with the parasite Theileria parva continuously secrete a growth factor that is essential for their proliferation in vitro and also constitutively express interleukin 2 receptors on their surface. Dilution of the secreted growth factor, caused by culturing cells at low density, results in retardation of culture growth. Human recombinant interleukin 2, however, effectively substitutes for the diluted growth factor by restoring normal growth rates and also allows Theileria-infected cells to be grown at low density without the use of feeder layers. Secretion of the growth factor and expression of the interleukin 2 receptor depend on the presence of the parasite in the cytoplasm of the host cell. Elimination of the parasite from the cell cytoplasm by the specific antitheilerial drug BW 720c results in the arrest of growth factor secretion and the disappearance of interleukin 2 receptors from the cell surface. This is accompanied by growth arrest and reversion of the infected cells to the morphology of resting lymphocytes. We propose that the continuous proliferation of infected cells in vitro is mediated by autocrine receptor activation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arya S. K., Wong-Staal F., Gallo R. C. T-cell growth factor gene: lack of expression in human T-cell leukemia-lymphoma virus-infected cells. Science. 1984 Mar 9;223(4640):1086–1087. doi: 10.1126/science.6320374. [DOI] [PubMed] [Google Scholar]
- Biffin M. E., Brown D. J., Sugimoto T. Aza-analogues of pteridine. I. 1,2,4,6,8-penta-azanaphthalene and some methyl, dihydro-, and 5-alkoxy-derivatives. J Chem Soc Perkin 1. 1970;1:139–145. doi: 10.1039/j39700000139. [DOI] [PubMed] [Google Scholar]
- Brown C. G., Stagg D. A., Purnell R. E., Kanhai G. K., Payne R. C. Letter: Infection and transformation of bovine lymphoid cells in vitro by infective particles of Theileria parva. Nature. 1973 Sep 14;245(5420):101–103. doi: 10.1038/245101a0. [DOI] [PubMed] [Google Scholar]
- Brown W. C., Logan K. S. Bovine T-cell clones infected with Theileria parva produce a factor with IL 2-like activity. Parasite Immunol. 1986 Mar;8(2):189–192. doi: 10.1111/j.1365-3024.1986.tb00844.x. [DOI] [PubMed] [Google Scholar]
- Chen S. J., Holbrook N. J., Mitchell K. F., Vallone C. A., Greengard J. S., Crabtree G. R., Lin Y. A viral long terminal repeat in the interleukin 2 gene of a cell line that constitutively produces interleukin 2. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7284–7288. doi: 10.1073/pnas.82.21.7284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett D. W., Doxsey S., Stagg D. A., Young A. S. The entry of sporozoites of Theileria parva into bovine lymphocytes in vitro. Electron microscopic observations. Eur J Cell Biol. 1982 Apr;27(1):10–21. [PubMed] [Google Scholar]
- Fawcett D. W., Stagg D. A. Passive endocytosis of sporozoites of Theileria parva in macrophages at 1-2 degrees C. J Submicrosc Cytol. 1986 Jan;18(1):11–19. [PubMed] [Google Scholar]
- Fawcett D., Musoke A., Voigt W. Interaction of sporozoites of Theileria parva with bovine lymphocytes in vitro. I. Early events after invasion. Tissue Cell. 1984;16(6):873–884. doi: 10.1016/0040-8166(84)90068-5. [DOI] [PubMed] [Google Scholar]
- Goddeeris B. M., Morrison W. I., Teale A. J., Bensaid A., Baldwin C. L. Bovine cytotoxic T-cell clones specific for cells infected with the protozoan parasite Theileria parva: parasite strain specificity and class I major histocompatibility complex restriction. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5238–5242. doi: 10.1073/pnas.83.14.5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson A. T., Randall A. W., Fry M., Ginger C. D., Hill B., Latter V. S., McHardy N., Williams R. B. Novel anti-malarial hydroxynaphthoquinones with potent broad spectrum anti-protozoal activity. Parasitology. 1985 Feb;90(Pt 1):45–55. doi: 10.1017/s0031182000049003. [DOI] [PubMed] [Google Scholar]
- Irvin A. D., Brown C. G., Kanhai G. K., Stagg D. A. Comparative growth of bovine lymphosarcoma cells and lymphoid cells infected with Theileria parva in athymic (nude) mice. Nature. 1975 Jun 26;255(5511):713–714. doi: 10.1038/255713a0. [DOI] [PubMed] [Google Scholar]
- Irvin A. D., Mwamachi D. M. Clinical and diagnostic features of East Coast fever (Theileria parva) infection of cattle. Vet Rec. 1983 Aug 27;113(9):192–198. doi: 10.1136/vr.113.9.192. [DOI] [PubMed] [Google Scholar]
- Irvin A. D., Ocama J. G., Spooner P. R. Cycle of bovine lymphoblastoid cells parasitised by Theileria parva. Res Vet Sci. 1982 Nov;33(3):298–304. [PubMed] [Google Scholar]
- Lang R. A., Metcalf D., Gough N. M., Dunn A. R., Gonda T. J. Expression of a hemopoietic growth factor cDNA in a factor-dependent cell line results in autonomous growth and tumorigenicity. Cell. 1985 Dec;43(2 Pt 1):531–542. doi: 10.1016/0092-8674(85)90182-5. [DOI] [PubMed] [Google Scholar]
- Mastro A. M., Pepin K. G. Suppression of lectin-stimulated DNA synthesis in bovine lymphocytes by the tumor promoter 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 1980 Sep;40(9):3307–3312. [PubMed] [Google Scholar]
- Morrison W. I., Buscher G., Murray M., Emery D. L., Masake R. A., Cook R. H., Wells P. W. Theileria parva: kinetics of infection in the lymphoid system of cattle. Exp Parasitol. 1981 Oct;52(2):248–260. doi: 10.1016/0014-4894(81)90080-1. [DOI] [PubMed] [Google Scholar]
- Newson J., Naessens J., Stagg D. A., Black S. J. A cell surface antigen associated with Theileria parva lawrencei-infected bovine lymphoid cells. Parasite Immunol. 1986 Mar;8(2):149–158. doi: 10.1111/j.1365-3024.1986.tb00841.x. [DOI] [PubMed] [Google Scholar]
- Pinder M., Kar S., Withey K. S., Lundin L. B., Roelants G. E. Proliferation and lymphocyte stimulatory capacity of Theileria-infected lymphoblastoid cells before and after the elimination of intracellular parasites. Immunology. 1981 Sep;44(1):51–60. [PMC free article] [PubMed] [Google Scholar]
- Reem G. H., Yeh N. H., Urdal D. L., Kilian P. L., Farrar J. J. Induction and upregulation by interleukin 2 of high-affinity interleukin 2 receptors on thymocytes and T cells. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8663–8666. doi: 10.1073/pnas.82.24.8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb R. J., Rusk C. M., Yodoi J., Greene W. C. Interleukin 2 binding molecule distinct from the Tac protein: analysis of its role in formation of high-affinity receptors. Proc Natl Acad Sci U S A. 1987 Apr;84(7):2002–2006. doi: 10.1073/pnas.84.7.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. A., Cantrell D. A. Interleukin 2 regulates its own receptors. Proc Natl Acad Sci U S A. 1985 Feb;82(3):864–868. doi: 10.1073/pnas.82.3.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg D. A., Dolan T. T., Leitch B. L., Young A. S. The initial stages of infection of cattle cells with Theileria parva sporozoites in vitro. Parasitology. 1981 Aug;83(Pt 1):191–197. doi: 10.1017/s0031182000050150. [DOI] [PubMed] [Google Scholar]
- Uchiyama T., Hori T., Tsudo M., Wano Y., Umadome H., Tamori S., Yodoi J., Maeda M., Sawami H., Uchino H. Interleukin-2 receptor (Tac antigen) expressed on adult T cell leukemia cells. J Clin Invest. 1985 Aug;76(2):446–453. doi: 10.1172/JCI111992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster P., Dobbelaere D. A., Fawcett D. W. The entry of sporozoites of Theileria parva into bovine lymphocytes in vitro. Immunoelectron microscopic observations. Eur J Cell Biol. 1985 Mar;36(2):157–162. [PubMed] [Google Scholar]