Summary
Direction-selective neurons, which respond selectively to motion in one direction, have been characterized in visual circuits across many species. Recently, the development of these directional neurons has been explored in both retina and primary visual cortex. The development of direction-selective cells in primary visual cortex requires visual experience. In contrast, direction-selective ganglion cells in retina are present at the age of the earliest light responses. The vision-independent signals guiding the asymmetric wiring underlying retinal direction selectivity remain unknown. The details of how retinal and cortical circuits extract motion information could explain their differing requirements for visual experience in development.
Introduction
Direction selectivity is a basic feature of visual systems that has fascinated neuroscientists for over 100 years [1]. Direction selective neurons give larger responses to motion in the preferred direction versus motion in the opposite, null direction. David Hubel first recorded the direction selective responses in primary visual cortex in the awake cat [2]. Soon after, Barlow and Levick characterized direction selective retinal ganglion cells (DSGCs) in the rabbit [3]. Directional preference implies an asymmetry in wiring, however this asymmetry manifests itself differently in retina versus cortex. While retinal direction selectivity relies heavily upon inhibition induced by motion in the null direction, cortical direction selectivity relies more upon the timing of excitatory and inhibitory inputs stimulated by motion in the preferred direction.
Here we compare the results of recent experiments that reveal a fundamental difference in the establishment of directional circuits in retina and visual cortex. Namely, retinal direction selectivity is established at eye-opening independent of visual experience, while direction selectivity in primary visual cortex (V1) requires visual experience during a critical period around the time of eye-opening. Though direction-selective responses have been found in many other locations throughout the nervous system, including subcortical visual pathways as well as high order cortical areas, we restrict our discussion to direction-selective responses in retina and primary visual cortex.
General circuit organization of direction-selective responses in retina and primary visual cortex
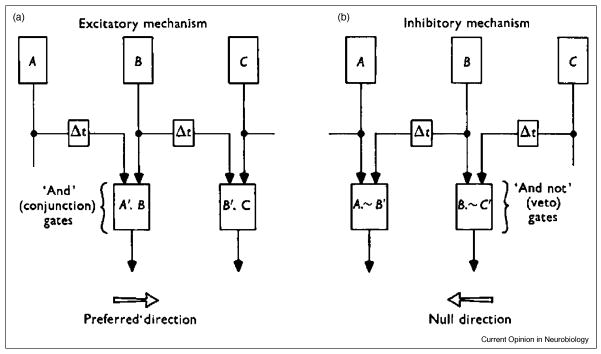
Models have long been used to guide and interpret the physiological studies exploring the underpinnings of direction selectivity [4]. The popular “Reichardt detector” exemplifies a correlation model of motion detection, in which a temporal correlation is computed to obtain the motion signal from the outputs of two spatially offset luminance receptors activated sequentially by a moving stimulus [5]. To compare the circuits that mediate direction selectivity in visual cortex and retina, here we present two other manifestations of the correlation model originally described by Barlow and Levick (Figure 1). A directional response is obtained by either facilitating the response to preferred motion and/or inhibiting the response to the null motion. In the Excitatory (Facilitation) model (Fig 1A), the outputs of two spatially offset receptors facilitate each other during preferred motion to produce a response that is greater than the linear sum of the responses generated by each receptor separately. In the Inhibitory model (Fig 1B), null motion elicits delayed inhibition that “vetoes” the excitatory inputs from subsequent receptors [6].
Figure 1. Two ways of implementing the correlation model of direction selectivity postulated by Barlow and Levick.
A The Excitatory (or Facilitation) model hypothesizes that the preferred motion elicits excitatory responses from receptor A whose delayed output combines with the excitatory output from B (via an ‘And’ conjunction gate) to produce a response that is greater than expected from the linear sum of the responses generated by the individual receptor inputs. B: The Inhibitory model relies upon delayed inhibition arising from null direction motion to “veto” the excitatory responses of subsequent receptors. Using apparent motion stimuli consisting of two bars flashed with a time delay in adjacent spatial locations, Barlow & Levick found that the apparent motion in the null direction strongly suppressed the response to the second bar. While they also detected facilitation in the preferred direction, the strong null inhibition led Barlow and Levick to favor the Inhibitory model as the primary source of directional responses in the retina.
The retina contains several types of direction-selective ganglion cells (DSGCs) that fall into three categories: On-DSGCs, which mediate the optokinetic reflex, Off-DSGCs, a recently described cell type with asymmetric dendrites, and the On-Off DSGCs originally described by Barlow and Levick [7,8]. We will limit our discussion to the On-Off DSGCs because they are the most well-studied. On-Off DSGCs have four subtypes, each preferring motion in one of the four cardinal directions (posterior, anterior, superior, inferior) that may map onto the four rectus muscles of the eye [9]. Based on their initial observations, Barlow and Levick concluded that the Inhibitory model was the dominant component of retinal direction selectivity. They predicted that the “symmetry-breaking” component of the circuit that provides null direction inhibition was based on asymmetric inputs from inhibitory interneurons onto excitatory cells. Indeed, several studies have identified a particular interneuron, the starburst amacrine cell (SBAC), as the inhibitory cell providing null-side inhibition to direction-selective ganglion cells [10,11]; for reviews see [12–14]. A surprising recent result revealed that SBACs themselves have an asymmetric response to motion, with motion away from their somas causing a larger calcium transient at their distal release sites than motion moving toward the soma [15]. This has led to the hypothesis that individual processes of SBACs, functioning as independent computational structures, selectively contact DSGC subtypes tuned to different directions of motion. Under this hypothesis, any model for the development of direction-selective circuits in the retina should include selective wiring of SBAC processes onto DSGCs of different preferred directions.
Direction-selective cells in V1 have been explained both with the Excitatory and Inhibitory correlation models described in Figure 1 [16]. V1 direction-selective neurons are organized into subcolumns within an iso-orientation column, with each subcolumn preferring motion in a different direction. Within a cortical column, V1 neurons show varying directional tuning ranging from a slight directional bias to a strong bias similar to that seen in the retina. This range of tuning may reflect a diverse set of mechanisms for generating directional responses in simple and complex cells [17].
In addition to the models above, direction-selective cells in V1 are often described using “Motion energy models” [18]. These models, which are formally equivalent to the correlational models [18,19], square and sum the outputs of non-directional receptive fields to create a directional receptive field [18,20,21]. These models formalize Hubel and Weisel’s intuition that cortical direction selectivity can arise from appropriate pooling of non-directional receptive fields [22]. Unlike retinal DSGCs, which receive asymmetric inhibition, some directional cells in V1 selectively gather excitatory inputs from non-directional neurons in cortex and/or lateral geniculate nucleus (LGN) to create directional preferences [23]. In fact, whole cell recordings from direction-selective cortical simple cells reveal that maximal inhibitory currents are elicited by the preferred direction of motion, though they lagged relative to the excitatory inputs [24]. These findings indicate that a model based on null-side inhibition is not likely to apply to this subset of V1 directional cells, and a facilitory model based on nonlinear summation of either geniculocortical inputs and/or intracortical circuitry is more appropriate.
Development of directional responses in retina occurs independent of vision
How does the selective wiring that underlies direction selectivity in the retina wire up during development? Three recent studies indicate that this process happens prior to eye-opening and independent of vision. (Similar results were found for On DSGCs [25]). Using either cell-attached recordings from single cells [26] or large-scale multi-electrode recordings from populations of DSGCs [27], robust directional responses were detected before and around the time eye opening in mice reared under control conditions or in the dark. At this young age, neither the glutamatergic inputs [28] nor the intrinsic excitability of retinal ganglion cells [26,29,30] are fully mature, and hence the firing rate in the preferred direction is lower than in adult animals. In contrast, the firing rate in the null direction is as low as in adult, indicating that the circuits mediating null-side inhibition are established early in development prior to the maturation of excitatory pathways. Similar results were found in rabbit retina, where DSGCs were detected at eye-opening and the strength of directional tuning was not altered by dark-rearing, although light deprivation did influence the development of other forms of inhibition [31].
The results above agree with an earlier study which attempted to bias the distribution of preferred directions for On-Off DSGCs in the rabbit retina [32]. Dark-reared rabbits were head-fixed daily from eye-opening to adulthood in a drum with vertical stripes rotating in one direction. The experimenters saw no change in the distribution of DSGC preferred directions in the adult rabbits, suggesting the training had little effect on the development of the DSGCs. Together, these results show that retinal direction selectivity develops independent of visual experience.
Development of directional responses in cortex depends on vision
In sharp contrast to the development of direction selectivity in the retina, direction selectivity in V1 does not emerge until several days after eye-opening [33]. Dark rearing ferrets from one week before until one to seven weeks after eye-opening prevented the formation of direction-selective responses. Visual experience provided later in development did not rescue direction selectivity, demonstrating that the establishment of direction selectivity in V1 requires visual experience during a critical period around eye-opening. This is in contrast to other neuronal response properties in primary visual cortex, such as orientation tuning and ocular dominance, which are detectable at eye-opening in ferret.
Recently, strong evidence has been provided that visual experience does not merely play a permissive role, but is a driving force behind the formation of direction selectivity in V1 [34]. Naïve ferrets, whose eyes had been open for less than 48 hours, were shown orientated gratings that moved back and forth along one axis. Remarkably, direction-selective responses could be detected after as few as 6 hours of training using calcium imaging to monitor responses in individual cortical neurons. In as little as 10 hours, direction-selective maps were induced as assayed by intrinsic signal imaging, a technique sensitive to changes in activity in contiguous neuronal populations with similar response properties. In the course of normal development, direction-selective maps would have become detectable 2–3 days later [33]. The rapid acquisition of direction-selective responses induced by the directional visual stimulus strongly suggests that vision plays an instructive role during normal development [35], possibly through mechanisms such as spike-timing dependent plasticity [36] (see below).
Proposed models for the development of direction selectivity
Why is the establishment of direction selectivity in V1 more sensitive to visual stimulation than the establishment of direction selectivity in retina? Here we explore the possibility that the answer lies in the details of the circuitry underlying direction selectivity in these two different parts of the visual system.
First consider the retina, where the computation of direction selectivity relies upon strong inhibition of inputs triggered by motion in the null direction. The timing of this inhibition is not critical so long as its duration is sufficient to suppress the subsequent excitatory inputs [3,37]. Wiring up null-side inhibition requires matching SBAC processes, which act as independent motion detectors, with DSGCs cells of appropriate directional preference. There are several scenarios under which this precise wiring can occur. In one scenario, molecular markers specific to each DSGC subtype ensure that DSGCs form synapses only with appropriately oriented SBAC processes. A second scenario is that there is an initial bias in the wiring of directional circuits that is reinforced by strengthening of appropriate synapses. In a third scenario, there is initially a uniform distribution of inhibitory synapses that are later pruned into directional circuits by eliminating synapses formed with inappropriate SBAC processes.
Given the current data, any of the above three scenarios are possible. All three rely upon the idea that there is a molecular signature that matches the four quadrants of SBAC processes with the four subtypes of DSGCs. Though there is recent evidence that other types of DSGCs have different molecular signatures [8,25,38], it remains unknown whether a single SBAC expresses four different markers on its processes as predicted by the model.
In the second and third scenarios, where synaptic strengthening or pruning is involved, a critical role is likely to be played by neural activity. Since retinal direction selectivity is established early in development, independent of visually driven activity, an intriguing possibility is that it relies on spontaneous activity present in the immature retina. This early patterned activity, termed retinal waves, can serve as a directional source of activity prior to the maturation of vision. Retinal waves provide important cues for the establishment of normal retinotopic and eye-specific maps of retinal projections [39]. Do retinal waves play a role in the establishment of DSGCs? During the first ten days after birth, retinal waves in mouse retina are mediated by a temporary cholinergic circuit comprised of retinal ganglion cells and SBACs, later replaced by a glutamatergic wave generation mechanism [40,41]. Using a mouse model with disrupted cholinergic waves (β2-nAChR-KO), we found DSGCs with robust tuning present at eye-opening [27]. However, recent studies indicate that under different physiological conditions, β2-nAChR-KO mice can exhibit non-cholinergic propagating waves [30], which, if present in vivo, may compensate for the absence of normal wave activity. In addition, glutamatergic waves appear 2–3 days earlier in β2-nAChR-KO mice compared to wild type mice. Therefore additional experiments are required to determine whether correlated spontaneous activity of the developing retina plays a role in the establishment of directional circuits.
In contrast to retinal DSGCs, directional responses in some cortical cells arise primarily from facilitating the excitatory inputs triggered by preferred motion, a nonlinear summation process that relies on the precise timing of those inputs [20]. White and colleagues [42] have postulated that development of directional responses in V1 requires two components: (1) maturation of receptive fields in the lateral geniculate nucleus (LGN), to ensure the precise timing of visual inputs; and (2) modification of synaptic strengths in cortex via an activity-dependent plasticity mechanism. The reliance upon retinotopic tuning of mature LGN receptive fields is consistent with the observations that dark rearing prevents the formation of robust center-surround receptive fields in LGN [43,44]. The plasticity mechanism required for the modification of synaptic strengths in V1 may come in the form of spike-timing dependent plasticity (STDP) [36]. STDP relies upon correlated firing of inputs, but more importantly, it relies upon the order of the activation of those inputs, and therefore provides a potential mechanism for encoding the timing of inputs induced by motion [45]. Indeed, STDP has been implicated in the establishment of direction responses induced in subcortical structures [46] by visual training. The lack of a detailed understanding of the cortical circuitry mediating direction-selectivity prevents more specific model predictions.
While our understanding of the circuitry behind direction selectivity has greatly improved since the earliest reports over 50 years ago, our journey in solving the developmental questions surrounding this remarkable feature of visual circuits is just beginning. Given the pace of recent discoveries, we may not have to wait another 50 years for the answers.
Acknowledgments
We wish to thank Dr. Anastacia Anishchenko and Dr. Yang Dan for critical feedback on the manuscript.
Abbreviations
- DSGC
direction selective ganglion cell
- RGC
retinal ganglion cell
- V1
primary visual cortex
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Exner S. I Teil. Leipzig; Deuticke: 1894. Entwurf zu Einer Physiologischen Erklärung der Psychischen Erscheinungen. [Google Scholar]
- 2.Hubel DH. Single unit activity in striate cortex of unrestrained cats. J Physiol (Lond) 1959;147:226–238. doi: 10.1113/jphysiol.1959.sp006238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barlow HB, Levick WR. The mechanism of directionally selective units in rabbit’s retina. J Physiol (Lond) 1965;178:477–504. doi: 10.1113/jphysiol.1965.sp007638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clifford CW, Ibbotson MR. Fundamental mechanisms of visual motion detection: models, cells and functions. Prog Neurobiol. 2002;68:409–437. doi: 10.1016/s0301-0082(02)00154-5. [DOI] [PubMed] [Google Scholar]
- 5.Reichardt W. Autocorrelation, a principle for the evaluation of sensory information by the central nervous system. In: Rosenblith W, editor. Sensory Communication. Wiley; 1961. pp. 303–317. [Google Scholar]
- 6.Torre V, Poggio T. A Synaptic Mechanism Possibly Underlying Directional Selectivity to Motion. Proceedings of the Royal Society of London. 1978 Series B. [Google Scholar]
- 7.Vaney DI, He S, Taylor R, Levick W. Direction-Selective Ganglion Cells in the Retina. In: Zanker JM, Zeil J, editors. Motion Vision: Computational, Neural, and Ecological Constraints. Springer; 2000. pp. 13–56. [Google Scholar]
- 8**.Kim I, Zhang Y, Yamagata M, Meister M, Sanes J. Molecular identification of a retinal cell type that responds to upward motion. Nature. 2008;452:478–482. doi: 10.1038/nature06739. A novel DSGC was identified using a transgenic mouse expressing GFP. The new DSGC had asymmetric dendrites, indicting a different model of computing direction selectivity in the retina independent of SBACs. It would be interesting to test whether these DSGCs acquire directionality using a facilitory model instead of relying upon null-side inhibition. [DOI] [PubMed] [Google Scholar]
- 9.Oyster CW, Barlow HB. Direction-selective units in rabbit retina: distribution of preferred directions. Science. 1967;155:841–842. doi: 10.1126/science.155.3764.841. [DOI] [PubMed] [Google Scholar]
- 10.Fried S, Münch T, Werblin F. Mechanisms and circuitry underlying directional selectivity in the retina. Nature. 2002;420:411–414. doi: 10.1038/nature01179. [DOI] [PubMed] [Google Scholar]
- 11.Fried SI, Munch TA, Werblin FS. Directional Selectivity Is Formed at Multiple Levels by Laterally Offset Inhibition in the Rabbit Retina. Neuron. 2005;46 :117–127. doi: 10.1016/j.neuron.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Zhou ZJ, Lee S. Synaptic physiology of direction selectivity in the retina. The Journal of Physiology. 2008;586:4371–4376. doi: 10.1113/jphysiol.2008.159020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fried SI, Masland RH. Image Processing: How the Retina Detects the Direction of Image Motion. Current Biology. 2007;17:R63–R66. doi: 10.1016/j.cub.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 14*.Demb JB. Cellular Mechanisms for Direction Selectivity in the Retina. Neuron. 2007;55:179–186. doi: 10.1016/j.neuron.2007.07.001. This is an excellent short review of the mechanisms underlying retinal direction selectivity. It incorporates many recent (and sometimes contradictory) findings about the role of starburst amacrine cells in generating directional responses. [DOI] [PubMed] [Google Scholar]
- 15.Euler T, Detwiler PB, Denk W. Directionally selective calcium signals in dendrites of starburst amacrine cells. Nature. 2002;418:845–852. doi: 10.1038/nature00931. [DOI] [PubMed] [Google Scholar]
- 16.Livingstone MS. Mechanisms of direction selectivity in macaque V1. Neuron. 1998;20:509–526. doi: 10.1016/s0896-6273(00)80991-5. [DOI] [PubMed] [Google Scholar]
- 17.Rust NC, Schwartz O, Movshon JA, Simoncelli E. Spatiotemporal elements of macaque v1 receptive fields. Neuron. 2005;46:945–956. doi: 10.1016/j.neuron.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 18.Adelson EH, Bergen JR. Spatiotemporal energy models for the perception of motion. Journal of the Optical Society of America A, Optics and image science. 1985;2:284–299. doi: 10.1364/josaa.2.000284. [DOI] [PubMed] [Google Scholar]
- 19.van Santen JP, Sperling G. Elaborated Reichardt detectors. Journal of the Optical Society of America A, Optics and image science. 1985;2:300–321. doi: 10.1364/josaa.2.000300. [DOI] [PubMed] [Google Scholar]
- 20.DeAngelis GC, Ohzawa I, Freeman RD. Receptive-field dynamics in the central visual pathways. Trends Neurosci. 1995;18:451–458. doi: 10.1016/0166-2236(95)94496-r. [DOI] [PubMed] [Google Scholar]
- 21.Livingstone MS. Directional inhibition: a new slant on an old question. Neuron. 2005;45:5–7. doi: 10.1016/j.neuron.2004.12.029. [DOI] [PubMed] [Google Scholar]
- 22.Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat’s visual cortex. J Physiol (Lond) 1962;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peterson MR, Li B, Freeman RD. The derivation of direction selectivity in the striate cortex. J Neurosci. 2004;24:3583–3591. doi: 10.1523/JNEUROSCI.5398-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Priebe NJ, Ferster D. Direction selectivity of excitation and inhibition in simple cells of the cat primary visual cortex. Neuron. 2005;45:133–145. doi: 10.1016/j.neuron.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 25*.Yonehara K, Ishikane H, Sakuta H, Shintani T, Nakamura-Yonehara K, Kamiji N, Usui S, Noda M, Iwaniuk A. Identification of Retinal Ganglion Cells and Their Projections Involved in Central Transmission of Information about Upward and Downward Image Motion. PLoS ONE. 2009;4:e4320. doi: 10.1371/journal.pone.0004320. A recent pair of studies, this one and [38], has characterized a transgenic mouse in which a subtype of On DSGCs, those preferring upward motion, expresses GFP. These results suggest there may be a molecular signature for different classes of DSGCs. In agreement with the dark rearing results for On-Off DSGCs, the authors also found that the development of the On DSGCs does not depend upon visual experience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26*.Chen M, Weng S, Deng Q, Xu Z, He S. Physiological properties of direction-selective ganglion cells in early postnatal and adult mouse retina. J Physiol (Lond) 2008 doi: 10.1113/jphysiol.2008.161240. This paper demonstrated that mice have robust directional tuning at the earliest retinal light responses, three days before eye-opening. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27**.Elstrott J, Anishchenko A, Greschner M, Sher A, Litke AM, Chichilnisky EJ, Feller MB. Direction selectivity in the retina is established independent of visual experience and cholinergic retinal waves. Neuron. 2008;58:499–506. doi: 10.1016/j.neuron.2008.03.013. This study utilized a large-scale multielectrode array to establish that two key aspects of retinal direction selectivity, robust null-side inhibition and 4 cardinal directions, are present at eye-opening independent of visual experience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tian N, Copenhagen DR. Visual deprivation alters development of synaptic function in inner retina after eye opening. Neuron. 2001;32:439–449. doi: 10.1016/s0896-6273(01)00470-6. [DOI] [PubMed] [Google Scholar]
- 29.Qu J, Myhr KL. The development of intrinsic excitability in mouse retinal ganglion cells. Devel Neurobio. 2008;68:1196–1212. doi: 10.1002/dneu.20653. [DOI] [PubMed] [Google Scholar]
- 30.Sun C, Warland DK, Ballesteros JM, van der List D, Chalupa LM. Retinal waves in mice lacking the beta2 subunit of the nicotinic acetylcholine receptor. Proc Natl Acad Sci USA. 2008;105:13638–13643. doi: 10.1073/pnas.0807178105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31*.Chan YC, Chiao CC. Effect of visual experience on the maturation of ON–OFF direction selective ganglion cells in the rabbit retina. Vision Res. 2008;48:2466–2475. doi: 10.1016/j.visres.2008.08.010. This is a thorough study on the development of On-Off DSGCs in rabbit retina. The authors showed that dark-rearing has no effect on the dendritic morphology, velocity-tuning, or directional tuning of DSGCs. [DOI] [PubMed] [Google Scholar]
- 32.Daw NW, Wyatt HJ. Raising rabbits in a moving visual environment: an attempt to modify directional sensitivity in the retina. The Journal of Physiology. 1974;240:309–330. doi: 10.1113/jphysiol.1974.sp010612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y, Fitzpatrick D, White LE. The development of direction selectivity in ferret visual cortex requires early visual experience. Nat Neurosci. 2006;9:676–681. doi: 10.1038/nn1684. [DOI] [PubMed] [Google Scholar]
- 34**.Li Y, Van Hooser S, Mazurek M, White LE, Fitzpatrick D. Experience with moving visual stimuli drives the early development of cortical direction selectivity. Nature. 2008;456:952–956. doi: 10.1038/nature07417. Several aspects of the role of vision in the establishment of direction selectivity in V1 were studied using both intrinsic signal imaging and calcium imaging. In addition to the results described in the text, the authors also found that the preferred direction of a direction-selective cell changed to match the preferred direction of its neighbor. This “local coherence” implies that local cortical interactions influence the final preferred direction of developing direction-selective cortical neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crair MC. Neuronal activity during development: permissive or instructive? Current Opinion in Neurobiology. 1999;9:88–93. doi: 10.1016/s0959-4388(99)80011-7. [DOI] [PubMed] [Google Scholar]
- 36.Caporale N, Dan Y. Spike timing-dependent plasticity: a hebbian learning rule. Annual Review of Neuroscience. 2008;31:25–46. doi: 10.1146/annurev.neuro.31.060407.125639. [DOI] [PubMed] [Google Scholar]
- 37.Taylor WR, Vaney DI. Diverse synaptic mechanisms generate direction selectivity in the rabbit retina. J Neurosci. 2002;22:7712–7720. doi: 10.1523/JNEUROSCI.22-17-07712.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yonehara K, Shintani T, Suzuki R, Sakuta H, Takeuchi Y, Nakamura-Yonehara K, Noda M. Expression of SPIG1 Reveals Development of a Retinal Ganglion Cell Subtype Projecting to the Medial Terminal Nucleus in the Mouse. PLoS ONE. 2008;3:e1533. doi: 10.1371/journal.pone.0001533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huberman AD, Feller MB, Chapman B. Mechanisms underlying development of visual maps and receptive fields. Annual Review of Neuroscience. 2008;31:479–509. doi: 10.1146/annurev.neuro.31.060407.125533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Firth SI, Wang CT, Feller MB. Retinal waves: mechanisms and function in visual system development. Cell Calcium. 2005;37:425–432. doi: 10.1016/j.ceca.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 41.Zheng J, Lee S, Zhou ZJ. A transient network of intrinsically bursting starburst cells underlies the generation of retinal waves. Nat Neurosci. 2006;9:363–371. doi: 10.1038/nn1644. [DOI] [PubMed] [Google Scholar]
- 42*.White LE, Fitzpatrick D. Vision and cortical map development. Neuron. 2007;56:327–338. doi: 10.1016/j.neuron.2007.10.011. A review of the role of vision in the establishment of cortical maps, highlighting the differences between direction selectivity and other features such as orientation and ocular dominance. The authors point out the limitations of the now popular model which states that the initial establishment of maps occurs independent of vision while the subsequent maintenance of these maps is vision-dependent. [DOI] [PubMed] [Google Scholar]
- 43.Tavazoie SF, Reid RC. Diverse receptive fields in the lateral geniculate nucleus during thalamocortical development. Nat Neurosci. 2000 doi: 10.1038/75786. [DOI] [PubMed] [Google Scholar]
- 44.Hooks BM, Chen C. Critical Periods in the Visual System: Changing Views for a Model of Experience-Dependent Plasticity. Neuron. 2007 doi: 10.1016/j.neuron.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 45.Mehta MR, Lee AK, Wilson MA. Role of experience and oscillations in transforming a rate code into a temporal code. Nature. 2002;417:741–746. doi: 10.1038/nature00807. [DOI] [PubMed] [Google Scholar]
- 46.Engert F, Tao HW, Zhang LI, Poo MM. Moving visual stimuli rapidly induce direction sensitivity of developing tectal neurons. Nature. 2002;419:470–475. doi: 10.1038/nature00988. [DOI] [PubMed] [Google Scholar]