Abstract
Caloric restriction (CR) is a daily reduction of total caloric intake without a decrease in micronutrients or disproportionate reduction of any one dietary component. CR can increase lifespan reliably in a wide range of species and appears to counteract some aspects of the aging process throughout the body. The effects on the brain are less clear, but moderate CR seems to attenuate age-related cognitive decline. Thus, we determined the effects of age and CR on key synaptic proteins in the CA3 region of the hippocampus and whether these changes were correlated with differences in behavior on a hippocampal-dependent learning and memory task. We observed an overall, age-related decline in the NR1, N2A and N2B subunits of the N-methyl-D-aspartate (NMDA)-type and the GluR1 and GluR2 subunits of the alpha-amino-3-hydroxy-5-methyl-4-isoxazole proprionic acid (AMPA)-type ionotropic glutamate receptors. Interestingly, we found that CR initially lowers the glutamate receptor subunit levels as compared to young AL animals, and then stabilizes the levels across lifespan. Synaptophysin, a presynaptic vesicle protein, showed a similar pattern. We also found that both CR and ad libitum (AL) fed animals exhibited age-related cognitive decline on the Morris water maze task. However, AL animals declined between young and middle-age, and between middle-age and old, whereas CR rats only declined between young and middle-age. Thus, the decrease in key synaptic proteins in CA3 and cognitive decline occurring across lifespan are stabilized by CR. This age-related decrease and CR-induced stabilization are likely to affect CA3 synaptic plasticity and, as a result, hippocampal function.
Keywords: Dietary Restriction, NMDA, AMPA, synapses, rat, Morris water maze
Introduction
A subset of age-related memory impairments is associated with compromised hippocampal function. Previously, it was thought that these behavioral changes were accompanied by hippocampal cell loss. However, current data demonstrate that neither significant neuron death nor synaptic loss occurs in the aged hippocampus, suggesting more subtle changes within synapses or neural circuits (West et al., 1991; Rapp and Gallagher, 1996; Rasmussen et al., 1996; Calhoun et al., 1998; Ganeshina et al., 2004). Evidence from genetic and pharmacological studies have demonstrated that both NMDA-type and AMPA-type ionotropic glutamate receptors are involved in hippocampal-dependent learning and memory (Kullmann et al., 2000; Riedel et al., 2003). Moreover, it has been proposed not only that alterations in hippocampal glutamate receptor levels contribute to the age-related cognitive decline, but also that these changes occur within specific regions of the hippocampus (Clark et al., 1992; Sonntag et al., 2000; Magnusson, 2000; Eckles-Smith et al., 2000; Adams et al., 2001; Clayton and Browning, 2001; Clayton et al., 2002). For example, the CA3 region of the hippocampus receives a convergence of excitatory input (Amaral and Witter, 1995) and is critically involved in spatial learning tasks (Jarrard, 1983; Stubley-Weatherly et al., 1996). Importantly, recent evidence indicates that spatial learning involving the CA3 region is dependent, at least in part, on NMDA receptors (Adams et al., 2001; Nakazawa et al., 2002; Nakazawa et al., 2003).
Caloric restriction (CR) is one method to effectively counteract the aging process throughout the body (Nicolas et al., 1999). The effects on the brain are less clear, but lifelong CR has been shown to attenuate age-related deficits on memory tasks that are hippocampal-dependent in some rodent strains, including Fischer 344 X Brown Norway (F344XBN) (Goodrick et al., 1983; Ingram et al., 1987; Idrobo et al., 1987; Stewart et al., 1989; Pitsikas et al., 1990; Pitsikas and Algeri, 1992; Markowska and Savonenko, 2002). The present study was designed to explore the possible contribution of age and CR-related alterations of NMDA and AMPA subunits in the CA3 region of the hippocampus to this improvement in cognitive performance. Accordingly, we assessed the relative protein levels of NMDA-type and AMPA-type glutamate receptor subunits in the CA3 region of CR and ad libitum (AL) fed young, middle-aged, and old animals. Additionally, we examined levels of a pre-synaptic vesicle protein, synaptophysin, as an indication of changes in synapse number or structure. We observed significant age-related decreases in both AMPA and NMDA receptor subunits, as well as synaptophysin. Moreover, although CR initially causes a decrease in these proteins in young animals as compared to young AL rats, it appears to prevent subsequent decreases in these synaptic proteins. A second cohort of age-matched AL and CR rats was tested on the Morris water maze, a hippocampal-dependent test of spatial learning and memory. We found that both AL and CR animals exhibited age-related decline during the training phase of this task. However, old CR rats performed significantly better than age-matched AL animals.
Materials and methods
Experimental Subjects
A total of 136 F1 male F344xBN hybrid rats was used in the present study. Sixty-four rats were in the cohort used for biochemical analysis and seventy-two rats in the behavioral cohort. The F344xBN hybrid is a widely-used model for studies of age and CR (Mayhew et al., 1998; Shi et al., 2002; Ramsey et al., 2004; Newton et al., 2005; Shi et al., 2007) and demonstrates CR-mediated protection of learning and memory after middle age (Markowska and Savonenko, 2002). Young (10-12 months), middle-aged (18-20 months), and old (29-32 months) rats were obtained from the NIA Caloric Restriction Colony (Harlan Industries) and were maintained in our facility on a 12-hour light/dark cycle for two months prior to sacrifice. Rats were housed individually in order to monitor daily food intake. Adequate measures were taken to minimize pain or discomfort. The animal facility at the Wake Forest University School of Medicine is fully accredited by the American Association for Accreditation of Laboratory Animal Care and complies with all Public Health Service, National Institutes of Health and institutional policies and standards for laboratory animal care. All experiments were conducted in accordance with Guidelines for the Care and Use of Experimental Animals, using protocols approved by the Institutional Animal Care and Use Committee at the Wake Forest University School of Medicine.
Caloric Restriction
In the NIA CR Colony, rats were fed AL (NIH-31 diet) until 14 weeks of age. The CR regimen then was initiated by incremental caloric reduction of 10% per week over four weeks, reaching full 60% caloric restriction by week seventeen. The vitamin-fortified NIH-31 diet fed to CR rats provided 60% of the calories and the 100% of the vitamins consumed by AL rats. After arriving at our facility, both AL and CR rats were fed daily, shortly before dark cycle onset, and all rats had free access to water. Rats were weighed weekly, prior to feeding.
Tissue Collection
One cohort of rats was used for the biochemical experiments. Animals were anesthetized with sodium pentobarbital (200 mg/kg, i.p.) and decapitated. The brains were removed rapidly and chilled for 4 minutes in cold artificial cerebrospinal fluid (140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 24 mM dextrose, 10 mM HEPES, pH 7.4). The hippocampi were removed from the brain and dissected on ice. While viewing with a dissecting microscope, the right hippocampus was bisected and the dorsomedial half was divided into four slabs perpendicular to the long axis of the hippocampus. Using anatomical landmarks, each slab was dissected further into dentate gyrus, CA3 and CA1 subregions (Newton et al., 2005); dissected samples were weighed, frozen and stored at -80 °C. The samples were homogenized using ground glass dounces in 60 mM TrisCl, pH 6.8 containing 10% glycerol, 2% SDS, 2 mM EDTA ad protease inhibitor cocktail (P8340, 1:250, Sigma-Aldrich, Inc, St. Louis, MO) at a dilution of 50 μl. Homogenates were heated to 70°C, centrifuged at 4°C, ad supernatants collected. The soluble protein concentration of each supernatant was determined using a BCA protein assay (Pierce Technology, Rockford, IL) and the supernatants were stored at -80°C.
Western Blot
For NMDA and AMPA receptor subunit analysis, 7.5 μg of each homogenized sample were loaded into wells on the left half of a 15 well 7.5% BioRad gel in a cohort of six (one sample per condition) (Fig. 1) that was duplicated on the right half of the gel. For synaptophysin analysis, 5.0 μg of each homogenized sample were loaded in a similar manner using 10% BioRad gels. Samples were separated under reducing conditions using SDS-PAGE and then electrophorectically transferred onto Immobilon membranes using a BioRad Mini Transfer Cell. Following incubation in Sigma blocking solution (B6429, Sigma-Aldrich, Inc., St. Louis, MO), membranes were incubated in rabbit polyclonal antibodies directed against the NMDA receptors subunits, NR1 (0.6 μg/ml, Chemicon International, Temencula, CA, USA), NR2A (0.07 mg/ml, Chemicon), NR2B (0.07 μg/ml, Chemicon), and AMPA receptor subunits, GluR1 (0.01 μg/ml, Upstate, Lake Placid, NY) and GluR2 (0.25 μg/ml, Chemicon), and a mouse monoclonal antibody for synaptophysin (0.004 μg/ml, Synaptic Systems, Gottingen, Germany). A mouse monoclonal antibody to actin (0.002 μg/ml, Chemicon) was used to control for the possible confound of unequal protein loading. Peroxidase-conjugated donkey anti-rabbit IgG (10 ng/ml, Jackson ImmunoResarch Laboratories, Inc., West Grove, PA) and peroxidase-conjugated donkey anti-mouse IgG (10 ng/ml, Jackson) secondary antibodies were used with the SuperSignal West Pico Chemiluminescent Substrate (Pierce Technology, Rockford, IL) for detection. Membranes were exposed to Kodak Biomax Film and quantitated densitometrically with BioRad VersaDoc and Quantity One analysis software (BioRad Laboratories, Hercules, CA) in order to determine the relative amounts of each protein. Primary antibody and sample protein loading concentrations were optimized for linear detection for each antibody prior to experimental analysis. The Gaussian trace optical density of each band was normalized to the average density of all of the matching bands within each cohort to permit comparison among blots and experiments. A repeated measurement was excluded if it was greater than two standard deviations from the mean. Each blot was repeated at least once, thus generating a minimum of four acceptable measurements per antibody per sample.
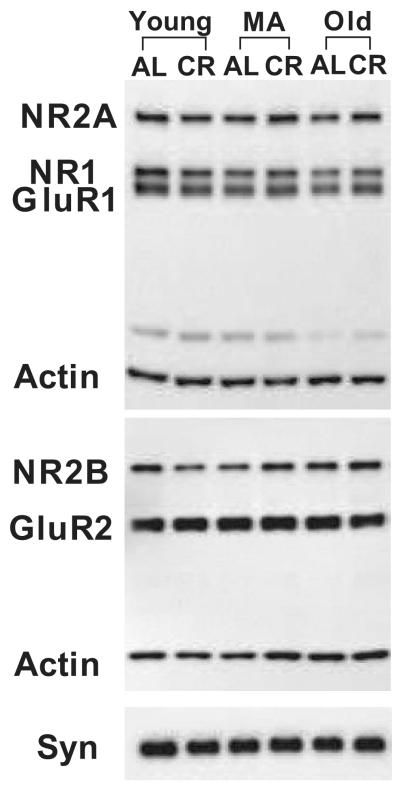
Figure 1. Representative Western Blots for Synaptic Proteins in the CA3 Region of Hippocampus.
Western blot analysis was performed to assess CA3 protein levels of the NMDA subunits NR2A, NR1, and NR2B; the AMPA subunits GluR1 and GluR2; and the essential synaptic protein, synaptophysin (Syn). Actin was assessed on each blot to insure equal protein loading. Experimental groups were young (10-12 months), middle-aged (MA; 18-20 months), and old (29-32 months) Fischer 344 × Brown Norway rats that were caloric restricted (CR) from 4 months of age or fed ad libitum (AL).
Morris Water Maze
A cohort of 72 rats was evaluated behaviorally on the Morris water maze (MWM) test of spatial learning (Morris, 1984). For this test, animals were placed in a white plastic tank (MacCourt Products, Denver, CO) (1.67 m in diameter and 0.76 m in height) containing an escape platform (Plexiglas, 10cm × 10 cm) 4 cm below the water surface. The tank was filled to a depth of 30 cm with water opacified with nontoxic, white powder tempura paint (Palmer Pain Products, Troy, MI). Spatial cues were provided by black patterns affixed to white curtains surrounding the perimeter of the maze at a distance of ∼40 cm. Animals were trained and tested on the spatial reference memory version of the MWM. For this task, the tank was divided into four imaginary quadrants of equal sizes (quadrants 1, 2, 3, 4) with the escape platform located in quadrant 4, 32 cm from the edge of the tank. The escape platform remained in the same location throughout the experiment and the rat was started randomly from quadrants 1, 2, or 3. Performance was recorded with an automated tracking system (Ethovision Observer, Nodulus, Netherlands). For training, 5 trials were conducted per day for 4 days with each trial lasting until the rat climbed on the platform with 3-5 minutes between trials. In each trial, if the platform was not located within 60 seconds, the rat was led to the platform and allowed to stay there for 30 seconds. The following parameters were recorded for the training trials: total distance to platform, or cumulative distance as defined by Gallagher et al., (Gallagher et al., 1993); path length; escape latency; and swimming velocity. For these parameters, better performance was indicated by a decrease in total distance, path length, and escape latency or an increase in swim velocity. The day after the completion of the training trials, a probe trial to assess spatial memory for the location of the escape platform was carried out. During the probe trial, the platform was removed and the rats were allowed to search in the pool for 30 seconds. The following parameters were recorded for the probe trial: mean distance to the previous location of the platform, or average proximity as defined by Gallagher et al., (Gallagher et al., 1993); platform crossings; and target quadrant (quadrant 4) preference. Better performance was indicated by an increase in platform crossings and a decrease in mean distance to platform. Finally, after the probe trial, swimming velocity was assessed during visible platform testing, consisting of two cued learning trials in which the platform was elevated above the water and a proximal cue hung directly above the visible platform.
Quantitative and Statistical Analysis
Statistical analyses were performed using GraphPad Prism (version 4.00 for Windows, GraphPad Software, San Diego, CA, USA) or SigmaStat (Systat Software, Inc., Richmond, CA). Potential effects of age and diet on protein levels were assessed using a two-way univariate analysis of variance with Bonferroni’s post-test. Significance was set at p < 0.05 and a Bonferroni adjustment was used for multiple comparisons to control for a type I error. Data from the training trials were analyzed using a 3-way ANOVA. Analysis of the probe trial data was done using a two-way ANOVA. Significance was set at p < 0.05 and a Bonferroni adjustment was used for multiple comparisons to control for a type I error.
Results
CR affects body weight
Food consumption was determined daily, and body weight was checked weekly to monitor health. The differences in mean weight across lifespan were significantly different between AL and CR rats (p < 0.001; F5,130 = 254.527; data not shown); the weights of the rats on the CR diet remained stable across ages, whereas the weights of those on the AL diet increased across lifespan. At no point did the weight of any CR rat exceed that of any AL rat.
Age and CR affect synaptic proteins in CA3
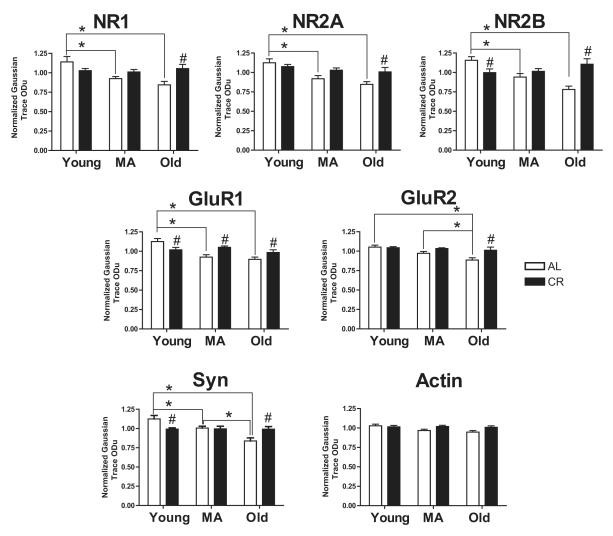
In order to assess changes in synaptic proteins, we examined the relative abundance of NMDA and AMPA receptor subunit levels in Western blots of the CA3 region of the hippocampus (Fig. 1). For the NR1 subunit proteins, the obligatory subunit of the NMDA receptor, we observed a significant main effect of age (F2,42 = 5.296; p < 0.009; Fig. 2) and a significant interaction between age and diet (F2,42 = 6.441; p < 0.009; Fig. 2). In AL animals, NR1 levels significantly decreased between young and middle age, as well as between young and old age (both p-values < 0.05). A comparison between AL and CR animals demonstrated that NR1 protein levels were significantly less in old AL rats than old CR animals (p < 0.05). For the NR2A and NR2B subunits, we also observed main effects of age and diet, as well as significant interactions between age and diet (NR2A: Age: F2,42 = 9.253; p < 0.001, Diet: F1,42 = 4.697; p < 0.036, Age × Diet: F2,42 = 3.530; p < 0.038; Fig. 2; NR2B: Age: F2,42 = 4.046; p < 0.025, Diet: F1,42 = 4.056; p < 0.05, Age × Diet: F2,42 = 12.260; p < 0.001; Fig. 2). In AL rats, both NR2A and NR2B significantly decreased between young and middle age, as well as between young and old age (all p-values < 0.05). In addition, both NR2A and NR2B subunit protein levels were significantly less in old AL rats than old CR animals (both p-values < 0.05). However, unlike NR2A, NR2B levels were significantly higher in young AL rats compared to young CR animals (p < 0.05).
Figure 2. Densitometric Analysis of Synaptic Protein Levels in CA3.
Protein levels of each subunit examined declined significantly with age in AL animals. In contrast, there were no age-related changes in CR animals. Although subunit protein levels in young animals were lower in the CR than in the AL group, those levels in CR animals were stabilized across lifespan. By old age, each subunit in the AL group decreased to a level significantly lower than that in the CR group. Levels of the synaptic protein synaptophysin (Syn) in AL and CR groups across lifespan demonstrate a similar pattern. Experimental groups were young (10-12 months), middle-aged (MA; 18-20 months), and old (29-32 months) F344 × BN rats that were caloric restricted (CR) from 4 months of age or fed ad libitum (AL). All measurements are reported as optical density units. An asterisk indicates an effect of age and # indicates an effect of diet. All significant differences are p < 0.05.
For the AMPA receptors, we observed significant main effects of age and significant interactions between age and diet for the GluR1 and GluR2 subunits of the AMPA receptor in CA3 (GluR1: Age: F2,42 = 9.716; p < 0.001, Age × Diet: F2,42 = 8.217; p < 0.001; Fig. 2; GluR2: Age: F2,42 = 7.741; p < 0.001, Age × Diet: F2,42 = 3.678; p < 0.05; Fig. 2). In addition, there was a significant main effect of diet on GluR2 levels in CA3 (Diet: F1,42 = 8.339; p < 0.01; Fig. 2). Our results also demonstrated significant declines in GluR1 protein levels in AL animals between young and middle age, as well as young and old (both p-values < 0.001). In addition, both middle age and old AL animals had significantly lower GluR1 protein levels than their age-matched CR groups (both p-values < 0.05), however, young AL rats had significantly more GluR1 in CA3 than young CR animals (p < 0.05). GluR2 levels declined between young and old AL animals, as well as middle and old age (both p-values < 0.05) and were higher in old CR than old AL animals (p < 0.001). We observed no significant changes in actin levels, suggesting that differences in subunit levels were not due to protein loading differences (all p-values > 0.05).
Alterations in glutamate receptor subunit levels may be associated with differences in synapse number or configuration. Although electron microscopic analysis is the only way to determine conclusively whether there are changes in synapse number or configuration, we used Western blot analysis of the synaptic vesicle protein, synaptophysin in the CA3 region to begin to evaluate overall changes in the population of synapses in that region. We observed significant main effects of age and significant interactions between age and diet on synaptophysin protein levels in CA3 (Age: F2,41 = 9.199; p < 0.001, Age × Diet: F2,41 = 8.976; p < 0.001; Fig. 2). Specifically, we observed a significant age-related decrease in synaptophysin levels in the AL animals between young and middle age, young and old age, and between middle and old age (all p-values < 0.05). Moreover, we observed significant effects of diet in young and old animals with synaptophysin levels significantly less in the young CR rats than the young AL animals; this effect was reversed in the old animals (both p-values < 0.01).
Age and CR affect behavioral performance
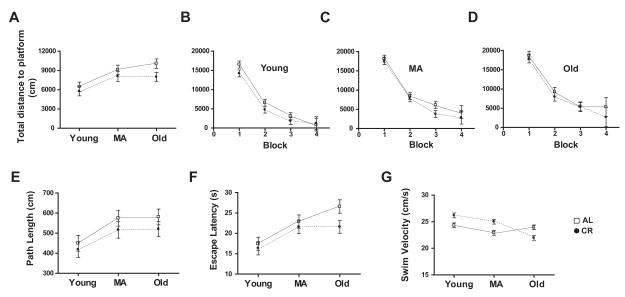
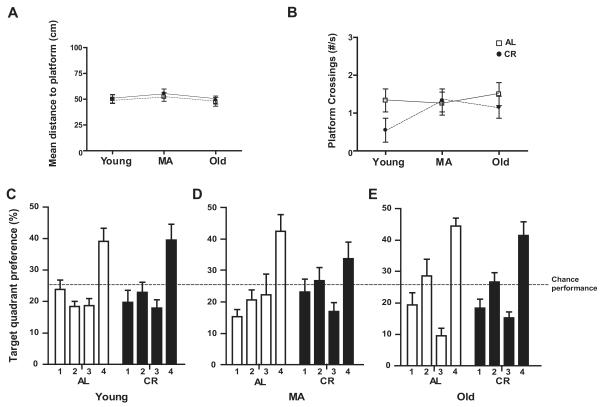
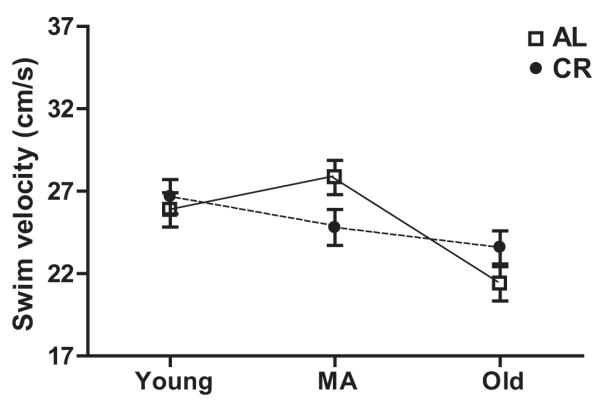
In light of our detection of both age and diet effects on synaptic proteins in CA3 and evidence in the literature that glutamate receptor levels in CA3 are linked to behavioral performance on the MWM (Magnusson, 1998; Adams et al., 2001; Nakazawa et al., 2003; El Bakri et al., 2004), we measured performance on this task in both AL and CR rats at young, middle, and old age. During the training trials of the MWM test of spatial learning, we observed main effects of age (F2,198 = 9.3, p<0.001), diet (F1, 198 = 4.43, p<0.05), and block (F 3,198 = 254.08, p<0.0001) for total distance to platform, but no group interactions (Fig. 3A). Performance of AL and CR animals on individual training trial blocks is shown in Figure 3B-D. The main effect of block suggests that all of the 6 groups (young AL and CR, middle age AL and CR, and old AL and CR) learned the task with the animals in each group becoming increasingly accurate in locating the escape platform. Post hoc tests indicate that the block mean for total distance to platform is significantly greater in middle-aged and old rats compared to young animals for both AL and CR animals (both p-values < 0.001). Nevertheless, although this measure increased significantly in both groups between young and middle age, between middle and old age only the AL group continued to increase significantly (all p-values < 0.001). In contrast, the CR group showed no significant change between middle and old age. Finally, post hoc tests revealed that lifelong CR resulted in a significantly lower block mean for total distance to platform in old rats compared to their AL counterparts (p < 0.001). No difference between the AL and CR groups was seen at either young or middle age. Additionally, we observed significant effects of age (F 2,198 = 5.58, p<0.001) and block (F 3,198 = 217.38, p<0.0001), but not diet (F 1,198 = 2.40, p=0.1229), on path length (Fig. 3E), as well as escape latency (age: F 2,198 = 10.62, p<0.0001; diet: F 1,198 = 3.42, p=0.0658; block: F 3,198 = 226.60, p<0.0001; Fig. 3F) and mean swim velocity (age: F2,198 = 14.924, p < 0.001; diet: F 1,198 = 3.675, p=0.0560; block: F 3,198 = 11.751, p<0.001; Fig. 3G). Post hoc comparisons indicate that the escape latency for old AL rats is significantly larger than for old CR rats and that the differences between AL and CR are significant at all ages for mean swim velocity. During the probe trial in which the escape platform was removed, there were no main effects of either age or diet on mean distance to platform or platform crossings (all p-values > 0.05; Fig. 4A and B). Moreover, all groups showed a significant preference for the target quadrant (quadrant 4; all p-values < 0.001; Fig. 4C-E). Visible platform testing revealed no effect of diet on the average swimming velocity (F 1, 66 = 0.0000948, p = 0.992; Fig. 5), however, a main effect of age was present (F 2, 66 = 9.075, p <0.001). Post-hoc tests revealed that this age effect is significant in AL rats, but not in CR animals (p-values < 0.05).
Figure 3. Morris Water Maze Performance during Training Trials in AL and CR Rats across Lifespan.
(A) The total distance to platform on the Morris water maze (MWM) increased between young and middle age for both AL (ad libitum fed) and CR (caloric restricted) rats, indicating a decline in performance (p<0.001). Between middle and old age, this measure increased significantly for AL rats (p<0.001), but the performance of CR rats did not change. At old age, AL rat swam significantly further than CR rats to reach the platform (p<0.001). (B-D) The improved performance of young, middle age, and old rats across training blocks on the total distance measure demonstrated that the animals in each group learned the task. (E) The path length to platform increased significantly for both AL and CR rats between young and middle age (p<0.001), but did not change between middle and old age. (F) Escape latency increased significantly for both AL and CR rats between young and middle age (p<0.001) and for AL rats between middle and old age (p<0.001). Escape latency did not change for CR rats between middle and old age, and old CR rats had a significantly smaller escape latency than did old AL rats (p<0.001). (G) Although AL and CR groups differed significantly on mean swim velocity across training trials at young, middle, and old age, this difference cannot explain the superior performance of old CR rats on the total distance to platform and escape latency measures (see text).
Figure 4. Morris Water Maze Probe Trial Performance in AL and CR Rats across Lifespan.
(A) The mean distance to platform on the probe trial of the Morris water maze (MWM) did not differ across life span or between AL (ad libitum fed) and CR (caloric restricted) groups. (B) Platform crossings, or the number of times the rats crossed the platform’s previous location, did not differ across life span or between AL and CR groups. (C-E) The percent of time young (C), middle age (MA, D), and old (E) rats spent in the target quadrant in which the platform was previously located (quadrant 4) did not differ between AL and CR groups. Each group spent significantly more time in quadrant 4 than in the other three quadrants (p<0.001).
Figure 5. Morris Water Maze Performance during Visible Platform Testing.
Average swimming velocity differed with age, but not diet during the visible platform testing phase of the Morris water maze (MWM; p <0.001). Post-hoc tests revealed that this effect of age is significant in AL (ad libitum fed; p<0.05), but not CR (caloric restricted) rats.
DISCUSSION
The present results revealed that both age and diet affect synaptic protein levels in the CA3 of the hippocampus and behavioral performance on a hippocampal-dependent task. We have shown significant age-related decreases in both AMPA and NMDA receptors, as well as the presynaptic vesicle protein synaptophysin. Moreover, we observed that CR appears to prevent these age-related decreases in the synaptic proteins. Although both AL and CR animals exhibited age-related cognitive decline across the training trials on the MWM task, CR animals perform significantly better than their AL counterparts at old age.
In the current study, we examined age- and diet-related changes in the protein levels of the NR1, NR2A, and NR2B subunits of the NMDA receptor, and the GluR1 and GluR2 subunits of the AMPA receptor in CA3. We found that in AL rats, all of the glutamate receptor subunits decline with age. Our data demonstrating age-related declines in these subunit levels are consistent with previous findings showing age-related decreases in NMDA and AMPA receptors (Clark et al., 1992; Gazzaley et al., 1996; Morrison and Hof, 1997; Sonntag et al., 2000; Wenk and Barnes, 2000; Magnusson, 2000; Eckles-Smith et al., 2000; Clayton and Browning, 2001; Clayton et al., 2002; Shi et al., 2007; Newton et al., 2007). Interestingly, we also found that CR appears to prevent this age-related loss of the NMDA and AMPA receptor subunits in CA3. This finding is consistent with other studies demonstrating that CR ameliorates age-related alterations in specific subunits of the NMDA receptor (Eckles-Smith et al., 2000; Monti et al., 2004). Importantly, however, those studies only evaluated subunit levels in young and old animals, whereas the present study determined subunit level changes across young, middle, and old ages. By assessing three time points across lifespan, were able to demonstrate that CR prevents the age-related subunit decline in most subunits by initially lowering the levels of the subunits in young animals to a level that then is maintained across lifespan.
NMDA and AMPA receptors are integrally associated with synaptic plasticity in the hippocampus. In addition to changes in these subunits, we also observed age- and diet-related changes in another synaptic protein, synaptophysin. For example, AL animals demonstrated an overall age-related decline in synaptophysin protein levels. In contrast, Nicolle et al., 1999 report no age-related changes in synaptophysin protein levels in the hippocampus. A likely explanation for this difference in findings is the inclusion of the whole hippocampus in that study rather than a region-specific subdissection used here. The presence of synaptic changes specific to hippocampal CA3 is in good agreement with other studies demonstrating circuit-specific or region-specific age-related changes (Smith et al., 2000). Specifically, an examination of synaptophysin levels in different projection zones of the hippocampal subregions revealed a decrease in synaptophysin in stratum lacunosum moleculare (SLM) of CA3 of learning impaired rats (Smith et al., 2000). However, that decrease was obscured when measurements were averaged across hippocampal subregions. With regard to diet, the present results reveal that the age-related decrease in synaptophysin protein levels in CA3 was absent in CR animals. Compared to AL animals, synaptophysin levels in CR animals were reduced by 10 months of age, but then remained stable across the lifespan. Thus, CR appears to ameliorate the age-related decline in synaptophysin levels in CA3 by stabilizing those levels across lifespan. A decline in synaptophysin protein levels could be due to a loss in synapse number and/or to an altered synaptic configuration. Although we demonstrated previously that the number of synapses is stable in the stratum lucidum of aged rats (Poe et al., 2001), the age-related synaptophysin decrease limited to SLM of CA3 (Smith et al., 2000) suggests the possibility of age-related synaptic changes in SLM that are ameliorated by CR. Serial reconstruction analyses of ultrastructurally identified synapses in SLM are currently ongoing in our laboratory in order to determine whether synapse number and/or configuration is changing with age and if so, whether CR stabilizes this change.
In light of our detection of effects of age and diet on synaptic proteins, we felt that it was important to demonstrate the behavioral effect on the MWM described by Markowska and Savonenko (2002) in our own animals. Accordingly, we assessed the effects of CR and age on learning and memory in a second cohort of rats. We tested young, middle age and old AL and CR rats on the MWM task, a test known to be sensitive to hippocampal damage (Morris et al., 1982) and age (Gallagher et al., 1993). Although both AL and CR animals exhibited age-related cognitive decline on this task across the training trials, the performance of CR rats was stable between middle and old age whereas old AL rats performed more poorly than the middle-aged group. Effect of CR on performance at old age was evident in both mean total distance to platform and escape latency. The shorter total distance to platform in old CR compared to old AL rats in Figure 3A is largely due to a difference in these two groups at Block 4 as shown in Figure 3D. Thus, our data suggest that there is a tendency for old CR rats to show learning-related improvements in performance much like the young animals. Although comparisons between AL and CR groups at young, middle, and old age indicate significant differences on mean swim velocity during training trials, we do not believe this can explain the CR effect in old age because i] the performance of young AL and CR rats did not differ on any measure and ii] AL rats swam faster than CR rats at old age, but had significantly a longer escape latency. Moreover, any difference in sensorimotor activity should not affect either the total distance to platform in training trial since this measure is independent of swimming velocity (Gallagher et al., 1993). The training trial data reported here corroborate the findings of Markowska and Savonenko (2002) in a similarly designed study. Unlike Markowska and Savonenko (2002), however, we observed no effect of age or diet on the probe trial. It may be that our experimental design of one probe trial at the end of training, rather than probe trials interspersed throughout training, obscured a memory impairment on the probe trial. It has been suggested that this experimental design may result in animals being over-trained at the time they begin the probe trial testing (see Gallagher et al., 1993).
The present results point to significant effects of age and diet on synaptic proteins in the CA3 region of the hippocampus and on behavioral performance linked to CA3, raising the possibility that the changes we observe in CA3 synaptic proteins underlie behavioral changes. Specifically, our data are consistent with the notions that i] the decline in MWM performance in AL animals across lifespan may be due, at least in part, to declines in CA3 glutamate receptor levels, and ii] the maintenance of MWM performance between middle and old age in CR animals may be associated with the stabilization in CA3 glutamate receptor levels in CR rats. Supporting this possibility are data that suggest MWM training performance depends, in part, on NMDA receptor levels in CA3 (Adams et al., 2001; Nakazawa et al., 2002). Moreover, these results are consistent with the notion that CA3 is a critical integrator of information processing in the hippocampus. The apical dendrites of the CA3 pyramidal cells receive a convergence of information (Amaral and Witter, 1995; Lisman, 1999) and computational models suggest that the CA3 region plays a critical role in the integration of spatial information (Shapiro et al., 1997; Lisman, 1999; Shapiro and Eichenbaum, 1999). The contribution of CA3 to hippocampal function is supported by several models in which deficits in spatial learning occur as a result of experimental manipulations. For example, lesions of the CA3 region produce spatial memory deficits in rats, including impaired performance in water maze tasks (Handelmann and Olton, 1981; Jarrard, 1983; Stubley-Weatherly et al., 1996). In addition, chronic stress causes an atrophy of CA3 apical dendrites through an NMDA receptor-dependent mechanism (Woolley et al., 1990; Magarinos et al., 1997; McEwen et al., 1999; McEwen and Magarinos, 2001) producing impairments in spatial learning and memory (Luine et al., 1994; Conrad et al., 1996; Sousa et al., 2000; Sandi, 2003). By contributing to the stability of excitatory neuronal connections in CA3, CR may play a role in the amelioration of age-related declines in hippocampal processing. While our data suggest that there is a relationship between CA3, glutamate receptors, age, diet, and cognition, however, we cannot exclude the possibility that CA1 also is involved in cognition. For example, CA3 lesions that cut the Schaffer collaterals leave CA1 place cell firing fields and spatial memory intact (Brun et al., 2002) and stress effects on CA3 may also include the CA1 region (for review see Jarrard, 2002). To this end, we also have examined the effects of CR on CA1 synaptic proteins (Shi et al., 2007). In that study, we observed a similar stabilization by CR of synaptic proteins in CA1, however, the effects were not as robust as those reported here in CA3.
Conclusion
Our results demonstrate that the progressive pattern of age-related declines in synaptic protein levels in CA3 and impairments on a hippocampal-dependent task of learning and memory can be prevented by lifelong CR. These findings are the first to demonstrate the effects of CR on excitatory connectivity in the CA3 region of hippocampus and provide important data relevant to the development of mimetics for preventing age-related declines in learning and memory.
Acknowledgements
This works was supported by NIH grants: NIA AG11370 and AG019886.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams MM, Smith TD, Moga D, Gallagher M, Wang Y, Wolfe BB, Rapp PR, Morrison JH. Hippocampal dependent learning ability correlates with N-methyl-D-aspartate (NMDA) receptor levels in CA3 neurons of young and aged rats. J. Comp Neurol. 2001;432:230–243. doi: 10.1002/cne.1099. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Witter MP. Hippocampal formation. In: Paxinos G, editor. The Rat Nervous System. 1995. pp. 443–495. [Google Scholar]
- Brun VH, Otnass MK, Molden S, Steffenach HA, Witter MP, Moser MB, Moser EI. Place cells and place recognition maintained by direct entorhinal-hippocampal circuitry. Science. 2002;296:2243–2246. doi: 10.1126/science.1071089. [DOI] [PubMed] [Google Scholar]
- Calhoun ME, Kurth D, Phinney AL, Long JM, Hengemihle J, Mouton PR, Ingram DK, Jucker M. Hippocampal neuron and synaptophysin-positive bouton number in aging C57BL/6 mice. Neurobiol. Aging. 1998;19:599–606. doi: 10.1016/s0197-4580(98)00098-0. [DOI] [PubMed] [Google Scholar]
- Clark AS, Magnusson KR, Cotman CW. In vitro autoradiography of hippocampal excitatory amino acid binding in aged Fischer 344 rats: relationship to performance on the Morris water maze. Behav. Neurosci. 1992;106:324–335. doi: 10.1037//0735-7044.106.2.324. [DOI] [PubMed] [Google Scholar]
- Clayton DA, Browning MD. Deficits in the expression of the NR2B subunit in the hippocampus of aged Fisher 344 rats. Neurobiol. Aging. 2001;22:165–168. doi: 10.1016/s0197-4580(00)00196-2. [DOI] [PubMed] [Google Scholar]
- Clayton DA, Mesches MH, Alvarez E, Bickford PC, Browning MD. A hippocampal NR2B deficit can mimic age-related changes in long-term potentiation and spatial learning in the Fischer 344 rat. J. Neurosci. 2002;22:3628–3637. doi: 10.1523/JNEUROSCI.22-09-03628.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, Galea LA, Kuroda Y, McEwen BS. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav. Neurosci. 1996;110:1321–1334. doi: 10.1037//0735-7044.110.6.1321. [DOI] [PubMed] [Google Scholar]
- Eckles-Smith K, Clayton D, Bickford P, Browning MD. Caloric restriction prevents age-related deficits in LTP and in NMDA receptor expression. Brain Res. Mol. Brain Res. 2000;78:154–162. doi: 10.1016/s0169-328x(00)00088-7. [DOI] [PubMed] [Google Scholar]
- El Bakri NK, Islam A, Zhu S, Elhassan A, Mohammed A, Winblad B, Adem A. Effects of estrogen and progesterone treatment on rat hippocampal NMDA receptors: relationship to Morris water maze performance. J. Cell Mol. Med. 2004;8:537–544. doi: 10.1111/j.1582-4934.2004.tb00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav. Neurosci. 1993;107:618–626. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- Ganeshina O, Berry RW, Petralia RS, Nicholson DA, Geinisman Y. Differences in the expression of AMPA and NMDA receptors between axospinous perforated and nonperforated synapses are related to the configuration and size of postsynaptic densities. J. Comp Neurol. 2004;468:86–95. doi: 10.1002/cne.10950. [DOI] [PubMed] [Google Scholar]
- Gazzaley AH, Weiland NG, McEwen BS, Morrison JH. Differential regulation of NMDAR1 mRNA and protein by estradiol in the rat hippocampus. J. Neurosci. 1996;16:6830–6838. doi: 10.1523/JNEUROSCI.16-21-06830.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrick CL, Ingram DK, Reynolds MA, Freeman JR, Cider NL. Effects of intermittent feeding upon growth, activity, and lifespan in rats allowed voluntary exercise. Exp. Aging Res. 1983;9:203–209. doi: 10.1080/03610738308258453. [DOI] [PubMed] [Google Scholar]
- Handelmann GE, Olton DS. Spatial memory following damage to hippocampal CA3 pyramidal cells with kainic acid: impairment and recovery with preoperative training. Brain Res. 1981;217:41–58. doi: 10.1016/0006-8993(81)90183-9. [DOI] [PubMed] [Google Scholar]
- Idrobo F, Nandy K, Mostofsky DI, Blatt L, Nandy L. Dietary restriction: effects on radial maze learning and lipofuscin pigment deposition in the hippocampus and frontal cortex. Arch. Gerontol. Geriatr. 1987;6:355–362. doi: 10.1016/0167-4943(87)90014-8. [DOI] [PubMed] [Google Scholar]
- Ingram DK, Weindruch R, Spangler EL, Freeman JR, Walford RL. Dietary restriction benefits learning and motor performance of aged mice. J. Gerontol. 1987;42:78–81. doi: 10.1093/geronj/42.1.78. [DOI] [PubMed] [Google Scholar]
- Jarrard LE. Selective hippocampal lesions and behavior: effects of kainic acid lesions on performance of place and cue tasks. Behav. Neurosci. 1983;97:873–889. doi: 10.1037//0735-7044.97.6.873. [DOI] [PubMed] [Google Scholar]
- Jarrard LE. Use of excitotoxins to lesion the hippocampus: update. Hippocampus. 2002;12:405–414. doi: 10.1002/hipo.10054. [DOI] [PubMed] [Google Scholar]
- Kullmann DM, Asztely F, Walker MC. The role of mammalian ionotropic receptors in synaptic plasticity: LTP, LTD and epilepsy. Cellular Molecular Life Sci. 2000;57:1551–1561. doi: 10.1007/PL00000640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE. Relating hippocampal circuitry to function: recall of memory sequences by reciprocal dentate-CA3 interactions. Neuron. 1999;22:233–242. doi: 10.1016/s0896-6273(00)81085-5. [DOI] [PubMed] [Google Scholar]
- Luine V, Villegas M, Martinez C, McEwen BS. Repeated stress causes reversible impairments of spatial memory performance. Brain Res. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, Verdugo JM, McEwen BS. Chronic stress alters synaptic terminal structure in hippocampus. Proc. Natl. Acad. Sci. U. S. A. 1997;94:14002–14008. doi: 10.1073/pnas.94.25.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson KR. Aging of glutamate receptors: correlations between binding and spatial memory performance in mice. Mech. Ageing Dev. 1998;104:227–248. doi: 10.1016/s0047-6374(98)00076-1. [DOI] [PubMed] [Google Scholar]
- Magnusson KR. Declines in mRNA expression of different subunits may account for differential effects of aging on agonist and antagonist binding to the NMDA receptor. J. Neurosci. 2000;20:1666–1674. doi: 10.1523/JNEUROSCI.20-05-01666.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowska AL, Savonenko A. Retardation of cognitive aging by life-long diet restriction: implications for genetic variance. Neurobiol. Aging. 2002;23:75–86. doi: 10.1016/s0197-4580(01)00249-4. [DOI] [PubMed] [Google Scholar]
- Mayhew M, Renganathan M, Delbono O. Effectiveness of caloric restriction in preventing age-related changes in rat skeletal muscle. Biochem. Biophys. Res. Commun. 1998;251:95–99. doi: 10.1006/bbrc.1998.9438. [DOI] [PubMed] [Google Scholar]
- McEwen BS, de Leon MJ, Lupien SJ, Meaney MJ. Corticosteroids, the Aging Brain and Cognition. Trends Endocrinol. Metab. 1999;10:92–96. doi: 10.1016/s1043-2760(98)00122-2. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Magarinos AM. Stress and hippocampal plasticity: implications for the pathophysiology of affective disorders. Hum. Psychopharmacol. 2001;16:S7–S19. doi: 10.1002/hup.266. [DOI] [PubMed] [Google Scholar]
- Monti B, Virgili M, Contestabile A. Alterations of markers related to synaptic function in aging rat brain, in normal conditions or under conditions of long-term dietary manipulation. Neurochem. Int. 2004;44:579–584. doi: 10.1016/j.neuint.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Hof PR. Life and death of neurons in the aging brain. Science. 1997;278:412–419. doi: 10.1126/science.278.5337.412. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Quirk MC, Chitwood RA, Watanabe M, Yeckel MF, Sun LD, Kato A, Carr CA, Johnston D, Wilson MA, Tonegawa S. Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science. 2002;297:211–218. doi: 10.1126/science.1071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K, Sun LD, Quirk MC, Rondi-Reig L, Wilson MA, Tonegawa S. Hippocampal CA3 NMDA receptors are crucial for memory acquisition of one-time experience. Neuron. 2003;38:305–315. doi: 10.1016/s0896-6273(03)00165-x. [DOI] [PubMed] [Google Scholar]
- Newton IG, Forbes ME, Legault C, Johnson JE, Brunso-Bechtold JK, Riddle DR. Caloric restriction does not reverse aging-related changes in hippocampal BDNF. Neurobiol. Aging. 2005;26:683–688. doi: 10.1016/j.neurobiolaging.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Newton IG, Forbes ME, Linville MC, Pang H, Tucker EW, Riddle DR, Brunso-Bechtold JK. Effects of aging and caloric restriction on dentate gyrus synapses and glutamate receptor subunits. Neurobiol. Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas A, Michaud M, Lavigne G, Montplaisir J. The influence of sex, age and sleep/wake state on characteristics of periodic leg movements in restless legs syndrome patients. Clin. Neurophysiol. 1999;110:1168–1174. doi: 10.1016/s1388-2457(99)00033-4. [DOI] [PubMed] [Google Scholar]
- Pitsikas N, Algeri S. Deterioration of spatial and nonspatial reference and working memory in aged rats: protective effect of life-long calorie restriction. Neurobiol. Aging. 1992;13:369–373. doi: 10.1016/0197-4580(92)90110-j. [DOI] [PubMed] [Google Scholar]
- Pitsikas N, Carli M, Fidecka S, Algeri S. Effect of life-long hypocaloric diet on age-related changes in motor and cognitive behavior in a rat population. Neurobiol. Aging. 1990;11:417–423. doi: 10.1016/0197-4580(90)90008-n. [DOI] [PubMed] [Google Scholar]
- Poe BH, Linville C, Riddle DR, Sonntag WE, Brunso-Bechtold JK. Effects of age and insulin-like growth factor-1 on neuron and synapse numbers in area CA3 of hippocampus. Neuroscience. 2001;107:231–238. doi: 10.1016/s0306-4522(01)00341-4. [DOI] [PubMed] [Google Scholar]
- Ramsey MM, Weiner JL, Moore TP, Carter CS, Sonntag WE. Growth hormone treatment attenuates age-related changes in hippocampal short-term plasticity and spatial learning. Neuroscience. 2004;129:119–127. doi: 10.1016/j.neuroscience.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Gallagher M. Preserved neuron number in the hippocampus of aged rats with spatial learning deficits. Proc. Natl. Acad. Sci. U. S. A. 1996;93:9926–9930. doi: 10.1073/pnas.93.18.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen T, Schliemann T, Sorensen JC, Zimmer J, West MJ. Memory impaired aged rats: no loss of principal hippocampal and subicular neurons. Neurobiol. Aging. 1996;17:143–147. doi: 10.1016/0197-4580(95)02032-2. [DOI] [PubMed] [Google Scholar]
- Riedel G, Platt B, Micheau J. Glutamate receptor function in learning and memory. Behav. Brain Res. 2003;140:1–47. doi: 10.1016/s0166-4328(02)00272-3. [DOI] [PubMed] [Google Scholar]
- Sandi C. [Glucocorticoid involvement in memory consolidation] Rev. Neurol. 2003;37:843–848. [PubMed] [Google Scholar]
- Shapiro ML, Eichenbaum H. Hippocampus as a memory map: synaptic plasticity and memory encoding by hippocampal neurons. Hippocampus. 1999;9:365–384. doi: 10.1002/(SICI)1098-1063(1999)9:4<365::AID-HIPO4>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Shapiro ML, Tanila H, Eichenbaum H. Cues that hippocampal place cells encode: dynamic and hierarchical representation of local and distal stimuli. Hippocampus. 1997;7:624–642. doi: 10.1002/(SICI)1098-1063(1997)7:6<624::AID-HIPO5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Shi L, Adams MM, Linville MC, Newton IG, Forbes ME, Long AB, Riddle DR, Brunso-Bechtold JK. Caloric restriction eliminates the aging-related decline in NMDA and AMPA receptor subunits in the rat hippocampus and induces homeostasis. Exp. Neurol. 2007;206:70–79. doi: 10.1016/j.expneurol.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Poe BH, Constance LM, Sonntag WE, Brunso-Bechtold JK. Caloric restricted male rats demonstrate fewer synapses in layer 2 of sensorimotor cortex. Brain Res. 2002;931:32–40. doi: 10.1016/s0006-8993(02)02249-7. [DOI] [PubMed] [Google Scholar]
- Smith TD, Adams MM, Gallagher M, Morrison JH, Rapp PR. Circuit-specific alterations in hippocampal synaptophysin immunoreactivity predict spatial learning impairment in aged rats. J. Neurosci. 2000;20:6587–6593. doi: 10.1523/JNEUROSCI.20-17-06587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag WE, Bennett SA, Khan AS, Thornton PL, Xu X, Ingram RL, Brunso-Bechtold JK. Age and insulin-like growth factor-1 modulate N-methyl-D-aspartate receptor subtype expression in rats. Brain Res. Bull. 2000;51:331–338. doi: 10.1016/s0361-9230(99)00259-2. [DOI] [PubMed] [Google Scholar]
- Sousa N, Lukoyanov NV, Madeira MD, Almeida OF, Paula-Barbosa MM. Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience. 2000;97:253–266. doi: 10.1016/s0306-4522(00)00050-6. [DOI] [PubMed] [Google Scholar]
- Stewart J, Mitchell J, Kalant N. The effects of life-long food restriction on spatial memory in young and aged Fischer 344 rats measured in the eight-arm radial and the Morris water mazes. Neurobiol. Aging. 1989;10:669–675. doi: 10.1016/0197-4580(89)90003-1. [DOI] [PubMed] [Google Scholar]
- Stubley-Weatherly L, Harding JW, Wright JW. Effects of discrete kainic acid-induced hippocampal lesions on spatial and contextual learning and memory in rats. Brain Res. 1996;716:29–38. doi: 10.1016/0006-8993(95)01589-2. [DOI] [PubMed] [Google Scholar]
- Wenk GL, Barnes CA. Regional changes in the hippocampal density of AMPA and NMDA receptors across the lifespan of the rat. Brain Res. 2000;885:1–5. doi: 10.1016/s0006-8993(00)02792-x. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat. Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J. Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]