Abstract
Caloric restriction (CR) attenuates aging-related degenerative processes throughout the body. It is less clear, however, whether CR has a similar effect in the brain, particularly in the hippocampus, an area important for learning and memory processes that often are compromised in aging. In order to evaluate the effect of CR on synapses across lifespan, we quantified synapses stereologically in the middle molecular layer of the dentate gyrus (DG) of young, middle aged, and old Fischer 344 X Brown Norway rats fed ad libitum (AL) or a CR diet from 4 months of age. The results indicate that synapses are maintained across lifespan in both AL and CR rats. In light of this stability, we addressed whether aging and CR influence neurotransmitter receptor levels by measuring subunits of NMDA (NR1, NR2A, and NR2B) and AMPA (GluR1, GluR2) receptors in the DG of a second cohort of AL and CR rats across lifespan. The results reveal that the NR1 and GluR1 subunits decline with age in AL, but not CR rats. The absence of an aging-related decline in these subunits in CR rats, however, does not arise from increased levels in old CR rats. Instead, it is due to subunit decreases in young CR rats to levels that are sustained in CR rats throughout lifespan, but that are reached in AL rats only in old age.
1. Introduction
Caloric restriction (CR) is regarded as a method of decelerating senescence because of its ability to extend lifespan and to protect against many aging-related degenerative processes and diseases throughout the body [44,55]. Although its effects on the brain are less clear, moderate CR has been shown to attenuate aging-related deficits in performance on hippocampus-dependent learning and memory tasks in some rodent strains, such as Fischer 344 x Brown Norway (F344xBN) rats [25,26,43,60,61,73]. This attenuation suggests that CR modulates neurobiological processes that subserve cognition and that a more complete understanding of the impact of CR on the neural basis of learning and memory during aging could lead to therapies for human memory impairment.
In view of recent stereological evidence that the number of principal neurons in the hippocampus does not decrease with age [63,65], attention has shifted to synapses as potential substrates for the aging-related changes that lead to deficits in learning and memory [17,19,67]. This possibility is supported by the positive correlation between learning and memory ability and the density of synaptic boutons and their spinous targets in the hippocampus [34,49,52,70]. Similarly, enlargement of the postsynaptic density (PSD) has been associated with enhanced synaptic efficacy. The PSD expands through an NMDA receptor-mediated insertion of AMPA receptors [14,24,38,42,62]. It has been proposed that the enlarged PSD then perforates and the postsynaptic spine ultimately divides to create a multiple synapse bouton (MSB) complex consisting of several synapses on a single presynaptic terminal contacting more than one postsynaptic element [24,38,57]. Because perforated and MSB synapses increase after long-term potentiation (LTP), learning, and environmental enrichment, they are believed to represent morphological correlates of enhanced synaptic efficacy [6,18,21,29,30,57,76,77].
In the hippocampus, NMDA and AMPA ionotropic glutamate receptors (NMDAR and AMPAR, respectively) are the chief mediators of excitatory synaptic transmission. While NMDARs mediate a major form of LTP, a functional correlate of learning and memory, AMPAR activation accounts for the fast postsynaptic responses to glutamate. Importantly, synaptic plasticity may rely upon AMPARs, since the absence of AMPARs at NMDAR-positive synapses renders them functionally silent at resting potential, and NMDAR-mediated LTP at these synapses involves the rapid insertion of AMPARs [3,35,37]. Moreover, genetic and pharmacological studies have demonstrated the critical involvement of these receptors in LTP and hippocampus-dependent learning and memory [66]. In fact, spatial memory ability varies directly with levels of these glutamate receptors and their constituent subunits [1,10,39,74]. In light of these data, evidence that NMDARs and AMPARs and their subunits decrease with age [9,15,40,41,71,81] has led to suggestions that this decline may contribute to the cognitive impairments observed in old animals. Evidence that CR not only prevents aging-related deficits in LTP but also prevents aging-related changes in the obligatory NR1 subunit of the NMDAR [6,18,21,29,30,57,76,77], suggests that CR also might prevent the aging-related decline in other glutamate receptor subunits. This question is important since maintenance of glutamate receptor subunits over lifespan represents a potential mechanism whereby CR could preserve learning and memory after middle age [43].
In the present study, we quantified synapses as well as relative protein levels of NMDAR and AMPAR subunits in the dentate gyrus (DG) of young, middle-aged and old rats fed ad libitum (AL) or a CR diet. The DG is the first subregion in the hippocampal trisynaptic pathway and has been shown in both lesion and pharmacological studies to play a critical role in hippocampus-dependent learning and memory [23,27,83]. Furthermore, stereologically quantified synapses in the DG have been reported to decrease across the lifespan [19]. Using unbiased stereological techniques, we determined the synapse per neuron ratio in the DG across life span in AL fed and CR rats to test the hypothesis that there are quantitative, aging-related changes in synapses that are attenuated by CR. In the second part of the study, we used Western blot analysis to address the possibility that CR affects aging-related synaptic changes at the receptor level. We specifically tested the hypotheses that NMDAR and AMPAR subunits decline with age in the DG of AL rats and that CR attenuates this decline. Our findings indicate that neither aging nor CR affects the number of DG synapses per neuron. In contrast with this stability, the NR1 and GluR1 glutamate receptor subunits in the DG demonstrate a decline across lifespan in AL rats. CR, however, decreases receptor subunits within 6 months to levels that are maintained across lifespan.
2. Methods
2.1. Animal model
The F344xBN F1 hybrid is a widely-used model for studies of aging and CR [36,46,69,82] and has been reported by Markowska and colleagues to demonstrate CR-mediated protection of learning and memory after middle age [43]. The central focus of the present study was to analyze synaptic structural and biochemical changes as a function of aging and CR. In order to avoid potential direct structural changes due to the environmental enrichment of the testing procedure itself, behavioral testing was not included in the present study. Nevertheless, the experimental design was modeled closely after the Markowska study to permit analysis of synaptic changes in a model of CR-induced amelioration of aging-related cognitive decline. For this study, adult male rats were obtained from the NIA Caloric Restriction Colony (Harlan Industries) and were maintained in our facility on a 12-hour light-dark cycle for two months prior to sacrifice. At sacrifice, groups consisted of young (10 months), middle age (18 months), and old (29 months) AL and CR rats with 6 rats per group for electron microscopy studies and 8 rats per group for Western blot analysis. Rats were housed individually in order to monitor daily food intake. The animal facility at the Wake Forest University School of Medicine is fully accredited by the American Association for Accreditation of Laboratory Animal Care and complies with all Public Health Service, National Institutes of Health, and institutional policies and standards for laboratory animal care. All procedures described herein were approved by the institutional Animal Care and Use Committee. The following notations (age/diet) are used in the Results section to refer to young, middle-aged, or old rats in AL or CR groups: 10AL, 18AL, 29AL, 10CR, 18CR, and 29CR.
2.2. Caloric Restriction
At the NIA facility, all rats were fed AL (NIH-31 diet) until 14 weeks of age, at which time the CR regimen was initiated by incremental reduction of 10% per week over four weeks, reaching full 60% restriction by 17 weeks of age. The vitamin-fortified NIH-31 diet fed to CR rats provided 60% of the calories and 100% of the vitamins consumed by AL rats (NIH-31). All rats were fed daily, shortly before dark cycle onset, and had free access to water. Daily monitoring of food consumption verified the caloric consumption by each group. Rats were weighed weekly, immediately prior to feeding.
2.3. Electron Microscopy
For electron microscopy studies, rats were anesthetized with ketamine/xylazine (80 mg/kg /12 mg/kg, i.m.) and then perfused transcardially with 1.3 M cacodylate buffer (pH 7.4, 20 ml), followed by fixative (2% paraformaledyde/ 2% glutaraldehyde), at a flow rate of 40 ml/min for a minimum of 30 minutes using a peristaltic pump. The brains were removed and post-fixed overnight at 4°C. The next day, the brain was sectioned coronally on a vibratome at 200 μm. A similar number of sections was derived through the hippocampus of all animals, indicating no gross evidence of a difference in hippocampal size across ages and diets. Sections were viewed with a dissecting microscope and a lightfield-darkfield base and trapezoids containing the DG were cut from sections containing the dorsomedial hippocampus (Bregma -2.8 through -4.0, [59]). Analysis was restricted to the dorsomedial hippocampus because, in contrast with the ventrolateral portion, this area is crucial for spatial memory acquisition and retrieval in the Morris water maze [31,50,51]. The six resulting blocks were washed thoroughly in 0.15M cacodylate buffer, postfixed in 2% osmium tetroxide, dehydrated, embedded in Araldite 502 plastic (Ted Pella). Two of the six blocks were selected randomly, resulting in an equal probability of sampling along the rostrocaudal axis and were trimmed so that the middle of the MML of the supragranular blade of the dentate gyrus was aligned along one edge of the trapezoid. Thin sections (700 Å) were cut on an LKB IV ultramicrotome, collected onto formvar-coated copper slot grids, stained with uranyl acetate and lead citrate, and viewed with a Zeiss 10-C electron microscope. Areas to be sampled were chosen in a systematically random fashion and photographed at 8,000X. Specifically, following random placement of the first photomicrograph site along the edge of the section that was aligned with the middle of the MML, subsequent photomicrographs were taken at regular intervals along the full extent of that edge. To obtain the serial pairs, comparable areas were photographed in the adjacent section.
Neurons were quantified stereologically in 1 μm sections at the light microscopic level (Nikon Labophot, 20X objective) using the physical disector method [72] (disector height = 4 μm). Counting frames of known area were superimposed on a field within the granule cell layer of the DG region. The counting units were neuronal nuclei and were included in the total if they appeared in only one of the counting frames in the physical disector pair and did not contact the two exclusion lines of the counting frame. Six section pairs were quantified per block, and two blocks were analyzed per animal.
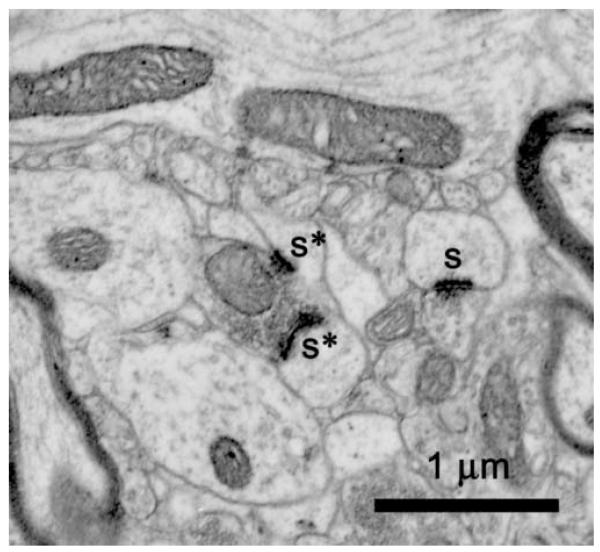
Synapses were quantified in sections from the same Araldite-embedded block used for neuron quantification to ensure that any changes in tissue volume during processing would affect neuron and synapse numbers equally. Synapses were quantified stereologically in 24 disector pairs per animal (12 disector pairs per block) comprising 48 electron micrographs from serial pairs of 700 Å thin sections. The thickness of the thin sections was determined by the Small fold method [80]. The number of blocks and disector pairs were determined a priori based on power analysis calculations using variance from preliminary studies to achieve a power of 80% to detect a 20% change with a p value less than 0.05. The electron micrographs were taken in the MML, midway between the two boundaries of the molecular layer, i] the hippocampal fissure and ii] the granule cell layer. Since the MML occupies the middle 40% of the molecular layer [64], all electron micrographs were taken well within the limits of the MML. Negatives were digitized for subsequent stereological quantification on a computer. Synapses were defined as having a PSD associated with a presynaptic element containing at least three vesicles (Figure 1, s), with the PSD considered as the counting object. MSB synapses were defined as those synapses that were part of multiple synapse bouton complexes; specifically, MSB synapses shared a single presynaptic terminal, but contacted different postsynaptic elements (Figure 1, asterisks). The numerical densities of all synapses and of MSB synapses in the MML of DG were determined in serial sections using the physical disector method and StereoInvestigator software (MicroBrightfield). Digitized images of the serial sections were viewed at a final magnification of 56,000X on the computer screen and superimposed with a counting box having 2 inclusion and 2 exclusion lines. First, all synapses within the box, but without PSDs (the counting objects) lying on the exclusion lines, were indicated with a marker on the reference section. Those markers then were temporarily removed by the software and the counting box was placed in the same position on the look-up section using fiduciary marks. Next, all synapses were indicated with a different marker on the look-up section. Finally, both sets of markers were revealed, and, only those synapses marked in either the look-up or reference sections, but not both, were counted. Evidence suggests that this method of counting MSB synapses underestimates their number, but generates relative differences between groups that are comparable to those generated by the serial section bouton reconstruction method [28,30]. This method of quantification was suitable for the present study, since the aim was not to determine actual number of MSB synapses, but instead, to examine relative changes in MSB synapses.
Figure 1.
Electron micrograph illustrating synapses (s), two of which are involved in an MSB complex (asterisks).
The numerical densities of neurons, synapses and MSB synapses were calculated for each animal using the equation Nv = ΣQ-/Σa·h, where Nv is the numerical density, Q- is the number of unique counting objects in a section, a is the counting frame area, and h is the height of the physical disector. In order to control for the possible confound of differential changes in tissue volume during processing, the numerical densities of synapses and MSB synapses were normalized by dividing by the numerical density of neurons in the same Araldite-embedded block of tissue, thus deriving the number of synapses per neuron. This normalized quantification of synapses is not intended to represent true number of synapses, but rather to provide a measure of the relative changes in synapses as a function of aging and caloric restriction. Finally, all quantification was performed blindly in order to eliminate investigator bias.
2.4. Western Blot
For Western blot analyses, rats were anesthetized with sodium pentobarbital (200 mg/kg, i.p.) and decapitated in accordance with the recommendations of the Panel on Euthanasia of the American Veterinary Medical Association. The brains were removed immediately and cooled for 2 minutes in chilled artificial cerebrospinal fluid (140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 24 mM dextrose, 10 mM HEPES, pH 7.4). The hippocampi then were dissected on ice. With the aid of a dissecting microscope, each hippocampus was bisected and the dorsomedial half was divided into four slabs perpendicular to the long axis of the hippocampus. As in the electron microscopy experiments, Western blot analysis was confined to the dorsomedial hippocampus due to its importance for Morris water maze learning and memory tasks [31,50,51]. Using anatomical landmarks, each slab was dissected further to isolate the DG; CA3 was separated from the DG with a cut connecting the ends of the supragranular and infragranular blades of the DG, and then the DG and CA1 were separated along the hippocampal fissure. Samples were weighed, frozen on dry ice, and stored at -80°C. Tissue from the right hippocampus was prepared for the present study and tissue from the left hippocampus was prepared for a separate study [54]. Samples were homogenized using a ground glass dounce with 50 volumes of Laemmli homogenization buffer (60 mM TrisCl, pH 6.8, 10% glycerol, 2% SDS, 200mM EDTA) per gram of tissue, with protease inhibitor cocktail (P8340, 1:250, Sigma-Aldrich, Inc., St. Louis, MI) and phosphatase-inhibitors (P5726, 0.5%, Sigma-Aldrich, Inc., St. Louis, MI). Use of this homogenization buffer has been reported to permit 100% solubilization of the NMDAR in each sample [4]. The soluble supernatant was stored at -80°C. The protein concentration of each supernatant was determined using a BCA protein assay (Pierce Technology, Rockford, IL).
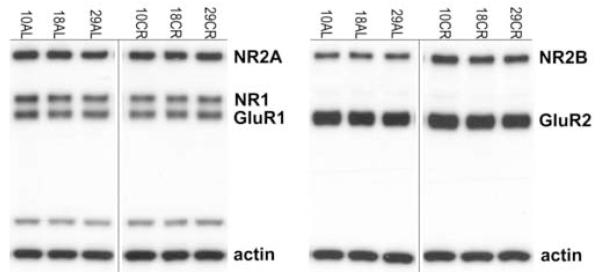
For NMDAR and AMPAR subunit analysis, 7.5μg of each homogenized sample were loaded into wells on the left half of a 15-well, 7.5% BioRad gel in a cohort of six (one sample per condition, 2 diets X 3 ages; Figure 2) that was duplicated on the right half of the gel. Samples were separated under reducing conditions using SDS-PAGE and then electrophorectically transferred onto Immobilon membranes using a BioRad Mini Transfer Cell. Rabbit polyclonal antibodies to the NR1 (0.6 μg/ml, Chemicon International, Temencula, CA), NR2A (0.07 μg/ml, Chemicon), NR2B (0.07 μg/ml, Chemicon), GluR1 (0.01 μg/ml, Upstate, Lake Placid, NY) and GluR2 (0.25 μg/ml, Chemicon) subunits were used for immunodetection. All of the antibodies were affinity purified and tested for specificity; no antibody cross-reacted with other receptor subunits in the family. A mouse monoclonal antibody to actin (0.002 μg/ml, Chemicon) was used to verify equal protein loading. Peroxidase-conjugated donkey anti-rabbit IgG (10 ng/ml, Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) and peroxidase-conjugated donkey anti-mouse IgG (10 ng/ml, Jackson) secondary antibodies were used with the SuperSignal West Pico Chemiluminescent Substrate (Pierce Technology, Rockford, IL) for detection. Blots were exposed on Kodak Biomax film and quantitated densitometrically with BioRad VersaDoc and Quantity One analysis software (BioRad Laboratories, Hercules, CA) in order to determine the relative amounts of each protein. The Gaussian trace optical density of each band was normalized to the average density of all of the matching bands within each cohort to permit comparison between blots and experiments. A repeated measurement was excluded if it was greater than two standard deviations away from the mean. Each blot was repeated at least once, thus generating a minimum of four acceptable measurements per antibody per sample.
Figure 2.
Representative Western blots showing glutamate receptor subunits NR2A, NR1, and GluR1 in the blot on the left and NR2B and GluR2 in the blot on the right for AL and CR rats at young (10 months), middle (18 months) and old (29 months) ages. Actin was included on all gels as a control for equal protein loading. Samples were processed in cohorts of six, one sample per age/diet group, which was repeated on the right half of the gel. The bands directly above actin on the left are nonspecific bands associated with the NR1 antibody.
2.5. Statistical Analysis
Statistical analyses were performed using GraphPad Prism (version 4.00 for Windows; GraphPad Software, San Diego, CA) and SigmaStat (Systat Software, Inc., Richmond, CA). The main effects and interaction of the two independent variables, age and diet, were evaluated using two-way univariate analysis of variance with Bonferroni’s post test. A two-tailed significance level for each of the main effects was held at 0.05; means are reported +/- standard deviation. A Bonferroni adjustment was used for multiple comparisons to control for a type I error. In the absence of a main effect of diet, AL and CR groups were combined to increase the power to detect change with age. In these cases, the effect of age on the dependent variables was determined using a one-way univariate analysis of variance with Tukey’s post test. Hartley’s F-max test was used to test the homogeneity of variances among combined AL and CR groups for the number of overall synapses and MSB synapses per neuron.
3. Results
3.1. CR rats weighed less than AL rats
All CR rats weighed less than age-matched AL rats (10CR, 275 g +/- 10 vs 10AL, 425 g +/- 26; 18CR, 300 g +/- 10 vs 18AL, 502 g +/- 25; 28CR, 311 g +/- 8 vs 28AL, 558 g +/- 20), but appeared healthy and had full coats of fur. The differences in mean weight rats varied significantly with both age (F(2,75) = 103.81, p <0.0001) and diet (F(1,75) = 1667.44, p<0.0001). For both AL and CR rats, the average weights at 18 and 29 months were higher than those at 10 months (p’s<0.001). Only in AL rats, however, was the average weight at 29 months significantly higher than that at 18 months (p<0.001). On average, the weight of CR rats was 40% lower than that of AL rats (35%, 40%, 44%, at 10, 18, and 29 months, respectively). At no point did the weight of any CR rat exceed that of any age-matched AL rat.
3.2. Effects of age and CR on DG synapses
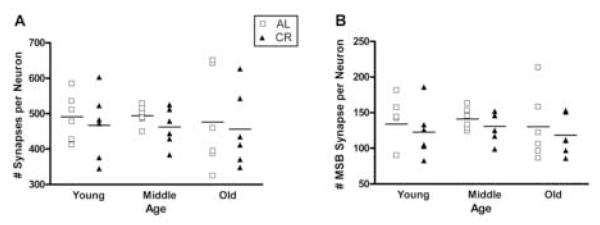
The numerical densities of neurons and synapses were determined stereologically (Table 1) and the overall synapses per neuron and MSB synapses per neuron were derived from these values. There were no main effects of age or diet on the overall number of synapses per neuron (age, F(2,30)=0.08, p=0.922; diet, F(1, 30)=0.73, p=0.401, Figure 3A) or the number of MSB synapses per neuron (age, F(2,30)= 0.40, p=0.676; diet, F(1, 30)=1.05, p=0.314, Figure 3B).
Table 1.
Numerical Density of Neurons, Synapses, and MSB Synapses
| Neurons/ mm3 | Synapses/mm3 | MSB Synapses/mm3 |
||
|---|---|---|---|---|
| 10AL N=6 |
mean | 3.10 × 106 | 1.52 × 109 | 4.17 × 108 |
| StDev | 0.09 × 106 | 0.20 × 109 | 1.10 × 108 | |
| SEM | 0.04 × 106 | 0.09 × 109 | 0.49 × 108 | |
| CE | 0.01 | 0.06 | 0.12 | |
| CV% | 3 | 13 | 26 | |
| 10CR N=6 |
mean | 3.04 × 106 | 1.41 × 109 | 3.69 × 108 |
| StDev | 0.18 × 106 | 0.21 × 109 | 0.90 × 108 | |
| SEM | 0.81 × 106 | 0.94 × 109 | 0.40 × 108 | |
| CE | 0.03 | 0.07 | 0.11 | |
| CV% | 6 | 15 | 24 | |
| 18AL N=6 |
mean | 3.20 × 106 | 1.58 × 109 | 4.50 × 108 |
| StDev | 0.11 × 106 | 0.09 × 109 | 0.64 × 108 | |
| SEM | 0.05 × 106 | 0.04 × 109 | 0.29 × 108 | |
| CE | 0.02 | 0.03 | 0.06 | |
| CV% | 4 | 6 | 14 | |
| 18CR N=6 |
mean | 3.17 × 106 | 1.45 × 109 | 4.07 × 108 |
| StDev | 0.32 × 106 | 0.11 × 109 | 0.46 × 108 | |
| SEM | 0.14 × 106 | 0.05 × 109 | 0.21 × 108 | |
| CE | 0.05 | 0.03 | 0.05 | |
| CV% | 10 | 8 | 11 | |
| 29AL N=6 |
mean | 3.16 × 106 | 1.48 × 109 | 4.04 × 108 |
| StDev | 0.19 × 106 | 0.34 × 109 | 1.25 × 108 | |
| SEM | 0.85 × 106 | 0.15 × 109 | 0.56 × 106 | |
| CE | 0.03 | 0.10 | 0.14 | |
| CV% | 6 | 23 | 31 | |
| 29CR N=6 |
mean | 3.25 × 106 | 1.46 × 109 | 3.79 × 108 |
| Stdev | 0.29 × 106 | 0.24 × 109 | 0.64 × 108 | |
| SEM | 0.13 × 106 | 0.11 × 109 | 0.29 × 108 | |
| CE | 0.04 | 0.07 | 0.08 | |
| CV% | 9 | 17 | 17 | |
Figure 3.
Scatter plot diagrams of the number of synapses per neuron (A) and the number of MSB synapses per neuron (B) for AL (open squares) and CR groups (filled triangles) at young (10 months), middle (18 months) and old (29 months) ages. The horizontal lines indicate the mean for each group. In no case did the means change as a function of age or diet.
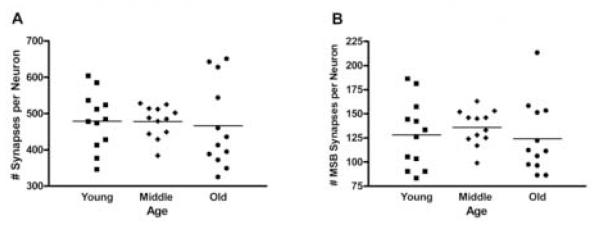
Because AL and CR groups did not differ statistically at any age, the data from those two groups were combined at each age to increase the power to detect potential changes across lifespan. Even with the resulting N of 12 per age, there was no significant main effect of age for either overall synapses per neuron (F(1,30)=0.087, p=0.917, Figure 4A) or MSB synapses per neuron (F(1,30)=0.422, p=0.659, Figure 4B). Although the mean number of synapses did not differ with age, Hartley’s F-max test revealed significantly larger variances in the number of synapses per neuron (p<0.05) at 29 months compared to 18 months as well as in the number of MSB synapses per neuron at 10 and 29 months compared to 18 months (p<0.05).
Figure 4.
Scatter plot diagrams of the number synapses per neuron (A) and the number of MSB synapses per neuron (B) for the AL and CR groups combined at young (10 months), middle (18 months) and old (29 months) ages. There are no significant differences in the means between any ages in either A or B; however, the variances at young and old ages in both are clearly greater than at middle age. The difference between variances was significant for synapses between middle and old age (A, p<0.05) and for MSB synapses between young and middle age and middle and old age (B, p<0.05 for each).
3.4. Effect of aging and CR on relative levels of NMDAR and AMPAR subunits
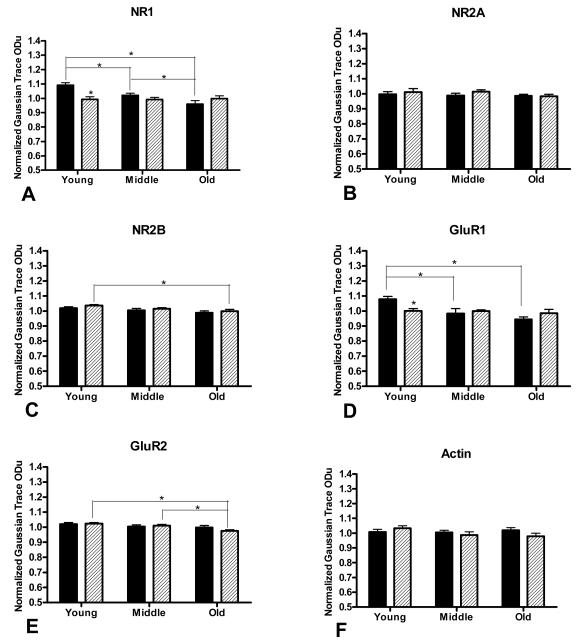
In the Western Blot experiments, we compared relative protein levels of the NR1, NR2A, and NR2B subunits of the NMDAR and of the GluR1 and GluR2 subunits of the AMPAR in young, middle aged, and old AL and CR rats. For NR1 (Figure 5A), there was a significant main effect of age (F(2,38)=6.359, p<0.01) and diet (F(1,38)=6.233, p<0.05) and a significant interaction between the two variables (F(2,38)=7.581, p<0.01). Levels of NR1 in both 18AL and 29AL groups were significantly lower than in the 10 AL group (5.8% decrease in mean, p<0.05; and 12.1% decrease, p<0.001; respectively) and 29AL levels were lower than 18AL levels (7% decrease, p<0.05). NR1 levels in the 10CR group also were significantly lower than in the 10AL group (9.1%, p<0.001), but did not differ between the two dietary conditions at 18 (p=0.059) or 29 months (p=0.139). NR1 levels in CR animals did not differ significantly among ages (all p’s>0.05). For NR2A (Figure 5B), there was no main effect of age (F(2,41)=0.927, p=0.404) or diet (F(1,41)=0.831, p=0.367) nor an interaction between the two variables (F(2,41)=0.391, p=0.679). Statistical analysis of NR2B levels (Figure 5C) demonstrated a main effect of age (F(2,39)=5.333, p<0.01), but not diet (F(1,39)=2.136, p =0.152), and no interaction between the two variables (F(2,39)=0.912, p =0.912). Within CR animals, NR2B levels in the 29CR group were slightly, yet significantly, lower than in the 10CR group (3.6% decrease, p<0.05). There were no other significant differences among age or diet groups for NR2B. There was a main effect of age (F(2,40)=6.080, p<0.01), but not diet (F(1,40)=0.138, p =0.712) on GluR1 levels (Figure 5D) as well as a significant interaction between the two variables (F(2,40)=4.331, p<0.05). Within the AL, but not CR groups, there were significant differences in GluR1 levels between individual age groups. Specifically, GluR1 levels were lower in both the 18AL and 29AL groups than in the 10AL group (8.9% decrease, p<0.01; and 12.4% decrease, p<0.001; respectively). Within the 10 month cohort, CR levels were significantly lower than the AL levels (7.2% decrease, p<0.05), but there were no differences between diet groups at 18 (p=0.570) or 29 months (p=0.178). Finally, for GluR2 (Figure 5E), there was a main effect of age (F(2,39)=6.612, p<0.01), but not diet (F(1,39)=0.291, p=0.593). No significant interaction between the variables was found (F(2,39)=1.315, p =0.280). Within the CR group, GluR2 levels in the 29 month group were lower than at 10 months (4.7% decrease, p<0.01) and 18 months (3.5% decrease, p<0.05). GluR2 levels did not differ among age groups in AL animals. For actin, there were no main effects of age or diet, nor was there an interaction between the two variables (all p’s>0.05, Figure 5F).
Figure 5.
Relative levels of NMDAR subunits NR1 (A), NR2A (B), and NR2B (C), and AMPAR subunits GluR1 (D) and GluR2 (E), and actin (F) as a function of age (young, 10 months; middle aged, 18 months; and old, 29 months) and diet (AL, filled bars; CR, patterned bars). Asterisks above the CR bars at young age for NR1 (A) and GluR1 (D) indicate significant differences compared to AL animals (p<0.05). Asterisks above connector lines indicate significant aging-related decreases in receptor subunits (p<0.05). These data represent the mean ± standard deviation of eight animals per experimental condition.
4. Discussion
The results of the present study demonstrate that the number of synapses per neuron in the MML of the DG region of hippocampus do not change across lifespan and are unaffected by CR at young, middle, and old age. In contrast to the that stability of synapses across ages and diets, glutamate receptor subunits in the DG decline as a function of age and with CR. Aging-related decreases in NR1 and GluR1 occur in AL rats. In young rats, levels of these subunits are significantly lower in CR than in AL groups, but the levels in CR rats then remain stable across lifespan. These data demonstrate that CR eliminates the aging-related decline in NR1 and GluR1 subunits by inducing and then maintaining stable, lower levels of these subunits across lifespan.
4.1. Mean number of synapses per neuron in the MML of the DG does not change with diet or age
Although CR-induced changes in dendritic spines and LTP have been associated with alterations in synapse structure in previous experiments [15,48], CR produced no significant changes in the number of synapses per neuron at any age in the present study. Specifically, CR had no impact either on overall synapses or MSB synapses normalized to neuron number. For AL and CR groups combined, the results also indicated that overall synapses and MSB synapses per neuron did not change across the three ages examined. Other studies have reported a similar preservation of DG synapses over lifespan based on synaptophysin labeling of synaptic boutons [7,56,70] and a stereologic study examining CA1 synapses at 6 and 27 months also showed no aging-related change [20]. The only other stereological investigation of synapses in the rodent DG reported a decline between the two ages examined in that study, 5 and 28 months [22]. The variation in findings between that study and the present one likely reflects the difference in animal ages. Although a 5 month old rat is sexually mature, it may well be that brain development continues beyond that age. Increases in dendritic extent observed in the supraoptic nucleus between 3 and 12 months of age have been interpreted to indicate the presence of ongoing maturational changes [16]. Moreover, synapses in CA3 have been demonstrated stereologically to decrease by 24% between 3 and 12 months of age [12]. Inclusion of intermediate ages would narrow the time period between 5 and 28 months during which synaptic loss occurs [11]. In light of the present results, we would predict a loss of synapses to occur between 5 and 10 months of age.
The present study used the physical disector to quantify synapses stereologically in the MML of the DG. Using this methodology, the disector height is determined based on the size of a specific counting object. In this study, the disector height was optimized for the quantification of simple synapse densities. Accordingly, the serial pairs of thin sections utilized here are not suitable for analysis of perforated synapses or entire MSB complexes. Such analysis requires serial reconstruction of these structures, and studies using this approach are currently underway in our laboratory. In the absence of a change in synapses per neuron, these studies may reveal subtle changes in synaptic structure.
4.2. Aging and caloric restriction modulate levels of glutamate receptor subunits
In light of the finding that neither aging nor CR affects the number of synapses per neuron in the MML of the DG, we used Western blot analysis to evaluate whether these variables affect synapses by influencing the levels of glutamate receptor subunits. The results indicated that subunit levels in AL rats declined (NR1 and GluR1) or did not change (NR2A, NR2B, GluR2) across lifespan; no subunit increased with age. These observations are consistent with the idea that a decline in one or more glutamate receptor subunits accompanies the aging-related learning and memory deficits. CR then might be expected to prevent aging-related declines in glutamate receptor subunits, since CR has been shown to prevent aging-related learning and memory deficits [25,26,43,60,61,73]. Interestingly, CR did not prevent the absolute declines in NR1 and GluR1 that occurred in AL rats. Instead, it resulted in significantly lower subunit levels by 10 months of age that then were maintained across lifespan. In comparison with NR1 and GluR1, levels of the other subunits were relatively stable across ages and dietary conditions. Even the CR-related declines in NR2B and GluR2 across age groups were so small as to be of questionable biological significance.
Several studies reported in the literature have used Western blot analysis to examine protein levels of NMDAR and AMPAR subunits in rat hippocampus across lifespan [1,9,15,47,71] and following manipulation of food intake [15,47]. The results of those studies, however, cannot be compared directly to the present findings. In none of those studies were NMDAR or AMPAR subunit levels measured in separate hippocampal subregions, and subunit levels in CA1 and/or CA3 could mask a unique response in the DG. In addition, the published data were collected from different rat strains, and the effect of CR on hippocampus-dependent learning and memory performance is known to vary among strains [43]. The ages examined in some of those studies also varied substantially from those examined in the present study. Finally, of the two studies that assessed the effects of dietary manipulation, only one used the same 60% CR regimen as in the present study [43]; the other employed an every-other-day feeding schedule [47] recognized to have distinct biological effects [2].
Western blot analysis in the present study assessed the overall levels of receptor subunit protein in the DG, but did not distinguish between levels in the synaptic and cytosolic compartments. Modulation of receptor subunits may occur as alterations in receptor composition, trafficking, or distribution within the synapse. Determining whether receptor subunits in the synaptic compartments specifically are affected by aging or CR requires ultrastructural localization of receptor subunits with postembedding immunogold electron microscopy. Although that technique could not be used in the present analysis as it requires tissue processing incompatible with the techniques used here, immunogold studies to localize receptor subunits that are underway in our laboratory will address this important issue.
In the present study, the only significant differences in relative protein levels of NMDAR and AMPAR subunits between CR and AL groups were in young animals; comparisons between middle aged and old animals revealed no differences between the two groups. In contrast, behavioral evaluation of spatial learning and memory in the same rat strain and at similar ages as in the present study revealed no difference between CR and AL groups at young or middle age [43]. In that study, only old CR animals demonstrated significantly better performance on learning and memory tasks as compared to old AL rats. These findings raise the critical question of whether CR-induced NMDAR and AMPAR subunit changes observed in young animals in the present study have effects restricted to early life or whether the early effects of CR may have important functional implications later in life.
4.3. Caloric restriction may promote biological stability
Considering the remarkable resistance of DG neurons to damage from acute insults such as ischemia or status epilepticus [33,58], it may not be surprising that aging and CR do not alter the number of synapses in the DG. Perhaps the mechanisms that exist to protect the DG from acute insults also act to buffer it against chronic, milder stressors such as aging and CR. CR is, in fact, a mild form of metabolic stress that induces some of the same changes that aging does, including increased glucocorticoids [53,68] and decreased levels of circulating insulin-like growth factor-I [5]. Results from our laboratory revealed a CR-induced increase in levels of brain-derived neurotrophic factor (BDNF) in CA1 similar to the aging-related BDNF increase that occurs in the DG and CA3 [54]. Given that BDNF is a neurotrophin that supports synapses and synaptic function [8,32,75,79], the aging-related increase in BDNF in the DG could contribute to the general stability of the number of synapses per neuron observed in the DG over lifespan. Thus, CR may not decelerate aging-related processes in the DG, but, instead, may stimulate natural protective mechanisms that occur normally during the aging process to help maintain homeostasis. This role for CR is consistent with the notion that CR is a form of “hormesis” [44,45,78], or a mild stressor that potentiates innate defenses that help to guard against subsequent, more egregious insults. If, in fact, the immediate benefits of CR are a consequence of stimulating adaptive responses normally triggered during the aging process, then the protection it affords during aging may reflect its ability to maintain these responses at stable levels over lifespan in order to preserve homeostasis. Such a scenario is consistent with the stability-longevity hypothesis [13] suggesting that the beneficial effects of CR are due to maintaining steady levels of metabolic byproducts. Although much investigation remains to be done to test the validity of this hypothesis, the data presented here are consistent with this concept of aging and the effects of CR.
4.4 Conclusion
In summary, the number of DG synapses per neuron does not change with aging or CR. Although some glutamate receptor subunit levels do decrease across lifespan in AL animals, CR does not prevent their decline. Instead, the levels of those subunits are reduced in CR animals early in life, by 10 months of age, after which they remain stable across lifespan. Further studies will be required to determine whether later introduction or more limited duration of CR will yield similar findings.
Acknowledgements
This work was supported by National Institute on Aging grants AG11370 and AG019886 (J.K.B-B. and D.R.R.). It was completed in partial fulfillment of the requirements for the Ph.D. degree in the Department of Neurobiology and Anatomy, Wake Forest University School of Medicine (I.G.N.). We thank Ashley Long and Dr. Lei Shi for assistance with statistical analysis and Dr. Michelle Adams for critical reading of the manuscript.
Footnotes
Disclosure Statement: The authors have had no actual or potential financial, personal, or other relationships with people or organizations within three years of beginning this work that could inappropriately influence or bias their work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- [1].Adams MM, Smith TD, Moga D, Gallagher M, Wang Y, Wolfe BB, Rapp PR, Morrison JH. Hippocampal dependent learning ability correlates with N-methyl-D-aspartate (NMDA) receptor levels in CA3 neurons of young and aged rats. J Comp Neurol. 2001;432:230–243. doi: 10.1002/cne.1099. [DOI] [PubMed] [Google Scholar]
- [2].Anson RM, Guo ZH, de Cabo R, Iyun T, Rios M, Hagepanos A, Ingram DK, Lane MA, Mattson MP. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:6216–6220. doi: 10.1073/pnas.1035720100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Barry MF, Ziff EB. Receptor trafficking and the plasticity of excitatory synapses. Curr Opin Neurobiol. 2002;12:279–286. doi: 10.1016/s0959-4388(02)00329-x. [DOI] [PubMed] [Google Scholar]
- [4].Blahos J, Wenthold RJ. Relationship between N-methyl-D-aspartate receptor NR1 splice variants and NR2 subunits. J Biol Chem. 1996;271:15669–15674. doi: 10.1074/jbc.271.26.15669. [DOI] [PubMed] [Google Scholar]
- [5].Breese CR, D’Costa A, Booze RM, Sonntag WE. Distribution of insulin-like growth factor 1 (IGF-1) and 2 (IGF-2) receptors in the hippocampal formation of rats and mice. Adv Exp Med Biol. 1991;293:449–458. doi: 10.1007/978-1-4684-5949-4_40. [DOI] [PubMed] [Google Scholar]
- [6].Buchs PA, Muller D. Induction of long-term potentiation is associated with major ultrastructural changes of activated synapses. Proc Natl Acad Sci U S A. 1996;93:8040–8045. doi: 10.1073/pnas.93.15.8040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Calhoun ME, Kurth D, Phinney AL, Long JM, Hengemihle J, Mouton PR, Ingram DK, Jucker M. Hippocampal neuron and synaptophysin-positive bouton number in aging C57BL/6 mice. Neurobiol Aging. 1998;19:599–606. doi: 10.1016/s0197-4580(98)00098-0. [DOI] [PubMed] [Google Scholar]
- [8].Causing CG, Gloster A, Aloyz R, Bamji SX, Chang E, Fawcett J, Kuchel G, Miller FD. Synaptic innervation density is regulated by neuron-derived BDNF. Neuron. 1997;18:257–267. doi: 10.1016/s0896-6273(00)80266-4. [DOI] [PubMed] [Google Scholar]
- [9].Clayton DA, Browning MD. Deficits in the expression of the NR2B subunit in the hippocampus of aged Fisher 344 rats. Neurobiol Aging. 2001;22:165–168. doi: 10.1016/s0197-4580(00)00196-2. [DOI] [PubMed] [Google Scholar]
- [10].Clayton DA, Mesches MH, Alvarez E, Bickford PC, Browning MD. A hippocampal NR2B deficit can mimic age-related changes in long-term potentiation and spatial learning in the Fischer 344 rat. J Neurosci. 2002;22:3628–3637. doi: 10.1523/JNEUROSCI.22-09-03628.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Coleman P, Finch C, Joseph J. The need for multiple time points in aging studies. Neurobiol Aging. 2004;25:3–4. doi: 10.1016/j.neurobiolaging.2003.10.001. [DOI] [PubMed] [Google Scholar]
- [12].DeGroot DMG, Bierman EPB. Numerical changes in rat hippocampal synapses. An effect of “ageing”? Acta Stereologica. 1987;6:53–58. [Google Scholar]
- [13].Demetrius L. Caloric restriction, metabolic rate, and entropy. J Gerontol A Biol Sci Med Sci. 2004;59:B902–B915. doi: 10.1093/gerona/59.9.b902. [DOI] [PubMed] [Google Scholar]
- [14].Desmond NL, Weinberg RJ. Enhanced expression of AMPA receptor protein at perforated axospinous synapses. Neuroreport. 1998;9:857–860. doi: 10.1097/00001756-199803300-00017. [DOI] [PubMed] [Google Scholar]
- [15].Eckles-Smith K, Clayton D, Bickford P, Browning MD. Caloric restriction prevents age-related deficits in LTP and in NMDA receptor expression. Brain Res Mol Brain Res. 2000;78:154–162. doi: 10.1016/s0169-328x(00)00088-7. [DOI] [PubMed] [Google Scholar]
- [16].Flood DG, Coleman PD. Dendritic regression dissociated from neuronal death but associated with partial deafferentation in aging rat supraoptic nucleus. Neurobiol Aging. 1993;14:575–587. doi: 10.1016/0197-4580(93)90042-a. [DOI] [PubMed] [Google Scholar]
- [17].Foster TC. Involvement of hippocampal synaptic plasticity in age-related memory decline. Brain Res Brain Res Rev. 1999;30:236–249. doi: 10.1016/s0165-0173(99)00017-x. [DOI] [PubMed] [Google Scholar]
- [18].Geinisman Y, Berry RW, Disterhoft JF, Power JM, Van der Zee EA. Associative learning elicits the formation of multiple-synapse boutons. J Neurosci. 2001;21:5568–5573. doi: 10.1523/JNEUROSCI.21-15-05568.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Geinisman Y, Detoledo-Morrell L, Morrell F, Heller RE. Hippocampal markers of age-related memory dysfunction: behavioral, electrophysiological and morphological perspectives. Prog Neurobiol. 1995;45:223–252. doi: 10.1016/0301-0082(94)00047-l. [DOI] [PubMed] [Google Scholar]
- [20].Geinisman Y, Ganeshina O, Yoshida R, Berry RW, Disterhoft JF, Gallagher M. Aging, spatial learning, and total synapse number in the rat CA1 stratum radiatum. Neurobiol Aging. 2004;25:407–416. doi: 10.1016/j.neurobiolaging.2003.12.001. [DOI] [PubMed] [Google Scholar]
- [21].Geinisman Y, Toledo-Morrell L, Morrell F, Heller RE, Rossi M, Parshall RF. Structural synaptic correlate of long-term potentiation: formation of axospinous synapses with multiple, completely partitioned transmission zones. Hippocampus. 1993;3:435–445. doi: 10.1002/hipo.450030405. [DOI] [PubMed] [Google Scholar]
- [22].Geinisman Y, Toledo-Morrell L, Morrell F, Persina IS, Rossi M. Age-related loss of axospinous synapses formed by two afferent systems in the rat dentate gyrus as revealed by the unbiased stereological dissector technique. Hippocampus. 1992;2:437–444. doi: 10.1002/hipo.450020411. [DOI] [PubMed] [Google Scholar]
- [23].Gilbert PE, Kesner RP, Lee I. Dissociating hippocampal subregions: double dissociation between dentate gyrus and CA1. Hippocampus. 2001;11:626–636. doi: 10.1002/hipo.1077. [DOI] [PubMed] [Google Scholar]
- [24].Hering H, Sheng M. Dendritic spines: structure, dynamics and regulation. Nat Rev Neurosci. 2001;2:880–888. doi: 10.1038/35104061. [DOI] [PubMed] [Google Scholar]
- [25].Idrobo F, Nandy K, Mostofsky DI, Blatt L, Nandy L. Dietary restriction: effects on radial maze learning and lipofuscin pigment deposition in the hippocampus and frontal cortex. Arch Gerontol Geriatr. 1987;6:355–362. doi: 10.1016/0167-4943(87)90014-8. [DOI] [PubMed] [Google Scholar]
- [26].Ingram DK, Weindruch R, Spangler EL, Freeman JR, Walford RL. Dietary restriction benefits learning and motor performance of aged mice. J Gerontol. 1987;42:78–81. doi: 10.1093/geronj/42.1.78. [DOI] [PubMed] [Google Scholar]
- [27].Jeltsch H, Bertrand F, Lazarus C, Cassel JC. Cognitive performances and locomotor activity following dentate granule cell damage in rats: role of lesion extent and type of memory tested. Neurobiol Learn Mem. 2001;76:81–105. doi: 10.1006/nlme.2000.3986. [DOI] [PubMed] [Google Scholar]
- [28].Jones TA. Multiple synapse formation in the motor cortex opposite unilateral sensorimotor cortex lesions in adult rats. J Comp Neurol. 1999;414:57–66. [PubMed] [Google Scholar]
- [29].Jones TA, Chu CJ, Grande LA, Gregory AD. Motor skills training enhances lesion-induced structural plasticity in the motor cortex of adult rats. J Neurosci. 1999;19:10153–10163. doi: 10.1523/JNEUROSCI.19-22-10153.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Jones TA, Klintsova AY, Kilman VL, Sirevaag AM, Greenough WT. Induction of multiple synapses by experience in the visual cortex of adult rats. Neurobiol Learn Mem. 1997;68:13–20. doi: 10.1006/nlme.1997.3774. [DOI] [PubMed] [Google Scholar]
- [31].Jung MW, Wiener SI, McNaughton BL. Comparison of spatial firing characteristics of units in dorsal and ventral hippocampus of the rat. J Neurosci. 1994;14:7347–7356. doi: 10.1523/JNEUROSCI.14-12-07347.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kang H, Schuman EM. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science. 1995;267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- [33].Kullmann DM, Asztely F, Walker MC. The role of mammalian ionotropic receptors in synaptic plasticity: LTP, LTD and epilepsy. Cellular and Molecular Life Sciences. 2000;57:1551–1561. doi: 10.1007/PL00000640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Leuner B, Falduto J, Shors TJ. Associative memory formation increases the observation of dendritic spines in the hippocampus. J Neurosci. 2003;23:659–665. doi: 10.1523/JNEUROSCI.23-02-00659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Liao WH, Van Den AT, Herman P, Frachet B, Huy PT, Lecain E, Marianowski R. Expression of NMDA, AMPA and GABA(A) receptor subunit mRNAs in the rat auditory brainstem. II. Influence of intracochlear electrical stimulation. Hear Res. 2000;150:12–26. doi: 10.1016/s0378-5955(00)00167-2. [DOI] [PubMed] [Google Scholar]
- [36].Lichtenwalner RJ, Forbes ME, Bennett SA, Lynch CD, Sonntag WE, Riddle DR. Intracerebroventricular infusion of insulin-like growth factor-I ameliorates the age-related decline in hippocampal neurogenesis. Neuroscience. 2001;107:603–613. doi: 10.1016/s0306-4522(01)00378-5. [DOI] [PubMed] [Google Scholar]
- [37].Luscher C, Frerking M. Restless AMPA receptors: implications for synaptic transmission and plasticity. Trends Neurosci. 2001;24:665–670. doi: 10.1016/s0166-2236(00)01959-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Luscher C, Nicoll RA, Malenka RC, Muller D. Synaptic plasticity and dynamic modulation of the postsynaptic membrane. Nat Neurosci. 2000;3:545–550. doi: 10.1038/75714. [DOI] [PubMed] [Google Scholar]
- [39].Magnusson KR. Aging of glutamate receptors: correlations between binding and spatial memory performance in mice. Mech Ageing Dev. 1998;104:227–248. doi: 10.1016/s0047-6374(98)00076-1. [DOI] [PubMed] [Google Scholar]
- [40].Magnusson KR. Declines in mRNA expression of different subunits may account for differential effects of aging on agonist and antagonist binding to the NMDA receptor. J Neurosci. 2000;20:1666–1674. doi: 10.1523/JNEUROSCI.20-05-01666.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Magnusson KR, Nelson SE, Young AB. Age-related changes in the protein expression of subunits of the NMDA receptor. Brain Res Mol Brain Res. 2002;99:40–45. doi: 10.1016/s0169-328x(01)00344-8. [DOI] [PubMed] [Google Scholar]
- [42].Malenka RC, Nicoll RA. Silent synapses speak up. Neuron. 1997;19:473–476. doi: 10.1016/s0896-6273(00)80362-1. [DOI] [PubMed] [Google Scholar]
- [43].Markowska AL, Savonenko A. Retardation of cognitive aging by life-long diet restriction: implications for genetic variance. Neurobiol Aging. 2002;23:75–86. doi: 10.1016/s0197-4580(01)00249-4. [DOI] [PubMed] [Google Scholar]
- [44].Masoro EJ. Hormesis and the antiaging action of dietary restriction. Exp Gerontol. 1998;33:61–66. doi: 10.1016/s0531-5565(97)00071-5. [DOI] [PubMed] [Google Scholar]
- [45].Masoro EJ. Caloric restriction and aging: an update. Experimental Gerontology. 2000;35:299–305. doi: 10.1016/s0531-5565(00)00084-x. [DOI] [PubMed] [Google Scholar]
- [46].Mayhew M, Renganathan M, Delbono O. Effectiveness of caloric restriction in preventing age-related changes in rat skeletal muscle. Biochem Biophys Res Commun. 1998;251:95–99. doi: 10.1006/bbrc.1998.9438. [DOI] [PubMed] [Google Scholar]
- [47].Monti B, Virgili M, Contestabile A. Alterations of markers related to synaptic function in aging rat brain, in normal conditions or under conditions of long-term dietary manipulation. Neurochem Int. 2004;44:579–584. doi: 10.1016/j.neuint.2003.10.007. [DOI] [PubMed] [Google Scholar]
- [48].Moroi-Fetters SE, Mervis RF, London ED, Ingram DK. Dietary restriction suppresses age-related changes in dendritic spines. Neurobiol Aging. 1989;10:317–322. doi: 10.1016/0197-4580(89)90042-0. [DOI] [PubMed] [Google Scholar]
- [49].Moser MB. Making more synapses: a way to store information? Cell Mol Life Sci. 1999;55:593–600. doi: 10.1007/s000180050317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Moser MB, Moser EI. Functional differentiation in the hippocampus. Hippocampus. 1998;8:608–619. doi: 10.1002/(SICI)1098-1063(1998)8:6<608::AID-HIPO3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- [51].Moser MB, Moser EI, Forrest E, Andersen P, Morris RG. Spatial learning with a minislab in the dorsal hippocampus. Proc Natl Acad Sci U S A. 1995;92:9697–9701. doi: 10.1073/pnas.92.21.9697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Moser MB, Trommald M, Andersen P. An increase in dendritic spine density on hippocampal CA1 pyramidal cells following spatial learning in adult rats suggests the formation of new synapses. Proc Natl Acad Sci U S A. 1994;91:12673–12675. doi: 10.1073/pnas.91.26.12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Nelson JF, Karelus K, Bergman MD, Felicio LS. Neuroendocrine involvement in aging: evidence from studies of reproductive aging and caloric restriction. Neurobiol Aging. 1995;16:837–843. doi: 10.1016/0197-4580(95)00072-m. [DOI] [PubMed] [Google Scholar]
- [54].Newton IG, Forbes ME, Legault C, Johnson JE, Brunso-Bechtold JK, Riddle DR. Caloric restriction does not reverse aging-related changes in hippocampal BDNF. Neurobiol Aging. 2005;26:683–688. doi: 10.1016/j.neurobiolaging.2004.06.005. [DOI] [PubMed] [Google Scholar]
- [55].Nicolas A, Michaud M, Lavigne G, Montplaisir J. The influence of sex, age and sleep/wake state on characteristics of periodic leg movements in restless legs syndrome patients. Clin Neurophysiol. 1999;110:1168–1174. doi: 10.1016/s1388-2457(99)00033-4. [DOI] [PubMed] [Google Scholar]
- [56].Nicolle MM, Gallagher M, McKinney M. No loss of synaptic proteins in the hippocampus of aged, behaviorally impaired rats. Neurobiol Aging. 1999;20:343–348. doi: 10.1016/s0197-4580(99)00054-8. [DOI] [PubMed] [Google Scholar]
- [57].Nikonenko I, Jourdain P, Alberi S, Toni N, Muller D. Activity-induced changes of spine morphology. Hippocampus. 2002;12:585–591. doi: 10.1002/hipo.10095. [DOI] [PubMed] [Google Scholar]
- [58].Opitz T, Grooms SY, Bennett MV, Zukin RS. Remodeling of alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor subunit composition in hippocampal neurons after global ischemia. Proc Natl Acad Sci U S A. 2000;97:13360–13365. doi: 10.1073/pnas.97.24.13360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 1st Edition Academic Press; San Diego, CA: 1986. [Google Scholar]
- [60].Pitsikas N, Algeri S. Deterioration of spatial and nonspatial reference and working memory in aged rats: protective effect of life-long calorie restriction. Neurobiol Aging. 1992;13:369–373. doi: 10.1016/0197-4580(92)90110-j. [DOI] [PubMed] [Google Scholar]
- [61].Pitsikas N, Carli M, Fidecka S, Algeri S. Effect of life-long hypocaloric diet on age-related changes in motor and cognitive behavior in a rat population. Neurobiol Aging. 1990;11:417–423. doi: 10.1016/0197-4580(90)90008-n. [DOI] [PubMed] [Google Scholar]
- [62].Racca C, Stephenson FA, Streit P, Roberts JD, Somogyi P. NMDA receptor content of synapses in stratum radiatum of the hippocampal CA1 area. J Neurosci. 2000;20:2512–2522. doi: 10.1523/JNEUROSCI.20-07-02512.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Rapp PR, Gallagher M. Preserved neuron number in the hippocampus of aged rats with spatial learning deficits. Proc Natl Acad Sci U S A. 1996;93:9926–9930. doi: 10.1073/pnas.93.18.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Rapp PR, Stack EC, Gallagher M. Morphometric studies of the aged hippocampus: I. Volumetric analysis in behaviorally characterized rats. J Comp Neurol. 1999;403:459–470. [PubMed] [Google Scholar]
- [65].Rasmussen T, Schliemann T, Sorensen JC, Zimmer J, West MJ. Memory impaired aged rats: no loss of principal hippocampal and subicular neurons. Neurobiol Aging. 1996;17:143–147. doi: 10.1016/0197-4580(95)02032-2. [DOI] [PubMed] [Google Scholar]
- [66].Riedel G, Platt B, Micheau J. Glutamate receptor function in learning and memory. Behav Brain Res. 2003;140:1–47. doi: 10.1016/s0166-4328(02)00272-3. [DOI] [PubMed] [Google Scholar]
- [67].Rosenzweig ES, Barnes CA. Impact of aging on hippocampal function: plasticity, network dynamics, and cognition. Prog Neurobiol. 2003;69:143–179. doi: 10.1016/s0301-0082(02)00126-0. [DOI] [PubMed] [Google Scholar]
- [68].Sabatino F, Masoro EJ, McMahan CA, Kuhn RW. Assessment of the role of the glucocorticoid system in aging processes and in the action of food restriction. Journal of Gerontology. 1991;46:B171–B179. doi: 10.1093/geronj/46.5.b171. [DOI] [PubMed] [Google Scholar]
- [69].Shi L, Poe BH, Constance LM, Sonntag WE, Brunso-Bechtold JK. Caloric restricted male rats demonstrate fewer synapses in layer 2 of sensorimotor cortex. Brain Res. 2002;931:32–40. doi: 10.1016/s0006-8993(02)02249-7. [DOI] [PubMed] [Google Scholar]
- [70].Smith TD, Adams MM, Gallagher M, Morrison JH, Rapp PR. Circuit-specific alterations in hippocampal synaptophysin immunoreactivity predict spatial learning impairment in aged rats. J Neurosci. 2000;20:6587–6593. doi: 10.1523/JNEUROSCI.20-17-06587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Sonntag WE, Bennett SA, Khan AS, Thornton PL, Xu X, Ingram RL, Brunso-Bechtold JK. Age and insulin-like growth factor-1 modulate N-methyl-D-aspartate receptor subtype expression in rats. Brain Res Bull. 2000;51:331–338. doi: 10.1016/s0361-9230(99)00259-2. [DOI] [PubMed] [Google Scholar]
- [72].Sterio DC. The unbiased estimation of number and sizes of arbitrary particles using the disector. J Microsc. 1984;134(Pt 2):127–136. doi: 10.1111/j.1365-2818.1984.tb02501.x. [DOI] [PubMed] [Google Scholar]
- [73].Stewart J, Mitchell J, Kalant N. The effects of life-long food restriction on spatial memory in young and aged Fischer 344 rats measured in the eight-arm radial and the Morris water mazes. Neurobiol Aging. 1989;10:669–675. doi: 10.1016/0197-4580(89)90003-1. [DOI] [PubMed] [Google Scholar]
- [74].Tang YP, Wang H, Feng R, Kyin M, Tsien JZ. Differential effects of enrichment on learning and memory function in NR2B transgenic mice. Neuropharmacology. 2001;41:779–790. doi: 10.1016/s0028-3908(01)00122-8. [DOI] [PubMed] [Google Scholar]
- [75].Thoenen H. Neurotrophins and neuronal plasticity. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- [76].Toni N, Buchs PA, Nikonenko I, Bron CR, Muller D. LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature. 1999;402:421–425. doi: 10.1038/46574. [DOI] [PubMed] [Google Scholar]
- [77].Toni N, Buchs PA, Nikonenko I, Povilaitite P, Parisi L, Muller D. Remodeling of synaptic membranes after induction of long-term potentiation. J Neurosci. 2001;21:6245–6251. doi: 10.1523/JNEUROSCI.21-16-06245.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Turturro A, Hass BS, Hart RW. Does caloric restriction induce hormesis? Human and Experimental Toxicology. 2000;19:320–329. doi: 10.1191/096032700678815981. [DOI] [PubMed] [Google Scholar]
- [79].Warner HR, Fernandes G, Wang E. A unifying hypothesis to explain the retardation of aging and tumorigenesis by caloric restriction. J Gerontol A Biol Sci Med Sci. 1995;50:B107–B109. doi: 10.1093/gerona/50a.3.b107. [DOI] [PubMed] [Google Scholar]
- [80].Weibel ER. Stereological methods. Academic Press; New York: 1979. [Google Scholar]
- [81].Wenk GL, Barnes CA. Regional changes in the hippocampal density of AMPA and NMDA receptors across the lifespan of the rat. Brain Res. 2000;885:1–5. doi: 10.1016/s0006-8993(00)02792-x. [DOI] [PubMed] [Google Scholar]
- [82].Wetter TJ, Gazdag AC, Dean DJ, Cartee GD. Effect of calorie restriction on in vivo glucose metabolism by individual tissues in rats. Am J Physiol. 1999;276:E728–E738. doi: 10.1152/ajpendo.1999.276.4.E728. [DOI] [PubMed] [Google Scholar]
- [83].Xavier GF, Oliveira-Filho FJ, Santos AM. Dentate gyrus-selective colchicine lesion and disruption of performance in spatial tasks: difficulties in “place strategy” because of a lack of flexibility in the use of environmental cues? Hippocampus. 1999;9:668–681. doi: 10.1002/(SICI)1098-1063(1999)9:6<668::AID-HIPO8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]