Abstract
Caloric restriction (CR) extends lifespan and ameliorates the aging-related decline in hippocampal-dependent cognitive function. In the present study, we compared subunit levels of NMDA and AMPA types of the glutamate receptor and quantified total synapses and multiple spine bouton (MSB) synapses in hippocampal CA1 from young (10 months), middle-aged (18 months), and old (29 months) Fischer 344 x Brown Norway rats that were ad libitum (AL) fed or caloric restricted (CR) from 4 months of age. Each of these parameters has been reported to be a potential contributor to hippocampal function. Western blot analysis revealed that NMDA and AMPA receptor subunits in AL animals decrease between young and middle age to levels that are present at old age. Interestingly, young CR animals have significantly lower levels of glutamate receptor subunits than young AL animals and those lower levels are maintained across life span. In contrast, stereological quantification indicated that total synapses and MSB synapses are stable across lifespan in both AL and CR rats. These results indicate significant aging-related losses of hippocampal glutamate receptor subunits in AL rats that are consistent with altered synaptic function. CR eliminates that aging-related decline by inducing stable NMDA and AMPA receptor subunit levels.
Keywords: Dietary restriction, Fischer 344 X Brown Norway rats, glutamate receptor, stratum radiatum, electron microscopy, multiple spine bouton (MSB) synapses
Introduction
Aging in rodents is associated with cognitive impairment as well as physiological, anatomical, and biochemical changes in the brain (Geinisman et al., 1995; Rosenzweig and Barnes, 2003). Aging-related cognitive and neural changes can be ameliorated by a variety of manipulations, including supplementation with trophic factors such as nerve growth factor and insulin-like growth factor 1 as well as the initiation of caloric restriction (CR) early in life (Ingram et al., 1987; Fischer et al., 1991; Tacconi et al., 1991; Sohal and Weindruch, 1996; Markowska et al., 1998; Sonntag et al., 1999). CR refers to the proportionate daily reduction of all dietary components and it can extend life expectancy by 20% to 40% in a broad range of species including rodents (Yu et al., 1985; Roth et al., 1995). CR also has been shown to maintain physiological parameters in different body systems at youthful levels and delays the onset of aging-associated diseases (Sohal and Weindruch, 1996; Major et al., 1997; Kalani et al., 2006).
Several studies have indicated that metabolic stability is a better predictor of longevity than metabolic rate. For example, it has been hypothesized that senescence-related loss of function is related to an impairment of homeostatic state and the capacity of an organism to maintain a steady metabolic state is a prime determinant of longevity (McCarter and McGee, 1989; Kirkwood and Shanley, 2005). As CR may enhance longevity by inducing metabolic stability (Demetrius, 2004), it may be that the ability of CR to prevent aging-related cognitive and neural changes is related to a CR-induced stability in the synaptic determinants of hippocampal function.
Earlier studies have revealed that principal neurons in the hippocampus are not lost in aged animals (Rapp and Gallagher, 1996; Morrison and Hof, 1997), suggesting that more subtle changes such as synaptic modifications may be occurring in the aging brain. Synapses are plastic structures and changes in the number of synapses or the ultrastructural composition of existing synapses can occur following microenvironmental alterations in the brain. Moreover, synaptic changes result in a continual refinement of neuronal circuitry (Adams et al., 2001a; Adams et al., 2001b; Nicholson et al., 2004; Tata et al., 2006). Synaptic plasticity is essential for information storage, experience-dependent learning and memory, as well as other phenomena associated with cognition (Martin et al., 2000; Burke and Barnes, 2006).
Previous reports have demonstrated aging-related changes in the structure, composition, and functional capacity of synapses in CA1, CA3 and DG even when total synapse number is maintained. Specifically, both synaptic transmission (Barnes, 1994; Foster and Norris, 1997) and long-term potentiation (LTP), the synaptic phenomena associated with spatial learning and memory, are compromised in aged rodents with cognitive impairments (Rosenzweig et al., 1997; Bach et al., 1999; Tombaugh et al., 2002). Interestingly, CR, which has been shown to protect neurons against excitotoxic, oxidative, and metabolic insults (Ingram et al., 1987; Sohal and Weindruch, 1996; Guo and Mattson, 2000), also ameliorates aging-related changes in synaptic plasticity and neurotransmitter systems. Particularly, it prevents the aging-related deficits in LTP induction in hippocampal CA1, and thus may lessen the impairment of synaptic function in old animals (Eckles-Smith et al., 2000; Okada et al., 2003). CR might exert such beneficial effects by inducing stability of synaptic function across life span similar to the systemic benefits of eliminating the metabolic changes (Yu and Chung, 2001; Koubova and Guarente, 2003). In the presence of life-long CR, a stable state in those parameters may be maintained across life span (Yu and Chung, 2001; Demetrius, 2004).
Glutamate is the major excitatory neurotransmitter in the brain and NMDA and AMPA subtypes of glutamate receptors are the primary mediators of excitatory synaptic transmission in the hippocampus (Hollmann and Heinemann, 1994). Both NMDA and AMPA receptor subunits are essential for LTP induction and maintenance (Hayashi et al., 2000; Clayton et al., 2002; Malinow and Malenka, 2002) and are required for hippocampal synaptic plasticity as well as spatial learning and memory (McHugh et al., 1996; Tsien et al., 1996; Adams et al., 2001b; Nakazawa et al., 2002; Riedel et al., 2003). Moreover, NMDA and AMPA receptors have been implicated in the structural changes associated with synaptic plasticity, including synapse formation, maintenance, and remodeling (Fischer et al., 2000; Luscher et al., 2000; Hering and Sheng, 2001). Studies in aging rodents have shown that functional impairments of these receptors are associated with spatial learning and memory deficits (Newcomer and Krystal, 2001; Clayton et al., 2002). CR can affect the aging-related loss and/or functional impairment of NMDA and AMPA receptors. Specifically, the CR-associated amelioration of hippocampal LTP deficits in old F344 rats have been suggested to result from enhanced NMDA-mediated transmission (Eckles-Smith et al., 2000; Okada et al., 2003). Moreover, the aging-related decrease in the NMDA receptor subunit NR1 was shown to be ameliorated by lifelong CR (Eckles-Smith et al., 2000; Magnusson, 2001).
Aging- and CR-induced changes in synaptic efficacy as well as in levels of NMDA and AMPA receptors may be reflected in modifications of specific features of synaptic morphology (Ziff, 1997; Luscher et al., 2000; Hering and Sheng, 2001). Particularly, multiple spine bouton (MSB) complexes, i.e., single presynaptic terminals contacting two or more postsynaptic targets, has been correlated with synaptic efficacy (Buchs and Muller, 1996; Toni et al., 2001) and the incidence of synapses in MSB complexes is an indicator of synaptic efficacy and thus plasticity (Sorra et al., 1998; Toni et al., 1999; Jones, 1999; Geinisman et al., 2001; Toni et al., 2001; Nikonenko et al., 2002). Moreover, MSBs increase after exposure to enriched environments (Jones et al., 1997) and LTP induction (Toni et al., 1999). Not only is the incidence of MSB synapses regulated by neuronal activity in a highly dynamic manner, this parameter also has been associated with more efficient synaptic transmission as well as enhanced learning and memory in rats (Sorra et al., 1998; Toni et al., 1999; Jones, 1999; Geinisman et al., 2001; Nikonenko et al., 2002),
The present study investigated the effect of life-long CR in young, middle-aged, and old Fischer 344 x Brown Norway rats on NMDA (NR1, NR2A, and NR2B) and AMPA (GluR1 and GluR2) subunits of glutamate receptors in hippocampal CA1, the output region of the hippocampal trisynaptic pathway. In addition, the effects of aging and CR on total synapses and MSB synapses in stratum radiatum of hippocampal CA1 were investigated. This is the first study to investigate the potential effect of CR on ultrastructurally identified synapses in hippocampal CA1 across life span.
Materials and methods
Animals and Caloric Restriction
A total of 84 ad libitum fed (AL) and CR Fischer 344 X Brown Norway (F344XBN) F1 hybrid male rats were acquired from the National Institute on Aging (NIA) Caloric Restriction Colony (Harlan Industries, Indianapolis, IN). Six groups of rats were included in the present study: AL and CR rats at 10 (young), 18 (middle-aged), and 29 months (old) (n=14/group). Cohorts of animals containing each of the 6 groups were used for either Western blot or electron microscopic analyses. Forty-eight (n = 8/group) rats were used for Western blot analyses of different NMDA and AMPA receptor subtypes and 36 (n = 6/group) rats were used for quantitative electron microscopic analyses of synapses. All rats were housed individually on a 12-hour light-dark cycle in the animal facility of Wake Forest University School of Medicine and housed for two months prior to sacrifice.
At the NIA facility, all rats were AL fed (NIH-31 diet) from weaning until 14 weeks of age, at which time the CR regime was initiated by incremental reduction of 10% food intake per week over a 4-week period until CR rats received 60% of caloric intake compared to AL animals. The vitamin-fortified NIH-31 diet that CR rats received provided 60% of the calories and 100% of the vitamins consumed by the AL rats (NIH-31). All rats were fed daily shortly before dark cycle onset, had free access to water, and were weighed weekly.
SDS-PAGE and Western Blot Analysis
Rats were deeply anesthetized with intraperitoneal injection of pentobarbital (150mg/kg) and decapitated with a guillotine. The brains were removed rapidly and immediately chilled by immersion for 4 minutes in ice-cold artificial cerebrospinal fluid (140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 24 mM dextrose, 10 mM HEPES, pH 7.4). The hippocampi were dissected from the cerebral hemispheres and the dorsomedial 2/3 of was divided into 4 equal-sized slabs perpendicular to the long axis of the hippocampus (Newton et al., 2005). Sampling was limited to this region because the dorsal hippocampus is associated more closely with spatial learning and memory performance in rats than is the ventral part (Moser et al., 1995; Moser and Moser, 1998; Oh et al., 2003). Using anatomical landmarks, each slab was dissected further into dentate gyrus, CA3 and CA1 subregions (Newton et al., 2005); dissected samples were weighed, frozen, and stored at -80°C. CA1 tissue samples from the right hemisphere were homogenized (50μl/mg) using a Laemmli-based buffer (60 mM Tris HCl, pH6.8, 10% glycerol, 2% SDS) supplemented with 2mM EDTA and protease inhibitor cocktail (P8340, 1:250, Sigma-Aldrich, Inc., St. Louis, MI). Homogenates were heated to 70°C for 10 minutes, centrifuged for 20 minutes at 1600×g and aliquots of the soluble supernatant were stored at -80°C. Protein concentrations were determined using the BCA method (Pierce Technology, Rockford, IL).
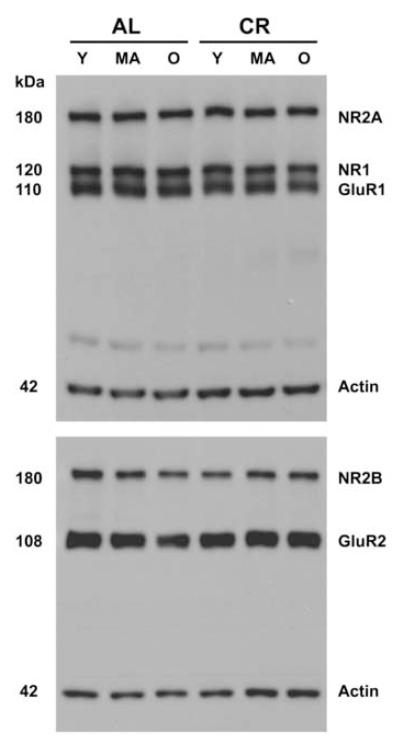
For analysis of NMDA and AMPA receptor subunits, 7.5 μg of CA1 supernatant protein was loaded into 15-well, 10% Tris-HCl Ready gels from Bio-Rad. Samples from a cohort of 6 rats (one each of: young AL, middle-aged AL, old AL, young CR, middle-aged CR and old CR) were run on the left side of the gel (Figure 1) and were repeated on the right side. Each gel was replicated once, allowing for 2 independent samples from each rat measured in duplicate. Samples were separated under reducing conditions using SDS-PAGE and transferred electrophorectically onto Immobilon membranes. The following primary antibodies were used for immunodetection: rabbit polyclonal antibody to the NR1 (0.6 μg/ml, Chemicon), NR2A (0.07 μg/ml, Chemicon), NR2B (0.07 μg/ml, Chemicon), GluR1 (0.01 μg/ml, Upstate, Placid, NY) and GluR2 (0.25 μg/ml, Chemicon). A mouse monoclonal antibody to actin (0.002 μg/ml, Chemicon) was used to control for protein loading. Peroxidase-conjugated donkey anti-rabbit IgG (10 ng/ml, Jackson Laboratory) and peroxidase-conjugated donkey anti-mouse IgG (10 ng/ml, Jackson Laboratory) secondary antibodies were used with the SuperSignal West Pico Chemiluminescent Substrate (Pierce Technology) for detection. Blots were exposed on Kodak Biomax film and Figure 1 shows the detected bands for individual glutamate receptor subunits as well as actin from AL and CR rats of different ages. Individual bands then were quantified with BioRad VersaDoc and Quantity One analysis software (BioRad Laboratories). The Gaussian trace optical densities of individual bands were normalized to the average of all the bands within that gel and represent the relative amount of each glutamate receptor subunit. The 4 normalized values of individual bands for NR1, NR2A, NR2B, GluR1, GluR2, and actin from each rat were averaged to derive an animal mean for each subunit. Finally, the mean normalized Gaussian trace optical densities of NR1, NR2A, NR2B, GluR1, GluR2, and actin were derived for the 6 groups of rats. These values represent the overall levels of three NMDA and two AMPA receptor subunits in CA1 of young, middle-aged, and old CR and AL rats, indicating which subunits and ages are most affected by CR. These results will direct the focus for our future immunogold experiments designed to quantify the synaptic distribution of the subunits most affected by CR.
Figure 1.
Representative Western blots showing glutamate receptor subunits NR2A, NR1, and GluR1 in the upper blot and NR2B and GluR2 in the lower blot for ad libitum fed (AL) and caloric restricted (CR) Fischer 344 x Brown Norway rats at young (Y, 10 months), middle (MA, 18 months) and old (O, 29 months) ages. Actin was included on all gels to control for equal protein loading. Samples were processed in cohorts of six, one sample of each age/diet group that were repeated on the right half of the gel and were in duplicates.
Electron Microscopic Analysis
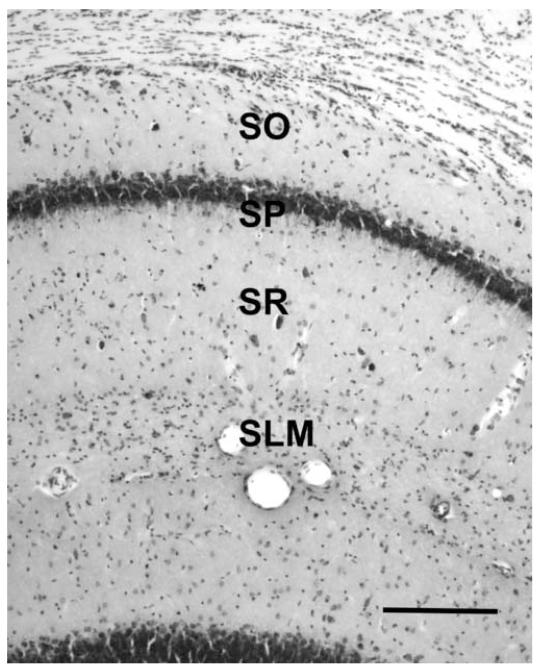
The details of tissue processing for electron microscopy and stereological quantification of synapses have been described previously (Shi et al., 2005). Rats were anesthetized with ketamine/xylazine (80 mg/kg /12 mg/kg, i.m.) and perfused transcardially with 1.3 M cacodylate buffer (pH 7.4) followed by fixative (2% paraformaldehyde/2% gluteraldehyde) in the same buffer. Brains were removed from the cranium, postfixed overnight, and sectioned coronally at 200 μm on a vibratome. Blocks containing the CA1 subregion were subdissected from sections through the dorsal hippocampus (Figure 2). As for Western blot analysis, electron microscopic analysis of synapses was limited to dorsal hippocampus (Bregma -2.3 to -4.3 mm, mediolateral 0∼2 mm; Paxinos and Watson, 1986). CA1 blocks were osmicated, dehydrated, embedded in araldite plastic, and trimmed to a face of 1 mm2 containing stratum radiatum and stratum pyramidale (Figure 2). Blocks were cut on an ultramicrotome in alternating segments of semithin (1 μm) and thin (700 Å) sections to permit quantification of neurons and synapses in the same blocks of tissue.
Figure 2.
Coronal section through the dorsal hippocampus demonstrating layers in CA1 region of Fischer 344 x Brown Norway rats. Quantifications of synapses and neurons were confined to stratum radiatum and stratum pyramidale respectively. SO: stratum oriens; SP: stratum pyramidale; SR: stratum radiatum; SLM: stratum lacunosum-moleculare. Scale bar = 400 μm.
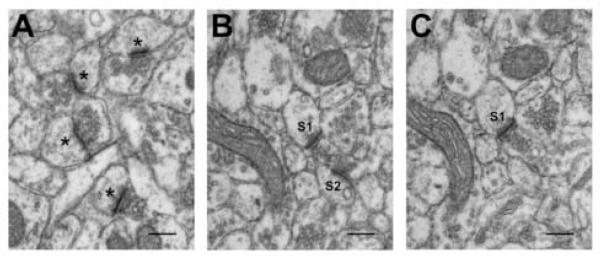
Stereological quantification of synapses was performed on a total of 24 physical disectors from 2 blocks per animal. Each physical disector consisted of a pair of photomicrographs (X8000; Zeiss 10-C transmission electron microscope) of serial sections of each block. Pairs from serial thin sections were selected in a systemically random fashion (Shi et al., 2005), consistent with the requirements for stereological analysis (West et al., 1991). The numerical density (Nv) of synapses in stratum radiatum of hippocampal CA1 was determined with physical disector technique (Sterio, 1984; Geinisman et al., 1996) using StereoInvestigator software (MicroBrightField, Inc., Colchester, VT). Synapses (Figure 3A; asterisks) were defined as having a presynaptic component with at least three synaptic vesicles and an obvious post-synaptic density (PSD). The mean Nv of synapses was calculated for each block. The same procedure was applied for quantification of MSB synapses (Figure 3B and 3C; S1 and S2).
Figure 3.
Electron micrograph illustrating synapses from a young, ad libitum fed Fischer 344 x Brown Norway rat. (A) Examples of simple synapses; synapses are defined by a presynaptic component containing at least three synaptic vesicles and a postsynaptic component with a clearly defined postsynaptic density. (B) Example of two synapses, S1 and S2, that are part of a multiple spine bouton (MSB) complexes; MSB complexes are comprised of a single presynaptic element that contacts more than one postsynaptic target. (B) and (C) are 700-Å serial sections that comprise a disector pair. S1 is defined as a MSB synapse on both (B) and (C) whereas S2 is on (B) but not (C); accordingly, only S2 is counted as a MSB synapse for this disector pair. Scale bar = 0.2 μm.
Stereological quantification of principal neurons in CA1 stratum pyramidale was performed using physical disector technique (Sterio, 1984; West et al., 1991; Shi et al., 2005) on serial sections cut from the semithin segment of each block with a disector height of 4 μm. A neuron was defined by the presence of a neuronal nucleus defined by a clear nuclear membrane. The Nv of pyramidal neurons was derived for individual disector pairs and the mean pyramidal neuron density for each block then was calculated. Neurons were quantified in semithin sections from each block in which synapses were quantified in thin sections.
The numerical densities of synapses and neurons were calculated using the following equation:
| (1) |
where Q- is the number of counting objects (synapses or neuronal nuclei) per counting frame, a is the area of the counting frame and h is the height of the physical disector. To avoid the possible confound of differential tissue shrinkage, the numerical densities of total and MSB synapses in CA1 stratum radiatum were normalized to the numerical density of CA1 pyramidal neurons in each block. Since neuronal and synaptic densities were derived from the same araldite -embedded blocks, any potential tissue shrinkage would contribute equally to these two sets of data. In this fashion, tissue volume was removed from the resulting synapse per neuron ratio and such a ratio represents a sensitive marker of net changes in synapse density (Jones et al., 1997; Jones, 1999; Jones et al., 1999; Mouton, 2002).
Statistical Analysis
The effects of the two independent variables, age and diet, on each of the dependent variables for the Western blot experiment (Gaussian trace optical densities for NR1, NR2A, NR2B, GluR1, GluR2, and actin) as well as for the electron microscopic experiment (synapse per neuron ratios for total and MSB synapses) were determined using a two-way ANOVA. Holm-Sidak’s post-hoc test (SigmaStat) was used for pair-wise comparison. A two-tailed significance level for each of the main effects was held at 0.05.
Results
Daily monitoring of food consumption verified that CR rats consumed 60% of the calories consumed by AL rats. Weekly measurement of body weight revealed that the average weight of CR rats was 41% lower than that of AL rats. All rats in the present study appeared healthy throughout the experiment, although it was evident that the old Cr rats were more active and appeared more youthful than their AL-fed counterparts.
Aging-related decreases in glutamate receptor subunits of AL rats are not seen in CR animals
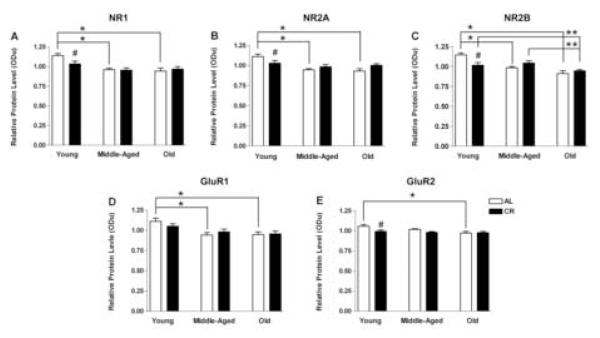
The present study evaluated the effects of age and diet on the relative protein levels of different NMDA and AMPA subunits in hippocampal CA1 and the results are shown in Figure 4. ANOVA revealed a significant main effect of age for each of the glutamate receptor subunits: NR1 (F(2, 42) = 12.024, p < 0.001; Figure 4A), NR2A (F(2, 42) = 10.479, p < 0.001; Figure 4B), NR2B (F(2, 42) = 15.769, p < 0.001; Figure 4C), GluR1 (F(2, 42) = 9.362, p < 0.001; Figure 4D), and GluR2 (F(2, 42) = 4.442, p = 0.018; Figure 4E). Post-hoc Holm-Sidak tests indicated that in AL rats, there is decreased level of all subunits except GluR2 between young and old age as well as between young and middle age (ps < 0.05), but not between middle and old age. In contrast, most glutamate receptor subunits in CR animals demonstrate stability across age in this study (ps > 0.05). NR2B is the only subunit that demonstrates a decline with age in CR rats (p < 0.05), decreasing significantly between young and old age as well as between middle and old age (Figure 4C). Comparisons of AL and CR rats at different ages indicated significantly lower protein levels (ps < 0.05) in young CR compared to young AL animals for all subunits except GluR1. None of the subunit levels differed between AL and CR groups at middle or old age (ps > 0.05). Finally, two-way ANOVA revealed no effect of age or diet on actin (ps >0.05). Taken together, there is a significant aging-related decline in glutamate receptor subunits in AL rats. CR eliminates that decline by producing an initial decrease in NMDA/AMPA subunits to levels that are maintained thereafter in CR animals.
Figure 4.
Relative protein levels of NMDAR subunits NR1 (A), NR2A (B), and NR2B (C), and AMPAR subunits GluR1 (D) and GluR2 (E), as a function of age (young, 10 months; middle-aged, 18 months; and old, 29 months) and diet (ad libitum fed, AL, open bars; caloric restricted, CR, filled bars). Note that (1) all subunits except for GluR2 decreased between young and middle age in AL rats and levels of those receptor subunits in young rats were significantly higher compared to middle-aged and old AL rats (*: p<0.05), (2) the only difference observed between AL and CR diet is in young rats in which all subunits levels except GluR1 are significantly lower in CR than AL animals (#: p<0.05), and (3) the only aging-related decrease in CR animals is between young and old and middle and old age for NR2B (**: p<0.05). These data represent the mean ± standard error of the mean of eight animals per experimental condition.
Effects of aging and CR on synaptic structures
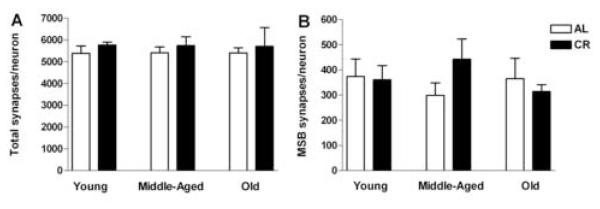
The age- and diet-induced changes in the levels of NMDA and AMPA receptor subunits raise the possibility that there may be corresponding changes in the number of CA1 synapses. Accordingly, we used stereological methods to quantify both the overall population of synapses (total synapses) and those involved in multiple spine bouton complexes (MSB synapses) across life span in both dietary groups. In addition, CA1 pyramidal neurons in AL and CR rats at young, middle, and old age were quantified stereologically. Neuron density did not differ with either age (F(2, 29) = 0.612, p = 0.549) or diet (F(1, 29) = 2.445, p = 0.129 (Table 1) and was used to normalize the numerical density of total as well as MSB synapses (Table 1), deriving the ratio of synapses per neuron for total and MSB synapses. ANOVA indicated no effect of age or diet on the ratio either of total synapses per neuron (age: F(2, 29) = 0.00155, p = 0.998; diet: F(1, 29) = 1.025, p = 0.320; Figure 5A) or of MSB synapses per neuron (age: F(2, 29) = 0.135, p = 0.875; diet: F(1, 29) = 0.248, p = 0.622; Figure 5B). Thus, total and MSB synapses in CA1 were maintained across life span and neither of these parameters was affected by life-long CR.
Table 1.
The numerical densities of neurons, total synapses and synapses in multiple spine bouton (MSB) complexes in stratum radiatum of hippocampal CA1 of Fischer 344 x Brown Norway Rats across life span.
| Neuronal density X 104/mm3 (± SEM) |
Total synapses X 107/mm3 (± SEM) |
MSB synapses X 107/mm3 (± SEM) |
|
|---|---|---|---|
| Young AL | 22.67 (± 0.44) | 123.16 (± 4.88) | 8.22 (± 1.43) |
| Young CR | 22.10 (± 0.62) | 127.38 (± 5.72) | 8.05 (± 1.37) |
| Middle-Aged AL | 23.10 (± 0.66) | 124.38 (± 7.58) | 7.05 (± 1.23) |
| Middle-Aged CR | 21.81 (± 0.93) | 123.26 (± 7.49) | 9.70 (± 1.85) |
| Old AL | 25.34 (± 0.59) | 119.39 (± 3.77) | 10.24 (± 3.71) |
| Old CR | 22.10 (± 1.43) | 116.61 (± 5.99) | 6.55 (± 0.64) |
Figure 5.
Synapse per neuron ratio in the CA1 stratum radiatum of Fischer 344 x Brown Norway rats across life span in ad libitum fed (AL, open bars) and caloric restricted (CR, filled bars) rats. Two-way ANOVA reveals no main effect of age or diet for total synapses (A) or multiple spine bouton (MSB) synapses (B) in this brain region (ps > 0.05), indicating a maintenance of total as well as MSB synapses throughout life for both CR and AL rats. These data are presented as mean ± standard error of the mean of six animals per experimental condition.
Discussion
The present study reveals for the first time that CR eliminates the aging-related decline in NMDA and AMPA subunits in CA1 not as a result of elevations in subunit levels in old animals, but rather as a result of a stabilization of subunit levels across the life span after an initial reduction compared to young AL rats. Despite these effects of CR and aging on glutamate receptor subunits, no quantitative changes were detected in the population of total or MSB synapses either during aging or following CR. Importantly, this is the first study to quantify ultrastructurally identified synapses in the CA1 region of hippocampus in CR animals, demonstrating that life-long CR does not affect synapse number in this region.
CR stabilizes NMDA and AMPA receptor subunits in hippocampal CA1
The present study reveals that all NMDA and AMPA receptor subunits examined in hippocampal CA1 decline significantly with age in AL rats. Earlier reports have shown similar decreases with age in NMDA and AMPA receptor subunits (Clayton et al., 2002; Magnusson et al., 2002). Other studies have investigated aging-related changes in hippocampal levels of NMDA and AMPA receptors in different animal models, ages, and regions, using a variety of technical approaches (Tamaru et al., 1991; Wenk et al., 1991; Magnusson and Cotman, 1993). Specifically, NMDA receptor binding in subdissected hippocampus of Sprague-Dawley rats revealed a decrease in NMDA receptors between young and old animals in CA1 and CA3, but not in DG (Wenk and Barnes, 2000). Furthermore, Western blot analysis revealed that in whole hippocampus, NMDA subunits NR1 (Clayton and Browning, 2001; Magnusson et al., 2002; Mesches et al., 2004), NR2A (Sonntag et al., 2000), and NR2B (Sonntag et al., 2000; Clayton and Browning, 2001; Clayton et al., 2002; Mesches et al., 2004) as well as the AMPA subunits GluR1 and GluR2 (Sonntag et al., 2000; Clayton and Browning, 2001; Clayton et al., 2002; Mesches et al., 2004) decline with age in different rodent strains including F344 rats, F344xBN rats, and C57B1/6 mice. Similar to the present findings in CA1, two recent studies indicated significant aging-related declines in NMDA and AMPA subunits across lifespan in CA3, and to a lesser degree in DG in subdissected hippocampus (Adams M.M. et al., 2005; Linville M.C. et al., 2005). Importantly, inclusion of a middle-aged group (18 mos) in addition to young (10 mos) and old groups (28 mos) in the present CA1 study revealed that, with the exception of NR2B in CA1, all of the aging-related subunit declines occurred not between middle and old age, but between young and middle age.
The loss of hippocampal NMDA and AMPA receptors in AL rats is likely to be associated with impaired glutamatergic transmission. Such aging-related loss and/or functional impairment of glutamate receptors has been shown to contribute to LTP deficits and impaired synaptic plasticity in the brains of aged animals (Geinisman et al., 1995; Bach et al., 1999; Clayton et al., 2002; Tombaugh et al., 2002; Rosenzweig and Barnes, 2003; Geinisman et al., 2004). Although declines in NMDA and/or AMPA subunits have been associated with the cognitive decline in aged rats (Newcomer and Krystal, 2001; Adams et al., 2001b; Mesches et al., 2004), results from the present study suggest that such a relationship in CA1 is either minimal or more complex than a simple correlation. Specifically, an earlier study on the effects of aging and CR on MWM performance in rats of the same strain and ages (Markowska and Savonenko, 2002) showed a more dramatic decline in performance between 18-month (middle aged) and 30-month (old) rats compared to the decline between 9-month (young) and 18-month rats whereas the present findings indicate a marked decline in glutamate receptor subunits between 10 and 18 months (young to middle age) in AL fed rats with little evidence of decline between 18 and 29 months (middle to old age). To elucidate the exact relationship between CR-induced changes in glutamate receptor subunit levels and neural function, electrophysiological studies to reveal NMDA and AMPA responses and electron microscopic studies to reveal the sub-cellular distribution of receptor subunits will be necessary.
Previous studies on the effects of CR on aging-related changes in glutamate receptors have yielded inconsistent findings. For example, levels of the NR1 subunit in the hippocampus have been reported to be higher (Eckles-Smith et al., 2000) or lower (Monti et al., 2004) in old CR compared to AL animals. Such discrepancies could be due to differences in rat strain, animal age, region sampled (whole hippocampus vs. dissected hippocampal subregions), or CR schedule (proportionate reduction of daily calorie intake vs. every-other-day feeding schedule). Because the present study included young, middle and old ages, we were able not only to assess the effect of CR on subunit levels in old animals, but also to evaluate changes in subunits levels across lifespan in CR rats. Importantly, had we compared CR and AL animals at old age only, we would have concluded that CR had no effect on subunit levels and we would have failed to detect that CR eliminated the aging-related subunit decline in AL animals. Subunit levels in CR animals were reduced in comparison to those in AL animals by 10 months of age, 6 months after initiation of CR; and subunits remained constant at that level across life span. Exactly when subunit levels begin to decline after the initiation of CR remains to be determined.
Thus, the present study suggests that CR induces a stability or homeostasis of NMDA and AMPA receptor subunits across life span. In general, homeostasis is held to be a prime determinant of longevity. For example, the capacity of an organism to maintain stable levels of free radicals is more important than how fast it produces them (McCarter and McGee, 1989; Kirkwood and Shanley, 2005). Senescence-related loss of biological function is due to impairment of a homeostatic state and CR enhances longevity by increasing metabolic stability (Demetrius, 2004), specifically by inducing a stable state of biological parameters that normally demonstrate aging-related declines (Yu and Chung, 2001; Koubova and Guarente, 2003). CR-induced homeostasis has been reported in a wide range of body systems and is characterized by enhanced somatic protection and repair mechanisms as well as delayed aging-related changes in protein turnover, serum corticosteroids, DNA repair activity, cytosolic antioxidants, and expression of heat shock proteins (Kirkwood and Shanley, 2005). The physiological response to CR has been described as comprising two phases: an initial adaptive period characterized by fluctuating of metabolic rate followed by a steady state period with a stable, altered metabolic state (Weindruch et al., 1988; McCarter and McGee, 1989; Koubova and Guarente, 2003). As a result, CR enhances the ability of an organism to withstand stress and imposed insults (Yu and Chung, 2001). In the present study, the decreased glutamate receptor subunit levels in young CR compared to young AL rats and the maintenance of those levels for the remainder of life span in CR animals may be a manifestation of those two phases of the CR response (Yu and Chung, 2001; Demetrius, 2004). Accordingly, we hypothesize that changes in critical neural parameters result in functional decline in the aging brain; and CR eliminates those changes by inducing a homeostatic state.
CR does not affect total synapses or MSB synapses
The present study revealed that neither the ratio of total synapses per neuron nor that of MSB synapses per neuron changed across life span in the stratum radiatum of hippocampal CA1. Moreover, there was no difference in the ratio of synapses per neuron between AL and CR groups at young, middle, or old age. Although no other studies have quantified the effect of CR on synapses in this region of hippocampus, previous reports have indicated that the number of synapses in CA1 does not change either across lifespan or as a function of cognitive performance (Smith et al., 2000; Geinisman et al., 2004). Specifically, the total number of ultrastructurally identified axospinous synapses in the stratum radiatum of CA1 remained constant among groups of young rats, aged rats with impaired spatial learning, and aged rats with intact spatial learning (Geinisman et al., 2004). Another stereological study investigating the effects of aging and IGF-1 on synapses in CA1 regions quantified synapses in old IGF-1 infused rats compared to young, middle-aged, and old saline infused control rats. In contrast to the stability of synapses across life span in the present study, that study reported a decline in the synapse to neuron ratio between middle and old age in the saline infused control groups (Shi et al., 2005). Importantly, all of the animals in that study received saline infusions via intracerebroventricular cannulae with mini-pumps for 28 days prior to sacrifice. The presence of the intracerebroventricular cannulae for 28 days prior to sacrifice and the repeated ketamine anesthesia for mini-pump placement and replacement in the previous study comprised a significant biological challenge (Castel-Barthe et al., 1996). It may be that a diminished capacity of the old animals to respond adequately to that challenge led to the loss of CA1 synapses in old animals. Particularly, ketamine, which is a competitive NMDA receptor blocker (Izquierdo and Medina, 1997; Tovar and Westbrook, 2002; Weeks et al., 2003) that interferes with LTP formation (Otani and Ben Ari, 1993) and abolishes the morphological changes of synapses following stimulation (Weeks et al., 2003), has been reported to induce more severe neurotoxicity in old compared to young animals (Jevtovic-Todorovic and Carter, 2005).
Summary
Synapses are highly labile structures. Nevertheless, the total number of ultrastructurally identified synapses is stable across life span in the absence of biological challenge. Instead, more subtle aspects of synapses such as the subunit composition of glutamate receptors are likely to underlie the functional changes that occur during aging. The capacity of an organism to maintain metabolic and physiological stability in the face of aging-related changes is a prime determinant of longevity and brain function. CR has been shown to extend lifespan and ameliorate aging-related functional impairments in a variety of body systems by imposing a homeostatic state. The present study indicates that CR also induces a homeostasis of glutamate receptor subunits by eliminating the aging-related decline of glutamate receptors.
Acknowledgements
This work was supported by National Institute on Aging grants AG11370 and AG019886 (J.K.B-B. and D.R.R).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams MM, Linville MC, Newton IG, Long A, Forbes ME, Riddle DR, Brunso-Bechtold JK. Effect of caloric restriction and age on synaptic proteins in hippocampal CA3. Society for Neuroscience 35th annual meeting abstract 731.13.2005. [Google Scholar]
- Adams MM, Shah RA, Janssen WG, Morrison JH. Different modes of hippocampal plasticity in response to estrogen in young and aged female rats. Proc. Natl. Acad. Sci. U. S. A. 2001a;98:8071–8076. doi: 10.1073/pnas.141215898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams MM, Smith TD, Moga D, Gallagher M, Wang Y, Wolfe BB, Rapp PR, Morrison JH. Hippocampal dependent learning ability correlates with N-methyl-D-aspartate (NMDA) receptor levels in CA3 neurons of young and aged rats. J. Comp Neurol. 2001b;432:230–243. doi: 10.1002/cne.1099. [DOI] [PubMed] [Google Scholar]
- Bach ME, Barad M, Son H, Zhuo M, Lu YF, Shih R, Mansuy I, Hawkins RD, Kandel ER. Age-related defects in spatial memory are correlated with defects in the late phase of hippocampal long-term potentiation in vitro and are attenuated by drugs that enhance the cAMP signaling pathway. Proc. Natl. Acad. Sci. U. S. A. 1999;96:5280–5285. doi: 10.1073/pnas.96.9.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CA. Normal aging: regionally specific changes in hippocampal synaptic transmission. Trends Neurosci. 1994;17:13–18. doi: 10.1016/0166-2236(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Buchs PA, Muller D. Induction of long-term potentiation is associated with major ultrastructural changes of activated synapses. Proc. Natl. Acad. Sci. U. S. A. 1996;93:8040–8045. doi: 10.1073/pnas.93.15.8040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat. Rev. Neurosci. 2006;7:30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- Castel-Barthe MN, Jazat-Poindessous F, Barneoud P, Vigne E, Revah F, Mallet J, Lamour Y. Direct intracerebral nerve growth factor gene transfer using a recombinant adenovirus: effect on basal forebrain cholinergic neurons during aging. Neurobiol. Dis. 1996;3:76–86. doi: 10.1006/nbdi.1996.0008. [DOI] [PubMed] [Google Scholar]
- Clayton DA, Browning MD. Deficits in the expression of the NR2B subunit in the hippocampus of aged Fisher 344 rats. Neurobiol. Aging. 2001;22:165–168. doi: 10.1016/s0197-4580(00)00196-2. [DOI] [PubMed] [Google Scholar]
- Clayton DA, Mesches MH, Alvarez E, Bickford PC, Browning MD. A hippocampal NR2B deficit can mimic age-related changes in long-term potentiation and spatial learning in the Fischer 344 rat. J. Neurosci. 2002;22:3628–3637. doi: 10.1523/JNEUROSCI.22-09-03628.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demetrius L. Caloric restriction, metabolic rate, and entropy. J. Gerontol. A Biol. Sci. Med. Sci. 2004;59:B902–B915. doi: 10.1093/gerona/59.9.b902. [DOI] [PubMed] [Google Scholar]
- Eckles-Smith K, Clayton D, Bickford P, Browning MD. Caloric restriction prevents age-related deficits in LTP and in NMDA receptor expression. Brain Res. Mol. Brain Res. 2000;78:154–162. doi: 10.1016/s0169-328x(00)00088-7. [DOI] [PubMed] [Google Scholar]
- Fischer M, Kaech S, Wagner U, Brinkhaus H, Matus A. Glutamate receptors regulate actin-based plasticity in dendritic spines. Nat. Neurosci. 2000;3:887–894. doi: 10.1038/78791. [DOI] [PubMed] [Google Scholar]
- Fischer W, Bjorklund A, Chen K, Gage FH. NGF improves spatial memory in aged rodents as a function of age. J. Neurosci. 1991;11:1889–1906. doi: 10.1523/JNEUROSCI.11-07-01889.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC, Norris CM. Age-associated changes in Ca(2+)-dependent processes: relation to hippocampal synaptic plasticity. Hippocampus. 1997;7:602–612. doi: 10.1002/(SICI)1098-1063(1997)7:6<602::AID-HIPO3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, Berry RW, Disterhoft JF, Power JM, Van der Zee EA. Associative learning elicits the formation of multiple-synapse boutons. J. Neurosci. 2001;21:5568–5573. doi: 10.1523/JNEUROSCI.21-15-05568.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geinisman Y, Detoledo-Morrell L, Morrell F, Heller RE. Hippocampal markers of age-related memory dysfunction: behavioral, electrophysiological and morphological perspectives. Prog. Neurobiol. 1995;45:223–252. doi: 10.1016/0301-0082(94)00047-l. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, Ganeshina O, Yoshida R, Berry RW, Disterhoft JF, Gallagher M. Aging, spatial learning, and total synapse number in the rat CA1 stratum radiatum. Neurobiol. Aging. 2004;25:407–416. doi: 10.1016/j.neurobiolaging.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Guo ZH, Mattson MP. Neurotrophic factors protect cortical synaptic terminals against amyloid and oxidative stress-induced impairment of glucose transport, glutamate transport and mitochondrial function. Cereb. Cortex. 2000;10:50–57. doi: 10.1093/cercor/10.1.50. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- Hering H, Sheng M. Dendritic spines: structure, dynamics and regulation. Nat. Rev. Neurosci. 2001;2:880–888. doi: 10.1038/35104061. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annu. Rev. Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Ingram DK, Weindruch R, Spangler EL, Freeman JR, Walford RL. Dietary restriction benefits learning and motor performance of aged mice. J. Gerontol. 1987;42:78–81. doi: 10.1093/geronj/42.1.78. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, Medina JH. Memory formation: the sequence of biochemical events in the hippocampus and its connection to activity in other brain structures. Neurobiol. Learn. Mem. 1997;68:285–316. doi: 10.1006/nlme.1997.3799. [DOI] [PubMed] [Google Scholar]
- Jevtovic-Todorovic V, Carter LB. The anesthetics nitrous oxide and ketamine are more neurotoxic to old than to young rat brain. Neurobiol. Aging. 2005;26:947–956. doi: 10.1016/j.neurobiolaging.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Jones TA. Multiple synapse formation in the motor cortex opposite unilateral sensorimotor cortex lesions in adult rats. J. Comp Neurol. 1999;414:57–66. [PubMed] [Google Scholar]
- Jones TA, Chu CJ, Grande LA, Gregory AD. Motor skills training enhances lesion-induced structural plasticity in the motor cortex of adult rats. J. Neurosci. 1999;19:10153–10163. doi: 10.1523/JNEUROSCI.19-22-10153.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TA, Klintsova AY, Kilman VL, Sirevaag AM, Greenough WT. Induction of multiple synapses by experience in the visual cortex of adult rats. Neurobiol. Learn. Mem. 1997;68:13–20. doi: 10.1006/nlme.1997.3774. [DOI] [PubMed] [Google Scholar]
- Kalani R, Judge S, Carter C, Pahor M, Leeuwenburgh C. Effects of caloric restriction and exercise on age-related, chronic inflammation assessed by C-reactive protein and interleukin-6. J. Gerontol. A Biol. Sci. Med. Sci. 2006;61:211–217. doi: 10.1093/gerona/61.3.211. [DOI] [PubMed] [Google Scholar]
- Kirkwood TB, Shanley DP. Food restriction, evolution and ageing. Mech. Ageing Dev. 2005;126:1011–1016. doi: 10.1016/j.mad.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Koubova J, Guarente L. How does calorie restriction work? Genes Dev. 2003;17:313–321. doi: 10.1101/gad.1052903. [DOI] [PubMed] [Google Scholar]
- Linville MC, Newton IG, Shi L, Forbes ME, Riddle DR, Brunso-Bechtold JK. Synapse number is unchanged across life span in calorically restricted Fischer 344 x Brown Norway rats. Society for Neuroscience 35th annual meeting abstract 848.14.2005. [Google Scholar]
- Luscher C, Nicoll RA, Malenka RC, Muller D. Synaptic plasticity and dynamic modulation of the postsynaptic membrane. Nat. Neurosci. 2000;3:545–550. doi: 10.1038/75714. [DOI] [PubMed] [Google Scholar]
- Magnusson KR. Influence of diet restriction on NMDA receptor subunits and learning during aging. Neurobiol. Aging. 2001;22:613–627. doi: 10.1016/s0197-4580(00)00258-x. [DOI] [PubMed] [Google Scholar]
- Magnusson KR, Cotman CW. Age-related changes in excitatory amino acid receptors in two mouse strains. Neurobiol. Aging. 1993;14:197–206. doi: 10.1016/0197-4580(93)90001-r. [DOI] [PubMed] [Google Scholar]
- Magnusson KR, Nelson SE, Young AB. Age-related changes in the protein expression of subunits of the NMDA receptor. Brain Res. Mol. Brain Res. 2002;99:40–45. doi: 10.1016/s0169-328x(01)00344-8. [DOI] [PubMed] [Google Scholar]
- Major DE, Kesslak JP, Cotman CW, Finch CE, Day JR. Life-long dietary restriction attenuates age-related increases in hippocampal glial fibrillary acidic protein mRNA. Neurobiol. Aging. 1997;18:523–526. doi: 10.1016/s0197-4580(97)00102-4. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu. Rev. Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Mooney M, Sonntag WE. Insulin-like growth factor-1 ameliorates age-related behavioral deficits. Neuroscience. 1998;87:559–569. doi: 10.1016/s0306-4522(98)00143-2. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Savonenko A. Retardation of cognitive aging by life-long diet restriction: implications for genetic variance. Neurobiol. Aging. 2002;23:75–86. doi: 10.1016/s0197-4580(01)00249-4. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu. Rev. Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- McCarter RJ, McGee JR. Transient reduction of metabolic rate by food restriction. Am. J. Physiol. 1989;257:E175–E179. doi: 10.1152/ajpendo.1989.257.2.E175. [DOI] [PubMed] [Google Scholar]
- McHugh TJ, Blum KI, Tsien JZ, Tonegawa S, Wilson MA. Impaired hippocampal representation of space in CA1-specific NMDAR1 knockout mice. Cell. 1996;87:1339–1349. doi: 10.1016/s0092-8674(00)81828-0. [DOI] [PubMed] [Google Scholar]
- Mesches MH, Gemma C, Veng LM, Allgeier C, Young DA, Browning MD, Bickford PC. Sulindac improves memory and increases NMDA receptor subunits in aged Fischer 344 rats. Neurobiol. Aging. 2004;25:315–324. doi: 10.1016/S0197-4580(03)00116-7. [DOI] [PubMed] [Google Scholar]
- Monti B, Virgili M, Contestabile A. Alterations of markers related to synaptic function in aging rat brain, in normal conditions or under conditions of long-term dietary manipulation. Neurochem. Int. 2004;44:579–584. doi: 10.1016/j.neuint.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Hof PR. Life and death of neurons in the aging brain. Science. 1997;278:412–419. doi: 10.1126/science.278.5337.412. [DOI] [PubMed] [Google Scholar]
- Moser MB, Moser EI. Functional differentiation in the hippocampus. Hippocampus. 1998;8:608–619. doi: 10.1002/(SICI)1098-1063(1998)8:6<608::AID-HIPO3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Moser MB, Moser EI, Forrest E, Andersen P, Morris RG. Spatial learning with a minislab in the dorsal hippocampus. Proc. Natl. Acad. Sci. U. S. A. 1995;92:9697–9701. doi: 10.1073/pnas.92.21.9697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton PR. Principles and practices of unbiased stereology. 2002.
- Nakazawa K, Quirk MC, Chitwood RA, Watanabe M, Yeckel MF, Sun LD, Kato A, Carr CA, Johnston D, Wilson MA, Tonegawa S. Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science. 2002;297:211–218. doi: 10.1126/science.1071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomer JW, Krystal JH. NMDA receptor regulation of memory and behavior in humans. Hippocampus. 2001;11:529–542. doi: 10.1002/hipo.1069. [DOI] [PubMed] [Google Scholar]
- Newton IG, Forbes ME, Legault C, Johnson JE, Brunso-Bechtold JK, Riddle DR. Caloric restriction does not reverse aging-related changes in hippocampal BDNF. Neurobiol. Aging. 2005;26:683–688. doi: 10.1016/j.neurobiolaging.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Nicholson DA, Yoshida R, Berry RW, Gallagher M, Geinisman Y. Reduction in size of perforated postsynaptic densities in hippocampal axospinous synapses and age-related spatial learning impairments. J. Neurosci. 2004;24:7648–7653. doi: 10.1523/JNEUROSCI.1725-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikonenko I, Jourdain P, Alberi S, Toni N, Muller D. Activity-induced changes of spine morphology. Hippocampus. 2002;12:585–591. doi: 10.1002/hipo.10095. [DOI] [PubMed] [Google Scholar]
- Oh MM, Kuo AG, Wu WW, Sametsky EA, Disterhoft JF. Watermaze learning enhances excitability of CA1 pyramidal neurons. J. Neurophysiol. 2003;90:2171–2179. doi: 10.1152/jn.01177.2002. [DOI] [PubMed] [Google Scholar]
- Okada M, Nakanishi H, Amamoto T, Urae R, Ando S, Yazawa K, Fujiwara M. How does prolonged caloric restriction ameliorate age-related impairment of long-term potentiation in the hippocampus? Brain Res. Mol. Brain Res. 2003;111:175–181. doi: 10.1016/s0169-328x(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Otani S, Ben Ari Y. Biochemical correlates of long-term potentiation in hippocampal synapses. Int. Rev. Neurobiol. 1993;35:1–41. doi: 10.1016/s0074-7742(08)60567-x. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Gallagher M. Preserved neuron number in the hippocampus of aged rats with spatial learning deficits. Proc. Natl. Acad. Sci. U. S. A. 1996;93:9926–9930. doi: 10.1073/pnas.93.18.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel G, Platt B, Micheau J. Glutamate receptor function in learning and memory. Behav. Brain Res. 2003;140:1–47. doi: 10.1016/s0166-4328(02)00272-3. [DOI] [PubMed] [Google Scholar]
- Rosenzweig ES, Barnes CA. Impact of aging on hippocampal function: plasticity, network dynamics, and cognition. Prog. Neurobiol. 2003;69:143–179. doi: 10.1016/s0301-0082(02)00126-0. [DOI] [PubMed] [Google Scholar]
- Rosenzweig ES, Rao G, McNaughton BL, Barnes CA. Role of temporal summation in age-related long-term potentiation-induction deficits. Hippocampus. 1997;7:549–558. doi: 10.1002/(SICI)1098-1063(1997)7:5<549::AID-HIPO10>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Roth GS, Ingram DK, Lane MA. Slowing ageing by caloric restriction. Nat. Med. 1995;1:414–415. doi: 10.1038/nm0595-414. [DOI] [PubMed] [Google Scholar]
- Shi L, Linville MC, Tucker EW, Sonntag WE, Brunso-Bechtold JK. Differential effects of aging and insulin-like growth factor-1 on synapses in CA1 of rat hippocampus. Cereb. Cortex. 2005;15:571–577. doi: 10.1093/cercor/bhh158. [DOI] [PubMed] [Google Scholar]
- Smith TD, Adams MM, Gallagher M, Morrison JH, Rapp PR. Circuit-specific alterations in hippocampal synaptophysin immunoreactivity predict spatial learning impairment in aged rats. J. Neurosci. 2000;20:6587–6593. doi: 10.1523/JNEUROSCI.20-17-06587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag WE, Bennett SA, Khan AS, Thornton PL, Xu X, Ingram RL, Brunso-Bechtold JK. Age and insulin-like growth factor-1 modulate N-methyl-D-aspartate receptor subtype expression in rats. Brain Res. Bull. 2000;51:331–338. doi: 10.1016/s0361-9230(99)00259-2. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Lynch CD, Cefalu WT, Ingram RL, Bennett SA, Thornton PL, Khan AS. Pleiotropic effects of growth hormone and insulin-like growth factor (IGF)-1 on biological aging: inferences from moderate caloric-restricted animals. J. Gerontol. A Biol. Sci. Med. Sci. 1999;54:B521–B538. doi: 10.1093/gerona/54.12.b521. [DOI] [PubMed] [Google Scholar]
- Sorra KE, Fiala JC, Harris KM. Critical assessment of the involvement of perforations, spinules, and spine branching in hippocampal synapse formation. J. Comp Neurol. 1998;398:225–240. [PubMed] [Google Scholar]
- Sterio DC. The unbiased estimation of number and sizes of arbitrary particles using the disector. J. Microsc. 1984;134(Pt 2):127–136. doi: 10.1111/j.1365-2818.1984.tb02501.x. [DOI] [PubMed] [Google Scholar]
- Tacconi MT, Lligona L, Salmona M, Pitsikas N, Algeri S. Aging and food restriction: effect on lipids of cerebral cortex. Neurobiol. Aging. 1991;12:55–59. doi: 10.1016/0197-4580(91)90039-m. [DOI] [PubMed] [Google Scholar]
- Tamaru M, Yoneda Y, Ogita K, Shimizu J, Nagata Y. Age-related decreases of the N-methyl-D-aspartate receptor complex in the rat cerebral cortex and hippocampus. Brain Res. 1991;542:83–90. doi: 10.1016/0006-8993(91)91001-h. [DOI] [PubMed] [Google Scholar]
- Tata DA, Marciano VA, Anderson BJ. Synapse loss from chronically elevated glucocorticoids: relationship to neuropil volume and cell number in hippocampal area CA3. J. Comp Neurol. 2006;498:363–374. doi: 10.1002/cne.21071. [DOI] [PubMed] [Google Scholar]
- Tombaugh GC, Rowe WB, Chow AR, Michael TH, Rose GM. Theta-frequency synaptic potentiation in CA1 in vitro distinguishes cognitively impaired from unimpaired aged Fischer 344 rats. J. Neurosci. 2002;22:9932–9940. doi: 10.1523/JNEUROSCI.22-22-09932.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toni N, Buchs PA, Nikonenko I, Bron CR, Muller D. LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature. 1999;402:421–425. doi: 10.1038/46574. [DOI] [PubMed] [Google Scholar]
- Toni N, Buchs PA, Nikonenko I, Povilaitite P, Parisi L, Muller D. Remodeling of synaptic membranes after induction of long-term potentiation. J. Neurosci. 2001;21:6245–6251. doi: 10.1523/JNEUROSCI.21-16-06245.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. Mobile NMDA receptors at hippocampal synapses. Neuron. 2002;34:255–264. doi: 10.1016/s0896-6273(02)00658-x. [DOI] [PubMed] [Google Scholar]
- Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996;87:1327–1338. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- Weeks AC, Ivanco TL, Leboutillier JC, Marrone DF, Racine RJ, Petit TL. Unique changes in synaptic morphology following tetanization under pharmacological blockade. Synapse. 2003;47:77–86. doi: 10.1002/syn.10113. [DOI] [PubMed] [Google Scholar]
- Weindruch R, Naylor PH, Goldstein AL, Walford RL. Influences of aging and dietary restriction on serum thymosin alpha 1 levels in mice. J. Gerontol. 1988;43:B40–B42. doi: 10.1093/geronj/43.2.b40. [DOI] [PubMed] [Google Scholar]
- Wenk GL, Barnes CA. Regional changes in the hippocampal density of AMPA and NMDA receptors across the lifespan of the rat. Brain Res. 2000;885:1–5. doi: 10.1016/s0006-8993(00)02792-x. [DOI] [PubMed] [Google Scholar]
- Wenk GL, Walker LC, Price DL, Cork LC. Loss of NMDA, but not GABA-A, binding in the brains of aged rats and monkeys. Neurobiol. Aging. 1991;12:93–98. doi: 10.1016/0197-4580(91)90047-n. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat. Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Yu BP, Chung HY. Stress resistance by caloric restriction for longevity. Ann. N. Y. Acad. Sci. 2001;928:39–47. doi: 10.1111/j.1749-6632.2001.tb05633.x. [DOI] [PubMed] [Google Scholar]
- Yu BP, Masoro EJ, McMahan CA. Nutritional influences on aging of Fischer 344 rats: I. Physical, metabolic, and longevity characteristics. J. Gerontol. 1985;40:657–670. doi: 10.1093/geronj/40.6.657. [DOI] [PubMed] [Google Scholar]
- Ziff EB. Enlightening the postsynaptic density. Neuron. 1997;19:1163–1174. doi: 10.1016/s0896-6273(00)80409-2. [DOI] [PubMed] [Google Scholar]