Abstract
Simple total syntheses of two Leucetta-derived marine alkaloids have been developed using position specific halogen-metal exchange of polyhaloimidazoles to introduce the benzyl substituted sidechains. Introduction of the C2 amine group by lithiation and trapping with tosyl azide provides amines on catalytic hydrogenation, which can be converted to naamidine G and 14-methoxynaamidine G using a procedure described in the literature.
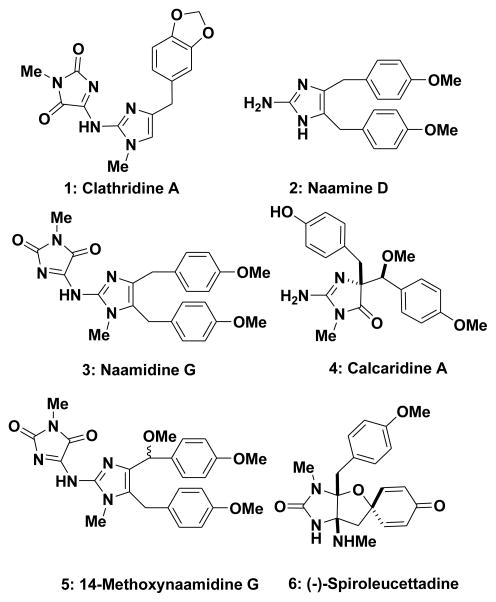
2-Aminoimidazole alkaloids have recently attracted the attention of the synthetic community, in particular several members of the oroidin family of alkaloids.1, 2 A less well explored group of 2-aminoimidazole-containing natural products are found in sponges of the Leucettidae family of sponges. The Leucetta family of alkaloids are characterized by the presence of a 2-aminoimidazole moiety which is substituted at various positions around the heterocycle and possess typically one or two benzylic fragments (1–6, Figure 1).1b They range in complexity from the fairly simple clathridine A (1)3 via naamine/naamidine type systems (e.g., naamine D (2), naamidine G (3), 14-methoxynaamidine G (5))4 to the more elaborate and highly oxygenated members such as calcaridine A (4) and spiroleucettadine (6).5 From a medicinal chemistry perspective, several of these alkaloids have been reported to exhibit activity as antibiotics,6 nitric oxide synthase inhibitors,7 and cytotoxicity,8 but in general their broad scale pharmacological evaluation has not been addressed. Although these molecules are not overly complex or challenging targets, they are potentially attractive scaffolds for evaluation in high throughput screening programs.1b
Figure 1.
Selected Leucetta Alkaloids
Our lab has developed a number of synthetic methods for the total synthesis of 2-aminoimidazole alkaloids using polyhaloimidazoles as the starting point, utilizing this strategy en route to the total synthesis of members of both the oroidin and Leucetta families of alkaloids.2,9 Following earlier reports,10 we and others have demonstrated that 4,5-dihaloimidazoles can be functionalized in a sequential and controlled manner in the order of C5→C4→C2 using Grignard reagents (for functionalization at the 4- and 5-positions) and n-BuLi (for C2).9,11,12 This manuscript describes the application of this strategy in the total syntheses of naamidine G (3) and 14-methoxynaamidine G (5).
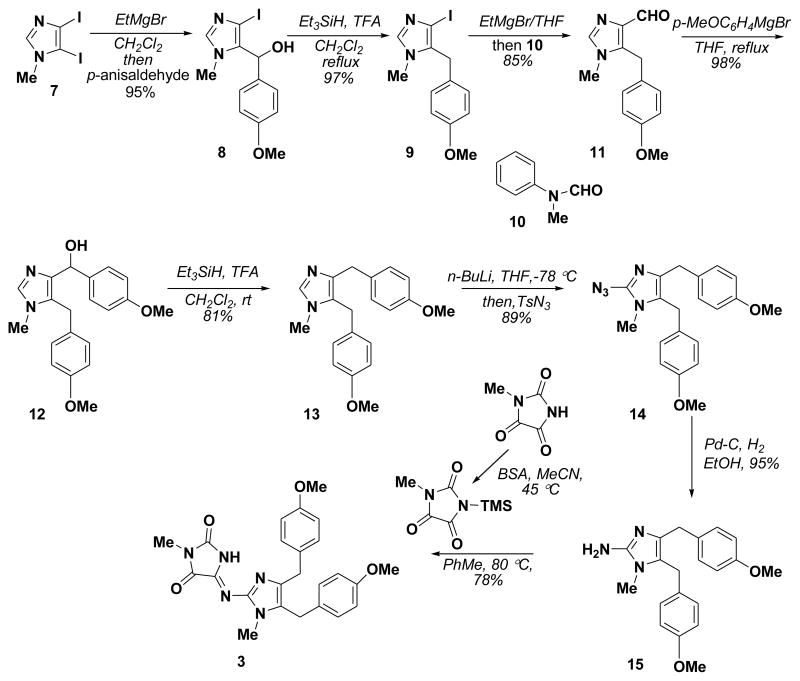
Naamidine G (3) and 14-methoxynaamidine G (5) were isolated and described by Pietra in 1995, but little in terms of biological activity of these natural products was reported.4b It is mentioned that the crude extract possess mild cytotoxicity (against KB cells) and activity against Candida albicans, but apparently the purified compounds were not further evaluated.4b Our approach to these natural products was patterned after a similar sequence of reactions used en route to calcaridine A9a starting from 4,5-diiodo-l-methylimidazole (7). Specifically, 7 was converted to the corresponding 4-iodoimidazole 8 by treatment with EtMgBr and then p-anisaldehyde (Scheme 1).9a After ionic reduction of the benzylic hydroxyl group in 8 with Et3SiH and TFA, the resulting 4-iodoimidazole was reacted with EtMgBr to effect formation of the imidazol-4-yl Grignard reagent which was treated with N-methylformanilide (10) to provide the aldehyde 11. Reaction of this aldehyde with p-MeOC6H4MgBr provided the desired alcohol 12, which is the key intermediate for both naamidine G and 14-methoxynaamidine G (Scheme 2). Removal of the doubly benzylic alcohol in 12 was achieved by reduction with Et3SiH and TFA at room temperature affording 13. Lithiation at C2 was accomplished with n-BuLi and the resulting organolithium species was treated with TsN3 to install an azide moiety, which was subjected to the catalytic hydrogenation to provide 1-methylnaamine D (15). This was then converted to naamidine G (3) using the procedure reported earlier with TMS-activated methyl parabanic acid13,9b to complete the total synthesis of 3 in 41% overall yields in 8 steps.
Scheme 1.
Total synthesis of naamidine G (3)
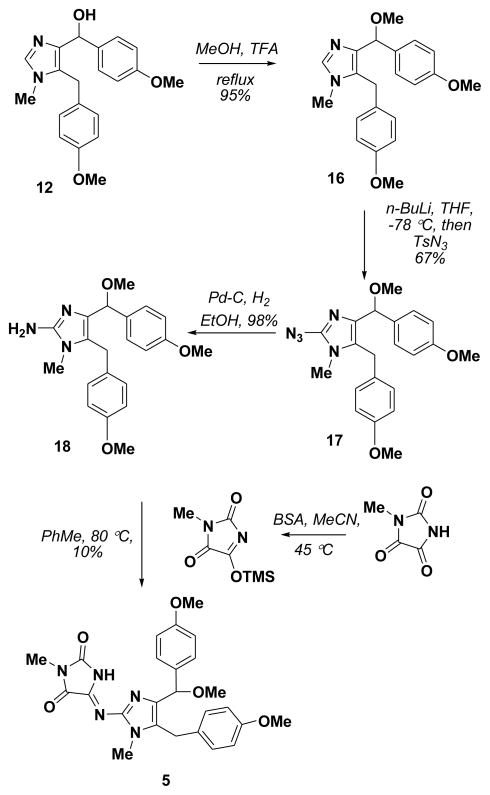
Scheme 2.
Total synthesis of 14-methoxynaamidine G (5)
The total synthesis of 14-methoxynaamidine G (5) commenced with the conversion of alcohol 12 to methyl ether 16 with methanol and TFA (Scheme 2). Introduction of C2 azide was accomplished by metalation and reaction with TsN3 provided 17, which was converted to amine 18 by catalytic hydrogenation. Following the same reaction as above, TMS-activated methyl parabanic acid provided 14-methoxynaamidine G (5) in low yield. Initially it was assumed that used TMS source might cause the decomposition of the starting material. Therefore, the introduction of hydantoin moiety was attempted with other methods reported in the literature.14 However, these methods failed to give the product and only the decomposition of the starting material was observed. The reason for the low yield from these reactions is still under investigation, but presumably is a result of competitive silylation and ionization of the methyl ether.
The spectroscopic data of the synthetic compounds were in agreement with the data reported for the natural products. However the melting point of the synthetic naamidine G was 195–196 °C in contrast to the natural product (94 °C).4b
In summary, we have developed high yielding eight-step syntheses of the Leucetta alkaloids naamidine G (3) and 14-methoxynaamidine G (5) using a sequential chemoselective halogen-magnesium exchange, permitting the selective installation of the C5 and C4-benzyl groups without protection of the more acidic C2-position. Subsequent lithiation at C2 and electrophilic trapping leads to introduction 2-amino moiety. We are actively exploring the synthesis of other members of the Leucetta family of alkaloids, and their analogs for medicinal chemistry programs.
Acknowledgments
This work was supported by the Robert A. Welch Foundation (Y-1362) and in part the NIH (GM066503). The NSF (CHE-9601771, CHE-0234811) is thanked for partial funding of the purchases of the NMR spectrometers used in this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arndt HD, Riedrich M. Angew Chem, Int Ed. 2008;47:4785. doi: 10.1002/anie.200801793.Sullivan JD, Giles RL, Looper RE. Curr Bioact Compd. 2009;5:39.See also Giles RL, Sullivan JD, Steiner AM, Looper RE. Angew Chem, Int Ed. 2009;48:3116. doi: 10.1002/anie.200900160.Int Ed. 2009;48:3116.
- 2.He Y, Du H, Sivappa R, Lovely CJ. Synlett. 2006:965. [Google Scholar]
- 3.(a) Ciminiello P, Fattorusso E, Magno S, Mangoni A. Tetrahedron. 1989;45:3873. [Google Scholar]; (b) Ciminiello P, Fattorusso E, Mangoni A, Di Blasio B, Pavone V. Tetrahedron. 1990;46:4387. [Google Scholar]
- 4.For isolation see: Dunbar DC, Rimoldi JM, Clark AM, Kelly M, Hamann MT. Tetrahedron. 2000;56:8795–8798.Mancini I, Guella G, Debitus C, Pietra F. Helv Chim Acta. 1995;78:1178.
- 5.(a) Edrada RA, Stessman CC, Crews P. J Nat Prod. 2003;66:939. doi: 10.1021/np020503d. [DOI] [PubMed] [Google Scholar]; (b) Ralifo P, Crews P. J Org Chem. 2004;69:9025. doi: 10.1021/jo048789+. [DOI] [PubMed] [Google Scholar]; (c) White KN, Amagata T, Oliver AG, Tenney K, Wenzel PJ, Crews P. J Org Chem. 2008;73:8719. doi: 10.1021/jo800960w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akee R, Carroll TR, Yoshida WY, Scheuer PJ, Stout TJ, Clardy J. J Org Chem. 1990;55:1944. [Google Scholar]
- 7.Dunbar DC, Rimoldi JM, Clark AM, Kelly M, Hamann MT. Tetrahedron. 2000;56:8795. [Google Scholar]
- 8.(a) Copp BR, Fairchild CR, Cornell L, Casazza AM, Robinson S, Ireland CM. J Med Chem. 1998;41:3909. doi: 10.1021/jm980294n. [DOI] [PubMed] [Google Scholar]; (b) Aberle NS, Catimel J, Nice EC, Watson KG. Bioorg Med Chem Lett. 2007;17:3741. doi: 10.1016/j.bmcl.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 9.(a) Koswatta PB, Sivappa R, Dias HV, Lovely CJ. Org Lett. 2008;10:5055–5058. doi: 10.1021/ol802018r. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Koswatta PB, Lovely CJ. Tetrahedron Lett. 2009;50:4998. doi: 10.1016/j.tetlet.2009.10.117. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Koswatta PB, Sivappa R, Dias HVR, Lovely CJ. Synthesis. 2009:2970. [Google Scholar]
- 10.Carver DS, Lindell SD, Saville-Stones EA. Tetrahedron. 1997;53:14481. [Google Scholar]
- 11.(a) Lovely CJ, Du H, Sivappa R, Bhandari MK, He Y, Dias HVR. J Org Chem. 2007;72:3741. doi: 10.1021/jo0626008. [DOI] [PubMed] [Google Scholar]; (b) Bhandari MR, Sivappa R, Lovely CJ. Org Lett. 2009;11:1535. doi: 10.1021/ol9001762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(a) Dehmel F, Abarbri M, Knochel P. Synlett. 2000:345. doi: 10.1021/jo000235t. [DOI] [PubMed] [Google Scholar]; (b) Abarbri M, Thibonnet J, Bérillon L, Dehmel F, Rottländer M, Knochel P. J Org Chem. 2000;65:4618. doi: 10.1021/jo000235t. [DOI] [PubMed] [Google Scholar]; (c) Knochel P, Dohle W, Gommermann N, Kneisel F, Kopp F, Korn T, Sapountzis I, Vu V. Angew Chem Int Ed. 2003;42:4302. doi: 10.1002/anie.200300579. [DOI] [PubMed] [Google Scholar]; (d) Yang X, Knochel P. Chem Commun. 2006:2170. doi: 10.1039/b603419e. [DOI] [PubMed] [Google Scholar]
- 13.Aberle NS, Lessene G, Watson KG. Org Lett. 2006;8:419. doi: 10.1021/ol052568o. [DOI] [PubMed] [Google Scholar]
- 14.(a) Kawasaki I, Taguchi N, Yoneda Y, Yamashita M, Ohta S. Heterocycles. 1996;43:1375. [Google Scholar]; (b) Ohta S, Tsuno N, Maeda K, Nakamura S, Taguchi N, Yamashita M, Kawasaki I. Tetrahedron Lett. 2000;41:4623. [Google Scholar]; (c) Nakamura S, Kawasaki I, Yamashita M, Ohta S. Heterocycles. 2003;60:583. [Google Scholar]