Abstract
The exact anti-neoplastic effects of calcium and vitamin D3 in the human colon are unclear. Animal and in vitro studies demonstrated that these two agents reduce oxidative stress, but these findings have never been investigated in humans. To address this, we conducted a pilot, randomized, double-blind, placebo-controlled, 2×2 factorial clinical trial to test the effects of calcium and vitamin D3 on a marker of oxidative DNA damage, 8-hydroxy-2’-deoxyguanosine (8-OH-dG), in the normal colorectal mucosa.
Patients (n=92) with at least one pathology-confirmed colorectal adenoma were treated with calcium 2 g/day and/or vitamin D3 800 IU/day vs. placebo over six months. Overall labeling and colorectal crypt distribution of 8-OH-dG in biopsies of normal-appearing rectal mucosa were detected by standardized automated immunohistochemistry and quantified by image analysis.
After six months treatment, 8-OH-dG labeling along the full lengths of colorectal crypts decreased by 22% (P=0.15) and 25% (P=0.10) in the calcium and vitamin D3 groups, respectively, but not in the calcium plus vitamin D3 group. The estimated treatment effects were strongest among participants with higher baseline colon crypt vitamin D receptor (VDR) expression (P=0.05).
Overall, these preliminary results indicate that calcium and vitamin D3 may decrease oxidative DNA damage in the normal human colorectal mucosa; support the hypothesis that 8-OH-dG labeling in colorectal crypts is a treatable oxidative DNA damage biomarker of risk for colorectal neoplasms; and provide support for further investigation of calcium and vitamin D3 as chemopreventive agents against colorectal neoplasms.
Keywords: vitamin D, calcium, 8-Hydroxy-2’-deoxyguanosine, randomized controlled trial, normal colorectal mucosa, colonic neoplasms
INTRODUCTION
Colorectal cancer, the second leading cause of cancer death in the U.S. (1), is a disease highly correlated with low vitamin D exposure, and with the Western-style diet, which is characterized by relatively low calcium consumption (2). Twenty-fold variations in international colon cancer rates, and migration studies showing acquired high risk within a generation, emphasize the importance of environmental exposures, especially diet and physical activity, in the etiology of colorectal cancer (2), and thus to its preventability. Currently, there is no complete agreement as to what dietary factors protect against or promote the development of colorectal cancer, nor any accepted pre-neoplastic biomarkers of risk. Further investigation of potential mechanisms whereby dietary agents lead to clinically relevant changes in normal colon tissue, and the development of biomarkers of risk derived from such mechanistic understanding, are urgently needed.
There is strong biological plausibility and animal experimental evidence for protection against colorectal cancer by calcium and vitamin D (3). Moreover, in epidemiologic studies, higher total calcium intakes have been consistently associated with reduced risk for colorectal neoplasms (4–8), and calcium supplementation reduced adenoma recurrence (9). Also, higher circulating 25-OH-vitamin D levels have been associated with reduced risk for colorectal neoplasms (8, 10). However, the anti-neoplastic effects of calcium and vitamin D on the normal colorectal epithelium remain unclear.
Proposed mechanisms of calcium against colorectal cancer include protection of colonocytes against free bile and fatty acids (11), direct effects on the cell cycle, and modulation of the APC colon carcinogenesis pathway (12). Beyond calcium homeostasis, vitamin D regulates cell cycle events; promotes bile acid degradation; influences growth factor signaling, cell adhesion, and DNA repair; and modulates more than 200 genes (12, 13). Recent evidence also indicates that vitamin D and the VDR (vitamin D receptor) are involved in protection against oxidative damage (14–16).
Despite the basic science evidence, there are no published human trials of the effects of vitamin D and/or calcium supplementation on markers of oxidative DNA damage, such as 8-hydroxy-2’-deoxyguanosine (8-OH-dG), in the normal-appearing colorectal mucosa. To address this, we conducted a pilot, randomized, double-blind, placebo-controlled, 2 × 2 factorial chemoprevention clinical trial of supplemental calcium and vitamin D3, alone and in combination vs. placebo over six months, to estimate the efficacy of these agents on a panel of biomarkers (including 8-OH-dG) in the normal colorectal mucosa. We hypothesized that calcium and vitamin D3, alone and in combination, decrease colorectal epithelial oxidative DNA damage.
PATIENTS AND METHODS
Participant Population
The detailed protocol of study recruitment and procedures was published previously (17). Briefly, eligible patients, 30–75 years of age, in general good health, capable of informed consent, with a history of at least one pathology-confirmed adenomatous colorectal polyp within the past 36 months, and no contraindications to calcium or vitamin D supplementation or rectal biopsy procedures and no medical conditions, habits, or medication usage that would otherwise interfere with the study were recruited from the patient population attending the Digestive Diseases Clinic at the Emory Clinic, Emory University. Detailed specific study exclusion criteria were presented elsewhere (17). This study was approved by the Emory University IRB. Written informed consent was obtained from each study participant.
Clinical Trial Protocol
Between April 2005 and January 2006, 522 patients passed initial chart screening for eligibility, and 224 (43%) patients were sent an introductory letter followed by a telephone interview. A total of 105 (47%) potential participants attended an eligibility visit during which there were interviewed, signed a consent form, completed questionnaires, provided a blood sample, and started a one-month placebo run-in period. Diet was assessed with a semiquantitative food frequency questionnaire (18). Medical and pathology records were reviewed. After a 30-day placebo run-in trial, 92 (88%) participants without significant perceived side effects and who had taken at least 80% of their tablets were eligible for randomized assignment. Eligible participants then underwent a baseline rectal biopsy and were randomly assigned to the following four treatment groups: a placebo control group, a 2.0 g elemental calcium (as calcium carbonate in equal doses twice daily) supplementation group, an 800 IU vitamin D3 supplementation group (400 IU twice daily), and a calcium plus vitamin D supplementation group taking 2.0 g elemental calcium plus 800 IU of vitamin D3 daily.
All study tablets were custom manufactured by Tishcon Corporation, NY, USA. The corresponding supplement and placebo pills were identical in size, appearance, and taste. The placebo was free of vitamin D, calcium, magnesium, and chelating agents. Additional details on the rationale for the doses and forms of calcium and vitamin D supplementation forms were previously described (17).
The treatment period was six months, and participants attended follow-up visits at 2 and 6 months after randomization and were contacted by telephone between the second and final follow-up visits. Pill-taking adherence was assessed by questionnaire, interview, and pill count. Participants were instructed to remain on their usual diet and not take any nutritional supplements not in use on entry into the study. At each of the follow-up visits participants were interviewed and filled out questionnaires. At the last visit all participants underwent venipuncture and a rectal biopsy procedure. All participants were asked to abstain from aspirin use for seven days prior to each biopsy visit. All visits for a given participant were scheduled at the same time of day to control for possible circadian variability in the outcome measures. Factors hypothesized to be related to 8-OH-dG levels in the normal colon mucosa (e.g., antioxidant micronutrient intakes) were assessed at baseline and at the final follow-up visit. Participants did not have to be fasting for their visits and did not take a bowel cleansing preparation or enema.
Tissue Collection and Processing
Six sextant 1.0 mm-thick biopsy specimens were taken from the rectal mucosa 10 cm proximal to the external anal aperture through a rigid sigmoidocsope with a jumbo cup flexible endoscopic forceps mounted on a semiflexible rod. The biopsies were then immediately placed in phosphate buffered saline, oriented under a dissecting microscope and placed in 10% normal buffered formalin, and then transferred to 70% ethanol 24 hours after initial placement in formalin. Within a week, the biopsies were processed and embedded in paraffin blocks with three biopsies per block.
Laboratory Methods
The paraffin blocks were cut into 3.0 µm-thick sections, with each level 40 µm apart. Five slides with four section levels per patient per biomarker were prepared for immunostaining. To uncover the epitope, heat-mediated antigen retrieval was used: slides were placed in a preheated Pretreatment Module (Lab Vision Corp., CA) with 100× Citrate Buffer pH 6.0 (DAKO S1699, DAKO Corp., Carpinteria, CA) and steamed for 40 minutes. Then, slides were placed in a DAKO Automated Immunostainer and immunohistochemically processed using a labeled streptavidin-biotin method for 8-OH-dG (mouse monoclonal antibody to 8-OH-dG manufactured by Abcam Inc., MA, clone number N45.1, at a concentration of 1:100 (19)). For each participant, baseline and follow-up biopsy slides were stained in the same batch, and each staining batch included a balance of participants from each treatment group. The slides were not counterstained. After staining, the slides were coverslipped with a Leica CV5000 Coverslipper (Leica Microsystems, Inc., IL). In each staining batch of slides, positive and negative control slides were included. Colon adenocarcinoma was used as a control tissue. The negative and the positive control slides were treated identically to the patients’ slides except that antibody diluent was used rather than primary antibody on the negative control slide. For vitamin D receptor (VDR), slides were processed as previously described, but using mouse monoclonal D-6 antibody raised against amino acids 344–424 of human VDR (SC-13133, Santa Cruz Biotechnology, Inc., CA) at a concentration of 1:7,500 (20, 21).
Image Analysis of Immunohistochemically Detected Biomarkers in Normal Colon Crypts
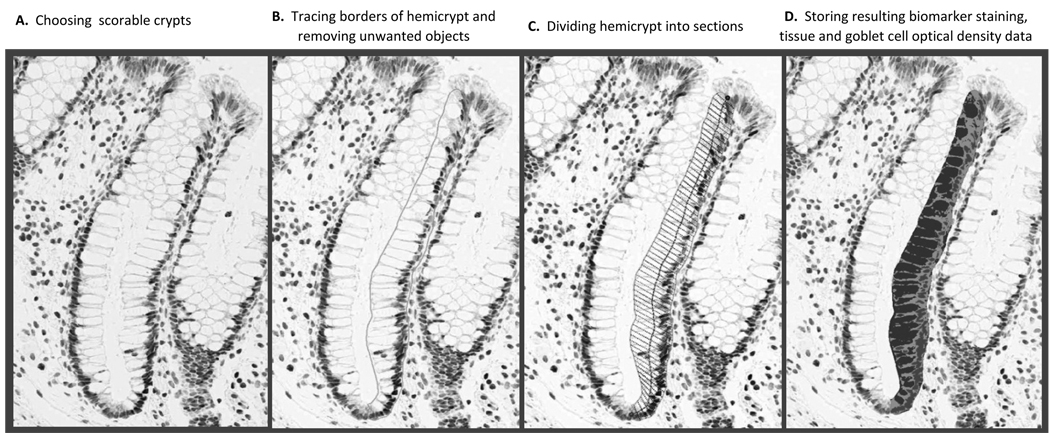
A quantitative image analysis method (“scoring”) was used to evaluate detected levels of the biomarkers in colon crypts, as depicted in Figure 1. The major equipment and software for the image analysis procedures were: Scanscope CS digital scanner (Aperio Technologies, Inc., CA), computer, digital drawing board, Matlab software (MathWorks, Inc., MA), CellularEyes Image Analysis Suite (DivEyes LLC, GA), and MySQL (Sun Microsystems Inc., CA). First, slides were scanned with the Aperio Scanscope CS digital scanner, then, electronic images were reviewed in the CellularEyes program to identify colon crypts acceptable for analysis. A “scorable” crypt was defined as an intact crypt extending from the muscularis mucosa to the colon lumen (17, 22). Before analysis, images of negative and positive control slides were checked for staining adequacy. Standardized settings were used on all equipment throughout the scoring procedures. The technician reviewed slides in the CellularEyes program and selected two of three biopsies with 16 to 20 “scorable” hemicrypts (one half of the crypt) per biopsy. Using the digital drawing board the borders of each selected hemicrypt were traced. The program then divided the outline into the equally spaced segments with the average widths of normal colonocytes. Finally, the program measured the background corrected optical density of the biomarker labeling across the entire hemicrypt as well as within each segment. The range of optical density for 8-OH-dG labeling was set between 0.04 and 0.20. All resulting data were automatically transferred into the MySQL database. Then, the technician moved to the next identified hemicrypt and repeated all the previously described analysis steps. A reliability control sample previously analyzed by the reader was re-analyzed during the course of the trial to determine intra-reader “scoring” reliability by intraclass correlation coefficient, which was 0.94 for 8-OH-dG.
Figure 1.
Quantitative image analysis using Aperio Scanscope and CellularEyes software to measure 8-OH-dG labeling in normal-appearing colorectal crypts.
Protocol for Measuring Serum Vitamin D Levels
All laboratory assays for serum 25-OH-vitamin D and 1,25-(OH)2-vitamin D were performed by Dr. Bruce Hollis at the Medical University of South Carolina using a radioimmunoassay method as previously described (23). Serum samples for baseline and follow-up visits for all subjects were assayed together, ordered randomly, and labeled to mask treatment group, follow-up visit, and quality control replicates. The average intra-assay coefficient of variation for serum 25-OH-vitamin D was 2.3%, and for 1,25-(OH)2-vitamin D, 6.2%.
Statistical Analysis
We assessed treatment groups for comparability of characteristics at baseline and at final follow-up by the Fisher’s exact test for categorical variables and analysis of variance (ANOVA) for continuous variables.
Several outcome variables were defined to estimate the overall labeling and within-crypt distributions of 8-OH-dG in the crypts. The mean optical density of 8-OH-dG labeling in the crypts was calculated for each patient at baseline and 6-months follow-up by summing all the densities from all analyzed crypts from the biopsy specimens and dividing by the number of crypts analyzed. Measures of the within-crypt distributions of the marker were calculated for each patient by taking the means of the biomarker densities in various zones of the crypt (e.g., the upper 40%, lower 60%).
Primary analyses were based on assigned treatment at the time of randomization, regardless of adherence status (intent-to-treat analysis). Mean biomarker densities were calculated for each treatment group for the baseline and 6-months follow-up visits. Treatment effects were evaluated by assessing the differences in the densities from baseline to the 6-months follow-up visit between patients in each active treatment group and the placebo group by a repeated measures linear MIXED effects model. The model included the intercept, follow-up visit effects (baseline and follow-up), and interactions between treatment groups and the follow-up visit effect (the absolute treatment effect). Since optical density is measured in arbitrary units, to provide perspective on the magnitude of the treatment effects we also calculated relative effects, defined as: [treatment group follow-up mean/treatment group baseline mean]/[placebo follow-up mean/placebo baseline mean]. The relative effect provides a conservative estimate of the proportional change in the treatment group relative to that in the placebo group. The interpretation of the relative effect is somewhat analogous to that of an odds ratio (e.g., a relative effect of 2.0 would mean that the proportional change in the treatment group was twice as great as that in the placebo group) (17, 24). Since the treatment groups were balanced on risk factors at baseline, no adjustment was made for other covariates in the primary intent-to-treat analyses.
The distributions of 8-OH-dG staining density were graphically evaluated using the LOESS procedure with smoothing parameter 0.5 and local quadratic fitting. First, the number of sections within a hemicrypt was standardized to 50. Then, the average for each section across all crypts was predicted by the LOESS model separately for each patient, and then for each treatment group by follow-up visit. The results were plotted in the graphs along with smoothing lines.
A questionnaire derived oxidative balance score (OBS) was calculated as described in (25, 26). Briefly, continuous variables that reflect pro-oxidant (saturated fat and total iron intake), and antioxidant (total tocopherol, carotenoid, vitamin C, lycopene, lutein/zeaxanthin, and β-cryptoxanthin intake) exposures were divided into high and low categories based on the median value among all participants at baseline. Participants with low (below median) exposure to a particular pro-oxidant were awarded 1 point, whereas those with high (above median) exposure to the same pro-oxidant were awarded 0 points. For antioxidant exposure, a point was awarded for each high-level (above median) exposure, and 0 points for each low-levels (below median) exposure. For dichotomous variables (“yes” vs. “no”), participants received one point for each antioxidant exposure (regular use of NSAIDs and/or aspirin, supplementation with selenium, and never smoker). Then the points assigned for each individual component of OBS were summed up to calculate the overall score. Lower OBS values indicate a higher prevalence of pro-oxidant exposures, whereas higher OBS values indicate a predominance of antioxidant exposures. The range of the baseline OBS in this study was between 3 and 10, and the median was 6. We dichotomized baseline OBS based on the median value, and assigned each participant to a high OBS (above median, “antioxidant”) or low OBS (below median, “pro-oxidant”) category. Similarly, continuous variables (e.g., age and VDR expression) were dichotomized (into high/low categories) based on the median value in all study participants at baseline. Then, stratified analyses were conducted to explore differential treatment effects by baseline age (<60 and ≥60 years), VDR expression (high/low), 8-OH-dG labeling (high/low), OBS (≤6 and >6), first-degree family history of colorectal cancer (yes/no), sex (male/female), regular NSAID use (yes/no), serum 25-OH-vitamin D levels (<22 and ≥22 ng/mL), and adherence to treatment (<80% or ≥80% treatment pills taken). Differences between categories were tested by including the category-intervention interaction term in the model.
Statistical analyses were done using SAS System software (version 9.1.3; SAS Institute, Inc., NC). A cutoff level of P ≤ 0.05 (2-sided) was used for assessing statistical significance.
RESULTS
Characteristics of Study Participants
Treatment groups did not differ significantly on participant characteristics measured at baseline (Table 1) or at the end of the study (data not shown). The mean age of participants was 61 years, 64% were men, 71% were White, and 20% had a family history of colorectal cancer in a first degree relative. Most participants were overweight, non-smokers, college graduates, and had a single small mildly dysplastic tubular adenoma (Table 1).
Table 1.
Selected Baseline Characteristics of the Study Participants* (n=92).
| Treatment Group |
|||||
|---|---|---|---|---|---|
| Placebo | Calcium | Vitamin D | Calcium + Vit. D | P-value** | |
| Characteristics | (n=23) | (n=23) | (n=23) | (n=23) | |
| Demographics, medical history, habits, anthropometrics | |||||
| Age, years | 58.5 (8.2) | 61.9 (8.2) | 60.2 (8.1) | 62.1 (7.5) | 0.39 |
| Men (%) | 70 | 70 | 70 | 70 | 1.00 |
| White (%) | 74 | 83 | 65 | 61 | 0.39 |
| College graduate (%) | 65 | 61 | 57 | 44 | 0.53 |
| History of colorectal cancer in 1° relative (%) | 17 | 30 | 17 | 13 | 0.60 |
| Take NSAID*** regularly§ (%) | 22 | 13 | 9 | 22 | 0.60 |
| Take aspirin regularly§ (%) | 22 | 52 | 30 | 56 | 0.05 |
| If woman (n = 28), taking estrogens (%) | 4 | 9 | 4 | 4 | 1.00 |
| Current smoker (%) | 9 | 4 | 0 | 0 | 0.61 |
| Take multivitamin (%) | 30 | 30 | 26 | 39 | 0.86 |
| Body mass index (BMI), kg/m2 | 30.6 (7.2) | 29.4 (5.5) | 28.9 (5.6) | 31.6 (6.0) | 0.44 |
| Mean dietary intakes | |||||
| Total energy intake, kcal/d | 1,596 (528) | 1,788 (691) | 1,848 (821) | 1,845 (752) | 0.59 |
| Total§§ calcium, mg/d | 618 (308) | 746 (335) | 843 (526) | 824 (714) | 0.41 |
| Total§§ vitamin D, IU/d | 277 (230) | 336 (202) | 360 (317) | 415 (316) | 0.40 |
| Total fat, gm/d | 67 (32) | 72 (35) | 70 (32) | 74 (28) | 0.59 |
| Dietary fiber, gm/d | 15 (7) | 17 (9) | 18 (9) | 17 (11) | 0.97 |
| Alcohol, gm/d | 9 (14) | 11 (15) | 14 (18) | 10 (20) | 0.84 |
| Oxidative balance score (OBS)¤ | 6 (2) | 7 (2) | 7 (2) | 7 (2) | 0.46 |
| Adenoma characteristic | |||||
| Multiple adenomas¤¤ (%) | 17 | 22 | 39 | 26 | 0.45 |
| Large adenoma ≥ 1 cm£ (%) | 19 | 32 | 17 | 9 | 0.32 |
| Villous/tubulovillous adenoma££ (%) | 4 | 9 | 9 | 4 | 1.00 |
| Mild dysplasia (%) | 100 | 96 | 100 | 100 | 1.00 |
Data are given as means (SD) unless otherwise specified.
By Fisher’s exact χ2 test for categorical variables, and ANOVA for continuous variables.
Nonsteroidal anti-inflammatory drug.
At least once a week.
Diet plus supplements.
See the “Statistical Analysis” section for details.
At least two adenomas.
At least one large adenoma.
At least one villous or tubulovillous adenoma.
Adherence to visit attendance averaged 92% and did not differ significantly among the four treatment groups. On average, at least 80% of pills were taken by 93% of participants at the first follow-up visit and by 84% at the final follow-up visit. There were no treatment or biopsy complications. Seven people (8%) were lost to follow-up due to perceived drug intolerance (n=2), unwillingness to continue participation (n=3), physician’s advice (n=1), and cardiovascular disease death (n=1). Dropouts included one person from the vitamin D supplementation group, and two persons from each of other three groups.
At baseline, there were no significant differences between the four study groups in serum 25-OH- or 1,25-(OH)2-vitamin D levels. At the study end, the vitamin D and calcium plus vitamin D groups had significantly higher levels of serum 25-OH-vitamin D (p<0.001), whereas the placebo and calcium groups had slight non-significant decreases in 25-OH-vitamin D levels (Table 2, A). As expected, serum levels of 1,25-(OH)2-vitamin D at the end of follow-up period did not differ significantly between study groups (17).
Table 2.
Serum 25-OH-vitamin D, and optical density of immunohistochemically detected 8-OH-dG in colorectal crypts at baseline and 6-months follow-up.
| Baseline |
6-Months Follow-up |
Absolute Rx Effect* |
Relative Effect § |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SE | P** | n | Mean | SE | P** | n | Mean | SE | P** | ||
| A. Serum vitamin D measurements | |||||||||||||
| 25-OH-vitamin D, ng/mL | |||||||||||||
| Placebo | 23 | 20.7 | 1.7 | 21 | 18.2 | 1.8 | 21 | 0 | 1.00 | ||||
| Calcium | 23 | 25.7 | 1.7 | 0.05 | 21 | 23.4 | 1.7 | 0.03 | 21 | 0.2 | 1.8 | 0.92 | 1.03 |
| Vitamin D | 23 | 21.0 | 1.7 | 0.81 | 22 | 29.5 | 1.7 | <0.0001 | 22 | 10.9 | 1.8 | <0.0001 | 1.59 |
| Calcium + Vit. D | 23 | 20.9 | 1.7 | 0.84 | 21 | 28.9 | 1.7 | <0.0001 | 21 | 10.5 | 1.8 | <0.0001 | 1.57 |
| B. 8-OH-dG$ labeling optical density in colorectal crypts | |||||||||||||
| Entire crypts | |||||||||||||
| Placebo | 23 | 2,360.8 | 193.2 | 21 | 2,509.0 | 202.1 | 21 | 0 | 1.00 | ||||
| Calcium | 23 | 2,349.4 | 193.2 | 0.97 | 21 | 1,946.2 | 202.1 | 0.05 | 21 | −551.5 | 374.4 | 0.14 | 0.78 |
| Vitamin D | 23 | 2,318.4 | 193.2 | 0.88 | 22 | 1,847.3 | 197.5 | 0.02 | 22 | −619.3 | 372.0 | 0.10 | 0.75 |
| Calcium + Vit. D | 23 | 2,347.8 | 193.2 | 0.96 | 21 | 2,642.6 | 202.1 | 0.64 | 21 | 146.5 | 264.8 | 0.70 | 1.06 |
| Upper 40% of crypts | |||||||||||||
| Placebo | 22 | 677.7 | 64.1 | 21 | 655.9 | 67.0 | 21 | 0 | 1.00 | ||||
| Calcium | 23 | 704.7 | 64.1 | 0.77 | 21 | 525.1 | 67.0 | 0.17 | 21 | −157.8 | 125.8 | 0.21 | 0.77 |
| Vitamin D | 23 | 684.4 | 64.1 | 0.94 | 22 | 505.9 | 65.5 | 0.11 | 22 | −156.8 | 125.0 | 0.21 | 0.76 |
| Calcium + Vit. D | 23 | 655.0 | 64.1 | 0.80 | 21 | 741.5 | 67.0 | 0.37 | 21 | 108.3 | 125.8 | 0.39 | 1.17 |
| Lower 60% of crypts | |||||||||||||
| Placebo | 22 | 1,418.7 | 112.0 | 21 | 1,459.7 | 117.2 | 21 | 0 | 1.00 | ||||
| Calcium | 23 | 1,431.8 | 112.0 | 0.93 | 21 | 1,201.5 | 117.2 | 0.12 | 21 | −271.3 | 213.5 | 0.21 | 0.82 |
| Vitamin D | 23 | 1,450.7 | 112.0 | 0.84 | 22 | 1,145.1 | 114.5 | 0.06 | 22 | −346.6 | 212.1 | 0.11 | 0.77 |
| Calcium + Vit. D | 23 | 1,390.0 | 112.0 | 0.86 | 21 | 1,556.8 | 117.2 | 0.56 | 21 | 125.8 | 213.5 | 0.56 | 1.09 |
Absolute treatment effect = [treatment group follow-up − treatment group baseline] − [placebo group follow-up − placebo group baseline].
P-value for difference between each active treatment group and placebo group from repeated measures Mixed model.
Relative effect = [(treatment group follow-up/treatment group baseline)/(placebo follow-up/placebo baseline)]; interpretation similar to that for an odds ratio (e.g., a relative effect of 1.7 indicates a proportional increase of 70% in the treatment group relative to that in the placebo group.
Biomarker detected immunohistochemically and then its labeling optical density quantified by image analysis (see text for details).
Graphical Assessment of Changes over Six Months in the Distribution of 8-OH-dG Labeling along Normal Colorectal Crypts
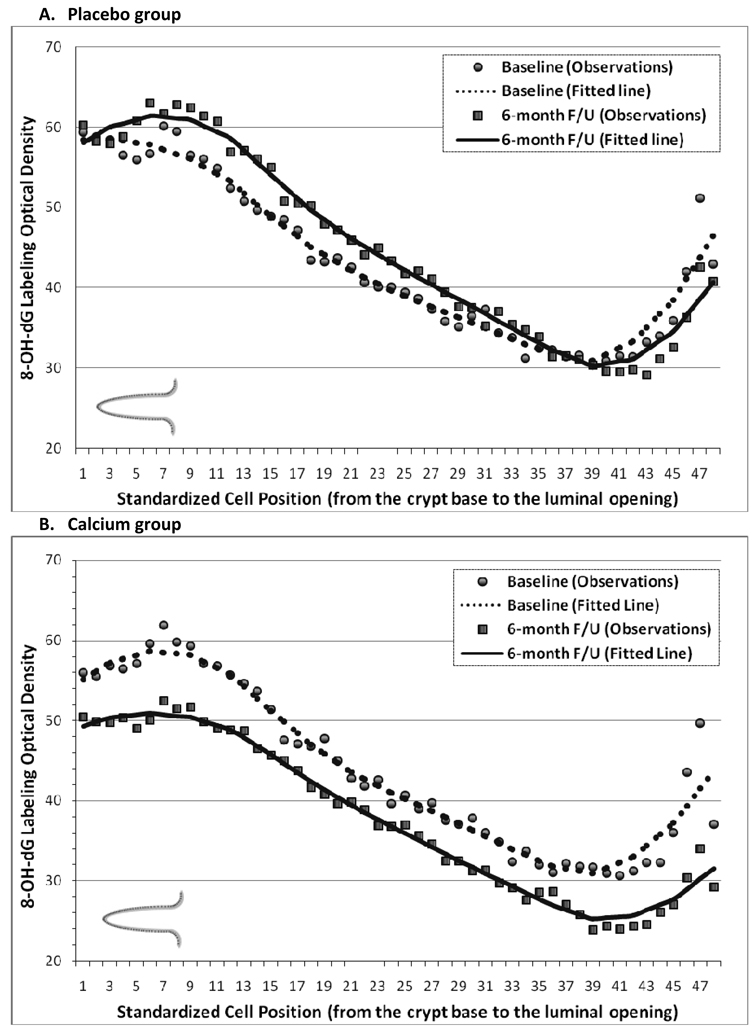
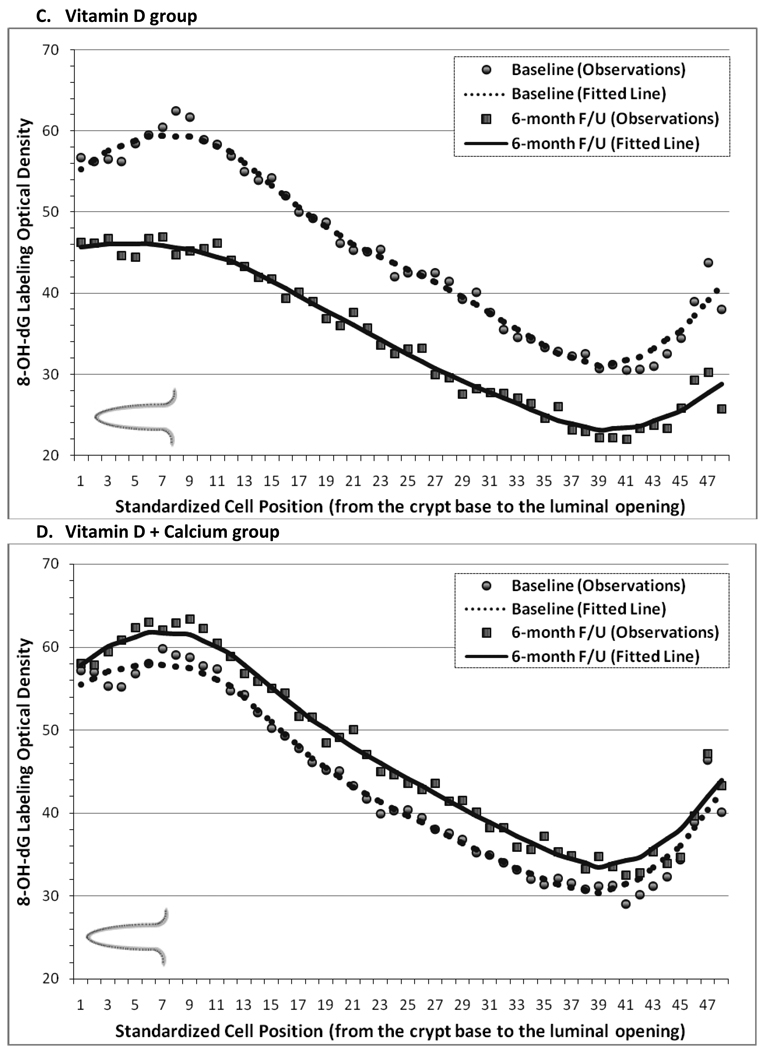
The distribution of 8-OH-dG staining optical density (“labeling”) along the colorectal crypts at the baseline and 6-months follow-up visits is shown in Figure 2. In each treatment group, 8-OH-dG labeling appeared to be the highest in the lower 20%–30% of the crypts, to decrease in the middle part of the crypts, and then to increase somewhat again toward the colon lumen. The baseline distribution of 8-OH-dG along the crypts in all four treatment groups appeared to be almost identical in shape and optical density range. In the placebo group, from baseline to follow-up 8-OH-dG labeling appeared to increase slightly in the middle part of the crypts (Figures 2, A). A large post-supplemental decrease in 8-OH-dG labeling along the full lengths of the crypt was noted in the calcium and vitamin D groups (Figure 2, B & C). In the vitamin D plus calcium group, similar to as in the placebo group, 8-OH-dG labeling slightly increased from baseline to follow-up (Figure 2, D).
Figure 2.
Distribution of 8-OH-dG staining optical densities along normal colorectal crypts by treatment group at baseline and follow-Up. A, Placebo group. B, Calcium Group. C, Vitamin D group. D, Vitamin D + Calcium group.
Effects of Calcium and/or Vitamin D Supplementation on 8-OH-dG Labeling in Normal Colorectal Crypts
At baseline, there were no differences in 8-OH-dG labeling along the full lengths of crypts among the four treatment groups. Relative to placebo, 8-OH-dG labeling along the full lengths of the crypts decreased by 22% (P=0.14) in the calcium group and by 25% (P=0.10) in the vitamin D group, and increased by 6% (P=0.70) in the calcium plus vitamin D group (Table 2, B). The findings for the upper 40% and the lower 60% of the crypts (the differentiation and proliferation zones, respectively; Table 2, B), and for the upper and lower 20% (areas closest to and furthest from colon lumen exposures, respectively; data not shown) did not differ substantially from those for the entire crypts.
Stratified Analyses
We investigated whether change in 25-OH-vitamin D levels, adherence to treatment, family history of colorectal cancer, sex, age, smoking, NSAID use, baseline oxidative balance score (OBS), and baseline batch-standardized VDR expression or 8-OH-dG labeling modified response to treatment; however, the sample size was too small for most of these results to be reliable. The effect of treatment on 8-OH-dG variables did not vary by age, smoking status, family history of colorectal cancer, NSAID use, or change in serum 25-OH-vitamin D levels (data not shown). In women, 8-OH-dG labeling decreased only in the calcium group (−25%, P=0.43); however, in men, 8-OH-dG labeling decreased in all three active treatment groups (Table 3). In those with a high (“anti-oxidant”) baseline OBS, 8-OH-dG labeling decreased in all three active treatment groups after 6-months treatment, whereas in those with low (“pro-oxidant”) baseline OBS, 8-OH-dG decreased only in the vitamin D group (−19%, P=0.40; Table 3).
Table 3.
8-OH-dG labeling in colorectal crypts stratified by sex, baseline oxidative balance score (OBS), and baseline colorectal crypt VDR expression.
| Absolute Rx Effect* |
Relative Effect§ |
P§§ | ||||
|---|---|---|---|---|---|---|
| n | Mean | SE | P** | |||
| Women | ||||||
| Placebo | 7 | 0 | 1.00 | |||
| Calcium | 7 | −577.4 | 714.7 | 0.43 | 0.75 | |
| Vitamin D | 6 | 253.3 | 729.4 | 0.73 | 1.11 | |
| Calcium + Vit. D | 7 | 508.3 | 714.7 | 0.48 | 1.23 | |
| Men | ||||||
| Placebo | 14 | 0 | 1.00 | |||
| Calcium | 14 | −526.1 | 422.9 | 0.22 | 0.80 | |
| Vitamin D | 16 | −959.0 | 414.3 | 0.02 | 0.62 | |
| Calcium + Vit. D | 14 | −26.2 | 422.9 | 0.95 | 0.99 | 0.35 |
| High oxidative balance score (OBS)& | ||||||
| Placebo | 8 | 0 | 1.00 | |||
| Calcium | 13 | −898.0 | 594.8 | 0.14 | 0.67 | |
| Vitamin D | 12 | −879.0 | 599.7 | 0.15 | 0.67 | |
| Calcium + Vit. D | 11 | −120.5 | 615.1 | 0.85 | 0.94 | |
| Low oxidative balance score (OBS)& | ||||||
| Placebo | 13 | 0 | 1.00 | |||
| Calcium | 8 | 25.1 | 509.7 | 0.96 | 1.02 | |
| Vitamin D | 9 | −421.6 | 498.6 | 0.40 | 0.81 | |
| Calcium + Vit. D | 10 | 383.2 | 478.1 | 0.43 | 1.19 | 0.71 |
| High baseline colorectal crypt VDR expression& | ||||||
| Placebo | 10 | 0 | 1.00 | |||
| Calcium | 10 | −827.0 | 477.0 | 0.09 | 0.68 | |
| Vitamin D | 8 | −1,626.1 | 506.7 | 0.003 | 0.46 | |
| Calcium + Vit. D | 11 | −443.6 | 462.5 | 0.34 | 0.83 | |
| Low baseline colorectal crypt VDR expression& | ||||||
| Placebo | 8 | 0 | 1.00 | |||
| Calcium | 9 | 264.6 | 532.4 | 0.62 | 1.11 | |
| Vitamin D | 13 | 63.5 | 498.8 | 0.90 | 1.00 | |
| Calcium + Vit. D | 8 | 1,420.4 | 551.5 | 0.02 | 1.75 | 0.05 |
Absolute treatment effect = [treatment group follow-up − treatment group baseline] − [placebo group follow-up − placebo group baseline].
P-value for difference between each active treatment group and placebo group from repeated measures Mixed model.
Relative effect = [(treatment group follow-up/treatment group baseline)/(placebo follow-up/placebo baseline)]; interpretation similar to that for an odds ratio (e.g., a relative effect of 1.7 indicates a proportional increase of 70% in the treatment group relative to that in the placebo group).
P-value for the category-intervention interaction term.
OBS and baseline colorectal crypt VDR expression were dichotomized into high/low categories based on the median value in all study participants at baseline. OBS was calculated as described in the ‘Statistical Analysis’ section. VDR detected immunohistochemically and then its labeling optical density was quantified by image analysis (see text for details).
There were no substantial differences in the estimated treatment effects according to baseline levels of 8-OH-dG labeling (data not shown). Among those with high baseline colorectal crypt VDR expression, 8-OH-dG labeling decreased by 35% (P=0.09) in the calcium group, 54% (P=0.003) in the vitamin D group, and 17% (P=0.34) in the calcium plus vitamin D group relative to the placebo; whereas there were no decreases seen in those with low baseline VDR expression, and there was a 75% increase in 8-OH-dG labeling in the calcium plus vitamin D group relative to the placebo. The test for interaction for treatment effect by VDR status was statistically significant (P=0.05; Table 3).
DISCUSSION
The results from this pilot, randomized, controlled clinical trial suggest that supplementation with calcium or vitamin D3, but not with both agents combined, may decrease oxidative DNA damage, as indicated by decreased 8-OH-dG immunohistochemical labeling, in the normal-appearing colorectal epithelium of sporadic adenoma patients. These findings are consistent with the hypothesis that high intakes of calcium or vitamin D3 may decrease oxidative stress and oxidative DNA damage in the colon, and, thus, reduce risk for colorectal neoplasms. Our findings also suggest that vitamin D3 combined with calcium may have either a lesser or no treatment effect on 8-OH-dG labeling than does either calcium or vitamin D alone. Consistent with existing animal data (15, 27), we found evidence that baseline VDR (vitamin D receptor) expression levels may modify treatment effects of calcium and vitamin D3, such that those with higher colorectal crypt VDR expression may be more strongly responsive to treatment. Finally, the treatment effect of calcium and vitamin D3 tended to be stronger in men and those with higher baseline anti-oxidant relative to pro-oxidant exposures.
Oxidative stress, a condition characterized by an imbalance of pro-oxidants to antioxidants which results in macromolecular damage and disruption of redox signaling and control (28), may play a role in colon carcinogenesis, inducing protein and DNA damage and lipid peroxidation, and impairing intracellular signaling. Under normal conditions, reactive oxygen species (ROS) have an important role as intracellular signaling molecules that regulate many genes (29). However, under inflammatory conditions, increased generation of ROS products leads to cell molecule damage such as oxidation of DNA (29). The most abundant product of oxidative DNA modifications by ROS is 8-hydroxy-2′-deoxyguanosine (8-OH-dG) (30). This oxidized base is a useful biomarker of oxidative stress that can be measured in urine, blood, and tissues (19, 31). Several studies demonstrated increased levels of oxidatively modified DNA in colorectal adenocarcinomas when compared to adenomas and adjacent normal epithelium (32, 33). This suggests that inhibition of oxidative stress in the normal colorectal epithelium may slow down or prevent carcinogenesis, and prompts the development of chemopreventive agents, such as calcium and vitamin D, that target oxidative stress in the colon.
There are several lines of evidence to support our hypotheses that calcium and vitamin D may act as antioxidants and DNA damage reducing agents in the colon. Bile acids damage cell membranes, at least in part through an oxidative mechanism (34, 35), provoking an inflammatory response and causing DNA damage (36), and both calcium and vitamin D can reduce the free bile acid load in the colon lumen. Calcium directly binds bile acids, rendering them inert (37). Vitamin D activation of the ubiquitous vitamin D receptor (VDR) in the colon up-regulates CYP3A4, which in turn catabolizes the secondary bile acid, lithocholic acid (38, 39). Furthermore, high blood 25-(OH)-vitamin D levels provide a pool of vitamin D that is available for various tissues, such as the colorectal epithelium. In colonocytes, vitamin D increases expression of enzymes involved in antioxidant response, inhibits iron-dependent lipid peroxidation in liposomes, lowers glutathione reductase levels, induces glutathione peroxidase and manganese dependent superoxide dismutase activity, and elevates glutathione levels ((16, 40), also reviewed in (14)), thereby decreasing oxidative stress in the colorectal epithelium. The results of this study, combined with the biological evidence, support calcium and vitamin D3 as oxidative DNA damage reducing agents.
Contrary to our original hypothesis and to what has been described in some epidemiologic and clinical studies (41–45), we did not observe a treatment effect in the calcium plus vitamin D group. We also previously reported that vitamin D combined with calcium may have lesser treatment effects on colorectal epithelial apoptosis and differentiation than does calcium or vitamin D separately (17, 46). There are several possible explanations for this finding. Considering the study’s small sample size, the lack of treatment effect in the calcium and vitamin D group may have been due to chance. It is also possible that the two agents may have attenuated the effects of one another. 1,25-(OH)2-vitamin D3 regulates calcium homeostasis (47). As calcium concentration decreases, the production of 1,25-(OH)2-vitamin D3 increases, which in turn increases intestinal calcium absorption (47). Elevated calcium in the diet may suppress 1,25-(OH)2-vitamin D3 synthesis at the cellular level, which in turn may also attenuate activation of vitamin D-responsive detoxifying enzymes. One animal study (48) found that calcium and vitamin D were more potent inhibitors of colon tumorigenesis when given separately, but several other animal studies reported synergistic effects with calcium and vitamin D combined (49, 50). A large adenoma recurrence trial also supported an enhanced chemopreventive effect of vitamin D with calcium (42). Taken altogether, the combined effect of calcium and vitamin D on oxidative DNA damage in colorectal epithelium is unclear and will require clarification via larger studies.
In contrast to as in men, there was no evidence for a treatment effect of vitamin D alone and in combination with calcium on colorectal crypt 8-OH-dG labeling levels in women. There are several possible explanations for this finding, including a very low statistical power to detect treatment effects due to the small sample size. Another possible explanation may be that women in our study may have had decreased estrogen levels as the majority of them were postmenopausal and not taking estrogens. The Women’s Health Initiative Hormone Replacement Therapy Trial (51) found that endogenous estrogen plus progestin therapy, but not estrogen alone therapy, reduced risk for colorectal cancer (52). However, one human study found that an estrogen intervention activated the VDR pathway, and downregulated inflammatory and immune signaling pathways in the rectal mucosa of postmenopausal women (53). So, the findings of our study are consistent with the hypothesis that low estrogen levels may interfere with VDR signaling in the colorectal mucosa, resulting in no changes in 8-OH-dG levels after supplementation with vitamin D; however, further studies are needed to clarify these issues.
Epidemiologic studies have not consistently found associations of colorectal neoplasms with individual pro- and anti-oxidant factors despite the strong biological rationale and basic science evidence. Several analyses (25, 26) suggested that oxidative stress, inflammation, diet, and other risk factors may synergistically or individually affect risk of colorectal neoplasia through multiple pathways. Therefore, we used the OBS to categorize patients into different “oxidative stress” profiles based on multiple determinants of oxidative stress. We hypothesized that patients with different “oxidative stress” profiles may respond differently to calcium and/or vitamin D. Those with a high baseline OBS (higher balance of anti- to pro-oxidant exposures) had greater estimated calcium and calcium plus vitamin D treatment effects on 8-OH-dG labeling than those with a low OBS. A low OBS reflects low total intakes of antioxidants such as vitamin C and carotene, combined with high pro-oxidant exposures such as high fat or iron intakes. In the colon lumen, free calcium directly binds bile acids (37), thereby reducing pro-carcinogenic effects of bile acids on the colorectal epithelium. Persons with high fat intake have higher colonic lumen levels of deoxycholic and lithocholic bile acids (36), and may require more calcium to neutralize the DNA damaging bile acids than do persons on a low-fat diet. Antioxidant enzymes in humans function in combination with low weight antioxidant compounds such as vitamin C, α-tocopherol, and β-carotene (54). In the colorectal epithelium, vitamin D activates the expression of antioxidant enzymes (14), which may not function properly in the antioxidant-depleted environment. Therefore, it is possible that calcium and vitamin D effects on the oxidative DNA damage marker, 8-OH-dG, are modified by the presence or absence of various pro- or antioxidant exposures. On the other hand, our findings may have been due to chance and further investigations are required to confirm them.
Since complete loss of the VDR significantly increased 8-OH-dG labeling in the mouse colon (15, 27), we hypothesized that different VDR expression levels in the normal-appearing colorectal mucosa modify vitamin D treatment effects. Consistent with this hypothesis, we observed substantial decreases in 8-OH-dG labeling in study participants with high, but not low, baseline VDR expression.
This study has several limitations. First, treatment effects of vitamin D and calcium on the oxidative DNA damage marker 8-OH-dG in parts of the colon other than the rectum are unclear, as we did not collect tissue biopsies from different parts of the colon and there are no published studies of 8-OH-dG labeling throughout the colon. Another potential limitation of this study is that it is not known whether oxidative stress markers are associated with risk for colon cancer in humans. However, substantial published literature supports the plausibility of an important role for increased oxidative DNA damage in colon carcinogenesis, especially for the transition from colorectal adenoma to carcinoma (32, 33). Persistent oxidative stress leads to protein and DNA damage and lipid peroxidation which can cause genetic and epigenetic alterations, and may facilitate the development of neoplasia from the normal colorectal mucosa (29). Therefore, 8-OH-dG in the normal colorectal mucosa may serve as a biomarker of risk for colorectal neoplasms. Finally, the most obvious limitation of the study is the small sample size, which may have increased the probability of chance findings in detecting or not detecting a treatment effect.
The strengths of this study include the randomized, double-blind, placebo-controlled trial design; high protocol adherence by study participants; examination of both the independent and combined effects of calcium and vitamin D3 on an oxidative stress marker; automated standardized biopsy handling and immunostaining procedures; and the use of cutting edge technologies to conduct the quantitative image analyses. Another strength of this study is that we used immunohistochemical detection of 8-OH-dG in the colorectal epithelium as it was important to detect 8-OH-dG in colonocytes, but not in infiltrating lymphocytes or other intermingled cells. Such detection was made possible by the development of a specific monoclonal antibody against 8-OH-dG (19), and our novel image analysis methods. HPLC (high-performance liquid chromatography), an alternative method of measuring 8-OH-dG in colon tissue, may overestimate oxidative DNA damage in the colonocytes, especially in the presence of inflammation. Finally, this study is the first human study to test the effect of calcium and/or vitamin D3 on an oxidative DNA damage marker in the normal-appearing colorectal mucosa.
Overall, these preliminary results from this pilot clinical trial suggest that calcium and vitamin D, given separately, may decrease oxidative DNA damage in the normal-appearing colorectal epithelium; the treatment effects of calcium and vitamin D on oxidative DNA damage marker 8-OH-dG may be strongest in those with higher vitamin D receptor expression in the colon; 8-OH-dG may be a modifiable biomarker of oxidative stress that can be used in colon cancer-related chemoprevention trials to assess treatment efficacy; and support further investigations of calcium and vitamin D as chemopreventive agents against colorectal neoplasms.
Acknowledgements
We thank Jill Joelle Woodard and Bonita Feinstein for managing the study, Dr. Bruce W. Hollis for conducting blood vitamin D assays, Vaunita Cohen and Eileen Veronica Smith for excellent technical assistance, Christopher Farino and Stuart Myerberg for development of the study database, John Melonakos and Tauseef Rehman from DivEyes LLC for development of the scoring software, the physicians of the Emory Clinic, GA for work on biopsy procurement, and all study participants for their time and dedication to the study.
Grant support: National Cancer Institute, National Institutes of Health (R01 CA104637, R03 CA136113 to R.M.B.); Georgia Cancer Coalition Distinguished Scholar award (to R.M.B.); the Franklin Foundation; Emory Graduate School (supplemental research funds to V.F.). The National Cancer Institute, the Georgia Cancer Coalition, the Franklin Foundation, and Emory Graduate School had no influence on the design of the study; the collection, analysis, and interpretation of the data; the decision to submit the manuscript for publication; or the writing of the manuscript.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009 doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Potter JD, Slattery ML, Bostick RM, Gapstur SM. Colon cancer: a review of the epidemiology. Epidemiol Rev. 1993;15:499–545. doi: 10.1093/oxfordjournals.epirev.a036132. [DOI] [PubMed] [Google Scholar]
- 3.Lipkin M, Lamprecht SA. Mechanisms of action of vitamin D: recent findings and new questions. Journal of medicinal food. 2006;9:135–137. doi: 10.1089/jmf.2006.9.135. [DOI] [PubMed] [Google Scholar]
- 4.Oh K, Willett WC, Wu K, Fuchs CS, Giovannucci EL. Calcium and vitamin D intakes in relation to risk of distal colorectal adenoma in women. American journal of epidemiology. 2007;165:1178–1186. doi: 10.1093/aje/kwm026. [DOI] [PubMed] [Google Scholar]
- 5.Kesse E, Boutron-Ruault MC, Norat T, Riboli E, Clavel-Chapelon F. Dietary calcium, phosphorus, vitamin D, dairy products and the risk of colorectal adenoma and cancer among French women of the E3N-EPIC prospective study. Int J Cancer. 2005;117:137–144. doi: 10.1002/ijc.21148. [DOI] [PubMed] [Google Scholar]
- 6.Peters U, Chatterjee N, McGlynn KA, et al. Calcium intake and colorectal adenoma in a US colorectal cancer early detection program. The American journal of clinical nutrition. 2004;80:1358–1365. doi: 10.1093/ajcn/80.5.1358. [DOI] [PubMed] [Google Scholar]
- 7.Cho E, Smith-Warner SA, Spiegelman D, et al. Dairy foods, calcium, and colorectal cancer: a pooled analysis of 10 cohort studies. J Natl Cancer Inst. 2004;96:1015–1022. doi: 10.1093/jnci/djh185. [DOI] [PubMed] [Google Scholar]
- 8.Bostick RM, Goodman M, Sidelnikov E. Calcium and vitamin D. In: Potter JD, Lindor NM, editors. Genetics of Colorectal Cancer. New York, NY: Springer Science + Business Media, LLC; 2009. pp. 277–296. [Google Scholar]
- 9.Weingarten MA, Zalmanovici A, Yaphe J. Dietary calcium supplementation for preventing colorectal cancer and adenomatous polyps. Cochrane Database Syst Rev. 2008;1:CD003548. doi: 10.1002/14651858.CD003548.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei MY, Garland CF, Gorham ED, Mohr SB, Giovannucci E. Vitamin D and prevention of colorectal adenoma: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2008;17:2958–2969. doi: 10.1158/1055-9965.EPI-08-0402. [DOI] [PubMed] [Google Scholar]
- 11.Newmark HL, Lipkin M. Calcium, vitamin D, and colon cancer. Cancer Res. 1992;52:2067s–2070s. [PubMed] [Google Scholar]
- 12.Lamprecht SA, Lipkin M. Chemoprevention of colon cancer by calcium, vitamin D and folate: molecular mechanisms. Nat Rev Cancer. 2003;3:601–614. doi: 10.1038/nrc1144. [DOI] [PubMed] [Google Scholar]
- 13.Ebert R, Schutze N, Adamski J, Jakob F. Vitamin D signaling is modulated on multiple levels in health and disease. Mol Cell Endocrinol. 2006;248:149–159. doi: 10.1016/j.mce.2005.11.039. [DOI] [PubMed] [Google Scholar]
- 14.Chatterjee M. Vitamin D and genomic stability. Mutation research. 2001;475:69–87. doi: 10.1016/s0027-5107(01)00080-x. [DOI] [PubMed] [Google Scholar]
- 15.Kallay E, Bareis P, Bajna E, et al. Vitamin D receptor activity and prevention of colonic hyperproliferation and oxidative stress. Food Chem Toxicol. 2002;40:1191–1196. doi: 10.1016/s0278-6915(02)00030-3. [DOI] [PubMed] [Google Scholar]
- 16.Kutuzova GD, DeLuca HF. 1,25-Dihydroxyvitamin D3 regulates genes responsible for detoxification in intestine. Toxicology and applied pharmacology. 2007;218:37–44. doi: 10.1016/j.taap.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Fedirko V, Bostick RM, Flanders WD, et al. Effects of vitamin D and calcium supplementation on markers of apoptosis in normal colon mucosa: a randomized, double-blind, placebo-controlled clinical trial. Cancer Prev Res. 2009;2:213–223. doi: 10.1158/1940-6207.CAPR-08-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willett WC, Sampson L, Browne ML, et al. The use of a self-administered questionnaire to assess diet four years in the past. American journal of epidemiology. 1988;127:188–199. doi: 10.1093/oxfordjournals.aje.a114780. [DOI] [PubMed] [Google Scholar]
- 19.Toyokuni S, Tanaka T, Hattori Y, et al. Quantitative immunohistochemical determination of 8-hydroxy-2'-deoxyguanosine by a monoclonal antibody N45.1: its application to ferric nitrilotriacetate-induced renal carcinogenesis model. Laboratory investigation; a journal of technical methods and pathology. 1997;76:365–374. [PubMed] [Google Scholar]
- 20.Hidalgo AA, Paredes R, Garcia VM, et al. Altered VDR-mediated transcriptional activity in prostate cancer stroma. J Steroid Biochem Mol Biol. 2007;103:731–736. doi: 10.1016/j.jsbmb.2006.12.072. [DOI] [PubMed] [Google Scholar]
- 21.Tishkoff DX, Nibbelink KA, Holmberg KH, Dandu L, Simpson RU. Functional vitamin D receptor (VDR) in the t-tubules of cardiac myocytes: VDR knockout cardiomyocyte contractility. Endocrinology. 2008;149:558–564. doi: 10.1210/en.2007-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bostick RM, Fosdick L, Lillemoe TJ, et al. Methodological findings and considerations in measuring colorectal epithelial cell proliferation in humans. Cancer Epidemiol Biomarkers Prev. 1997;6:931–942. [PubMed] [Google Scholar]
- 23.Hollis BW. Quantitation of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D by radioimmunoassay using radioiodinated tracers. Methods Enzymol. 1997;282:174–186. doi: 10.1016/s0076-6879(97)82106-4. [DOI] [PubMed] [Google Scholar]
- 24.Bostick RM, Fosdick L, Wood JR, et al. Calcium and colorectal epithelial cell proliferation in sporadic adenoma patients: a randomized, double-blinded, placebo-controlled clinical trial. J Natl Cancer Inst. 1995;87:1307–1315. doi: 10.1093/jnci/87.17.1307. [DOI] [PubMed] [Google Scholar]
- 25.Goodman M, Bostick RM, Dash C, Flanders WD, Mandel JS. Hypothesis: oxidative stress score as a combined measure of pro-oxidant and antioxidant exposures. Annals of epidemiology. 2007;17:394–399. doi: 10.1016/j.annepidem.2007.01.034. [DOI] [PubMed] [Google Scholar]
- 26.Goodman M, Bostick RM, Dash C, Terry P, Flanders WD, Mandel J. A summary measure of pro- and anti-oxidant exposures and risk of incident, sporadic, colorectal adenomas. Cancer Causes Control. 2008;19:1051–1064. doi: 10.1007/s10552-008-9169-y. [DOI] [PubMed] [Google Scholar]
- 27.Kallay E, Pietschmann P, Toyokuni S, et al. Characterization of a vitamin D receptor knockout mouse as a model of colorectal hyperproliferation and DNA damage. Carcinogenesis. 2001;22:1429–1435. doi: 10.1093/carcin/22.9.1429. [DOI] [PubMed] [Google Scholar]
- 28.Sies H, Jones DP. Oxidative Stress. In: Fink G, editor. Encyclopedia of Stress. Elsevier; 2007. pp. 45–48. [Google Scholar]
- 29.Roessner A, Kuester D, Malfertheiner P, Schneider-Stock R. Oxidative stress in ulcerative colitis-associated carcinogenesis. Pathol Res Pract. 2008;204:511–524. doi: 10.1016/j.prp.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 30.Evans M, Cooke M. Oxidative Damage to Nucleic Acids. Springer; 2007. [Google Scholar]
- 31.Kasai H. Analysis of a form of oxidative DNA damage, 8-hydroxy-2'-deoxyguanosine, as a marker of cellular oxidative stress during carcinogenesis. Mutation research. 1997;387:147–163. doi: 10.1016/s1383-5742(97)00035-5. [DOI] [PubMed] [Google Scholar]
- 32.Kondo S, Toyokuni S, Iwasa Y, et al. Persistent oxidative stress in human colorectal carcinoma, but not in adenoma. Free Radic Biol Med. 1999;27:401–410. doi: 10.1016/s0891-5849(99)00087-8. [DOI] [PubMed] [Google Scholar]
- 33.Ribeiro ML, Priolli DG, Miranda DD, Arcari DP, Pedrazzoli J, Jr, Martinez CA. Analysis of oxidative DNA damage in patients with colorectal cancer. Clin Colorectal Cancer. 2008;7:267–272. doi: 10.3816/CCC.2008.n.034. [DOI] [PubMed] [Google Scholar]
- 34.Venturi M, Hambly RJ, Glinghammar B, Rafter JJ, Rowland IR. Genotoxic activity in human faecal water and the role of bile acids: a study using the alkaline comet assay. Carcinogenesis. 1997;18:2353–2359. doi: 10.1093/carcin/18.12.2353. [DOI] [PubMed] [Google Scholar]
- 35.Babbs CF. Free radicals and the etiology of colon cancer. Free Radic Biol Med. 1990;8:191–200. doi: 10.1016/0891-5849(90)90091-v. [DOI] [PubMed] [Google Scholar]
- 36.Bernstein H, Bernstein C, Payne CM, Dvorakova K, Garewal H. Bile acids as carcinogens in human gastrointestinal cancers. Mutation research. 2005;589:47–65. doi: 10.1016/j.mrrev.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 37.Newmark HL, Wargovich MJ, Bruce WR. Colon cancer and dietary fat, phosphate, and calcium: a hypothesis. J Natl Cancer Inst. 1984;72:1323–1325. [PubMed] [Google Scholar]
- 38.Harris DM, Go VL. Vitamin D and colon carcinogenesis. J. Nutr. 2004;134:3463S–3471S. doi: 10.1093/jn/134.12.3463S. [DOI] [PubMed] [Google Scholar]
- 39.Lamprecht SA, Lipkin M. Cellular mechanisms of calcium and vitamin D in the inhibition of colorectal carcinogenesis. Ann. N. Y. Acad. Sci. 2001;952:73–87. doi: 10.1111/j.1749-6632.2001.tb02729.x. [DOI] [PubMed] [Google Scholar]
- 40.Sardar S, Chakraborty A, Chatterjee M. Comparative effectiveness of vitamin D3 and dietary vitamin E on peroxidation of lipids and enzymes of the hepatic antioxidant system in Sprague--Dawley rats. Int. J. Vitam. Nutr. Res. 1996;66:39–45. [PubMed] [Google Scholar]
- 41.Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 2007;85:1586–1591. doi: 10.1093/ajcn/85.6.1586. [DOI] [PubMed] [Google Scholar]
- 42.Grau MV, Baron JA, Sandler RS, et al. Vitamin D, calcium supplementation, and colorectal adenomas: results of a randomized trial. J Natl Cancer Inst. 2003;95:1765–1771. doi: 10.1093/jnci/djg110. [DOI] [PubMed] [Google Scholar]
- 43.Peters U, McGlynn KA, Chatterjee N, et al. Vitamin D, calcium, and vitamin D receptor polymorphism in colorectal adenomas. Cancer Epidemiol Biomarkers Prev. 2001;10:1267–1274. [PubMed] [Google Scholar]
- 44.Wu K, Willett WC, Fuchs CS, Colditz GA, Giovannucci EL. Calcium intake and risk of colon cancer in women and men. J Natl Cancer Inst. 2002;94:437–446. doi: 10.1093/jnci/94.6.437. [DOI] [PubMed] [Google Scholar]
- 45.Zheng W, Anderson KE, Kushi LH, et al. A prospective cohort study of intake of calcium, vitamin D, and other micronutrients in relation to incidence of rectal cancer among postmenopausal women. Cancer Epidemiol Biomarkers Prev. 1998;7:221–225. [PubMed] [Google Scholar]
- 46.Fedirko V, Bostick RM, Flanders WD, et al. Effects of Vitamin D and Calcium on Proliferation and Differentiation in Normal Colon Mucosa: A Randomized Clinical Trial. Cancer Epidemiol Biomarkers Prev. 2009 doi: 10.1158/1055-9965.EPI-09-0239. (accepted for publication). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quarles LD. Endocrine functions of bone in mineral metabolism regulation. J Clin Invest. 2008;118:3820–3828. doi: 10.1172/JCI36479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pence BC, Buddingh F. Inhibition of dietary fat-promoted colon carcinogenesis in rats by supplemental calcium or vitamin D3. Carcinogenesis. 1988;9:187–190. doi: 10.1093/carcin/9.1.187. [DOI] [PubMed] [Google Scholar]
- 49.Sitrin MD, Halline AG, Abrahams C, Brasitus TA. Dietary calcium and vitamin D modulate 1,2-dimethylhydrazine-induced colonic carcinogenesis in the rat. Cancer Res. 1991;51:5608–5613. [PubMed] [Google Scholar]
- 50.Beaty MM, Lee EY, Glauert HP. Influence of dietary calcium and vitamin D on colon epithelial cell proliferation and 1,2-dimethylhydrazine-induced colon carcinogenesis in rats fed high fat diets. J Nutr. 1993;123:144–152. doi: 10.1093/jn/123.1.144. [DOI] [PubMed] [Google Scholar]
- 51.Chlebowski RT, Wactawski-Wende J, Ritenbaugh C, et al. Estrogen plus progestin and colorectal cancer in postmenopausal women. N Engl J Med. 2004;350:991–1004. doi: 10.1056/NEJMoa032071. [DOI] [PubMed] [Google Scholar]
- 52.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 53.Protiva P, Cross HS, Hopkins ME, et al. Chemoprevention of colorectal neoplasia by estrogen: potential role of vitamin D activity. Cancer Prev Res (Phila Pa) 2009;2:43–51. doi: 10.1158/1940-6207.CAPR-08-0103. [DOI] [PubMed] [Google Scholar]
- 54.Harris ED. Regulation of antioxidant enzymes. FASEB J. 1992;6:2675–2683. doi: 10.1096/fasebj.6.9.1612291. [DOI] [PubMed] [Google Scholar]