Abstract
Heparan sulfate proteoglycans (HSPGs) are potent regulators of vascular remodeling and repair. Heparanase is the major enzyme capable of degrading heparan sulfate in mammalian cells. Here we examined the role of heparanase in controlling arterial structure, mechanics and remodeling. In vitro studies supported that heparanase expression in endothelial cells serves as a negative regulator of endothelial inhibition of vascular smooth muscle cell (vSMC) proliferation. Arterial structure and remodeling to injury were also modified by heparanase expression. Transgenic mice overexpressing heparanase had increased arterial thickness, cellular density and mechanical compliance. Endovascular stenting studies in Zucker rats demonstrated increased heparanase expression in the neointima of obese, hyperlipidemic rats in comparison to lean rats. The extent of heparanase expression within the neointima strongly correlated with the neointimal thickness following injury. To test the effects of heparanase overexpression on arterial repair, we developed a novel murine model of stent injury using small diameter self-expanding stents. Using this model we found that increased neointimal formation and macrophage recruitment occurs in transgenic mice overexpressing heparanase. Taken together, these results support a role for heparanase in the regulation of arterial structure, mechanics and repair.
Keywords: heparanase, vascular remodeling, restenosis, stenting, arterial compliance
Introduction
Vascular smooth muscle cell (vSMC) proliferation and hypertrophy are common pathophysiological mechanisms underlying clinical cardiovascular disorders including hypertension, atherosclerosis and restenosis1–3. Arterial structure is maintained by a dynamic interplay between growth inhibitory factors produced primarily by endothelial cells and growth stimulatory factors produced principally by vSMCs, inflammatory cells, and dysfunctional endothelial cells4. Disease states can compromise endothelial function and disturb this balance leading to local inflammation, thrombosis and vasoconstriction5. Heparan sulfate proteoglycans (HSPGs) derived from endothelial cells are potent regulators of vSMC growth and vascular remodeling6, 7. Both heparin and endothelial cell-derived HSPGs are potent inhibitors of vSMC proliferation and mitogenesis8–10. This regulation is dependent on the overall health and growth state of the endothelial cells. Subconfluent cultures of endothelial cells stimulate vSMC growth whereas postconfluent cultures inhibit vSMC growth6. Further, these endothelial-derived HSPGs are essential to inhibiting and resolving the neointimal response to vascular injury7.
While there is evidence that HSPGs participate in the control of vascular remodeling, it remains unclear how these molecules are regulated in context of disease and injury. Heparanase is the major mammalian enzyme capable of digesting heparan sulfate chains. This enzyme is an endo-β-D-glucuronidase that cleaves at a specific motif in the heparan sulfate chains to create fragments 10–20 sugar units long and biologically active11, 12. Heparanase has been intensely studied for its role in angiogenesis and cancer metastasis13, 14, yet the role of this enzyme in regulating arterial structure and remodeling remains poorly defined. We hypothesized that heparanase plays a key role in regulating arterial structure, mechanics and vascular remodeling. In the present work, we show that heparanase expression in endothelial cells serves as a negative feedback regulator of paracrine inhibition of vSMC proliferation. Mice overexpressing the human heparanase transgene had increased arterial thickening, cellularity and arterial compliance. We found that stent-induced vascular injury in obese, hyperlipidemic rats had increased neointimal heparanase expression and that the magnitude of this increase directly related to the extent of neointimal expansion. Finally, we developed a novel minimally-invasive murine arterial stenting model that demonstrated increased neointimal formation in response to endovascular stenting in transgenic heparanase mice.
Materials and Methods
Cell Culture
Human aortic vSMCs and human umbilical cord vascular endothelial cell (HUVEC) lines were purchased from Cambrex (Walkersville, MD). Cells were maintained in MCDB-131 media (Invitrogen, Carlsbad, CA) supplemented with EGM-2 growth supplements (Cambrex). Cells were cultured at 37°C under 5% CO2 and were used between passages three and five.
Transfection and siRNA Vectors
Four short hairpin RNA expression vectors were screened for gene silencing activity towards the heparanase gene. The following sequences were used in the pRS expression vector (Origene, Rockville, MD): (1) TTATGTGGCTGGATAAATTGGGCCTGTCA, (2) GTGGTGATGAGGCAAGTATTCTTTGGAGC, (3) TCGTTCCTGTCCGTCACCATTGACGCCAA, (4) GTTCAAGAACAGCACCTACTCAAGAAGCT. Overexpression of heparanase and syndecan was performed using vectors with constitutive gene expression under control of the CMV promoter (Origene).
Cell Lysis and Western Blotting
Cells were lysed in 1 ml of lysis buffer containing 20 mM Tris, 150 mM NaCl, 1% Triton X-100, 1% deoxycholate, 0.1% SDS, 1 mM sodium orthovanadate, 50mM NaF, 2 mM PMSF, and complete protease inhibitor cocktail (Roche, Nutley, NJ). After 10 minutes of incubation, the plates were scraped and the lysates pipetted into a centrifuge tube. The samples were then centrifuged at 14,000 g for 15 min prior to western blotting. Samples were run on 4–15% polyacrylamide gradient gels and transferred to PVDF membranes. The membranes were blocked for 1 hr in 5% non-fat milk in PBS with 0.01% tween-20 (PBST) and exposed to a heparanase primary antibody (1:200, Cell Sciences, Canton, MA) at 4°C overnight in 1% non-fat milk. The membranes were washed with PBST, incubated at room temperature for 2 hrs with a 1:3500 dilution of a horse radish peroxidase linked secondary antibody and detected using chemiluminescence (Perkin Elmer, Boston, MA).
Immunocytochemical Staining
Cells were washed three times in PBS and fixed for 5 min with methanol at −20°C. The cultures were then blocked with 20% goat serum in PBS for 45 min at room temperature. A primary antibody heparanase (Cell Science, Canton, MA) was added at 1:100 dilution in PBS containing 1% BSA. A secondary antibody conjugated with Alexa Fluor 594 or Oregon Green dyes (Invitrogen) was added at 1:200 dilution in PBS-BSA solution. The plates were then coverslipped, mounted using a DAPI containing mounting medium (Vector Laboratories, Burlingame, CA) and visualized with fluorescence microscopy.
Metabolic Labeling, Isolation and Analysis of Proteoglycans
To metabolically label the glycosaminoglycans, 80 μCi of 3H-glucosamine was added to each plate. The conditioned media was collected and combined with guanidine-HCl to a final concentration of 4 M. The cell layers were then washed three times with cold PBS and lysed. This solution was then brought to a final concentration of 4 M guanidine-HCl. To obtain extracellular matrix bound proteoglycans, the plates were washed three times in PBS and extracted for 48 hrs with a solution of 50 mM sodium acetate (pH = 6.0), 4 M guanidine-HCl, 2% triton x-100 and protease inhibitors.
The isolated proteoglycans from conditioned media, extracellular matrix and cell surface were desalted into Buffer A (20 mM Tris, 8 M Urea, pH = 8.0) using a HiTrap desalting column (Amersham Biosciences, Piscataway, NJ). The proteoglycans were separated from other proteins by fractionation on a 1 ml HiTrap Q ion exchange column (Amersham) with a linear salt gradient from 0 to 2 M NaCl. One ml fractions were collected and aliquots of these samples were counted using liquid scintillation. Aliquots were subjected to digestion with 0.6 U/ml of protease free chondroitinase ABC (Seikagaku, Japan) for 4 hours. Control samples were subjected to digestion conditions without the addition of enzyme.
Immunohistochemical Staining
The abdominal aorta from rats was formalin fixed and sectioned using standard methods. The sections were heated for ten minutes in a 60°C oven, deparaffinized in xylene, and rehydrated. Antigen retrieval was performed by placing the slides in 10mM citrate buffer (pH = 6.0) and heating in the microwave for 10 min. The samples were allowed to cool for 20 min and were then incubated in 3% hydrogen peroxide for 10 min. The samples were rinsed 3 times with PBS with 0.01% tween-20 (PBST) between each of the following steps. The sections were blocked with 20% normal goat serum for 45 minutes at room temperature. Primary antibodies were diluted in PBS containing 1% BSA, applied to slides, and incubated in a humid chamber overnight at 4°C. Secondary antibody staining at detection was performed using the LSAB 2 kit (DakoCytomation, Carpinteria, CA) according to the manufacturer’s instructions. An AEC substrate (DakoCytomation) was used for detection of the HRP conjugate. The samples were counterstained in Mayer’s hematoxylin for 3 min, washed with tap water, and mounted in aqueous mounting medium (DakoCytomation).
Histochemical Analysis
Aortae were harvested from wild type and heparanase transgenic mice. The aortae were fixed in neutral buffered formalin at 4°C overnight, paraffin embedded and sectioned using standard methods. The aortae were stained with hemotoxylin and eosin or with an elastin Movat stain as previously described15. Arterial thickness, circumference and nuclei were counted on three aortic sections from four mice for each group of animals using Adobe Photoshop (Adobe, San Jose, CA).
Smooth Muscle Cell Proliferation Assay
Human smooth muscle cells were passaged into 48-well plates at low density. Smooth muscle cells were serum starved in 0.5% calf serum for 24 hours. The cells were then washed twice in culture media with no growth supplements and endothelial cell conditioned media was applied. After 24 hours 1 μCi of 3H-thymidine was added. Twenty-four hours later, the cells were washed three times with PBS at 4°C and then incubated with 10% TCA for 30 min. The cultures were then washed twice in 95% ethanol and solubilized in 1 ml of 0.25 M NaOH with 0.1% SDS for 1 hour. The samples were added to scintillation cocktail and radioactivity was measured using a liquid scintillation counter.
Animal Models of Endovascular Stent Injury
All experimental procedures and protocols used in this investigation were reviewed and approved by the Animal Care and Use Committee of the Massachusetts Institute of Technology and conformed to the “Guiding Priniciples in the Care and Use of Animals” of the American Physiological Society and the NIH Guide for the Care and Use of Laboratory Animals. Zucker obese (Crl: (ZUC)-faBR) and Zucker lean rats were used in an animal model of vascular injury and stenting in the presence of metabolic syndrome and insulin resistance16. At the time of stenting the rats were 12 and 14 weeks old for the obese and lean rats, respectively. The rats were anesthetized using isofluorane, given a 100 U/kg dose of heparin, and a small incision was made to expose the right femoral artery. This artery was ligated and an arteriotomy was performed proximal to the ligature. A 0.014” angioplasty guidewire was passed into the aorta and an 9-mm long endovascular stent (Nirflex; Medinol Inc., Tel Aviv, Israel) mounted on a 15 × 2.5-mm angioplasty balloon (Crossail; Guidant Inc., Santa Clara, CA) was passed into the abdominal aorta. The stent was deployed with a 15 second inflation at 8 atm inflation pressure. Post-stenting the animals were given aspirin via drinking water at an approximate dose of 5 mg/kg/day16. After 14 days the stents were harvested for analysis.
Wild-type and heparanase transgenic mice were derived directly from those previously described17. The animals were given aspirin via drinking water for 24 hrs prior to stent implantation and thereafter. Immediately prior to stenting the mice were given a subcutaneous dose of heparin. Vascular access was obtained through the right femoral artery in a manner similar to that used for the rat studies. A coronary stent system with a 1.5 mm constrained diameter and 12 mm length was used (CardioMind, Sunnyvale, CA). The catheter was passed into the abdominal aorta through the femoral artery. The stent was deployed by removing an external sheath, allowing the self expanding stent to deploy. The average diameter of the aortic lumen in the mice was 1.3 mm and the deployed stent diameter is 1.5 mm, leading to 15.4% dilation in on stent deployment. After seven days the stents were explanted and analysed by histochemical and immunochemical staining as previously described16, 18. To ensure that the differences in response to injury were not a result of altered blood pressure, the arterial blood pressure in the mice was measured using a tail cuff blood pressure monitor (Holliston, MA). The average blood pressure for each group was 113.6±4.2 and 106.8±3.6 mmHg for wild type and heparanase transgenic mice, respectively (n = 4, p = ns).
Mechanical Testing of Aortae
Prior to mechanical testing aortae from heparanase transgenic and wild-type mice were harvested and adventitial tissue removed. The longitudinal compliance and ultimate tensile strength of whole arteries was measured using an ElectroForce Biodynamic Test Instrument (Bose Corporation, Framingham, MA). The samples were measured in uniaxial tensile configuration using a 22 N load cell (Interface Inc., Scottsdale, AZ) with a displacement rate of 0.05 mm/s and maximal displacement of 4.0 mm. WinTest 3.0 software (Bose) was used to record data during the testing. Circumferential testing was performed on aortic ring samples cannulated with two sutures and tested using the Biodynamic Test Instrument. To examine mechanical properties of the elastin networks we digested aortae in 0.1N NaOH at 75°C for 1 hour. The solution was neutralized and removed with multiple washes with PBS. This procedure has been shown to remove cellular components and extracellular matrix but leave the elastin network intact19.
Statistics
All results are shown as mean ± standard error of the mean. An ANOVA followed by Student-Newman–Keuls post hoc test. A two-tailed p value <0.05 was considered statistical significant was used to make comparisons between groups of continuous variables. The Pearson product moment correlation statistic was used as a measure of correlation between variables.
Results
Heparanase provides negative feedback control to paracrine growth inhibition of vSMCs by endothelial cells
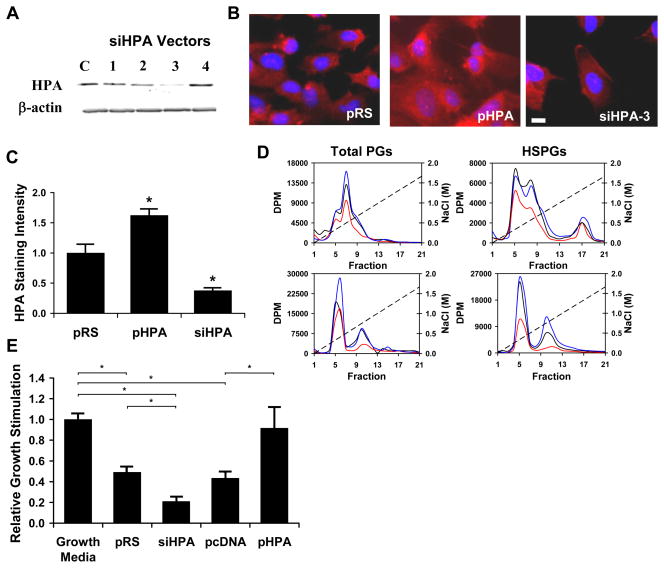
Endothelial cells were transfected with an expression vector for heparanase (pHPA), one of four siRNA vectors targeted to the heparanase (siHPA-1 to -4), a siRNA control vector (pRS) or an empty overexpression vector (pcDNA). After 2 days in culture, the cells were lysed and assayed for heparanase by immunoblotting to confirm alterations in heparanase expression (Figure 1a). The most effective siRNA sequence (siHPA-3) was selected and used in further studies. This siRNA reduced heparanase expression to 13.2±7.2% from 100.0±18.7% for control cells (n=3, p<0.05). Transfected cells under similar conditions were fixed, immunostained for heparanase and imaged using fluorescent microscopy (Figure 1b and Figure 1c). The effect of heparanase on the glycosaminoglycans of endothelial cells was measured through metabolic labeling and analysis by ion exchange chromatography. In these graphs the early peaks represent predominantly protein while later peaks are from highly charged glycosaminoglycans (i.e. fractions 9 through 20). These studies demonstrated that enhanced endothelial expression of heparanase led to reduction in both cell surface and soluble heparan sulfate glycosaminoglycans (Figure 1d). For the surface HSPGs the relative area of the glycosaminoglycan peak was 100.0±6.4%, 36.2±3.3% and 154.9±4.9% for pRS, pHPA and siHPA transfected cells, respectively (p<0.05 for comparisons between all groups). Conversely, reduction of heparanase expression increased heparan sulfate in cell surface and conditioned media fractions. Notably, there was also a large reduction in cell surface protein with heparanase overexpression, likely due to heparanase-induced shedding of syndecans20.
Figure 1.
Heparanase knock down and overexpression modulates endothelial inhibition of vascular smooth muscle cell (vSMC) proliferation. (a) Expression of heparanase identified by Western blotting in cells transfected with a vector expressing siRNA specific for mammalian heparanase (siHPA). Four siHPA were evaluated and the one that most effectively reduced heparanase was used in all subsequent experiments. A scrambled sequence (pRS) served as control. (b) Immunocytochemically identified heparanase (Bar = 10 μm) in cells transfected with pHPA, siHPA or pRS. (c) Quantification of cellular staining for heparanase in transfected cells. Values are based on measurement of intensity of the average of ten cells from five independent experiments (n=5). *Statistically significant difference from control group (p < 0.05). (d) Measurement of heparanase’s effect on total glycosaminoglycans and heparan sulfate in endothelial cells. Endothelial cell HSPGs were metabolically labeled using 3H-glucosamine. Cell lysates and conditioned media were then analyzed for glycosaminoglycan content using ion exchange chromatography. Heparanase overexpression caused a reduction in cellular and soluble HSPGs in endothelial cells. Conversely, reduction of heparanase expression in endothelial cells by siRNA led to an increase in both cellular and soluble HSPGs. Plots are the average of three independent analyses. Black line = pRS; red line = pHPA; blue line = siHPA. (e) Heparanase regulates endothelial inhibition of vSMC proliferation. Conditioned media from endothelial cells that were transfected with pcDNA, pHPA, siHPA or pRS vectors was harvested and applied to cultures of vSMCs. Bars represent the relative growth of vSMC in the presence of conditioned media of endothelial cells transfected with the indicated vectors as measured by 3H-thymidine incorporation. *Statistically significant difference between groups (p < 0.05).
Endothelial cells produce soluble factors that inhibit vSMC proliferation. This process is essential for arterial health and requires endothelial cell derived HSPGs6, 7. We took conditioned media from endothelial cells and applied it to vSMCs in culture to examine the role of heparanase in controlling endothelial inhibition of vSMC proliferation. Endothelial cells transfected with a control vector inhibited vSMC proliferation to about half of the baseline vSMC proliferation in growth media (Figure 1e). This effect was absent in cells overexpressing heparanase and markedly enhanced in cells with reduced heparanase levels (Figure 1e). Previous studies have implicated the release of soluble factors, in particular FGF-2, as an effector mechanism of heparanase tissue action21, 22. However, in our experiments FGF-2 levels remained relatively constant irrespective of heparanase expression (data not shown).
Heparanase overexpression leads to arterial thickening, increased arterial compliance and incidence of spontaneous aneurysm
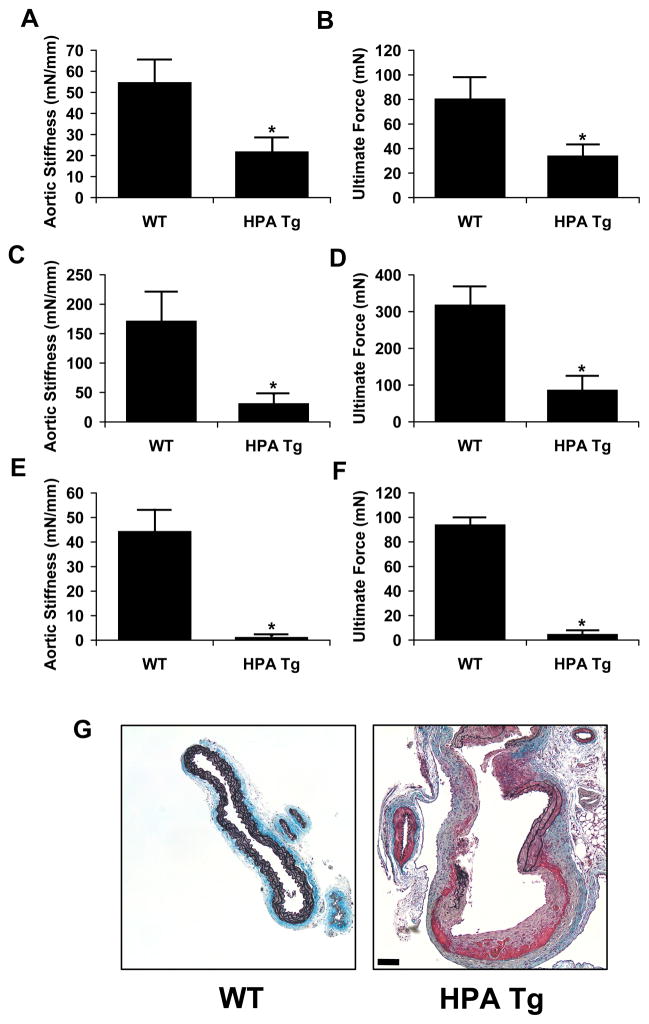
Transgenic mice overexpressing heparanase were used to examine the effects of heparanase excess on arterial structure and mechanics. The aortae of mice with the heparanase transgene had increased medial thickness in comparison to wild-type controls (Figure 2a and 2b). This thickening appeared to be due to enhanced cellular density rather than hypertrophy in the heparanase transgenic animals when compared to their wild-type counterparts (Figure 2c). Aortae were harvested from both groups and mechanical properties of the aorta were measured using a mechanical testing device with environmental control. Heparanase overexpression led to a profound alteration in the the force-displacement relationship of the aorta. This led to a nearly three-fold reduction in longitudinal arterial stiffness (Figure 3a) and ultimate tensile strength (Figure 3b). We also measured circumferential stiffness and ultimate strength and found a similar reduction in arterial mechanical properties for the heparanase transgenic mice (Figure 3c and 3d). To test whether heparanase expression affected the integrity of the elastin network, arteries were digested with NaOH at elevated temperature. This treatment has been shown to destroy most cellular and extracellular matrix components while leaving the elastin network intact. We mechanical tested the remaining elastin networks and found that the heparanase transgenic mice had elastin with dramatically reduced mechanical integrity (Figure 3e and 3f). Consistent with the reduced stiffness and reduction in ultimate strength, localized spontaneous aneurysms were found in the transgenic heparanase mice but not in the wild type (Figure 3g).
Figure 2.
Transgenic mice overexpressing heparanase demonstrate altered arterial structure and cellular density. Thoracic aortae were harvested from transgenic and wild-type animals (n = 8 for each group), formalin fixed, paraffin embedded and sectioned. Aortic sections were taken from four animals of each group at three different locations on the aorta. (a) Histological analysis of heparanase transgenic (HPA Tg) and wild-type (WT) mice stained with H&E and elastic Movat pentachrome stain (EMP). (b) Aortic thickness is increased in heparanase transgenic mice. *Statistically significant difference between samples (n = 4, p < 0.05). Bar = 50 μm.
Figure 3.
Overexpression of heparanase alters the mechanical compliance and ultimate strength of the mouse aorta. The thoracic aortae were harvested from transgenic and wild-type animals and mechanical properties measured using a Bose BioDynamic test instrument. (a) Aortic stiffness expressed as the slope of the force-displacement curve was reduced in HPA Tg animals. (b) Average aortic ultimate strength was reduced in mice expressing the heparanase transgene. (c) Circumferential aortic stiffness of wild type and HPA Tg animals. (d) Circumferential ultimate force for aortae isolated from wild type and HPA Tg animals. (e) Circumferential stiffness of the elastin network remaining after digestion with NaOH solution. (f) Circumferential ultimate strength of the elastin network remaining after digestion with NaOH solution. (g) Example of a local, spontaneous aneurysm found in the aorta of a transgenic heparanase mouse compared to a wild type control. The sample was stained with elastic Movat’s pentachrome stain. Bar = 50 μm.
Heparanase expression is increased in stent injury on obese, hyperlipidemic Zucker rats and is associated with increased intimal thickness
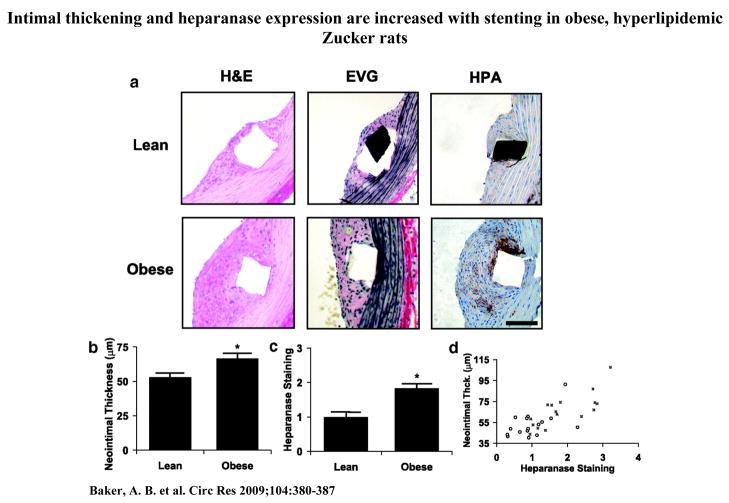
In the clinical setting, diabetic patients are at increased risk of atherosclerosis and restenosis with both bare metal and drug-eluting stents23–25. We examined the expression of heparanase in stent induced restenosis by implanting stents in the aortae of normal and obese Zucker rats using minimally invasive femoral access surgery16. The obese Zucker rat is a model of type II diabetes with obesity, insulin resistance, hyperinsulinemia, hypertriglyceridemia and hypercholesterolemia. Following stent injury and restenosis we found that obese Zucker rats had increased neointimal thickness and expression of heparanase (Figure 4a–4c). The expression of heparanase within the neointima correlated strongly with the final neointimal area, giving a correlation of R=0.50 (p < 0.001) for lean rats and R=0.77 (p < 0.001) for fatty rats (Figure 4d).
Figure 4.
Intimal thickening and heparanase expression are increased with stenting in obese, hyperlipidemic Zucker rats. Endovascular stenting was performed through minimally invasive femoral access. (a) Histochemical staining and immunohistochemical staining of lean and obese Zucker rat aorta, 14 days after stent placement (n=16). EVG = Elastic Verhoff Van Geisen staining. Bar = 50 μm. (b) Analysis of neointimal thickness in lean and obese Zucker rats. (c) Quantitative analysis of heparanase in the neointima of lean and obese Zucker rats. (d) Heparanase expression and neointimal proliferation in response to endovascular stenting are enhanced in obese Zucker rats (■) compared to lean controls (○).The expression of heparanase within the neointima correlated strongly with the final neointimal area, giving a correlation of R=0.50 (p < 0.001) for lean rats and R=0.77 (p < 0.001) for fatty rats. *Statistically significant difference between samples (p < 0.05).
Heparanase overexpression enhances neointimal proliferation and macrophage recruitment in response to endovascular stent implantation
To examine arterial repair in the presence of heparanase overexpression we developed a minimally invasive method of stent placement in mice. Using a novel small diameter stenting system (CardioMind, Sunnyvale, CA) we performed minimally invasive endovascular stenting of transgenic mice overexpressing heparanase. This method takes advantage of self expanding stents that have a diameter identical to that of the catheter wire prior to deployment. Nitinol self expanding stents were deployed in the abdominal aorta of wild type and heparanase transgenic mice via femoral access (Figure 5a). In the heparanase transgenic mouse the neointimal lesion was rich in macrophages, staining strongly for the Mac-1 surface marker (Figure 5b). Heparanase transgenic mice had markedly increased neointimal formation with increased neointimal area (Figure 5c) and intima to media ratio (Figure 5d) with no significant change in medial area (Figure 5e). We examined release of monocyte chemoattractant protein-1 (MCP-1) one hour after vascular injury and found increased amounts of MCP-1 in the heparanase transgenic mice (Figure 5f).
Figure 5.
Abdominal stenting of heparanase transgenic and wild type mice was performed using femoral access with small diameter self-expanding stents. Stents were harvested after 14 days and processed for histological analysis in resin sections. (a) Lateral x-ray of stent placement in the abdominal aorta of the mouse. The stent is visible just below the spine (marked with arrow). (b) Neointimal formation in response to vascular injury with endovascular staining is increased in heparanase transgenic mice. Staining for Mac-1 was increased in heparanase transgenic mice. Bar = 50 μm. (c–e) Morphological analysis of the stented arteries showed increased intimal area, intima to medial ratio and no change in media area for heparanase transgenic mice. (f) Concentration of MCP-1 in arterial lysates as measured by ELISA assay. The arteries were harvested one hour following arterial injury (n=4). *Statistically significant difference between all samples (p < 0.05).
Discussion
Despite the discovery of heparanase-like activity in endothelial cells nearly two decades ago26, the functional role of this expression in arterial biology has been explored by only a limited number of studies. Previous studies have shown that heparanase is expressed in endothelial cells and can be regulated by high glucose27 and oxidized lipoproteins28. However, the functional significance of these findings to arterial biology was unknown. The current study adds to these findings by defining a functional role for heparanase in controlling paracrine inhibition of vSMCs by endothelial cells. Endothelial inhibition of vSMCs is an essential process for maintaining arterial health and its loss is a precursor to atherosclerosis, restenosis and arterial thickening. We and others have shown that HSPGs, specifically perlecan, are essential for endothelial inhibition of vSMC proliferation8–10. In this study, we demonstrated that overexpression of heparanase can abolish this effect and, further, that knock down of heparanase expression enhances endothelial cell inhibition of vSMC growth. We found that heparanase expression within endothelial cells led to degradation of surface and soluble heparan sulfate chains. The heparan sulfate motif recognized by heparanase may be critical to inhibition of vSMCs. Thus, even with small changes in total HSPGs, the inhibitory properties may be dramatically reduced. This finding implies that inhibitors of heparanase may be potential therapeutics for diseases that compromise endothelial dysfunction and induce aberrant vascular remodeling.
One known mechanism of heparanase activity is the cleavage of extracellular matrix HSPGs leading to a release of matrix bound growth factors13. Heparanase isolated from platelets has been shown to release FGF-2 and increase cell proliferation22. Here we examined the effects of endothelial cell expression of heparanase in contrast to direct application of the active enzyme. This allows for the endogenous control mechanism for regulating heparanase activity in endothelial cells to remain intact while increasing gene expression. The levels of FGF-2 in the conditioned media of samples under the various treatments were constant, implying a differential mechanism in between to the direct application of active heparanase protein in comparison to increased gene expression.
Diabetes, infection and inflammation can lead to arterial states with heightened heparanase expression27, 28. We examined the functional outcome of increased heparanase expression on the arterial structure and mechanical properties. Transgenic mice overexpressing heparanase had aortic thickening and increased aortic cellular density. In addition, aortic stiffness and ultimate strength were both decreased and this was accompanied by increased incidence of spontaneous aneurysm. These demonstrate that excessive heparanase expression can compromise the mechanical integrity of the aorta. Arterial stiffness is maintained by elastin fiber integrity whereas ultimate strength is predominantly a function of the collagen network within the artery. Alterations in both of these properties would suggest a multifactorial mechanism for heparanase within this system. Heparanase can act on the cell surface HSPG syndecan-1 which serves as an adhesion receptor for collagen I29. In addition, HSPGs are important for elastin and collagen fiber assembly30 and heparanase may compromise both of these processes. We digested the aortae to leave on the elastin network intact and then performed circumferential stress testing. These results reveal that there is a reduction in stiffness of the elastin network with heparanase overexpression.
We examined the role of heparanase in two models of stent induced vascular injury and repair. Endovascular stent placement in the obese Zucker rat led to increased neointimal thickness and neointimal heparanase expression. Our studies revealed a strong correlation between neointimal heparanase expression and neointimal thickness. Previous studies have made use of potential heparanase inhibitors in the context of neointimal formation. Heparin and PI-88 (a synthetic heparin analogue) have been used in animal models to reduce neointimal formation31, 32. Both of these compounds can inhibit heparanase activity but also have other activities including growth factor binding and direct activity on vSMCs. A neutralizing antibody to heparanase has also been used to reduce neointima formation following carotid balloon injury33. In our studies, we used a novel murine stent injury model which provided deep vascular injury, permanent stretch and in-dwelling device providing injury similar to clinical interventions. Our results showed increased macrophage infiltration in heparanase transgenic mice in concert with enhanced intimal formation. Consistent with these findings, we also found that the concentration of MCP-1 was increased in the transgenic heparanase mice following acute vascular injury. The cellular production of MCP-1 is decreased by heparin/heparan sulfate34 and this may be a potential mechanism for heparanase causing an increase in MCP-1 following vascular injury. The murine model of endovascular stenting represents a significant advancement in the animal models of vascular injury, allowing for clinically relevant stent-induced injury in the powerful genetic models available in mice. Stent injury provides deeper vascular injury, thus generating a distinct inflammatory response and endothelial dysfunction not present in balloon or wire injury models.
Taken together, our results demonstrate that heparanase is a potent regulator of vascular remodeling, both on the level of paracrine regulation of vascular homeostasis and as an effector molecule in vascular response to injury. Our study suggests that heparanase can act through multiple mechanisms to alter vascular remodeling and response to injury. Heparanase can serve as a control point allowing endothelial cells to modulate between inhibition and stimulation of vascular smooth muscle cells. Further, overexpression of heparanase can alter elastin fiber integrity as well as increase response to vascular injury through enhancing macrophage recruitment. A common unifying feature of these processes is the involvement of heparan sulfate and, thus, vulnerability to disruption by heparanase. Consequently, aberrant heparanase may serve as a common pathophysiological mechanism governing vascular remodeling under different pathological disease states. While effective and specific small molecule inhibitors of heparanase have long been sought after for the treatment of cancer, our study indicates that these molecules would also be useful in disease states leading to pathophysiologic arterial remodeling.
Acknowledgments
We gratefully acknowledge the technical assistance of G. Wong and CardioMind Corporation for the kind donation of small caliber endovascular stents.
Sources of Funding
This work was supported by US National Institutes of Health (grant R01 HL67246) to E.R.E as well as Whitaker Foundation and Philip Morris Postdoctoral Research Fellowships to A.B.B.
Footnotes
Disclosures: None.
References
- 1.Cohen JD. Overview of physiology, vascular biology, and mechanisms of hypertension. J Manag Care Pharm. 2007;13:S6–8. doi: 10.18553/jmcp.2007.13.s5.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Libby P. Atherosclerosis: disease biology affecting the coronary vasculature. Am J Cardiol. 2006;98:3Q–9Q. doi: 10.1016/j.amjcard.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 3.Weintraub WS. The pathophysiology and burden of restenosis. Am J Cardiol. 2007;100:3K–9K. doi: 10.1016/j.amjcard.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Karnovsky MJ, Edelman ER. Heparin/heparan sulphate regulation of vascular smooth muscle behaviour. In: Page CP, Black JL, editors. Airways and Vascular Remodeling in Asthma and Cardiovascular Disease: Implications for Therapeutic Intervention. New York: Academic Press; 1994. pp. 45–70. [Google Scholar]
- 5.Michiels C. Endothelial cell functions. J Cell Physiol. 2003;196:430–443. doi: 10.1002/jcp.10333. [DOI] [PubMed] [Google Scholar]
- 6.Ettenson DS, Koo EW, Januzzi JL, Edelman ER. Endothelial heparan sulfate is necessary but not sufficient for control of vascular smooth muscle cell growth. J Cell Physiol. 2000;184:93–100. doi: 10.1002/(SICI)1097-4652(200007)184:1<93::AID-JCP10>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 7.Nugent MA, Nugent HM, Iozzo RV, Sanchack K, Edelman ER. Perlecan is required to inhibit thrombosis after deep vascular injury and contributes to endothelial cell-mediated inhibition of intimal hyperplasia. Proc Natl Acad Sci U S A. 2000;97:6722–6727. doi: 10.1073/pnas.97.12.6722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castellot JJ, Jr, Addonizio ML, Rosenberg R, Karnovsky MJ. Cultured endothelial cells produce a heparinlike inhibitor of smooth muscle cell growth. J Cell Biol. 1981;90:372–379. doi: 10.1083/jcb.90.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edelman ER, Nugent MA, Karnovsky MJ. Perivascular and intravenous administration of basic fibroblast growth factor: vascular and solid organ deposition. Proc Natl Acad Sci U S A. 1993;90:1513–1517. doi: 10.1073/pnas.90.4.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nugent MA, Karnovsky MJ, Edelman ER. Vascular cell-derived heparan sulfate shows coupled inhibition of basic fibroblast growth factor binding and mitogenesis in vascular smooth muscle cells. Circ Res. 1993;73:1051–1060. doi: 10.1161/01.res.73.6.1051. [DOI] [PubMed] [Google Scholar]
- 11.Elkin M, Ilan N, Ishai-Michaeli R, Friedmann Y, Papo O, Pecker I, Vlodavsky I. Heparanase as mediator of angiogenesis: mode of action. Faseb J. 2001;15:1661–1663. doi: 10.1096/fj.00-0895fje. [DOI] [PubMed] [Google Scholar]
- 12.Vlodavsky I, Goldshmidt O, Zcharia E, Metzger S, Chajek-Shaul T, Atzmon R, Guatta-Rangini Z, Friedmann Y. Molecular properties and involvement of heparanase in cancer progression and normal development. Biochimie. 2001;83:831–839. doi: 10.1016/s0300-9084(01)01318-9. [DOI] [PubMed] [Google Scholar]
- 13.Vlodavsky I, Abboud-Jarrous G, Elkin M, Naggi A, Casu B, Sasisekharan R, Ilan N. The impact of heparanese and heparin on cancer metastasis and angiogenesis. Pathophysiol Haemost Thromb. 2006;35:116–127. doi: 10.1159/000093553. [DOI] [PubMed] [Google Scholar]
- 14.Vlodavsky I, Friedmann Y, Elkin M, Aingorn H, Atzmon R, Ishai-Michaeli R, Bitan M, Pappo O, Peretz T, Michal I, Spector L, Pecker I. Mammalian heparanase: gene cloning, expression and function in tumor progression and metastasis. Nat Med. 1999;5:793–802. doi: 10.1038/10518. [DOI] [PubMed] [Google Scholar]
- 15.Baker AB, Ettenson DS, Jonas M, Nugent MA, Iozzo RV, Edelman ER. Endothelial cells provide feedback control for vascular remodeling through a mechanosensitive autocrine TGF-beta signaling pathway. Circ Res. 2008;103:289–297. doi: 10.1161/CIRCRESAHA.108.179465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jonas M, Edelman ER, Groothuis A, Baker AB, Seifert P, Rogers C. Vascular neointimal formation and signaling pathway activation in response to stent injury in insulin-resistant and diabetic animals. Circ Res. 2005;97:725–733. doi: 10.1161/01.RES.0000183730.52908.C6. [DOI] [PubMed] [Google Scholar]
- 17.Zcharia E, Metzger S, Chajek-Shaul T, Aingorn H, Elkin M, Friedmann Y, Weinstein T, Li JP, Lindahl U, Vlodavsky I. Transgenic expression of mammalian heparanase uncovers physiological functions of heparan sulfate in tissue morphogenesis, vascularization, and feeding behavior. Faseb J. 2004;18:252–263. doi: 10.1096/fj.03-0572com. [DOI] [PubMed] [Google Scholar]
- 18.Simon DI, Dhen Z, Seifert P, Edelman ER, Ballantyne CM, Rogers C. Decreased neointimal formation in Mac-1(−/−) mice reveals a role for inflammation in vascular repair after angioplasty. J Clin Invest. 2000;105:293–300. doi: 10.1172/JCI7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vesely I. The role of elastin in aortic valve mechanics. J Biomech. 1998;31:115–123. doi: 10.1016/s0021-9290(97)00122-x. [DOI] [PubMed] [Google Scholar]
- 20.Yang Y, Macleod V, Miao HQ, Theus A, Zhan F, Shaughnessy JD, Jr, Sawyer J, Li JP, Zcharia E, Vlodavsky I, Sanderson RD. Heparanase enhances syndecan-1 shedding: a novel mechanism for stimulation of tumor growth and metastasis. J Biol Chem. 2007;282:13326–13333. doi: 10.1074/jbc.M611259200. [DOI] [PubMed] [Google Scholar]
- 21.Ishai-Michaeli R, Eldor A, Vlodavsky I. Heparanase activity expressed by platelets, neutrophils, and lymphoma cells releases active fibroblast growth factor from extracellular matrix. Cell Regul. 1990;1:833–842. doi: 10.1091/mbc.1.11.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Myler HA, West JL. Heparanase and platelet factor-4 induce smooth muscle cell proliferation and migration via bFGF release from the ECM. J Biochem (Tokyo) 2002;131:913–922. doi: 10.1093/oxfordjournals.jbchem.a003182. [DOI] [PubMed] [Google Scholar]
- 23.Cutlip DE, Chauhan MS, Baim DS, Ho KK, Popma JJ, Carrozza JP, Cohen DJ, Kuntz RE. Clinical restenosis after coronary stenting: perspectives from multicenter clinical trials. J Am Coll Cardiol. 2002;40:2082–2089. doi: 10.1016/s0735-1097(02)02597-4. [DOI] [PubMed] [Google Scholar]
- 24.Lemos PA, Hoye A, Goedhart D, Arampatzis CA, Saia F, van der Giessen WJ, McFadden E, Sianos G, Smits PC, Hofma SH, de Feyter PJ, van Domburg RT, Serruys PW. Clinical, angiographic, and procedural predictors of angiographic restenosis after sirolimus-eluting stent implantation in complex patients: an evaluation from the Rapamycin-Eluting Stent Evaluated At Rotterdam Cardiology Hospital (RESEARCH) study. Circulation. 2004;109:1366–1370. doi: 10.1161/01.CIR.0000121358.26097.06. [DOI] [PubMed] [Google Scholar]
- 25.Mathew V, Gersh BJ, Williams BA, Laskey WK, Willerson JT, Tilbury RT, Davis BR, Holmes DR., Jr Outcomes in patients with diabetes mellitus undergoing percutaneous coronary intervention in the current era: a report from the Prevention of REStenosis with Tranilast and its Outcomes (PRESTO) trial. Circulation. 2004;109:476–480. doi: 10.1161/01.CIR.0000109693.64957.20. [DOI] [PubMed] [Google Scholar]
- 26.Godder K, Vlodavsky I, Eldor A, Weksler BB, Haimovitz-Freidman A, Fuks Z. Heparanase activity in cultured endothelial cells. J Cell Physiol. 1991;148:274–280. doi: 10.1002/jcp.1041480213. [DOI] [PubMed] [Google Scholar]
- 27.Han J, Woytowich AE, Mandal AK, Hiebert LM. Heparanase upregulation in high glucose-treated endothelial cells is prevented by insulin and heparin. Exp Biol Med (Maywood) 2007;232:927–934. [PubMed] [Google Scholar]
- 28.Chen G, Wang D, Vikramadithyan R, Yagyu H, Saxena U, Pillarisetti S, Goldberg IJ. Inflammatory cytokines and fatty acids regulate endothelial cell heparanase expression. Biochemistry. 2004;43:4971–4977. doi: 10.1021/bi0356552. [DOI] [PubMed] [Google Scholar]
- 29.Beauvais DM, Burbach BJ, Rapraeger AC. The syndecan-1 ectodomain regulates alphavbeta3 integrin activity in human mammary carcinoma cells. J Cell Biol. 2004;167:171–181. doi: 10.1083/jcb.200404171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buczek-Thomas JA, Chu CL, Rich CB, Stone PJ, Foster JA, Nugent MA. Heparan sulfate depletion within pulmonary fibroblasts: implications for elastogenesis and repair. J Cell Physiol. 2002;192:294–303. doi: 10.1002/jcp.10135. [DOI] [PubMed] [Google Scholar]
- 31.Khachigian LM, Parish CR. Phosphomannopentaose sulfate (PI-88): heparan sulfate mimetic with clinical potential in multiple vascular pathologies. Cardiovasc Drug Rev. 2004;22:1–6. doi: 10.1111/j.1527-3466.2004.tb00127.x. [DOI] [PubMed] [Google Scholar]
- 32.Edelman ER, Adams DH, Karnovsky MJ. Effect of controlled adventitial heparin delivery on smooth muscle cell proliferation following endothelial injury. Proc Natl Acad Sci U S A. 1990;87:3773–3777. doi: 10.1073/pnas.87.10.3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Myler HA, Lipke EA, Rice EE, West JL. Novel heparanase-inhibiting antibody reduces neointima formation. J Biochem (Tokyo) 2006;139:339–345. doi: 10.1093/jb/mvj061. [DOI] [PubMed] [Google Scholar]
- 34.Douglas MS, Ali S, Rix DA, Zhang JG, Kirby JA. Endothelial production of MCP-1: modulation by heparin and consequences for mononuclear cell activation. Immunology. 1997;92:512–518. doi: 10.1046/j.1365-2567.1997.00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]