Introduction
Overweight and obesity are a leading cause of preventable death, and an estimated 65% of U.S. adults are overweight or obese [1-3]. Obesity has been linked to increased overall mortality [4-6], and decreased life expectancy [7]. The impact of obesity is deleterious, and many of the harmful effects are in part related to the increased morbidity risks associated with cardiovascular disease (CVD) [8-16]. The prevalence of hypertension, diabetes, and dyslipidemia is 1.5-2.9 times greater among adults who are overweight compared to normal weight adults [17-19], and the costs of obesity and related conditions are astounding [5, 20-22]. The annual U.S. medical expenditures attributable to obesity are estimated to be $75 billon in 2003 dollars, and total direct and indirect costs related to obesity and its consequences amounted to $117 billion in the year 2000 [23]. As the prevalence of obesity and comorbid CVD continues to rise, as well as their attributable costs, controlling the obesity epidemic has become one of the nation's top health priorities [17, 24].
Fortunately, the effect of obesity and overweight on CVD risk factors is reversible. Research has shown that short-term weight loss can be reasonably attained, and can attenuate CVD risk factors. Relatively modest amounts of weight loss can lower blood pressure [25-28], as well as having health-improving benefits on blood lipids [29]. Long-term (greater than 3 years) maintenance of weight loss results in maintained blood pressure reduction [30]. Similarly, weight loss lowers blood glucose levels in diabetic and non-diabetic individuals, and recent randomized trials suggest that weight loss can prevent the development of type 2 diabetes [31, 32]. However, a major factor contributing to the rise in obesity is weight regain after initial weight loss. There is extensive evidence suggesting that weight loss can be achieved over the short term, however weight regain is unfortunately common. The most promising strategies to prevent regain involve continued behavioral interventions with frequent contacts.
National recommendations emphasize weight loss/control as a key component of treatment guidelines for hypertension, dyslipidemia, and type 2 diabetes. Behavioral strategies for weight loss and reduction of CVD risk factors have also been recommended in various national guidelines and consensus statements [1, 33-35] These guidelines emphasize the need for combined treatment of reduced energy intake, improved dietary choices, increased physical activity, and behavior therapy. Many of these reports acknowledge the difficulty of sustaining long-term weight loss, and further suggest that weight loss programs be followed by a prolonged weight maintenance program. Despite this recommendation, little data are available on the needed content, structure, and mode of delivery for successful maintenance programs. Although long-term weight loss trials are available in the literature, few studies have tested alternative strategies designed to sustain weight loss once it has been achieved [36-38]. In most studies, intensive intervention leads to weight loss over a relatively short period of time (usually 6 months or less). This intensive phase is generally followed by a less intensive period consisting of support only, and is associated with steady weight regain [27]. Even studies designed to provide a long-term intervention other than support show a similar pattern of behavior [39]. Those studies that have tested long-term weight management interventions have typically done so for the purposes of achieving some other endpoint of interest (e.g., blood pressure or diabetes control) [27, 28]. Direct comparisons of interventions specifically designed to maintain weight loss are needed.
Many national health organizations have stressed the need for comprehensive research aimed at maintenance of lifestyle modifications with particular focus on factors which enhance the maintenance of behavior change for weight loss (e.g., dietary intake and physical activity, behavioral strategies, and the frequency and duration of intervention contacts) [1, 36, 40-42]. Recent reviews on weight maintenance suggest that weight loss efforts may require different behavioral approaches than maintenance efforts [43-45]. Specifically, nutritional skill building and education may be more critical for weight loss, while relapse prevention, problem-solving, and motivational enhancement techniques may be more germane for weight maintenance. Furthermore, review of the weight loss literature consistently demonstrates that longer duration of initial intensive intervention and higher frequency of intense follow-up contact appear to augment long-term effectiveness [37, 46-49].
Despite the positive influence that multi-component behavioral approaches have on weight loss and maintenance, most studies have tended to have a limited repertoire of delivery approaches. Less traditional approaches have been shown effective in applying the principles of behavior modification to obese populations [50]. Telephone contacts have been shown to improve adherence to physical activity [51], promote self-monitoring of dietary intake and physical activity [52], and improve diabetes indicators [53]. The telephone provides an alternative delivery method of behavioral interventions that may prove helpful for long-term intensive follow-up due to low participant burden (e.g., flexible scheduling and no need to travel). Additionally, alternative communication technologies (e.g., internet) may also have potential [54-57]. A recent study comparing weight loss between an internet-based intervention group and a therapist-led group found that weight loss was similar in the two groups [58].
The existing research and national health recommendations emphasize the need for research on strategies for maintaining weight loss. The Weight Loss Maintenance Trial (WLM) will compare the effects of two maintenance interventions (e.g., Personal Contact and Interactive Technology) to a self-directed usual care control group. WLM is a unique trial: (1) it specifies maintenance as the primary outcome, (2) focuses on a high-risk population (CVD risk factors), and (3) emphasizes innovative behavioral strategies delivered through novel methods. Additionally, the maintenance strategies being tested in this trial are designed to maximize the potential for rapid translation into the public health sector and the medical care environment.
Methods
Overview
The Weight Loss Maintenance Trial (WLM) is a multicenter randomized trial which will test two maintenance strategies: a Personal Contact (PC) and Interactive Technology (IT) intervention. The Personal Contact (PC) condition provides monthly contacts with an interventionist primarily via telephone and supplemented with face-to-face visits. The Interactive Technology (IT) intervention provides frequent, individualized contact through a tailored, internet website. These two maintenance strategies will be compared to a self-directed (SD/UC) control group. Participating institutions in the trial include four clinical centers (Johns Hopkins University in Baltimore, MD; Pennington Biomedical Research Center in Baton Rouge, LA; Duke University Medical Center in Durham, NC; and the Kaiser Permanente Center for Health Research in Portland, OR), the Coordinating Center (Kaiser Permanente Center for Health Research located in Portland, OR), and the NHLBI Project Office. The study was approved by the Institutional Review Board at each clinical site. Study funding began in January of 2003. Recruitment began in April 2003 and final data collection will be completed in July 2007.
Study Population
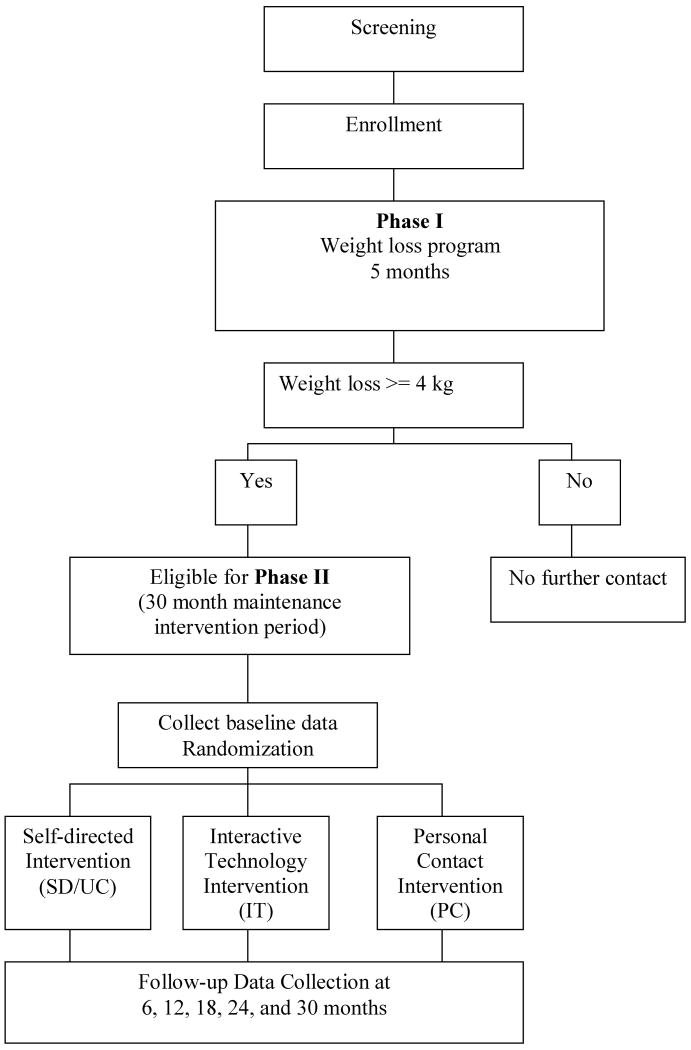
The WLM has two phases, an initial weight loss program (Phase I) and a weight loss maintenance program (Phase II). Eligibility criteria for Phase I included overweight or obese adults who are taking medication for hypertension and/or dyslipidemia, and are able to lose at least 4 kg during an initial 5-month weight loss program. Eligibility criteria for inclusion into the initial 5-month intensive weight-loss program are summarized in Table 1. After completion of the intensive weight loss program, participants will be required to meet a second set of eligibility requirements. The primary inclusion criteria for randomization into Phase II is the loss of at least 4 kg compared to entry weight. Eligibility criteria for Phase II are summarized in Table 2.
Table 1. Eligibility Criteria (Phase I).
| Inclusion criteria for Phase I |
| BMI of 25-45 kg/m2 |
| Age 25 or older |
| Taking prescription medication for hypertension and/or dyslipidemia |
| Personal physician permission required if diabetes present or if participant had a prior CVD event (CVD event within past 12 months is an exclusion) |
| Non-positive stress test or other diagnostic test for CAD required if diabetic or if history of CVD. The stress test can be waived for participants who enter WLM currently engaging in vigorous physical activity |
| Willing not to use weight loss medications for the duration of the trial |
| Able and willing to give informed consent and participate in the interventions |
| Access to telephone and the internet |
| Demonstrated ability to access a specific web site |
| Demonstrated ability to receive and respond to email |
| Able to keep a complete 5-day food record during screening |
| Exclusion criteria for Phase I |
| Medical Exclusions |
| Contraindication to weight loss (e.g., malignancy or other serious illness) |
| Cardiovascular event within the past 12 months |
| Current symptoms of angina, unless we have both physician permission and a non-positive stress test or other diagnostic test for CAD |
| Evidence of active cancer diagnosis (except for non-melanoma skin cancer) or treatment in past 2 years, defined as any diagnosis or any treatment within the past 2 years |
| Medication treated diabetes. |
| If unmedicated diabetes, eligible if HgbA1C <8 and with both MD permission and a non-positive stress test or other diagnostic test for CAD. The stress test can be waived for participants who enter WLM currently engaging in vigorous physical activity |
| Self-reported history of renal disease (other than kidney stones) |
| Psychiatric hospitalization within the last 2 years |
| Consumption of more than 21 alcoholic drinks per week or binge drinking |
| Weight loss of > 20 lbs in the last 3 months |
| Any history of gastric bypass surgery or fundoplication for obesity, or scheduled surgery for this purpose |
| Liposuction in the past 12 months |
| Medications |
| Use of prescription weight loss medications in the 3 months prior to screening. |
| Current use of medications for treatment of psychosis or manic-depressive illness |
| Other |
| Planning to leave the area within three years |
| Pregnant, breast feeding, or planning pregnancy prior to the end of participation |
| Current participation in another clinical trial |
| Weight Loss Maintenance Trial staff member or household member of a Weight Loss Maintenance Trial staff person |
Table 2. Eligibility Criteria (Phase II).
| Inclusion criteria for Phase II |
| Weight loss of at least 4 kg during Phase I |
| Willing not to use weight loss medications for the duration of the trial |
| Willing to be randomized to maintenance phase |
| Able and willing to give informed consent and participate in the interventions |
| Exclusion criteria for Phase II |
| Medical History |
| Contraindication to weight loss (e.g., malignancy or other serious illness) |
| Cardiovascular event since entry |
| Current symptoms of angina, unless we have both physician permission and a non-positive stress test or other diagnostic test for CAD. |
| Evidence of active cancer diagnosis (except for non-melanoma skin cancer) or treatment in past 2 years, defined as any diagnosis or any treatment within the past 2 years |
| Self-reported history of renal disease (other than kidney stones) since entry |
| Psychiatric hospitalization since entry |
| Gastric bypass surgery or fundoplication for obesity since entry, or scheduled surgery for this purpose |
| Liposuction since entry, or scheduled liposuction |
| Medications |
| Use of prescription weight loss medications |
| Current use of medications for treatment of psychosis or manic-depressive illness |
| Current use of medication for the treatment of diabetes |
| Other |
| Planning to leave the area within 30 months |
| Pregnant, breast feeding, or planning pregnancy prior to the end of participation |
| Weight Loss Maintenance Trial staff member or household member of a Weight Loss Maintenance Trial staff person |
Recruitment
The primary source of participants was from mass mailings. Additionally, each clinical center used other strategies such as printed advertisements in local newspapers, radio advertisements, e-mail broadcasts, screening events, and word-of-mouth.
There is a high prevalence of overweight and cardiovascular disease in African Americans [59]. Most behavioral intervention studies have shown that African Americans lose less weight than non-African Americans and do so at a slower rate [60]. Therefore, a major goal of this trial will be to randomize a population sample consisting of 40% African Americans. In order to ensure our randomization goal, African Americans will be over-recruited during the first two cohorts of the trial. Minority specific recruiting efforts include community-based screening events, public service announcements to radio stations, and newspapers serving minority populations. A two stage consent process is used for the trial; initial consent obtained prior to the intensive weight loss program, and a second consent at randomization.
Screening
A prescreening questionnaire is administered via telephone to all potential participants to determine preliminary eligibility and interest. Those persons meeting prescreening eligibility are invited to attend a series of screening visits prior to beginning Phase I. The purpose of these screening visits is to determine eligibility and to collect entry measurements. Screening visit measurements include weight, height, physical activity (e.g., accelerometry data), a fasting blood specimen, and a series of questionnaires and interviews to help assess eligibility. After screening is completed, eligible participants begin Phase I, the 5-month intensive weight-loss program.
Randomization
Participants who lose a minimum of 4 kg at the completion of the initial weight-loss program (e.g., Phase I) are eligible to be randomized to Phase II. Prior to randomization, study staff (1) reassess the participants' eligibility based on inclusion/exclusion criteria (see Tables 1 and 2), (2) reviews study requirements with the participant, (3) administers the randomization consent, and (4) collects baseline data. Phase II participants are then randomly assigned to one of the three maintenance conditions: (1) Personal Contact (PC), (2) Interactive Technology (IT), or (3) Self-directed/Usual Care (SD/UC) (Figure 1). Randomization assignments are generated using computer software developed by the Coordinating Center. Randomization assignments are stratified by clinic site, race (e.g., African-American vs. non African-American), and weight loss during Phase I, and are also blocked to provide a balance in treatment assignments over time.
Figure 1. WLM Study Design.
Masking
Study participants and the clinical center intervention staff are aware of participants' treatment assignments. All clinical center staff involved in follow-up data collection are masked to participants' intervention assignments. Participants are informed of their baseline weight measurements, and will also receive a summary of other clinical and laboratory measurements at the conclusion of the trial.
Interventions
The theoretical basis of the behavioral interventions in Phase I and II are derived from social cognitive theory [61], behavioral self-management techniques [62], the transtheoretical model (e.g., stages of change theory) [63, 64], and motivational enhancement techniques [65, 66]. These approaches stress the importance of regulating individual behavior through developing behavior change plans that are individual-specific, and incorporate goal setting, self-monitoring, and skill development.
Phase I: Weight Loss Program
Prior to receiving the maintenance intervention, all eligible persons participate in an intensive five month weight loss program. This intervention promotes weight loss through decreased calorie consumption and increased physical activity. Participants are encouraged to follow the DASH (Dietary Approaches to Stop Hypertension) eating pattern. This dietary approach has demonstrated reductions in blood pressure and desirable effects on lipids [67]. The dietary goals of the DASH eating pattern are to increase the consumption of fruits, vegetables, and low-fat dairy, and reduce intake of saturated and total fat. The high-fiber and low-fat content of the DASH eating pattern make it particularly appropriate for weight loss. Additional intervention suggestions are based on the National High Blood Pressure Education Program recommendations which include a reduction in sodium, increased physical activity, and limiting alcohol intake [68].
The Phase I weight loss program consists of 20 weekly group sessions. Group sessions are designed to be participant-centered and interactive rather than didactic, and are based on standardized program materials and guides. Sessions are approximately one and a half to two hours in duration with 18-25 participants per group. Guided physical activity or food demonstrations are included in many of the group sessions. Supplemental individual contacts by phone or in person are permissible in order to assist participants with weight loss and behavior change. These contacts focus on enhancing motivation, identifying specific behavior change goals, and problem-solving. The weight loss goal is to achieve a minimum weight loss of 4.5-6.8 kg. Notably, this amount of weight loss corresponds with significant improvements in CVD risk factors [1, 25, 69]. Information in the Phase I groups is based on current clinical practice guidelines for obesity treatment, and for individuals with increased risk for CVD [1]. Specifically, the weight loss intervention is designed to achieve weight loss through dietary change and increased energy expenditure. Behavioral goals for Phase I include engaging in 180 minutes per week of moderate physical activity, and following a healthy diet (e.g., the DASH eating pattern) [67]. The Phase I weight loss program includes strategies which focus on frequent contacts, social support, goal-setting, decision-making, and problem-solving. All interventions are culturally-sensitive and special attention is given to maintain the cultural appropriateness of the interventions for African Americans.
Additional behavioral strategies include self-monitoring and personalized plans for diet and physical activity, increased physical activity, portion control, moderate caloric restriction, reduction in sodium intake, increased fruits and vegetable consumption, contingency planning for undesired behavior, visual graphing of individual weight and behavioral progress, and developing core food-choice competencies.
Phase II: Maintenance
During the maintenance phase, the goals for physical activity, dietary pattern, and calorie levels are similar across all three maintenance conditions. All participants are provided with individualized goals for caloric intake based on personalized needs for weight maintenance or additional weight loss. Calorie ranges will be determined using standard formulas and will be tailored to participants' physical activity level [70]. The physical activity goal for Phase II is to increase moderate intensity physical activity to an average of at least 45 minutes per day at least five times each week (i.e., > 225 minutes per week). This is an increase of 45 minutes/week over the Phase I goals which encourage an average goal of 180 min/week. The increase in physical activity during the maintenance phase is based on research findings suggesting that higher levels of physical activity are required for maintaining weight loss [71].
The comparison condition is a self-directed/usual care program which provides advice to participants on maintaining their weight loss [54, 72]. The two active maintenance interventions (PC and IT) are based on the same theoretical foundations as the initial weight-loss program, however, they also incorporate specific constructs of maintenance (e.g., motivation, support, problem-solving, and relapse prevention). The active maintenance interventions are based on previous research findings supporting the use of approaches associated with sustained weight loss (e.g., frequent contacts, self-monitoring, regular physical activity, accountability), and innovative counseling methods (e.g., motivational interviewing) designed to enhance participant involvement and adherence [46, 73]. Care is being taken to make the two active interventions as similar as possible.
Self-Directed/Usual Care (SD/UC) condition
Participants randomized to this arm receive support and encouragement with a minimal contact schedule. At the randomization visit, participants meet with a study interventionist to discuss the assignment, and receive encouragement for attending future measurement visits. Participants in this arm receive printed lifestyle guidelines with diet and physical activity recommendations. At the 12-month and 30-month follow-up visits, participants meet with a study interventionist for a brief meeting to discuss their continued participation in the study. Although the number of contacts exceeds what is typically viewed as usual care, we decided we needed the extra contact to ensure continued participation over a lengthy maintenance phase.
Personal Contact (PC) intervention
Overview of PC
The Personal Contact (PC) intervention offers one-on-one guidance and support in maintaining weight loss. This approach is geared toward overcoming barriers that typically sabotage maintenance efforts. The PC intervention is founded on the principle that weight maintenance is difficult, and success is more likely when a case management approach is utilized. This arm uses brief, individual contacts on a monthly basis which emphasize core elements of weight maintenance. Monthly contacts with an interventionist occur for 30 months after the end of Phase I. Face-to-face contacts occur approximately every four months (e.g., three annually), with telephone contacts occurring every month between FTF contacts. One of the three annual face-to-face contacts is a group session with other PC participants. Two supplemental contacts per year (face-to-face or by phone) may be conducted at the interventionist's discretion. We are considering a special holiday group session to help plan for high risk holiday eating. Frequency of visits for this trial is based on information from previous studies examining contact frequency to enhance maintenance [46], disease management programs [74], and suggested frequency of contacts from the Medicare Medical Nutrition Therapy Amendment Act of 2001 [75]. Standardized materials and procedures including topical discussions and case scenarios for problem-solving specific situations and barriers guide the PC contacts.
The key components of the PC intervention include methods to assist participants in adhering to health behaviors for maintaining weight loss. These strategies include frequent contacts, self-monitoring (e.g., weekly weighing, food and fitness diaries), tailored reinforcement, social support, problem-solving (e.g., troubleshooting barriers to success), and relapse prevention (e.g., anticipating “high risk” situations). Motivational Interviewing (MI) techniques are used to facilitate and augment the more traditional behavioral strategies.
Telephone contacts
These contacts consist of an interval of rapport building, followed by a review of the diet and activity efforts for the previous month. Particular attention is given to identifying barriers to success encountered during the past month. After identifying an obstacle, problem-solving techniques are used to develop a plan to overcome the obstacle.
Face-to-face (FTF) contacts
FTF contacts are approximately 45 minutes in duration, and include a weigh-in, a review of self-monitoring and goals, and a brief review of the previous month's performance. High-risk situations are discussed followed by problem-solving tailored to the participant's choice and need. Brief vignettes focusing on practical maintenance elements (e.g., staying motivated with exercise) are chosen based on the needs of the participant and then reviewed. Each session ends with setting goals for weight, dietary intake, and physical activity for the upcoming month.
Interactive Technology (IT) intervention
Overview of IT
Similar to the PC intervention, the IT intervention is also founded on key behavior change components. However, this maintenance intervention utilizes internet and automated phone technology to enhance the frequency and timeliness of feedback. Several website features (e.g., weekly tips and polls, personal profiles, a bulletin board for communication with other participants, links to reliable health and weight loss related information, and printable program materials and resources) are used to maintain participant interest. The WLM website is password protected and utilizes state of the art security measures.
At randomization, all IT participants receive a face-to-face orientation explaining the website. During this orientation, participants develop a personal action plan regarding weight, physical activity, and dietary goals using the interactive website. The website utilizes collaborative goal setting and problem-solving strategies to identify contingent action plans for perceived obstacles to success. In order to tailor the intervention to the needs of the participant, the action plan can be updated anytime and as frequently as desired by the individual. Participants are encouraged to input data on weight, food records, physical activity, and goals on a weekly basis. Participants are not restricted to logging on to the website only once a week. Rather, participants have the option of logging on to the website to enter data, communicate with other participants, or to seek other information, as frequently as they wish. Additional efforts at tailoring the intervention include: automated feedback in the form of weight and exercise graphs, personalized messages, and tailored progress summary reports. In addition to the face-to-face orientation at randomization, all IT participants will be invited to attend a group reorientation visit following the end of the 12-month data collection. The purpose of this visit will be to familiarize the participants with new features on the website, and reenergize their maintenance efforts.
Continued use of the website is maintained with automated email and telephone prompts. Participants who miss their scheduled log-in are sent an automated email reminding them to check-in on the website. If they do not log-in within a week, a second email message is sent. If they do not respond to this email after another week, they will receive an automated telephone prompt reminding them to check-in. A second automated call is made if there is no response within another week. If the participant does not respond to the second automated call they are contacted directly by a project staff member and encouraged to return to the website.
The core behavioral elements of the IT intervention are similar to those in the PC intervention (e.g., frequent contacts, self-monitoring, social support, problem-solving and relapse prevention). The self-monitoring module consists of a calendar-style data form. Participants are prompted to enter current weight, and food and fitness records, and increased use is encouraged during times of relapse. Participants are then prompted to choose their next check-in date. Email prompts are automatically sent to the participants on the next check-in date indicated by the participant in order to reinforce the use of the WLM website. Each email reminder includes a direct link to the website in order to simplify the data entry process.
A motivational module modifies the relapse prevention and problem-solving materials used in the PC intervention and adapts them for interactive use on the internet. Participants proceed through an interactive process of assessing the situation, identifying the problem, determining a strategy, and creating a plan. Social support and problem-solving are facilitated via a message board. Participants can post questions and communicate with other participants at all four clinical sites.
Summary of Interventions
The two active maintenance interventions (PC and IT) were chosen as two practical applications that could be readily translated and adopted in a variety of health care settings. These interventions investigate new innovations for improving weight loss maintenance by focusing on communication technologies: either through frequent personal contact, primarily through the use of telephone calls, or through the use of unlimited access to interactive technology using the internet and automated phone based technology. Each of the interventions is based on the same theoretical constructs, are individually tailored to the specific needs of the participant, and employ a toolbox of state-of-the-art behavioral strategies. Table 3 provides a description of the 30 month Phase II randomized interventions.
Table 3. Description of 30-Month Phase II Randomized Intervention Arms.
| Intervention Arm | Self Directed Usual Care | Personal Contact PC | Interactive Technology IT |
|---|---|---|---|
| Description and Approach | Advice and Information | Behavioral counseling using Motivational Interviewing, delivered through personal phone calls and in-person visits | Web-based, individually tailored behavioral intervention. supplemented with automated phone call reminders. |
| Frequency and Type of Contacts | |||
| Overall Frequency of Study-initiated Contacts | Annually | Monthly | Continuously available, encourage at least weekly contacts |
| Types of Contacts | |||
| Internet-Web site | 0 | 0 | Self-management and self-assessment modules and record-keeping tools. |
| 0 | 0 | Welcome messages and reminders to return to website, if needed | |
| VR Phone Calls | 0 | 0 | Reminders to return to website, if needed |
| Personal Phone Calls | 0 | 9/year* | Prompts to return to website, if needed |
| In-person Visits | ∼1/year | 3/year | 1 orientation at randomization; annual booster sessions |
| Contact flexibility | N/A | Scheduled personal | Unlimited electronic |
| Goals | |||
| Physical Activity | 225 min/wk moderate intensity activity | ||
| Total Calorie Intake | Reduced, individually tailored to maintain prior weight loss | ||
| Diet | DASH Dietary Pattern | ||
| Core Components | |||
| Self-Monitoring | None | F&F diaries, report during monthly contacts | F&F diaries with Web entry of weight, minutes of exercise, total daily calories and number of F&F diaries kept, at least weekly |
| Feedback | None | Quarterly reports, Motivational Interviewing and support at monthly contacts | Printable progress reports and graphs of self-reported data, tailored welcome messages, reminder prompts (e-mail and phone) based on website usage. |
| Problem solving | None | Tailored responses to problems identified at monthly contacts | Customizable action plans from interactive problem-solving, self-management modules. |
| Relapse prevention | None | Skills provided at monthly contacts | Customizable action plans from interactive relapse prevention, self-management modules. |
| Social Support | None | Phone | Bulletin board |
| Information on Diet and Physical Activity | Provided at annual visit | Available at each contact | Available on the Web site |
IVR = Interactive Voice Response Telephone
F&F = Food and Fitness Diary for monitoring diet, physical activity, and weight
15-minute calls
Study Measurements
Table 4 displays the types and schedule of measurements. The primary outcome variable for the trial is change in weight from baseline to end of follow-up. Weight is measured on two separate days at the following timepoints: entry, baseline, 12, and 30 months. Weight is measured once at clinic visits occurring at 6, 18, and 24 months after randomization. Participants are weighed in light, indoor clothes without shoes, using a high-quality digital scale. The scales are calibrated annually by the local Bureau of Weights and Standards, and quarterly by trained staff personnel using standard weights. All weight measurements are performed by study personnel who are trained and certified per the study protocol guidelines. The first entry weight taken at screening is used to compute BMI for eligibility into Phase I. Height is measured once at entry using a calibrated, wall-mounted stadiometer. The second entry weight serves as the initial weight for the Phase I weight loss eligibility. Both baseline weights must be at least 4 kg less than the second entry weight in order for the participant to be eligible for randomization into Phase II. The second baseline weight serves as the variable for analysis of post-randomization weight loss maintenance.
Table 4. Schedule of WLM Measurements.
| Entry Measures | Phase I Intervention | Baseline Measures | Follow-up Measures | |||||
| Phase I | Phase I | End of Phase I/Start of Phase II | Phase II (time since randomization) | |||||
| Screening | Phase I | Randomization | 6 mo | 12 mo | 18 mo | 24 mo | 30 mo | |
| Info consent | X | X1 | ||||||
| Demographics | X | |||||||
| Internet ques | X | |||||||
| Medical Hx ques | X | |||||||
| Height | X | |||||||
| Weight | X2 | X2 | X | X2 | X | X | X2 | |
| Rose angina ques | X | X1 | X | X | X | X | X | |
| Medication ques | X | X1 | X | X | ||||
| FFQ* | X | X1 | X | X | ||||
| Accelerometry | X | X1 | X | X | ||||
| Blood Pressure3 | X1 | X | X | |||||
| Fasting blood4,5 | X4 | X1 | X | X | ||||
| Adverse Event ques | X | X | X | X | X | X | ||
| Psychosocial ques | X | X1 | X | X | ||||
| Perceptions ques | X6 | |||||||
| Beliefs ques | X7 | X7 | ||||||
| Interv adherence** | X | X8 | ||||||
only collected for those eligible for Phase II. Weight and adverse event questionnaire are collected for everyone
weight is collected on two separate days at entry, baseline, 12 and 30 months
blood pressure is collected on two separate occasions for each measurement point
includes serum for analysis of glucose and lipids. Aliquots of plasma, serum, and buffy coat are collected for long-term storage
only analyzed on individuals who are subsequently randomized to Phase II
once, preferably during screening, but may be collected later if unable to obtain
once during screening, but can be completed up to 4 months after the participant has enrolled in Phase I
PC and IT participants
FFQ = Food Frequency Questionnaire
adherence measures including attendance, food and activity records
Secondary outcome measures include process measures (attendance and self-monitoring), measures of behavior change (total energy intake and minutes of physical activity), psychosocial variables (social support, quality of life, perceived stress), costs, and the control, prevalence, and medication treatment of CVD risk factors. Blood pressure is measured on two separate days at baseline, 12 and 30 month clinic visits. Blood pressure is measured twice using an ambulatory blood pressure monitor while the participant rests quietly in the seated position. Lipids and glucose are measured from plasma collected by venipuncture after an overnight fast at the following timepoints: entry, baseline, 12, and 30 months. Aliquots of plasma and serum are stored for future investigation of putative risk factors related to CVD and other chronic disease contingent upon future independent funding.
Eligibility and demographic data are collected during screening visits prior to entry into Phase I. Baseline data are collected at the end of Phase I for those individuals who qualify for entry to Phase II. Comprehensive follow-up data are collected at 12 and 30 months post-randomization. Additional weight and safety measurements are obtained at 6, 18, and 24 month clinic visits.
Analysis and Power
Analysis
The planned primary analysis is intent-to-treat (ITT) with the primary outcome variable being change in weight from baseline to month 30 (WR-F). Weight is measured six times: (1) at baseline/randomization (WR: which is defined by the second baseline weight), (2) at the end of follow-up after 30 months (WF), and (3) at 6-month intervals in between randomization and follow-up (6, 12, 18, 24 months). The primary aim of the trial is to compare the effectiveness of each of two active interventions (PC and IT) versus self-directed/usual care (SD) in maintaining weight loss from randomization to the end of Phase II maintenance. We use E to denote an entry measure (beginning of weight loss program, or Phase I), e.g. WE.
The full model for the primary hypothesis test is:
WR-F = β0 + β1PC + β2IT + β3WE-R + β4WE+ ΣαiSi + ΣδiEi + eij
For this model, PC is an indicator of the personal contact intervention, IT is an indicator of the web-based intervention, WE-R indicates the change in weight from Entry to Randomization, and WE is the entry weight. The Si terms are indicators of the performance sites, the Ei terms are planned additional explanatory variables, and eij is a random “error” term assumed to be normally distributed with a mean of zero.
The primary null hypothesis is: The PC and IT interventions do not differ from SD/UC in the change in weight over the 30 month maintenance period. This is a joint hypothesis with two parts: β1 = 0 and β2 = 0. Alternative hypotheses are: (1) The PC intervention differs from SD/UC in the amount of weight change over the 30 month maintenance period: β1 ≠ 0 (2-sided test), and (2) The IT intervention differs from SD/UC in the amount of weight change over the 30 month maintenance period: β1 ≠ 0 (2-sided test). It is hypothesized that both PC and IT will be different from SD/UC as a means to sustain weight loss. However, there is less certainty regarding the relative effects of PC and IT. The greater frequency of contact may benefit IT, while the personal contacts of PC may favor PC. Therefore, contrast between the PC and IT interventions is a secondary aim. A test of the null hypothesis that β2-β1 = 0 will be executed only if at least one of the previous two null hypotheses is rejected. Ultimately, the real life utility of both interventions will be a function of both efficacy and costs of implementation. Therefore, the study plan includes a cost analysis.
In order to preserve the experiment-wide Type I error rate at .05 for the primary outcome analysis, we will apply the Holm sequential testing procedure to the hypotheses β1 = 0 and β2 = 0 at overall level .05 (all two-sided tests) [76, 77]. Additional explanatory (Es) factors using the same model presented above include: age, female gender, African-American ethnicity, and gender × ethnicity interaction.
Power Considerations
The sample size for the trial (n=800 randomized participants) was designed to provide adequate power for the overall study comparisons, as well as for subgroup comparisons. Since randomization occurs after an initial 5 month weight loss phase, the sample size calculations are based on the number randomized into Phase II, rather the number who enter the trial initially. In order to randomize 800 participants, we anticipate that we require 1600 participants to enter Phase I. This estimate assumes a 10% attrition rate during the 5 month weight loss phase, that 35% of participants will complete Phase I but fail to lose at least the 4 kg of weight required for randomization, and 5% who are eligible but decline to be randomized. These estimates are based on the experience in the PREMIER study in which 6 month follow-up rates were 94%, and 50% of participants had lost 4 kg or more at the 6 month visit. With a sample size of 800 randomized participants, the study has 90% power to detect approximately a 2.0 kg difference in weight change between either of the active intervention groups (PC or IT) and the self-directed group overall. Additionally, there is 80% power to detect treatment differences of approximately 2.7 kg among African Americans participants.
Discussion
Obesity has become a major public health concern and is increasingly recognized as a national health priority particularly given the contribution of CVD that often accompanies it. Short-term weight loss success using behavioral strategies is well-documented in the literature. However, weight regain is disappointingly common. Given the disparity between short-term and long-term weight loss success, national guidelines have emphasized the need for maintenance of weight loss effects. Multi-component behavioral approaches have shown promise with maintenance efforts, however, continued intervention through frequent contacts appears to be crucial to success.
Although sustained weight loss is important for all persons who are overweight or obese, certain populations require more urgency. Individuals with CVD risk factors associated with obesity are most at risk for the consequences of overweight/obesity. Maintenance research on high-risk CVD (e.g., hypertension and/or dyslipidemia) overweight/obese populations has significant research and public health implications. Therefore, the benefits of the WLM trial could be substantial. The WLM trial carries with it several innovations to the existing research: (1) it denotes maintenance as the primary outcome rather than as a secondary endpoint, (2) it utilizes innovative behavioral strategies through two different communication approaches with frequent maintenance contacts, and (3) it targets high-risk populations with minimal existing data on maintenance (e.g., CVD risk and African Americans). Specifically, African-Americans are at a disproportionate risk for obesity [17], a greater prevalence and severity of obesity-related CVD risk factors [20, 78], and have obtained less benefit from existing weight loss and maintenance strategies [30, 79]. Therefore, the development of new strategies for maintaining weight loss which may potentially curtail obesity in African Americans is noteworthy.
There are several additional innovations of this trial that are notable. In addition to the frequency and mode of contact for the behavioral interventions, the interventions will take into account the dynamic processes often associated with behavior change. Data suggest that the tailoring of materials is critical to success with health behavior change [80-82]. The WLM interventions utilize a toolbox approach which permits individual tailoring of behavioral tools as needed. Furthermore, a nested study within this trial will examine the effect racial composition of groups in an intensive behavioral weight loss program has on weight loss, attendance at group sessions, improvement in dietary patterns and physical activity.
Additionally, there were notable “lessons learned” from this trial that may prove beneficial to other studies utilizing innovative strategies (e.g., overcoming rapid start-up, use of coordinated cohorts across all sites). However, the most striking lesson has been the importance of extensive screening for computer ability when utilizing a web-based program. In this trial, each clinical site screened for participant access to the internet and self-reported proficiency of computer use. Overall screening procedures worked well, however, each site encountered a few participants who embellished their computer proficiency and/or access to a computer with internet. This serves as a poignant reminder that screening procedures are critical to the success of a clinical trial, and that motivation to participate in a state-of-the-art weight maintenance program is high among the overweight population.
Conclusion
In order to win the battle against obesity, clinicians and health care systems must have effective options which are feasible, cost-effective, and easily disseminated to large numbers of individuals. Therefore, the purpose of this study is to exam the efficacy of two maintenance interventions which should meet the need for rapid translation into the public health and medical domains, as well as complement continuing efforts to stem the obesity epidemic and prevent obesity-related CVD.
References
- 1.National Institutes of Health. NHLBI clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults, executive summary. U.S. Department of Health and Human Services; 1998. [Google Scholar]
- 2.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999-2000. JAMA. 2002;288(14):1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 3.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the Third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 4.Seidel JC, Visscher TLS, Hoogeveen RT. Overweight and obesity in the mortality rate data: current evidence and research issues. Med Sci Sports Exerc. 1999;31(suppl):S597–S601. doi: 10.1097/00005768-199911001-00018. [DOI] [PubMed] [Google Scholar]
- 5.Allison DB, Fontaine KR, Manson JE, Stevens J, Van Itallie TB. Annual deaths attributable to obesity in the United States. JAMA. 1999;282:1530–1538. doi: 10.1001/jama.282.16.1530. [DOI] [PubMed] [Google Scholar]
- 6.Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. Years of life lost due to obesity. JAMA. 2003;289:187–193. doi: 10.1001/jama.289.2.187. [DOI] [PubMed] [Google Scholar]
- 7.Olshansky SJ, Passaro DJ, Hershow RC, Layden J, Carnes BA, Brody J, Hayflick L, Butler RN, Allison DB, Ludwig DS. A Potential Decline in Life Expectancy in the United States in the 21st Century. New Engl J Med. 2005;352:1138–1145. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- 8.Kannel WB, D'Agostino RB, Cobb JL. Effect of weight on cardiovascular disease. Am J Clin Nutr. 1996;63(3 Suppl):419S–422S. doi: 10.1093/ajcn/87.6.1602. [DOI] [PubMed] [Google Scholar]
- 9.Pi-Sunyer FX. Medical hazards of obesity. Ann Intern Med. 1993;119(7 Pt 2):655–660. doi: 10.7326/0003-4819-119-7_part_2-199310011-00006. [DOI] [PubMed] [Google Scholar]
- 10.King AC, Haskell WL, Young DR, Oka RK, Stefanick ML. Long-term effects of varying intensities and formats of physical activity on participation rates, fitness, and lipoproteins in men and women aged 50 to 65 years. Circulation. 1995;91(10):2596–2604. doi: 10.1161/01.cir.91.10.2596. [DOI] [PubMed] [Google Scholar]
- 11.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282(16):1523–1529. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 12.Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med. 1995;122:481–486. doi: 10.7326/0003-4819-122-7-199504010-00001. [DOI] [PubMed] [Google Scholar]
- 13.Dickey RA, Janick JJ. Lifestyle modifications in the prevention and treatment of hypertension. Endocr Pract. 2001;7:392–399. doi: 10.4158/EP.7.5.392. [DOI] [PubMed] [Google Scholar]
- 14.Dubbert PM, Carithers T, Sumner AE, et al. Obesity, physical inactivity, and risk for cardiovascular disease. Am J Med Sci. 2002;324:116–126. doi: 10.1097/00000441-200209000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Mann JI. Diet and risk of coronary heart disease and type 2 diabetes. Lancet. 2002;360(9335):783–789. doi: 10.1016/s0140-6736(02)09901-4. [DOI] [PubMed] [Google Scholar]
- 16.Visscher TLS, Seidell JC. The public health impact of obesity. Annu Rev Public Health. 2001;22:355–375. doi: 10.1146/annurev.publhealth.22.1.355. [DOI] [PubMed] [Google Scholar]
- 17.Mokdad AH, Serdula MK, Dietz WH, Bowman BA, Marks JS, Koplan JP. The spread of the obesity epidemic in the United States, 1991-1998. JAMA. 1999;282(16):1519–1522. doi: 10.1001/jama.282.16.1519. [DOI] [PubMed] [Google Scholar]
- 18.Wing RR. Obesity and related eating and exercise behaviors in women. Ann Behav Med. 1993;15:124–134. [Google Scholar]
- 19.Garrison RJ, Kannel WB, Stokes J, III, Castelli WP. Incidence and precursors of hypertension in young adults: the Framingham Offspring Study. Prev Med. 1987;16(2):235–251. doi: 10.1016/0091-7435(87)90087-9. [DOI] [PubMed] [Google Scholar]
- 20.Thompson D, Brown JB, Nichols GA, Elmer PJ, Oster G. Body mass index and future health-care costs: A retrospective cohort study. Obes Res. 2001:210–218. doi: 10.1038/oby.2001.23. [DOI] [PubMed] [Google Scholar]
- 21.Allison DB, Zannolli R, Narayan KM. The direct health care costs of obesity in the United States. Am J Public Health. 1999;89(8):1194–1199. doi: 10.2105/ajph.89.8.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Musich SA, Lu C, McDonals T, Champagne LJ, Edington DW. Association of additional health risks on medical charges and prevalence of diabetes within body mass index categories. Am J Health Promot. 2004;18(3):264–268. doi: 10.4278/0890-1171-18.3.264. [DOI] [PubMed] [Google Scholar]
- 23.Finkelstein EA, Fiebelkorn IC, Wang G. State-level estimates of annual medical expenditures attributable to obesity. Obes Res. 2004;12(1):18–24. doi: 10.1038/oby.2004.4. [DOI] [PubMed] [Google Scholar]
- 24.Kuczmarski RJ, Flegal KM, Campbell SM, Johnson CL. Increasing prevalence of overweight among US adults. The National Health and Nutrition Examination Surveys, 1960 to 1991. JAMA. 1994;272(3):205–211. doi: 10.1001/jama.272.3.205. [DOI] [PubMed] [Google Scholar]
- 25.Elmer PJ, Grimm R, Jr, Laing B, Grandits G, Svendsen K, Van Heel N, et al. Lifestyle intervention: results of the Treatment of Mild Hypertension Study (TOMHS) Prev Med. 1995;24(4):378–388. doi: 10.1006/pmed.1995.1062. [DOI] [PubMed] [Google Scholar]
- 26.Whelton PK, Appel LJ, Espeland MA, Applegate WB, Ettinger WH, Jr, Kostis JB, et al. Sodium reduction and weight loss in the treatment of hypertension in older persons: a randomized controlled trial of nonpharmacologic interventions in the elderly (TONE). TONE Collaborative Research Group. JAMA. 1998;279(11):839–846. doi: 10.1001/jama.279.11.839. published erratum appears in JAMA 1998 Jun 24;279(24):1954. [DOI] [PubMed] [Google Scholar]
- 27.The Trials of Hypertension Prevention Collaborative Research Group. Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in overweight people with high-normal blood pressure. The Trials of Hypertension Prevention, phase II. Arch Intern Med. 1997;157(6):657–667. [PubMed] [Google Scholar]
- 28.TOHP Research Group. The effects of nonpharmacologic interventions on blood pressure of persons with high normal levels. Results of the Trials of Hypertension Prevention, Phase I. JAMA. 1992;267(9):1213–1220. doi: 10.1001/jama.1992.03480090061028. published erratum appears in JAMA 1992 May 6;267(17):2330. [DOI] [PubMed] [Google Scholar]
- 29.Wood PD, Stefanick ML, Dreon DM, Frey-Hewitt B, Garay SC, Williams PT, et al. Changes in plasma lipids and lipoproteins in overweight men during weight loss through dieting as compared with exercise. N Engl J Med. 1988;319(18):1173–1179. doi: 10.1056/NEJM198811033191801. [DOI] [PubMed] [Google Scholar]
- 30.Stevens VJ, Obarzanek E, Cook NR, Lee IM, Appel LJ, Smith D, et al. Long-term weight loss and changes in blood pressure: Results of the trials of hypertension prevention, phase II. Ann Intern Med. 2001;(134):1–11. doi: 10.7326/0003-4819-134-1-200101020-00007. [DOI] [PubMed] [Google Scholar]
- 31.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 32.Hogan JW, Roy J, Korkontzelou C. Handling drop-out in longitudinal studies. Stat Med. 2004;23(9):1455–1497. doi: 10.1002/sim.1728. [DOI] [PubMed] [Google Scholar]
- 33.National Institutes of Health. The fifth report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure (JNC V) Arch Intern Med. 1993;153(2):154–183. [PubMed] [Google Scholar]
- 34.National Institutes of Health. The sixth report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Arch Intern Med. 1997;157(21):2413–2446. doi: 10.1001/archinte.157.21.2413. published erratum appears in Arch Intern Med 1998 Mar 23;158(6):573. [DOI] [PubMed] [Google Scholar]
- 35.National Institutes of Health. NHLBI report of the conference on socioeconomic status and cardiovascular health and disease. U.S. Department of Health and Human Services; 1995. [Google Scholar]
- 36.Wing RR. Maintenance of behavior change in cardiorespiratory risk reduction: introduction to proceedings from the National Heart, Lung, and Blood Institute conference. Health Psychol. 2000;19(Suppl 34):3–4. [Google Scholar]
- 37.Orleans C. T. Promoting the maintenance of health behavior change: Recommendations for the next generation of research and practice Health Psychol. 2000;19:76–83. doi: 10.1037/0278-6133.19.suppl1.76. [DOI] [PubMed] [Google Scholar]
- 38.Marcus BH, Dubbert PM, Forsyth LH, McKenzie TL, Stone EJ, Dunn AL, et al. Physical activity behavior change: issues in adoption and maintenance. Health Psychol. 2000;19(1 Suppl):32–41. doi: 10.1037/0278-6133.19.suppl1.32. [DOI] [PubMed] [Google Scholar]
- 39.Writing Group for the Activity Counseling Trial Research Group. Effects of physical activity counseling in primary care: the Activity Counseling Trial: a randomized controlled trial. JAMA. 2001;286(6):677–687. doi: 10.1001/jama.286.6.677. [DOI] [PubMed] [Google Scholar]
- 40.National Institutes of Health. NHLBI strategic plan. U.S. Department of Health and Human Services; 2000. [Google Scholar]
- 41.U.S. Department of Health and Human Services. Physical activity and health: a report of the surgeon general. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; 1996. [Google Scholar]
- 42.NIH Consensus Development Panel on Physical Activity and Cardiovascular Health. Physical activity and cardiovascular health. JAMA. 1996;276(3):241–246. [PubMed] [Google Scholar]
- 43.Perri MG, Sears SF, Jr, Clark JE. Strategies for improving maintenance of weight loss. Toward a continuous care model of obesity management. Diabetes Care. 1993;16(1):200–209. doi: 10.2337/diacare.16.1.200. [DOI] [PubMed] [Google Scholar]
- 44.Rothman AJ. Toward a theory-based analysis of behavioral maintenance. Health Psychol. 2000;19(1 Suppl):64–69. doi: 10.1037/0278-6133.19.suppl1.64. [DOI] [PubMed] [Google Scholar]
- 45.Andersen RE, Wadden TA, Bartlett SJ, Zemel B, Verde TJ, Franckowiak SC. Effects of lifestyle activity vs structured aerobic exercise in obese women: a randomized trial. JAMA. 1999;281(4):335–340. doi: 10.1001/jama.281.4.335. [DOI] [PubMed] [Google Scholar]
- 46.Jeffery RW, Drewnowski A, Epstein LH, Stunkard AJ, Wilson GT, Wing RR, et al. Long-term maintenance of weight loss: current status. Health Psychol. 2000;19(1 Suppl):5–16. doi: 10.1037/0278-6133.19.suppl1.5. [DOI] [PubMed] [Google Scholar]
- 47.Pierce JP, Faerber S, Wright FA, Newman V, Flatt SW, Kealey S, et al. Feasibility of a randomized trial of a high-vegetable diet to prevent breast cancer recurrence. Nutr Cancer. 1997;28(3):282–288. doi: 10.1080/01635589709514589. [DOI] [PubMed] [Google Scholar]
- 48.Perri MG, Nezu AM, Patti ET, McCann KL. Effect of length of treatment on weight loss. J Consult Clin Psychol. 1989;57(3):450–452. [PubMed] [Google Scholar]
- 49.Wadden TA, Foster GD, Wang J, Pierson RN, Yang MU, Moreland K, et al. Clinical correlates of short- and long-term weight loss. Am J Clin Nutr. 1992;56(1 Suppl):271S–274S. doi: 10.1093/ajcn/56.1.271S. [DOI] [PubMed] [Google Scholar]
- 50.Berkel LA, Carlos Poston WS, Reeves RS, Foreyt JP. Behavioral interventions of obesity. J Am Diet Assoc. 2005;105(5 Pt 2):35–43. doi: 10.1016/j.jada.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 51.King AC, Frey-Hewitt B, Dreon DM, Wood PD. The effects of minimal intervention strategies on long-term outcomes in men. Arch Intern Med. 1989;149:2741–2746. doi: 10.1001/archinte.149.12.2741. [DOI] [PubMed] [Google Scholar]
- 52.Wing RR, Jeffery RW, Hellerstedt WL, Burton L. Effect of frequent phone contacts and optional food provision on maintenance of weight loss. Annals Behav Med. 1996;18:172–176. doi: 10.1007/BF02883394. [DOI] [PubMed] [Google Scholar]
- 53.Meneghini LF, Albisser AM, Goldberg RB, Mintz DH. An electronic case manager for diabetes control. Diabetes Care. 1998;21(4):591–596. doi: 10.2337/diacare.21.4.591. [DOI] [PubMed] [Google Scholar]
- 54.U.S. Department of Health and Human Services. Science Panel on Interactive Communication and Health. In: Eng TR, Gustafson DH, editors. Wired for Health and Well-Being: the Emergence of Interactive Health Communication. Washington, DC: U.S. Government Printing Office; 1999. pp. i–41. [Google Scholar]
- 55.Revere D, Dunbar PJ. Review of Computer-generated Outpatient Health Behavior Interventions: Clinical Encounters “in Absentia”. J Am Med Inform Assoc. 2001;8(1):62–79. doi: 10.1136/jamia.2001.0080062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jimison H, Adler L, Coye M, Mulley A, Jr, Eng TR. Health care providers and purchasers and evaluation of interactive health communication applications. Science Panel on Interactive Communication and Health. Am J Prev Med. 1999;16(1):16–22. doi: 10.1016/s0749-3797(98)00105-6. [DOI] [PubMed] [Google Scholar]
- 57.Harvey-Berino J, Pintauro S, Buzzell P, Gold EC. Effect of internet support on long-term maintenance of weight loss. Obes Res. 2004;12(2):320–329. doi: 10.1038/oby.2004.40. [DOI] [PubMed] [Google Scholar]
- 58.Harvey-Berino J, Pintauro SJ, Gold EC. The feasibility of using Internet support for the maintenance of weight loss. Behav Modif. 2002;26(1):103–116. doi: 10.1177/0145445502026001006. [DOI] [PubMed] [Google Scholar]
- 59.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among U.S. children, adolescents, and adults, 1999-2002. JAMA. 2004;291:2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 60.Kumanyika SK. Obesity treatment in minorities. In: Wadden TA, Stunkard AJ, editors. Handbook of obesity treatment. Vol. 2002. New York, NY: Guilford Press; 2002. pp. 416–446. [Google Scholar]
- 61.Bandura A. Social foundations of thought and action: A social cognitive theory. Englewood Cliffs, NJ: Prentice Hall; 1986. [Google Scholar]
- 62.Watson DL, Tharp RG. Self-directed behavior: Self-modification for personal adjustment. 7th. Belmont, CA: Brooks/Cole; 1997. [Google Scholar]
- 63.Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol. 1983;51(3):390–395. doi: 10.1037//0022-006x.51.3.390. [DOI] [PubMed] [Google Scholar]
- 64.Bock BC, Marcus BH, Pinto BM, Forsyth LH. Maintenance of physical activity following an individualized motivationally tailored intervention. Ann Behav Med. 2001;23(2):79–87. doi: 10.1207/S15324796ABM2302_2. [DOI] [PubMed] [Google Scholar]
- 65.Rollnick S, Mason P, Butler C. Health Behavior Change: A Guide for Practitioners. London: Churchill Livingston; 1999. [Google Scholar]
- 66.Miller WR, Rollnick S. Motivational Interviewing: Preparing People to Change Addictive Behavior. New York, NY: The Guilford Press; 1991. [Google Scholar]
- 67.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336(16):1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 68.Working Group on Primary Prevention of Hypertension. National High Blood Pressure Education Program: Working Group Report on Primary Prevention of Hypertension. 1993 NIH Publication No. 93-2669. [PubMed] [Google Scholar]
- 69.Dattilo AM, Kris-Etherton PM. Effects of weight reduction on blood lipids and lipoproteins: a meta-analysis. Am J Clin Nutr. 1992;56:320–328. doi: 10.1093/ajcn/56.2.320. [DOI] [PubMed] [Google Scholar]
- 70.Harris J, Benedict F. A biometric study of basal metabolism in man. Carnegie Institution; Washington, DC: 1919. Publication #279. [Google Scholar]
- 71.Klem ML, Wing RR, McGuire MT, Seagle HM, Hill JO. A descriptive study of individuals successful at long-term maintenance of substantial weight loss. Am J Clin Nutr. 1997;66(2):239–246. doi: 10.1093/ajcn/66.2.239. [DOI] [PubMed] [Google Scholar]
- 72.McBride CM, Rimer BK. Using the telephone to improve health behavior and health service delivery. Patient Educ Couns. 1999;37(1):3–18. doi: 10.1016/s0738-3991(98)00098-6. [DOI] [PubMed] [Google Scholar]
- 73.Wadden TA, Butryn ML, Byrne KJ. Efficacy of lifestyle modification for long-term weight control. Obes Res. 2004;12(32 Suppl):151S–162S. doi: 10.1038/oby.2004.282. [DOI] [PubMed] [Google Scholar]
- 74.Wagner EH, Glasgow RE, Davis C, Bonomi AE, Provost L, McCulloch D, et al. Quality improvement in chronic illness care: a collaborative approach. J Comm J Qual Improv. 2001;27(2):63–80. doi: 10.1016/s1070-3241(01)27007-2. [DOI] [PubMed] [Google Scholar]
- 75.Medicare medical nutrition therapy amendment act of 2001. 2001 Jun 7; HR 2117. [Google Scholar]
- 76.Aickin M, Gensler H. Adjusting for multiple testing when reporting research results: the Bonferroni vs Holm methods. Am J Public Health. 1996;86(5):726–728. doi: 10.2105/ajph.86.5.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Proschan MA, Waclawiw MA. Practical guidelines for multiplicity adjustment in clinical trials. Control Clin Trials. 2000;21(6):527–539. doi: 10.1016/s0197-2456(00)00106-9. [DOI] [PubMed] [Google Scholar]
- 78.Cooper RS. Health and the social status of blacks in the United States. Ann Epidemiol. 1993;3(2):137–144. doi: 10.1016/1047-2797(93)90126-o. [DOI] [PubMed] [Google Scholar]
- 79.Kumanyika SK, Obarzanek E, Stevens VJ, Hebert PR, Whelton PK. Weight-loss experience of black and white participants in NHLBI-sponsored clinical trials. Am J Clin Nutr. 1991;53(6 Suppl):1631S–1638S. doi: 10.1093/ajcn/53.6.1631S. [DOI] [PubMed] [Google Scholar]
- 80.Bouchard C, Shephard R, Stephens T. Consensus statement. In: Bouchard C, Shephard R, Stephens T, editors. Physical activity, fitness, and health: International proceedings and consensus statement. Champaign, IL: Human Kinetics Books; 1994. pp. 9–76. [Google Scholar]
- 81.DeBourdeaudhuij I, Brug J. Tailoring dietary feedback to reduce fat intake: an intervention at the family level. Health Educ Res. 2000;15(4):449–462. doi: 10.1093/her/15.4.449. [DOI] [PubMed] [Google Scholar]
- 82.Campbell MK, DeVellis BM, Strecher VJ, Ammerman AS, DeVellis RF, Sandler RS. Improving dietary behavior: the effectiveness of tailored messages in primary care settings. Am J Public Health. 1994;84(5):783–787. doi: 10.2105/ajph.84.5.783. [DOI] [PMC free article] [PubMed] [Google Scholar]