Abstract
Objective
The purpose of the present study is to test the hypothesis that stellate ganglia ablation can reduce the incidences of atrial arrhythmias in a canine model of pacing-induced heart failure (HF).
Background
There is an association between autonomic nerve discharges and atrial arrhythmias (including both bradycardia and tachycardia) in ambulatory dogs with pacing-induced HF.
Methods
We performed cryoablation of the caudal half of left and right stellate ganglia and T2–4 thoracic sympathetic ganglia in 6 dogs (Experimental Group). We then continuously recorded left upper stellate ganglia nerve activity (SGNA), vagal nerve activity (VNA) and electrocardiogram using an implanted radiotransmitter.
Results
After two weeks of baseline recording, rapid right ventricular pacing (28 ± 4 days) was used to induce HF. Control Group (N=6) underwent the same procedures except for cryoablation. The Experimental Group had no paroxysmal atrial tachyarrhythmia (PAT) episodes (P<0.0001 compared with Control). Cryoablation significantly (p=0.0097) reduced prolonged (> 3 s) sinus pause (PSP) episodes from 5±6 to 0 in day 1, from 250 ± 424 to 11±11 in day 7 and from 123 ± 206 to 30±33 in day 14 after induction of HF. In experimental group only, VNA may occur alone without concomitant SGNA. However, these isolated VNA episodes did not result in PSP. Histological studies confirmed successful cryoablation of the caudal half of the stellate ganglia.
Conclusions
We conclude that cryoablation of bilateral stellate and T2–T4 thoracic ganglia significantly reduced PAT and PSP episodes induced by sympathetic discharges in dogs with pacing-induced HF.
Heart failure (HF) is associated with significant electrophysiological remodeling and increased incidence of cardiac arrhythmia, including both atrial and ventricular tachyarrhythmias.1 In addition, HF is also associated with a high incidence of atrial fibrillation (AF)2 and significant sinus node dysfunction.3 Because AF and HF both directly induce sinus node dysfunction,4,5 patients with HF and AF may be particularly prone to sick sinus (tachybrady) syndrome, manifested by intermittent tachycardia followed by bradycardia.3 We6 previously reported that simultaneous sympathovagal discharges preceded the onset of atrial tachyarrhythmias in a canine model of pacing-induced HF. In that study, we also documented frequent prolonged sinus pause (PSP) episodes after, but not before, the induction of HF. Over half of these PSP episodes were preceded by bursts of sympathetic (but not vagal) activation and tachycardia. These findings suggested that in addition to being a model of tachyarrhythmias, this canine model of pacing-induced HF is also a model of tachybrady syndrome. Sympathetic (rather than vagal) discharges appear to be a major cause of the tachybrady episodes. Because stellate ganglion discharges precedes the onset of tachybrady syndrome, stellate ganglion ablation may be a novel approach to controlling these arrhythmias. The purpose of the present study was to perform cryoablation of bilateral stellate ganglia and thoracic ganglia T2–T4 to test the hypothesis that reduction of cardiac sympathetic outflow can effectively eliminate both paroxysmal atrial tachyarrhythmia (PAT) and the tachybrady episodes in dogs with pacing-induced HF.
Methods
The experimental protocols were performed with approval of the Institutional Animal Care and Use Committee of the Cedars-Sinai Medical Center, Los Angeles. The control group had been previously published.6 The experimental group underwent the same surgical procedures except for cryoablation of the stellate ganglia as described below. The conclusions were supported by the comparison between the results of these two groups.
Surgical preparations of the Experimental Group
A right thoracotomy was performed under isoflurane general anesthesia. We performed cryoablation of the caudal half of the right stellate ganglion (RSG) and the T2–T4 thoracic sympathetic ganglia 7 with a 7-cm SurgiFrost® probe (CryoCath Technologies Inc., Montreal). We then implanted a Data Sciences International (DSI) D70EEE transmitter to record from unablated (upper) half of the LSG, the left vagal nerve above the aortic arch and subcutaneous ECG.6 A pacing lead was implanted in the right ventricular (RV) apex and connected to a Medtronic Itrel neurostimulator for chronic rapid ventricular pacing.6 After two weeks of continuous (24 hr/day, 7 days/wk) baseline ambulatory monitoring, the RV was paced at 150 bpm for 3 days, at 200 bpm for 3 days and then at 250 bpm for 3 weeks to induce HF. The pacemaker was then turned off to allow an additional two weeks of ambulatory monitoring. Echocardiograms were performed before and after rapid pacing. NT-proBNP levels were measured by Veterinary Diagnostics Institute, Irvine, California. The plasma norepinephrine levels were measured by ELISA kits from Rocky Mountain Diagnostics (Colorado Springs, Colorado). The dogs were euthanized at the end of 2 week monitoring period.
Data Analyses
Two types of methods were used to analyze nerve recordings. The first one was manual review. Long episodes of paroxysmal atrial tachycardia (PAT) was identified when there was an abrupt (>50 bpm/s) increase in the atrial rate to > 160 bpm and persisted for at least 10 s. We also identified premature atrial contractions (PACs) and prolonged (>3 s) sinus pauses (PSP) by manual analyses.6 The PSP episodes were identified in one baseline day (the day before the commencement of pacing) and in days 1, 7 and 14 after cessation of rapid pacing. High amplitude spike discharge activities (HASDA) is identified manually. HASDA was characterized by high amplitude spikes with relatively regular intervals. Frequency, number of spikes and amplitude of HASDA were manually counted by the investigator. A second method of analysis was to use a custom-designed software. The activation cycle lengths (RR intervals) were automatically determined. The nerve activity was high-pass (125 Hz) filtered, rectified and summed over fixed time segments to represent the total integrated nerve activity.6
Statistical Analyses
We compared the results obtained in the present study (Experimental Group) with a historical Control Group of 6 dogs that did not have cryoablation.6 All dogs in the Control Group underwent the same procedure, received the same hardware implantation and underwent the same pacing protocol to induce HF as the dogs in the present study. The only difference is that the present 6 dogs also received bilateral stellate ganglia cryoablation. In addition, the control dogs underwent right heart catheterization immediately before euthanasia. Data were expressed as mean ± standard deviation (SD). In data sets with large SD (PSP episodes and plasma norepinephrine levels), we performed log transform before statistical comparisons. Values between two groups were compared with student's two tailed t-tests whereas those of multiple groups were compared using analyses of variance (ANOVA), followed by Newman-Keuls post-hoc analysis. Multivariate repeated measure ANOVA was used to determine the effects of HF duration and the cryoablation on the occurrence of PSP. Cosinor tests were used to determine if there were significant circadian variations. A p value of ≤ 0.05 was considered statistically significant.
Results
Recording Duration
All dogs survived the first surgery without significant complications. The dogs were followed up for a total of 63 ± 6 days. During this time interval, a mean of 28 ± 4 days were used for rapid pacing.
Evidence for Heart Failure
The echocardiographic parameters at baseline and during heart failure are summarized in Table 1. The Table shows comparable severity of left ventricular dysfunction between these two groups of animals during HF, although the Experimental Group had a higher baseline ejection fraction than the Control Group. In experimental group, the NT-proBNP levels (normal range ≤ 566 pmol/L) significantly increased immediately after termination of rapid pacing (3818.5±906.7 pmol/L, range 2857–5057 pmol/L) compared to baseline (401.7±237.9 pmol/L, range 166–683 pmol/L, p<0.01). The NT-proBNP levels then significantly decreased to 1265.07±863.4 pmol/L (range 273–2320 pmol/L, p<0.01) after two weeks of recovery. The norepinephrine concentration was 115±68 pg/ml at baseline, 367±391 pg/ml immediately after induction of HF and 206±155 pg/ml two weeks after induction of HF (P=NS). The NT-proBNP and norepinephrine levels in the control group also showed elevation at the end of pacing and reduction at day 14 of recovery.6 Invasive cardiac catheterization was performed in Control Group immediately prior to euthanasia. The pulmonary artery (PA) wedge pressure, systolic main PA pressure, diastolic main PA pressure, systolic right ventricle (RV) pressure, diastolic RV pressure and RA pressure were 8.7±4.8 mmHg, 19.0±4.9 mmHg, 9.7±2.0 mmHg, 20.3±5.6 mmHg, 5.5±2.6 mmHg and 3.7±1.4 mmHg, respectively. These data suggest a rapid resolution of heart failure 14 days after cessation of rapid pacing.
Table 1.
Echocardiographic measurements of the left ventricular function
| Baseline | Heart Failure | |||||
|---|---|---|---|---|---|---|
| Control | Experimental | P value | Control | Experimental | P value | |
| LVEDV (ml) | 41.5± 9.5 | 40.0±13.8 | NS | 67.8±11.2*** | 73.5±6.6*** | NS |
| LVESV (ml) | 17.5± 4.3 | 11.8±4.9 | 0.06 | 50.7±13.5* | 52.5±10.9* | NS |
| LVEF (%) | 57.8± 4.2 | 70.8±5.3 | 0.0008 | 26.2± 7.8* | 29.0±12.7** | NS |
| LVWT (cm) | 0.88± 0.14 | 0.83±0.07 | NS | 0.79±0.07 | 0.74±0.13 | NS |
LVEDV, left ventricle end diastolic volume; LVEF, left ventricle ejection fraction; LVESV, left ventricle end systolic volume; LVWT, left ventricle wall thickness.
P values between Control and Experimental groups are shown in the right column.
P<0.0001
P<0.001
P<0.01, compared with baseline in the same groups.
Prolonged Sinus Pauses
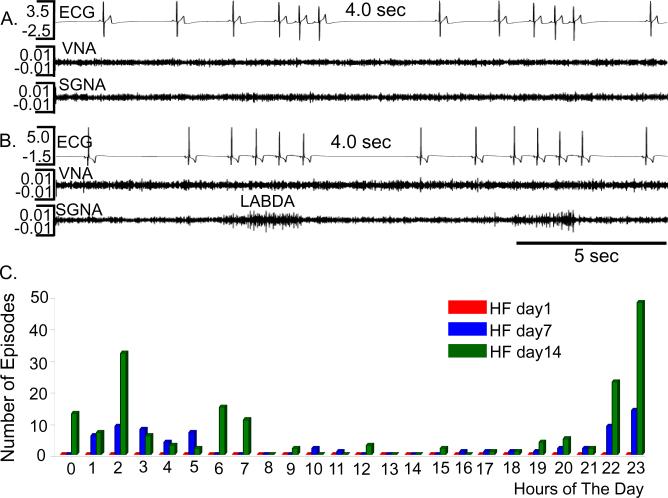
A major effect of cryoablation is a reduction of PSP episodes. The PSP episode per dog in Control Group and Experimental Group were (respectively) 5±6 and 0 episodes in day 1, 250 ± 424 to 11±11 in day 7 and 123 ± 206 to 30±33 in day 14 after cessation of rapid pacing that induced HF. Both time of HF (p=0.0118) and the cryoablation (p=0.0097) were significantly associated with PSP episodes. Among the PSP episodes in Experimental Group, 228 of the 248 (84.3%) occurred between 6 PM and 6 AM. In 63 PSP episodes, no obvious changes of SGNA or VNA were observed before and after PSP (Figure 1A). In the remaining 185 episodes (74.6%), the PSP occurred following short bursts of SGNA in the form of low amplitude burst discharge activity (LABDA) (Figure 1B), similar to that in the Control Group.6 Figure 1C shows the occurrence of PSP during a 24 hr period at HF day 1, 7 and 14.
Figure 1.
PSP episodes in experimental group. A and B show typical examples of PSP that occurred without and with, respectively, preceding SGNA. No VNA preceded any of the PSP episodes. C shows the 24-hr distribution of PSP episodes at different days after cessation of rapid pacing. There were no PSP episodes at day1.
Paroxysmal Atrial Tachycardia
A second major effect of cryoablation is the elimination of PAT episodes. In control group, we6 documented a total of 568 episodes of long (> 10 s) PAT (95±21 episodes/dog) at one baseline day. The number of long PAT episodes were 195 (33±25/dog), 283 (47±27/dog) and 374 (62±43/dog), respectively in days 1, 7 and 14 after the cessation of rapid pacing (p=0.0129 by ANOVA). However, in the present study, there were no PAT episodes either at baseline (p<0.0001) or during HF (p<0.0001).
Ventricular Arrhythmias
We6 have identified 35 episodes of premature ventricular contractions at baseline in the Control Group (5.8±14.3 episodes/dog/day) but none in the Experimental Group (0 /dog/day, p=NS). After the induction of HF, the number of premature ventricular contractions was 12±14 episodes/dog/day in the Control Group and 1±3/dog/day in the Experimental Group (p=NS). The coupling interval between premature contraction and preceding sinus beat in the Experimental group was significantly longer than that in the Control group during HF (327±47 ms vs. 277±43 ms, p<0.01).
Autonomic Nerve Activity and Heart Rate
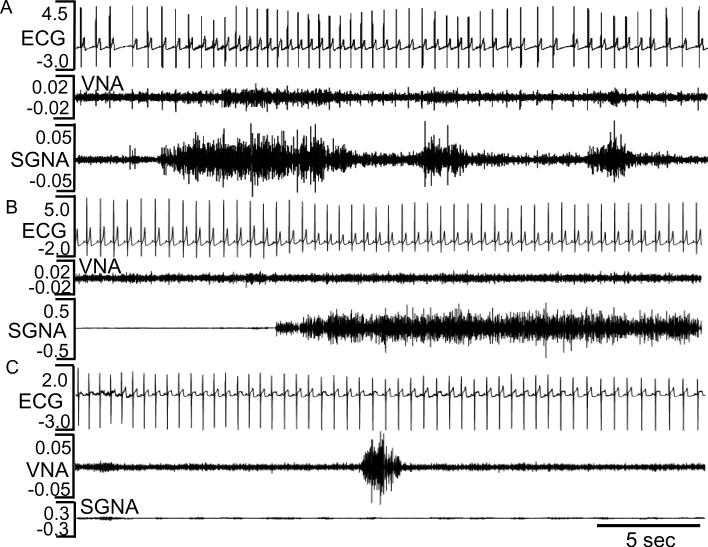
Table 2 shows the nerve activity and heart rate in control and experimental group. The HASDA values were manually analyzed. Cryoablation in the Experimental Group resulted in a reduction of HASDA frequency, number of spikes and the amplitude both before and after the induction of HF. We also calculated the integrated nerve activity by the following methods: The nerve activity as shown in Figure 2 shows signals both above and below 0 mV. We rectified the negative values to positive values. Because we sampled at 1000/s, there were 10,000 data points per 10 s. For this study, we chose to integrate the data over 10 s segments, resulting in a unit of mV-10s. Hourly averages of these values are shown in Figure 3. The 24-hr average is shown in Table 2.There is a significant reduction in integrated SGNA at baseline and HF day 1, and integrated VNA amplitude at baseline and during heart failure. In spite of bilateral stellate ganglia ablation, bursts of sympathetic discharges in the form of low amplitude burst discharge activity (LABDA) along with increased vagal nerve activity can occasionally induce heart rate acceleration (Figure 2 A). In most cases, however, SGNA (Figure 2B) or VNA (Figure 2C) failed to immediately change heart rates. The Experimental Group has a longer RR interval at baseline and again at day 14 of HF than the Control Group. The heart rate variability measured by standard deviation of RR intervals (SDRR) is increased at baseline in experimental group, consistent with reduced sympathetic output. However, due to a reduction of PSPs, the SDRR was lower in the experimental than control group at day 1 of HF (Table 2). The maximum daily heart rates (Experimental and Control) at baseline were 158±14 bpm and 219±13 bpm, respectively (p<0.0001) and were 160±7 bpm and 225±13 bpm, respectively (p<0.0001) at day 1, 159±6 bpm and 217±21 bpm, respectively (p<0.0001) at day 7 and 151±10 bpm and 225±13 bpm, respectively (p<0.0001) at day 14 after cessation of rapid pacing. The reduced maximum heart rate in the Experimental Group as compared with the Control Group is compatible with a reduction of sympathetic outflow due to successful stellate and thoracic ganglia ablation.
Table 2.
Patterns of Nerve Activity in Control and Experimental Groups
| Control Group | Experimental Group | P Value | |
|---|---|---|---|
| Number of HASDA at baseline | 4.3 ± 4.4 / hour | 1.6 ±1.5/hour | P=0.0072 |
| Number of HASDA at HF day 1 | 4.6 ± 4.7 / hour | 2.4 ± 2.0 / hour | P=0.04 |
| Spikes per HASDA at baseline | 6.7 ± 2.2 | 4.0 ± 1.0 | P<0.0001 |
| Spikes per HASDA at HF day 1 | 5.9 ± 1.7 | 3.9 ± 0.9 | P<0.0001 |
| Frequency of HASDA at baseline | 5.8 ± 0.7 (Hz) | 5.7 ± 1.0 (Hz) | P=0.92 |
| Frequency of HASDA at HF day 1 | 6.6 ± 0.6 (Hz) | 6.8 ± 0.8 (Hz) | P=0.17 |
| HASDA Amplitude at baseline | 0.6 ± 0.2 (mV) | 0.4 ±1.0 (mV) | P<0.0001 |
| HASDA Amplitude at HF day 1 | 1.4 ± 0.8 (mV) | 0.4 ±1.0 (mV) | P<0.0001 |
| Integrated SGNA at baseline | 5.9 ± 1.7 (mV-10s) | 3.1± 1.2 (mV-10s) | P<0.0001 |
| Integrated SGNA at HF day 1 | 7.8 ± 1.9 (mV-10s) | 5.2 ± 2.2 (mV-10s) | P<0.0001 |
| Integrated SGNA at HF day 14 | 6.1 ± 2.3 (mV-10s) | 5.5 ± 3.0 (mV-10s) | P=0.46 |
| Integrated VNA at baseline | 2.8 ± 0.4 (mV-10s) | 1.3 ± 0.1 (mV-10s) | P<0.0001 |
| Integrated VNA at HF day 1 | 3.5 ± 0.4 (mV-10s) | 1.3 ± 0.1 (mV-10s) | P<0.0001 |
| Integrated VNA at HF day14 | 3.4 ± 0.4 (mV-10s) | 1.3 ± 0.1 (mV-10s) | P<0.0001 |
| RR interval at baseline | 588 ± 74 (ms) | 745 ± 65 (ms) | P<0.0001 |
| RR interval at HF day 1 | 654 ± 83 (ms) | 685 ± 67 (ms) | P=0.21 |
| RR interval at HF day14 | 788 ± 137(ms) | 900 ± 93 (ms) | P=0.0018 |
| SDRR at baseline | 182 ± 62 (ms) | 213 ± 50 (ms) | P=0.06 |
| SDRR at HF day 1 | 172 ± 63 (ms) | 122 ± 45 (ms) | P=0.003 |
| SDRR at HF day 14 | 292 ± 106 (ms) | 314 ± 77 (ms) | P=0.41 |
HF, heart failure; SDRR, standard deviation of RR intervals;
Figure 2.
Autonomic nerve activity and heart rate after cryoablation. A shows modest increase of heart rate during SGNA and VNA activation at baseline. B and C show SGNA and VNA activities, respectively, one day after cessation of rapid pacing. There were no obvious changes of heart rates in response to the isolated SGNA and VNA.
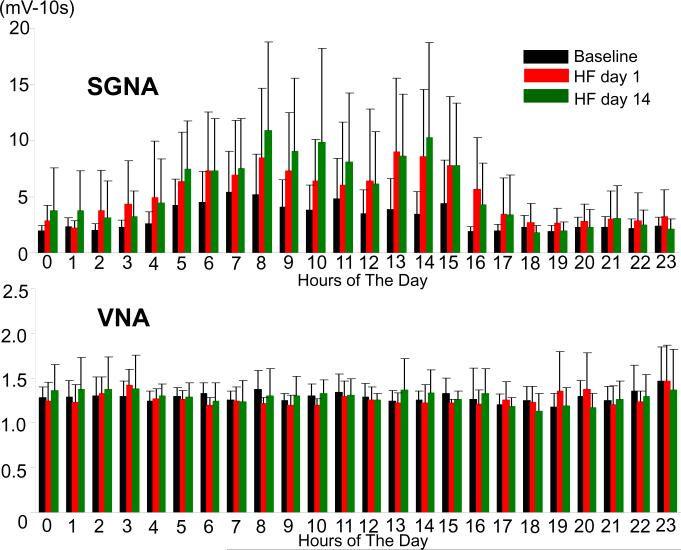
Figure 3.
The 24-hr average SGNA and VNA in dogs with cryoablation. Panel A shows a significant circadian variation of the 24-hour SGNA. SGNA at day1 and day 14 were both significantly higher than that at baseline. Panel B shows that VNA had no significant circadian variation either at baseline or during HF. There were no significant differences in VNA between baseline and day 1 or day 14 after cessation of rapid pacing.
Effects of Cryoablation on VNA
In the Control Group of dogs, we found no incidence of isolated VNA. All VNA activation occurred along with simultaneous sympathetic activations. However, in the Experimental Group only, we found intermittent isolated VNA, defined by the absence of SGNA within 2 s prior to VNA activation (Figure 2C). We manually analyzed isolated VNA at midnight (0 am to 4 am) in all dogs studied at baseline and again at 1, 7 and 14 days after cessation of rapid pacing. We found that there were 2.3±0.5, 4.5±0.5, 3.3±1.2 and 4.5±3.3 episodes of isolated VNA activity (P<0.0001 compared with no VNA activity at baseline). The isolated VNA was not associated with PSP episodes.
Circadian Variation
Figure 3A summarizes the SGNA of all dogs in Experimental Group. The average of 24-hour SGNA had significant circadian variation at baseline, day 1 and day 14 after cessation of rapid pacing (p<0.0001, p<0.01 and p<0.01, respectively). SGNA at day1 and day 14 were both significantly higher than that at baseline (p<0.01, respectively). Figure 3B summarizes VNA of all dogs studied. The average of 24-hr VNA had no significant circadian variation either at baseline or during HF. There were no significant differences in VNA between baseline and day 1 or day 14 after cessation of rapid pacing.
Effects of Cryoablation on High Amplitude Spike Discharge Activity
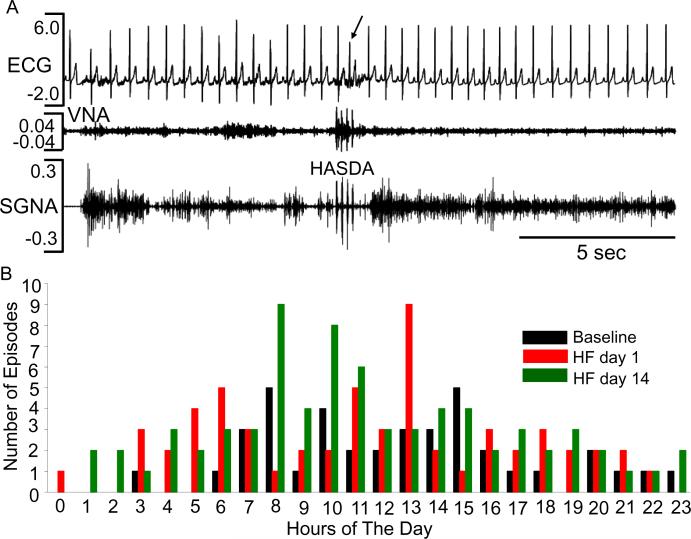
In the Experimental Group, we identified 170 runs of HASDA from the baseline day (n=39) and day1 (n=58) and day 14 (n=73) during HF (by manual analyses). Table 2 shows that the number of high amplitude spike discharge activity (HASDA), the spikes per HASDA episode and the HASDA amplitude are significantly reduced by cryoablation both during baseline and after induction of HF. HASDA episodes did not occur in isolation; rather, they occurred in association with increased SGNA. As in the Control Group, HASDA episodes may precede the occurrence of PAC (Figure 4A). Overall, 24 of 170 (14%) of HASDA induced PAC. Most HASDA episodes occurred in the day time (from 6 AM to 6 PM) at baseline (33/39) as well as during day 1 (41/58) and day 14 (54/73) (Figure 4B).
Figure 4.
HASDA after cryoablation. A shows a premature atrial contraction (arrow) followed by HASDA on both SGNA and VNA at day1 after cessation of rapid pacing in dog#2. B shows the 24-hr distribution of HASDA at baseline and during HF.
Histological Studies
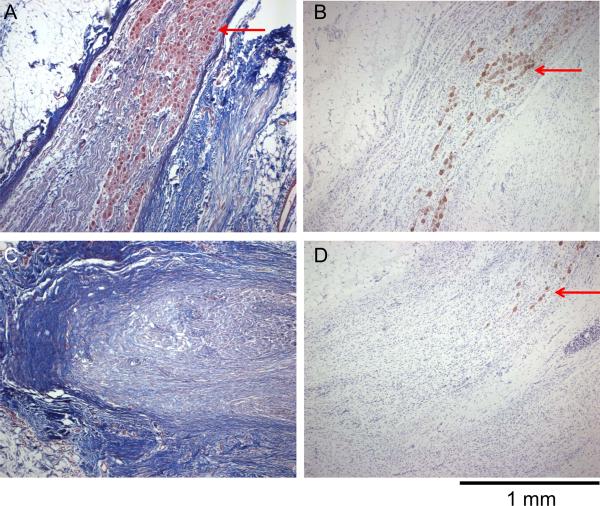
Successful cryoablation of the caudal portion of the stellate ganglia was documented by trichrome staining and tyrosine hydroxylase staining. The latter staining identifies sympathetic nerve structures. Figure 5 shows an example of a cryoablated stellate ganglion. The ablated tissue was replaced by dense scar tissue that stained blue. Arrows point to surviving ganglion cells in trichrome staining (Figures 5A and 5C) and in tyrosine hydroxylase staining (Figures 5B and 5D).
Figure 5.
Cryoablated left stellate ganglion stained with trichrome (A and C) and with tyrosine hydroxylase (B and D). A and B show the cranial (unablated) portion of the left stellate ganglion. The fibrotic tissues stained blue with trichrome (Panel A). There were surviving ganglion cells (arrows) in both Panels A and B. C and D show the caudal (ablated) portion of the stellate ganglion. There were dense fibrosis and a few surviving sympathetic ganglion cells (arrow) in D. Objective lens: 4X.
Discussion
Bilateral satellite ganglia ablation significantly reduced PAT and PSP episodes in dogs with HF. These findings confirm a causal relationship between stellate ganglion nerve activity and atrial arrhythmias (including both tachycardia and bradycardia) in a canine model of HF. A clinical implication of this study is that reducing sympathetic outflow from the stellate ganglia or blocking sympathetic receptors on the myocardium may be a novel approach to control not only the tachycardia but also the bradycardia associated with HF.
Heart Failure, AF and Sick Sinus Syndrome
Wang et al2 reported that the total proportion of HF subjects with AF at some time was 41%. Similarly, 42% of AF subjects had HF at some point during their lifetime. Because AF and HF can both independently lead to sinus node dysfunction,4,5 it is not surprising that there is an association between heart failure, AF and sick sinus syndrome.8 Among all patients who receive pacemaker implantation, approximately 6% had heart failure at implant. This percentage may go up to 50% among survivors during follow up. The canine model of pacing-induced HF partially reproduced both types of the atrial arrhythmia, although the incidence of tachybrady episodes are higher than that expected for patients with heart failure.6 A possible explanation of this discrepancy is that these dogs had untreated severe heart failure while most of the human patients have been actively managed with beta blockers and other medications. However, the severity of HF alone is not the only factor determining the incidence of PSP, as the peak incidence of PSP occurred 7 days (rather than 24 hrs) after cessation of rapid pacing. The latter findings suggest that the mechanisms of PSP in this model may be in part related to the rapid heterogeneous reverse remodeling after cessation of pacing in this model. In contrast, rapid recovery from HF is uncommon in humans. Multiple events occur during rapid recovery of pacing-induced HF, including increased immunostained tyrosine hydroxylase and norepinephrine profile in one week, adenylyl cyclase responses to agonists and norepinephrine uptake activity in two weeks and normalization of myocardial beta-adrenoceptor density in four weeks.9 In addition to heterogeneous recovery of innervation, significant changes of Ca handling proteins and membrane ion currents also occur during HF and these changes could undergo heterogeneous recovery after cessation of rapid pacing. Pogwizd et al10 proposed that changes in INaCaX and IK1, and residual beta adrenoceptor responsiveness conspire to greatly increase the propensity for triggered arrhythmias in HF. The same interaction could occur to promote tachybradycardia when there is rapid and uneven recovery of the neural and electrophysiological remodeling after cessation of rapid pacing. We propose that the presence of increased incidence of tachybrady episodes in pacing-induced HF might be a result of rapid and heterogeneous reverse remodeling after cessation of rapid pacing rather than due to a lack of applicability of pacing-induced HF to human patients.
Calcium Clock Malfunction and the Mechanisms of Tachybrady Syndrome
Recent studies showed that rhythmic spontaneous SR Ca release (Ca clock) work synergistically with membrane ionic currents (membrane clock) to generate sinoatrial node automaticity.11–13 Heart failure is associated with a reduction of intra-SR free Ca content 14 and a concomitant downregulation of pacemaker current.15,16 It is possible that short bursts of sympathetic discharges and the resultant tachycardia have aggravated the Ca handling abnormalities associated with HF, resulting in Ca clock malfunction and PSP upon tachycardia termination. Cryoablation of the LSG prevented the massive catecholamine surge induced by sympathetic nerve discharges, hence significantly reduced the incidences of both tachycardia and PSP episodes. The findings of the present study also suggest that the contractile dysfunction and the sinus node dysfunction share the same mechanism, i.e., abnormalities of Cai handling.
Vagal Nerve Activity and Tachybrady Syndrome
Our previous study6 showed that both sympathetic and vagal nerve activities are increased during HF. The VNA activity was often associated with bradycardia and increased heart rate variability. After cryoablation, an overall increase of VNA was no longer observed in HF. This negative finding suggests that the reduced sympathetic outflow indirectly reduced VNA in cryoablated animals. An additional interesting finding is that after cryoablation, VNA occasionally activated alone without preceding sympathetic inputs. Although VNAs were unopposed by sympathetic activation, PSP did not follow. Levy et al17,18 reported that the vagal response to sympathetic stimulation is potentiated during increased cardiac sympathetic activity (accentuated antagonism). It is possible that in addition to reduced VNA, reduced accentuated antagonism due to decreased sympathetic discharges also contributed to the elimination of PSP by cryoablation. A limitation of this latter hypothesis is that there were no VNAs before the PSP episodes.6 However, we only measured VNA in the extrinsic cardiac nervous system. The activity of ganglionated plexi, which contains both sympathetic and vagal nerves,19 were not recorded. Choi et al20 recently developed new techniques to directly record from the ganglionated plexi. We hope that these new methods will help us determine if vagal tone from the intrinsic cardiac nervous system played a role in PSPs.
Study Limitations
The definition of PAT in the present and a previous study6 included abrupt-onset , long duration and high heart rate. With this definition, cryoablation completely eliminated all PAT episodes in HF. However, because abrupt onset and 10 s duration was used to define PAT, the results of the study may not be applicable to all PAT episodes in HF. There are only infrequent episodes of premature ventricular contractions and ventricular tachycardia in dogs with pacing-induced HF (Control Group).6 Therefore, we were not able to test the hypothesis that cryoablation of stellate ganglion can significantly reduce the number of ventricular arrhythmias in this model. We did not perform invasive cardiac catheterization at different time points during recovery to measure the hemodynamic parameters. However, we have performed echocardiography to noninvasively assess LV function. As shown in Table 1, the severity of HF in Control and Experimental groups were similar. We propose that the reduced tachybrady episodes in the experimental group are better explained by the cryoablation of the stellate ganglia than by the differential severity of heart failure.
Acknowledgements
We thank Elaine Lebowitz, Avile McCullen, Lei Lin, Jian Tang and Stephanie Plummer for their assistance.
Funding source: This study was supported by a Japan Medtronic fellowship, International Research Fund for Subsidy of Kyushu University and International Research Funds for Subsidy of Fukuoka University School of Medicine Alumni, NIH Grants P01 HL78931, R01 HL78932, 58533, 71140, AHA Established Investigator Award, AHA Western Region Fellowship Award, Piansky family, Pauline and Harold Price and Medtronic-Zipes Endowments.
Relation to Industry: We also thank Xiaohong Zhou of Medtronic Inc. for providing the pacemaker and the CryoCath Technologies, Inc, for providing SurgiFrost® probe used in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Janse MJ. Electrophysiological changes in heart failure and their relationship to arrhythmogenesis. Cardiovasc Res. 2004;61:208–217. doi: 10.1016/j.cardiores.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 2.Wang TJ, Larson MG, Levy D, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107:2920–2925. doi: 10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- 3.Dobrzynski H, Boyett MR, Anderson RH. New insights into pacemaker activity: promoting understanding of sick sinus syndrome. Circulation. 2007;115:1921–1932. doi: 10.1161/CIRCULATIONAHA.106.616011. [DOI] [PubMed] [Google Scholar]
- 4.Elvan A, Wylie K, Zipes DP. Pacing-induced chronic atrial fibrillation impairs sinus node function in dogs--electrophysiological remodeling. Circulation. 1996;94:2953–2960. doi: 10.1161/01.cir.94.11.2953. [DOI] [PubMed] [Google Scholar]
- 5.Sanders P, Kistler PM, Morton JB, et al. Remodeling of sinus node function in patients with congestive heart failure: reduction in sinus node reserve. Circulation. 2004;110:897–903. doi: 10.1161/01.CIR.0000139336.69955.AB. [DOI] [PubMed] [Google Scholar]
- 6.Ogawa M, Zhou S, Tan AY, et al. Left stellate ganglion and vagal nerve activity and cardiac arrhythmias in ambulatory dogs with pacing-induced congestive heart failure. J Am Coll Cardiol. 2007;50:335–343. doi: 10.1016/j.jacc.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz PJ, Priori SG, Cerrone M, et al. Left cardiac sympathetic denervation in the management of high-risk patients affected by the long-QT syndrome. Circulation. 2004;109:1826–1833. doi: 10.1161/01.CIR.0000125523.14403.1E. [DOI] [PubMed] [Google Scholar]
- 8.Sutton R, Bourgeois I. Cost benefit analysis of single and dual chamber pacing for sick sinus syndrome and atrioventricular block. An economic sensitivity analysis of the literature. Eur Heart J. 1996;17:574–582. doi: 10.1093/oxfordjournals.eurheartj.a014911. [DOI] [PubMed] [Google Scholar]
- 9.Kawai H, Mohan A, Hagen J, et al. Alterations in cardiac adrenergic terminal function and beta-adrenoceptor density in pacing-induced heart failure. Am J Physiol Heart Circ Physiol. 2000;278:H1708–H1716. doi: 10.1152/ajpheart.2000.278.5.H1708. [DOI] [PubMed] [Google Scholar]
- 10.Pogwizd SM, Bers DM. Cellular basis of triggered arrhythmias in heart failure. Trends Cardiovasc Med. 2004;14:61–66. doi: 10.1016/j.tcm.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Lipsius SL, Huser J, Blatter LA. Intracellular Ca2+ release sparks atrial pacemaker activity. News Physiol Sci. 2001;16:101–106. doi: 10.1152/physiologyonline.2001.16.3.101. [DOI] [PubMed] [Google Scholar]
- 12.Maltsev VA, Vinogradova TM, Lakatta EG. The emergence of a general theory of the initiation and strength of the heartbeat. J Pharmacol Sci. 2006;100:338–369. doi: 10.1254/jphs.cr0060018. [DOI] [PubMed] [Google Scholar]
- 13.Joung B, Tang L, Maruyama M, et al. Intracellular calcium dynamics and the acceleration of sinus rhythm by {beta}-adrenergic stimulation. Circulation. 2009;119:788–796. doi: 10.1161/CIRCULATIONAHA.108.817379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo T, Ai X, Shannon TR, et al. Intra-sarcoplasmic reticulum free [Ca2+] and buffering in arrhythmogenic failing rabbit heart. Circ Res. 2007;101:802–810. doi: 10.1161/CIRCRESAHA.107.152140. [DOI] [PubMed] [Google Scholar]
- 15.Zicha S, Fernandez-Velasco M, Lonardo G, et al. Sinus node dysfunction and hyperpolarization-activated (HCN) channel subunit remodeling in a canine heart failure model. Cardiovasc Res. 2005;66:472–481. doi: 10.1016/j.cardiores.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 16.Verkerk AO, Wilders R, Coronel R, et al. Ionic remodeling of sinoatrial node cells by heart failure. Circulation. 2003;108:760–766. doi: 10.1161/01.CIR.0000083719.51661.B9. [DOI] [PubMed] [Google Scholar]
- 17.Levy MN, ngM, Martin P, et al. Sympathetic and parasympathetic interactions upon the left ventricle of the dog. Circ Res. 1966;19:5–10. [Google Scholar]
- 18.Levy MN, Zieske H. Effect of enhanced contractility on the left ventricular response to vagus nerve stimulation in dogs. Circ Res. 1969;24:303–311. doi: 10.1161/01.res.24.3.303. [DOI] [PubMed] [Google Scholar]
- 19.Tan AY, Li H, Wachsmann-Hogiu S, et al. Autonomic innervation and segmental muscular disconnections at the human pulmonary vein-atrial junction: implications for catheter ablation of atrial-pulmonary vein junction. J Am Coll Cardiol. 2006;48:132–143. doi: 10.1016/j.jacc.2006.02.054. [DOI] [PubMed] [Google Scholar]
- 20.Choi E-K, Han W-W, Joung B, et al. A novel method to measure intracardiac autonomic nerve activity at the ligament of Marshall. Heart Rhythm. 2009;6:S237. [Google Scholar]