1. Introduction to the Ladder Polyethers
1.1. Biological Importance
Red tides are massive and harmful algal blooms (HABs) that can wreak tremendous environmental destruction. They poison and devastate fish, mammal, bird, and other marine populations, crippling the fishing, tourism, and other industries that depend on their continued health. The scientific community continues to investigate both the long-term and acute causes of red tides and strives to predict when these disasters will strike.i Remarkable detective work over the last 30 years by both chemists and biologists has implicated members of the ladder polyether family of natural products as the poisons responsible for the ill effects of many of these red tides.ii Ladder polyether toxins have been identified off the coasts of regions as disparate as Tahiti, Japan, Vietnam, and New Caledonia, and they are a regular threat to the west coast of Florida.iii
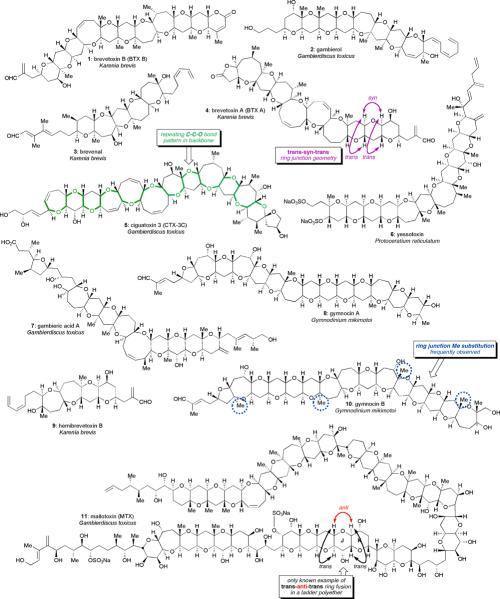
In addition to their importance as extraordinarily potent neurotoxins and agents of ecological destruction, ladder polyether natural products have long captivated organic chemists because of their fascinating structures; a few of the dozens of known compounds are shown in Figure 1.iv Because the toxicity of the larger members of the ladder polyether family arises from their very high affinity for binding to voltage-gated sodium, potassium, or calcium ion transport channel proteins, some of these natural products have proven useful as probes of protein structure and function, including the brevetoxins (1 and 4), the ciguatoxins (e.g., ciguatoxin 3C (5)), and gambierol (2).2,4,v
Figure 1.
Representative structures from the ladder polyether family of natural products.
Although they are believed to share a common polyketide origin,vi,vii there is considerable diversity among ladder polyether natural products. Their complexity derives in part from the wide variety of oxacyclic ring sizes encountered, from five- up to nine-membered cyclic ethers. Individual rings may be fused together into ladders in numbers as few as four (as in the compact hemibrevetoxin B (9, Figure 1)) or as many as 15 (as in gymnocin B (10)). The truly massive maitotoxin (11), which represents the single largest nonbiopolymeric structure from nature to be characterized to date (as well as one of the most toxic),viii contains some 32 rings in four separate ladders. Across the family of structures, rings are occasionally decorated with methyl or hydroxyl substituents, while larger rings can contain a cis-alkene. While most ring junctions are substituted with two hydrogens, many bear a methyl substituent in place of one of the hydrogens (see gymnocin B (10)).
Despite the wide geographic dispersion of these poisons, the considerable phylogenetic variety of the dinoflagellates that produce them, and the remarkable size and complexity of these compounds, they also show a surprising structural uniformity. All ladder polyethers share a conserved C-C-O bond motif that can be traced through the length of the ladder (see ciguatoxin 3C (5), Figure 1). Furthermore, the relative stereochemistry of the ladder ring junctions is almost invariably trans-syn-trans (see brevetoxin A (4), Figure 1), with the sole exception found in maitotoxin.6,ix
1.2. Total Synthesis of Ladder Polyethers
Due to their unique and fascinating structures, the ladder polyethers have attracted great attention from the synthetic community.4,x,xi The tremendous size and stereochemical complexity of the ladder polyethers have tested the limits of what organic chemistry can achieve. Their synthesis has invariably demanded monumental endeavors; witness the Nicolaou group's landmark total synthesis of brevetoxin B,xii which involves more than 100 individual synthetic operations. In the course of these total synthesis efforts, many new and powerful synthetic methodologies have been developed,xiii primarily for the construction of six membered-tetrahydropyran, seven memberedoxepane and oxepene, and eight membered-oxocane and oxocene rings, and for the controlled fusion of these rings in the appropriate trans-syn-trans geometry. As in all complex molecule synthesis, convergence has been emphasized, and, specifically, the prevailing paradigm has been that the convergent assembly of preformed ring systems is the most efficient route to the ladder polyethers. That is to say, the most common strategy is ring formation via cyclization or annulation steps that construct one or two rings at a time, followed by the piecing together of these ring systems, usually two to five rings at a time.
2. Biosynthesis and Biomimetic Synthesis of Ladder Polyethers
2.1. Nakanishi's Hypothesis and endo-Selective Epoxide-Opening Cascades
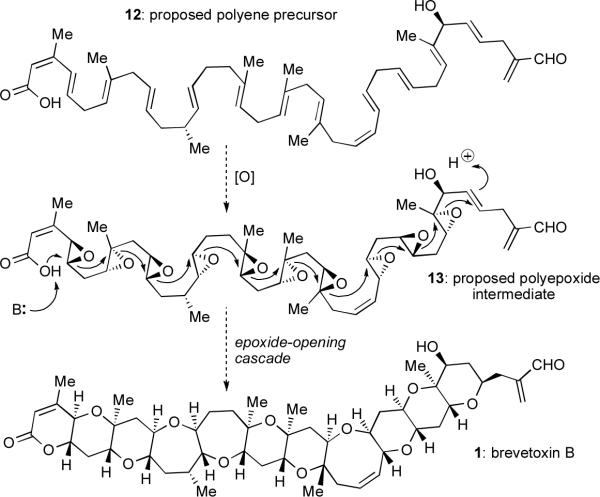
Despite its proven utility, the aforementioned synthetic strategy is conceptually quite distant from the proposed biosynthesis of the ladder polyethers. On the basis of carbon labeling and feeding experiments, Nakanishi and Shimizu suggested a polyketide origin of the carbon skeleton of these compounds,6 and later studies from Rein and coworkers have corroborated this hypothesis.7 Still, much remains unknown about their biosynthesis, including major questions like how oxygen is incorporated and how the cyclic ethers are formed. This is not to suggest, of course, that chemists have not speculated on these unsolved problems. With Occam's razor in hand, Nakanishi dissected the unique structural regularity of the ladder polyethers and made an educated guess about the final steps of their biogenesis. His proposal has become known as the Nakanishi hypothesis (although similar prospective biosyntheses were advanced contemporaneously by Shimizuxiv and Nicolaou4,12). The hypothesis remains highly appealing in its elegance and concision despite the rather limited experimental evidence in its favor.
In this hypothesis, the distinctive repeating C-C-O pattern and trans-syn-trans ring junction stereochemistry of brevetoxin B are neatly explained by a domino or cascade reaction of multiple epoxide openings (Scheme 1). If each epoxide opening in such a cascade proceeded stereospecifically, with clean inversion of stereochemistry, then the all-(R,R)-polyepoxide 13 is the requisite cascade substrate. Remarkably, all eleven rings of brevetoxin B would thus be assembled in this one cascade, with no further manipulation of the cascade product required. Nakanishi proposed that 13 could arise from epoxidation of polyene precursor 12; in theory, a single (R,R)-selective epoxidase could catalyze this transformation,9 setting 20 of the natural product's 23 stereocenters.
Scheme 1.
Nakanishi's proposed biosynthesis of brevetoxin B.
2.2. The Nakanishi Hypothesis as Inspiration to Synthetic Chemists
Even as the details of ladder polyether biosynthesis remain very much a mystery, Nakanishi's proposal has been inspirational to synthetic chemists. Cascade reactions in general are outstanding tools for improving the economy and efficiency of synthesis.xv Even among cascades, however, the proposed two-step conversion of polyene 13 to brevetoxin B (1) scores remarkably highly in terms of key synthetic principles such as step economyxvi and atom economy.xvii
The in vitro emulation of this proposal for the rapid, one-pot assembly of multiple rings would greatly streamline the synthesis of ladder polyethers. Emulation of the penultimate step, the exhaustive stereoselective epoxidation of isolated E olefins, can be achieved thanks to the powerful and versatile Shi asymmetric epoxidation.xviii The epoxide-opening cascade remains more elusive. Such a cascade method must achieve very high yields for each ring formed in order to overcome the “arithmetic demon,”xix the precipitous decrease in yields inherent to one-pot processes involving many consecutive reactions.
2.3. Methods for endo-Regioselective Epoxide Opening
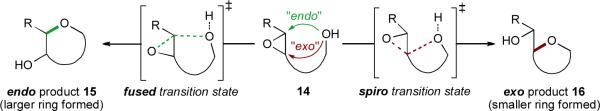
The sticking point is that achieving a good yield of the desired product in even a single epoxide-opening cyclization has historically been quite challenging. Intramolecular epoxide opening by an internal, pendent nucleophile (e.g., an alcohol, as shown in Scheme 2) can proceed via two distinct pathways, to afford regioisomeric products. These two pathways are frequently referred to as exo and endo processes, in reference to Baldwin's rules for cyclization.xx,xxi The climactic cascade of polyepoxide 13 to 1 involves no fewer than ten endo epoxide openings. Unfortunately for those seeking to mimic this cascade in vitro, pioneering work from Coxon and coworkersxxii and a battery of examples sincexxiii have shown that cyclizations of simple epoxy alcohols typically proceed through the spiro transition state to afford the exo product.
Scheme 2.
Regiochemical possibilities for intramolecular epoxide opening.
In simple epoxy alcohol cyclizations – “simple” implying that the epoxides involved are trans-disubstituted (as are the majority of epoxides in the proposed biosynthetic intermediates) and therefore electronically relatively unbiased – preferential formation of the smaller-ring exo product over the alternative, larger-ring endo product has been documented in manifold examples. Under basic, neutral, and acidic conditions, 5-exo cyclization generally predominates over 6-endo, so as to provide tetrahydrofuran (THF) rather than tetrahydropyran (THP) products.23 Likewise, 6-exo epoxy alcohol cyclization has been shown to proceed in preference to 7-endo under acidic promotion, affording THP rather than 7-membered oxepane products.xxiv
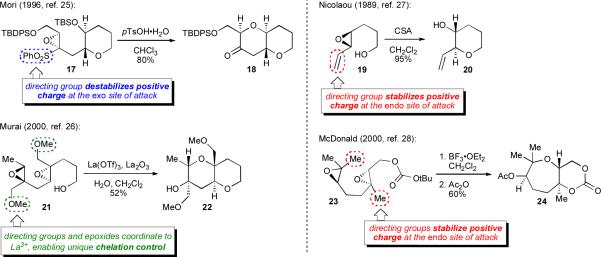
To overcome this “intrinsic” selectivity, a number of ingenious and highly useful methods have been developed to enforce endo-selective epoxide-opening cyclization. Most of these methods rely on electronically biasing the epoxide with a directing group, either by discouraging reactivity through the spiro transition state en route to exo opening, as in the work of Morixxv and Murai,xxvi or by stabilizing the fused transition state en route to endo opening, with key instances from the Nicolaou,xxvii McDonald,xxviii and Morimotoxxix groups, as well as a report from our group.xxx Scheme 3 provides a few representative examples of these methods. The majority of methods effect cyclization via acidic promotion (either Brønsted or Lewis); cyclizations in basic and neutral media have been studied less extensively.
Scheme 3.
Directed endo-selective methods for epoxide-opening cyclization.
For epoxides that are not strongly electronically biased, the prevailing wisdom has been that enzymatic control is necessary. A seminal report on protein-promoted endo epoxide opening was disclosed by Janda and coworkers,xxxi and recent work from Leadlayxxxii and Oikawaxxxiii has clearly shown that the epoxide hydrolase Lsd19 is essential to achieve endo-selective epoxide opening in the biosynthesis of lasalocid. It seemed that directing group-free epoxide-opening cascades would not be possible without such enzymatic control, even though epoxide hydrolase enzymes analogous to Lsd19 have yet to be identified in the organisms that produce ladder polyethers.
3. Development of Water-Promoted Directing Group-Free Epoxide-Opening Cascades
3.1. Cascades Promoted by a Disappearing Directing Group
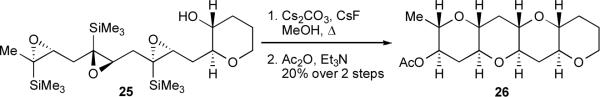
Before we began investigation of directing group-free cascades, our group developed a cascade method for ladder synthesis that was base-promoted and directed by trimethylsilyl (TMS) groups at each epoxide.30 We found that these TMS groups could act as disappearing directing groups, protiodesilylated in situ with the aid of cesium fluoride to afford tetrahydropyran (THP) tetrad 26, a characteristic subunit of ladder polyether natural products (Scheme 4).
Scheme 4.
Trimethylsilyl-directed epoxide-opening cascade.
While TMS directing groups are notable in that they can be efficiently removed during the cascade, their removal complicated the cascade conditions, and their stereocontrolled introduction made the synthesis of 25 rather challenging. Furthermore, the installation of a disappearing TMS group at a given position precludes the placement of a methyl group at that site, hindering the incorporation of methyl groups at ring junctions. To avoid some of the limitations associated with these directing groups, and to more closely emulate the Nakanishi hypothesis, we therefore sought to explore whether an endo-selective cascade might be possible without them.
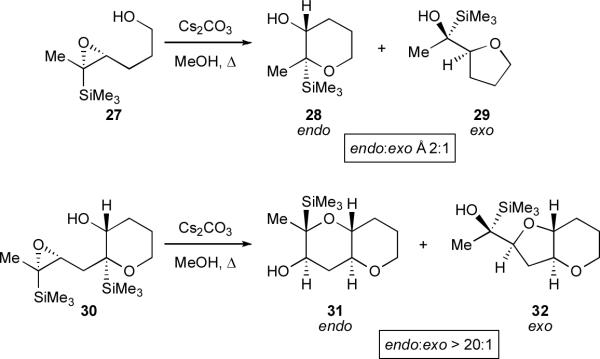
In retrospect, an early clue towards what would become such a directing group-free method was observed in the course of our studies of TMS direction. Modifying the substrate from linear epoxy alcohol 27 to compound 30, in which one THP ring has already been formed, resulted in a dramatic increase in endo regioselectivity (Scheme 5).xxxiv
Scheme 5.
Early suggestions of a template effect.
3.2. Development of Directing Group-Free endo-Selective Epoxide-Opening Cyclizations
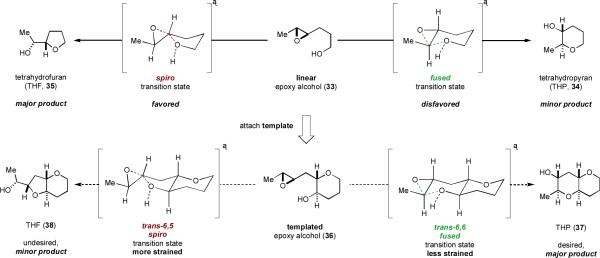
Abandoning directing groups reduces the number of possible approaches for control of regioselectivity in intramolecular epoxide-opening cyclization. While strong substrate control via powerful electronic directing groups on the epoxide electrophile would no longer be possible, we hoped that some element of substrate control could still play a role. The approach taken by our group stemmed from a rather simple analysis of the potential factors governing the regioselectivity of epoxide opening in these reactions.
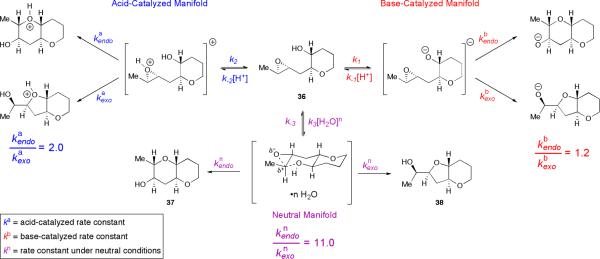
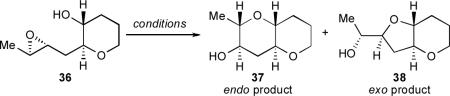
We reasoned that pre-organization of the substrate in an appropriate fashion could encourage, or “template,” the cyclization towards the endo pathway. We will herein use the term “template” to describe a molecular architecture that induces otherwise atypical reactivity and/or selectivity. Our initial working hypothesis was that a molecular template could alter the approach of the alcohol nucleophile to the epoxide electrophile and bias the substrate towards endo cyclization. In retrospect, such an effect would explain the unusual reactivity of 30. Our initial analysis was predicated on a single assumption – that the greater stability of the desired 6,6-fused (endo) product 37 as compared to the undesired 6,5-fused (exo) product 38 could, with judicious design of the template, be translated into a kinetic selectivity (Scheme 6). We conjectured that the reaction of 36, in which one THP is already in place and the epoxy alcohol appeared “primed” for cyclization, might go through more product-like transition states. Furthermore, preliminary calculations suggested that the predicted trans-bicyclo[4.4.0]decane transition state en route to 37 should be substantially less strained than the trans-bicyclo [4.3.0]nonane transition state proposed en route to 38. Were this difference in developing ring strain reflected in the transition states, then the desired THP product might be favored in this templated system under the appropriate reaction conditions. Later mechanistic studies have revealed that the basis of THP template effect is considerably more complex than our initial analysis assumed, but this germinal idea has proved quite fruitful nonetheless, as it led to the discovery of a powerful cooperative effect between the THP template and water as solvent (vide infra).
Scheme 6.
Initial analysis and design of a THP template.
To evaluate this hypothesis, we prepared simple THP-templated epoxy alcohol 36.xxxv Cyclization reactions of 36 were monitored under a variety of conditions (Table 1).
Table 1.
Cyclization studies of templated epoxy alcohol 36.
| entry | condition | product ratio (37:38) |
|---|---|---|
| 1 | Cs2CO3, MeOH | 1:2.7 |
| 2 | BF3•OEt2, CH2Cl2 | 1.4:1 |
| 3 | AcOH, PhMe | 1.6:1 |
| 4 | H2O (rt or 60 °C) | >10:1 |
| 5 | ethylene glycol | 9:1 |
| 6 | methanol | 8:1 |
Based on our experience with trimethylsilyl-substituted epoxy alcohols 27 and 30, we initially imagined that relatively forcing conditions would be necessary. Unfortunately, upon cyclization of 36 under the strongly basic conditions (Cs2CO3 in MeOH, Table 1, entry 1) that proved ideal for 27 and 30, the undesired THF product 38 predominated. However, slight preference for the desired larger ring in THP 37 was observed under both Lewis and Brønsted acidic conditions (e.g., Table 1, entries 2 and 3).
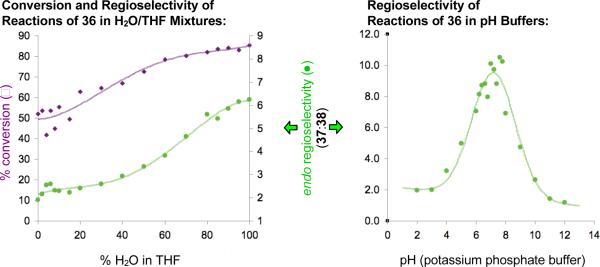
Intrigued by this turnover in selectivity upon shifting from basic to acidic promotion, we moved reactions of 36 into aqueous media, wherein buffers allow for careful control of acid and base concentrations. Regioselectivity was examined in potassium phosphate buffers. These experiments revealed an unexpected and exciting trend. While the low endo:exo regioselectivity in strong aqueous acid and base (i.e., below pH 4 and above pH 10) was consistent with that observed in organic solvents, selectivity increased sharply on moving towards neutrality, with greater than 10:1 selectivity observed at pH 7 (Figure 2). The balance of acid and base found at neutral pH appeared essential, but the buffer clearly was not, as simple deionized water also promoted cyclization with greater than 10:1 endo:exo regioselectivity (Table 1, entry 4). Evidently, water is the key promoter, not potassium phosphate.
Figure 2.
Regioselectivity of cyclization of 36 in H2O/THF mixtures and at various pH.
Several lines of evidence clearly indicate that water effects both a rate acceleration and an increase in selectivity in these reactions. In less polar solvents (e.g., CH2Cl2 and toluene), cyclization of 36 is slow, and in polar aprotic solvents (e.g., CH3CN, dimethyl sulfoxide (DMSO), and N,N′-dimethylformamide (DMF)) some cyclization occurs, but the selectivity is greatly reduced (≤3:1 37:38). Intriguingly, the only neutral organic solvents that approach the selectivity and rate possible in water are ethylene glycol and methanol (Table 1, entries 5 and 6). Like water, both of these solvents are excellent hydrogen bond donors and acceptors, but conversion in these solvents was lower than that observed in aqueous media. Conversion and regioselectivity were lower still in bulkier alcohols like EtOH and iPrOH. Studies in water/THF mixtures showed that both increased endo:exo selectivity and increased conversion of 36 correlate with increased water content in the reaction medium (Figure 2).
Further evidence for the critical role of the THP template was provided by the recent work of Qu and coworkers,xxxvi who observed that the regioselectivity of epoxide opening in untemplated epoxy alcohol 33 in water (6:1 exo:endo regioselectivity, Scheme 7) was dramatically different than the 10:1 endo regioselectivity observed with the analogous, templated 36. This attests to a synergistic relationship between THP template and water that is necessary to overturn the “intrinsic” exo regioselectivity normally observed in epoxide-opening cyclizations.
Scheme 7.
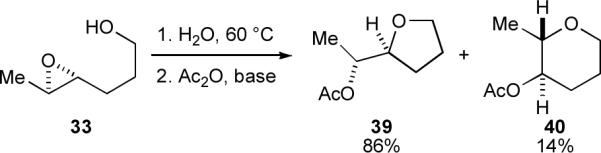
Exo-selective cyclization of linear epoxy alcohol 33.
3.3. Mechanistic Analysis of Water-Promoted endo-Selective Epoxide-Opening Cyclization
Our attention was piqued by the power of water, in conjunction with a THP template, to promote endo opening. Exploration of the literature revealed that efforts directed towards understanding the reaction mechanism of aqueous intramolecular cyclizations of aliphatic epoxy alcohols have been mostly limited to theoretical studies.xxxvii,xxxviii However, the analogous intermolecular variant, epoxide solvolysis, has been studied more extensively.xxxix The most relevant of these studies, with seminal work from Long and Pritchartxl and more recent reports by Pocker and coworkers,xli use product distributions, reaction kinetics, and solvent isotope effects to show that epoxide hydrolysis reactions can proceed by any one of three mechanisms depending on pH: an acid-catalyzed mechanism at low pH, a base-catalyzed mechanism at high pH, and a pH-independent or neutral mechanism at intermediate pH. Importantly, unsymmetrically substituted epoxides displayed different regioselectivities depending on reaction conditions. For example, propylene oxide (41) in isotopically labeled water undergoes hydrolysis at both sites of the epoxide. The ratio of 18O incorporation at the secondary (42) versus the primary (43) position varies, with selectivities (42:43) of 2:1, 1:2, and 1:3 observed for reactions carried out under acidic, neutral, and basic conditions, respectively (Scheme 8).40
Scheme 8.
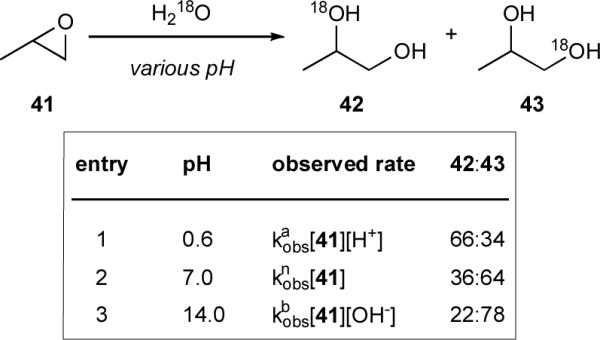
Hydrolysis of propylene oxide under acidic, neutral, and basic conditions.
This pH-dependent regioselectivity was explained by the relatively greater ability of the secondary carbon to stabilize positive charge in the acid-catalyzed mechanism and by the relatively greater steric accessibility of the primary carbon in the base-catalyzed mechanism. In the neutral mechanism, both factors may contribute, leading to intermediate regioselectivity. Intriguingly, Pocker, among others, has suggested that the transition state for epoxide opening in the neutral manifold may have considerable zwitterionic character.41
Something similar was observed for the cyclization of epoxy alcohol 36. Regioselectivities at high and low pH reflect those inherent to acid- and base-catalyzed mechanisms, while regioselectivities observed under neutral conditions apparently reflect selectivities inherent to a pH-independent mechanism (Scheme 9). A striking difference between hydrolysis reactions of propylene oxide and cyclizations of 36, however, is that the cyclization of 36 under neutral conditions is surprisingly selective despite the fact that 36 is a 1,2-disubstituted epoxide with no obvious electronic or steric bias. Furthermore, as previously stated, the endo-selectivity observed under neutral conditions is markedly higher than the regioselectivity normally observed for epoxy alcohol cyclizations.
Scheme 9.
Proposed pathways for cyclization of 36 in aqueous solutions at various pH.
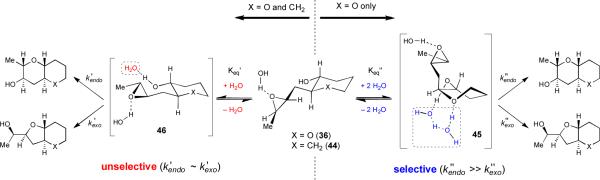
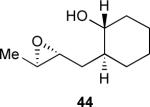
These observations suggested an interplay between the tetrahydropyran template and neutral water that was not well understood, and they prompted us to undertake a more detailed mechanistic study of epoxy alcohol cyclizations in neutral water.xlii A surprising finding during these studies was that the carbocyclic analog (44) of the original template reacted to give a nearly equal quantity of endo and exo cyclization products (Table 2). Furthermore, reaction kinetics revealed cyclization rates for 44 that are an order of magnitude faster than for 36. A series of experiments determined that both reactions are kinetically controlled and occur in solution rather than on the surface of water or in micelles. Solvent isotope effects for both reactions are consistent with those measured for the pH-independent hydrolysis of epoxides, implicating a neutral mechanism.
Table 2.
Summary of kinetic experiments carried out for the cyclizations of 36 and 44 in water at 45 °C (pH 7, 0.1 M potassium phosphate buffer).
 |
 |
|
|---|---|---|
| endo:exo | 11.0 | 0.73 |
| kobs (10−4, S−1) | 0.47 | 7.16 |
| t1/2 (h) | 4.11 | 0.27 |
| kH2O/kD2O | 1.33 | 1.47 |
| kinetic order in watera | 1st and 2nd b | 1st |
determined in DMSO-d6/D2O mixtures.
70 °C.
Moreover, kinetic experiments carried out in water-rich dimethyl sulfoxide/water mixtures revealed a first-order dependence on the concentration of water for cyclizations of 44, while cyclizations of 36 displayed evidence for two competing mechanisms that are first and second order in water, respectively. These studies of reaction rate and selectivity in water/DMSO mixtures were broadly consistent with what we had previously observed in water/THF mixtures (Figure 2). We must emphasize here that these experiments do not conclusively identify the absolute number of water molecules present around 36 in the transition state but simply indicate the relative excess in the number of water molecules required to promote reaction, in addition to those water molecules already in place around 36, to solvate the epoxy alcohol. We surmised that the pathway that is first-order in water for THP 36 is similar to what is observed in cyclohexane 44 and is therefore unselective. To account for the high selectivity in cyclizations of 36, we concluded that the pathway for 44 that is second order in water must be very selective for the endo product 37.
From these experiments we hypothesized that epoxy alcohol cyclizations in water occur for hydrated conformations that are situated appropriately for reaction (Scheme 10). The presence of the oxygen in the template of 36 likely serves two functions. First, its inductive electron-withdrawing effect electronically biases the epoxy alcohol towards endo cyclization both by decreasing the nucleophilicity of the alcohol and by discouraging the development of positive charge at the nearby exo site of attack on the epoxide.
Scheme 10.
Proposed mechanism for the cyclizations of 36 and 44 in neutral water. Proton transfer step(s) are omitted for clarity. The highlighted water molecules indicate the number and not necessarily the identity of water molecules that are kinetically relevant. Other water molecules represent generic waters of solvation.
Second, the THP oxygen may also facilitate reaction through the putative endo-selective pathway that is second order in water, possibly via an intermediate with the tetrahydropyran template in a twist-boat conformation such as 45 (although 45 is depicted in Scheme 10 with three water molecules, it is possible that one of these molecules originates from the solvated ground state). If a conformer like 45 is indeed involved, then the observed synergistic relationship between the THP template and water as solvent could be partially explained by water's ability to encourage the twist boat conformation via hydrogen bonding. Water may also be essential to facilitate proton transfer from the alcohol to the epoxide oxygen during the reaction.
Reaction through conformer 45 significantly alters the trajectory of nucleophilic attack by the epoxy alcohol as compared to the reactive conformer in the first-order pathway, presumably one with the template in a chair conformation (46, Scheme 10). This fact is particularly relevant because theoretical studies carried out by Houk37 and Coxon38 indicate that the most important factor dictating regioselectivity in epoxy alcohol cyclizations is the angle with which the alcohol approaches the epoxide, with an incidence angle of 100° being optimal. Such a trajectory is ideal because it allows for maximum overlap between the hydroxyl lone pair and the C–Oepox σ* orbital. The critical role of nucleophile trajectory in determining the regioselectivity of epoxide-opening cyclization was also examined empirically by Stork and Cohen in their pioneering report on epoxynitrile cyclizations.xliii
Whatever the precise rationale, it is clear from these studies that an oxygen in the template is critical for endo-selective cyclization and that the conformational and electronic advantages that such a substitution engenders is amplified by interactions with neutral water molecules. It is important to note that the mechanistic studies carried out by our group were limited to monoepoxides and are, therefore, not necessarily applicable to cascade reactions of multiple epoxides. Nevertheless, we believe that many important factors required for endo-selective cyclizations have been identified, and we are currently applying lessons learned from this mechanistic work towards the rational design of more highly regioselective cascade templates.
3.4 Water-Promoted Epoxide-Opening Cascades
Having developed a directing group-free epoxide-opening cyclization, we were excited to explore the extension of this method to cascades of multiple epoxides. For the synthesis of cascade substrates, we again turned to Nakanishi's biogenetic hypothesis for inspiration. Emulating the penultimate step of the Nakanishi hypothesis, we began with an attempted exhaustive and stereoselective epoxidation of a polyene.
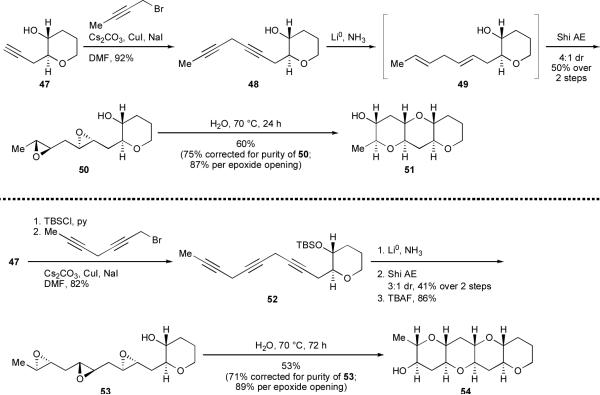
We found that bishomopropargylic alcohol 47 and its silyl ether derivative could be converted to skipped diyne 48 and skipped triyne 52 in high yield via alkylation with the appropriate propargylic bromides (Scheme 10).xliv Dissolving metal reduction of 48 and 52 afforded unstable skipped polyenes that were immediately subjected to Shi's asymmetric epoxidation method,18 which capably effected one-pot exhaustive and stereoselective epoxidation to afford diepoxide 50 and triepoxide 53. Notably, in the conversion of 52 to 53, three epoxidations are performed in a single step, in 3:1 overall dr (3:1 being the ratio of the desired compound to the sum of all other diastereomers). Of the eight stereogenic centers contained in THP tetrad 54, six are set in this one operation.
Stirring diepoxy alcohol 50 in deionized water for 24 hours at 70°C afforded THP triad 51 in 60% isolated yield (75% yield when corrected for the purity of 50). Similarly, THP tetrad 54, a characteristic structural subunit of the majority of known ladder polyethers, was obtained in 53% isolated yield (71% when corrected for starting material purity) upon the reaction of 53 in water.
These water-promoted transformations are notable for two reasons. First, epoxide opening proceeds with clean inversion, such that the requisite trans-syn-trans ladder polyether geometry is generated with complete stereospecificity. Second, after adjustment for the purity of cascade substrates 50 and 53, the yields per epoxide opening of THP triad 51 and tetrad 54, 87% and 89%, respectively, are quite good. In fact, the yields of these directing group-free cascades are markedly better than those observed in cascades guided by directing groups.26,28,30 Even with silyl protection and deprotection steps adding to the total count, the assembly of THP tetrad 54 is notably concise; given that alkyne 47 can be synthesized in five steps from 2,3-dihydropyran,xlv polyether 54 is only eleven steps from commercially available starting materials.
3.5. Water-Promoted Epoxide-Opening Cascades Accommodate Methyl Substitution
Methyl groups are the only substituents other than hydrogen found at ladder polyether ring junctions. Indeed, at least one methyl-substituted junction is found in every member of this large family of natural products, and approximately one quarter of ring junctions across the whole set of structures are methylated (see Figure 1). For example, brevetoxin B (1) bears methyl substituents at five of its ten ring junctions. Methyl substituents also appear in 12 and 13, Nakanishi's hypothesized polyene and polyepoxide precursors to brevetoxin B. Critically, the methyl groups that adorn the trisubstituted epoxides of 13 are encountered both distal (Med, Scheme 12) and proximal (Mep) to the internal nucleophile. We herein refer to distal epoxides as being methyl-substituted at the far side of the epoxide with respect to that pendent nucleophile, at the endo site of attack, while proximal epoxides bear methyl substituents at the near side of the epoxide, on the exo site of attack (see inset, Scheme 12).
Scheme 12.
Two proposed biosyntheses of brevetoxin B.
In Nakanishi's putative cascade of all (R,R)-polyepoxide 13, a generic base deprotonates the leftmost carboxylic acid to generate a nucleophilic species. There is some discussion in the literature over whether 13 or all (S,S)-polyepoxide 55 is more likely a polyepoxide precursor to brevetoxin B; some contend that an activated carboxylate species (as shown in 55) is more likely to serve as the terminal electrophile of the cascade.9,12
Again, the key point is that regardless of which direction the epoxide-opening cascade proceeds, both varieties of epoxide substitution will be encountered in the cascade, as both 13 and 55 contain a mixture of distal and proximal trisubstituted epoxides. Indeed, nearly all ladders bearing more than one methyl group, including the brevetoxins, maitotoxin, gambierol, and gymnocin B, are proposed to arise from similar polyepoxides bearing an “out-of-register” mixture of both distally and proximally substituted epoxides.
This point is quite important, as the methyl group can exert a rather powerful directing effect on the regioselectivity of epoxide opening, particularly under acidic activation – witness the acid-promoted endo-selective cascade methods developed by McDonald,28 as well as methyl-directed endo cyclization methods from Morimoto29 and Floreancigxlvi and the highly exo-selective methyl-directed epoxide-opening cascade in Corey's total synthesis of glabrescol.xlvii
When we began our work, there were no examples of the endo-selective opening of epoxides with methyl or other simple alkyl substituents proximal to the pendent nucleophile (at the exo site of attack), except under enzymatic catalysis33 or in systems with a stronger electronic directing group at the endo site of attack.xlviii Acid-activated endo-selective cascades accommodate distal substitution and, to a lesser extent, trans-disubstituted epoxides28,46 but presumably cannot tolerate proximally trisubstituted epoxides. We conjectured that cascades of THP-templated epoxy alcohols promoted by neutral water could prove less sensitive to the electronic perturbation of methyl substitution.
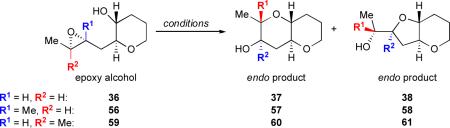
We prepared monoepoxy alcohols 56 and 59, the trisubstituted analogs of the disubstituted 36, which respectively bear a methyl group proximal to the pendent hydroxyl nucleophile and a methyl group distal to the internal nucleophile (Table 3).45 Upon cyclization of these compounds under Brønsted basic, Brønsted acidic, Lewis acidic, and aqueous promotion, we found that only water afforded the desired endo product for all three substrates. Furthermore, endo selectivity was highest for each substrate in neutral water, with 4.9:1 endo regioselectivity observed for the challenging proximally trisubstituted 56 and better than 20:1 selectivity achieved with distally trisubstituted 59. Investigations of these systems in pH buffers indicated that peak endo selectivity occurs near neutral pH for both 56 and 59, as it did for the disubstituted 36.
Table 3.
Effect of Methyl Substitution on the Regioselectivity of Epoxide Opening under Various Conditions.
| conditions and regioselectivity (endo:exo) |
||||||
|---|---|---|---|---|---|---|
| epoxy alcohol | R1 | R2 | Cs2CO3, MeOH | CSA, CH2Cl2 | BF3•OEt2, CH2Cl2 | H2O |
| 36 | H | H | 1 :2.7 | 1 : 1.2 | 1.4 : 1 | 10 : 1 |
| 56 | Me | H | 3.0 : 1 | 1 : 5.2 | 1 : 11 | 4.9 : 1 |
| 59 | H | Me | 1 : 17 | 5.8 : 1 | >20 : 1 | >20 : 1 |
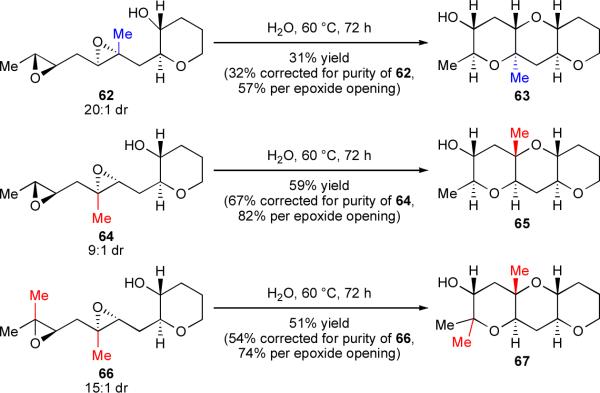
The unique ability of water to promote the endo-selective opening of all three types of epoxides encountered in the Nakanishi hypothesis (trans-disubstituted, proximally methyl-substituted, and distally methyl-substituted) spurred us to attempt the cascade reactions of variously methyl-substituted diepoxy alcohols 62, 64, and 66 (Scheme 13). Water again proved successful, enabling the rapid construction of methylated THP triads 63, 65, and 67 in modest to good yields. While acidic activation of 64 and 66, both of which bear distal methyl substituents, also afforded the desired product in both cases (in yields competitive with those obtained in water), only cascades in water provided any of THP triad 63 from diepoxide 62. To the best of our knowledge, the transformation of 62 to 63 represents the first endo-selective epoxide-opening cascade to accommodate a proximal methyl substituent.
Scheme 13.
Endo-selective cascades of methyl-substituted epoxides.
Recall that yields per epoxide opening in cascades of all-disubstituted polyepoxides 50 and 53 approached 90% (vide supra, Scheme 11), consistent with the 10:1 regioselectivity predicted by monoepoxide model system 36. However, yields per epoxide in reactions of methylated diepoxides 62, 64, and 66 were substantially lower than expected, in light of the good regioselectivities observed with trisubstituted monoepoxides 56 and 59. We submit that suppressing side reactions during cascades becomes more challenging with trisubstituted epoxides. Such epoxides are potentially both more nucleophilic, due to electronic donation by the methyl substituent, and more electrophilic at their tertiary centers in the presence of an acidic activator like the trace of hydronium ion in neutral water. This heightened reactivity could be a vulnerability, making undesired epoxide-on-epoxide activation competitive with the desired cascade pathway initiated by the templated alcohol. We have isolated side products from cascade reactions of 62, 64, and 66 that are consistent with epoxide-on-epoxide opening; such products have not been detected in reactions of 50 and 53.35 It is also possible that the mechanism of cascade reactions is simply more complex than we currently understand. Cascades require considerably higher temperatures and/or longer reaction times than single-epoxide cyclizations, a property we cannot fully explain but are currently studying.
Scheme 11.
Directing group-free epoxide-opening cascades (Shi AE = Shi asymmetric epoxidation).
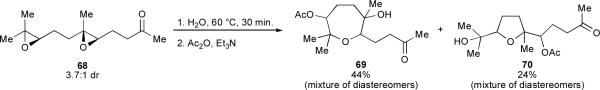
Another recent example of an endo-selective epoxide-opening reaction of trisubstituted epoxides in water was disclosed by Qu and coworkers.36 They found that diepoxy ketone 68 cyclized in water to afford the endo oxepane product 69 as the major product, along with a smaller quantity of the exo THF product 70 (Scheme 14). This regioselectivity is remarkably different from the high exo regioselectivity of intramolecular epoxide opening normally observed for linear systems in water (see Scheme 7); the difference may arise from a directing effect of the distal methyl substituents on the epoxides of 68. Distal substitution could stabilize the development of carbocationic character on the endo sites of attack in those epoxides, which may be critical if zwitterionic transition states are indeed involved in neutral water-promoted epoxide opening.
Scheme 14.
Water-promoted, endo-selective epoxide opening in a linear system.
4. Epoxide-Opening Cascades in Total Synthesis
4.1. Seminal Work on Exo-Selective Opening
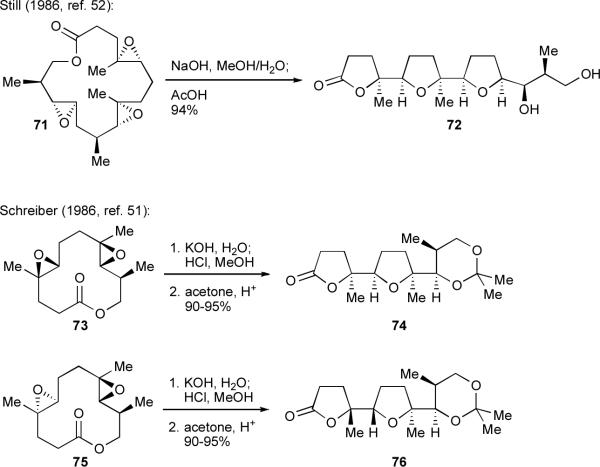
A number of total syntheses involve epoxide-opening cascades, but the vast majority are exo-selective.23 For example, a number of efficient and beautiful epoxide-opening cascades have been applied to syntheses of polyether ionophores and squalene-derived polyethers. Many of these cascades closely mimic the Cane-Celmer-Westley proposal for polyether ionophore biogenesis.xlix Key demonstrations of such cascades have been reported by Hoye,l Schreiber,li Still,lii Paterson,liii Evans,liv and Corey,47 with their reports among a number of landmark achievements in the development of exo-selective epoxide-opening cascades dating back into the 1980s (two canonical examples of such cascades, from Still and Schreiber, are shown in Scheme 15).
Scheme 15.
Exo-selective cascades applied to the synthesis of subunits of monensin.
4.2. Endo-Selective Epoxide-Opening in the Total Synthesis
Given the difficulties associated with forging endo-selective epoxide-opening cascades, on the other hand, examples of their application to total synthesis have been scarce until quite recently. Discrete endo-cyclization steps have been employed in total synthesis contexts: for example the Nicolaou group made frequent and gainful use of its vinyl epoxide cyclization protocol (Scheme 3) in its historic syntheses of hemibrevetoxin B,12 brevetoxin B,12 and brevetoxin A.lv Scheme 16 shows characteristic examples of this method being used to form THP rings in high yield en route to brevetoxin B.
Scheme 16.
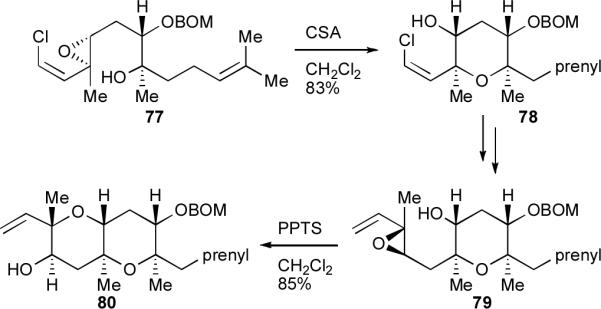
Endo-selective epoxide-opening cyclizations in the synthesis of the FG rings of brevetoxin B.
Mori and coworkers effectively implemented their own method for endo-selective cyclization onto sulfone-substituted epoxides (Scheme 3) in an impressive formal total synthesis of hemibrevetoxin B (Scheme 17).lvi
Scheme 17.
Endo-selective epoxide-opening cyclization in the synthesis of hemibrevetoxin B.
Each of these reactions is a cyclization of a single epoxide, however, not a cascade; notably, it was not until 2003 that Holton and coworkers became the first to incorporate an endo-selective epoxide opening into a selenonium-induced cascade of cyclizations in their synthesis of hemibrevetoxin B.lvii
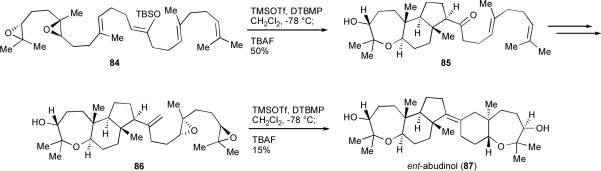
Extensive application of endo-selective epoxide-opening cascades to total synthesis was reported by the McDonald group, who used two hybrid oxa/carbacyclization cascades in a closely biomimetic and remarkably concise synthesis of the enantiomer of the terpenoid abudinol (87, Scheme 18).lviii These Lewis acid-promoted cascades, each of which involves the opening of multiple epoxides, build on earlier studies from the McDonald group on the cascade synthesis of fused oxepane systems.28 An early cascade reaction of diepoxide 84 rapidly assembles tricycle 85 in 50% yield (79% average yield per ring), and the two remaining rings of ent-abudinol (87) are constructed in short order via a second cascade of diepoxy alkene 86, in 15% yield (39% average per ring).
Scheme 18.
Biomimetic synthesis of ent-abudinol (DTBMP = 2,6-di-t-butyl-4-methylpyridine).
Our group recently applied a bromonium-induced, endo-selective epoxide-opening cascade (conceptually related to those of Holton and McDonald) to the total synthesis of ent-dioxepandehydrothyrsiferol.lix While these cascades are not in water and therefore fall outside the scope of this review, they, along with earlier examples of epoxide-opening cyclization applied to the synthesis of natural products, serve as encouragement and as instructive comparisons for our own explorations into the utility of water-promoted epoxide opening in total synthesis.
4.3. An Application of Water-Promoted Epoxide-Opening Cascades towards Total Synthesis
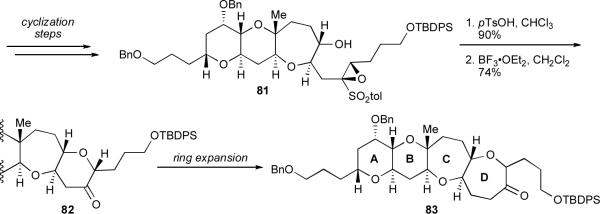
We have thus far discussed how THP rings can effectively template epoxide-opening cascades in water to rapidly construct ladder polyether subunits. Unfortunately, because the THP templates used in all of these studies were bereft of substitution, the triand tetracyclic cascade products thereof could not be easily elaborated into larger natural products. As straightforward product derivatization is an indispensable criterion for any effective method in total synthesis, we consequently sought to replace the undecorated THP template with one more substituted, one where judicious choice of substituents could facilitate rapid elaboration of the cascade product into a natural product.
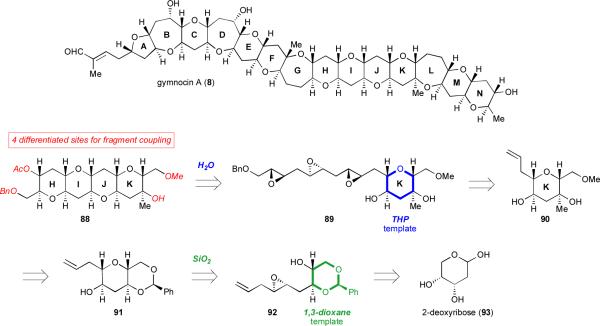
The substituted template we ultimately chose to investigate is ring K of the polyether gymnocin A (8).lx This highly cytotoxic natural product is one of the largest of the polyethers; at 14 consecutive rings gymnocin A is among the longest continuous ladders yet identified. We envisioned that a cascade of epoxide-openings initiated by the secondary alcohol on ring K, a highly functionalized THP (89), could construct three additional rings (HIJ) of the natural product. The HIJK fragment formed (88) would possess two sites for functionalization at each end of the ladder, making it a potentially synthetically versatile intermediate primed for elaboration into the full natural product (Scheme 19).lxi
Scheme 19.
Retrosynthetic analysis of the HIJK ring system of gymnocin A.
Our retrosynthetic analysis began with this water-promoted cascade of triepoxy alcohol 89, a cascade in which ring K would template the transformation (Scheme 15). Triepoxy alcohol 89 could, in turn, arise from cross-metathesis62 of alkene 90 with another epoxy alkene. THP 90 could be liberated on cleavage of the acetal 91, which could itself be prepared by cyclization of epoxy alcohol 92. Significantly, the cyclization of 92 would involve a novel benzylidene acetal template (more generically referred to as a 1,3-dioxane template). We anticipated that this template would be “removable”; that is, it could be cleaved after cyclization to reveal handles for further synthetic manipulation.
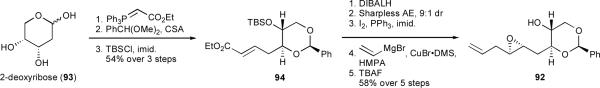
Having never investigated benzylidene acetals as templates, we began with the synthesis and evaluation of 92 in this context. Inexpensive 2-deoxyribose (93) was transformed into epoxy alcohol 92 in 8 steps and 31% overall yield (Scheme 20).
Scheme 20.
Synthesis of epoxy alcohol 92.
Remarkably, epoxy alcohol 92 did not cyclize in water at ambient temperature, and heating the reaction led to hydrolysis of the acetal (Scheme 21). Clearly, the benzylidene acetal template behaves differently from the THP template. We conjecture that one reason that 92 does not cyclize appreciably in water at room temperature is the reduced nucleophilicity of the alcohol, a presumable result of the presence of a second inductively electron-withdrawing oxygen in the template. Consequently, we needed to identify a new promoter for this new template, and we gratifyingly found that silica gel (SiO2) and its hydrated sibling, silicic acid (SiO3H2), serve as excellent promoters, promoting the cyclization with greater than 9:1 endo selectivity to afford the desired 6,6-fused bicycle 91 in good yield. We believe that silica, like water, can serve as both hydrogen bond donor and hydrogen bond acceptor and may thereby facilitate proton transfer during cyclization of the epoxy alcohol. Silica may represent a flexible and valuable scaffold for the design of new cyclization promoters. Other amphoteric oxides similar to silica, such as alumina, zinc oxide, and a wide variety of mixed metal and metalloid oxides, also marry Brønsted acidity and basicity, and achieving a balance between these properties could be essential for promoting opening with even higher regioselectivity.
Scheme 21.
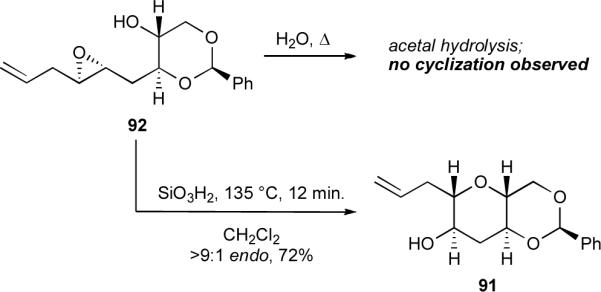
Cyclization of 1,3-dioxane-templated epoxy alcohol 92.
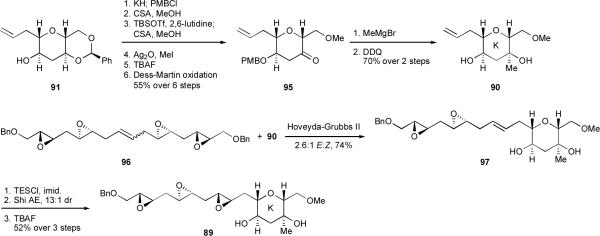
In order to transform diad 91 into the ring K template, installation of an axial methyl group at the 3-position of the THP ring remained. This proved straightforward (Scheme 22), showcasing the synthetic flexibility of the benzylidene acetal and providing (in the form of 90) the fully functionalized K ring of gymnocin A. Cross metathesislxii of 90 with an excess of tetraepoxyalkene 96 and catalyzed by Hoveyda-Grubbs 2nd generation catalystlxiii afforded 97 and completed the assembly of the entire carbon framework of the HIJK rings. Such a cross metathesis tactic represents an alternative method for the efficient construction of skipped polyepoxide chains, one complementary to the strategy described earlier of skipped polyyne reduction under dissolving metal conditions and subsequent epoxidation (see Scheme 10). Alkene 97 was epoxidized via one final Shi asymmetric epoxidation,18 and deprotection of the THP-templated secondary alcohol yielded triepoxy alcohol 89, ready for cascade studies.
Scheme 22.
Synthesis of the K ring of gymnocin A.
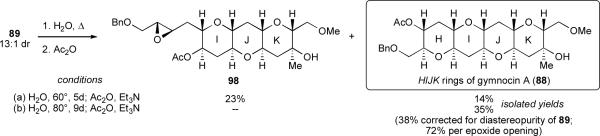
As our studies with cyclohexane-templated epoxy alcohols clearly indicated that template composition can radically affect the regioselectivity of epoxide opening, we could not be certain a priori whether ring K in 89, which bears a methoxymethyl substituent at the 2-position as well as a tertiary alcohol and axial methyl group at the 3-position, would template the reaction as well as the undecorated THP template of 53 (Scheme 11). We were therefore pleased to discover that incubation of 89 in H2O at 60 °C for 5 days followed by acetylation afforded some of the desired THP tetrad 88 (Scheme 23). We were surprised, however, to isolate a larger quantity of compound 98, in which rings IJ had formed but the final epoxide remained intact.
Scheme 23.
Cascade synthesis of the HIJK rings of gymnocin A.
The cascade reaction of 89 was intriguing. It appeared to proceed substantially more slowly than the cascade of analogous triepoxy alcohol 53 in water, wherein complete conversion was observed after 3 days at 70 °C. Moreover, prior to the isolation of 98 we had not observed any epoxide-containing intermediates en route to the final cascade products. The attenuated reactivity of the surviving epoxide of 98 is hypothesized to arise from the presence of the oxygen atom in the neighboring benzyl ether. An inductive electron-withdrawing effect could destabilize the formation of positive charge at the site of epoxide opening, an effect that could be pivotal if epoxide opening proceeds through a transition state with significant zwitterionic character. Deactivation of a given epoxide toward opening is a potentially useful feature that may find application in future cascades. A higher temperature and longer reaction time (80 °C, 9 d) overcame this stalled cascade and, upon acetylation, afforded 88, the desired HIJK fragment of gymnocin A in 35% yield (38% adjusted for the purity of 89), corresponding to approximately 70% yield per newly formed ring.
Notably, THP tetrad 88 bears four differently substituted pendent hydroxyl groups ready for synthetic elaboration. Its formation from 89 marked the first endo-selective cascade from a highly decorated THP template, a template of a form more likely to be encountered in nature than the simpler 2,3-disubstituted tetrahydropyranols we had studied previously. Still, we hesitate to speculate too much on what this result implies for the biosynthesis of gymnocin A (and other ladder polyether natural products). The success of this cascade clearly does not prove the Nakanishi hypothesis – one obvious incongruity is that marine dinoflagellates are unlikely to heat up to 90 °C or more for days on end in order to synthesize their secondary metabolites!
5. Conclusion
We have herein recounted the development of endo-selective epoxide-opening cascades in water, a method useful for the rapid assembly of fused tetrahydropyran structures found in ladder polyether natural products. Throughout our work we have been guided and inspired by Nakanishi's famous hypothesized biosynthesis of these fascinating and forbidding natural products. While we cannot claim to be rigorously testing the Nakanishi hypothesis, we strive to emulate its concision. Our intellectual journey has in a sense carried us ever more closely towards this biogenetic hypothesis, as we have progressed from trimethylsilyl-directed cascades in organic solvent to directing group-free cascades in water, the biological reaction medium, and finally to a cascade templated by a “real” template, the K ring of gymnocin A. Adding to the intrigue is the recent isolation of the tetrahydropyranol brevisamide from Karenia brevis by Wright, Baden, and coworkers,lxiv who suggest that the compound might point to the existence of a tetrahydropyran template in nature.
Many outstanding challenges remain. Not least among these are the invention of methods for seven- and eight-membered ring formation via endo-selective epoxide opening and the development of new templates and small molecule catalysts to expedite epoxide-opening cascades. We continue our ongoing mission: to seek out new cascade reactions that can reduce the staggering complexity of ladder polyethers to simpler problems.
Footnotes
Part of the rapid formation of molecular complexity in organic synthesis themed issue.
References
- i.Sellner KG, Doucette GJ, Kirkpatrick GJ. J. Ind. Microbiol. Biotechnol. 2003;30:383. doi: 10.1007/s10295-003-0074-9. [DOI] [PubMed] [Google Scholar]
- ii.Yasumoto T, Murata M. Chem. Rev. 1993;93:1897. [Google Scholar]
- iii.For an article on the economic and environmental impact of red tides on the Gulf Coast of Florida, see: Schrope M. Nature. 2008;452:24. doi: 10.1038/452024a.
- iv.Nicolaou KC, Frederick MO, Aversa RJ. Angew. Chem. Int. Ed. 2008;47:7182. doi: 10.1002/anie.200801696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- v.For a few examples of the use of ladder polyethers as probes of ion transport channel protein function, see: Baden DG, Bourdelais AJ, Jacocks H, Michelliza S, Naar J. Environ. Health Perspect. 2005;113:621. doi: 10.1289/ehp.7499.. Matile S, Nakanishi K. Angew. Chem. Int. Ed. Engl. 1996;35:757.. Ghiaroni V, Sasaki M, Fuwa H, Rossini GP, Scalera G, Yasumoto T, Pietra P, Bigiani A. Toxicol. Sci. 2005;85:657. doi: 10.1093/toxsci/kfi097.
- vi.(a) Lee MS, Qin G.-w., Nakanishi K, Zagorski MG. J. Am. Chem. Soc. 1989;111:6234. [Google Scholar]; (b) Chou H-N, Shimizu Y. J. Am. Chem. Soc. 1987;109:2184. [Google Scholar]
- vii.(a) Snyder RV, Gibbs PDL, Palacios A, Abiy L, Dickey R, Lopez JV, Rein KS. Mar. Biotechnol. 2003;5:1. doi: 10.1007/s10126-002-0077-y. [DOI] [PubMed] [Google Scholar]; (b) Snyder RV, Guerrero MA, Sinigalliano CD, Winshell J, Perez R, Lopez JV, Rein KS. Phytochemistry. 2005;66:1767. doi: 10.1016/j.phytochem.2005.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- viii.Murata M, Naoki H, Iwashita T, Matsunaga S, Sasaki M, Yokoyama A, Yasumoto T. J. Am. Chem. Soc. 1993;115:2060. [Google Scholar]
- ix.Gallimore AR, Spencer JB. Angew. Chem. Int. Ed. 2006;45:4406. doi: 10.1002/anie.200504284. [DOI] [PubMed] [Google Scholar]
- x.Nakata T. Chem. Rev. 2005;105:4314. doi: 10.1021/cr040627q. [DOI] [PubMed] [Google Scholar]
- xi.At least three additional total syntheses of ladder polyethers have been reported since the publication of ref. 4. Crimmins MT, Zuccarello JL, Ellis JM, McDougall PJ, Haile PA, Parrish JD, Emmitte KA. Org. Lett. 2009;11:489. doi: 10.1021/ol802710u. Brevetoxin A.. Hamajima A, Isobe M. Angew. Chem. Int. Ed. 2009;48:2941. doi: 10.1002/anie.200805996. Ciguatoxin.. Takamura H, Kikuchi S, Nakamura Y, Yamagami Y, Kishi T, Kadota I, Yamamoto Y. Org. Lett. 2009;11:2531. doi: 10.1021/ol900769d. Brevenal.
- xii.Nicolaou KC. Angew. Chem. Int. Ed. Engl. 1996;35:589. and references therein. [Google Scholar]
- xiii.Inoue M. Chem. Rev. 2005;105:4379. doi: 10.1021/cr0406108. [DOI] [PubMed] [Google Scholar]
- xiv.Shimizu Y. In: Natural Toxins: Animal, Plant, and Microbial. Harris JB, editor. Clarendon; Oxford: 1986. p. 123. [Google Scholar]
- xv.Nicolaou KC, Edmonds DJ, Bulger PG. Angew. Chem. Int. Ed. 2006;45:7134. doi: 10.1002/anie.200601872. [DOI] [PubMed] [Google Scholar]
- xvi.Wender PA, Miller BL. In: Organic Synthesis: Theory and Applications. Hudlicky T, editor. vol. 2. JAI; Greenwich: 1993. pp. 27–66. [Google Scholar]
- xvii.Trost BM. Science. 1991;254:1471. doi: 10.1126/science.1962206. [DOI] [PubMed] [Google Scholar]
- xviii.Wang Z-X, Tu Y, Frohn M, Zhang J-R, Shi Y. J. Am. Chem. Soc. 1997;119:11224. [Google Scholar]
- xix.Crispino GA, Ho PT, Sharpless KB. Science. 1993;259:64. doi: 10.1126/science.8418495. [DOI] [PubMed] [Google Scholar]
- xx.Baldwin JE. J. Chem. Soc. Chem. Commun. 1976;18:734. [Google Scholar]
- xxi.Technically, since the breaking C–O bond is outside the newly formed ring in the transition states to both “endo” product 15 and “exo” product 16, both processes are formally exo. A more rigorous description of 15 and 16 would be “large ring” product and “small ring” product, respectively. Alternatively, 15 may be described as the product arising from a fused transition state, while 16 arises from a spiro transition state. However, we will use the “endo” and “exo” terminology throughout this article, as it is deeply ingrained in the literature and serves as a convenient shorthand.
- xxii.Coxon JM, Hartshorn MP, Swallow WH. Aust. J. Chem. 1973;26:2521. [Google Scholar]
- xxiii.Vilotijevic I, Jamison TF. Angew. Chem. Int. Ed. 2009;48:5250. doi: 10.1002/anie.200900600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xxiv.Iimori T, Still WC, Rheingold AL, Staley DL. J. Am. Chem. Soc. 1989;111:3439. [Google Scholar]
- xxv.Mori Y, Furuta H, Takase T, Mitsuoka S, Furukawa H. Tetrahedron Lett. 1999;40:8019. and references therein. [Google Scholar]
- xxvi.Tokiwano T, Fujiwara K, Murai A. Chem. Lett. 2000:272. and references therein. [Google Scholar]
- xxvii.Nicolaou KC, Prasad CVC, Somers PK, Hwang CK. J. Am. Chem. Soc. 1989;111:5330. [Google Scholar]
- xxviii.Valentine JC, McDonald FE. Synlett. 2006;12:1816. and references therein. [Google Scholar]
- xxix.Morimoto Y, Nishikawa Y, Ueba C, Tanaka T. Angew. Chem. Int. Ed. 2006;45:810. doi: 10.1002/anie.200503143. [DOI] [PubMed] [Google Scholar]
- xxx.(a) Heffron TP, Jamison TF. Org. Lett. 2003;5:2339. doi: 10.1021/ol0347040. [DOI] [PubMed] [Google Scholar]; (b) Simpson GL, Heffron TP, Merino E, Jamison TF. J. Am. Chem. Soc. 2006;128:1056. doi: 10.1021/ja057973p. [DOI] [PubMed] [Google Scholar]
- xxxi.Janda KD, Shevlin CG, Lerner RA. Science. 1993;277:936. doi: 10.1126/science.8424171. [DOI] [PubMed] [Google Scholar]
- xxxii.Smith L, Hong H, Spencer JB, Leadlay PF. ChemBioChem. 2008;9:2967. doi: 10.1002/cbic.200800585. [DOI] [PubMed] [Google Scholar]
- xxxiii.Shichijo Y, Migita A, Oguri H, Watanabe M, Tokiwano T, Watanabe K, Oikawa H. J. Am. Chem. Soc. 2008;130:12230. doi: 10.1021/ja8040543. [DOI] [PubMed] [Google Scholar]
- xxxiv.Heffron TP. Ph.D. thesis. Massachusetts Institute of Technology; 2005. [Google Scholar]
- xxxv.Vilotijevic I, Jamison TF. Science. 2007;317:1189. doi: 10.1126/science.1146421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xxxvi.Wang Z, Cui Y-T, Xu Z-B, Qu J. J. Org. Chem. 2008;73:2270. doi: 10.1021/jo702401t. [DOI] [PubMed] [Google Scholar]
- xxxvii.Na J, Houk KN, Shevlin CG, Janda KD, Lerner RA. J. Am. Chem. Soc. 1993;115:8453. [Google Scholar]
- xxxviii.Coxon JM, Thorpe AJ. J. Org. Chem. 1999;64:5530. doi: 10.1021/jo990364d. [DOI] [PubMed] [Google Scholar]
- xxxix.Whalen DM. In: Advances in Physical Organic Chemistry. Richard JP, editor. Vol. 40. Elsevier; Boston: 2005. p. 247. [Google Scholar]
- xl.Long FA, Pritchard JG. J. Am. Chem. Soc. 1956;78:2663. [Google Scholar]
- xli.Pocker Y, Ronald BP, Anderson KW. J. Am. Chem. Soc. 1988;110:6492. and references therein. [Google Scholar]
- xlii.Byers JA, Jamison TF. J. Am. Chem. Soc. 2009;131:6383. doi: 10.1021/ja9004909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xliii.Stork G, Cohen JF. J. Am. Chem. Soc. 1974;96:5270. [Google Scholar]
- xliv.Jeffery T, Gueugnot S, Linstrumelle G. Tetrahedron Lett. 1992;33:5757. [Google Scholar]
- xlv.Morten CJ, Jamison TF. J. Am. Chem. Soc. 2009;131:6687. doi: 10.1021/ja9025243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xlvi.Wan S, Gunaydin H, Houk KN, Floreancig PE. J. Am. Chem. Soc. 2007;129:7915. doi: 10.1021/ja0709674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xlvii.Xiong Z, Corey EJ. J. Am. Chem. Soc. 2000;122:9328. [Google Scholar]
- xlviii.Matsukura H, Morimoto M, Koshino H, Nakata T. Tetrahedron Lett. 1997;38:5545. [Google Scholar]
- xlix.Cane DE, Celmer WD, Westley JW. J. Am. Chem. Soc. 1983;105:3594. [Google Scholar]
- l.(a) Hoye TR, Suhadolnik JC. J. Am. Chem. Soc. 1985;107:5312. [Google Scholar]; (b) Hoye TR, Witowski NE. J. Am. Chem. Soc. 1992;114:7291. and references therein. [Google Scholar]
- li.Schreiber SL, Sammakia T, Hulin B, Schulte G. J. Am. Chem. Soc. 1986;108:2106. [Google Scholar]
- lii.Still WC, Romero AG. J. Am. Chem. Soc. 1986;108:2105. [Google Scholar]
- liii.Paterson I, Craw PA. Tetrahedron Lett. 1989;30:5799. and references therein. [Google Scholar]
- liv.Evans DA, Ratz AM, Huff BE, Sheppard GS. J. Am. Chem. Soc. 1995;117:3448. [Google Scholar]
- lv.Nicolaou KC, Yang Z, Shi G-Q, Gunzer JL, Agrios KA, Gartner P. Nature. 1998;392:264. doi: 10.1038/32623. [DOI] [PubMed] [Google Scholar]
- lvi.Mori Y, Yaegashi K, Furukawa H. J. Org. Chem. 1998;63:6200. doi: 10.1021/jo980320p. and references therein. [DOI] [PubMed] [Google Scholar]
- lvii.Zakarian A, Batch A, Holton RA. J. Am. Chem. Soc. 2003;125:7822. doi: 10.1021/ja029225v. [DOI] [PubMed] [Google Scholar]
- lviii.Tong R, McDonald FE. Angew. Chem. Int. Ed. 2008;47:4377. doi: 10.1002/anie.200800749. [DOI] [PubMed] [Google Scholar]
- lix.Tanuwidjaja J, Ng S-S, Jamison TF. J. Am. Chem. Soc. 2009;131:12084. doi: 10.1021/ja9052366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- lx.For the isolation and initial toxicity studies of gymnocin A, see: Satake M, Shoji M, Oshima Y, Naoki H, Fujita T, Yasumoto T. Tetrahedron Lett. 2002;43:5829.. One total synthesis of 3 has been reported to date: Tsukano C, Sasaki M. J. Am. Chem. Soc. 2003;125:14294. doi: 10.1021/ja038547b. and references therein.
- lxi.Van Dyke AR, Jamison TF. Angew. Chem. Int. Ed. 2009;48:4430. doi: 10.1002/anie.200900924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- lxii.(a) Blackwell HE, O'Leary DJ, Chatterjee AK, Washenfelder RA, Bussmann DA, Grubbs RH. J. Am. Chem. Soc. 2000;122:58. [Google Scholar]; (b) O'Leary DJ, Blackwell HE, Washenfelder RA, Grubbs RH. Tetrahedron Lett. 1998;39:7427. [Google Scholar]
- lxiii.Garber SB, Kingsbury JS, Gray BL, Hoveyda AH. J. Am. Chem. Soc. 2000;122:8168. [Google Scholar]
- lxiv.Satake M, Bourdelais AJ, Van Wagoner RJ, Baden DG, Wright JLC. Org. Lett. 2008;10:3465. doi: 10.1021/ol801243n. [DOI] [PMC free article] [PubMed] [Google Scholar]