Abstract
This study examined the relationship between airway blood flow (Q̇aw), ventilation (V̇E) and cardiac output (Q̇tot) during exercise in healthy humans (n = 12, mean age 34 ± 11 yr). Q̇aw was estimated from the uptake of the soluble gas dimethyl ether while V̇E and Q̇tot were measured using open circuit spirometry. Measurements were made prior to and during exercise at 34 ± 5W (Load 1) and 68 ± 10 W (Load 2) and following the cessation of exercise (recovery). Q̇aw increased in a stepwise fashion (P < 0.05) from rest (52.8 ± 19.5 µl min−1 ml−1) to exercise at Load 1 (67.0 ± 20.3 µl min−1 ml−1) and Load 2 (84.0 ± 22.9 µl min−1 ml−1) before returning to pre-exercise levels in recovery (51.7 ± 13.2 µl min−1 ml−1). Q̇aw was positively correlated with both Q̇tot (r = 0.58, P < 0.01) and V̇E (r = 0.50, P < 0.01). These results demonstrate that the increase in Q̇aw is linked to an exercise related increase in both Q̇tot and V̇E and may be necessary to prevent excessive airway cooling and drying.
Keywords: Bronchial blood flow, Cardiac output, Ventilation
1. Introduction
During exercise there is a dynamic increase in the dimensions of the conducting airways resulting in a decrease in the resistance to airflow and an increase in alveolar ventilation (de Bisschop et al., 2003). While these dynamic changes in airway dimensions are well characterized, changes in the blood flow to the conducting airways themselves (i.e., airway blood flow, Q̇aw) are not so well defined.
In humans, the majority of the blood flow to the conducting airways is via the bronchial artery with the remaining flow arising from bronchopulmonary anastomoses (Deffebach et al., 1987). Airway blood flow represents only about 1% of the total pulmonary blood flow (Q̇tot) (Coleridge and Coleridge, 1994), however changes in Q̇aw may be important during exercise to maintain airway function by preventing excessive airway cooling and drying (Parsons et al., 1989). For example, in humans, Kim et al. (1996) demonstrated that Q̇aw increased during eucapneic hyperventilation (V̇E) with frigid air. Similarly there is evidence that Q̇aw increases during eucapneic hyperventilation in sheep (Parsons et al., 1989).
To date, the results of studies that have examined changes in Q̇aw during exercise in animals remain controversial. Two studies by Manohar and coworkers (Manohar, 1990; Manohar et al., 1992) have reported that Q̇aw, measured using microspheres, increased during exercise in ponies. In contrast, Bishop et al. (2007) reported that Q̇aw, measured using a flowmeter mounted on the bronchial artery, decreased during incremental exercise in sheep.
While these differential responses in Q̇aw during exercise may be species specific (Coleridge and Coleridge, 1994), to date no study has examined changes in Q̇aw during exercise in humans. One reason for the failure to examine these changes during exercise in humans is the invasive nature of the techniques required to measure Q̇aw. The development of the non-invasive method using the soluble gas dimethyl ether (DME) has made the measurement of Q̇aw far more practical in humans (Wanner et al., 1988). This technique, which has been validated against accepted invasive techniques in animals (Scuri et al., 1995), is relatively easy to use and can be performed during low to moderate-intensity, steady-state exercise in humans.
Therefore, the aim of this study was to measure Q̇aw in healthy untrained subjects at different levels of exercise intensity and to determine the relationship between changes in Q̇aw, Q̇tot and V̇E. We hypothesized that a progressive rise in Q̇aw would occur as exercise intensity was increased. Moreover, we hypothesized that the rise in Q̇aw, with a stepwise increase in submaximal exercise intensity, would be associated with a corresponding rise in Q̇tot and V̇E.
2. Methods
2.1. subject details and exercise protocol
Twelve healthy subjects (7 females and 5 males) participated in this study. subject details are provided in Table 1. Spirometry results indicate that subjects were free of lung disease. subjects were also normotensive and not taking any prescription medications for control of blood pressure or bronchial asthma. The Mayo Clinic Institutional review board approved the study and all subjects signed a written consent form prior to commencement.
Table 1.
Physical characteristics, spirometry results and resting blood pressure of subjects
| Age (yr) | 34 ± 11 |
| Male/female | 5/7 |
| BMI (kg m−2) | 24.3 ± 3.0 |
| FEV1 (% predicted) | 101 ± 11 |
| FVC (% predicted) | 101 ± 15 |
| FEV1/FVC (%) | 84 ± 6 |
| SBP (mmHg) | 107 ± 9 |
| DBP (mmHg) | 66 ± 12 |
Values are mean ± S.D.; BMI: body mass index; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; SBP: systolic blood pressure; DBP: diastolic blood pressure.
Q̇aw and Q̇tot were measured pre-exercise, at each of two different exercise Loads (exercise Load 1 and Load 2) and post-exercise. For male subjects, exercise Load 1 and Load 2 typically corresponded to cycling on a stationary ergometer (Corival 400, St. Paul, MN, USA) at 40 and 80 W, respectively. The two exercise loads for female subjects typically corresponded to cycling at 30 and 60 W respectively. Exercise loads were determined following pilot testing and familiarization to determine each subject’s ability to perform breath-holding required for measuring Q̇aw.
Subjects exercised for 10 min at each of the corresponding workloads. Dual measures of Q̇tot were made at 3 and 5 min of exercise, while the measurement of Q̇aw was obtained between minutes 6 and 10 of exercise. Recovery measures of Q̇tot were made 3 and 5 min post exercise, while the recovery measurement of Q̇aw was obtained between minutes 6 and 10 after the cessation of exercise.
Systolic and diastolic blood pressure measurements were made using auscultation prior to and during each of the exercise loads and during the recovery period. Mean arterial pressure was calculated by summing the diastolic blood pressure and one third of the pulse pressure.
2.2. Determination of airway blood flow
Airway blood flow was determined using the modified soluble, gas DME technique that has previously been described (Scuri et al., 1995; Wanner et al., 2006). DME has a high solubility in tissue and blood (Bunsen solubility coefficient of DME, α, in tissue and blood is 9 ml ml−1 at 37° C). When inhaled into the pulmonary dead space (VD) DME has a biphasic pattern of uptake following equilibration. The first rapid phase of uptake will occur when DME dissolves in the tissue. The slower second phase of uptake occurs as the blood flow in the bronchial capillaries take up DME. Assuming rapid equilibration of DME within the airways and tissue, then the steady-state uptake of the DME (V̇DME) over a defined segment of the conducting airway will be proportional with Q̇aw (Scuri et al., 1995). Airway blood flow can be calculated using mass balance as:
where FDME represents the mean fractional concentration of DME and α the solubility coefficient of DME in blood. V̇DME can be calculated from the slope of the mean expired DME concentration over time multiplied by the volume of the defined airway segment in the conducting airways (VS). The DME concentration was determined from phase 1 of the expired nitrogen (N2) washin curve. In the current study, the defined airway segment (VS) was determined as:
where VD,min is the minimal dead space volume, determined from the volume exhaled to the point of initial departure of exhaled FN2 from room air. Twenty milliliters represent the first 20 ml exhaled at the end of breath-hold and represents the oropharyngeal volume.
In the equation, α is defined as the volume of gas at standard, temperature and pressure dry (STPD) dissolved in 1 ml of liquid at 760 mmHg measured at body temperature (37° C or 310 K) where the water vapour pressure is 47 mmHg. All other elements in the equation are corrected accordingly. VS was measured under body temperature pressure saturated (BTPS) conditions. When corrected, the STPD factor for VS is [(760 − 47) × 273]/[760 × 310] or 0.83.
Hence Q̇aw = 0.83(VD,min − 20 ml) × (slope of mean expired DME concentration)/(αFDME).
Normalising the measurement of Q̇aw to the dead space volume from which DME uptake is measured (i.e., VD,min − 20 ml), this equation reduces to:
where Q̇aw is expressed in microliters per minute per milliliter of dead space.
2.3. Technique for measurement of airway blood flow
See Fig. 1 for a schematic representation of the experimental setup. To measure Q̇aw, the subject breathed through a mouthpiece connected in series to a heated pneumotachometer (Custom Series 3700, Hans Rudolph, Kansas City, MO, USA), a shutter valve (Series 4260A, Hans Rudolph, Kansas City, MO, USA) and a 3-way slide valve (Series 8560A, Hans Rudolph, Kansas City, MO, USA). The 3-way slide valve was connected to a reservoir tank containing 9% DME in a balance of N2. The total dead space in this system was less than 44 ml. Concentrations of DME and N2 were sampled at the mouth using a mass spectrometer (MGA 1100, Perkins Elma, MA Tech, St Louis, MO, USA). The signals from the pneumotachometer and the mass spectrometer were time aligned and sent to a computer via an analogue-to-digital board (Measurement Computing, PCI DAS 6034, Norton, MA, USA) where they were processed using custom written software.
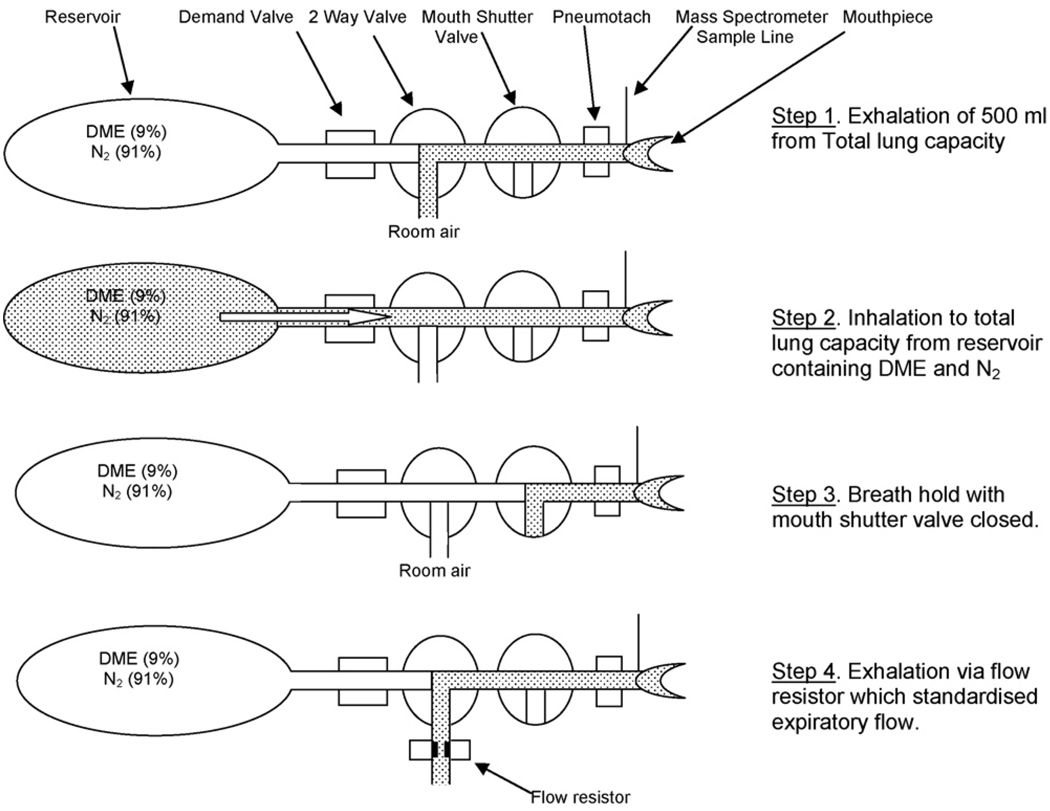
Fig. 1.
Experimental apparatus and technique for measuring airway blood flow showing the four steps used both at rest and during exercise. Pneumotach: pneumotachometer; DME: dimethyl ether; N2: nitrogen.
To measure Q̇aw, subjects performed a four-step maneuver (Fig. 1). In the first step, the subject breathed out 500 ml (Fig. 1a) from total lung capacity (TLC). In the second step, the slide valve port to the reservoir tank containing DME and N2 was opened and the subject rapidly inhaled back up to TLC (Fig. 1b) via a demand valve (01DV2000, O-Two Medical Technologies, Mississauga, CA, USA). In the third step, the subject breath-held for a pre-determined period during which time the shutter valve was closed (Fig. 1c). In the fourth step, the subject breathed out through a flow resistor designed to standardize expiratory flow (Fig. 1d).
For each measurement of Q̇aw, each subject performed maneuvers with two sets of breath-hold times of 5, 8 and 10s. In our laboratory, the test-retest reliability for determining Q̇aw is 0.98 in 8 healthy subjects (mean age 31 ± 9 yr) with a coefficient of variation of 3.8%.
2.4. Measurement of oxygen uptake
Oxygen uptake (V̇O2), V̇E and end-tidal CO2 (PETCO2) were measured breath-by-breath using a metabolic measuring system (CPX-D, Medical Graphics Corporation, St. Paul, MN, USA). In the present study PETCO2 was measured to provide a non-invasive estimate of PaCO2 (Weinger and Brimm, 1987). Metabolic data were collected for a 3-min period at rest, during each of the exercise loads and during recovery.
2.5. Measurement of cardiac output
Q̇tot was measured using the open-circuit acetylene washin technique. This technique has been validated using the direct Fick method in healthy adults and is not influenced by breathing pattern and does not create further breathlessness by a build up of CO2 (no rebreathing, Johnson et al., 2000). The average of the two repeated measures was reported as the Q̇tot.
2.6. Calculation of bronchial vascular resistance
Bronchial vascular resistance (BVR) was determined from the ratio of bronchial perfusion pressure and Q̇aw. In the current study we did not obtain a direct measurement of bronchial perfusion pressure, rather we estimated perfusion pressure from mean arterial pressure.
2.7. Statistical analysis
All data are presented as mean ± standard deviation (S.D.). The change in Q̇aw, from rest, to Load 1, Load 2 and back to rest were assessed using a repeated measures analysis of variance (ANOVA). Where a significant difference was found, Bonferroni post hoc tests were used to determine where this effect occurred. Relationships between Q̇aw and other dependent measures were evaluated using linear regression analysis. Statistical significance was set at P < 0.05.
3. Results
The metabolic, cardiovascular and Q̇aw responses measured at rest, during exercise and recovery are shown in Table 2. There was a significant, stepwise increase in both the measured metabolic (V̇O2, V̇E) and cardiovascular responses (HR, Q̇tot and mean arterial pressure) to exercise. Each of the measured metabolic and cardiovascular responses returned to pre-exercise levels during recovery.
Table 2.
Physiological responses measured pre-exercise, during exercise and in recovery
| Pre-exercise | Load 1 | Load 2 | Recovery | |
|---|---|---|---|---|
| Power (W) | Rest | 34 ± 5 | 68 ± 10 | Rest |
| V̇O2 (l min−1) | 0.37 ± 0.08 | 0.92 ± 0.18* | 1.32 ± 0.17* | 0.41 ± 0.08 |
| V̇e (l min−1) | 13.2 ± 3.6 | 22.4 ± 5.4* | 32.2 ± 6.7* | 13.9 ± 4.7 |
| PETCO2 (mmHg) | 33 ± 4 | 37 ± 3* | 38 ± 4* | 33 ± 3 |
| HR (beats min−1) | 74 ± 9 | 89 ± 8* | 106 ± 9* | 76 ± 13 |
| Q̇tot (l min−1) | 5.3 ± 1. 3 | 7.6 ± 1.2* | 9.8 ± 1. 3 * | 5.1 ± 1.1 |
| MAP (mmHg) | 80 ± 10 | 85 ± 9* | 93 ± 9* | 84 ± 10 |
| Q̇aw (µlml−1 min−1) | 52.8 ± 19.5 | 67.0 ± 20.3* | 84.0 ± 22.9* | 51.7 ± 13.2 |
| BVR (units) | 1.7 ± 0.7 | 1.4 ± 0.6* | 1.2 ± 0.3* | 1. 7 ± 0.4 |
Results are mean ± S.D. VO2: oxygen uptake; V̇E: minute ventilation. PETCO2: endtidal carbon dioxide; HR: heart rate; Q̇tot: cardiac output; MAP: mean arterial pressure; Q̇aw: airway blood flow. AVR (units): BVR (mmHg (µl ml−1 min−1)−1).
P < 0.05, different from pre-exercise mean.
We also found a significant, stepwise increase in Q̇aw during exercise at Load 1 and Load 2. Q̇aw returned pre-exercise levels during recovery. Q̇aw represented 130 ± 7% and 179 ± 26% of preexercise values during exercise at Load 1 and Load 2 respectively. Accompanying the stepwise increase in Q̇aw we found a progressive fall in BVR during exercise at Load 1 and Load 2 which returned to the pre-exercise levels during recovery.
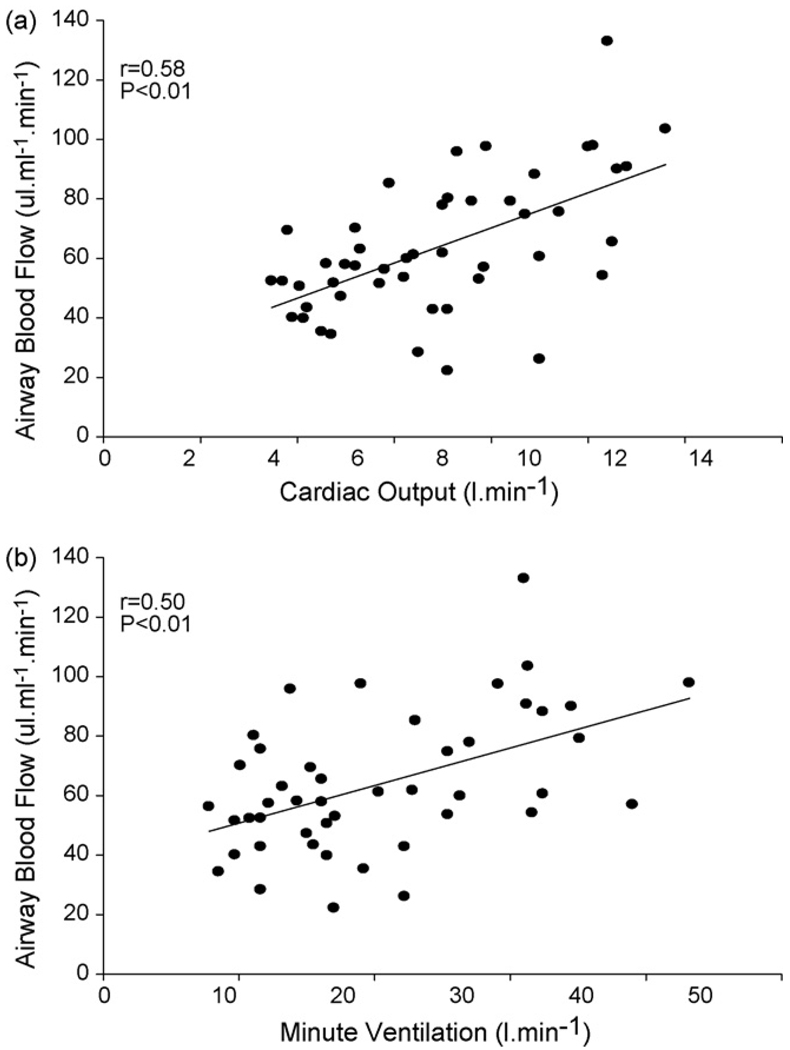
Univariate correlation coefficients were calculated for the relationships between Q̇aw and Q̇tot, V̇E and PETCO2. Significant univariate correlation coefficients (P < 0.01) were found for the relationship between Q̇aw and Q̇tot (r = 0.58) and between Q̇aw and VE (r = 0.50) (Fig. 2a and b respectively). No significant correlation was found between Q̇aw and PETCO2 (r = 0.24, P = 0.15).
Fig. 2.
Relationship between airway blood flow (Q̇aw) and cardiac output (Q̇tot) (a) and minute ventilation (V̇E) (b).
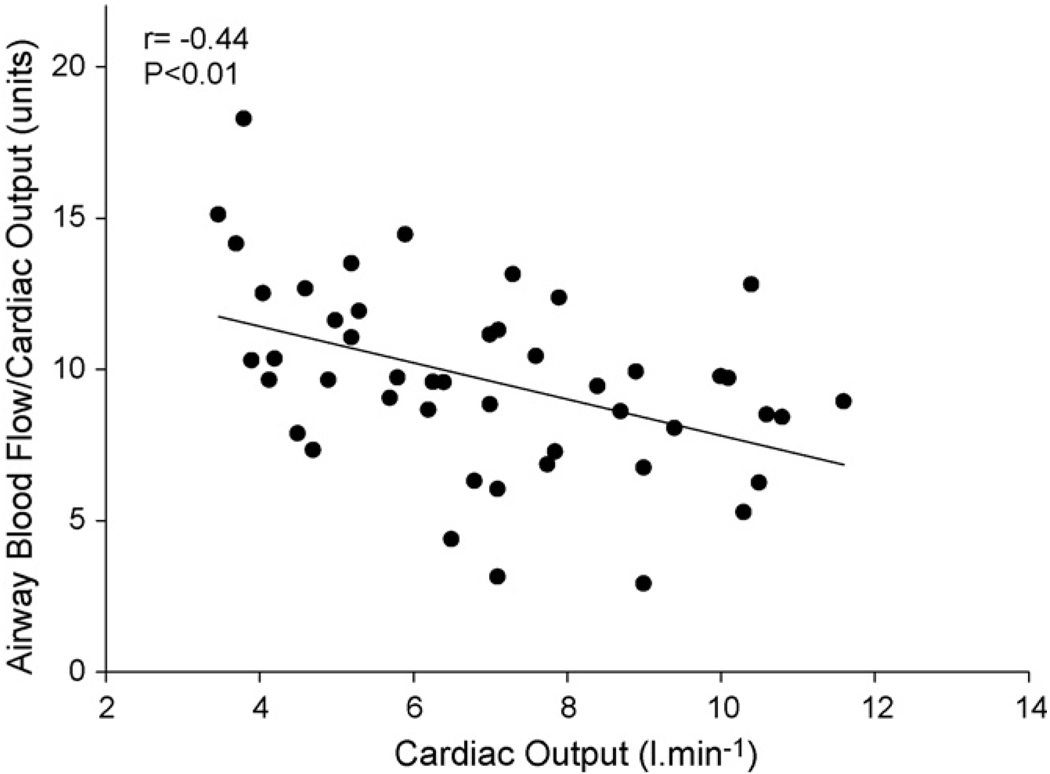
To determine the relative change in Q̇aw per unit change in cardiac output (Q̇aw/Q̇tot) we plotted Q̇aw/Q̇tot vs Q̇tot (Fig. 3). The relative change in Q̇aw was inversely related to Q̇tot (r = −0.44, P < 0.01).
Fig. 3.
Relationship between airway blood flow per unit cardiac output (Q̇aw/Q̇tot) and cardiac output.
4. Discussion
This is the first study to examine simultaneous changes in Q̇aw, Q̇tot and V̇E during submaximal exercise in humans. Using noninvasive gas techniques to independently measure Q̇aw and Q̇tot, we found that Q̇aw increased during exercise and that these changes were related to the rise in both Q̇tot and V̇E.
We found that after approximately 10 min of submaximal exercise at Load 1 (34 W) Q̇aw had risen significantly and that an additional 10 min of exercise at a higher exercise intensity (68 W) resulted in a further rise in Q̇aw. In the present study, we examined changes in Q̇aw during submaximal exercise loads which could be best classified as being low to moderate (less than 80 W) intensity rather than heavy. We were unable to examine changes in Q̇aw at higher exercise intensities as subjects would have difficulty sustaining breath-holds for longer than 10 s under higher-intensity exercise conditions.
Our results suggest that the changes in airway circulation are related to changes in cardiac output during exercise (Fig. 2a). This result is similar to that of Elsasser et al. (1991) who reported a positive correlation between hypoxemia induced changes in Q̇aw and changes in Q̇tot in anaesthetized sheep. However when we examined the relative change in Q̇aw (i.e. the change in Q̇aw relative to the change in Q̇tot) the rise in Q̇aw during exercise tended to be less than the rise Q̇tot as shown by the inverse relationship between Q̇aw/Q̇tot and Q̇tot (Fig. 3). Therefore, our results suggest that while there is an increase Q̇aw and Q̇tot during exercise, the proportion of Q̇tot which is directed towards the airways tends to decrease.
Hemodynamically, the rise in Q̇aw during exercise was due to both an increase in the perfusion pressure (as measured by an increase in mean arterial pressure) and a decrease in the BVR (Table 2). We did not obtain a direct measurement of bronchial perfusion pressure, which is estimated from the difference in mean arterial pressure and pulmonary artery pressure, i.e., bronchial perfusion pressure = mean arterial pressure – pulmonary artery pressure (Charan et al., 1998). However, given the low to moderate intensity of exercise employed in the current study, we would predict that the changes in PAP from rest to exercise would have been negligible (Stickland et al., 2004) and hence the approximation of perfusion pressure using the mean arterial pressure would be representative.
The fall in BVR (Table 2) suggests that there is a vasodilation of the bronchial vasculature during exercise. The vascular smooth muscle of the bronchial arteries has both α1 and β2 adrenergic receptors, with α1 adrenergic receptors tending to predominate (Coleridge and Coleridge, 1994; Onorato et al., 1994; White et al., 2003). Brieva and Wanner (2001) showed that inhalation of an α1 receptor agonist resulted in an decrease in resting Q̇aw, while inhalation of a β2 receptor agonist resulted in an increase. Apart from these adrenergic receptor mediated responses, there is evidence that Q̇aw is also regulated by shear stress and the release of endothelial related vasodilators (especially nitric oxide–NO) (White et al., 2003) and the release of local vasodilatory prostaglandins or neuropeptides associated with changes in local airway temperature (Baile et al., 1990).
Bronchial blood flow has been shown to be extremely labile and may be increased significantly by factors such as changes in temperature and hyperventilation (Coleridge and Coleridge, 1994). In the current study, we also found that the increase in Q̇aw was related to an increase in V̇E. Several animal studies have found a rise in Q̇aw associated with an increase in eucapneic hyperventilation (Baile et al., 1987, 1990; Parsons et al., 1989). In addition, hypercapnia has been associated with a marked increase Q̇aw (Baile and Pare, 1983). We measured PETCO2 as an indication of the changes in PaCO2 during exercise (Weinger and Brimm, 1987). During exercise there was a significant rise in the PETCO2, however this small increase remained well within a normal range. Given this, it could be argued that as PETCO2 remained unchanged and that the ventilatory response in the current study was essentially eucapneic hyperpnoea.
In animal studies, the rise in Q̇aw in response to an increase in V̇E appears to be an important mechanism to maintain airway temperature and to mitigate airway cooling and drying (Parsons et al., 1989). During eucapnic hyperventilation at 20 and 40 l min−1 with warm dry air in ventilated sheep, Parsons et al. (1989) showed there was a stepwise increase in Q̇aw. Similarly in humans, Kim et al. (1996) reported that eucapnic hyperventilation (40 l min−1) with frigid air resulted in an increase in Q̇aw whereas the same level of ventilation with room air failed to result in any change in Q̇aw. These investigators concluded that the magnitude of change in of increase in Q̇aw may reflect the magnitude of the thermal stress on the airway rather than the level of V̇E.
The temperature of the airway surface itself reflects a dynamic balance between heat loss (through evaporation, conduction and convection) and heat delivery by way of the bronchial circulation (Baile et al., 1985). Airway surface temperature will fall if there is an increase in heat loss through evaporation, conduction and convection without any concomitant increase in heat delivery by way of the bronchial circulation (i.e., an increase in Q̇aw). During exercise, increased airflow over the airway as a result of an increase in V̇E will result in greater evaporative and convective heat loss. In turn, Q̇aw would need to increase if the airway is to minimize the degree of cooling and drying that may occur during exercise (Parsons et al., 1989). Unfortunately the current study did not measure the temperature and humidity of the expired air during exercise and we acknowledge that further discussion on the change in airway surface temperature as a stimulus for the increase in Q̇aw during exercise remains beyond the scope of the current paper.
The results of the present study are in contrast to those reported by Bishop et al. (2007). These investigators found that Q̇aw, measured using a flowmeter mounted on the bronchial artery, decreased during moderate-intensity incremental exercise in four sheep. Bishop et al. (2007) also reported that airway diameter actually decreased during incremental exercise. These results were supported by a study by the same group (Quail et al., 2007) published at the same time. These studies (Bishop et al., 2007; Quail et al., 2007) concluded that at the onset of exercise there was a stimulus dependent constrictor effect in both the airway and the vascular beds.
The difference in these results to those of the present study may be due in part to the exercise protocol used to study changes in Q̇aw. Where we examined changes in Q̇aw during steady-state exercise, Bishop et al. (2007) used a rapid incremental protocol (i.e., 1-min exercise bouts) consisting of short exercise bouts. These researchers have suggested that the rapid incremental protocol may have resulted in a constrictor rather than a dilatory response in both the airways and the vascular beds of the airways due to the fact that secondary factors such as thermal stress and the build up of metabolic by products of exercise that would have resulted in a dilation of the bronchial artery, may not have had time to take effect.
There are some limitations to the current study. Firstly the exercise intensities were low to moderate at best and we were unable to have subjects exercise at higher exercise intensities because of the breath holding requirement of the Q̇aw technique. Exercise at higher intensities may have provided information on whether Q̇aw continued to rise or plateaued at higher intensities. Secondly, given that we used a soluble gas technique to measure Q̇aw, there is the potential that the increase in Q̇aw may have been the result of an alveolar uptake of the DME. We believe this is unlikely as we were clearly able to define phase 1 of the expired N2 washin curve in our analysis. Using this method we were clearly able to detect a DME concentration during expiration that excluded any alveolar gas mixing. In our validation of the technique we sampled the expired DME concentration earlier during the phase 1 expiratory phase (i.e. from a more proximal point in the deadspace) and we found that this did not significantly alter the measured Q̇aw. Thirdly we note that the sample size of 12 is small and may represent a limitation to the interpretation of our results. While we found that changes in Q̇aw, Q̇tot and V̇E and the relationships between these changes were relatively homogenous during exercise, we acknowledge that a larger powered study would be warranted.
In conclusion, this study found a significant stepwise rise in Q̇aw with an increase in exercise intensity that was associated with an increase in both Q̇tot and V̇E. Our results suggest that the increase in Q̇aw is mediated by both an increase in perfusion pressure and a decrease in BVR. The increase in Q̇aw may in turn be necessary to prevent excessive airway cooling and drying during exercise.
Acknowledgments
The authors of this study would like to thank Kathy O’Malley for her assistance in the data collection and management of this project. This work was supported in part by National Institute of Health grant HL71478 and Griffith University.
References
- Baile EM, Dahlby RW, Wiggs BR, Pare PD. Role of tracheal and bronchial circulation in respiratory heat exchange. J. Appl. Physiol. 1985;58(1):217–222. doi: 10.1152/jappl.1985.58.1.217. [DOI] [PubMed] [Google Scholar]
- Baile EM, Dahlby RW, Wiggs BR, Parsons GH, Pare PD. Effect of cold and warm dry air hyperventilation on canine airway blood flow. J. Appl. Physiol. 1987;62(2):526–532. doi: 10.1152/jappl.1987.62.2.526. [DOI] [PubMed] [Google Scholar]
- Baile EM, Godden DJ, Pare PD. Mechanism for increase in tracheobronchial blood flow induced by hyperventilation of dry air in dogs. J. Appl. Physiol. 1990;68(1):105–112. doi: 10.1152/jappl.1990.68.1.105. [DOI] [PubMed] [Google Scholar]
- Baile EM, Pare PD. Response of the bronchial circulation to acute hypoxemia and hypercarbia in the dog. J. Appl. Physiol. 1983;55(5):1474–1479. doi: 10.1152/jappl.1983.55.5.1474. [DOI] [PubMed] [Google Scholar]
- Bishop R, Mcleod D, McIlveen S, Blake R, Gunther R, Davis J, Talken L, Cottee D, Quail A, Parsons G, White S. Effects of graded exercise on bronchial blood flow and airway dimensions in sheep. Pulm. Pharmacol. Ther. 2007;20(2):178–189. doi: 10.1016/j.pupt.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Brieva J, Wanner A. Adrenergic airway vascular smooth muscle responsiveness in healthy and asthmatic subjects. J. Appl. Physiol. 2001;90(2):665–669. doi: 10.1152/jappl.2001.90.2.665. [DOI] [PubMed] [Google Scholar]
- Charan NB, Carvalho P, Johnson SR, Thompson WH, Lakshminarayan S. Effect of aerosolized acetylcholine on bronchial blood flow. J. Appl. Physiol. 1998;85(2):432–436. doi: 10.1152/jappl.1998.85.2.432. [DOI] [PubMed] [Google Scholar]
- Coleridge HM, Coleridge JCG. Neural regulation of airway blood flow. Respir. Physiol. 1994;98:1–13. doi: 10.1016/0034-5687(94)90032-9. [DOI] [PubMed] [Google Scholar]
- de Bisschop C, Pichon A, Guenard H, Denjean A. Accounting for flow dependence of respiratory resistance during exercise. Respir. Physiol. Neurobiol. 2003;136(1):65–76. doi: 10.1016/s1569-9048(03)00106-x. [DOI] [PubMed] [Google Scholar]
- Deffebach ME, Charan NB, Lakshminarayan S, Butler J. The bronchial circulation. Small, but a vital attribute of the lung. Am. Rev. Respir. Dis. 1987;135(2):463–481. doi: 10.1164/arrd.1987.135.2.463. [DOI] [PubMed] [Google Scholar]
- Elsasser S, Long WM, Baier HJ, Chediak AD, Wanner A. Independent control of mucosal and total airway blood flow during hypoxemia. J. Appl. Physiol. 1991;71(1):223–228. doi: 10.1152/jappl.1991.71.1.223. [DOI] [PubMed] [Google Scholar]
- Johnson BD, Beck KC, Proctor DN, Miller J, Dietz NM, Joyner MJ. Cardiac output during exercise by the open circuit acetylene washin method: comparison with direct. Fick J. Appl. Physiol. 2000;88(5):1650–1658. doi: 10.1152/jappl.2000.88.5.1650. [DOI] [PubMed] [Google Scholar]
- Kim HH, LeMerre C, Demirozu CM, Chediak AD, Wanner A. Effect of hyperventilation on airway mucosal blood flow in normal subjects. Am. J. Respir. Crit. Care Med. 1996;154(5):1563–1566. doi: 10.1164/ajrccm.154.5.8912781. [DOI] [PubMed] [Google Scholar]
- Manohar M. Tracheobronchial perfusion in ponies. J. Appl. Physiol. 1990;68(5):2182–2185. doi: 10.1152/jappl.1990.68.5.2182. [DOI] [PubMed] [Google Scholar]
- Manohar M, Duren SE, Sikkes BP, Day J, Baker JP. Bronchial circulation during prolonged exercise in ponies. Am. J. Vet. Res. 1992;53(6):925–929. [PubMed] [Google Scholar]
- Onorato DJ, Demirozu MC, Breitenbucher A, Atkins ND, Chediak AD, Wanner A. Airway mucosal blood flow in humans. Response to adrenergic agonists. Am. J. Respir. Crit. Care Med. 1994;149(5):1132–1137. doi: 10.1164/ajrccm.149.5.8173752. [DOI] [PubMed] [Google Scholar]
- Parsons GH, Pare PD, White DA, Baile EM. Airway blood flow response to eucapnic dry air hyperventilation in sheep. J. Appl. Physiol. 1989;66(3):1443–1447. doi: 10.1152/jappl.1989.66.3.1443. [DOI] [PubMed] [Google Scholar]
- Quail A, McIlveen S, Bishop R, Mcleod D, Gunther R, Davis J, Talken L, Cottee D, Parsons G, White S. Autonomic control of bronchial blood flow and airway dimensions during strenuous exercise in sheep. Pulm. Pharmacol. Ther. 2007;20(2):190–199. doi: 10.1016/j.pupt.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Scuri M, McCaskill V, Chediak AD, Abraham WM, Wanner A. Measurement of airway mucosal blood flow with dimethylether: validation with microspheres. J. Appl. Physiol. 1995;79(4):1386–1390. doi: 10.1152/jappl.1995.79.4.1386. [DOI] [PubMed] [Google Scholar]
- Stickland MK, Welsh RC, Haykowsky MJ, Petersen SR, Anderson WD, Taylor DA, Bouffard M, Jones RL. Intra-pulmonary shunt and pulmonary gas exchange during exercise in humans. J. Physiol. 2004;561(Pt 1):321–329. doi: 10.1113/jphysiol.2004.069302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner A, Barker JA, Long WM, Mariassy AT, Chediak AD. Measurement of airway mucosal perfusion and water volume with an inert soluble gas. J. Appl. Physiol. 1988;65(1):264–271. doi: 10.1152/jappl.1988.65.1.264. [DOI] [PubMed] [Google Scholar]
- Wanner A, Mendes ES, Atkins ND. A simplified noninvasive model for measuring airway blood flow in humans. J. Appl. Physiol. 2006;100:1674–1678. doi: 10.1152/japplphysiol.01349.2005. [DOI] [PubMed] [Google Scholar]
- Weinger MB, Brimm JE. End-tidal carbon dioxide as a measure of arterial carbon dioxide during intermittent mandatory ventilation. J. Clin. Monit. 1987;3(2):73–79. doi: 10.1007/BF00858353. [DOI] [PubMed] [Google Scholar]
- White S, McIlveen S, Parsons G, Quail A, Cottee D, Gunther R, Bishop R, McLeod D, Blake R. Neural control of the bronchial circulation. Arch. Physiol. Biochem. 2003;111(4):305–308. doi: 10.3109/13813450312331337450. [DOI] [PubMed] [Google Scholar]