Abstract
Velo-cardio-facial syndrome is one of the names that has been attached to one of the most common multiple anomaly syndromes in humans. The labels DiGeorge sequence, 22q11 deletion syndrome, conotruncal anomalies face syndrome, CATCH 22, and Sedlačková syndrome have all been attached to the same disorder. Velo-cardio-facial syndrome has an expansive phenotype with more than 180 clinical features described that involve essentially every organ and system. The syndrome has drawn considerable attention because a number of common psychiatric illnesses are phenotypic features including attention deficit disorder, schizophrenia, and bipolar disorder. The expression is highly variable with some individuals being essentially normal at the mildest end of the spectrum, and the most severe cases having life-threatening and life-impairing problems. The syndrome is caused by a microdeletion from chromosome 22 at the q11.2 band. Although the large majority of affected individuals have identical 3 megabase deletions, less than 10% of cases have smaller deletions of 1.5 or 2.0 megabases. The 3 megabase deletion encompasses a region containing 40 genes. The syndrome has a population prevalence of approximately 1:2,000 in the U.S., although incidence is higher. Although initially a clinical diagnosis, today velo-cardio-facial syndrome can be diagnosed with extremely high accuracy by fluorescence in situ hybridization (FISH) and several other laboratory techniques. Clinical management is age dependent with acute medical problems such as congenital heart disease, immune disorders, feeding problems, cleft palate, and developmental disorders occupying management in infancy and preschool years. Management shifts to cognitive, behavioral, and learning disorders during school years, and then to the potential for psychiatric disorders including psychosis in late adolescence and adult years. Although the majority of people with velo-cardio-facial syndrome do not develop psychosis, the risk for severe psychiatric illness is 25 times higher for people affected with velo-cardio-facial syndrome than the general population. Therefore, interest in understanding the nature of psychiatric illness in the syndrome remains strong.
Keywords: velo-cardio-facial syndrome, chromosome 22, microdeletion syndrome, 22q11 deletion syndrome, DiGeorge syndrome, neurodevelopmental disorder
INTRODUCTION
Velo-cardio-facial syndrome (VCFS) is one of the most common multiple anomaly syndromes in humans. The syndrome’s pattern of inheritance was confirmed to be autosomal dominant in reports in the 1980s [Shprintzen et al., 1981; Williams et al., 1985] and its specific genetic cause was found in 1992 when a microdeletion of chromosome 22 at band q11.2 was described [Scambler et al., 1992]. Several other reports followed that confirmed the microdeletion [Driscoll et al., 1992; Kelly et al., 1993]. VCFS has several other labels that have been applied in a variety of literature sources around the world. As a result, some clinicians and researchers mistakenly believe that each label represents a separate and specific syndrome each caused by the same deletion from chromosome 22 [Robin and Shprintzen, 2005]. These disorders include DiGeorge sequence (often mistakenly labeled as DiGeorge syndrome), Sedlačková syndrome, conotruncal anomalies face syndrome, and Cayler syndrome [Robin and Shprintzen, 2005; Shprintzen, 2005b and c]. Other names that have been applied include 22q11 deletion syndrome and CATCH 22. Although 22q11 deletion syndrome has been adopted by some clinicians and researchers, it has not gained universal acceptance in much the same way that trisomy 21 is used less frequently than Down syndrome to describe that disorder. Velo-cardio-facial syndrome is descriptive of the clinical presentations and is preferred in many circles. CATCH 22 has been decried as an attempt at humor at the expense of affected individuals. Catch 22 is the title of a black humor novel by Joseph Heller and is a term reserved for a “no-win” situation, or an impossible circumstance with no solution [Robin and Shprintzen, 2005], something that is clearly inappropriate for a syndrome like VCFS that can often be treated very effectively.
As discussed by Robin and Shprintzen [2005], an individual with a deletion from chromosome 22 at band q11.2 has VCFS (22q11 deletion syndrome or whatever other label you choose) and if there is no deletion, then the individual does not have the syndrome. It is also true that any diagnostic label that involves the same 22q11.2 deletion represents exactly the same disorder regardless of the name [Robin and Shprintzen, 2005; Shprintzen, 2005b and c]. Although this may seem to be a trivial point, it is not at all. For example, Opitz syndrome (also known as B/GGG syndrome) was reported erroneously to be caused by the same deletion of chromosome 22 as VCFS [McDonald-McGinn et al., 1995; Fryburg et al., 1996]. The distinction between the two syndromes is important because the diagnostic and treatment implications are significant. Opitz syndrome has specific anomalies of the larynx and esophagus that do not occur in VCFS, but VCFS has anomalies of the larynx different from those in Optiz syndrome [Chegar et al., 2006]. The diagnosis and management of laryngeal cleft or tracheoesophageal fistula, which do occur in Opitz syndrome but do not in VCFS, would be completely different than the management of laryngeal web and laryngomalacia that occur in VCFS. The interpretation of “esophageal abnormalities” as consistent with VCFS is erroneous in nearly all cases. Anomalies of the internal carotid arteries do occur in VCFS, but not in Opitz syndrome. Most importantly, a strong link to psychiatric illness has been established in VCFS, but not in Opitz syndrome. Therefore, diagnostic errors can result in potentially dangerous decisions about management based on the presumed phenotypic spectrum of the syndrome. Although feeding difficulties occur in both VCFS and Opitz syndrome, the structural malformations that lead to them are entirely different.
The use of different names for the same condition is not new in the field of clinical genetics. Besides the previously mentioned Down syndrome and trisomy 21, many other nosologic variations exist. There is no specific system of taxonomy in the naming of syndromes. There are many examples of disorders that have eponyms, including Wolf-Hirschhorn syndrome, Williams syndrome, Angelman syndrome, Prader-Willi syndrome, and Kallmann syndrome to name a few. On occasion, a single syndrome will have multiple eponyms, including Williams syndrome (also called Beuren-Williams syndrome by some), or Treacher Collins syndrome (also known as Franceschetti syndrome), but VCFS may be unique in having five eponyms assigned to it (Shprintzen syndrome, DiGeorge syndrome, Cayler syndrome, Takao syndrome, and Sedlačková syndrome). Some syndromes have their names linked to their causes, although this has caused some problems with syndromes that are etiologically heterogeneous. Besides 22q11 deletion syndrome, there is trisomy 13, trisomy 18, amnion rupture sequence, fetal alcohol syndrome, retinoic acid embryopathy, homocystinuria, and phenylketonuria. It is curious that most microdeletion syndromes have escaped this classification system (so that Williams syndrome and Prader-Willi syndrome are not called 7q11.23 deletion syndrome or 15q11 – 13 deletion syndrome respectively), but for some reason VCFS has not. Other syndromes are named symptomatically (often with acronyms), including branchio-oto-renal syndrome (BOR), ectrodactyly, ectodermal dysplasia and clefting syndrome (EEC), and cardio-facial-cutaneous syndrome (CFC). Many syndromes have names that cover two or more of these nosologic systems so that their names may be either symptom based, eponyms, or causation, such as branchio-oto-renal syndrome (also known as Melnick-Fraser syndrome), Treacher Collins syndrome (also known as mandibulofacial dysostosis), neurofibromatosis type 1 (von Recklinghausen disease), and Apert syndrome (acrocephalosyndactyly); but VCFS covers all of those categories and within the eponym category has multiple names. The differences relate to place of training (calling it what your professor called it), geographic location (it is more likely to be Sedlačková syndrome in the Czech Republic and conotruncal anomalies face syndrome in Japan), and politics (not using the name your competitor uses). Regardless, all of these names refer to exactly the same syndrome, and the confusion over the nosology has unfortunately resulted in clinical confusion, as well. As a personal bias (keeping in mind that one of the eponyms includes my difficult to spell and difficult to pronounce last name) VCFS is simply easier to say and write and communicate than any of the other labels and its use should therefore be encouraged.
Diagnostic Criteria
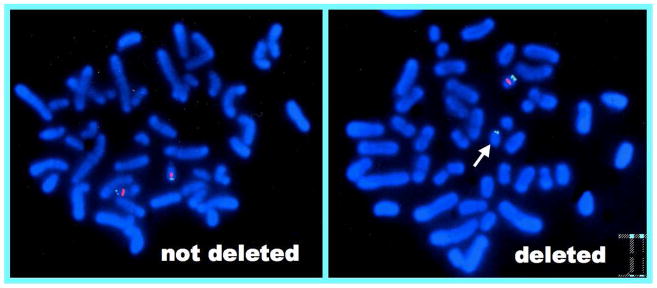
VCFS has an extremely expansive phenotypic spectrum. More than 180 clinical features, both physical and behavioral, have been described [Robin and Shprintzen, 2005; Shprintzen, 2005a and b; VCFS Educational Foundation, 2007]. No single clinical feature occurs in 100% of cases and there is no reported case of the syndrome that has all or even most of the clinical findings. The phenotype therefore shows markedly variable expression. The diagnosis is therefore defined by the deletion of DNA from chromosome 22 at the q11.2 band spanning the region that is regarded as the critical region (see below). The diagnostic procedures used to identify the deletion are highly reliable and for all practical purposes can be regarded as close to 100% reliable [Robin and Shprintzen, 2005; Shprintzen, 2005b and c]. At this time, FISH (Fluorescence In Situ Hybridization) is the most commonly used and most easily accessible diagnostic procedure. FISH is a procedure that uses DNA probes to determine if a specific region of the genome, in this case the 22q11.2 region, is present in two copies in a chromosome preparation obtained from peripheral blood that has been denatured to allow hybridization of a probe specific to the site in question. As noted in Figure 1, only one copy of the chromosome 22q11.2 region is present. FISH for a 22q11.2 deletion is essentially accurate 100% of the time [Robin and Shprintzen, 2005]. If a deletion is not detected, then the patient does not have VCFS, and if a deletion is present, then the patient does have VCFS. Other molecular genetics tests will clearly become widely available in the near future, such as microarray analysis and MLPA, but at the current time, FISH is widely available, relatively cost effective, and highly accurate.
Figure 1.
Fluorescence In Situ Hybridization (FISH) of someone with VCFS (left) compared to a normal inidivual (right). The FISH on the left shows that one copy of chromosome 22 has only one fluorescent signal (the control probe) compared to the normal case that has two signals on each chromosome (control probe and the probe specific to 22q11.2).
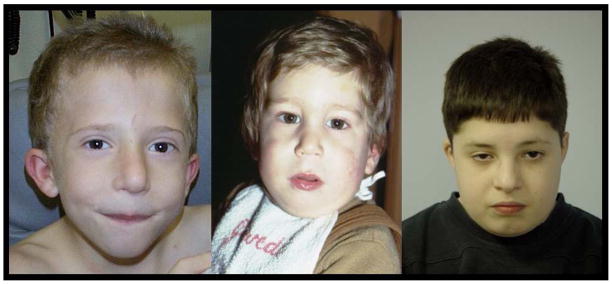
Some clinicians rely on minimal diagnostic criteria or a probability approach to diagnosing genetic syndromes. Using this type of approach, it is possible that someone who has a tetralogy of Fallot, cleft palate, mental retardation, immune disorder, hypotonia, developmental delay, and small ears with overfolded helices could have VCFS. Unfortunately, I was describing an individual with Down syndrome. There are multiple syndromes that present with this combination of anomalies, and even if the list were increased in complexity, there would still be significant phenotypic overlap between a large number of syndromes. Syndromic diagnosis, or even syndromic suspicion is dependent on experience and the ability to use sound deductive reasoning to reach a conclusion, much the same way that Sherlock Holmes solved mysteries. Although the diagnosis depends on the positive FISH test for the deletion, one must still use deductive reasoning to refer for the test unless all patients with a particular condition, such as tetralogy of Fallot, are screened for VCFS. Because VCFS does not involve facial dysmorphism in most cases (Figure 2), there is not as distinctive a facial appearance as in Down syndrome or Williams syndrome. When originally published, the facial appearance was called “typical” meaning that the original dozen cases resembled each other, but not that the appearance was abnormal, per se. Therefore, children with VCFS do not call attention to themselves as do cases with noticeable facial dysmorphisms. It is important for clinicians to become familiar with the “gestalt” of the diagnosis of VCFS unlike Down syndrome that is easily recognized by even inexperienced clinicians.
Figure 2.
Typical facial appearance in VCFS. Note that although the children in these photos have a characteristic facial appearance, it is not abnormal.
The decision to perform FISH for the diagnosis of VCFS depends on clinical suspicion. Screening of newborns with interrupted aortic arch, type B, tetralogy of Fallot, and truncus arteriosus are clearly indicated because of the high yield these anomalies will produce. However, one must keep in mind that the most common heart anomaly in VCFS is ventricular septal defect (VSD) although VCFS cases make up a small percentage of people with VSD. Therefore, children with VSD should be examined by an experienced clinical geneticist to determine if FISH testing is indicated. Among children with hypernasal speech or cleft palate, the yield will be somewhat lower than for major heart anomalies. Therefore, it is necessary to determine if other anomalies are present that would indicate the presence of a multiple anomaly syndrome that might be consistent with VCFS. The presence of asymmetric facial animation, characteristic ears, hypotonia, or any of the other anomalies consistent with VCFS would increase the level of suspicion. The more anomalies that are present, the higher the probability that FISH will be positive. However, there is no substitute for clinical judgment and the power of solid deductive reasoning.
The Prevalence and Incidence of VCFS
There have been a number of reports citing the prevalence or incidence of VCFS with estimates ranging from approximately 1:2000 to approximately 1:7000. Data relating to population statistics are highly dependent on the method of ascertainment of the disease. A large percentage of previous reports were based on birth records, reports of infant examinations, or reports of anomalies from multiple sources that were not controlled for quality or reliability [Ryan et al., 1997; Óskarsdóttir et al., 2004]. Because VCFS is a developmental disorder with clinical findings that are not apparent until later in life, many of the major findings are not evident or detectable at birth or in infancy. At least 30% of cases do not manifest cardiac malformations, and many of those with heart anomalies have silent anomalies, such as right-sided aortic arch that cannot be detected without specifically looking for them. It is probable that at least one-third if not nearly one-half of cases are not diagnosed until later in life and that many cases go undetected.
It is also likely that population prevalence varies with place of birth. Because of the high frequency of severe and life-threatening congenital heart disease associated with VCFS, it is likely that many children born with the syndrome in areas where there is not good access to good surgical care and neonatal intensive care units that many babies with VCFS do not survive the neonatal period. In most developed and medically sophisticated countries, a very high percentage of children with even very severe heart malformations survive. Therefore, population prevalence in nations with access to pediatric cardiothoracic surgeons and intensive care units might have twice the population prevalence compared to developing or undeveloped nations where such care is not uniformly available.
It is also true that population prevalence for VCFS is not as high as incidence of the syndrome. It is known that VCFS triggers several developmental sequences that are incompatible with life, including Potter sequence and holoprosencephaly sequence [Devriendt et al., 1997; Wraith et al., 1985]. Some neonates with VCFS who have pulmonary atresia in association with major heart anomalies also do not survive [Michielon et al., 2006]. Some fetal death associated with these factors is also to be expected. Therefore, the overall frequency of nonsurviving pregnancies, although not known, may be relatively high.
Over the past several years, it has become clear to us that the frequency of VCFS among all surviving newborns in our area is at least 1:2000. This can be stated with a high degree of certainty because our entire region of Central New York has one pediatric cardiology group, one pediatric cardiothoracic surgeon, and one craniofacial center and these practitioners send all of their patients to The VCFS International Center. Based on these data alone with approximately 10,000 births per year in the region covered by our institution, and an average of five neonates per year with VCFS identified from that sample, the ascertainment would be 1:2,000. However, if we factor in babies without congenital heart disease, without overt or obvious submucous cleft palate, there are likely to be nearly one-quarter of the cases that are not detected in infancy (based on the frequency of heart and palate anomalies in VCFS). Therefore, our estimate would put the true population prevalence at approximately 1:1600. This statistic is approximately consistent with our previous report of 1:2000 [Shprintzen, 2005b].
Clinical Features and the VCFS Phenotype
Because VCFS is so common among genetic disorders, many cases have been seen and the phenotype studied from a variety of disciplines including heart, craniofacial, behavioral, vascular, central nervous system and more. More than 180 distinct clinical phenotypes have been described and reported impacting nearly every organ system and developmental function. Table 1 lists the clinical phenotype by category. None of the findings are obligatory, and some occur with low frequency as noted in the table. However, the low frequency anomalies occur at a rate higher than seen in the general population.
There are certain clinical features that can be used as strong indications for suspecting VCFS as the diagnosis that would prompt clinicians to refer their patients for diagnostic lab procedures. Congenital heart disease is present in approximately 70% of cases and the type of heart anomalies may lead to stronger suspicion of VCFS than any other diagnosis. VCFS constitutes a high percentage of conotruncal heart anomalies including more than half of all cases of interrupted aortic arch, type B. More than 15% of newborns with tetralogy of Fallot, slightly less than half of truncus arteriosus cases, and approximately one-third of posterior malalignment ventricular septal defects [Goldmuntz et al., 1998]. The most common heart anomaly found in individuals with VCFS is ventriculoseptal defect, but because VSD is such a common malformation, the percentage of people with VSD who have VCFS is small. Given the high frequency of VCFS among individuals with conotruncal anomalies, newborns with these types of heart anomalies should be screened with FISH if they do not present with another obvious syndrome such as Down syndrome or trisomy 13. It has now been confirmed that hemizygosity for the gene TBX1 is responsible for the cardiac phenotypes in VCFS [Merscher et al., 2001].
VCFS is also common among children with palatal anomalies. Although initially reported as a common finding in children with cleft palate [Shprintzen et al., 1978], the most common forms of palatal anomalies in VCFS are submucous cleft palate and occult submucous cleft palate [Shprintzen, 1982, 2000b,Shprintzen, 2005]. These clefts can be very difficult to identify without state-of-the-art diagnostic procedures such as nasopharyngoscopy. It is for this reason that several reports have significantly underestimated the frequency of clefting, especially when data regarding anomalies has been ascertained from birth records or multiple sources with varying levels of diagnostic expertise [Ryan et al., 1997; Óskarsdóttir et al., 2004]. More specifically, occult submucous cleft palate is an anomaly largely unknown to clinicians who do not specialize in the diagnosis of palatal anomalies. It is for this reason that many reports suggest that individuals with VCFS have “short palates” or hypernasal speech in the absence of a cleft. However, occult submucous cleft palate is an identifiable anomaly that is a form of cleft palate that often goes undetected because it requires endoscopic examination of the nasal surface of the velum [Shprintzen, 1982, 2000b,Shprintzen, 2005]. It has also been found that there is a high frequency of palatal and pharyngeal asymmetry in VCFS that can also only be determined by endoscopic assessment [Chegar et al., 2006]. Among the anomalies seen in the pharynx are major vascular anomalies including abnormal placement of the internal carotid arteries [MacKenzie-Stepner et al., 1987; Mitnick et al., 1996; Tatum et al., 2002] and tortuous or kinked vertebral arteries [Mitnick et al., 1996]. These vascular anomalies have been cited as potentially dangerous risks when pharyngeal surgery is being planned [MacKenzie-Stepner et al., 1987; Mitnick et al., 1996; Tatum et al., 2002]. It has also been reported that the muscle tissue in the pharynx has abnormally small fiber size and abnormal distribution of fiber type [Zim et al., 2003]. All of these factors have been cited as risk factors for the development of velopharyngeal insufficiency and hypernasal speech.
The vascular anomalies of the pharynx may represent one of a number of vascular anomalies associated with VCFS. Major anomalies of the chest vessels [Shprintzen et al., 1978; Young et al., 1980; Shprintzen, 2005b; VCFS Educational Foundation, 2007], neck vessels [MacKenzie-Stepner et al., 1987; Mitnick et al., 1996; Shprintzen, 2000b; Tatum et al., 2002], and brain vasculature [Shprintzen, 2000a] have all been reported in individuals with VCFS. It has been hypothesized that the large number of vascular anomalies in VCFS in major vessels may result in a number of developmental sequences [Shprintzen et al., 1997], a notion supported by reports of Robin sequence [Shprintzen, 1988; Shprintzen and Singer, 1992], DiGeorge sequence [Robin and Shprintzen, 2005; Shprintzen, 2005b], Potter sequence [Devriendt et al., 1997] and holoprosencephaly sequence [Wraith et al., 1985] in individuals with VCFS.
A large number of other structural anomalies have been reported in association with VCFS including ocular anomalies [Mansour et al., 1987], cranial anomalies [Arvystas and Shprintzen, 1984], limb anomalies [Ming et al., 1998], meningomyelocele [Nickel et al., 1994], anal anomalies [Worthington et al., 1997], platelet anomalies [Van Geet et al., 1998], brain anomalies [Worthington et al., 2000; Eliez et al., 2001; Kates et al., 2004; Antshel et al., 2005; Kates et al., 2006a and b; Schaer et al., 2006; Debbane et al., 2006], renal anomalies [Czarnecki et al., 1998; Shprintzen, 2005b], spine and skeletal anomalies [Ricchetti et al., 2004], thyroid disorders [Kawame et al., 2001], and hypoparathyroidism [Hieronimus et al., 2006]. In all, over 180 separate anomalies or developmental sequences have been reported in association with VCFS covering nearly every organ and system [Robin and Shprintzen, 2005; Shprintzen, 2005b; Shprintzen, 2000a; VCFS Educational Foundation, 2007]. However, the anomalies that have attracted the greatest amount of attention are the behavioral and developmental disorders common to VCFS.
The initial report of psychiatric disorders in VCFS was published in 1992 [Shprintzen et al., 1992] and coincided with the identification of the deletion from 22q11.2 [Scambler et al., 1992]. This coincidence sparked substantial research interest because of the notion that a gene that would influence psychiatric phenotypes resided in the deleted region. The search for this influence has continued unabated ever since with focus on a number of candidate genes, including but not limited to COMT [Lachman et al., 1996; Graf et al., 2001; Gothelf et al., 2005] and PRODH [Shprintzen, 2005a; Li et al., 2004]. There has also been substantial research focusing on brain structure and function in VCFS [Worthington et al., 2000; Eliez et al., 2001; Kates et al., 2004; Antshel et al., 2005; Kates et al., 2006a and b; Schaer et al., 2006; Debbane et al., 2006]. Reductions in both grey and white matter volumes have been documented with anomalous characteristics of the corpus callosum, the amygdala, the caudate nucleus, and temporo-parietal regions of the brain. It is not yet clear what biologic basis of mental illness in VCFS, but it is clear that the syndrome presents an excellent model for understanding psychiatric disorders, especially psychosis, in humans. The rate of psychosis in VCFS is more than 25 times greater than it is in the general population. It is therefore almost certain that a genomic factor or factors in the deleted region exert some control over brain development and/or function. Because the large majority of individuals with VCFS have 40 genes deleted, the process of determining the specific factor involved is complicated and the search is continuing. There have, however, been some promising outcomes that may have broader implications for the general population.
One gene that may play an important role is COMT (catechol-O-methyltransferase). COMT is responsible for degrading catecholamines, including dopamine and epinephrine, two major neural transmitters. Once released into the synapses of the brain, catecholamines must be catabolized in order to avoid having an elevated level of these substances that could interfere with normal brain function. COMT sits close to the middle of the deleted region in VCFS and one copy of COMT is deleted in all individuals with VCFS. Polymorphisms in COMT may also play a role in the severity of psychiatric illness. Two polymorphisms that have been studied include a low activity allele (COMT158met) and a high activity allele (COMT158val) [Lachman et al., 1996] and the association of the most severe form of mental illness in adults with VCFS has been reported to occur when a single copy of the COMT158met allele is present [Lachman et al., 1996; Graf et al., 2001]. However, the samples studied to date have been relatively small and more data is required to determine the contribution of COMT and its polymorphisms to the development of mental illness.
Speech and language impairment are also common manifestions of VCFS with approximately 75% of affected individuals having hypernasal speech and a high percentage having severe articulation impairment [Golding-Kushner et al., 1985; Golding-Kushner, 2000; Shprintzen, 2005b; Golding-Kushner, 2007]. Speech onset is usually mildly delayed and receptive language abilities exceed expressive [Golding-Kushner et al., 1985] although some of the expressive disorders are related to unintelligible speech and incorrect management of these disorders in early years [Golding-Kushner, 2000]. However, remediation of these problems has led to excellent prognosis in the large majority of cases.
Immune disorders are also relatively common, but few cases have severe immunodeficiency. In the large majority of cases with immune problems, early childhood is marked by frequent respiratory infections. In most cases, these illnesses present as frequent upper respiratory infections, middle ear effusions, and sinusitis. In the more severe cases, pneumonia and bronchitis may present early in life and persist through childhood. The early onset of pneumonia in VCFS can be confused with aspiration, a rare problem in VCFS [Shprintzen, 2005b]. Children with VCFS often have problems with emesis in infancy and many clinicians mistakenly believe that emesis is “reflux” and that it can lead to aspiration.
Early feeding problems are also common in VCFS. These problems are multifactorial in etiology, prompted by hypotonia, congenital heart disease, endocrine disorders, and airway obstruction secondary to a retruded lower jaw and low muscle tone. Although swallowing disorders have been reported in VCFS [Eicher et al., 2000], there has been no unifying or satisfactory explanation for their origin. Although many children with the syndrome have been treated by long-term gavage feeding and gastrostomy, clinical experience has shown that these techniques are rarely necessary.
Etiology: Specific or Heterogeneous?
As mentioned earlier, it has now been firmly established that all individuals with VCFS have a microdeletion from chromosome 22q11.2 [Robin and Shprintzen 2005; Shprintzen, 2005b]. Although two studies have reported that approximately 20% of individuals clinically diagnosed with VCFS do not have detectable deletions [Driscoll et al., 1993; Morrow et al., 1995], both of these studies were flawed by uncontrolled ascertainment of subjects [Robin and Shprintzen, 2005a]. Both studies accepted blood samples for DNA analysis from multiple clinicians at multiple sites based on their clinical diagnosis without assessing the clinician’s accuracy or expertise. It is likely that the cases reported to have VCFS who were found to be absent the deletion were simply clinical errors of judgment.
There is also some degree of diagnostic finesse involved in differentiating syndromic diagnoses when there are overlapping clinical features. For example, it has been suggested that the same deletion from chromosome 22 can result in a number of distinctive, separate syndromes, such as Opitz syndrome [McDonald-McGinn et al., 1995; Fryburg et al., 1996] in addition to VCFS. There is little doubt that clinical diagnosis is as much an art as a science and as such it is possible that the molecular science that confirms the diagnosis (such as FISH) is more definitive than clinical categorization.
The deletion of DNA from 22q11.2 is somewhat variable in the population of individuals with VCFS. Approximately 90% of affected individuals have a 3 Mb deletion with identical breakpoints at each end [Morrow et al., 1995; Edelmann et al., 1999]. Fewer than 10% of individuals with VCFS have smaller nested deletions with the same proximal breakpoint, but a distal breakpoint that encompasses only 1.5 Mb. It has been reported that there is no difference in the clinical expression between the 3 Mb and 1.5 Mb deletion [Morrow et al., 1995], but it may be that subtle differences do exist that have not been studied sufficiently because of the expansiveness of the phenotype and the number of genes in the deletion.
It is also interesting to note that the variability of expression of VCFS relates even to anomalies that have had their genomic causes identified. Deletion of the gene TBX1 has been shown to cause the same spectrum of heart malformations in the mouse as is found in humans. However, at least 25% of humans with VCFS do not have structural heart anomalies although they are deleted for TBX1. Is it sufficient to chalk this up to variable expression without offering an explanation of possible mechanisms that will explain it? It is possible that the deletion of TBX1 contributes to the heart anomalies but is not the putative cause. It is also possible that TBX1 is involved in a more complex mechanism of heart development that involves downstream regulation of other genes or complex genetic interactions of multiple genes. There is good evidence that TBX1 interacts several genes important to embryogenesis including several homeobox genes that are highly involved in the regional development of the pharyngeal arches and pouches that form the structures of the head and neck and portions of the heart and its vascular system (Merscher et al., 2001; Kelly et al., 2004; Stalmans et al., 2003; Shih et al., 2007).
Genetic Counseling
VCFS has been established to have an autosomal dominant mode of inheritance. Penetrance is 100% with highly variable expression. Variable expression is evident even in cases with the same deletion. The large majority of cases are de novo mutations. Neither parent is affected in over 90% of cases. This indicates that 22q11.2 is a highly mutable region of the human genome and that the area is prone to rearrangement. The fact that so many cases represented new mutations pointed to the possibility of some type of recombination event during stage 1 of meiosis. This was confirmed and related to a unique evolutionary arrangement of chromosome 22 at the q11.2 band that had four series of low copy repeat DNA sequences within the typically deleted region (Edelmann et al., 1999). More than 90% of people with VCFS have a deletion that spans 3 Mb (Morrow et al., 1995). At each end of this typical deletion are identical sets of low copy repeats (LCRs). The presence of these two sets of LCRs coinciding with the breakpoints for the deletion directed researchers to discover the mechanism of the deletion to be either an interchromosomal recombination error during stage 1 meiosis during gametogenesis or an intrachromosomal event resulting in material being spliced out of one copy of the chromosome (Edelmann et al., 1999). The interchromosomal event was found to be more common (Edelmann et al., 1999). Fewer than 10% of individuals with VCFS inherited the deletion from an affected parent in our series of more than 1,000 cases.
The variability in the expression of individual traits may have a number of contributing factors, including polymorphisms in the copies of the genes within the 22q11.2 region on the nondeleted chromosome. It is also probable that there is downstream and upstream interaction between genes in the deleted region and genes elsewhere in the genome. Several of these interactions and regulatory functions have already been reported, including the interactions of TBX1, a gene always deleted in VCFS, with VEGF, PITX, and FGF10 [Stalmans et al., 2003; Kelly et al., 2004; Shih et al., 2007]. The role of gene dosage within the 22q11.2 region has been the subject of many studies within the past decade because of the hope that one or more of the deleted genes will be found to contribute to psychiatric illness and/or cognitive impairment. The hope has been that finding this type would lead to a better understanding of mental illness in general, and potential treatments once the mechanism of mental functioning has been determined. This research continues, but the possibility that upstream and downstream regulation may modify deleted genes has complicated the picture considerably.
Management
With over 180 phenotypes associated with VCFS, clinical management is obviously complex. To date, there has been no indication that congenital heart disease should be treated any differently in VCFS than other disorders. Success rates are good and seem to be worse in VCFS only when pulmonary stenosis/atresia are present [Michielon et al., 2006].
Early immune disorders are rarely very severe and in most cases, live viral vaccines can be administered [Sullivan, 2004]. Illnesses are best treated symptomatically with most infections being viral. Therefore, overuse of antibiotics should be avoided in order to prevent the development of opportunistic infections.
Feeding disorders represent a major problem in many infants with VCFS and poor outcomes for typical treatments may lead to the recommendation for gastrostomy or gavage feedings. The phenotypic factors that lead to feeding problems in VCFS are hypotonia, vascular anomalies in the chest, airway compromise, slow clearing of the digestive tract with chronic constipation, chronic illness, weakness from heart disease, and temperament issues. It is rarely necessary to perform a gastrostomy in VCFS if appropriate feeding techniques are implemented, including upright positioning during feedings, increasing the flow from the bottle by enlarging the hole in the nipple, treating constipation vigorously, and resolving the airway issues.
Hypernasal speech almost always requires surgical intervention. When cleft palate is present, it is rare for speech disorders to be successfully resolved with palate repair alone. Secondary reconstructive surgery is almost always necessary. It has been demonstrated that pharyngeal flap surgery can be highly effective [Tatum et al., 2002], but an anomalous course of the internal carotid arteries presents a major risk factor [Mitnick et al., 1996; Tatum et al., 2002]. The use of preoperative magnetic resonance angiography to locate the internal carotids has been recommended in children with VCFS [Mitnick et al., 1996; Tatum et al., 2002].
The problem that most concerns parents of children with VCFS is the treatment of behavioral and psychiatric disorders. The most common early psychiatric disorder in VCFS is ADD. Early data suggest that low doses of methylphenidate are effective in most cases for controlling ADD [Gothelf et al., 2003]. There have also been reports that psychosis in VCFS may be treated effectively using antidopaminergic medications, including metyrosine [Graf et al., 2001] and L-methyldopa [O’Hanlon et al., 2003]. To date, outcome studies have been limited to anecdotal reports, but having a human model for genetically caused mental illness continues to focus attention on VCFS, and the possibility that COMT plays a major role may help to focus attention on the dopaminergic system.
Prognosis
The prognosis for the resolution of heart, speech, and immune problems in VCFS is good. The large majority of babies with VCFS have successful corrections of their heart disease and will live normal life spans. Immune problems subside with time, and endocrine problems tend to be intermittent and treatable with appropriate medications. Speech problems respond well to speech therapy and surgery. Educational issues in VCFS have just begun to receive some attention in the literature [Landsman, 2007].
There is not yet a substantial data set on the treatment of psychiatric disorders, but the majority of patients with VCFS do not require medication to treat behavioral disorders. To date, reports of significant successes with treatments designed to interrupt the abnormal dopaminergic pathway in VCFS have been limited to a small number of anecdotal reports [Graf et al., 2001; O’Hanlon et al., 2003]. However, these few reports have been exciting and positive in their outcomes and will hopefully lead to larger and better controlled studies.
There is not sufficient data as yet to determine the life span of individuals with VCFS. Because the “modern era” of study of this syndrome is less than two decades old, and the delineation of the syndrome dates at its earliest to cases described by Sedlačková in 1955, there has not been an opportunity to study large samples of adults who have reached advanced age. Relying solely on anecdotal data, our clinical sample contains a number of adults with VCFS in their seventh decade of life, and we have historical information on a dozen families with three generations of affected individuals with the first documented case in the family living into the eighth decade of life. Intuitively, if one were to consider some of the health related issues in VCFS that might cause early death, such as severe congenital heart disease, severe immune deficiency, and possible stroke or bleeding disorders from Bernard-Soulier syndrome, an actuarial table might demonstrate that in aggregate (i.e., mean age of death), people with VCFS do not have the same life span as nonaffected individuals, but it is probable that people who live into their early adult years who have not had serious health concerns up to that point will live a normal life span.
Acknowledgments
The data presented in this paper was supported in part by funding from the Campaign to Establish the VCFS International Center, The Joseph and Annette Cooper Fund, and grants from the National Institutes of Health 5R01MH064824-03 and 1R01MH065481-01A2, Wendy Kates, Ph.D., P.I. and 1R01HL084410-01A1, Bernice Morrow, Ph.D., P.I.
References
- Antshel KM, Conchelos J, Lanzetta G, et al. Behavior and corpus callosum morphology relationships in velocardiofacial syndrome (22q11.2 deletion syndrome) Psychiatry Res. 2005;138:235–45. doi: 10.1016/j.pscychresns.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Arvystas M, Shprintzen RJ. Craniofacial morphology in the velo-cardio-facial syndrome. J Craniofac Genet Devel Biol. 1984;4:39–45. [PubMed] [Google Scholar]
- Brondum-Nielsen K, Stewart F, Van Essen T, et al. Spectrum of clinical features associated with interstitial chromosome 22q11 deletions: a European collaborative study. J Med Genet. 1997;34:798–804. doi: 10.1136/jmg.34.10.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chegar BE, Tatum SA, Marrinan E, et al. Upper airway asymmetry in velo-cardio-facial syndrome. Int J Pediatr Otorhinolaryngol. 2006;70:1375–1381. doi: 10.1016/j.ijporl.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Cutler-Landsman D. Educating Children with Velo-Cardio-Facial Syndrome. San Diego: Plural Publishing; 2007. [Google Scholar]
- Czarnecki PM, Van Dyke DL, Vats S, et al. A mother with VCFS and unilateral dysplastic kidney and her fetus with multicystic dysplastic kidneys: additional evidence to support the association of renal malformations and VCFS. J Med Genet. 1998;35:348. doi: 10.1136/jmg.35.4.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debbane M, Schaer M, Farhoumand R, et al. Hippocampal volume reduction in 22q11.2 deletion syndrome. Neuropsychologia. 2006;44:2360–2365. doi: 10.1016/j.neuropsychologia.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Devriendt K, Moerman P, Van Schoubroeck D, Vandenberghe K, et al. Chromosome 22q11 deletion presenting as the Potter sequence. J Med Genet. 1997;34:423–425. doi: 10.1136/jmg.34.5.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll DA, Salvin J, Sellinger B. Prevalence of 22q11 microdeletions in DiGeorge and velocardiofacial syndromes: implications for genetic counseling and prenatal diagnosis. J Med Genet. 1993;30:813–817. doi: 10.1136/jmg.30.10.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll DA, Spinner NB, Budarf ML, et al. Deletions and microdeletions of 22q11.2 in velo-cardio-facial syndrome. Am J Med Genet. 1992;44:261–268. doi: 10.1002/ajmg.1320440237. [DOI] [PubMed] [Google Scholar]
- Edelmann L, Pandita RK, Spiteri E, et al. A common molecular basis for rearrangement disorders on chromosome 22q11. Hum Mol Genet. 1999;8:1157–1167. doi: 10.1093/hmg/8.7.1157. [DOI] [PubMed] [Google Scholar]
- Eicher PS, McDonald-Mcginn DM, Fox CA, et al. Related Articles, Links Dysphagia in children with a 22q11.2 deletion: unusual pattern found on modified barium swallow. J Pediatr. 2000;137:158–64. doi: 10.1067/mpd.2000.105356. [DOI] [PubMed] [Google Scholar]
- Eliez S, Blasey CM, Schmitt EJ, et al. Velocardiofacial syndrome: are structural changes in the temporal and mesial temporal regions related to schizophrenia? Am J Psychiatry. 2001;158:447–453. doi: 10.1176/appi.ajp.158.3.447. [DOI] [PubMed] [Google Scholar]
- Fryburg JS, Lin KY, Golden WL. Chromosome 22q11.2 deletion in a boy with Opitz (G/BBB) syndrome. Am J Med Genet. 1996;62:274–275. doi: 10.1002/(SICI)1096-8628(19960329)62:3<274::AID-AJMG13>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Golding-Kushner KJ. Therapy Techniques for Cleft Palate Speech and Related Disorders. San Diego: Singular Publishing Group; 2000. [Google Scholar]
- Golding-Kushner The Velo-Cardio-Facial Syndrome Educational Foundation, Inc. web site, PowerPoint Presentations. 2007 http://www.vcfsef.org/powerpoint.html.
- Golding-Kushner K, Weller G, Shprintzen RJ. Velo-cardio-facial syndrome: Language and psychological profiles. J Craniofac Genet Devel Biol. 1985;5:259–266. [PubMed] [Google Scholar]
- Goldmuntz E, Clark BJ, Mitchell LE, Jawad AF, et al. Frequency of 22q11 deletions inpatients with conotruncal defects. J Am Coll Cardiol. 1998;32:492–498. doi: 10.1016/s0735-1097(98)00259-9. [DOI] [PubMed] [Google Scholar]
- Gothelf D, Eliez S, Thompson T, et al. COMT genotype predicts longitudinal cognitive decline and psychosis in 22q11.2 deletion syndrome. Nat Neurosci. 2005;8:1500–1502. doi: 10.1038/nn1572. [DOI] [PubMed] [Google Scholar]
- Gothelf D, Gruber R, Presburger G, et al. Methylphenidate treatment for attention-deficit/hyperactivity disorder in children and adolescents with velocardiofacial syndrome: an open-label study. J Clin Psychiatry. 2003;64:1163–1169. doi: 10.4088/jcp.v64n1004. [DOI] [PubMed] [Google Scholar]
- Graf WD, Unis AS, Yates CM, et al. Catecholamines in patients with 22q11.2 deletion syndrome and the low-activity COMT polymorphism. Neurology. 2001;57:410–416. doi: 10.1212/wnl.57.3.410. [DOI] [PubMed] [Google Scholar]
- Hieronimus S, Bec-Roche M, Pedeutour F, et al. The spectrum of parathyroid gland dysfunction associated with the microdeletion 22q11. Eur J Endocrinol. 2006;155:47–52. doi: 10.1530/eje.1.02180. [DOI] [PubMed] [Google Scholar]
- Kates WR, Antshel KM, Abdulsabur N, et al. A gender-moderated effect of a functional COMT polymorphism on prefrontal brain morphology and function in velo-cardio-facial syndrome (22q11.2 deletion syndrome) Am J Med Genet B Neuropsychiatr Genet. 2006;14:274–280. doi: 10.1002/ajmg.b.30284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates WR, Burnette CP, Bessette BA, et al. Frontal and caudate alterations in velocardiofacial syndrome (deletion at chromosome 22q11.2) J Child Neurol. 2004;19:337–342. doi: 10.1177/088307380401900506. [DOI] [PubMed] [Google Scholar]
- Kates WR, Miller AM, Abdulsabur N, et al. Temporal lobe anatomy and psychiatric symptoms in velocardiofacial syndrome (22q11.2 deletion syndrome) J Am Acad Child Adolesc Psychiatry. 2006;45:587–595. doi: 10.1097/01.chi.0000205704.33077.4a. [DOI] [PubMed] [Google Scholar]
- Kawame H, Adachi M, Tachibana K, et al. Graves’ disease in patients with 22q11.2 deletion. J Pediatr. 2001;139:892–895. doi: 10.1067/mpd.2001.119448. [DOI] [PubMed] [Google Scholar]
- Kelly D, Goldberg R, Wilson D, et al. Velo-cardio-facial syndrome associated with haplo-insufficiency of genes at chromosome 22q11. Am J Med Genet. 1993;45:308–312. doi: 10.1002/ajmg.1320450306. [DOI] [PubMed] [Google Scholar]
- Kelly RG, Jerome-Majewska LA, Papaioannou VE. The del22q11.2 candidate gene Tbx1 regulates branchiomeric myogenesis. Hum Molec Genet. 2004;13:2829–2840. doi: 10.1093/hmg/ddh304. [DOI] [PubMed] [Google Scholar]
- Lachman HM, Morrow B, Shprintzen RJ, et al. Association of codon 108/158 catechol-o-methyl transferase gene polymorphism with the psychiatric manifestations of VCFS. Amer J Med Genet. 1996;67:468–472. doi: 10.1002/(SICI)1096-8628(19960920)67:5<468::AID-AJMG5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Li T, Ma X, Sham PC, et al. Evidence for association between novel polymorphisms in the PRODH gene and schizophrenia in a Chinese population. Am J Med Genet. 2004;129B:13–15. doi: 10.1002/ajmg.b.30049. [DOI] [PubMed] [Google Scholar]
- MacKenzie-Stepner K, Witzel MA, Stringer DA, et al. Abnormal carotid arteries in the velocardiofacial syndrome: A report of three cases. Plast Reconstr Surg. 1987;80:347–351. doi: 10.1097/00006534-198709000-00002. [DOI] [PubMed] [Google Scholar]
- Mansour A, Wang F, Goldberg R, et al. Ocular findings in the velo-cardio-facial syndrome. J Pediatr Ophthalmol. 1987;24:263–266. doi: 10.3928/0191-3913-19870901-16. 1987. [DOI] [PubMed] [Google Scholar]
- Merscher S, Funke B, Epstein JA, et al. TBX1 is responsible for cardiovascular defects in velo-cardio-facial/DiGeorge syndrome. Cell. 2001;104:619–629. doi: 10.1016/s0092-8674(01)00247-1. [DOI] [PubMed] [Google Scholar]
- McDonald-McGinn DM, Driscoll DA, Bason L, et al. Autosomal dominant “Opitz” GBBB syndrome due to a 22q11.2 deletion. Am J Med Genet. 1995;59:103–13. doi: 10.1002/ajmg.1320590122. [DOI] [PubMed] [Google Scholar]
- Michielon G, Marino B, Formigari R, et al. Genetic syndromes and outcome after surgical correction of tetralogy of Fallot. Ann Thorac Surg. 2006;81:968–975. doi: 10.1016/j.athoracsur.2005.09.033. [DOI] [PubMed] [Google Scholar]
- Ming JE, McDonald-McGinn DM, Megerian TE, et al. Skeletal anomalies and deformities in patients with deletions of 22q11. Am J Med Genet. 1998;72:210–215. doi: 10.1002/(sici)1096-8628(19971017)72:2<210::aid-ajmg16>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Mitnick RJ, Bello JA, Golding-Kushner KJ, et al. The use of magnetic resonance angiography prior to pharyngeal flap surgery in patients with velo-cardio-facial syndrome. Plast Reconstr Surg. 1996;97:908–919. doi: 10.1097/00006534-199604001-00005. [DOI] [PubMed] [Google Scholar]
- Morrow B, Goldberg R, Carlson C, et al. Molecular definition of the 22q11 deletions in velo-cardio-facial syndrome. Am J Hum Genet. 1995;56:1391–1403. [PMC free article] [PubMed] [Google Scholar]
- Nickel RE, Pillers D-AM, Merkens M, et al. Velo-cardial-facial syndrome and DiGeorge sequence with meningomyelocele and deletions of the 22q11 region. Am J Med Genet. 1994;52:445–449. doi: 10.1002/ajmg.1320520410. [DOI] [PubMed] [Google Scholar]
- O’Hanlon JF, Ritchie RC, Smith EA, et al. Replacement of antipsychotic and antiepileptic medication by L-alpha-methyldopa in a woman with velocardiofacial syndrome. Int Clin Psychopharmacol. 2003;18:117–119. doi: 10.1097/00004850-200303000-00010. [DOI] [PubMed] [Google Scholar]
- Óskarsdóttir S, Vujic M, Fasth A. Incidence and Prevalence of the 22q11 Deletion Syndrome:A Population-based Study in Western Sweden. Arch Dis Child. 2004;89:148–51. doi: 10.1136/adc.2003.026880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricchetti ET, States L, Hosalkar HS, et al. Radiographic study of the upper cervical spine in the 22q11.2 deletion syndrome. J Bone Joint Surg Am. 2004;86-A:1751–1760. doi: 10.2106/00004623-200408000-00020. [DOI] [PubMed] [Google Scholar]
- Robin NH, Shprintzen RJ. Defining the clinical spectrum of deletion 22q11.2. J Pediatr. 2005;147:90–96. doi: 10.1016/j.jpeds.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Ryan AK, Goodship JA, Wilson DI, et al. Velo-cardio-facial syndrome associated with chromosome 22 deletions encompassing the DiGeorge locus. Lancet. 1992;339:1138–1139. doi: 10.1016/0140-6736(92)90734-k. [DOI] [PubMed] [Google Scholar]
- Schaer M, Schmitt JE, Glaser B, et al. Abnormal patterns of cortical gyrification in velo-cardio-facial syndrome (deletion 22q11.2): an MRI study. Psychiatry Res. 2006;146:1–11. doi: 10.1016/j.pscychresns.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Sedlačková E. The syndrome of the congenitally shortening of the soft palate. Cas Lek Ces. 1955;94(12):1304–1307. [Google Scholar]
- Shih HP, Gross MK, Kioussi C. Cranial muscle defects of Pitx2 mutants result from specification defects in the first branchial arch. Proc Natl Acad Sci U S A. 2007;104:5907–5912. doi: 10.1073/pnas.0701122104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shprintzen RJ. Palatal and pharyngeal anomalies in craniofacial syndromes. Birth Defects. 1982;18(1):53–78. [PubMed] [Google Scholar]
- Shprintzen RJ. Pierre Robin, micrognathia, and airway obstruction: the dependency of treatment on accurate diagnosis. Int Anesthesiol Clin. 1988;26:84–91. doi: 10.1097/00004311-198802610-00014. [DOI] [PubMed] [Google Scholar]
- Shprintzen RJ. Velo-cardio-facial syndrome: a distinctive behavioral phenotype. Mental Retardation Developmental Disabilities Research Reviews. 2000a;6:142–147. doi: 10.1002/1098-2779(2000)6:2<142::AID-MRDD9>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Shprintzen RJ. Velocardiofacial syndrome. Otolaryngol Clin North Am. 2000b;33:1217–1240. doi: 10.1016/s0030-6665(05)70278-4. [DOI] [PubMed] [Google Scholar]
- Shprintzen RJ. Velo-cardio-facial syndrome. Current Opinions in Pediatrics. 2005a;17:725–730. doi: 10.1097/01.mop.0000184465.73833.0b. [DOI] [PubMed] [Google Scholar]
- Shprintzen RJ. Velo-cardio-facial syndrome. In: Cassidy SB, Allanson J, editors. Management of Genetic Syndromes. 2. New York: Wiley-Liss; 2005b. pp. 615–632. [Google Scholar]
- Shprintzen RJ. Velo-cardio-facial syndrome. Prog Pediatr Cardiol. 2005c;20:187–193. [Google Scholar]
- Shprintzen RJ, Goldberg R, Golding-Kushner KJ, et al. Late-onset psychosis in the velo-cardio-facial syndrome. Am J Med Genet. 1992;42:141–142. doi: 10.1002/ajmg.1320420131. [DOI] [PubMed] [Google Scholar]
- Shprintzen RJ, Goldberg RB, Lewin ML, et al. A new syndrome involving cleft palate, cardiac anomalies, typical facies, and learning disabilities: velo-cardio-facial syndrome. Cleft Palate J. 1978;15:56–62. [PubMed] [Google Scholar]
- Shprintzen RJ, Goldberg R, Young D, et al. The velo-cardio-facial syndrome: a clinical and genetic analysis. Pediatrics. 1981;67:167–172. [PubMed] [Google Scholar]
- Shprintzen RJ, Morrow B, Kucherlapati R. Vascular anomalies may explain many of the features of velo-cardio-facial syndrome (Abstract) Am J Hum Genet. 1997;61:34A. [Google Scholar]
- Shprintzen RJ, Singer L. Upper airway obstruction and the Robin sequence. Int Anesthesiol Clin. 1992;30:109–114. [PubMed] [Google Scholar]
- Stalmans I, Lambrechts D, De Smet F, et al. VEGF: a modifier of the del22q11 (DiGeorge) syndrome? Nature Med. 2003;9:173–182. doi: 10.1038/nm819. [DOI] [PubMed] [Google Scholar]
- Sullivan KE. The clinical, immunological, and molecular spectrum of chromosome 22q11.2 deletion syndrome and DiGeorge syndrome. Curr Opin Allergy Clin Immunol. 2004:4505–4512. doi: 10.1097/00130832-200412000-00006. [DOI] [PubMed] [Google Scholar]
- Tatum SA, III, Chang J, Havkin N, Shprintzen RJ. Pharyngeal flap and the internal carotid in velo-cardio-facial syndrome. Arch Facial Plast Surg. 2002;4:73–80. doi: 10.1001/archfaci.4.2.73. [DOI] [PubMed] [Google Scholar]
- Van Geet C, Devriendt K, Eyskens B, et al. Velocardiofacial syndrome patients with a heterozygous chromosome 22q11 deletion have giant platelets. Pediat Res. 1998;44:607–611. doi: 10.1203/00006450-199810000-00023. [DOI] [PubMed] [Google Scholar]
- The Velo-Cardio-Facial Syndrome Educational Foundation, Inc. web site. 2007 http://www.vcfsef.org/vcfs.html.
- Williams MA, Shprintzen RJ, Goldberg RB. Male-to-male transmission of the velo-cardio-facial syndrome: A case report and review of 60 cases. J Craniofac Genet Devel Biol. 1985;5:175–180. [PubMed] [Google Scholar]
- Worthington S, Colley A, Fagan K, et al. Anal anomalies: an uncommon feature of velocardiofacial (Shprintzen) syndrome? J Med Genet. 1997;34:79–82. doi: 10.1136/jmg.34.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthington S, Turner A, Elber J, et al. 22q11 deletion and polymicrogyria--cause or coincidence? Clin Dysmorphol. 2000;9:193–197. doi: 10.1097/00019605-200009030-00008. [DOI] [PubMed] [Google Scholar]
- Wraith JE, Super M, Watson GH, Phillips M. Velo-cardio-facial syndrome presenting as holoprosencephaly. Clin Genet. 1985;27:408–410. doi: 10.1111/j.1399-0004.1985.tb02284.x. [DOI] [PubMed] [Google Scholar]
- Young D, Shprintzen RJ, Goldberg R. Cardiac malformations in the velo-cardio-facial syndrome. Am J Cardiol. 1980;46:43–48. doi: 10.1016/0002-9149(80)90515-9. [DOI] [PubMed] [Google Scholar]
- Zim S, Schelper R, Kellman R, et al. Thickness and histologic and histochemical properties of the superior pharyngeal constrictor muscle in velocardiofacial syndrome. Arch Facial Plas Surg. 2003;5:503–507. doi: 10.1001/archfaci.5.6.503. [DOI] [PubMed] [Google Scholar]