Abstract
The development of cancer is a major problem in immunosuppressed patients, particularly after solid organ transplantation. We have recently shown that calcineurin inhibitors (CNI) used to treat transplant patients may play a critical role in the rapid progression of renal cancer. To examine the intracellular signaling events for CNI-mediated direct tumorigenic pathway(s), we studied the effect of CNI on the activation of proto-oncogenic Ras in human normal renal epithelial cells (REC) and renal cancer cells (786-0 and Caki-1). We found that CNI treatment significantly increased the level of activated GTP-bound form of Ras in these cells. In addition, CNI induced the association of Ras with one of its effector molecules Raf, but not with Rho and PI-3K; CNI treatment also promoted the phosphorylation of the Raf kinase inhibitory protein, and the downregulation of carabin, all of which may lead to the activation of the Ras-Raf pathway. Blockade of this pathway through either pharmacological inhibitors or gene-specific siRNA significantly inhibited CNI-mediated augmented proliferation of renal cancer cells. Finally, it was observed that CNI treatment increased the growth of human renal tumors in vivo, and the Ras-Raf pathway is significantly activated in the tumor tissues of CNI-treated mice. Together, targeting the Ras-Raf pathway may prevent the development/progression of renal cancer in CNI-treated patients.
Keywords: Calcineurin inhibitor, Cancer, Ras, Raf
INTRODUCTION
Immunosuppressive medications are essential for the prevention of allograft rejection, and also for the treatment of different inflammatory diseases (1, 2). However, patients receiving immunosuppressive therapy have a greater risk of developing cancer compared with the general population (3–8). Recent studies have established that calcineurin inhibitors (CNI) may play a major role for the increased incidence of cancers in these patients (9–13).
CNI, such as cyclosporine (CsA) and tacrolimus (FK506) may suppress the immune system, so that the immune surveillance mechanism is impaired (6, 9, 14). Immunosuppression predisposes patients to a variety of viral infections that lead to malignant transformations of different tissues (7, 15). In addition, they may also have direct tumorigenic effects. CNI can promote tumor growth through transforming growth factor-β (TGF-β) production (9, 11, 16). We and others have reported that CNI may induce tumor growth through overexpression of the angiogenic cytokine vascular endothelial growth factor (VEGF) (10, 17). Recently, we have shown that CNI may promote the proliferation of human renal cancer cells through altered expression of the chemokine receptor CXCR3 (18). Although several effector molecules have been identified to be responsible for CNI-mediated tumor growth, the intracellular signaling mechanism(s) for these direct tumorigenic pathways have not been defined.
The major and classical function of CNI is to suppress T cell activation responses through downregulation of the calcineurin-NFAT pathway (19). Although the calcineurin-NFAT pathway is well characterized in immune cells, components of this signaling pathway exist in many other cell types (20). The tumorigenic effects of CNI may be mediated either through suppression of the calcineurin-NFAT pathway in T cells or through some other direct mechanism(s) independent of immune cells. In support of direct mechanism(s), it has been shown that the treatment of tumor xenografts in T- and B-cell deficient SCID mice with CNI enhanced tumor growth and progression (9). CNI may also activate/inhibit genes important for cell-cycle regulation, apoptosis, and oncogene/tumor-suppressor function (21). There are some reports that oncogenes may become activated in immunosuppressed transplant patients (21–23). However, very little is known about the signaling mechanism(s) of CNI-mediated direct tumorigenic pathways.
The ras family of proto-oncogenes encodes small proteins that transduce mitogenic signals from tyrosine kinase receptors (24, 25). Ras proteins act as molecular switches that cycle between active GTP-bound and inactive GDP-bound forms (26–28). The three isoforms of Ras, H-Ras, K-Ras, and N-Ras are ubiquitously expressed in mammalian cells (29). The hyperactive Ras can promote the growth of cancer cells without being mutated, where it may be activated by persistent upstream signaling events (30–32). Activated Ras proteins transmit their signals to a cascade of protein kinases that have MAP kinase kinase (MEK) as the substrate, such as MEK kinase, c-Raf-1, and B-Raf, culminating in the activation of MAP kinase (MAPK) (33). Upon activation, Ras may primarily function to promote the translocation of Raf-1 from the cytosol to the plasma membrane, where subsequent Ras-independent events trigger Raf-1 kinase activation (34). However, Ras may also mediate its action through Raf-independent pathways, including Rho- and phosphatidylinositol 3-kinase (PI-3K)-pathways (35–38).
In the present study, we show a novel tumorigenic pathway in which CNI promotes the activation of Ras and its downstream effector molecules in human renal cancer cells. CNI-mediated Ras activation plays a critical role in renal cancer cell proliferation.
MATERIALS AND METHODS
Reagents
CsA (Novartis) and FK506 (Astellas) were purchased from Children’s Hospital Boston pharmacy. Rapamycin was gifted to the laboratory by Wyeth-Ayerst Research. Raf-1 kinase inhibitor I (5-iodo-3-[(3,5-dibromo-4-hydroxy-phenyl)methylene]-2-indolinone) and Farnesyl Transferase Inhibitor (FTI) were purchased from Calbiochem. The gene-specific small interfering RNAs (siRNA) for H-Ras, K-Ras, N-Ras and carabin along with their controls were purchased from Qiagen.
Antibodies
The antibodies for Ras, H-Ras, K-Ras, N-Ras, Raf, RKIP and phospho-RKIP were purchased from Santa Cruz Biotechnology. The Rho antibody was purchased from Upstate. The carabin antibody was purchased from ProSci Inc. The antibodies for PI-3K, ERK and phospho-ERK were purchased from Cell Signaling Technology Inc. The β-Actin antibody was obtained from Sigma.
Cell Culture
The human renal cancer cell lines (786-0 and Caki-1) were obtained from American Type Culture Collection. The cells were grown in RPMI 1640 supplemented with 10% fetal bovine serum (Hyclone Laboratories). Human renal proximal tubular epithelial cells (REC) were purchased from Clonetics and were cultured in complete epithelial medium (REGM Bulletkit).
Measurement Of Active/GTP-Bound Ras
The active/GTP-bound form of Ras in the cell lysates was measured by utilizing an EZ-detect Ras activation kit (Pierce). This kit utilizes specific Ras-binding domain (RBD) of Raf-1 that can specifically bind active GTP-bound form of Ras. The cell lysates were incubated with GST-Raf-1-RBD and a swellgel immobilized glutathione disc. The eluted samples were separated by SDS-polyacrylamide gel electrophoresis, transferred to a polyvinylidene difluoride (PVDF) membrane (NEN Life Sciences Product, Inc), and probed with either anti-Ras or isoform-specific Ras antibody.
Immunoprecipitation Assays
Immunoprecipitations were performed with 0.5 mg of total protein at antibody excess. Immunocomplexes were captured with protein A-Sepharose beads (Amersham Pharmacia Biotech), and bead-bound proteins were subjected to Western blot analysis using specific antibody.
Western Blot analysis
Protein samples were run on SDS-polyacrylamide gel and transferred to a PVDF membrane. The membrane was probed with specific primary antibody, and subsequently incubated with peroxidase-linked secondary antibody. The reactive band was detected by chemiluminescent substrate (Pierce).
Cell Proliferation Assay
Cells (5 × 103) were seeded and grown in 96-well plates. [3H]thymidine (0.5 μCi/well) was added for the final 15 h before cell harvesting. [3H]thymidine incorporation was measured using a microplate scintillation and luminescence counter (Perkin Elmer/Wallac).
In Vivo Tumor Development
Human renal cancer cells (786-0) were injected subcutaneously either in immunodeficient nude (nu/nu) mice or in SCID-Beige mice. Either CsA (10 mg/kg/day) or the vehicle was then administered intraperitoneally to these mice. Tumor volume was measured using a digital caliper at regular intervals. The volume was estimated by following standard method (18), using the formula V = Π/6 × a2 × b, where a is the short and b is the long tumor axis. Mice were killed at designated times after injection. All animal works were approved by the animal care and use committee at Children’s Hospital Boston.
Statistical Analyses
Statistical evaluation for data analysis was determined either by t test for two groups of data or by one-way ANOVA for three or more groups. Differences with P < 0.05 were considered statistically significant.
RESULTS
Calcineurin Inhibitors Promote The Activation Of Ras In Human Renal Epithelial Cells
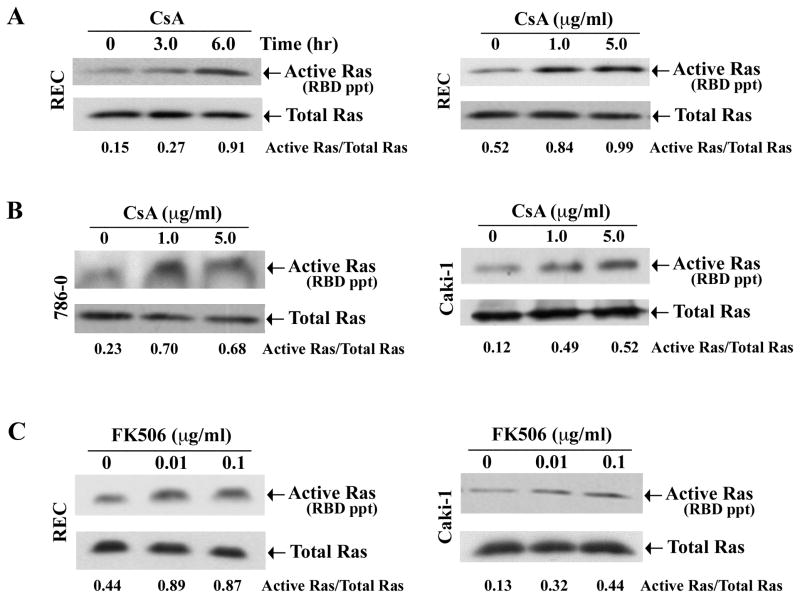
We first evaluated whether calcineurin inhibitors (CNI) can induce Ras activation in human renal tubular epithelial cells (REC) in vitro. We treated REC with CsA in both time (1-μg/ml for 3-and 6-hours)- and dose (1- and 5-μg/ml for 6-hours)-dependent manner. Vehicle-treated cells served as controls. To measure the activation status of Ras in these cell lysates, we performed affinity pull-down reactions with the Ras-binding domain (RBD) of Raf that can specifically bind active GTP-bound form of Ras as described in methods section. The eluted samples were subjected to Western blot analysis using Ras-specific antibody. CsA treatment markedly induced the activation of Ras in both time- and dose-dependent manner compared with controls (Figure 1A, upper panels; and Supplementary Figure 1A). However, there was no change in the expression of total Ras following CsA treatment (Figure 1A, lower panels).
Figure 1. Calcineurin inhibitors promote the activation of Ras.
A, REC were treated either with CsA (1.0 μg/ml) for 3 and 6 hours (left panel) or with different concentrations (1.0 and 5.0μg/ml) of CsA for 6 hours (right panel). The vehicle-treated cells served as control. B, 786-0 and Caki-1 cells were treated with CsA (1.0 and 5.0 μg/ml) or with the vehicle for 6 hours. C, REC and Caki-1 cells were treated with FK506 (0.01 and 0.1 μg/ml) or with the vehicle for 6 hours. Cells (A–C) were lysed, and the active GTP-bound form of Ras in the cell lysates (with equal amount of proteins) were measured by affinity pull-down reactions with GST-Raf-1-RBD, followed by Western blot analysis using Ras-specific antibody (upper panels). The amount of total Ras in the cell lysates were measured by direct Western blot analysis, using Ras-specific antibody (lower panels). The relative intensity of each active Ras band to that of total Ras was measured by densitometry, and the values are listed under the blots. Representative of three independent experiments with similar findings.
Next, we examined whether CsA can activate Ras in human renal cancer cells as observed in REC. 786-0 and Caki-1 cells were treated with either increasing concentrations of CsA or vehicle. We found that, similar to REC, CsA treatment markedly increased the activation of Ras in both 786-0 and Caki-1 cells compared with vehicle-treated controls (Figure 1B, upper panels; and Supplementary Figure 1A). There was no change in the expression of total Ras (Figure 1B, lower panels).
We also evaluated the effect of FK506 (another CNI) on the activation of Ras. We treated REC and Caki-1 cells with either increasing concentrations of FK506 or vehicle. As observed with CsA treatment, FK506 also increased the activation of Ras in both the cell types (Figure 1C and Supplementary Figure 1A); there was no change in the level of total Ras following FK506 treatment. Together, these results suggest that CNI play a major role in the activation of Ras in human normal renal epithelial and renal cancer cells.
Calcineurin Inhibitors Primarily Activate H-Ras In Vitro
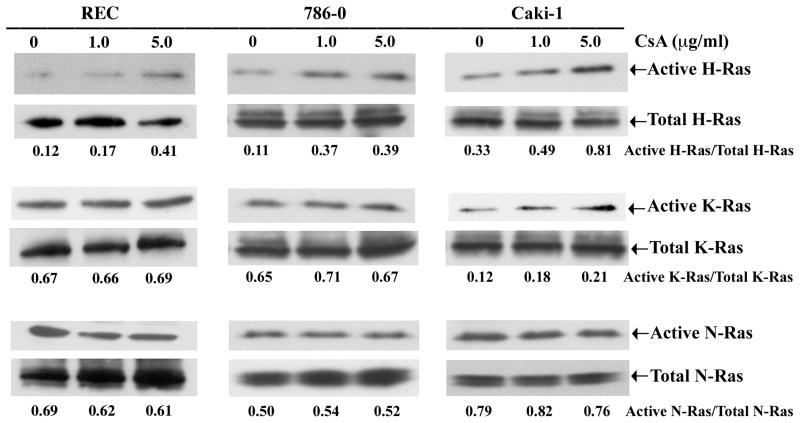
Here, we set out to determine which particular isoform(s) of Ras is activated following CNI treatment. REC, 786-0 and Caki-1 cells were treated with either increasing concentrations of CsA or vehicle, and the activation status of Ras isoforms (H-, K- and N-Ras) in these cells were measured by affinity pull-down reactions using RBD of Raf, followed by Western blot analysis using isoform-specific Ras antibody. As shown in Figure 2 (upper panels) and Supplementary Figure 1B, CsA markedly induced the activation of H-Ras compared with vehicle-treated controls; there was minimal change in the activation of K-Ras and N-Ras. There was no change in the amount of total Ras isoforms (H, K and N) in these cells following CsA treatment (Figure 2, lower panels). These results suggest that CNI primarily activates H-Ras in human renal epithelial and renal cancer cells in vitro.
Figure 2. CsA primarily activates H-Ras in vitro.
REC, 786-0 and Caki-1 cells were treated with CsA (1.0 and 5.0 μg/ml) or with the vehicle (control) for 6 hours. The active GTP-bound form of isoform-specific Ras (H, K or N) in the cell lysates were measured by affinity pull-down reactions with GST-Raf-1-RBD, followed by Western blot analysis using isoform-specific Ras antibodies (upper panels). To measure the amount of total Ras isoforms in the cell lysates, the extracts were immunoprecipitated with Ras-specific antibody, followed by Western blot analysis using isoform-specific Ras antibodies (lower panels). The relative intensity of each active Ras band to that of total Ras was measured by densitometry, and the values are listed under the blots. Representative of three independent experiments with similar findings.
Calcineurin Inhibitor-Induced And Ras-Mediated Signals Are Channeled Through The Raf Pathway
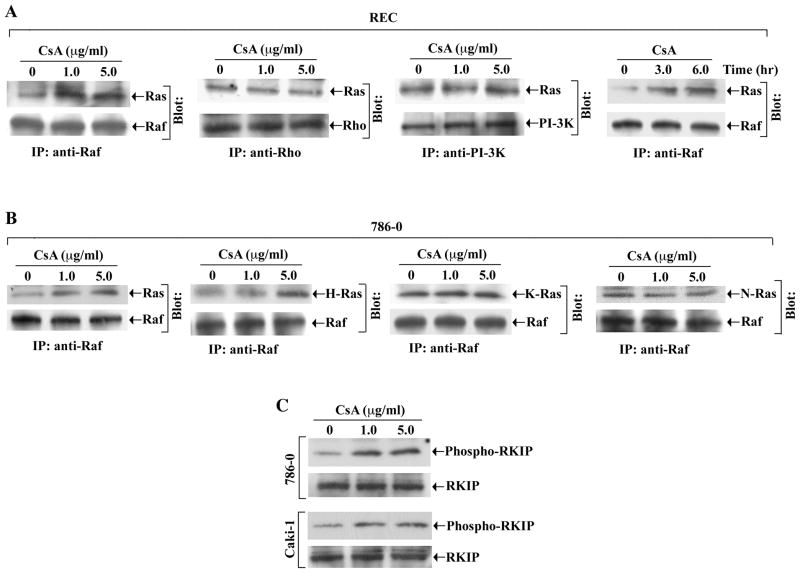
We evaluated whether CNI could promote the association between Ras and one of its effector molecules (Raf, Rho and PI-3K). We treated REC with increasing concentrations of CsA. By immunoprecipitation, we observed that CsA treatment markedly increased the association between Ras and Raf compared with vehicle-treated control (Figure 3A, left panel). However, there was no significant change in Ras-Rho or Ras-PI-3K association following CsA treatment (Figure 3A, middle two panels). We confirmed that CsA treatment also increased the Ras-Raf association in these cells in a time-dependent manner (Figure 3A, right panel).
Figure 3. CsA promotes the association between Ras and Raf, and increases the phosphorylation of RKIP.
A, REC were treated either with different concentrations of CsA (1.0 and 5.0 μg/ml) for 6 hours (panels 1–3), or with CsA (1.0 μg/ml) for 3 and 6 hours (panel 4). The vehicle-treated cells served as control. The cell extracts were immunoprecipitated with anti-Raf (panels 1 and 4), anti-Rho (panel 2), or anti-PI-3K (panel 3); and Western blots (Blot) were performed with anti-Ras (panels 1–4), anti-Raf (panels 1 and 4), anti-Rho (panel 2), or anti-PI-3K (panel 3). B, 786-0 were treated with CsA (1.0 and 5.0 μg/ml) or with vehicle for 6 hours. The cell extracts were immunoprecipitated with anti-Raf; and Western blots (Blot) were performed with anti-Ras (panel 1), anti-H-Ras (panel 2), anti-K-Ras (panel 3), anti-N-Ras (panel 4), or anti-Raf (panels 1–4). C, 786-0 and Caki-1 cells were treated with CsA (1.0 and 5.0 μg/ml) or with vehicle for 6 hours. The amounts of phospho-RKIP (upper panels) and total RKIP (lower panels) in the cell lysates were measured by Western blot analysis using anti-phosphorylated RKIP and anti-RKIP respectively. A–C is representative of three independent experiments with similar findings.
We observed that similar to REC, CsA treatment increased the association between Ras and Raf in 786-0 renal cancer cells (Figure 3B, first left panel). We also found that CsA treatment primarily increased the association between H-Ras and Raf in these cells (Figure 3B, second left panel); however, there was no significant change in K-Ras-Raf or N-Ras-Raf associations (Figure 3B, two right panels). A similar finding in terms of CsA-induced H-Ras-Raf association was observed in REC (data not shown).
It has been established that Raf kinase inhibitory protein (RKIP) acts as an endogenous inhibitor of the Raf-1/MEK pathway (39). Non-phosphorylated RKIP normally inhibits Raf and therefore blocks Raf-mediated signaling events (40). To examine the effect of CNI on RKIP, we treated 786-0 and Caki-1 cells with CsA, and measured the phosphorylation status of the protein by Western blot analysis. CsA treatment increased the phosphorylation of RKIP in both cell types compared with vehicle-treated control (Figure 3C); however, there was no change in the expression of total RKIP. Together, these experiments suggest that the Raf pathway may play a critical role in channeling CNI-induced and Ras-mediated signals in renal epithelial cells. Also, CNI may inhibit RKIP through its increased phosphorylation, and thereby may induce the Raf-mediated signaling pathway.
Calcineurin Inhibitor-Induced Renal Cancer Cell Proliferation Is Mediated Through The Ras-Raf pathway
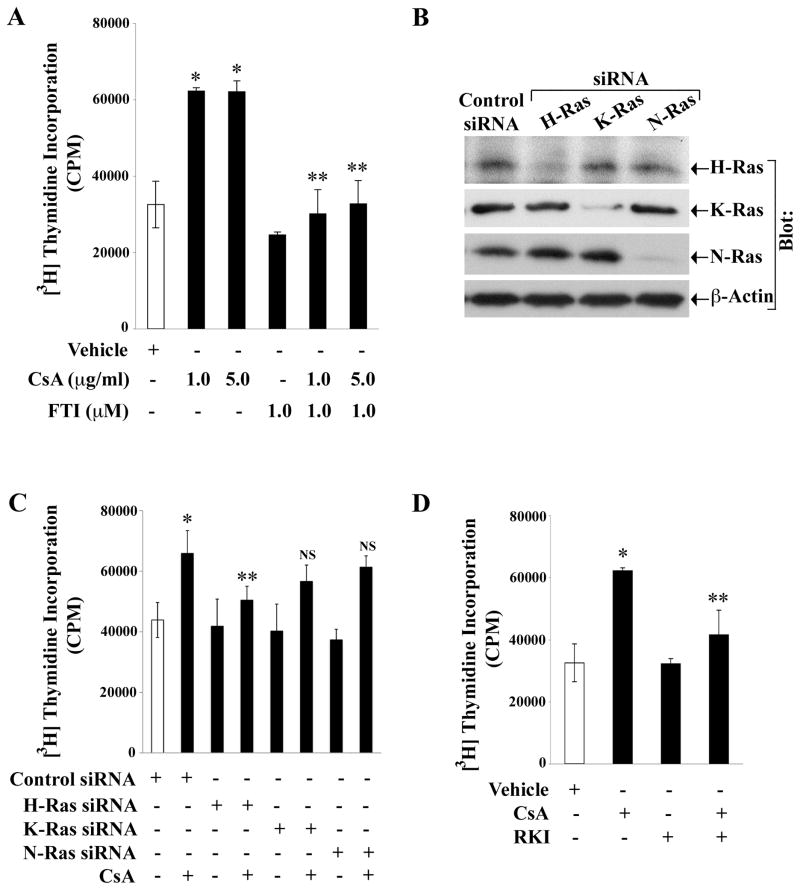
We have reported that human renal cancer cells undergo increased proliferation following CNI treatment (18). Here, we examined the role of Ras activation on CNI-induced renal cancer cell proliferation in vitro. The 786-0 cells were treated either with CsA or with vehicle in the absence or presence of pharmacological Ras inhibitor - Farnesyl Transferase Inhibitor (FTI), and then subjected to a cell proliferation assay. CsA increased the proliferation of renal cancer cells compared with vehicle-treated control, and FTI significantly reduced CsA-induced cell proliferation (Figure 4A). Similar to CsA, FK506 also increased the proliferation of renal cancer cells, and FTI treatment inhibited FK506-mediated cell proliferation (Supplementary Figure 2A). To examine whether an mTOR inhibitor can counteract the effect of CNI, we observed that rapamycin significantly inhibited CsA-mediated renal cancer cell proliferation (Supplementary Figure 2B).
Figure 4. CsA promotes renal cancer cell proliferation through the activation of Ras.
A, 786-0 cells were treated with FTI (1μM) or with the vehicle for 12 hours, and then treated with CsA (1 and 5 μg/ml) or with vehicle (control) for 72 hours. B, 786-0 cells were transfected with 25 nM of the control siRNA, H-Ras siRNA, K-Ras siRNA, or N-Ras siRNA for 48 hours. The expression of H-Ras, K-Ras, N-Ras and β-actin in the cell lysates were measured by Western blot analysis using isoform-specific Ras antibodies or anti-β-actin. Representative of three independent experiments. C, 786-0 cells were transfected with 25 nM of either control siRNA, H-Ras siRNA, K-Ras siRNA or N-Ras siRNA for 48 hours, and then treated with CsA (1 μg/ml) or vehicle alone for 48 hours. D, 786-0 cells were treated with RKI (1μM) for 12 hours, and then treated either with CsA (1 μg/ml) or vehicle for 48 hours. In A, C, and D, the cells were subjected to cell proliferation assay by measuring (3H) thymidine incorporation within the cells. Data reflect three independent experiments. Columns are average of triplicate readings (cpm) of the samples; bars are +/− SD. In A, *P<0.01 versus vehicle-treated cells, and **P<0.01 versus CsA-treated and FTI-untreated cells. In C, *P<0.05 versus control siRNA-transfected cells, and **P<0.05 versus control siRNA-transfected and CsA-treated cells; NS, not statistically significant versus control siRNA-transfected and CsA-treated cells. In D, *P<0.01 versus vehicle-treated cells, and **P<0.05 versus CsA-treated and RKI-untreated cells.
Next, we evaluated the role of specific Ras isoform(s) in CNI-induced renal cancer cell proliferation in vitro. We utilized gene-specific siRNAs for H-, K- and N-Ras that specifically and significantly knock down the respective genes (Figure 4B). The 786-0 cells were transfected with H-, K- or N-Ras-specific siRNA, and then treated with either CsA or vehicle to assess cell proliferation. As shown in Figure 4C, H-Ras siRNA significantly decreased CsA-induced cell proliferation; however, there was very little decrease in CsA-induced cell proliferation following transfection with either K-Ras-siRNA or N-Ras-siRNA. We observed a similar result in REC and Caki-1 cells where CsA-induced cell proliferation is also primarily mediated through the H-Ras signaling pathway (Supplementary Figure 3A and 3B).
To examine if Raf is critical in CNI-induced renal cancer cell proliferation, we treated 786-0 cells with either CsA or vehicle in the absence or presence of Raf-1 kinase inhibitor I (RKI). RKI significantly inhibited CsA-induced cell proliferation (Figure 4D). Together, these results suggest that the Ras-Raf pathway plays a major role in CNI-induced renal cancer cell proliferation.
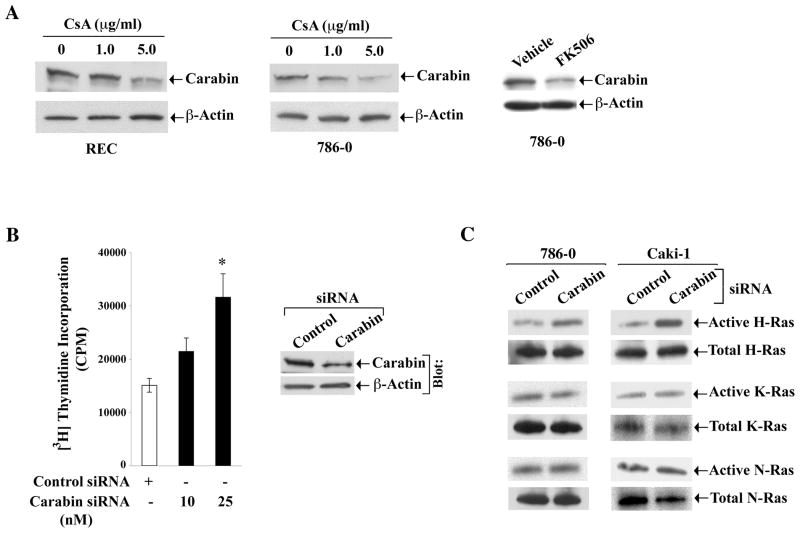
Calcineurin Inhibitors Downregulate The Expression Of Carabin, A Negative Regulator Of The Ras Pathway
It has been reported that a novel molecule called carabin can inhibit not only the calcineurin pathway, but also the Ras signaling pathway (41). Thus, the level of expression of carabin may play a significant role in regulating the cellular status of Ras activation. To explore the possible mechanism(s) of CNI-induced Ras activation, we first investigated the effect of CNI on the expression of carabin. REC and 786-0 cells were treated with either CsA or vehicle, and the expression of carabin was evaluated by Western blot analysis. In both cell types, the expression level of carabin was decreased following CsA treatment compared with vehicle-treated control (Figure 5A, first two panels). Similarly, we found that the expression of carabin was also downregulated in 786-0 cells following FK506 treatment (Figure 5A, right panel).
Figure 5. Calcineurin inhibitors downregulate the expression of carabin.
A, REC and 786-0 cells were treated with CsA (1 and 5 μg/ml) (left and middle panels), and 786-0 cells were treated with FK506 (0.1μg/ml) (right panel) for 6 hours. Vehicle-treated cells served as controls. The expression of carabin (upper panels) and β-actin (lower panels) in the cell lysates were measured by Western blot analysis using anti-carabin and anti-β-actin respectively. Representative of three independent experiments. B, 786-0 cells were transfected with either carabin siRNA (10 and 25 nM) or control siRNA for 72 hours, and the cells were subjected to cell proliferation assay by measuring (3H) thymidine incorporation within the cells. Data reflect three independent experiments. Columns are average of triplicate readings (cpm) of the samples; bars are +/− SD. *P<0.05 versus control siRNA-transfected cells. The knockdown of carbin by siRNA (25 nM) was confirmed by Western blot analysis using anti-carabin (right panel). C, 786-0 and Caki-1 cells were transfected with either carabin siRNA (25 nM) or control siRNA for 72 hours. The active GTP-bound form of Ras isoforms, and also the level of total Ras isoforms in the cell lysates were measured by immunoprecipitaion and Western blot analysis as described in Figure 2. Representative of three independent experiments.
Next, we examined the effect of carabin knockdown on the proliferation of renal cancer cells in vitro. The 786-0 cells were transfected with either the gene-specific siRNA of carabin or the control siRNA, and then subjected to a cell proliferation assay. The knockdown of carabin increased the proliferation of renal cancer cells compared with controls (Figure 5B). The knockdown of carabin was confirmed by Western blot analysis (Figure 5B, right panel). We also observed that the knockdown of carabin primarily increased the level of active H-Ras in 786-0 and Caki-1 cells (Figure 5C); there was very minimal change in the activation of K-Ras and N-Ras. Together, carabin may act as a negative regulator of cancer cell proliferation. We suggest that CsA-induced and Ras-mediated increase in renal cancer cell proliferation may involve downregulation of carabin.
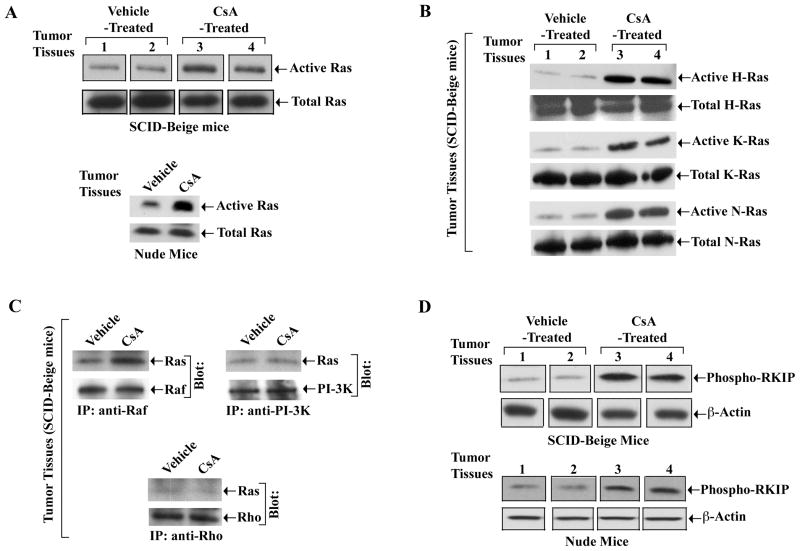
The Calcineurin Inhibitor CsA Promotes Activation Of The Ras-Raf Pathway In Human Renal Tumor Tissues In Vivo
We have recently shown that in immunodeficient (nu/nu) mice, the growth of human renal tumors (786-0) was significantly accelerated following CsA treatment (10 mg/kg/day, a dose that can significantly prolong allograft survival in Balb/c mice) compared with vehicle treated controls (10). We have also observed that CsA promoted an enhanced growth of human renal tumors in SCID-Beige mice that do not have any residual NK cells (Supplementary Figure 4). Here, we examined the status of the Ras-Raf pathway in the tumor tissues from CsA-treated mice. We observed that similar to our in vitro findings, CsA treatment in both SCID-beige and nude mice significantly increased the activation of Ras in the tumors compared with vehicle-treated controls (Figure 6A). However, in contrast to our in vitro findings, we observed that the activation status of all the Ras isoforms (H-, K- and N-Ras) were increased in tumor tissues following CsA treatment (Figure 6B); this is possibly due to in vivo microenvironment, where CsA can regulate multiple factors in tumor cells as well as in other cell types that may result in the activation of all the Ras isoforms.
Figure 6. CsA activates the Ras-Raf signaling pathway in tumor tissues in vivo.
1.0 × 106 human renal cancer cells (786-0) were injected subcutaneously either in SCID-Beige mice (n = 5 in each group) or in nude (nu/nu) mice (n = 10 in each group), and they were treated either with CsA (10mg/kg/day) or with vehicle as control. Tumors were harvested at day 28 (SCID-Beige mice) and day 30 (nu/nu mice) following tumor injection. A, The active GTP-bound form of Ras in the tissue lysates (with equal amount of proteins) were measured by affinity pull-down reactions with GST-Raf-1-RBD, followed by Western blot analysis using Ras-specific antibody (upper panels). The amounts of total Ras were measured by direct Western blot analysis using Ras-specific antibody (lower panels). B, The active GTP-bound form of Ras isoforms (upper panels), and also the level of total Ras isoforms (lower panels) in the tissue lysates were measured by immunoprecipitaion and Western blot analysis as described in Figure 2. C, The tissue lysates were immunoprecipitated with anti-Raf (panel 1), or anti-PI-3K (panel 2), or anti-Rho (panel 3); and Western blots (Blot) were performed with anti-Ras (panels 1–3), anti-Raf (panel 1), anti-PI-3K (panel 2), or anti-Rho (panel 3). D, The amount of phospho-RKIP (upper panels) and β-actin (lower panels) in the tissue lysates were measured by Western blot analysis using anti-phosphorylated RKIP and anti-β-actin respectively. (A–D) Representative of three different tissue samples of both CsA- and vehicle-treated groups.
Next, we wished to evaluate which Ras effector molecules are involved in CsA-induced Ras activation in these tumor tissues. We observed that CsA treatment increased the association of Ras and Raf compared with vehicle-treated control (Figure 6C); however, there was no significant change in the association of either Ras-Rho or Ras-PI-3K in the tumor tissues. We also measured the phosphorylation status of RKIP in these tissues. As shown in Figure 6D, CsA treatment significantly increased the phosphorylation of RKIP compared with vehicle-treated controls.
As ERK is a potential downstream target of the Ras-Raf pathway (33), we examined the phosphorylation status of this kinase in these tumor tissues. CsA treatment significantly increased the level of phospho-ERK compared with control (Supplementary Figure 5). There was no change in the level of total ERK. Together, these experiments suggest that CsA treatment may promote accelerated growth of human renal tumors in vivo likely through the induction of the Ras-Raf-ERK signaling cascade.
DISCUSSION
Calcineurin inhibitors (CNI) may promote cancer development through tumorigenic pathways. In the present study, we show a novel mechanism by which CNI can promote the growth of human renal cancer through the activation of the proto-oncogene ras.
Kidney cancer has been reported to be one of the major cancers among transplant recipients (8). Nephrotoxicity is the most common and limiting side effect of CNI treatment in transplant patients, and gives rise to both acute and chronic kidney damage (42). It has been demonstrated that CsA can induce some proto-oncogenes (c-fos and c-jun), which may partially be responsible for long-term nephrotoxicity (23). However, there was no correlation between the proto-oncogenic activation, nephrotoxicity and renal tumor development.
Ras proteins play a key role in tumor development (25, 28). Here, for the first time we have shown that CNI can activate the proto-oncogene ras in human renal epithelial and renal cancer cells, without altering the expression of total Ras. It is possible that CNI may directly/indirectly modualte the expression/activity of either guanine nucleotide exchange factors or GTPase activating proteins that regulate Ras activity (24, 25). Our in vitro studies suggest that CNI primarily activate H-Ras, and induce renal cancer cell proliferation. Although the Ras isoforms are ubiquitously expressed and highly conserved, they may exhibit different biological functions (29, 43). In support of our findings, Best and colleagues (44) demonstrated that H-Ras-transformed renal epithelial cells have an altered growth pattern and morphology that correlates with the characteristics of renal cell carcinoma. Chin and colleagues (45) showed that H-Ras activation is sufficient in both the genesis and maintenance of solid tumors. However, in contrast to our in vitro findings, we observed that all the Ras isoforms (H- K- and N-Ras) are activated in tumor tissues from CNI-treated mice. We suggest that within in vivo microenvironment, CNI can regulate multiple factors that may act in autocrine/paracrine manner to promote activation of all the Ras isoforms.
There is a possible crosstalk between the calcineurin and the Ras pathway, and it has been demonstrated that a novel molecule named carabin may act as an endogenous inhibitor for both the pathways (41). In this study, we have observed that CNI may downregulate the expression of carabin in renal epithelial and renal cancer cells, and can promote cell proliferation. We suggest that CNI-mediated downregulation of carabin may act as one of the possible mechanisms for Ras activation in renal epithelial cells following CNI treatment. In future, it will be interesting to examine if knockdown of carabin can promote tumor growth in vivo through activation of Ras.
The activation of the Ras-Raf-MEK-ERK pathway contributes to the development of different types of human tumors, including renal cancer (25, 46). We have observed that CNI-induced and Ras-mediated signals are channeled through the Raf pathway. A prerequisite for Raf-1 activation in many signaling pathways is an interaction with the ras proto-oncogene product (34). Recent studies suggest that the Ras-Raf pathway is assembled by some specific scaffolding proteins, such as the kinase suppressor of Ras (KSR) and connector enhancer of KSR (CNK) (47). RKIP is a member of the phosphatidylethanolamine-binding proteins (PEBPs), and represents a new class of modulators for the Ras-Raf-MEK signaling cascade (39). It can interrupt the Ras-mediated signaling by dissociating the interaction between Raf-1 and its substrate MEK (40).
More recently, RKIP has emerged as an important suppressor of metastatsis (48). In this study, we have found that CsA promotes an increased association between Ras and Raf in human renal cancer cells, and it also inactivates RKIP through increased phosphorylation of the protein. We suggest that CNI-mediated inactivation of RKIP may play a major role in channeling the Ras signals through the Raf pathway to promote augmented tumor growth.
We have recently shown that CsA can induce the overexpression of VEGF in human renal cancer cells, and promote a rapid progression of post-transplantation cancer (10). It is now established that the activation of Ras is one of the critical factors for VEGF overexpression (49, 50). Thus, our findings in this study suggest that CsA-induced Ras activation might be one of the key factors for VEGF overexpression in renal cancer cells.
In summary, our study identifies the activation of the proto-oncogenic ras as a central mediator of CNI-induced renal cancer progression. Also, it shows that the Raf-MEK-ERK pathway acts as the key downstream effector for the CNI-induced and Ras-mediated signals. Thus, targeting this pro-tumorigenic pathway may allow new opportunities for the development of novel anti-cancer drugs, particularly for the treatment of transplant patients.
Supplementary Material
Acknowledgments
This work was supported by a John-Merrill Grant (ASN-AST) and NIH Grant R01 CA131145 to S. Pal.
References
- 1.Ponticelli C. Cyclosporine: from renal transplantation to autoimmune diseases. Ann N Y Acad Sci. 2005;1051:551–8. doi: 10.1196/annals.1361.099. [DOI] [PubMed] [Google Scholar]
- 2.Kaufman DB, Shapiro R, Lucey MR, Cherikh WS, RTB, Dyke DB. Immunosuppression: practice and trends. Am J Transplant. 2004;4 (Suppl 9):38–53. doi: 10.1111/j.1600-6135.2004.00397.x. [DOI] [PubMed] [Google Scholar]
- 3.Dantal J, Soulillou JP. Immunosuppressive drugs and the risk of cancer after organ transplantation. N Engl J Med. 2005;352(13):1371–3. doi: 10.1056/NEJMe058018. [DOI] [PubMed] [Google Scholar]
- 4.Webster AC, Craig JC, Simpson JM, Jones MP, Chapman JR. Identifying high risk groups and quantifying absolute risk of cancer after kidney transplantation: a cohort study of 15,183 recipients. Am J Transplant. 2007;7(9):2140–51. doi: 10.1111/j.1600-6143.2007.01908.x. [DOI] [PubMed] [Google Scholar]
- 5.Kasiske BL, Snyder JJ, Gilbertson DT, Wang C. Cancer after kidney transplantation in the United States. Am J Transplant. 2004;4(6):905–13. doi: 10.1111/j.1600-6143.2004.00450.x. [DOI] [PubMed] [Google Scholar]
- 6.Jamil B, Nicholls K, Becker GJ, Walker RG. Impact of acute rejection therapy on infections and malignancies in renal transplant recipients. Transplantation. 1999;68(10):1597–603. doi: 10.1097/00007890-199911270-00027. [DOI] [PubMed] [Google Scholar]
- 7.Gutierrez-Dalmau A, Campistol JM. Immunosuppressive therapy and malignancy in organ transplant recipients: a systematic review. Drugs. 2007;67(8):1167–98. doi: 10.2165/00003495-200767080-00006. [DOI] [PubMed] [Google Scholar]
- 8.Wimmer CD, Rentsch M, Crispin A, et al. The janus face of immunosuppression - de novo malignancy after renal transplantation: the experience of the Transplantation Center Munich. Kidney Int. 2007;71(12):1271–8. doi: 10.1038/sj.ki.5002154. [DOI] [PubMed] [Google Scholar]
- 9.Hojo M, Morimoto T, Maluccio M, et al. Cyclosporine induces cancer progression by a cell-autonomous mechanism. Nature. 1999;397(6719):530–4. doi: 10.1038/17401. [DOI] [PubMed] [Google Scholar]
- 10.Basu A, Contreras AG, Datta D, et al. Overexpression of vascular endothelial growth factor and the development of post-transplantation cancer. Cancer Research. 2008;68(14):5689–98. doi: 10.1158/0008-5472.CAN-07-6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maluccio M, Sharma V, Lagman M, et al. Tacrolimus enhances transforming growth factor-beta1 expression and promotes tumor progression. Transplantation. 2003;76(3):597–602. doi: 10.1097/01.TP.0000081399.75231.3B. [DOI] [PubMed] [Google Scholar]
- 12.Koehl GE, Andrassy J, Guba M, et al. Rapamycin protects allografts from rejection while simultaneously attacking tumors in immunosuppressed mice. Transplantation. 2004;77(9):1319–26. doi: 10.1097/00007890-200405150-00002. [DOI] [PubMed] [Google Scholar]
- 13.Ohsawa I, Murakami T, Uemoto S, Kobayashi E. In vivo luminescent imaging of cyclosporin A-mediated cancer progression in rats. Transplantation. 2006;81(11):1558–67. doi: 10.1097/01.tp.0000209448.50238.de. [DOI] [PubMed] [Google Scholar]
- 14.Bustami RT, Ojo AO, Wolfe RA, et al. Immunosuppression and the risk of post-transplant malignancy among cadaveric first kidney transplant recipients. Am J Transplant. 2004;4(1):87–93. doi: 10.1046/j.1600-6135.2003.00274.x. [DOI] [PubMed] [Google Scholar]
- 15.Molmenti EP, Nagata DE, Roden JS, et al. Post-transplant lymphoproliferative syndrome in the pediatric liver transplant population. Am J Transplant. 2001;1(4):356–9. doi: 10.1034/j.1600-6143.2001.10411.x. [DOI] [PubMed] [Google Scholar]
- 16.Khanna A, Cairns V, Hosenpud JD. Tacrolimus induces increased expression of transforming growth factor-beta1 in mammalian lymphoid as well as nonlymphoid cells. Transplantation. 1999;67(4):614–9. doi: 10.1097/00007890-199902270-00021. [DOI] [PubMed] [Google Scholar]
- 17.Guba M, von Breitenbuch P, Steinbauer M, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8(2):128–35. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- 18.Datta D, Contreras AG, Grimm M, Waaga-Gasser AM, Briscoe DM, Pal S. Calcineurin inhibitors modulate CXCR3 splice variant expression and mediate renal cancer progression. J Am Soc Nephrol. 2008;19(12):2437–46. doi: 10.1681/ASN.2008040394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu J, Farmer JD, Jr, Lane WS, Friedman J, Weissman I, Schreiber SL. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66(4):807–15. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- 20.Crabtree GR, Olson EN. NFAT signaling: choreographing the social lives of cells. Cell. 2002;109 (Suppl):S67–79. doi: 10.1016/s0092-8674(02)00699-2. [DOI] [PubMed] [Google Scholar]
- 21.Tiu J, Li H, Rassekh C, van der Sloot P, Kovach R, Zhang P. Molecular basis of posttransplant squamous cell carcinoma: the potential role of cyclosporine a in carcinogenesis. Laryngoscope. 2006;116(5):762–9. doi: 10.1097/01.mlg.0000205170.24517.28. [DOI] [PubMed] [Google Scholar]
- 22.Skalkeas GD, Spandidos DA, Kostakis A, et al. K-ras oncogene mutations in neoplasias of kidney transplanted patients: preliminary results with a new technique. Anticancer Res. 1991;11(6):2091–3. [PubMed] [Google Scholar]
- 23.Saggi SJ, Andoh TF, Safirstein R, Bennett WM. Cyclosporin induces renal proto-oncogene RNA message and increased transforming growth factor-beta prior to renal fibrosis: Modification by calcium channel blockade in the salt replete rat. Nephrology (Carlton) 2004;9(2):58–64. doi: 10.1111/j.1440-1797.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- 24.Schlessinger J. How receptor tyrosine kinases activate Ras. Trends Biochem Sci. 1993;18(8):273–5. doi: 10.1016/0968-0004(93)90031-h. [DOI] [PubMed] [Google Scholar]
- 25.Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3(1):11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 26.Settleman J, Albright CF, Foster LC, Weinberg RA. Association between GTPase activators for Rho and Ras families. Nature. 1992;359(6391):153–4. doi: 10.1038/359153a0. [DOI] [PubMed] [Google Scholar]
- 27.Boguski MS, McCormick F. Proteins regulating Ras and its relatives. Nature. 1993;366(6456):643–54. doi: 10.1038/366643a0. [DOI] [PubMed] [Google Scholar]
- 28.Bourne HR, Sanders DA, McCormick F. The GTPase superfamily: a conserved switch for diverse cell functions. Nature. 1990;348(6297):125–32. doi: 10.1038/348125a0. [DOI] [PubMed] [Google Scholar]
- 29.Omerovic J, Hammond DE, Clague MJ, Prior IA. Ras isoform abundance and signalling in human cancer cell lines. Oncogene. 2008;27(19):2754–62. doi: 10.1038/sj.onc.1210925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mo L, Zheng X, Huang HY, et al. Hyperactivation of Ha-ras oncogene, but not Ink4a/Arf deficiency, triggers bladder tumorigenesis. J Clin Invest. 2007;117(2):314–25. doi: 10.1172/JCI30062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Datta D, Flaxenburg JA, Laxmanan S, et al. Ras-induced modulation of CXCL10 and its receptor splice variant CXCR3-B in MDA-MB-435 and MCF-7 cells: relevance for the development of human breast cancer. Cancer Research. 2006;66(19):9509–18. doi: 10.1158/0008-5472.CAN-05-4345. [DOI] [PubMed] [Google Scholar]
- 32.Eckert LB, Repasky GA, Ulku AS, et al. Involvement of Ras activation in human breast cancer cell signaling, invasion, and anoikis. Cancer Research. 2004;64(13):4585–92. doi: 10.1158/0008-5472.CAN-04-0396. [DOI] [PubMed] [Google Scholar]
- 33.Crespo P, Xu N, Simonds WF, Gutkind JS. Ras-dependent activation of MAP kinase pathway mediated by G-protein beta gamma subunits. Nature. 1994;369(6479):418–20. doi: 10.1038/369418a0. [DOI] [PubMed] [Google Scholar]
- 34.Leevers SJ, Paterson HF, Marshall CJ. Requirement for Ras in Raf activation is overcome by targeting Raf to the plasma membrane. Nature. 1994;369(6479):411–4. doi: 10.1038/369411a0. [DOI] [PubMed] [Google Scholar]
- 35.Qiu RG, Chen J, Kirn D, McCormick F, Symons M. An essential role for Rac in Ras transformation. Nature. 1995;374(6521):457–9. doi: 10.1038/374457a0. [DOI] [PubMed] [Google Scholar]
- 36.Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70(3):389–99. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez-Viciana P, Warne PH, Dhand R, et al. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature. 1994;370(6490):527–32. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- 38.Khosravi-Far R, White MA, Westwick JK, et al. Oncogenic Ras activation of Raf/mitogen-activated protein kinase-independent pathways is sufficient to cause tumorigenic transformation. Mol Cell Biol. 1996;16(7):3923–33. doi: 10.1128/mcb.16.7.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yeung K, Seitz T, Li S, et al. Suppression of Raf-1 kinase activity and MAP kinase signalling by RKIP. Nature. 1999;401(6749):173–7. doi: 10.1038/43686. [DOI] [PubMed] [Google Scholar]
- 40.Hagan S, Garcia R, Dhillon A, Kolch W. Raf kinase inhibitor protein regulation of raf and MAPK signaling. Methods Enzymol. 2006;407:248–59. doi: 10.1016/S0076-6879(05)07021-7. [DOI] [PubMed] [Google Scholar]
- 41.Pan F, Sun L, Kardian DB, Whartenby KA, Pardoll DM, Liu JO. Feedback inhibition of calcineurin and Ras by a dual inhibitory protein Carabin. Nature. 2007;445(7126):433–6. doi: 10.1038/nature05476. [DOI] [PubMed] [Google Scholar]
- 42.Naesens M, Kuypers DR, Sarwal M. Calcineurin Inhibitor Nephrotoxicity. Clin J Am Soc Nephrol. 2009;4(2):481–508. doi: 10.2215/CJN.04800908. [DOI] [PubMed] [Google Scholar]
- 43.Esteban LM, Vicario-Abejon C, Fernandez-Salguero P, et al. Targeted genomic disruption of H-ras and N-ras, individually or in combination, reveals the dispensability of both loci for mouse growth and development. Mol Cell Biol. 2001;21(5):1444–52. doi: 10.1128/MCB.21.5.1444-1452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Best CJ, Tanzer LR, Phelps PC, et al. H-ras-transformed NRK-52E renal epithelial cells have altered growth, morphology, and cytoskeletal structure that correlates with renal cell carcinoma in vivo. In Vitro Cell Dev Biol Anim. 1999;35(4):205–14. doi: 10.1007/s11626-999-0028-2. [DOI] [PubMed] [Google Scholar]
- 45.Chin L, Tam A, Pomerantz J, et al. Essential role for oncogenic Ras in tumour maintenance. Nature. 1999;400(6743):468–72. doi: 10.1038/22788. [DOI] [PubMed] [Google Scholar]
- 46.Schoffski P, Dumez H, Clement P, et al. Emerging role of tyrosine kinase inhibitors in the treatment of advanced renal cell cancer: a review. Ann Oncol. 2006;17(8):1185–96. doi: 10.1093/annonc/mdj133. [DOI] [PubMed] [Google Scholar]
- 47.Claperon A, Therrien M. KSR and CNK: two scaffolds regulating RAS-mediated RAF activation. Oncogene. 2007;26(22):3143–58. doi: 10.1038/sj.onc.1210408. [DOI] [PubMed] [Google Scholar]
- 48.Eves EM, Shapiro P, Naik K, Klein UR, Trakul N, Rosner MR. Raf kinase inhibitory protein regulates aurora B kinase and the spindle checkpoint. Mol Cell. 2006;23(4):561–74. doi: 10.1016/j.molcel.2006.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pal S, Datta K, Khosravi-Far R, Mukhopadhyay D. Role of protein kinase Czeta in Ras-mediated transcriptional activation of vascular permeability factor/vascular endothelial growth factor expression. J Biol Chem. 2001;276(4):2395–403. doi: 10.1074/jbc.M007818200. [DOI] [PubMed] [Google Scholar]
- 50.Rak J, Mitsuhashi Y, Bayko L, et al. Mutant ras oncogenes upregulate VEGF/VPF expression: implications for induction and inhibition of tumor angiogenesis. Cancer Research. 1995;55(20):4575–80. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.