Abstract
Background and Purpose
Stress is an important risk factor for cardiovascular disease; however, most of the research on this topic has focused on incidence rather than outcome. The goal of this study was to determine the effects of prior exposure to chronic stress on ischemia-induced neuronal death, microglial activation, and anxiety-like behavior.
Methods
In Experiment 1, mice were exposed to 3 weeks of daily restraint (3 hrs), then subjected to either 8 min of cardiac arrest/cardiopulmonary resuscitation (CA/CPR) or SHAM surgery. Anxiety-like behavior, microglial activation, and neuronal damage were assessed on post-ischemic day 4. In Experiment 2, mice were infused icv with minocycline (10 μg/day) to determine the effect of inhibiting post-CA/CPR microglial activation on the development of anxiety-like behavior and neuronal death.
Results
CA/CPR precipitated anxiety-like behavior and increased microglial activation and neuronal damage within the hippocampus relative to SHAM. Prior exposure to stress exacerbated these measures among CA/CPR mice, but had no significant effect on SHAM-operated mice. Treatment with minocycline reduced both neuronal damage and anxiety-like behavior among CA/CPR animals. Anxiety-like behavior was significantly correlated with measures of microglial activation but not neuronal damage.
Conclusions
A history of stress exposure increases the pathophysiological response to ischemia and anxiety-like behavior, whereas inhibiting microglial activation reduces neuronal damage and mitigates the development of anxiety-like behavior after CA/CPR. Thus, modulating inflammatory signaling after cerebral ischemia may be beneficial in protecting the brain and preventing the development of affective disorders.
Keywords: inflammation, anxiety, microglia, stress, cardiac arrest
Introduction
Exposure to stressful life events can increase the probability of cardiac arrest (CA)1, 2, and may complicate recovery. Indeed, prolonged exposure to stress or glucocorticoids decreases neuronal viability and increases microglial reactivity3, 4. Priming of microglia by stress could impact their response to CNS injury; upon activation, microglia release pro-inflammatory cytokines, proteolytic enzymes, and nitric oxide and can become phagocytic5, 6. Global ischemia potently activates microglia7, 8, but the effects of prior exposure to stressors on post-ischemic microglial activation have not been determined.
The role of microglial activation in modulation of affect behavior also is not well-characterized, though stimulating reactive microglia in otherwise healthy rats increases anxiety-like behavior9. Given that increases in anxiety are reported in both people10, 11 and rodents12–14 that survive CA, and that cerebral ischemia reliably activates microglia, there may be a role for post-ischemic microglial activation in the development of anxiety after CA. Suppressing microglial activation with minocycline reduces neuronal damage in other models of cerebral ischemia15, 16 and ameliorates anxiety-like behavior after neonatal hypoxia-ischemia17. The current study examines the influence of chronic stress on CA/CPR-induced microglial activation, neurodegeneration, and anxiety-like behavior, and also determines the necessity of activated microglia for the generation of CA/CPR-induced anxiety-like behavior.
Materials and Methods
Animals
Adult male C57BL/6 mice were randomly assigned to groups. Experiment 1 consisted of four groups: (1) SHAM (n=8), (2) SHAM+STRESS (n=9), (3) CA/CPR (n=8), and (4) CA/CPR+STRESS (n=9). In Experiment 2, mice were treated ICV with minocycline (MIN) or its vehicle (VEH); they were assigned to: (1) SHAM+VEH (n = 5), (2) SHAM+MIN (n = 5), (3) CA/CPR+VEH (n = 7), or (4) CA/CPR+MIN (n = 7). This study was approved by The Ohio State University Animal Care and Use Committee, and conforms to guidelines provided by NIH for the care and use of animals. All surgeries were conducted under sterile conditions and mice were not returned to the colony after surgery until mobile. They were visually monitored twice daily afterwards.
Restraint
Mice were placed in well-ventilated polypropylene tubes (9.7 cm long, 2.8 cm ID) that allow postural adjustments but not turns for 3 h/day for 3 weeks during the light cycle. The final restraint session occurred 24 hrs prior to surgery. This method of daily restraint reliably elicits a corticosterone response for up to 6 weeks of exposure18.
Cardiac Arrest Procedure
Mice were anesthetized with halothane, and intubated. Brain and core body temperature were assessed using temperature probes placed in the temporalis muscle (methodological validation14) and rectum, respectively. Head and body temperature were independently controlled through water-filled coils. A PE10 catheter was inserted into the right jugular vein for potassium chloride (KCL) and epinephrine (EPI) administration. A blood pressure transducer (Columbus Instruments, Columbus, OH) was connected to a right femoral artery cannula. Mice were ventilated at a tidal volume of 120 μl and a respiratory rate of 160 breaths per minute (Columbus Instruments, Columbus, OH). Blood pressure and temperatures were recorded at one min intervals during a 10 min acclimation period (Figure 1). Body temperature was decreased to 27°C to prevent peripheral organ damage14, 19. Head temperature was maintained at 37°C. To induce cardiac arrest, cold KCl (50.0 μl, 0.5 M, 4°C) was infused and the mouse was detached from the ventilator. Re-warming began when body temperature reached 27oC after approximately 4 min of arrest. At 7 min 45 sec into the arrest period, the mouse was reattached to the ventilator and began to receive 100% oxygen. At 8 min, 8 μg of EPI in 0.5 cc saline was injected and chest compressions (approximately 300/min) initiated. Additional EPI was administered in increments of 0.5 μg/30 sec until mice were resuscitated. Mice were maintained on 100% oxygen for 15 min, then extubated, followed by the removal of catheters and suturing of wounds.
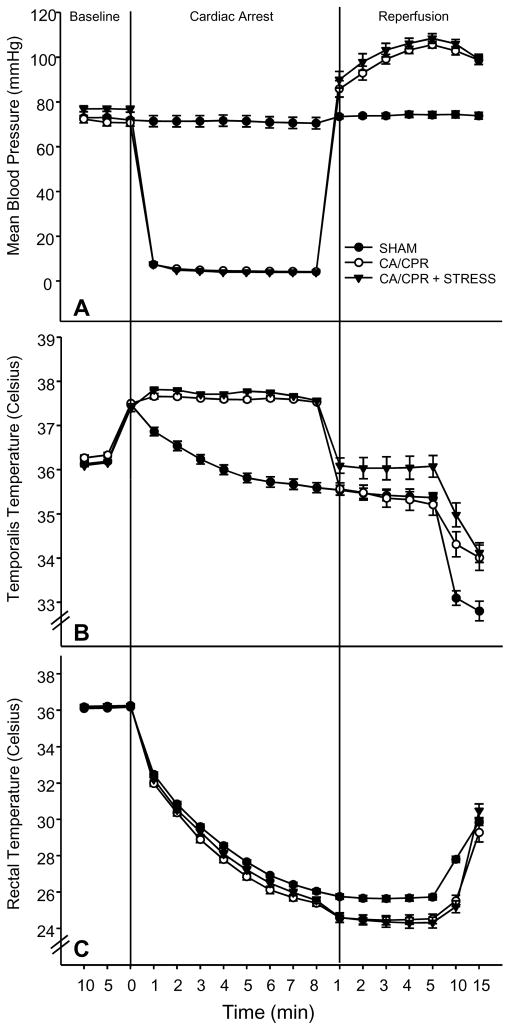
Figure 1.
A) Mean blood pressure was reduced by the cardiac arrest procedure (CA/CPR), and following resuscitation mean blood pressure was transiently elevated relative to SHAM. Restraint had no effect on mean blood pressure among CA/CPR or SHAM mice. B) Temporalis temperature was lower among CA/CPR than SHAM groups, but there was no effect of restraint. C) Core body temperature decreased during both the SHAM and CA/CPR procedures as compared to basal body temperatures. Data are presented as mean ± SEM.
The surgical preparations, anesthetic exposure, and temperature modulation described above were similar for SHAM mice, except that they received injections of isotonic saline instead of KCl and EPI, and were not given chest compressions.
Minocycline
Minocycline, a tetracycline derivative with anti-inflammatory properties, prevents microglial activation after neurological insults20. In Experiment 2, a stereotaxic apparatus was used to implant a cannula into the left lateral ventricle of anesthetized mice (isoflurane) three days prior to CA/CPR (cannula position: +0.02 posterior and −0.95 lateral to bregma, extending 2.75 mm below the skull; Plastics One, Roanoke, VA). The cannula was connected via tubing to an Alzet minipump (Model 1002, Durect, Cupertino, CA) that was implanted subcutaneously in the scapular region and delivered artificial cerebrospinal fluid (aCSF, the vehicle) or minocycline at a rate of 0.25μL/hour. The minocycline group received 10μg of the drug per day beginning 12 hrs prior to CA/CPR; the minipump tubing and cannula were primed to deliver aCSF for the first 2.5 days after implantation. The cannula and pump were implanted during a single surgery, three days prior to CA/CPR or SHAM, in an attempt to minimize surgical stress. Cannula placement was verified with cresyl violet staining after tissue collection.
Behavioral Testing
Total locomotor activity, rearing, and central tendency were assessed for 60 min in an open field (40 cm × 40 cm × 37.5 cm; San Diego Instruments, CA) one day prior to surgery and 4 days after surgery for both experiments. Central tendency is the percent time spent in a 90 cm2 zone in the center of the apparatus. Habituation can occur with repeated exposure to the open field as the novelty of the open field diminishes over time21, but the anxiogenic properties of the center do not decrease22. Because the CA/CPR+STRESS group in Experiment 1 and the CA/CPR+MIN group in Experiment 2 had two levels of treatment, the use of a baseline measure (after stress or minocycline but before CA/CPR) allowed within subjects control for the combined condition when using ANOVA with repeated measures.
Histology
On day 5 post-surgery, mice were transported one at a time from the colony, deeply anesthetized (pentobarbital) and perfused with 0.1 M phosphate buffered saline (PBS) followed by perfusion, then post-fixing, with 4% paraformaldehyde in 0.1 M PBS. Brains were cryoprotected in 30% sucrose in 0.2 M PBS. The region containing the hippocampus was cut into 10 μM sections and thaw-mounted onto slides. The slides were incubated in 0.2 M phosphate buffer with 0.1% Tris and blocked with 4% rabbit serum. Anti-mouse MAC-1 (1:100; Serotec, Oxford, England) was then added to the slides and incubated overnight at 4°C. Then, the slides were rinsed in 0.5 M Tris buffer and incubated overnight at 4°C in biotinylated rabbit anti-rat (Vector, Burlingame, CA) in 4% rabbit serum. Slides were then rinsed in 0.5 M Tris buffer and visualized with DAB and counterstained with 0.1% cresyl violet. Finally, slides were dehydrated through a series of graded ethanol solutions followed by xylene, and then coverslipped.
Histological measures were collected by an individual who was not was not aware of group assignment. The degree of microglia activation was qualitatively analyzed by the summation of two scores. The first score was assigned based on the degree of microglial activation in the hippocampus. The scale was as follows: 0= no glial activation, 1= mild CA1/CA2 glial activation, 2= moderate glial activation throughout the hippocampus, 3= pronounced glial activation throughout the hippocampus. The second score described glial activation outside the hippocampus (e.g. cortex or caudate/putamen) and was as follows: 0= no glial activation, 1= mild activation, 2= pronounced activation, 3= pronounced activation in more than one region. The two scales were summed for each mouse and then group medians determined. Alternate sections were stained with hemotoxylin and eosin, dehydrated, and coverslipped14. Pyknotic cells were counted at 600X in the dorsal hippocampus proper and dentate gyrus of each hemisphere, then averaged.
Statistical Analyses
Behavioral parameters were assessed via three-way repeated measures ANOVAs using the factors of time (pre-CA/CPR vs. post-CA/CPR), surgery (SHAM vs. CA/CPR), and stress (control vs. restraint) in Experiment 1 and the factors of time (pre-CA/CPR vs. post-CA/CPR), surgery (SHAM vs. CA/CPR), and drug (VEH vs. MIN) in Experiment 2. Neuronal damage was assessed via two-way ANOVAs using the factors of surgery (SHAM vs. CA/CPR) and stress (control vs. restraint) in Experiment 1 and the factors of surgery (SHAM vs. CA/CPR) and drug (VEH vs. MIN) in Experiment 2. Post-hoc analysis was used to further distinguish among groups, and all differences were considered statistically significant if p < 0.05. Microglial activation was analyzed using a Mann-Whitney Rank Sum Test because the data did not meet the criteria for parametric analysis. Correlations between microglial activation or cell death and central tendency were assessed using the non-parametric Spearman Rank Order Correlation. α = 0.05 for all parametric and non-parametric statistical analyses.
Results
Surgical Parameters
Neither stress nor minocycline altered blood pressure, temperature, surgical time, or resuscitation time (Figure 1). As expected, CA/CPR decreased mean blood pressure, as compared to SHAM during the period between KCl and EPI administration (F(3,56) = 104.3, p < 0.05; Figure 1A). Temporalis temperature was higher among CA/CPR than SHAM mice (F(3,56) = 34.1, p < 0.05; Figure 1B). Core body temperature was lower among CA/CPR than SHAM mice (F(3,56) = 9.8, p < 0.05; Figure 1C).
Behavioral Testing
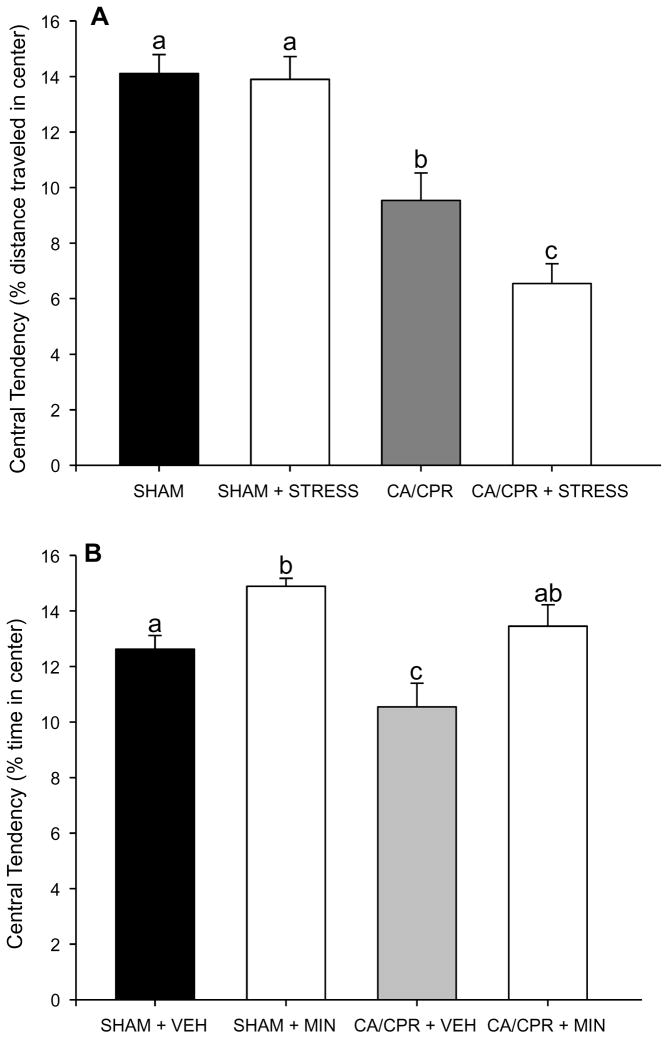
In Experiment 1, groups did not differ in general locomotor activity during pre-surgical or post-surgical testing (p > 0.05), and there was no significant change in total activity between these two timepoints for any group (p > 0.05). In contrast, central tendency was similar among groups during pre-surgical testing, but reduced among CA/CPR groups during post-surgical testing (F(1,33) = 57.23, p < 0.05; Figure 2A). Furthermore, CA/CPR+STRESS further increased anxiety-like behavior relative to the CA/CPR group (p < 0.05). Restraint did not impact anxiety-like behavior among SHAMs (p >0.05).
Figure 2.
A) Central tendency, a measure of anxiety-like behavior, was decreased in all mice exposed to CA/CPR relative to SHAM. CA/CPR+STRESS further decreased central tendency relative to CA/CPR. Restraint had no effect among SHAM mice. B) In Experiment 2, CA/CPR reduced central tendency relative to SHAM, and this effect was blocked by administration of minocycline. Data are presented as mean ± SEM; bars with different letters are statistically different (p < 0.05).
In Experiment 2 there were no significant pre or post-surgical group differences in general locomotor activity or rearing (p > 0.05), and no significant change in these measures between pre and post-surgical testing for any group (p > 0.05). There were no group differences in central tendency at presurgical testing (p > 0.05), but the CA/CPR+VEH group reduced central tendency relative to the other three groups on post-surgical day 4 (F(1,19) = 13.59, p < 0.05). In contrast, central tendency was similar for CA/CPR+MIN and the two SHAM groups (t(11) = 2.55, p < 0.05; Figure 2B). Minocycline also increased central tendency in SHAM mice compared to the vehicle group (F(1,9) = 16.053, p < 0.05; Figure 2B).
Microglial Analysis
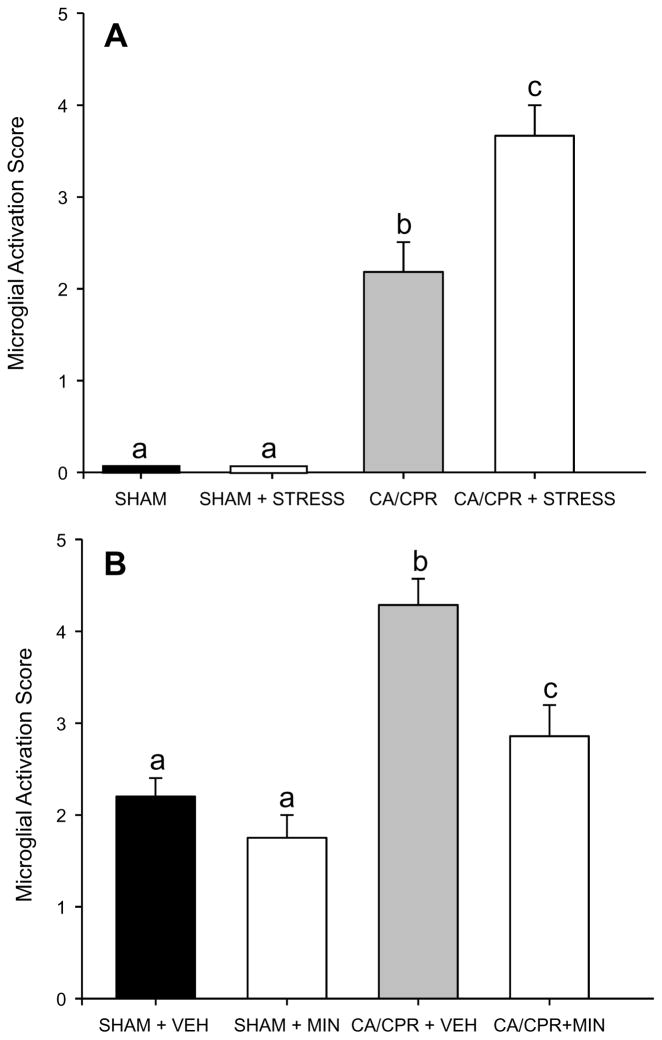
In Experiment 1, there was no microglial activation in SHAM brains (Figure 3A and B). CA/CPR caused activation of microglia, primarily in the hippocampus (Figure 3C and D). CA/CPR+STRESS increased both the level of microglial activation and its spatial distribution relative to CA/CPR (T(11) = 87.5, p < 0.05; Figure 3E and F, Figure 5A).
Figure 3.
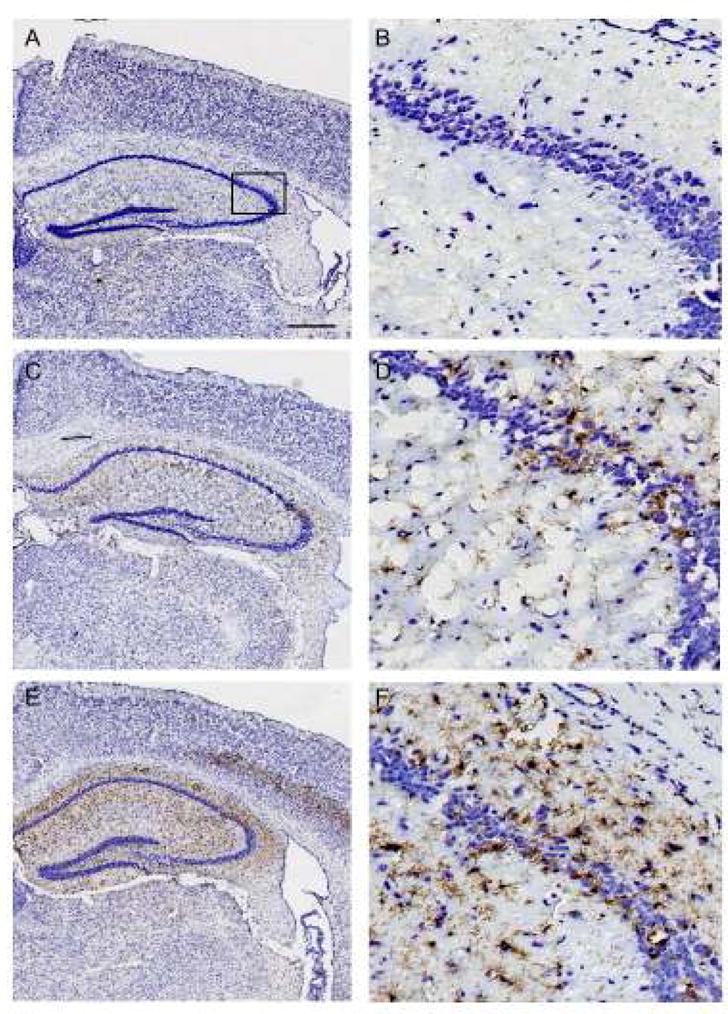
Representative photomicrographs showing morphological and phenotypic differences of microglia in the hippocampus of mice in the SHAM (A,B), cardiac arrest and cardiopulmonary resuscitation (CA/CPR) (C,D), and chronic restraint and CA/CPR (E,F) groups. Microglial activation is particularly enhanced at the border of the CA1 and CA2 regions of the hippocampus following CA/CPR (box in C). Microglial activation is enhanced further in the hippocampus and in the adjacent cortex (arrows in E) of CA/CPR+STRESS groups (E,F). Scale in A = 250 μM for A, C, E and 50 μM for B, D, F.
Figure 5.
Qualitative assessment of microglial activation in the brain following CA/CPR or SHAM surgery. A) CA/CPR increased microglial activation relative to SHAM, an effect that was further exacerbated by prior exposure to restraint. B) VEH+CA/CPR increased microglial activation relative to SHAM, whereas MINO+CA/CPR reduced microglial activation to a level that was no longer significantly different from SHAM Data are presented as mean ± SEM; bars with different letters are statistically different (p < 0.05).
In Experiment 2, CA/CPR activated microglia in both vehicle and minocycline treated mice (F(3,22) = 13.6, p < 0.001; Figure 4 and 5B) relative to SHAM. Post-hoc analysis revealed that CA/CPR+VEH mice had significantly greater microglial activation then SHAM mice, but microglial activation was significantly reduced among CA/CPR+MIN mice, which did not differ from SHAM (p > 0.05).
Figure 4.
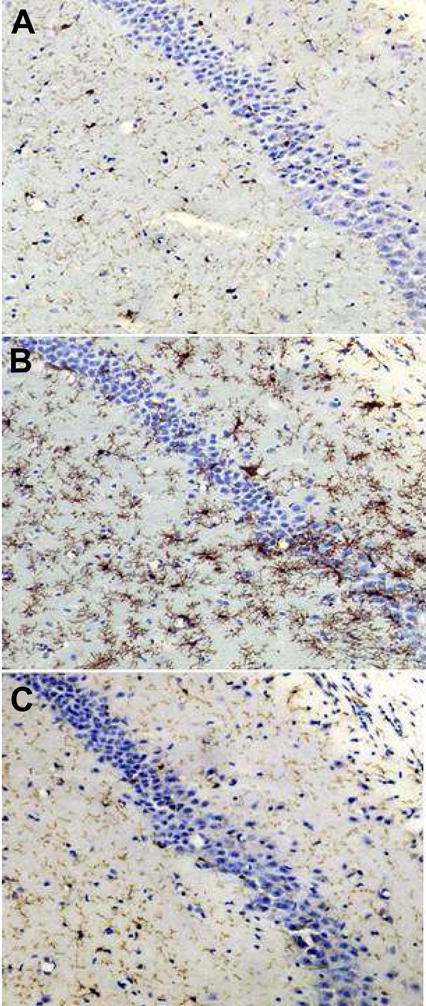
Representative photomicrographs showing morphological and phenotypic differences of microglia in the hippocampus of the mice in the SHAM (A), cardiac arrest and cardiopulmonary resuscitation (CA/CPR) (B), and CA/CPR + minocycline (C) groups. Microglial activation is inhibited by icv administration of minocycline (panel C as compared to panel B). Scale = 250 μM.
Spearman rank order correlation demonstrated that microglial activation was correlated with anxiety-like behavior (r2 = −0.45, p < 0.05).
Assessment of Pyknotic Cells
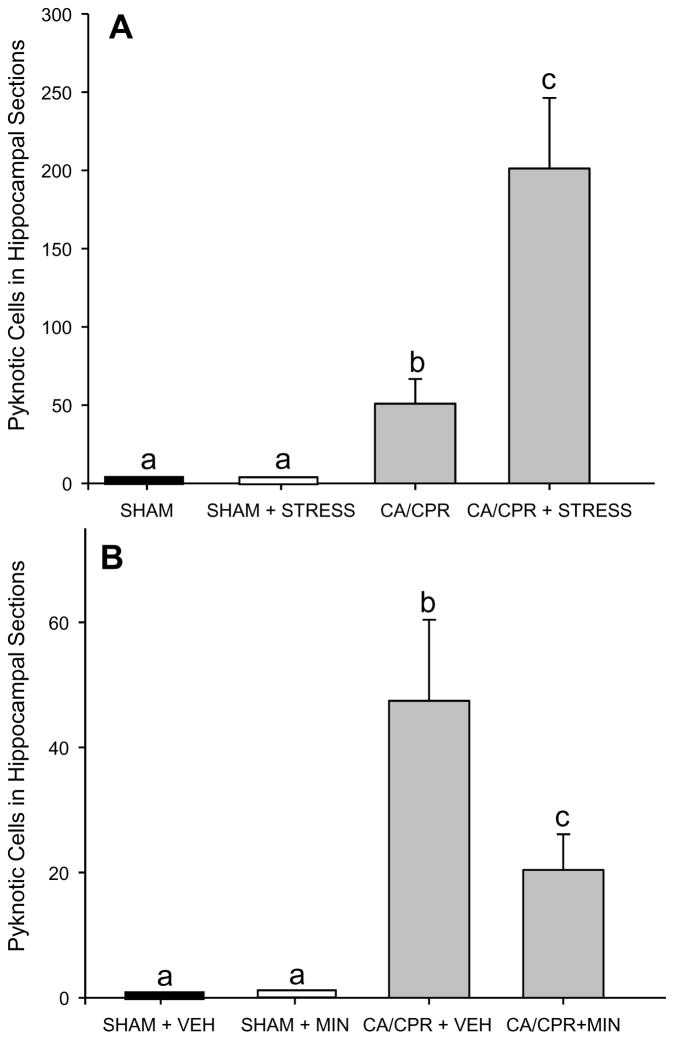
In Experiment 1, there was no cell death in SHAM brains (p > 0.05). CA/CPR caused a significant increase in pyknotic cells in the hippocampus (Figure 6A; H(2) = 17.8, p < 0.05) relative to SHAM. CA/CPR+STRESS increased rating of pyknotic cells in the hippocampus relative to CA/CPR alone (t(15) = 2.281, p < 0.05).
Figure 6.
Quantification of pyknotic cells from representative sections of the hippocampus. A) CA/CPR induces pyknotic cells, the number of which is increased by prior exposure to three weeks of restraint. B) The number of pyknotic cells after CA/CPR is reduced among mice treated with minocycline (MIN) as compared to VEH. SHAMs had no pyknotic cells in either A or B. Data are presented as mean ± SEM; bars with different letter are statistically different (p < 0.05).
In Experiment 2 there was no cell death in SHAM brains, and CA/CPR significantly increased the number of pyknotic cells (F(2,23) = 21.820, p < 0.05; Figure 6B) in the hippocampus. A post-hoc analysis revealed that CA/CPR+VEH mice had more pyknotic cells than SHAM operated mice, however, CA/CPR+MIN significantly reduced the number of pyknotic cells relative to CA/CPR+VEH (p < 0.05).
Spearman rank order correlation demonstrated that the number of pyknotic cells was not correlated with anxiety-like behavior (p > 0.05).
Discussion
Collectively, these data demonstrate that a history of stress exposure exacerbates post-CA/CPR anxiety-like behavior and augments microglial activation and neuronal cell death. In addition, ischemia-induced anxiety-like behavior can be prevented by minocycline, which attenuates both microglial activation and neuronal death. Anxiety-like behavior was significantly correlated with microglial activation, but not neuronal damage. These data confirm that surviving cardiac arrest precipitates the development of anxiety, and suggests that minocycline administration may be an effective treatment.
These data confirm previous studies indicating that global ischemia induced in rodents by CA/CPR results in neuronal cell death14, 19, microglial activation7, 8, and increased anxiety-like behavior12–14. However, the indication that these three measures are exacerbated when exposure to chronic restraint precedes CA/CPR provides new evidence for the importance of stress as a risk factor for cardiovascular disease. Stress is a known risk factor for the onset of cardiac arrest2, but the current data suggest that it also affects outcome among survivors. Prior exposure to stress altered the pattern of microglial activation following CA/CPR but not SHAM surgery, thereby suggesting that the morphological effects of stress on microglia are only apparent following injury. Furthermore, stress altered the spatial distribution of activated microglia following CA/CPR; in the absence of prior stress, microglial activation occurred exclusively in the hippocampus, with preferential involvement of the pyramidal cell layer at the border of CA1 and CA2. In contrast, the combination of stress and CA/CPR lead to additional microglial activation in the cortex and caudate/putamen (Figure 3E and 3F). Although the mechanisms may differ, a similar negative effect of stress on neuronal damage and cognitive function has been demonstrated following experimental stroke23.
The association of increased microglial activation with increased neuronal death and anxiety-like behavior after CA/CPR in the current study suggests that activated microglia are contributing to early post-ischemic neuropathology and behavioral changes. However, because microglia can improve survival of metabolically impaired neurons24 under other circumstances, the relationship between microglia and neurons may be dynamic. Whether the role of microglia in brains exposed to CA/CPR changes over time, as the initial wave of neuronal death passes, remains to be determined.
Consistent with previous reports17, 25, minocycline administration inhibited microglial activation following CA/CPR, decreased cell death, and prevented post-CA/CPR anxiety-like behavior. However, the correlation between microglial activation and anxiety-like behavior suggests that the anxiolytic effects of minocycline may be associated with inhibition of microglial activation. The absence of a significant correlation between neuronal damage and behavioral outcome is consistent with previous studies in which cell death did not predict post-ischemic behavioral outcome26. However, given that minocycline decreased both cell death and microglial activation following CA/CPR, we cannot rule out the potential for cell survival to contribute to the changes in behavior. Regardless of the precise mechanism, minocycline did effectively prevent post-CA/CPR anxiety-like behavior and decrease cell death.
Summary
Exposure to stress augments CA/CPR-induced anxiety-like behavior and increases microglia activation and neuronal death in the hippocampus. Minocycline, which has been effective in minimizing damage in a clinical stroke study27, inhibited microglial activation, cell death, and anxiety-like behavior following CA/CPR in the current mouse study. These data complement a growing body of literature documenting the impact of neuroinflammation on affective behavior. Identifying the post-ischemic physiology that underlies changes in affective behavior is important for improving the quality of life of cardiac arrest survivors.
Acknowledgments
This work was supported by grants from the National Institutes of Health (NS 40267, NS20020, NS746703) and American Heart Association.
Footnotes
Financial Disclosures
None.
References
- 1.Grippo AJ, Johnson AK. Stress, depression and cardiovascular dysregulation: A review of neurobiological mechanisms and the integration of research from preclinical disease models. Stress. 2009;12:1–21. doi: 10.1080/10253890802046281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coumel P. The role of stress in sudden death: Can it be prevented? In: Aliot E, Clementy J, Prystowsky EN, editors. Fighting sudden cardiac death: A worldwide challenge. Armonk, NY: Futura; 2000. pp. 567–579. [Google Scholar]
- 3.Nair A, Bonneau RH. Stress-induced elevation of glucocorticoids increases microglia proliferation through NMDA receptor activation. J Neuroimmunol. 2006;171:72–85. doi: 10.1016/j.jneuroim.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 4.Ramos-Remus C, Gonzalez-Castaneda RE, Gonzalez-Perez O, Luquin S, Garcia-Estrada J. Prednisone induces cognitive dysfunction, neuronal degeneration, and reactive gliosis in rats. J Investig Med. 2002;50:458–464. doi: 10.1136/jim-50-06-06. [DOI] [PubMed] [Google Scholar]
- 5.Stoll G, Jander S. The role of microglia and macrophages in the pathophysiology of the CNS. Prog Neurobiol. 1999;58:233–247. doi: 10.1016/s0301-0082(98)00083-5. [DOI] [PubMed] [Google Scholar]
- 6.Amantea D, Nappi G, Bernardi G, Bagetta G, Corasaniti MT. Post-ischemic brain damage: Pathophysiology and role of inflammatory mediators. Febs J. 2009;276:13–26. doi: 10.1111/j.1742-4658.2008.06766.x. [DOI] [PubMed] [Google Scholar]
- 7.Clemens JA, Stephenson DT, Smalstig EB, Roberts EF, Johnstone EM, Sharp JD, Little SP, Kramer RM. Reactive glia express cytosolic phospholipase a2 after transient global forebrain ischemia in the rat. Stroke. 1996;27:527–535. doi: 10.1161/01.str.27.3.527. [DOI] [PubMed] [Google Scholar]
- 8.Sugawara T, Lewen A, Noshita N, Gasche Y, Chan PH. Effects of global ischemia duration on neuronal, astroglial, oligodendroglial, and microglial reactions in the vulnerable hippocampal ca1 subregion in rats. J Neurotrauma. 2002;19:85–98. doi: 10.1089/089771502753460268. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez-Perez O, Ramos-Remus C, Garcia-Estrada J, Luquin S. Prednisone induces anxiety and glial cerebral changes in rats. J Rheumatol. 2001;28:2529–2534. [PubMed] [Google Scholar]
- 10.Parnia S, Spearpoint K, Fenwick PB. Near death experiences, cognitive function and psychological outcomes of surviving cardiac arrest. Resuscitation. 2007;74:215–221. doi: 10.1016/j.resuscitation.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 11.Wachelder EM, Moulaert VR, van Heugten C, Verbunt JA, Bekkers SC, Wade DT. Life after survival: Long-term daily functioning and quality of life after an out-of-hospital cardiac arrest. Resuscitation. 2009 doi: 10.1016/j.resuscitation.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 12.Yan B, He J, Xu H, Zhang Y, Bi X, Thakur S, Gendron A, Kong J, Li XM. Quetiapine attenuates the depressive and anxiolytic-like behavioural changes induced by global cerebral ischemia in mice. Behav Brain Res. 2007;182:36–41. doi: 10.1016/j.bbr.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Dhooper A, Young C, Reid KH. Ischemia-induced anxiety following cardiac arrest in the rat. Behav Brain Res. 1997;84:57–62. doi: 10.1016/s0166-4328(96)00133-7. [DOI] [PubMed] [Google Scholar]
- 14.Neigh GN, Kofler J, Meyers JL, Bergdall V, La Perle KM, Traystman RJ, DeVries AC. Cardiac arrest/cardiopulmonary resuscitation increases anxiety-like behavior and decreases social interaction. J Cereb Blood Flow Metab. 2004;24:372–382. doi: 10.1097/01.WCB.0000112323.75217.B4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cai ZY, Yan Y, Sun SQ, Zhang J, Huang LG, Yan N, Wu F, Li JY. Minocycline attenuates cognitive impairment and restrains oxidative stress in the hippocampus of rats with chronic cerebral hypoperfusion. Neurosci Bull. 2008;24:305–313. doi: 10.1007/s12264-008-0324-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peeling J, Yan H, Buist R, Sitar DS, Corbett D. Protective effect of minocycline treatment on striatal ischemia. J Stroke Cerebrovasc Dis. 2006;15:101–105. doi: 10.1016/j.jstrokecerebrovasdis.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Fan LW, Lin S, Pang Y, Rhodes PG, Cai Z. Minocycline attenuates hypoxia-ischemia-induced neurological dysfunction and brain injury in the juvenile rat. Eur J Neurosci. 2006;24:341–350. doi: 10.1111/j.1460-9568.2006.04918.x. [DOI] [PubMed] [Google Scholar]
- 18.Neigh GN, Bowers SL, Pyter LM, Gatien ML, Nelson RJ. Pyruvate prevents restraint-induced immunosuppression via alterations in glucocorticoid responses. Endocrinology. 2004 doi: 10.1210/en.2003-1748. [DOI] [PubMed] [Google Scholar]
- 19.Kofler J, Hattori K, Sawada M, DeVries AC, Martin LJ, Hurn PD, Traystman RJ. Histopathological and behavioral characterization of a novel model of cardiac arrest and cardiopulmonary resuscitation in mice. J Neurosci Methods. 2004;136:33–44. doi: 10.1016/j.jneumeth.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 20.Zemke D, Majid A. The potential of minocycline for neuroprotection in human neurologic disease. Clin Neuropharmacol. 2004;27:293–298. doi: 10.1097/01.wnf.0000150867.98887.3e. [DOI] [PubMed] [Google Scholar]
- 21.Fowler H. Curiousity and exploratory behavior. New York: Macmillan; 1965. [Google Scholar]
- 22.Adams LM, Geyer MA. A proposed animal model for hallucinogens based on lsd’s effects on patterns of exploration in rats. Behav Neurosci. 1985;99:881–900. doi: 10.1037//0735-7044.99.5.881. [DOI] [PubMed] [Google Scholar]
- 23.Sugo N, Hurn PD, Morahan MB, Hattori K, Traystman RJ, DeVries AC. Social stress exacerbates focal cerebral ischemia in mice. Stroke. 2002;33:1660–1664. doi: 10.1161/01.str.0000016967.76805.bf. [DOI] [PubMed] [Google Scholar]
- 24.Garden GA, Moller T. Microglia biology in health and disease. J Neuroimmune Pharmacol. 2006;1:127–137. doi: 10.1007/s11481-006-9015-5. [DOI] [PubMed] [Google Scholar]
- 25.Yrjanheikki J, Keinanen R, Pellikka M, Hokfelt T, Koistinaho J. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci U S A. 1998;95:15769–15774. doi: 10.1073/pnas.95.26.15769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neigh GN, Glasper ER, Kofler J, Traystman RJ, Mervis RF, Bachstetter A, DeVries AC. Cardiac arrest with cardiopulmonary resuscitation reduces dendritic spine density in ca1 pyramidal cells and selectively alters acquisition of spatial memory. Eur J Neurosci. 2004;20:1865–1872. doi: 10.1111/j.1460-9568.2004.03649.x. [DOI] [PubMed] [Google Scholar]
- 27.Lampl Y, Boaz M, Gilad R, Lorberboym M, Dabby R, Rapoport A, Anca-Hershkowitz M, Sadeh M. Minocycline treatment in acute stroke: An open-label, evaluator-blinded study. Neurology. 2007;69:1404–1410. doi: 10.1212/01.wnl.0000277487.04281.db. [DOI] [PubMed] [Google Scholar]