Abstract
Objective
Apolipoprotein E (apoE), a key protein in lipid metabolism, is highly expressed in adipose tissues. Studies have shown that human APOE*4 is associated with a lower body mass index but with a greater risk of coronary heart disease compared with other APOE alleles. To define the isoform-specific role of apoE in regulating the expandability and functionality of adipose tissues, we investigated the effects of diet-induced obesity in mice whose endogenous Apoe gene has been replaced by either the human APOE*3 or APOE*4 allele.
Results
After 8 weeks on a Western-type high-fat diet, male APOE4 mice displayed impaired tolerance to glucose and fat overload compared with APOE3 mice. Subcutaneous fat tissues in APOE4 and APOE3 mice after high fat feeding were not different. In contrast, although epididymal fat tissues in APOE4 mice gained 30% less weight during the high fat feeding than in APOE3 mice, they showed impaired insulin-stimulated glucose uptake ex vivo. Epididymal APOE4 adipocytes were larger in size than APOE3 adipocytes, and expressed reduced levels of mRNA for peroxisome proliferator-activated receptor γ2 and adiponectin, important markers of adipocyte functionality. Adenoviral expression of apoE3 in apoE-null culture adipocytes induced adiponectin mRNA in a dose-dependent manner, but the induction was significantly blunted in cells overexpressing apoE4. However, in contrast to the apoE3-expressing cells, Glut1, but not Glut4, expression levels were positively correlated with increased apoE4 mRNA, suggesting that apoE4 expression in adipocyte interferes in insulin-sensing pathways.
Conclusion
Dysfunctional epididymal adipose tissues contribute to the accelerated impairment of glucose tolerance in APOE4 mice fed a Western-type diet. Our results underscore the importance of functionality of individual fat depots rather than total fat mass as a determinant for metabolic disturbance during diet-induced obesity.
Keywords: apolipoprotein E, apoE3, apoE4, diet-induced obesity, adipocyte functionality
Introduction
Dysregulated adipose tissue, as seen in cases of obesity and lipoatrophy, raises the risk of morbidity from hypertension, dyslipidemia, type 2 diabetes and cardiovascular disease.1,2 Explanations for this increased risk involve the concomitant presence of impaired insulin sensitivity and chronic inflammation with subsequent ectopic fat accumulation.3 Although adipocytes have long been considered as an inert storage compartment for triglycerides, they are now thought to be master regulators of energy metabolism. Adipocytes communicate with other organs through the secretion of an array of cytokines (adipokines) and other proteins, one of which is apolipoprotein E (apoE).4 A wide range of investigations indicate that human apoE may also play a significant role in both adipocyte function and body fatness.5–7
The 34-kDa apoE, protein, associates with lipoprotein particles and mediates their binding to receptors for subsequent endocytosis.8 In humans, the APOE gene is polymorphic and has three alleles, APOE*2, APOE*3 and APOE*4, with frequencies of 7, 77 and 15%, respectively, in the general population.9 The Heritage Family Study showed a pleiotropic quantitative trait locus for triglycerides and adiposity on ch19q13 where APOE is located,7 and the Atherosclerosis Risk in Communities (ARIC) Study has reported that the apoE isoforms are associated with increasing body mass index in the order: apoE2>apoE3>apoE4.6 However, despite apoE4 carriers being the leanest group, epidemiologic studies have clearly shown that the presence of at least one APOE*4 allele is associated with a greater risk of coronary artery disease.10,11 This increased risk has been attributed to elevated plasma low-density lipoprotein (LDL) cholesterol, a well-established risk factor. Although a direct association between the APOE*4 allele and insulin resistance has not been established,12,13 associations of APOE*4 with both higher levels, and accelerated changes over time of fasting glucose levels have been demonstrated in the Baltimore Longitudinal Study of Aging.14 Additionally, Elosua et al.5 have reported that male subjects carrying the APOE*4 allele had significantly higher fasting insulin and glucose plasma concentrations only in the presence of obesity.
Our current study was aimed to test the hypothesis that the most common polymorphic variations, APOE*3 and APOE*4, which account for more than 90% of human population, can differentially modulate the expandability and functionality of adipocytes, thereby influencing overall glucose tolerance under the stress produced by a high-fat diet. To this end, we have used mice in which their endogenous Apoe gene has been replaced by the human APOE*3 or APOE*4 allele. These mice retain the murine Apoe regulatory sequences and solely produce human apoE3 or apoE4 proteins at physiological levels.8 We found that when these mice are stressed by a high-fat diet, adipocytes expressing apoE4 fail to buffer postprandial lipids and glucose completely. This dysfunctional adipocyte, produced by an interaction between genotype and the environment, reconciles the paradox of diminished body fat mass but increased atherogenic risk described in APOE*4 carriers.
Methods
Mice
Mice homozygous for replacement of the endogenous apoE gene with the human APOE*3 (APOE3) or APOE*4 (APOE4) allele were backcrossed onto a C57BL/6 genetic background. 8,15 Male mice were fed ad libitum either regular chow containing 5.3% fat and 0.019% cholesterol (Prolab Isopro RMH 3000, ref 5P76; Agway Inc., Syracuse, NY, USA) or a high-fat Western-type diet (WD) containing 21% (w/w) fat and 0.2% (w/w) cholesterol (TD88137; Teklad, Madison, WI, USA) over the period indicated in each experiment. Animals were fasted for 4 h and oral glucose tolerance tests or intraperitoneal insulin tolerance tests were performed with 2gkg−1 body weight glucose load by oral gavage and with intraperitoneal injections of 0.5 IU kg−1 of insulin, respectively. Approximately 30 μl of blood was collected at times indicated and assayed for glucose. Oral fat tolerance test (OFTT) was carried out after overnight fasting by gavaging olive oil (10 ml kg−1), by assaying plasma triglycerides at time points indicated. Mice were denied access to food during the course of these tolerance tests. The animals were handled under protocols approved by the Institutional Animal Care and Use Committees of the University of North Carolina––Chapel Hill.
Biochemical determinations
Plasma concentration of non-esterified free fatty acids (NEFAs), glucose and cholesterol was determined by commercial kits from Wako (Richmond, VA, USA) according to the manufacturer’s instructions. Triglyceride and insulin concentrations were determined using commercial kits from Stanbio (San Antonio, TX, USA) and Crystal Chem Inc., (Chicago, IL, USA) respectively. Pooled plasma samples (100 μl) were fractionated by fast protein liquid chromatography using a Superose 6 HR10/30 column (GE Healthcare, Piscataway, NJ, USA). apoE and plasma adiponectin were measured using an ELISA with antibodies specific for human apoE (Calbiochem, La Jolla, CA, USA) and murine adiponectin (Sigma, St Louis, MO, USA) as previously described.16
Fat explants
Subcutaneous inguinal and visceral epididymal adipose tissues were cut with scissors into small pieces (20–40mg) under aseptic conditions and incubated in high glucose Dulbecco’s modified Eagle’s medium with or without insulin. After 24h, glucose reduction in the medium was measured and the result was normalized according to fat weight. The fat tissues were fixed in 10% formalin. Paraffin sections (10 μm thickness) were stained with hematoxylin and eosin. The cross-sectional area of adipocytes was measured using Image J (National Institutes of Health (NIH)).
Adenoviruses and cell culture
Recombinant adenovirus harboring human apoE3-GFP or apoE4-GFP was made using the AdEasy system (Stratagene, La Jolla, CA, USA) from cytomegalovirus promoter-driven cDNA for fusion proteins, apoE3-GFP or apoE4-GFP, provided by Dr Robert DeKroon at Duke University.17,18 Mouse embryonic fibroblasts isolated from ApoE−/− embryos were obtained essentially as described,19 seeded in 12-well plates (105 cells per well) and maintained in 10% fetal bovine serum–Dulbecco’s modified Eagle’s medium. At 1 day post-confluence, 107 or 2×107 p.f.u. adenoviruses were added to the medium. After 24 h, cells were placed in adipocyte differentiation medium (Zen-Bio, Research Triangle Park, NC, USA). The medium was replaced 48 h later with 10% fetal bovine serum–Dulbecco’s modified Eagle’s medium containing 5 μg insulin. Mature adipocytes were lysed 7 days later using Nucleic Acid Purification Solution (Applied Biosystems, Foster City, CA, USA).
Gene expression
mRNA was purified using an Automated Nucleic Acid Workstation ABI 6700, and real-time PCR was performed in an ABI PRISM 7700 Sequence Detector (Applied Biosystems). β-Actin mRNA was used for normalization. Sequences for primers and probes are available on request.
Statistical analysis
Results are expressed as means±s.e. Two-way analysis of variance with genotype and time as factors followed by Student’s t-test for post hoc analysis were used unless otherwise stated. To test the strength of the correlations between variables, the Pearson’s correlation test was used. All statistical analyses were performed using SPSS software, version 11.0 (SPSS Inc., Chicago, IL, USA).
Results
Allele-specific effects of a high-fat diet on adipose tissues
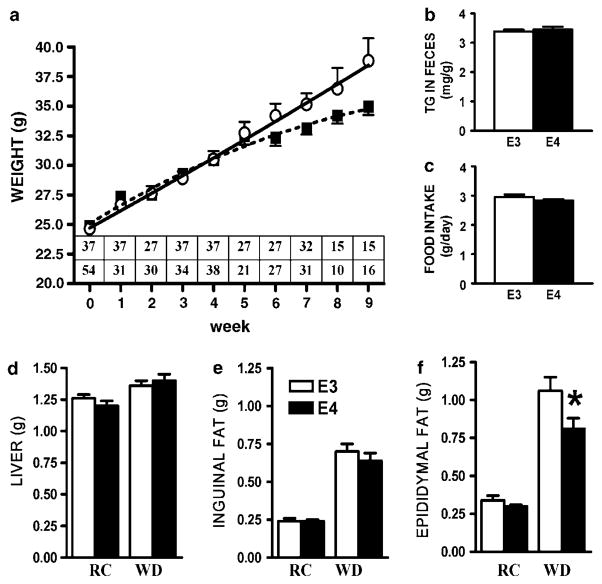
Male mice (2 month old) were placed on WD and their body weights were monitored for 8 weeks. Both APOE3 and APOE4 mice displayed similar body weight gain until 4 weeks on WD, after which time the body weight of APOE4 mice increased more slowly than that of APOE3 mice (Figure 1a). Repeated measures two-way analysis of variance matching both alleles showed that APOE3 mice maintained significantly higher body weight over the course of the experiment (P = 0.02). Similarly, the net body weight gain of APOE4 mice (10.2±0.6 g) was less than that of APOE3 mice (13.2±1.0 g; P<0.05) after 2 months on WD. There were no differences in food intake and excretion of fecal fat (Figures 1b and c).
Figure 1.
The effect of Western-type diet (WD) on body weight in APOE3 (○) or APOE4 (■) mice over the experimental period (a), fecal fat excreted (b) and food intake (c). Weights of liver (d), subcutaneous adipose tissue (e) and epididymal adipose tissue (f) of 4-month-old mice fed regular chow (RC) or WD. (a) Statistical analysis was carried out by repeated measurements analysis of variance (ANOVA) using unweighted median analysis. Inset displays the number of mice weighed at each time point, APOE3 (upper row), APOE4 (bottom row). Analysis between subjects showed difference between alleles (P = 0.02). (b–f) Data are means±s.e.; n = 12–16, *P = 0.03 for the difference between groups.
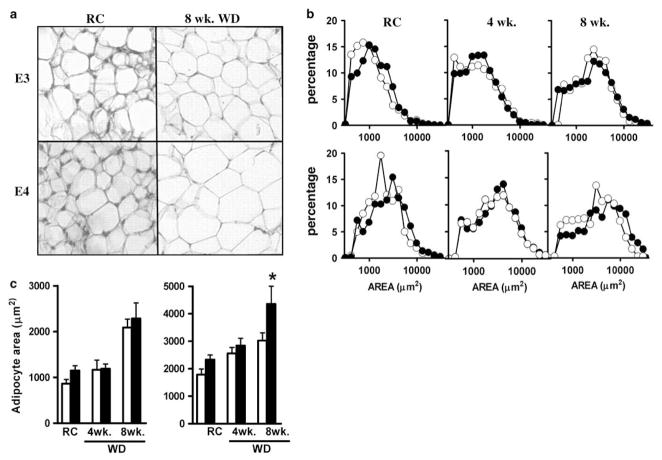
Fat and liver weights were not significantly different when animals were maintained on regular chow (Figures 1d–f). However, a large increase was seen during the second 4 weeks on WD. Subcutaneous fat and liver mass did not differ between the two genotypes, but epididymal fat was significantly less in APOE4 mice compared with APOE3 mice. Histological examination of adipose tissue pads revealed striking differences with respect to average adipocyte size and distribution. Subcutaneous fat from both APOE3 and APOE4 mice on WD displayed a similar and progressive enlargement of cell size (Figure 2b, upper panel). In contrast, epididymal adipose tissues from APOE4 animals on WD contained fewer small adipocytes and more enlarged cells than those from APOE3 mice (Figure 2b, lower panel; Figure 2a). Mean cross-sectional area of epididymal fat cells in APOE4 mice (4358±642 μm2) was significantly greater than in APOE3 mice (3028±280 μm2; P<0.05; Figure 2c). As the total fat weight is less in APOE4 mice, we conclude that adipocytes in epididymal fat pads of these mice are larger in size but fewer in number than those in APOE3 mice. This highlights the limited ability of APOE4 mice to produce new adipocytes capable of storing the surplus of triglycerides produced by this diet.
Figure 2.
Adipose tissue in APOE4 mice. (a) Morphology of epididymal adipose tissues from mice fed regular chow (RC) or Western-type diet (WD) over 8 weeks (8 week WD). Magnification ×40. (b) Size distribution of adipocytes in inguinal (upper panel) and epididymal fat (lower panel) from APOE3 (○) or APOE4 (●) mice fed RC, 4 weeks WD and 8 weeks WD (c) Cross-sectional areas of adipocytes from subcutaneous (left panel) and epididymal adipose tissues (right panel) of APOE3 (white bars) or APOE4 mice (black bars) fed different diets. Data are means±s.e.; *P = 0.05, n = 5–8 mice; 200–300 cells were randomly selected and scored in each fat depot of each mice. All mice were 12–15 weeks of age.
Selective postprandial effects of WD on plasma lipids
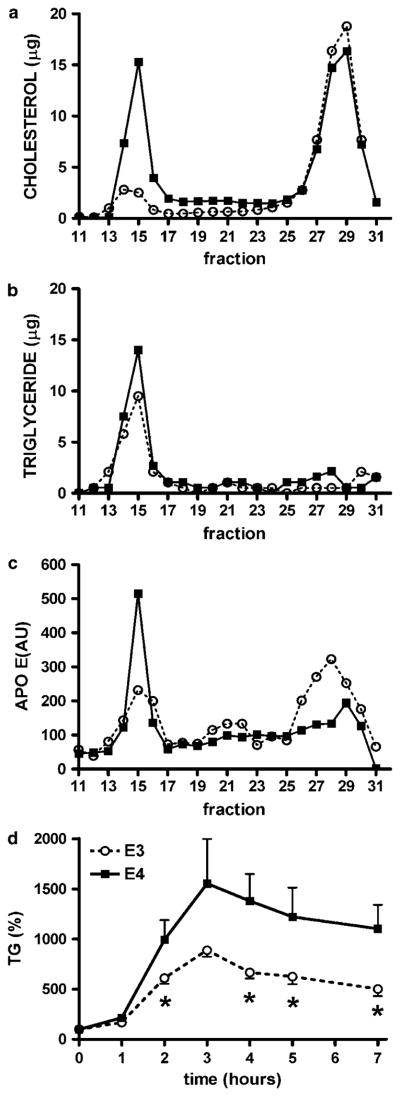
One of the major functions of adipose tissue is to buffer lipids after a high-fat meal, minimizing postprandial chylomicron and very LDL (VLDL) residence time in plasma,20 and thereby preventing ectopic lipid accumulation and subsequent lipotoxicity.21 Fast protein liquid chromatography fractionation of plasma from non-fasted APOE3 mice fed WD showed a typical mouse lipoprotein distribution in which most of the cholesterol and apoE are in the high-density lipoprotein fraction and triglycerides in the VLDL fraction (Figures 3a–c). In contrast, plasma from APOE4 mice had increased levels of large cholesterol-rich and triglyceride-rich particles with apoE bound to these larger particles. When mice were challenged with an oral fat load (100% olive oil), there was a significant rise in plasma triglyceride concentrations in both genotypes (Figure 3d). However, APOE4 mice showed a higher absorption peak and slower clearance of triglycerides from the circulation than APOE3 mice.
Figure 3.
Plasma distribution of cholesterol (a), triglycerides (b) and apolipoprotein E (c) in APOE3 and APOE4 mice. Postprandial lipoproteins of mice fed Western-type diet (WD) were fractionated by fast protein liquid chromatography (FPLC) and results are presented as micrograms of lipids in each fraction. Fractions 11–17 corresponded to TRL (very low-density lipoprotein (VLDL) and Chylomicrons), 17–25 to LDL and TRL remnants and 25–31 to high-density lipoprotein (HDL). (d) Variation of plasma triglyceride (TG) levels measured during lipid challenge in 5-month-old APOE3 (○) or APOE4 (■) mice fed 3 months WD. Data are the means±s.e.; n = 4–7, *P<0.05 for the difference between genotypes in each condition.
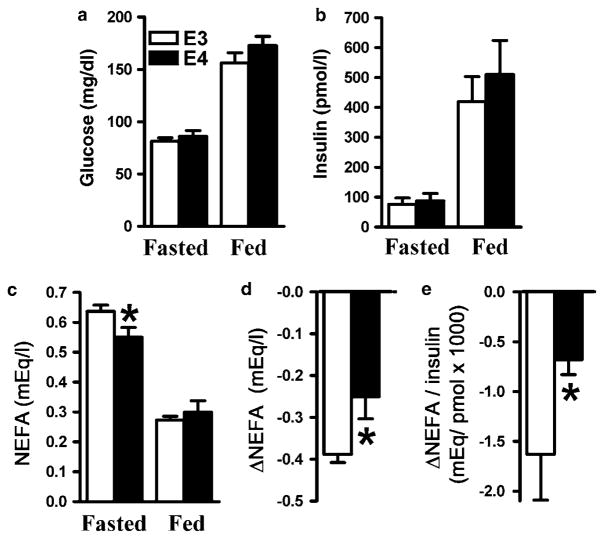
In the fasted state, lipolysis of triglycerides stored in adipocytes fuels the heart, liver and skeletal muscle with non-esterified free fatty acids (NEFAs). In the postprandial state, insulin suppresses NEFA release and promotes the switch to dietary fat and glucose as energy source.22 The levels of insulin and glucose in APOE3 and APOE4 mice fasted overnight were not significantly different and increased to a similar degree in both genotypes 1 h after a single WD meal (Figures 4a and b). In contrast, reduction of plasma NEFA after feeding was significantly less in APOE4 than in APOE3 mice (Figures 4c and d). Impaired insulin action to suppress NEFA release was reflected in the smaller reduction of the NEFA to insulin ratio Δ(NEFA/I) in APOE4 mice compared with their APOE3 peers (Figure 4e), indicating that the latter needed more insulin to suppress NEFA release.
Figure 4.
Plasma glucose (a), insulin (b), non-esterified free fatty acid (NEFA) (c), change of NEFA (d) and NEFA to insulin ratio (e). Plasma was isolated from overnight fasted APOE3 (○) or APOE4 (■) mice fed Western-type diet (WD) at 1 h after a single high-fat meal. Data are the means±s.e.; n = 8–10, *P<0.05 for the difference between genotypes in each condition.
Impaired carbohydrate metabolism in APOE4 mice during metabolic challenges
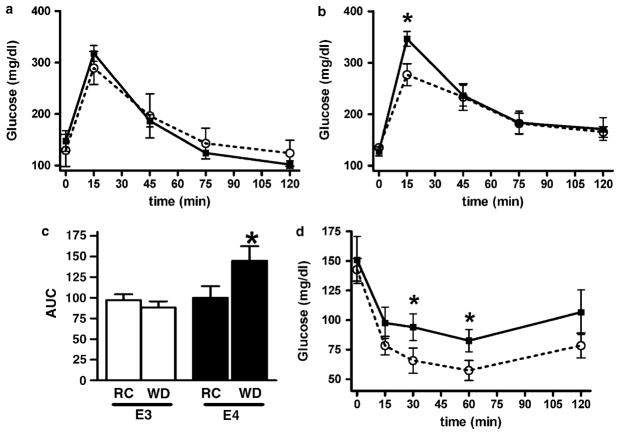
During the oral glucose tolerance test, both APOE3 and APOE4 mice on regular chow tolerated a glucose overload equally, exhibiting similar plasma glucose peaks at 15 min and similar recovery to basal levels by 120 min (Figure 5a). However, after animals were fed WD for 2 months, APOE4 mice displayed a higher 15 min peak level of post-challenge plasma glucose than APOE3 mice (Figure 5b). The changes in the areas under the curve of these oral glucose tolerance test experiments suggest that impaired glucose tolerance is a unique characteristic of APOE4 mice after 2 months of WD, as glucose tolerance was not significantly altered in APOE3 mice on WD, despite a clear increase in their body weight (Figure 5c). Whole-body insulin sensitivity measured by an insulin tolerance test also demonstrated that APOE4 mice had a reduced ability to lower glucose levels at 30 and 60 min after the intraperitoneal injection of insulin, indicating that the hypoglycemic response to insulin was blunted in these mice (Figure 5d).
Figure 5.
Oral glucose tolerance tests (OGTTs) (a, b), area under the glucose curves (AUC) (c) and intraperitoneal insulin tolerance test (ITT) (d) in 4-month-old APOE3 (○) or APOE4 (■) mice fed regular chow (a) or Western-type diet (WD) for 2 months. (b, d). Data are the means±s.e., n = 5–8. *P< 0.05 for the difference between genotypes in each condition.
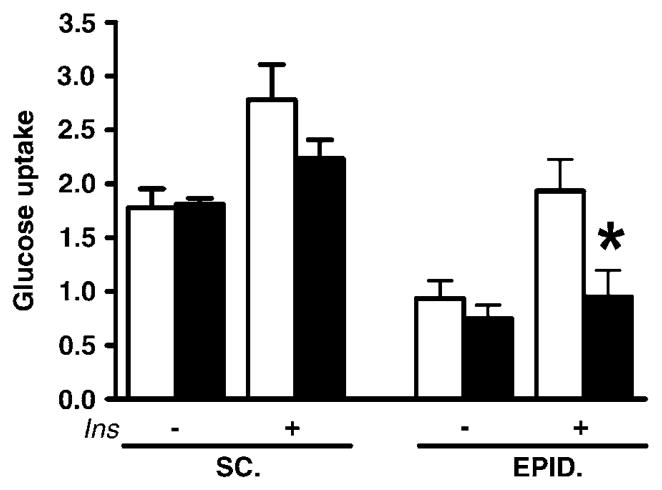
To test whether the altered epididymal fat in APOE4 mice on WD might underlie the associations between the APOE4 allele and insulin resistance, we determined basal and insulin-stimulated glucose uptake in both visceral and subcutaneous fat explants. Glucose uptake from a high-glucose medium (350mg 100 ml−1) was similar in subcutaneous as well as epididymal fat explants from APOE3 and APOE4 mice (Figure 6). When insulin was added to the incubation medium to mimic high circulating glucose and high insulin levels during the physiological postprandial state, glucose uptake was similarly stimulated in subcutaneous fat of APOE3 and APOE4 mice. In epididymal fat, however, the increase in insulin-stimulated glucose uptake was significantly blunted in APOE4 mice (126±32% of the basal uptake, not significant) when compared with APOE3 mice (206±31%; P<0.05) (Figure 6).
Figure 6.
Explants of subcutaneous inguinal (SC) and epididymal (EPID) fat were incubated for 24 h at 37 °C in triplicate, with or without insulin, and glucose uptake was determined as described in Methods. Results are milligram of glucose taken up by milligram of tissue protein. Values are means±s.e.; n = 5, *P ≤ 0.05 vs for the difference between groups.
These findings suggest that APOE4 mice on WD develop impaired glucose and insulin tolerance only after they become obese and that reduced tolerance was associated with decreased glucose clearance mediated by insulin in epididymal fat tissue.
Adipose tissue gene expression
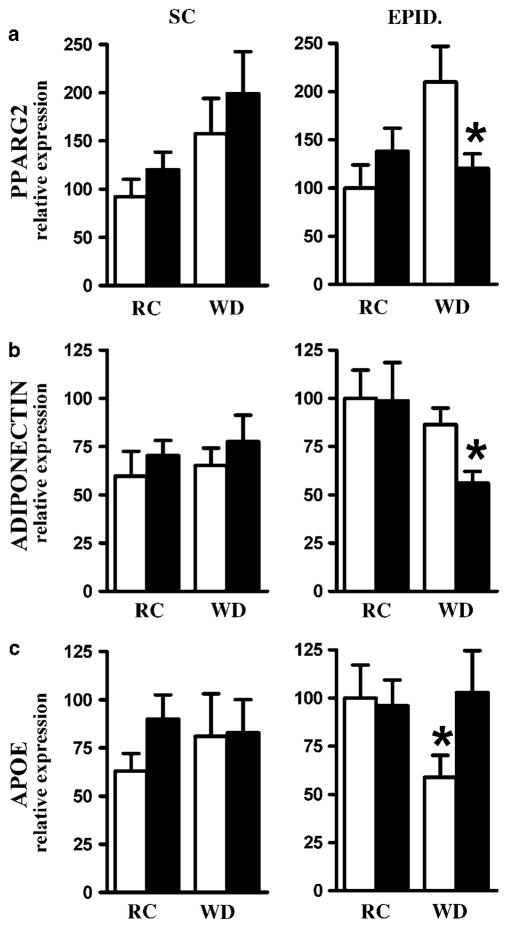
Reduced expression of peroxisome proliferator-activated receptor γ2 (PPARγ2) and adiponectin is associated with obesity, insulin resistance and type 2 diabetes.23–25 Feeding a WD for 2 months increased PPARγ2 expression twofold in the subcutaneous fat of both APOE3 and APOE4 mice compared with mice fed regular chow (Figure 7a, left). In epididymal fat tissues, a similar increase in PPARγ2 mRNA occurred in APOE3 mice but not in APOE4 mice (Figure 7a, right). In contrast, neither apoE genotype nor diet had significant effects on the expression of adiponectin gene in subcutaneous fat depots (Figure 7b, left). Although plasma adiponectin in APOE4 mice fed WD (31.6±1.5 μmol l−1) did not differ from APOE3 mice, WD significantly reduced adiponectin mRNA levels by 50% in epididymal fat of apoE4 mice compared with a small, nonsignificant reduction in APOE3 mice (Figure 7b, right). Consequently, both PPARγ2 mRNA and adiponectin mRNA levels in the epididymal fat of APOE4 mice on WD were significantly lower than those of APOE3 mice. Expression of apoE gene was not altered by diet or by apoE genotype in subcutaneous fat (Figure 7c, left). In epididymal fat, however, WD significantly lowered apoE expression in APOE3 mice but not in APOE4 mice (Figure 7c, right).
Figure 7.
Effects of APOE allele and high-fat feeding on expression of PPARγ2 (a), ADIPONECTIN (b) or APOE (c) genes in subcutaneous (SC) inguinal fat (left panels) and epididymal (EPID) visceral fat (right panels) in 4-month-old APOE3 (○) or APOE4 (■) mice. Mice were fed regular chow (RC) or Western-type diet (WD) during 2 months. Values are means±s.e.; n = 6–8. Group comparisons were by Mann–Whitney U-test; *P ≤ 0.05 for the difference between genotypes in each condition.
Increased adiposity is associated with macrophage infiltration into adipose tissue.26 The expression of macrophage-specific markers, Cd68 and F4/80, did not differ significantly between APOE3 and APOE4 fat depots, ruling out a differential infiltration of macrophage as a cause for the different epididymal fat tissue mass of APOE3 and APOE4 mice.
Overexpression of human apoE3 and apoE4 in mouse embryonic fibroblast-derived apoE−/− adipocytes
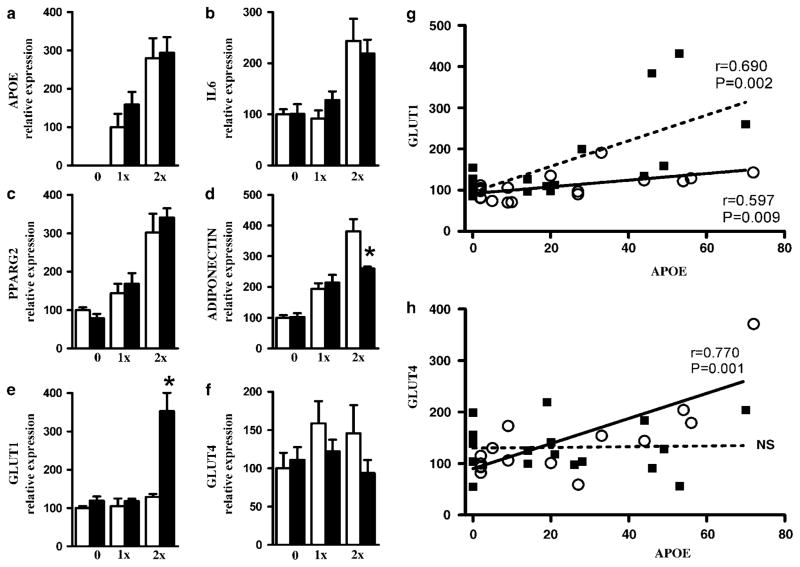
To determine whether isoform expression of apoE in adipocytes can directly influence its expression of metabolic genes in an isoform-specific manner, fibroblasts isolated from apoE-deficient embryos were transfected with adenoviral vector encoding human apoE3 or apoE4. Cells differentiated into mature adipocytes showed a dose-dependent increase of apoE mRNA levels (Figure 8a). Expression of interleukin-6 increased in cells received the high dose (2×107 p.f.u. of vectors), but the inflammatory effects were modest (twofold) and genotype independent (Figure 8b). PPARγ2 mRNA increased significantly in a dose-dependent but genotype-independent manner (Figure 8c). Overexpression of apoE3 also led to a dose-dependent increase of adiponectin mRNA, but adiponectin expression was blunted in cells transfected with 2×107 p.f.u. of ad-apoE4 (Figure 8d). Conversely, cells transfected with 2×107 p.f.u. of ad-apoE4 contained threefold more Glut1 mRNA than cells with ad-apoE3 (Figure 8e). Expression of Glut4 was higher in apoE3-expressing cells than in apoE4-expressing cells, but there were no statistical differences (Figure 8f). Correlation analyses between the expression of glucose transporters and apoE in individual mRNA samples showed that both apoE3 and apoE4 expression had a positive effect on Glut1 expression, but the effect was much stronger with apoE4 (Figure 8g). In contrast, Glut4 expression was strongly correlated with apoE3 expression but not with apoE4 expression (Figure 8h). These isoform-specific effects on glucose transporters demonstrate that whereas APOE3 increases insulin-dependent glucose transporters in adipocytes, APOE4 adipocytes depend more on insulin-independent uptake.
Figure 8.
mRNA levels of APOE (a), interleukin-6 (IL6) (b), PPARγ2 (c), ADIPONECTIN (d), GLUT1 (e) and GLUT4 (f) genes in adipocytes differentiated in vitro. Embryonic fibroblasts isolated from apoE-deficient mouse were treated with 0, 1×107 (1×) and 2×107 (2×) p.f.u. of adenoviruses encoding APOE3 (○) or APOE4 (■). Values are means±s.e.; n = 6 replicates in each condition and genotype. Group comparisons were by Mann–Whitney U-test; *P ≤ 0.05 vs for the difference between genotypes in each condition. Relationships between relative expressions of GLUT1 (g) and GLUT4 (h).
Discussion
Our results showed that male APOE4 mice fed a WD gained less body weight but were more susceptible to the development of impaired tolerance to glucose and fat overload than APOE3 mice. Insulin-stimulated glucose uptake in the epididymal fat of APOE4 mice was significantly lowered. Overexpression of apoE3 and apoE4 in adipocytes differentiated in culture revealed that these two apoE isoforms differentially affected the expression of genes for glucose transporters and adiponectin, providing a direct link between the apoE isoform expressed in adipose tissue with the accelerated metabolic impairment seen in the APOE4 mice.
Mounting evidence points toward apoE as a key player in adiposity, insulin sensitivity and glucose metabolism. For example, apoE-deficient mice have less body fat, and the adipocytes in their white adipose tissues are smaller than those in wild-type mice when both are fed a high-fat diet.27,28 apoE deficiency also improves glucose tolerance in obese Ay mice.29 The improved insulin sensitivity and protection from diet-induced obesity of apoE-deficient mice have been attributed to decreased lipid delivery to insulin-sensitive tissues, including liver, muscle and adipose tissue.30 However, adipose tissues also synthesize and secrete substantial amounts of apoE,4 and Huang et al.,28 using cultured adipocytes and adipose tissues isolated from apoE-deficient mice, showed that apoE present in triglyceride-rich lipoproteins cannot substitute for the endogenous adipocyte apoE to facilitate triglyceride accumulation. Thus, expression of apoE in adipocytes likely modulates cellular lipid metabolism by facilitating the uptake of triglyceride-rich lipoproteins to adipocytes as well as the efflux of cholesterol from these cells.
As apoE is found in three isoforms, with APOE*3 and APOE*4 alleles accounting for more than 90% of the population, it is important to elucidate the manner in which apoE3 and apoE4 isoforms differentially modulate adipose tissue expandability and functionality. The two isoforms, apoE3 and apoE4, have significantly different influence on human lipid homeostasis and lipid-related diseases;11,31,32 apoE4 has a somewhat higher affinity for the LDL receptor than apoE3,8 and cholesterol efflux from cells expressing apoE4, such as macrophages and neurons, is less efficient than cells expressing other isoforms.33,34 In addition, apoE4 has a reduced ability to protect cells from oxidative stress compared with apoE3.35,36 These characteristics of apoE4 could alter lipid metabolism of adipocytes and render them more susceptible to dysfunction than apoE3. Several studies have suggested a correlation between membrane lipid composition and insulin sensitivity through lipid-enriched microdomains that control many intracellular pathways.37 Although further studies are necessary, we speculate that lipid influx into the APOE4 adipocytes selectively alters membrane lipid composition, thereby changing cholesterol-enriched microdomains, and ultimately resulting in impaired PPARγ2 expression. This would decrease the capability of epididymal adipose tissue to promote new adipocyte differentiation and force the existing cells to enlarge to accumulate the surplus of lipids coming from a high-fat diet.
Whole-body glucose homeostasis is maintained by complex interactions from multiple organs, including the liver and muscle as well as adipose tissue. We are aware that blunted insulin-stimulated glucose uptake by epididymal fat explants from APOE4 mice could be a result of overall changes in lipid metabolism primarily in the liver. However, our study also showed that expression of apoE4 in adipocytes affects metabolism in these cells directly and differentially from the expression of apoE3. Thus, adipocytes differentiated from apoE-null mouse embryonic fibroblast38,39 and transfected with human apoE-GFP adenovirus revealed a positive correlation between the expression of the gene for Glut4, an insulin-dependent glucose transporter, with apoE3 expression, but not with apoE4 expression. Conversely, Glut1, whose expression and membrane localization are independent of insulin signaling, was significantly increased in parallel to the apoE4 expression. Decreased Glut4 expression with simultaneous increased level of Glut1 expression in adipocytes has been observed in the insulin resistance of atrophic adipose tissue due to trans-10, cis-12-conjugated linoleic acid intake.40 This suggests that cells overexpressing apoE4 are forced to increase Glut1 to maintain glucose uptake because of the reduced responsiveness of Glut4. This difference in glucose transporter usage is related to insulin insensitivity in vivo41–43 and provides a potential explanatory mechanism for the impaired glucose tolerance in the APOE4 mice.
Increased GLut1 mRNA may also be explained by the increase in the number of undifferentiated cells in culture, which rely exclusively on this transporter. Alternatively, a selective increase in pro-inflammatory cytokines could cause an induction of Glut1. However, we can rule out these alternative hypotheses as the expression of PPARγ2, a marker of adipocyte differentiation, and interleukin-6, a marker of inflammatory status, reached similar levels on transfection with either apoE3- or apoE4-containing viruses. The accumulation of subcutaneous and visceral (intra-abdominal) fat is associated with an increased risk of insulin resistance and type 2 diabetes.44 However, the association is weak in some studies,45,46 and many obese individuals can remain normoglycemic. Indeed, expansion of adipose tissue has also been associated with improved metabolic profiles.23,25,47–49 The demonstration of a smaller body weight gain but an impaired carbohydrate/lipid metabolism in APOE4 mice during metabolic challenges re-emphasizes that disturbed energy metabolism is not simply due to an increase in fat mass, but instead to alterations in fat tissue functionality.50 In concordance with the postulate that apoE is involved in surplus fat accumulation but does not play a major role in fat functionality until a certain threshold is reached,5,29 visceral adipocytes in APOE4 mice fail to increase in cell number but increase in cell volume leading to dysfunction only when they are dietary challenged with a WD.
Taken together, our results provide new insights to reconcile the seemingly paradoxical associations of the APOE*4 allele with a lower body mass index and an increased cardiovascular disease risk in general human populations. Our results also underscore an important interaction between the APOE*4 allele and diet in determining diabetogenic and atherogenic risks of human individuals.
Acknowledgments
We thank Drs Theodore Mazzone and Jesus Osada for their helpful comments. Dr Kumar Pandya, Avani Pendse and Anna Garcia for their critical reading of our paper. This study was supported by NIH grants; HL42630 and HL87946. JMA-M was partially supported by a Medicina Regenerativa fellowship from the Instituto Aragones de Ciencias de la Salud.
References
- 1.Garg A. Lipodystrophies. Am J Med. 2000;108:143–152. doi: 10.1016/s0002-9343(99)00414-3. [DOI] [PubMed] [Google Scholar]
- 2.Haslam DW, James WP. Obesity. Lancet. 2005;366:1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 3.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zechner R, Moser R, Newman T, Fried S, Breslow J. Apolipoprotein E gene expression in mouse 3T3-L1 adipocytes and human adipose tissue and its regulation by differentiation and lipid content. J Biol Chem. 1991;266:10583–10588. [PubMed] [Google Scholar]
- 5.Elosua R, Demissie S, Cupples LA, Meigs JB, Wilson PWF, Schaefer EJ, et al. Obesity modulates the association among APOE genotype, insulin, and glucose in men. Obesity Res. 2003;11:1502–1508. doi: 10.1038/oby.2003.201. [DOI] [PubMed] [Google Scholar]
- 6.Volcik KA, Barkley RA, Hutchinson RG, Mosley TH, Heiss G, Sharrett AR, et al. Apolipoprotein E polymorphisms predict low density lipoprotein cholesterol levels and carotid artery wall thickness but not incident coronary heart disease in 12 491 ARIC study participants. Am J Epidemiol. 2006;164:342–348. doi: 10.1093/aje/kwj202. [DOI] [PubMed] [Google Scholar]
- 7.Feitosa MF, Rice T, North KE, Kraja A, Rankinen T, Leon AS, et al. Pleiotropic QTL on chromosome 19q13 for triglycerides and adiposity: the HERITAGE Family Study. Atherosclerosis. 2006;185:426–432. doi: 10.1016/j.atherosclerosis.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 8.Knouff C, Hinsdale ME, Mezdour H, Altenburg MK, Watanabe M, Quarfordt SH, et al. Apo E structure determines VLDL clearance and atherosclerosis risk in mice. J Clin Invest. 1999;103:1579–1586. doi: 10.1172/JCI6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davignon J, Gregg R, Sing C. Apolipoprotein E polymorphism and atherosclerosis. Arterioscler Thromb Vasc Biol. 1988;8:1–21. doi: 10.1161/01.atv.8.1.1. [DOI] [PubMed] [Google Scholar]
- 10.Wilson PWF, Schaefer EJ, Larson MG, Ordovas JM. Apolipoprotein E alleles and risk of coronary disease: a meta-analysis. Arterioscler Thromb Vasc Biol. 1996;16:1250–1255. doi: 10.1161/01.atv.16.10.1250. [DOI] [PubMed] [Google Scholar]
- 11.Bennet AM, Di Angelantonio E, Ye Z, Wensley F, Dahlin A, Ahlbom A, et al. Association of apolipoprotein E genotypes with lipid levels and coronary risk. JAMA. 2007;298:1300–1311. doi: 10.1001/jama.298.11.1300. [DOI] [PubMed] [Google Scholar]
- 12.Shriver M, Boerwinkle E, Hewett-Emmett D, Hanis C. Frequency and effects of apolipoprotein E polymorphism in Mexican-American NIDDM subjects. Diabetes. 1991;40:334–337. doi: 10.2337/diab.40.3.334. [DOI] [PubMed] [Google Scholar]
- 13.Meigs J, Ordovas J, Cupples L, Singer D, Nathan D, Schaefer E, et al. Apolipoprotein E isoform polymorphisms are not associated with insulin resistance: the Framingham Offspring Study. Diabetes Care. 2000;23:669–674. doi: 10.2337/diacare.23.5.669. [DOI] [PubMed] [Google Scholar]
- 14.Scuteri A, Najjar SS, Muller D, Andres R, Morrell CH, Zonderman AB, et al. apoE4 allele and the natural history of cardiovascular risk factors. Am J Physiol Endocrinol Metab. 2005;289:E322–E327. doi: 10.1152/ajpendo.00408.2004. [DOI] [PubMed] [Google Scholar]
- 15.Sullivan PM, Mezdour H, Aratani Y, Knouff C, Najib J, Reddick RL, et al. Targeted replacement of the mouse apolipoprotein E gene with the common human APOE3 allele enhances diet-induced hypercholesterolemia and atherosclerosis. J Biol Chem. 1997;272:17972–17980. doi: 10.1074/jbc.272.29.17972. [DOI] [PubMed] [Google Scholar]
- 16.Arbones-Mainar JM, Navarro MA, Acin S, Guzman MA, Arnal C, Surra JC, et al. Trans-10, cis-12- and cis-9, trans-11-conjugated linoleic acid isomers selectively modify HDL-apolipoprotein composition in apolipoprotein E knockout mice. J Nutr. 2006;136:353–359. doi: 10.1093/jn/136.2.353. [DOI] [PubMed] [Google Scholar]
- 17.Dekroon RM, Armati PJ. Endocytosis of apoE-EGFP by primary human brain cultures. Cell Biol Int. 2002;26:761–770. doi: 10.1016/s1065-6995(02)90930-3. [DOI] [PubMed] [Google Scholar]
- 18.Altenburg M, Arbones-Mainar J, Johnson L, Wilder J, Maeda N. Human LDL receptor enhances sequestration of ApoE4 and VLDL remnants on the surface of hepatocytes but not their internalization in mice. Arterioscler Thromb Vasc Biol. 2008;28:1104–1110. doi: 10.1161/ATVBAHA.108.164863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conner DA. Mouse embryo fibroblast (MEF) feeder cell preparation. Curr Protoc Mol Biol. 2001;Chapter 23(Unit 232) doi: 10.1002/0471142727.mb2302s51. [DOI] [PubMed] [Google Scholar]
- 20.Bickerton AST, Roberts R, Fielding BA, Hodson L, Blaak EE, Wagenmakers AJM, et al. Preferential uptake of dietary fatty acids in adipose tissue and muscle in the postprandial period. Diabetes. 2007;56:168–176. doi: 10.2337/db06-0822. [DOI] [PubMed] [Google Scholar]
- 21.Unger RH. Minireview: weapons of lean body mass destruction: the role of ectopic lipids in the metabolic syndrome. Endocrinology. 2003;144:5159–5165. doi: 10.1210/en.2003-0870. [DOI] [PubMed] [Google Scholar]
- 22.Sniderman AD. Postprandial hypertriglyceridemia(s): time to enlarge our pathophysiologic perspective. Eur J Clin Invest. 2000;30:935–937. doi: 10.1046/j.1365-2362.2000.00733.x. [DOI] [PubMed] [Google Scholar]
- 23.Medina-Gomez G, Gray SL, Yetukuri L, Shimomura K, Virtue S, Campbell M, et al. PPAR gamma 2 prevents lipotoxicity by controlling adipose tissue expandability and peripheral lipid metabolism. PLoS Genet. 2007;3:e64. doi: 10.1371/journal.pgen.0030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 25.Kim J-Y, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiba T, Nakazawa T, Yui K, Kaneko E, Shimokado K. VLDL induces adipocyte differentiation in ApoE-dependent manner. Arterioscler Thromb Vasc Biol. 2003;23:1423–1429. doi: 10.1161/01.ATV.0000085040.58340.36. [DOI] [PubMed] [Google Scholar]
- 28.Huang ZH, Reardon CA, Mazzone T. Endogenous ApoE expression modulates adipocyte triglyceride content and turnover. Diabetes. 2006;55:3394–3402. doi: 10.2337/db06-0354. [DOI] [PubMed] [Google Scholar]
- 29.Gao J, Katagiri H, Ishigaki Y, Yamada T, Ogihara T, Imai J, et al. Involvement of apolipoprotein E in excess fat accumulation and insulin resistance. Diabetes. 2007;56:24–33. doi: 10.2337/db06-0144. [DOI] [PubMed] [Google Scholar]
- 30.Hofmann SM, Perez-Tilve D, Greer TM, Coburn BA, Grant E, Basford JE, et al. Defective lipid delivery modulates glucose tolerance and metabolic response to diet in apolipoprotein E deficient mice. Diabetes. 2008;57:5–12. doi: 10.2337/db07-0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weisgraber K, Mahley R. Human apolipoprotein E: the Alzheimer’s disease connection. FASEB J. 1996;10:1485–1494. doi: 10.1096/fasebj.10.13.8940294. [DOI] [PubMed] [Google Scholar]
- 32.Sima A, Iordan A, Stancu C. Apolipoprotein E polymorphism—a risk factor for metabolic syndrome. Clin Chem Lab Med. 2007;45:1149–1153. doi: 10.1515/CCLM.2007.258. [DOI] [PubMed] [Google Scholar]
- 33.Michikawa M, Fan Q-W, Isobe I, Yanagisawa K. Apolipoprotein E exhibits isoform-specific promotion of lipid efflux from astrocytes and neurons in culture. J Neurochem. 2000;74:1008–1016. doi: 10.1046/j.1471-4159.2000.0741008.x. [DOI] [PubMed] [Google Scholar]
- 34.Lucic D, Huang ZH, Gu DS, Altenburg MK, Maeda N, Mazzone T. Regulation of macrophage apoE secretion and sterol efflux by the LDL receptor. J Lipid Res. 2007;48:366–372. doi: 10.1194/jlr.M600259-JLR200. [DOI] [PubMed] [Google Scholar]
- 35.Miyata M, Smith JD. Apolipoprotein E allele-specific antioxidant activity and effects on cytotoxicity by oxidative insults and beta-amyloid peptides. Nat Genet. 1996;14:55–61. doi: 10.1038/ng0996-55. [DOI] [PubMed] [Google Scholar]
- 36.Altenburg M, Johnson L, Wilder J, Maeda N. Apolipoprotein E4 in macrophages enhances atherogenesis in a low density lipoprotein receptor-dependent manner. J Biol Chem. 2007;282:7817–7824. doi: 10.1074/jbc.M610712200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen AW, Hnasko R, Schubert W, Lisanti MP. Role of caveolae and caveolins in health and disease. Physiol Rev. 2004;84:1341–1379. doi: 10.1152/physrev.00046.2003. [DOI] [PubMed] [Google Scholar]
- 38.Cohen AW, Razani B, Schubert W, Williams TM, Wang XB, Iyengar P, et al. Role of caveolin-1 in the modulation of lipolysis and lipid droplet formation. Diabetes. 2004;53:1261–1270. doi: 10.2337/diabetes.53.5.1261. [DOI] [PubMed] [Google Scholar]
- 39.Kim S, Huang L-W, Snow KJ, Ablamunits V, Hasham MG, Young TH, et al. A mouse model of conditional lipodystrophy. Proc Natl Acad Sci USA. 2007;104:16627–16632. doi: 10.1073/pnas.0707797104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.LaRosa PC, Miner J, Xia Y, Zhou Y, Kachman S, Fromm ME. Trans-10, cis-12 conjugated linoleic acid causes inflammation and delipidation of white adipose tissue in mice: a microarray and histological analysis. Physiol Genomics. 2006;27:282–294. doi: 10.1152/physiolgenomics.00076.2006. [DOI] [PubMed] [Google Scholar]
- 41.Nadler ST, Stoehr JP, Schueler KL, Tanimoto G, Yandell BS, Attie AD. The expression of adipogenic genes is decreased in obesity and diabetes mellitus. Proc Natl Acad Sci USA. 2000;97:11371–11376. doi: 10.1073/pnas.97.21.11371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garvey WT, Maianu L, Huecksteadt TP, Birnbaum MJ, Molina JM, Ciaraldi TP. Pretranslational suppression of a glucose transporter protein causes insulin resistance in adipocytes from patients with non-insulin-dependent diabetes mellitus and obesity. J Clin Invest. 1991;87:1072–1081. doi: 10.1172/JCI115068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bloch-Damti A, Bashan N. Proposed mechanisms for the induction of insulin resistance by oxidative stress. Antioxid Redox Signal. 2005;7:1553–1567. doi: 10.1089/ars.2005.7.1553. [DOI] [PubMed] [Google Scholar]
- 44.Carr DB, Utzschneider KM, Hull RL, Kodama K, Retzlaff BM, Brunzell JD, et al. Intra-abdominal fat is a major determinant of the national cholesterol education program adult treatment panel III criteria for the metabolic syndrome. Diabetes. 2004;53:2087–2094. doi: 10.2337/diabetes.53.8.2087. [DOI] [PubMed] [Google Scholar]
- 45.Abate N, Garg A, Peshock RM, Stray-Gundersen J, Grundy SM. Relationships of generalized and regional adiposity to insulin sensitivity in men. J Clin Invest. 1995;96:88–98. doi: 10.1172/JCI118083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vega GL, Adams-Huet B, Peshock R, Willett D, Shah B, Grundy SM. Influence of body fat content and distribution on variation in metabolic risk. J Clin Endocrinol Metab. 2006;91:4459–4466. doi: 10.1210/jc.2006-0814. [DOI] [PubMed] [Google Scholar]
- 47.Tansey JT, Sztalryd C, Gruia-Gray J, Roush DL, Zee JV, Gavrilova O, et al. Perilipin ablation results in a lean mouse with aberrant adipocyte lipolysis, enhanced leptin production, and resistance to diet-induced obesity. Proc Natl Acad Sci USA. 2001;98:6494–6499. doi: 10.1073/pnas.101042998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fonseca V. Effect of thiazolidinediones on body weight in patients with diabetes mellitus. Am J Med. 2003;115 (Suppl 8A):42S–48S. doi: 10.1016/j.amjmed.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 49.Konrad D, Rudich A, Schoenle E. Improved glucose tolerance in mice receiving intraperitoneal transplantation of normal fat tissue. Diabetologia. 2007;50:833–839. doi: 10.1007/s00125-007-0596-1. [DOI] [PubMed] [Google Scholar]
- 50.Laclaustra M, Corella D, Ordovas JM. Metabolic syndrome pathophysiology: the role of adipose tissue. Nutr Metab Cardiovasc Dis. 2007;17:125–139. doi: 10.1016/j.numecd.2006.10.005. [DOI] [PubMed] [Google Scholar]