Abstract
A vaccine comprised of recombinant cytomegalovirus (CMV) envelope glycoprotein B (gB) with MF59 adjuvant developed in the 1990s recently was recently found to have efficacy for prevention of CMV infection in a phase 2 clinical trial in young mothers. This review briefly considers the rationale for gB as a vaccine antigen, the history of this CMV gB vaccine and the data supporting vaccine efficacy.
Keywords: cytomegalovirus vaccine, glycoprotein B, congenital CMV infection, MF59
1. Introduction
The importance of congenital cytomegalovirus (CMV) infection as a cause of mental, motor, auditory and visual disabilities has been known for decades.1–3 Development of vaccines for prevention of congenital cytomegalovirus disease is recognized as an important public health priority largely because of the conclusions of a committee of the Institute of Medicine of the National Academy of Sciences which reviewed priorities for vaccine development.4 The committee concluded that in terms of health care dollars saved and improvement in quality adjusted life years, a vaccine for prevention of congenital CMV infection should be among the highest priorities for the United States.
Results of clinical trials aimed at initial evaluation of immunogenicity and safety have been reported for a variety of CMV vaccine formats.5–12 Although more than 20 years ago Towne CMV was shown to have efficacy for prevention of CMV disease in seronegative renal transplant patients who received a graft from a CMV seropositive donor,13 a more recent clinical trial reported no efficacy for prevention of infection in parents of CMV shedding children.14 This review will focus on the development on a subunit vaccine comprised of recombinant CMV envelope glycoprotein B (gB) with a novel adjuvant, MF59, which was recently shown to have efficacy for prevention of maternal CMV infection.15
2. Rationale for gB as vaccine antigen
Glycoprotein B is present on all human cytomegaloviruses and is necessary for viral infectivity. It mediates attachment and entry to infected cells, cell to cell transmission of virus and syncytium formation.16–19 A significant proportion of neutralizing antibody to CMV in human serum is specific for epitopes on gB and essentially all CMV infected humans have antibody to gB.20–22 Key epitopes for neutralizing antibody on gB are highly conserved among human CMV strains. Studies with murine CMV showed that both passive and active immunization with agents specific for gB provided protection from challenge.23 In the guinea pig model of congenital CMV infection, immunization with gB either as a plasmid DNA vaccine or a recombinant protein decreased rates of fetal loss and fetal infection with CMV.24
3. Development of CMV gB vaccine
Towne CMV was the source of the UL55 (gB) gene used for the recombinant protein vaccine developed at Chiron.25 The native gene was modified to facilitate in vitro production of antigen in Chinese hamster ovary cell culture. In a series of phase I and phase 2 studies performed during the 1990’s, CMV gB vaccine safety, immunogenicity, antigen dose and immunization schedule were studied.7, 8, 26, 27 In addition MF59 a novel, proprietary adjuvant was compared with alum. MF59 is a squalene in water emulsion which has been studied in a number of investigational vaccines; MF59 is licensed for use with an inactivated influenza vaccine in Europe.28 Immunogenicity of CMV gB vaccine was superior with MF59 compared with alum.7, 8 Peak levels of antibody to gB were around 5-fold higher in recipients of 3 injections of CMV gB/MF59 than those in persons with past CMV infection. Peak levels of neutralizing antibody were similar to those measured in sera from persons with past infection. A small study in preschool aged children showed that they achieved peak levels of antibody to gB and neutralizing antibody that were much higher than those in adult vaccinees.26 In the dozen or so clinical trials sponsored by Chiron, over 700 subjects (mostly healthy seronegative adults plus a limited number of seronegative children and seropositive adults) received CMV gB vaccine. Safety and reactogenicity data were reassuring. There were no serious adverse events associated with vaccine. Local and systemic reactions were mostly mild and of short duration (<48 hours) and appeared to be similar in frequency, severity and duration to those associated with many licensed vaccines. Based on results of these early studies, an antigen dose of 25 micrograms of gB and injection schedule of 0, 1 and 6 months were selected for a phase 2 efficacy trial. Most of the studies of CMV gB/MF59 performed in the 1990s did not include populations considered at increased risk for CMV infection and none of them were designed to test vaccine efficacy. The rights to CMVgB vaccine were obtained by Aventis Pasteur (now Sanofi Pasteur), in 2000.
4. Choosing a study population for a CMV vaccine efficacy trial
A study of obstetric patients in Birmingham, Alabama, strengthened the rationale for vaccine prevention of congenital CMV infection and provided the data required to estimate the sample size needed for a phase 2 efficacy trial. Results showed that seronegative mothers acquired CMV between deliveries at a rate of ~6% per year and that past CMV infection reduced the congenital infection rate by around 67% in subsequent pregnancies compared with the rate in newborns of women who were seronegative when first tested.29, 30 Seronegative women from this obstetric population were an attractive group for an efficacy trial because of their relatively high rate of incident CMV infection and because if enrolled near the time of birth of a newborn, many of them would have another newborn within three years. In addition, approaching women on postpartum wards offered the opportunity to meet them face to face in order to obtain consent and to obtain serum for screening without the discomfort of venipuncture. Blood samples from all obstetric patients are submitted to hospital blood banks and remnant maternal or cord serum can be obtained from the hospital laboratory with consent of the patient and appropriate local approvals.
5. A phase 2 clinical trial to test efficacy of CMV gB vaccine
5.1 Study Population and Design
A phase 2, randomized, double blind, placebo controlled clinical trial of CMV gB/MF59 vaccine in young mothers was initiated in 1999. The primary endpoint was time to CMV infection. Secondary goals included determining the congenital CMV infection rate in babies born to trial participants, assessment of vaccine safety, immunogenicity and comparison of viral shedding and viremia between vaccine and placebo groups. A sample size of 400 (randomized 1;1, vaccine:placebo) was expected to provide the ability to test a hypothesis of 50% efficacy with the probability of a type I error of ≤ 0.05 and power ≥ 0.80 with an estimated CMV infection rate of 20% over 3.5 years in placebo recipients. None of the investigators involved in the planning or execution of this clinical trial had confidence that immunization with CMV gB/MF59 vaccine would be able to prevent maternal infection. In large measure, the rationale for the efficacy trial was based on the need to learn more about the biology of primary CMV infection in healthy young women and to test a trial model based on ability to accurately predict both maternal and congenital CMV infection rates.
Written consent to screen women for CMV antibody was obtained from subjects on postpartum hospital wards and serum was obtained from the hospital blood bank for that purpose. Subjects were screened for IgG antibody to CMV and seronegative women in good health who lived in the Birmingham metropolitan area were invited to enroll in the clinical trial within one year of the birth of a newborn if they met inclusion and exclusion criteria. Women who were breastfeeding were excluded because of the lack of data on vaccine safety for lactating women and breastfed infants. Enrollment was completed in April 2006. Additional subjects (exceeding 400) were enrolled to replace those who were randomized but not did not receive study vaccine (due to discovery of an exclusion), or were found to be seropositive on the day of enrollment, or did not receive all three study vaccines.
5.2 Endpoint detection
It is very unlikely that any clinical manifestations will herald the onset of CMV infection in healthy adults or children, and therefore it is necessary to use repeated laboratory testing to find infections in clinical trial participants. If antibody assays are used for this purpose, one must find a way to distinguish the antibody response to infection from that induced by vaccine. In addition, testing subjects for antibody to CMV could potentially unblind laboratory personnel. Although testing subjects for the presence of CMV in blood, urine or other body fluids by real time PCR or other means avoids the problems inherent in serologic testing for infection, knowledge of the prevalence, magnitude and duration of viremia or viral shedding in normal healthy subjects is limited. Virologic events that appear and then clear between follow-up dates could be missed. A simple and rapid method of screening subjects for antibody to CMV proteins other than the vaccine antigen, gB, was developed and validated using sera from persons with naturally acquired CMV infection and from participants in past clinical trials of CMV gB/MF59 vaccine 31, 32. Sera from vaccine clinical trial participants were preabsorbed with gB (vaccine antigen provided by Sanofi Pasteur for laboratory use) and then tested for CMV IgG antibody with a standard, commercial assay. Preabsorption with gB removes antibody to CMV from sera of subjects who have only vaccine induced immunity while subjects who have naturally acquired infection remain CMV antibody positive. This method does not unblind technicians performing the assay; vaccine recipients are negative unless CMV infection has occurred. A positive gB absorbed CMV IgG result triggered confirmation of infection by detection of virus in body fluids (urine, saliva, vaginal swab or whole blood) by culture or real time PCR or if no virus was detected, by a commercial immunoblot assay (Mikrogen Recomblot CMV, Neuried, Germany).
5.3 Results of the phase 2 efficacy trial
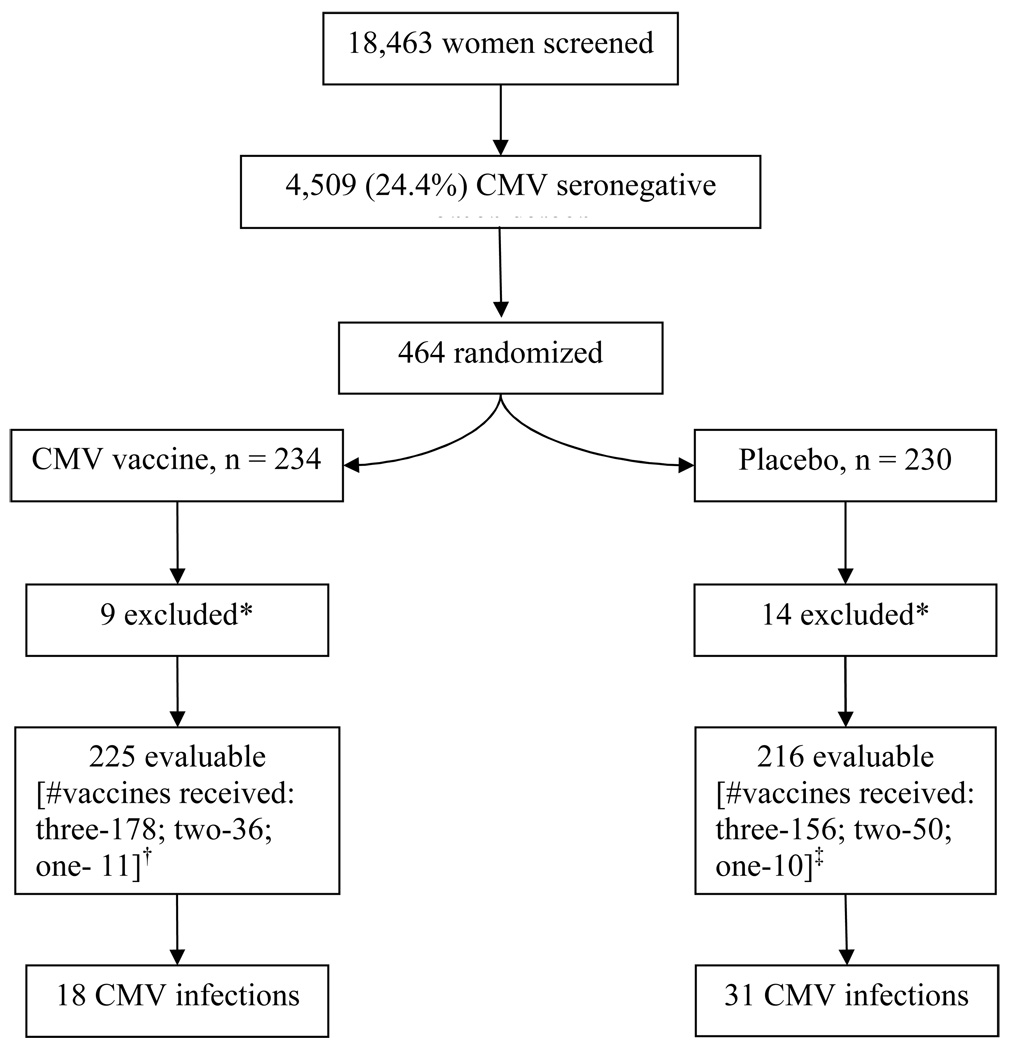
The clinical trial results were recently published, providing a more detailed exposition of methods as well as safety and efficacy results than can be presented here15. Figure 1 shows the numbers of subjects screened, enrolled, and excluded from data analysis and the accrual of endpoints for both CMV gB vaccine and placebo groups at the time data was analyzed. After the second scheduled review of efficacy when 75% of expected endpoints had occurred, the DSMB reported that vaccine was superior to placebo and that a preset boundary for efficacy had been crossed. The DSMB recommended continuing the clinical trial per protocol until all subjects had completed at least 6 months follow-up after the third injection of study vaccine.
Figure 1.
Study population and endpoint accrual.
*After randomization 3 subjects assigned to CMV vaccine and 4 assigned to placebo were found to meet an exclusion criterion and were not immunized. Serum antibody results from the day of randomization (not available on that day) showed that 6 CMV vaccine recipients and 10 placebo recipients were not seronegative and therefore did not meet inclusion criteria.
†CMV gB group, reasons for less than 3 vaccines: lost to follow-up (4), adverse event (5), noncompliance (7), withdrew from study (5), CMV infection (2), pregnancy (6), vaccine not available (18)
‡Placebo group, reasons for less than 3 vaccines: lost to follow-up (16), adverse event (2), noncompliance (4), withdrew from study (4), CMV infection (4), pregnancy (13), vaccine not available (17)
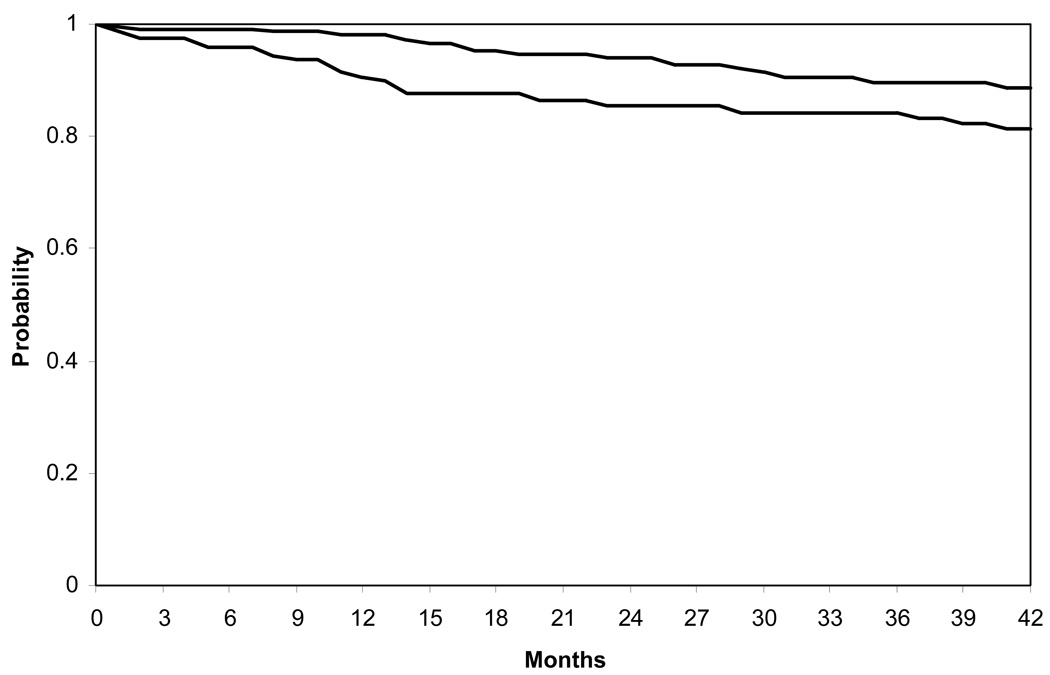
There were no statistically significant differences between CMV gB vaccine and placebo recipients in key demographic and personal variables.15 CMV infections occurred in 31/216 (14%) placebo recipients and 18/225 (8%) CMV gB vaccine recipients. Forty-seven infections were confirmed by detection of virus in body fluids and two infections in CMV gB vaccine recipients were confirmed by western blot. Infection rates were compared using Kaplan-Meir curves expressing the probability of remaining uninfected with passage of time, Figure 2. Results show that CMV gB vaccine recipients were more likely to remain uninfected over the 42 months interval of follow-up, P = 0.02. The rate of CMV infection per 100 person-years was 6.6 in placebo recipients compared with 3.3 in vaccine recipients, an overall efficacy of 50% (95% confidence interval, 7; 73). Proportional hazards multivariate analysis showed that vaccine regimen was the only exposure that was significantly associated with infection; the hazard ratio for CMV gB vaccine was 0.51 (95% confidence interval, 0.29; 0.92), P = 0.02.
Figure 2.
Kaplan-Meier curves comparing probability of remaining CMV uninfected; subjects at risk at each interval are shown in the accompanying table. Up to 42 months from enrollment, CMV gB vaccine recipients (N = 225) were more likely to remain uninfected than placebo recipients (N = 216), P = 0.02.
Congenital CMV infection occurred in 1/81(1%) and 3/97 (3%) babies born respectively to CMV gB vaccine and placebo recipients. The infected baby born to a vaccine recipient was normal at birth and has remained free of sequelae. One of the infected babies born to placebo recipients was severely affected with clinical and laboratory signs of congenital infection at birth and significant developmental disability; the other two were normal at birth and have remained free of sequelae. All four congenital infections were the result of maternal infections that occurred during pregnancy. These numbers are too small to allow any conclusions regarding the vaccine’s ability to prevent congenital infection beyond its efficacy for prevention of maternal infection.
Pain, warmth, induration and erythema at the injection site occurred more often in CMV gB vaccine recipients than in placebo recipients. Fever, rash, headache, nausea and fatigue occurred with similar frequency among vaccine and placebo recipients. Arthralgias (6%), myalgias (16%) and chills (8%) occurred more often among CMV gB recipients and differences compared with rates in placebo recipients were statistically significant after one or more of the vaccine injections15. Local and systemic reactions to CMVgB/MF59 immunization were generally mild and of short (< 1 day duration). There was no difference between vaccine and placebo groups in the overall rate of adverse events, serious adverse events or serious adverse events involving babies born during the clinical trial. Adverse events considered possibly related to test article occurred in 16/231 (7%) CMV gB vaccine recipients and in 4/226 (2%) placebo recipients, P = 0.01; most of these events were considered mild.
5.4 Anticipated results
Although the trial was unblinded and subjects were informed of their study vaccine assignments, all active subjects will be followed until they have completed the planned 3.5 years of follow-up. Completion of all study visits will increase the number of pregnancies and newborns studied, provide a large number of samples from which to study persistence of antibody to gB and neutralizing antibody over an extended period of time and will provide valuable data on CMV viremia and virus shedding in infected subjects.
6. The future of CMV gB vaccine
It is hoped that continued evaluation of CMV gB vaccine will lead to a phase 3 clinical trial that will provide a robust test of the vaccine’s ability to prevent CMV infection in women of childbearing potential. Although some might question the need for a vaccine with less than optimal efficacy, when there is no alternative means of preventing maternal and congenital CMV infection, 50% efficacy could be very attractive, especially to women at high risk of CMV infection. It is worth noting that our trial was performed in a population that appears to have relatively intense exposure to CMV and a high rate of congenital CMV infection due to reinfection in women with immunity to CMV33. One could speculate that in other populations with less intense exposure to CMV, efficacy might be higher. Could the vaccine’s efficacy for prevention of congenital CMV infection exceed the efficacy for prevention of maternal infection? A phase 3 clinical trial with congenital infection as the primary endpoint would answer this question.
Acknowledgement
Funding: This work was supported by grants from the National Institutes of Health (NIAID/DMID PO1 AI043681 and U01 AI063565 and National Center for Research Resources, M01 RR00032) and from Sanofi Pasteur, Marcy L’Etoile, France. Competing interests: Dr. Pass reports serving as a consultant to Vical, AlphaVax, Merck and MedImmune within the past two years and having partial interest in a relevant patent. Dr. Pass is a recipient of research grants from Sanofi Pasteur.
Abbreviations
- CMV
cytomegalovirus
- gB
glycoprotein B
- DNA
deoxyribonucleic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest Statements
Dr. Pass reports serving as a consultant to Vical, AlphaVax, Merck and MedImmune within the past two years and having partial interest in a relevant patent. Dr. Pass is a recipient of research grants from Sanofi Pasteur.
References
- 1.McCracken GJ, Shinefield HR, Cobb K, Rausen AR, Dische MR, Eichenwald HF. Congenital cytomegalic inclusion disease. A longitudinal study of 20 patients. Am J Dis Child. 1969;117:522–539. doi: 10.1001/archpedi.1969.02100030524005. [DOI] [PubMed] [Google Scholar]
- 2.Reynolds DW, Stagno S, Stubbs KG, et al. Inapparent congenital cytomegalovirus infection with elevated cord IgM levels: causal relationship with auditory and mental deficiency. N Engl J Med. 1974;209:291–296. doi: 10.1056/NEJM197402072900601. [DOI] [PubMed] [Google Scholar]
- 3.Hanshaw JB, Scheiner AP, Moxley AW, Gaev L, Abel V, Scheiner B. School failure and deafness after "silent" congenital cytomegalovirus infection. N Engl J Med. 1976;295:468–470. doi: 10.1056/NEJM197608262950902. [DOI] [PubMed] [Google Scholar]
- 4.Stratton K, Durch J, Lawrence R. Vaccines for the 21st Century: A Tool for Decisionmaking. Washington, DC: National Academy Press; 2001. [PubMed] [Google Scholar]
- 5.Elek SD, Stern H. Development of a vaccine against mental retardation caused by cytomegalovirus infection in utero. Lancet. 1974;1:1–5. doi: 10.1016/s0140-6736(74)92997-3. [DOI] [PubMed] [Google Scholar]
- 6.Plotkin SA, Farquhar J, Hornberger E. Clinical trials of immunization with the Towne 125 strain of human cytomegalovirus. J Infect Dis. 1976;134:470–475. doi: 10.1093/infdis/134.5.470. [DOI] [PubMed] [Google Scholar]
- 7.Pass RF, Duliege A-M, Boppana S, et al. A subunit cytomegalovirus vaccine based on recombinant envelope glycoprotein B and a new adjuvant. J Infect Dis. 1999;180:970–975. doi: 10.1086/315022. [DOI] [PubMed] [Google Scholar]
- 8.Frey SE, Harrison H, Pass RF, et al. Effects of antigen dose and immunization regimens on antiboby responses to a cytomegalovirus glycoprotein B subunit vaccine. J Infect Dis. 1999;180:1700–1703. doi: 10.1086/315060. [DOI] [PubMed] [Google Scholar]
- 9.Berencsi K, Gyulai Z, Gonczol E, et al. A canarypox vector-expressing cytomegalovirus (CMV) phosphoprotein 65 induces long-lasting T cell responses in human CMV-seronegative subjects. J Infect Dis. 2001;183:1171–1179. doi: 10.1086/319680. [DOI] [PubMed] [Google Scholar]
- 10.Bernstein DI, Schleiss MR, Berencsi K, et al. Effect of previous or simultaneous immunization with canarypox expressing cytomegalovirus (CMV) glycoprotein B (gB) on response to subunit gB vaccine plus MF59 in healthy CMV-seronegative adults. J Infect Dis. 2002;185:686–690. doi: 10.1086/339003. [DOI] [PubMed] [Google Scholar]
- 11.Heineman TC, Schleiss M, Bernstein DI, et al. A phase 1 study of 4 live, recombinant human cytomegalovirus Towne/Toledo chimeric vaccines. J Infect Dis. 2006;193:1350–1356. doi: 10.1086/503365. [DOI] [PubMed] [Google Scholar]
- 12.Wloch MK, Smith LR, Souphaphone B, et al. Safety and immunogenicity of a bivalent cytomegalovirus DNA vaccine in healthy adult subjects. J Infect Dis. 2008;197:1634–1642. doi: 10.1086/588385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plotkin SA, Starr SE, Friedman HM, et al. Effect of Towne live virus vaccine on cytomegalovirus disease after renal transplant. A controlled trial. Ann Intern Med. 1991;114:525–531. doi: 10.7326/0003-4819-114-7-525. [DOI] [PubMed] [Google Scholar]
- 14.Adler SP, Starr SE, Plotkin SA, et al. Immunity induced by primary human cytomegalovirus infection protects against secondary infection among women of childbearing age. J Infect Dis. 1995;171:26–32. doi: 10.1093/infdis/171.1.26. [DOI] [PubMed] [Google Scholar]
- 15.Pass RF, Zhang C, Evans A, et al. Vaccine prevention of maternal cytomegalovirus infection. N Engl J Med. 2009;360:1191–1199. doi: 10.1056/NEJMoa0804749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Compton T, Nowlin DM, Cooper NR. Initiation of human cytomegalovirus infection requires initial interaction with cell surface haparan sulfate. Virology. 1993;193:834–841. doi: 10.1006/viro.1993.1192. [DOI] [PubMed] [Google Scholar]
- 17.Navarro D, Paz P, Tugizov S, Topp K, et al. Glycoprotein B of human cytomegalovirus promotes virion penetration into cells, transmission of infection from cell to cell, and fusion of infected cells. Virology. 1993;197:143–158. doi: 10.1006/viro.1993.1575. [DOI] [PubMed] [Google Scholar]
- 18.Britt W, Mach M. Human cytomegalovirus glycoproteins. Intervirology. 1996;39:401–412. doi: 10.1159/000150510. [DOI] [PubMed] [Google Scholar]
- 19.Tugizov S, Navarro D, Paz P, Wang Y, Qadri I, Pereira L. Function of human cytomegalovirus glycoprotein B: syncytium formation in cells constitutively expressing gB is blocked by virus-neutralizing antibodies. Virology. 1994;201:263–276. doi: 10.1006/viro.1994.1291. [DOI] [PubMed] [Google Scholar]
- 20.Britt WJ, Vugler L, Butfiloski EJ, Stephens EB. Cell surface expression of human cytomegalovirus (HCMV) gp55-116 (gB): use of HCMV-vaccinia recombinant virus infected cells in analysis of the human neutralizing antibody response. J Virol. 1990;64:1079–1085. doi: 10.1128/jvi.64.3.1079-1085.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marshall GS, Rabalais GP, Stout GG, Waldeyer SL. Antibodies to recombinant-derived glycoprotein B after natural human cytomegalovirus infection correlate with neutralizing activity. J Infect Dis. 1992;165:381–384. doi: 10.1093/infdis/165.2.381. [DOI] [PubMed] [Google Scholar]
- 22.Navarro D, Lennette E, Tugizov S, Pereira L. Humoral immune response to functional regions of human cytomegalovirus glycoprotein B. J Med Virol. 1997;52:451–459. [PubMed] [Google Scholar]
- 23.Rapp M, Messerle M, Lucin P, Koszinowski UH. In vivo protection studies with MCMV glycoproteins gB and gH expressed by vaccinia virus. In: Michelson S, Plotkin SA, editors. Multidisciplinary Approach to Understanding Cytomegalovirus Disease. Amsterdam: Excerpta Medica; 1993. pp. 327–332. [Google Scholar]
- 24.Schleiss MR. Comparison of vaccine strategies against congenital CMV infection in the guinea pig model. J Clin Virol. 2008;41:224–230. doi: 10.1016/j.jcv.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Spaete RR. A recombinant subunit vaccine approach to HCMV vaccine development. Transplant Proc. 1991;23:90–96. [PubMed] [Google Scholar]
- 26.Mitchell DK, Holmes SJ, Burke RL, Adler SP. Immunogenicity of a recombinant human cytomegalovirus (CMV) gB vaccine in seronegative toddlers. Pediatr Inf Dis J. 2002;21:133–138. doi: 10.1097/00006454-200202000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Wang JB, Adler SP, Hempfling S, et al. Mucosal antibodies to human cytomegalovirus glycoprotein B occur following both natural infection and immunization with human cytomegalovirus vaccines. J Infect Dis. 1996;174:387–392. doi: 10.1093/infdis/174.2.387. [DOI] [PubMed] [Google Scholar]
- 28.Schultze V, D'Agosto V, Wack A, Novicki D, Zorn J, Hennig R. Safety of MF59 adjuvant. Vaccine. 2008;26:3209–3222. doi: 10.1016/j.vaccine.2008.03.093. [DOI] [PubMed] [Google Scholar]
- 29.Fowler KB, Stagno S, Pass RF. Maternal immunity and prevention of congenital cytomegalovirus infection. JAMA. 2003;289:1008–1011. doi: 10.1001/jama.289.8.1008. [DOI] [PubMed] [Google Scholar]
- 30.Fowler KB, Stagno S, Pass RF. Interval between births and risk of congenital cytomegalovirus infection. Clin Infect Dis. 2004;38:1035–1037. doi: 10.1086/382533. [DOI] [PubMed] [Google Scholar]
- 31.Zhang C, Buchanan H, Andrews W, Evans A, Pass R. Detection of cytomegalovirus infection during a vaccine clinical trial in healthy young women: seroconversion and viral shedding. J Clin Virol. 2006;35:338–342. doi: 10.1016/j.jcv.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 32.Zhang C, Pass RF. Detection of cytomegalovirus infection during clinical trials of glycoprotein B vaccine. Vaccine. 2004;23:507–510. doi: 10.1016/j.vaccine.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 33.Boppana SB, Rivera LB, Fowler KB, Mach M, Britt WJ. Intrauterine transmission of cytomegalovirus to infants of women with preconceptional immunity. N Engl J Med. 2001;344:1366–1371. doi: 10.1056/NEJM200105033441804. [DOI] [PubMed] [Google Scholar]