Abstract
Purpose
Fractionated whole brain irradiation (WBI) is used to treat primary brain tumors and brain metastases, but also is associated with late-delayed cognitive impairment. Previously, impaired performance was demonstrated in Fischer 344 x Brown Norway (F344xBN) rats on a hippocampal-dependent learning and memory test 12 months after fractionated WBI at middle age (1). The present study investigated whether there also is an affect on hippocampal neuron number or volume in that animal model.
Methods and Materials
Twelve month-old F344xBN male rats were divided into WBI and control (CON) groups (n=6/group). Anesthetized WBI rats received 45 Gy of 137Cs γ rays delivered as nine 5 Gy fractions twice per week for 4.5 weeks. CON rats were anesthetized, but not irradiated. Twelve months following WBI completion, all rats were anesthetized, perfused with paraformaldehyde, and hippocampal sections were immunostained with the neuron-specific antibody, NeuN. Using unbiased stereology, total neuron number and the volume of the neuronal and neuropil layers were determined in the dentate gyrus (DG), CA3, and CA1 subregions of hippocampus.
Results
No differences in tissue integrity or neuron distribution were observed between the WBI and CON groups. Moreover, quantitative analys is demonstrated that neither total neuron number nor the volume of neuronal or neuropil layers differed between the two groups for any subregion.
Conclusions
Impairment on a hippocampal-dependent learning and memory test occurs one year following fractionated WBI at middle-age. The same WBI regimen, however, does not lead to a loss of neurons or a reduction in the volume of hippocampus.
Keywords: fractionated WBI, middle-age, hippocampus, neuron number, Fischer 344 x Brown Norway rats
INTRODUCTION
Large-field partial or whole brain irradiation (WBI) is used for the treatment of primary and metastatic brain tumors as well as for prophylaxis to prevent metastases from peripheral malignancies (2,3). As many as 200,000 patients receive large-field partial or WBI every year (4). Unfortunately, radiation therapy also can cause brain injuries that have been associated with cognitive impairment. With the number of long-term survivors receiving WBI increasing, radiation-induced brain injury is emerging as a major risk factor following therapy (5,6). In fact, debilitating cognitive decline occurs in as many as 50% of brain tumor patients who live longer than 6 months after treatment (7,8,9). Hippocampal-dependent functions of learning, memory, and spatial information processing are preferentially affected by radiation therapy (10,11,12,13,14). Late-delayed, radiation-induced brain injuries typically begin 6 months or more after WBI and are believed to be irreversible and progressive. These late effects are the main contributors to the morbidity and mortality of radiation-induced brain injury. (15). Radiation-induced brain injury is a particular problem at middle-age when the incidence of cancers that commonly metastasize to the brain, such as lung and breast tumors and melanoma, increases significantly (3,16,17). This increase leads to a corresponding rise in the number of middle-age patients undergoing WBI (16).
One important goal of radiation research is to investigate the late-delayed neural changes following WBI. Accordingly, our group at Wake Forest Medical School has developed an animal model in which Fischer 344 X Brown Norway (F344xBN) rats demonstrate impaired spatial learning and memory 12 months following fractionated WBI at middle-age (1). Importantly, because spatial learning and memory depend on the integrity of the hippocampus (18,19,20), ongoing investigations have addressed the neural changes associated with WBI-induced learning and memory impairments in this forebrain structure. Although other studies have demonstrated impairments on several hippocampal-dependent tasks following fractionated WBI with different ages and rodent strains (21,22,23), the full spectrum of biological effects of a clinically relevant dose of fractionated WBI on the hippocampus is not yet understood (24).
Late-delayed, radiation-induced brain injury does not occur as a single, instantaneous event. Instead, it is a dynamic, multifaceted process operating over an extended period of time and is characterized by a variety of pathological changes to the vasculature (25,26,27) and myelin (28,29,24). Nevertheless, other components of the brain as well as critical cellular interactions also may contribute to the radiation-induced neural changes (15). Particularly, recent evidence suggests that synaptic composition, specifically the balance among different subunits of the NMDA type of glutamate receptor, is altered following WBI (1). However, the long-term population response of neurons, the cellular mediators of all neural processing in the brain, after irradiation has received relatively little attention.
Neurological effects such as ataxia, memory loss, and even dementia have been reported following brain irradiation (8,30), consistent with a direct effect of radiation therapy on neurons. Several animal studies also have indicated that neurons may be sensitive to radiation at clinically relevant doses (15,31). Accordingly, the present study used unbiased stereological techniques to determine whether hippocampal neurons are lost 12 months after middle-age rats received a fractionated course of WBI that is expected to be biologically equivalent to the regimens used clinically in the treatment of brain tumors (32). Specifically, the numbers of principal neurons in the dentate gyrus (DG), CA3, and CA1 subregions of the hippocampus were counted and the volumes of the neuronal and neuropil layers in each subregion were measured. Contrary to expectations, the results indicate that neither a loss of neurons nor a decrease in volume in the DG, CA3, or CA1 subregions of the hippocampus occurs 12 months after a clinically relevant course of WBI.
METHODS AND MATERIALS
Animals and Irradiation Procedure
Male Fischer 344 x Brown Norway F1 (F344xBN) rats were acquired from the National Institute on Aging at 11 months of age and divided randomly into whole brain irradiated (WBI) and control (CON) groups (6/group) at 12 months of age. The animal protocol for this study (A06-064) conforms to NIH guidelines and was approved by the Animal Care and Use Committee of Wake Forest University Health Sciences (Winston-Salem, NC). Rats were housed singly in a climate-controlled environment with a 12-hour light/dark cycle and were provided food and water ad libitum. Rats were weighed weekly to monitor their health.
A total dose of 45 Gy was delivered to the whole brain as 9 fractions of 5 Gy, twice per week for 4.5 weeks. The irradiation procedure has been described in detail previously (33,1). Briefly, the WBI rats were anesthetized lightly with ketamine/xylazine (26.5 mg/kg ketamine, 5.4 mg/kg xylazine) and irradiated in a 444-TBq self-shielded 137Cs irradiator using lead and Cerrobend shielding devices to collimate the beam so that the whole brain was irradiated. To ensure that each rat received the same midline brain dose, the dose was delivered to alternate sides of the head for alternate fractions. CON rats were anesthetized, but not irradiated. Both CON and WBI rats were allowed to survive for 12 months and were euthanized by overdose of ketamine/xylazine anesthesia at 24 months of age. All subsequent experimental procedures were carried out by investigators blinded to the irradiation status of the individual rat brains.
Tissue Preparation and Immunocytochemistry
Tissue preparation and immunocytochemistry were performed as reported previously (34). All rats were anesthetized with an intraperitoneal injection of pentobarbital (200 mg/kg) and perfused transcardially with saline followed by 4% paraformaldehyde in phosphate buffer. The brains were dissected from the cranial vault and blocked by two coronal cuts, one just caudal to the olfactory bulb (Bregma 6.5; 64) and a second at the level of the brainstem (Bregma -15; 64). Tissue blocks were post-fixed overnight, cryoprotected in a graded concentration series of sucrose (10%, 20%, and 30%), frozen in Tissue-Tek OCT (Optimal Cutting Temperature Compound; Sakura Finetek) on dry ice, and stored at -80°C. Brain sections were cut coronally at 40 μm on a freezing microtome, collected into antifreeze, and stored at -80°C until processing. For each brain, every 12th section through the hippocampus (Bregma -1.8 to -6.8) was immunoreacted with the anti-neuronal nuclei mouse monoclonal antibody, NeuN (Chemicon). Briefly, sections were rinsed in phosphate buffered saline (PBS), blocked in Triton X and normal horse serum (NHS), and immunoreacted with the NeuN primary antibody (1:4500) in Triton X and NHS. The sections then were treated with biotinylated, rat-adsorbed horse anti-mouse IgG secondary antibody (1:1000, Vector) and visualized with diaminobenzidine (Elite Kit, Vector). Finally, sections were washed, mounted, dehydrated, cleared, and coverslipped. As a control, one section from each animal was processed according to the same protocol without the primary antibody.
Stereological Quantifications
Stereological quantification is superior to traditional, single-section estimation techniques as the result is not biased by the shape, size, and orientation of the counting objects or by volumetric variation (35). Using the stereological optical fractionator technique (35), the total number of neurons in the principal cell layers of the DG, CA3, and CA1 subregions of hippocampus was estimated with the StereoInvestigator system (MicroBrightField, Williston, VT) and a Nikon Labophot microscope connected to a digital video camera (Hitachi, HV-C20) as described previously (34,36). For each section to be analyzed, the borders of the granular cell layer in DG and the pyramidal cell layers in CA3 and CA1 were defined, and NeuN-immunoreactive (NeuN-IR) cells were quantified in counting boxes that were distributed in a systematically random manner by the software. All NeuN-IR cells to be counted were visualized by focusing through the dissector height, and the numerical density (Nv, number per mm3) of NeuN-IR cells was calculated. Table 1 shows the stereological parameters and the coefficient of error (CE) for each subregion. The volumes of the granular cell layer in DG and the pyramidal cell layers in CA3 and CA1 were derived and the total number of neurons in each layer was determined by multiplying Nv by the layer volume. In addition, the volume of the neuropil layers was determined for each subregion, and the total volume of the neuronal and neuropil layers in the three hippocampal subregions was calculated using the StereoInvestigator system.
Table 1.
Stereological Parameters
| Region | Object | Disector Size (μm2) |
Disector Height (μm) |
Sampling Grid Size (μm2) |
1Mean Objects Counted |
2Mean CE Schmitz-Hof |
|---|---|---|---|---|---|---|
| Dentate Gyrus | Granular cells | 20 × 20 | 15 | 240 × 240 | 334 ± 11 | 5.49 |
| CA3 | Pyramidal cells | 25 × 25 | 15 | 140 × 140 | 492 ± 27 | 4.56 |
| CA1 | Pyramidal cells | 25 × 25 | 15 | 120 × 120 | 552 ± 22 | 4.24 |
The mean objects counted for individual animals are shown ± SEM.
Coefficient of error (CE) is the mean CE (Schmitz-Hof) for individual estimates.
GAD: glutamic acid decarboxylase; IR: immunoreactive; PV: parvalbumin.
Statistical Analysis
All data were analyzed using SigmaStat software (Systat Software, Inc., Richmond, CA). All statistical comparisons between WBI and CON groups were performed using a two-tailed Student’s t-test. A p value of < 0.05 was considered statistically significant.
RESULTS
Figure 1 shows representative NeuN-immunoreacted sections through the hippocampus from a CON (A) and WBI (B) rat at low magnification. The general histological appearance of the DG, CA3, and CA1 hippocampal subregions was similar in both groups of rats. No evidence of degeneration was detected in any of the sections from WBI rats. Figures 1C - H illustrates higher magnification photomicrographs of NeuN-immunoreacted neurons in the hippocampus demonstrating the neuronal layers (granule cell layer in DG; and stratum pyramidale in CA3 and CA1) and the adjacent neuropil layers. These sections are from animals with pyramidal neuron numbers close to the mean for their respective groups and demonstrate similar packing density and morphological appearance of the neurons in CON and WBI rats.
Figure 1.
Photomicrographs of NeuN-immunoreactive (NeuN-IR) cells in the hippocampus of Fischer 344 x Brown Norway rats 12 months after receiving whole-brain irradiation (WBI) at middle-age (12 months age). A and B: NeuN-immunostained coronal sections through the hippocampus of control (CON; A) and WBI (B) rats. Layers of darkly stained cells as well as cell-sparse neuropil regions in the dentate gyrus (DG), CA3, and CA1 subregions of hippocampus can be identified. C-H: NeuN-IR cells were quantified stereologically in the granular cell layer (GCL) of the DG (C, F) and in the stratum pyramidale (SP) of CA3 (D, G) and CA1 (E, H) of CON and WBI rats. Scale Bar = 750 μm in A and B; 75 μm in C - H.
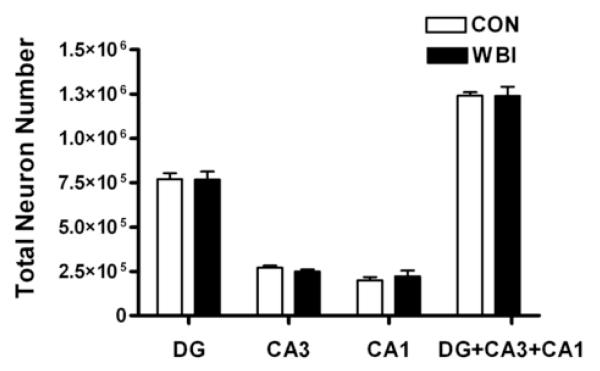
Quantitative results from the stereological analysis of total neuron number in the principal cell layers of DG, CA3, and CA1 of CON and WBI rats are illustrated in Figure 2. Consistent with previous estimates (37), there were approximately 770,000 neurons in the granular cell layer of DG, 260,000 neurons in the pyramidal cell layer of CA3, and 210,000 neuron in the pyramidal cell layer of CA1 in CON rats. Neuron number did not differ after a clinically relevant dose of fractionated WBI in the DG (p > 0.9), CA3 (p > 0.2), or CA1 (p > 0.5) subregions. It is noteworthy that the quantitative techniques used here were sufficiently sensitive to detect even small WBI-induced changes in neuron number. Specifically, the coefficient of error provides a standardized statistic for evaluating the precision of neuron number estimates derived with modern stereological techniques (38). Averaged across animals in both CON and WBI groups, this parameter is 5.49% for DG, 4.56% for CA3, and 4.34% for CA1 (Table 1) and suggests that systemic sampling error in estimating hippocampal neuron number does not account for the absence of WBI-induced cell loss. Accordingly, these results indicate that a significant loss of principal neurons does not occur in any of the three primary hippocampal subregions 12 months after fractionated WBI at middle-age.
Figure 2.
Total number of NeuN-immunoreactive (IR) cells in the dentate gyrus, CA3, and CA1 of control (CON) and whole brain irradiated (WBI) Fischer 344 x Brown Norway rats. There is no significant difference (p > 0.05) in the total number of neurons in DG, CA3, or CA1 subregions of CON and WBI rats 12 months after WBI at middle-age rats.
The volumes of the principal cell layers in DG, CA3, and CA1 are shown in Table 2. As would be expected from the stereological results presented above, these volumes do not differ between WBI and CON rats. In order to evaluate whether the neuropil layers of the hippocampus were affected by WBI, the volume of those layers in each subregion was determined as well. The results reveal that the volumes of the neuropil layers in the DG, CA3 and CA1 subregions of hippocampus do not differ between WBI and CON rats 12 months after a fractionated course of WBI at middle-age (Table 2). Finally, neither the total volume (neuronal layers plus neuropil layers) of DG, CA3 and CA1 nor the volume of the entire hippocampus (all subregions) differs between WBI and CON groups (Table 2; all p values > 0.05).
Table 2.
The Volume of Different Hippocampal Subregions between Control and Whole-brain Irradiated Fischer 344 x Brown Norway Rats.
| VOLUME (mm3) | ||||
|---|---|---|---|---|
| Dentate Gyrus | CA3 | CA1 | DG + CA3 + CA1 | |
| CON | ||||
| Principal Cell Layer | 3.13 ± 0.12 | 2.40 ± 0.07 | 1.64 ± 0.18 | 7.17 ± 0.13 |
| Neuropil Layers | 12.65 ± 0.49 | 9.26 ± 0.61 | 11.61 ± 0.50 | 33.52 ± 1.41 |
| Total Volume | 15.78 ± 0.46 | 11.64 ± 0.59 | 12.98 ± 0.53 | 40.40 ± 1.38 |
| WBI | ||||
| Principal Cell Layer | 2.91 ± 0.14 | 2.25 ± 0.12 | 1.67 ± 0.20 | 6.83 ± 0.31 |
| Neuropil Layers | 12.08 ± 0.40 | 8.72 ± 0.18 | 10.56 ± 0.69 | 31.36 ± 1.14 |
| Total Volume | 14.99 ± 0.50 | 11.00 ± 0.17 | 11.95 ± 0.72 | 37.94 ± 1.14 |
DISCUSSION
The present study provides the first evidence that a clinically relevant course of fractionated WBI, which has been shown to lead to spatial learning and memory impairment (1), does not affect hippocampal neuron number or volume. Previous clinical reports have documented cognitive impairments (39,40,41) as well as deterioration of brain structures (42,43) months to years after WBI. The occurrence of both of these changes following WBI has led to the assumption that a causal link may exist between them. This causal relationship derives from the perspective that severe necrosis of brain tissue, whether due primarily to the presence of tumor or secondarily to radiation exposure, can lead to alterations in neural processing. However, the present findings suggest that the cognitive impairment 12 months after fractionated WBI at middle-age is not associated with a decrease in either hippocampal neuron number or hippocampal volume.
Earlier in vitro studies have shown that irradiation can lead to neuron death (44,45,46). However, examination of brain sections in the present study revealed no evidence of neuronal death 12 months after WBI in vivo. Although the possibility that some neurons are lost after each radiation fraction and then replaced by newly generated neurons cannot be ruled out, a more likely explanation for the stability of hippocampal neuron number in the present study is the survival of those neurons following WBI. Neurogenesis in the rodent hippocampus does continue into adulthood (47,48,49), but decrease significantly with age (50,51,52,53). In fact, DG neurogenesis decreases by 85 – 90% between 7 and 25 months of age in Long Evans rats (54) and by 52% between 7 and 17 months of age in Fisher 344 x Brown Norway rats, the strain used here (55). The rats in the present study received WBI at 12 months of age when the level of DG neurogenesis is reduced substantially from that present in young animals. Moreover, neurogenesis has been reported to decrease significantly after WBI not only in young (56,57), but also in middle-age and old rats (55). Taken together, these findings strongly suggest that hippocampal neuron number in the present study remains stable not as a result of newly generated neurons replacing the neuronal cell loss following fractionated WBI, but instead because WBI simply does not lead to a significant neuron loss in DG, CA3, or CA1.
The present results further reveal that neither the volume of the hippocampal subregions nor the volume of the whole hippocampus is altered 12 months following fractionated WBI at middle-age. In light of the stability reported here in hippocampal neuron number following WBI, it is not surprising that the volume of the neuronal layers is unchanged. Significantly, however, the volume of the neuropil layers of the DG, CA1, and CA3 subregions also does not differ between WBI and CON groups. The neuropil layers contain the dendritic arborizations that receive neural input. Accordingly, these layers integrate incoming synaptic information as well as convey that information to the neuronal bodies. The maintenance of neuropil volume suggests that these signal transduction regions in the hippocampus do not degenerate following WBI, and that radiation-induced cognitive impairment is likely to be associated with more subtle cellular and molecular changes. The absence of change in the volume of both the neuronal and neuropil layers in the hippocampus one year after WBI at middle-age is consistent with maintenance of the gross integrity of the structural elements on which neural connectivity depends.
The absence of radiation-induced neuron loss or volume decrease is somewhat unexpected since neuronal degeneration would be consistent with the increased frequency of neurocognitive deficits after treatment of brain tumors (15,58,59). Moreover, the stability of hippocampal neuron number and volume in this study is in the same rodent model with radiation-induced performance deficits on the hippocampal-dependent Morris water maze test of learning and memory (1). Nevertheless, a review of earlier radiation studies reveals that radiation-induced cognitive impairment often can be observed in animals and humans prior to, or in the absence of gross histological or radiographic changes (60,22,61). Consequently, factors other than a loss of neurons or neuropil must underlie radiation-induced deficits in learning and memory performance. These factors are likely to include subcelluar or molecular changes such as the change in receptor subunits for the excitatory neurotransmitter glutamate in the hippocampus (1) or other changes in synaptic function. These changes may be induced primarily by WBI, or be secondary to a radiation-induced capillary deficiency (25) or other well-documented vascular changes (62,63,26).
In summary, the present study provides compelling evidence that neither a loss of neurons, nor a decrease in volume occur in the hippocampus 12 months following fractionated WBI at middle-age. Moreover, WBI-induced impairments in hippocampal-dependent spatial learning and memory are not associated with neuronal cell death. The preservation of neuron number after WBI represents the essential foundation for further characterization of synaptic as well as other, more subtle neurobiological changes that may underlie the observed radiation-induced cognitive decline.
Acknowledgements
This work was presented in abstract form at the 2006 annual meeting of the Society for Neuroscience and was supported by NIH grants CA119990 and CA112593.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST NOTIFICATION: No actual or potential conflicts of interest exist.
REFERENCES
- 1.Shi L, Adams MM, Long A, et al. Spatial learning and memory deficits after whole-brain irradiation are associated with changes in NMDA receptor subunits in the hippocampus. Radiat Res. 2006;166:892–899. doi: 10.1667/RR0588.1. [DOI] [PubMed] [Google Scholar]
- 2.Brandes AA. State-of-the-art treatment of high-grade brain tumors. Semin Oncol. 2003;30:4–9. doi: 10.1053/j.seminoncol.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 3.Soffietti R, Ruda R, Mutani R. Management of brain metastases. J Neurol. 2002;249:1357–1369. doi: 10.1007/s00415-002-0870-6. [DOI] [PubMed] [Google Scholar]
- 4.Stone HB, Moulder JE, Coleman CN, et al. Models for Evaluating Agents Intended for the Prophylaxis, Mitigation and Treatment of Radiation Injuries Report of an NCI Workshop, December 3-4, 2003. Radiat Res. 2004;162:711–728. doi: 10.1667/rr3276. [DOI] [PubMed] [Google Scholar]
- 5.Sheline GE, Wara WM, Smith V. Therapeutic irradiation and brain injury. Int J Radiat Oncol Biol Phys. 1980;6:1215–1228. doi: 10.1016/0360-3016(80)90175-3. [DOI] [PubMed] [Google Scholar]
- 6.Leibel SA, Gutin PH, Wara WM, et al. Survival and quality of life after interstitial implantation of removable high-activity iodine-125 sources for the treatment of patients with recurrent malignant gliomas. Int J Radiat Oncol Biol Phys. 1989;17:1129–1139. doi: 10.1016/0360-3016(89)90518-x. [DOI] [PubMed] [Google Scholar]
- 7.Imperato JP, Paleologos NA, Vick NA. Effects of treatment on long-term survivors with malignant astrocytomas. Ann Neurol. 1990;28:818–822. doi: 10.1002/ana.410280614. [DOI] [PubMed] [Google Scholar]
- 8.Crossen JR, Garwood D, Glatstein E, et al. Neurobehavioral sequelae of cranial irradiation in adults: a review of radiation-induced encephalopathy. J Clin Oncol. 1994;12:627–642. doi: 10.1200/JCO.1994.12.3.627. [DOI] [PubMed] [Google Scholar]
- 9.Johannesen TB, Lien HH, Hole KH, et al. Radiological and clinical assessment of long-term brain tumour survivors after radiotherapy. Radiother Oncol. 2003;69:169–176. doi: 10.1016/s0167-8140(03)00192-0. [DOI] [PubMed] [Google Scholar]
- 10.Armstrong CL, Corn BW, Ruffer JE, et al. Radiotherapeutic effects on brain function: double dissociation of memory systems. Neuropsychiatry Neuropsychol Behav Neurol. 2000;13:101–111. [PubMed] [Google Scholar]
- 11.Armstrong CL, Hunter JV, Ledakis GE, et al. Late cognitive and radiographic changes related to radiotherapy: initial prospective findings. Neurology. 2002;59:40–48. doi: 10.1212/wnl.59.1.40. [DOI] [PubMed] [Google Scholar]
- 12.Roman DD, Sperduto PW. Neuropsychological effects of cranial radiation: current knowledge and future directions. Int J Radiat Oncol Biol Phys. 1995;31:983–998. doi: 10.1016/0360-3016(94)00550-8. [DOI] [PubMed] [Google Scholar]
- 13.Abayomi OK. Pathogenesis of irradiation-induced cognitive dysfunction. Acta Oncol. 1996;35:659–663. doi: 10.3109/02841869609083995. [DOI] [PubMed] [Google Scholar]
- 14.Shaw EG, Rosdhal R, D’Agostino RB, Jr., et al. Phase II study of donepezil in irradiated brain tumor patients: effect on cognitive function, mood, and quality of life. J Clin Oncol. 2006;24:1415–1420. doi: 10.1200/JCO.2005.03.3001. [DOI] [PubMed] [Google Scholar]
- 15.Tofilon PJ, Fike JR. The radioresponse of the central nervous system: a dynamic process. Radiat Res. 2000;153:357–370. doi: 10.1667/0033-7587(2000)153[0357:trotcn]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 16.Jemal A, Tiwari RC, Murray T, et al. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 17.Patchell RA, Regine WF. The rationale for adjuvant whole brain radiation therapy with radiosurgery in the treatment of single brain metastases. Technol Cancer Res Treat. 2003;2:111–115. doi: 10.1177/153303460300200206. [DOI] [PubMed] [Google Scholar]
- 18.Moser E, Moser MB, Andersen P. Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. J Neurosci. 1993;13:3916–3925. doi: 10.1523/JNEUROSCI.13-09-03916.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morris RG, Schenk F, Tweedie F, et al. Ibotenate Lesions of Hippocampus and/or Subiculum: Dissociating Components of Allocentric Spatial Learning. Eur J Neurosci. 1990;2:1016–1028. doi: 10.1111/j.1460-9568.1990.tb00014.x. [DOI] [PubMed] [Google Scholar]
- 20.Broadbent NJ, Squire LR, Clark RE. Spatial memory, recognition memory, and the hippocampus. Proc Natl Acad Sci U S A. 2004;101:14515–14520. doi: 10.1073/pnas.0406344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lamproglou I, Chen QM, Boisserie G, et al. Radiation-induced cognitive dysfunction: an experimental model in the old rat. Int J Radiat Oncol Biol Phys. 1995;31:65–70. doi: 10.1016/0360-3016(94)00332-F. [DOI] [PubMed] [Google Scholar]
- 22.Yoneoka Y, Satoh M, Akiyama K, et al. An experimental study of radiation-induced cognitive dysfunction in an adult rat model. Br J Radiol. 1999;72:1196–1201. doi: 10.1259/bjr.72.864.10703477. [DOI] [PubMed] [Google Scholar]
- 23.Madsen TM, Kristjansen PE, Bolwig TG, et al. Arrested neuronal proliferation and impaired hippocampal function following fractionated brain irradiation in the adult rat. Neuroscience. 2003;119:635–642. doi: 10.1016/s0306-4522(03)00199-4. [DOI] [PubMed] [Google Scholar]
- 24.Belka C, Budach W, Kortmann RD, et al. Radiation induced CNS toxicity-- molecular and cellular mechanisms. Br J Cancer. 2001;85:1233–1239. doi: 10.1054/bjoc.2001.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown WR, Blair RM, Moody DM, et al. Capillary loss precedes the cognitive impairment induced by fractionated whole-brain irradiation: a potential rat model of vascular dementia. J Neurol Sci. 2007;257:67–71. doi: 10.1016/j.jns.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 26.Calvo W, Hopewell JW, Reinhold HS, et al. Time- and dose-related changes in the white matter of the rat brain after single doses of X rays. Br J Radiol. 1988;61:1043–1052. doi: 10.1259/0007-1285-61-731-1043. [DOI] [PubMed] [Google Scholar]
- 27.Reinhold HS, Calvo W, Hopewell JW, et al. Development of blood vessel-related radiation damage in the fimbria of the central nervous system. Int J Radiat Oncol Biol Phys. 1990;18:37–42. doi: 10.1016/0360-3016(90)90264-k. [DOI] [PubMed] [Google Scholar]
- 28.van der Maazen RW, Kleiboer BJ, Verhagen I, et al. Repair capacity of adult rat glial progenitor cells determined by an in vitro clonogenic assay after in vitro or in vivo fractionated irradiation. Int J Radiat Biol. 1993;63:661–666. doi: 10.1080/09553009314450861. [DOI] [PubMed] [Google Scholar]
- 29.Hopewell JW, van der Kogel AJ. Pathophysiological mechanisms leading to the development of late radiation-induced damage to the central nervous system. Front Radiat Ther Oncol. 1999;33:265–275. doi: 10.1159/000061239. [DOI] [PubMed] [Google Scholar]
- 30.Maire JP, Coudin B, Guerin J, et al. Neuropsychologic impairment in adults with brain tumors. Am J Clin Oncol. 1987;10:156–162. doi: 10.1097/00000421-198704000-00052. [DOI] [PubMed] [Google Scholar]
- 31.Pellmar TC, Lepinski DL. Gamma radiation (5-10 Gy) impairs neuronal function in the guinea pig hippocampus. Radiat Res. 1993;136:255–261. [PubMed] [Google Scholar]
- 32.Fowler JF. Brief summary of radiobiological principles in fractionated radiotherapy. Semin Radiat Oncol. 1992;2:16–21. [Google Scholar]
- 33.Brown WR, Thore CR, Moody DM, et al. Vascular damage after fractionated whole-brain irradiation in rats. Radiat Res. 2005;164:662–668. doi: 10.1667/rr3453.1. [DOI] [PubMed] [Google Scholar]
- 34.Shi L, Argenta AE, Winseck AK, et al. Stereological quantification of GAD-67-immunoreactive neurons and boutons in the hippocampus of middle-aged and old Fischer 344 x Brown Norway rats. J Comp Neurol. 2004;478:282. doi: 10.1002/cne.20303. [DOI] [PubMed] [Google Scholar]
- 35.West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- 36.Shi L, Pang H, Linville MC, et al. Maintenance of inhibitory interneurons and boutons in sensorimotor cortex between middle and old age in Fischer 344 X Brown Norway rats. J Chem Neuroanat. 2006;32:46–53. doi: 10.1016/j.jchemneu.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 37.Rapp PR, Gallagher M. Preserved neuron number in the hippocampus of aged rats with spatial learning deficits. Proc Natl Acad Sci U S A. 1996;93:9926–9930. doi: 10.1073/pnas.93.18.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- 39.Laukkanen E, Klonoff H, Allan B, et al. The role of prophylactic brain irradiation in limited stage small cell lung cancer: clinical, neuropsychologic, and CT sequelae. Int J Radiat Oncol Biol Phys. 1988;14:1109–1117. doi: 10.1016/0360-3016(88)90386-0. [DOI] [PubMed] [Google Scholar]
- 40.Meyers CA, Smith JA, Bezjak A, et al. Neurocognitive function and progression in patients with brain metastases treated with whole-brain radiation and motexafin gadolinium: results of a randomized phase III trial. J Clin Oncol. 2004;22:157–165. doi: 10.1200/JCO.2004.05.128. [DOI] [PubMed] [Google Scholar]
- 41.Johnson BE, Becker B, Goff WB, et al. Neurologic, neuropsychologic, and computed cranial tomography scan abnormalities in 2- to 10-year survivors of small-cell lung cancer. J Clin Oncol. 1985;3:1659–1667. doi: 10.1200/JCO.1985.3.12.1659. [DOI] [PubMed] [Google Scholar]
- 42.Mikhael MA. Radiation necrosis of the brain: correlation between computed tomography, pathology, and dose distribution. J Comput Assist Tomogr. 1978;2:71–80. [PubMed] [Google Scholar]
- 43.De Reuck J, Vander EH. The anatomy of the late radiation encephalopathy. Eur Neurol. 1975;13:481–494. doi: 10.1159/000114704. [DOI] [PubMed] [Google Scholar]
- 44.Enokido Y, Araki T, Tanaka K, et al. Involvement of p53 in DNA strand break-induced apoptosis in postmitotic CNS neurons. Eur J Neurosci. 1996;8:1812–1821. doi: 10.1111/j.1460-9568.1996.tb01325.x. [DOI] [PubMed] [Google Scholar]
- 45.Gobbel GT, Bellinzona M, Vogt AR, et al. Response of postmitotic neurons to X-irradiation: implications for the role of DNA damage in neuronal apoptosis. J Neurosci. 1998;18:147–155. doi: 10.1523/JNEUROSCI.18-01-00147.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Noel F, Tofilon PJ. Astrocytes protect against X-ray-induced neuronal toxicity in vitro. Neuroreport. 1998;9:1133–1137. doi: 10.1097/00001756-199804200-00032. [DOI] [PubMed] [Google Scholar]
- 47.Schmitz C, Grolms N, Hof PR, et al. Altered spatial arrangement of layer V pyramidal cells in the mouse brain following prenatal low-dose X-irradiation. A stereological study using a novel three-dimensional analysis method to estimate the nearest neighbor distance distributions of cells in thick sections. Cereb Cortex. 2002;12:954–960. doi: 10.1093/cercor/12.9.954. [DOI] [PubMed] [Google Scholar]
- 48.Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- 49.Caviness VS., Jr. Time of neuron origin in the hippocampus and dentate gyrus of normal and reeler mutant mice: an autoradiographic analysis. J Comp Neurol. 1973;151:113–120. doi: 10.1002/cne.901510203. [DOI] [PubMed] [Google Scholar]
- 50.Merrill DA, Karim R, Darraq M, et al. Hippocampal cell genesis does not correlate with spatial learning ability in aged rats. J Comp Neurol. 2003;459:201–207. doi: 10.1002/cne.10616. [DOI] [PubMed] [Google Scholar]
- 51.van Praag H, Shubert T, Zhao C, et al. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Merrill DA, Chiba AA, Tuszynski MH. Conservation of neuronal number and size in the entorhinal cortex of behaviorally characterized aged rats. J Comp Neurol. 2001;438:445–456. doi: 10.1002/cne.1327. [DOI] [PubMed] [Google Scholar]
- 53.Lichtenwalner RJ, Forbes ME, Bennett SA, et al. Intracerebroventricular infusion of insulin-like growth factor-I ameliorates the age-related decline in hippocampal neurogenesis. Neuroscience. 2001;107:603–613. doi: 10.1016/s0306-4522(01)00378-5. [DOI] [PubMed] [Google Scholar]
- 54.Bizon JL, Gallagher M. Production of new cells in the rat dentate gyrus over the lifespan: relation to cognitive decline. Eur J Neurosci. 2003;18:215–219. doi: 10.1046/j.1460-9568.2003.02733.x. [DOI] [PubMed] [Google Scholar]
- 55.Schindler MK, Forbes ME, Riddle DR. Aging-dependent Changes in Normal Brain Tissue Response to Whole Brain Irradiation. Int J Radiat Oncol Biol Phys. doi: 10.1016/j.ijrobp.2007.10.054. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rola R, Raber J, Rizk A, et al. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol. 2004;188:316–330. doi: 10.1016/j.expneurol.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 57.Raber J, Rola R, LeFevour A, et al. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 2004;162:39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- 58.Laack NN, Brown PD. Cognitive sequelae of brain radiation in adults. Semin Oncol. 2004;31:702–713. doi: 10.1053/j.seminoncol.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 59.Armstrong CL, Gyato K, Awadalla AW, et al. A critical review of the clinical effects of therapeutic irradiation damage to the brain: the roots of controversy. Neuropsychol Rev. 2004;14:65–86. doi: 10.1023/b:nerv.0000026649.68781.8e. [DOI] [PubMed] [Google Scholar]
- 60.Akiyama K, Tanaka R, Sato M, et al. Cognitive dysfunction and histological findings in adult rats one year after whole brain irradiation. Neurol Med Chir (Tokyo) 2001;41:590–598. doi: 10.2176/nmc.41.590. [DOI] [PubMed] [Google Scholar]
- 61.Hodges H, Katzung N, Sowinski P, et al. Late behavioural and neuropathological effects of local brain irradiation in the rat. Behav Brain Res. 1998;91:99–114. doi: 10.1016/s0166-4328(97)00108-3. [DOI] [PubMed] [Google Scholar]
- 62.Li YQ, Chen P, Jain V, et al. Early radiation-induced endothelial cell loss and blood-spinal cord barrier breakdown in the rat spinal cord. Radiat Res. 2004;161:143–152. doi: 10.1667/rr3117. [DOI] [PubMed] [Google Scholar]
- 63.Rubin P, Gash DM, Hansen JT, et al. Disruption of the blood-brain barrier as the primary effect of CNS irradiation. Radiother Oncol. 1994;31:51–60. doi: 10.1016/0167-8140(94)90413-8. [DOI] [PubMed] [Google Scholar]
- 64.Paxino G, Watson C. Academic Press; New York: 1982. The rat brain in stereotaxic coordinates. [Google Scholar]