Abstract
Studies in experimental models and controlled patient trials indicate that opioids are effective in managing neuropathic pain. However, side effects secondary to their central nervous system actions present barriers to their clinical use. Therefore, we examined whether activation of the peripheral mu-opioid receptors (MORs) could effectively alleviate neuropathic pain in rats after L5 spinal nerve ligation (SNL). Systemic loperamide hydrochloride (0.3–10 mg/kg, s.c.), a peripherally acting MOR-preferring agonist, dose-dependently reversed the mechanical allodynia at day 7 post-SNL. This anti-allodynic effect produced by systemic loperamide (1.5 mg/kg, s.c.) was blocked by systemic pretreatment with either naloxone hydrochloride (10 mg/kg, i.p.) or methyl-naltrexone (5 mg/kg, i.p.), a peripherally acting MOR-preferring antagonist. It was also blocked by ipsilateral intraplantar pretreatment with methyl-naltrexone (43.5 µg/50 µl) and the highly selective MOR antagonist CTAP (5.5 µg/50 µl). However, this anti-allodynic effect of systemic loperamide was not blocked by intraplantar pretreatment with the delta-opioid receptor antagonist naltrindole hydrochloride (45.1 µg/50 µl). The anti-allodynic potency of systemic loperamide varied with time after nerve injury, with similar potency at days 7, 28, and 42 post-SNL, but reduced potency at day 14 post-SNL. Ipsilateral intraplantar injection of loperamide also dose dependently (10-100 µg/50 µl) reversed mechanical allodynia on day 7 post-SNL. We suggest that loperamide can effectively attenuate neuropathic pain, primarily through activation of peripheral MORs in local tissue. Therefore, peripherally acting MOR agonists may represent a promising therapeutic approach for alleviating neuropathic pain.
1. Introduction
Chronic nonmalignant pain that develops after a peripheral nerve injury is often challenging to treat and refractory to current pharmacotherapies. Nevertheless, several studies in experimental models and controlled patient trials suggest that mu-opioid receptor (MOR) agonists are effective at attenuating neuropathic pain [30, 35,36,38,50]. However, side effects (respiratory depression, sedation, cognitive dysfunction, addiction, and abuse) and toxicity secondary to their central nervous system (CNS) actions present substantial barriers to their clinical use, especially with high doses or chronic use [15, 30, 39].
Opioid receptors synthesized in dorsal root ganglion (DRG) neurons are transported to their central and peripheral terminals in the superficial dorsal horn and peripheral tissues, respectively [8, 17, 50]. Although MORs located in the CNS are the primary sites for the anti-nociceptive actions of systemically administered morphine, peripheral MORs may become increasingly important for the anti-allodynic/anti-hyperalgesic actions of morphine under chronic pain conditions. For example, a growing body of evidence suggests that peripheral MORs can be important effectors of systemic opioids in alleviating persistent inflammatory pain [11, 23, 34, 47]. However, the roles of peripheral opioidergic mechanisms in management of neuropathic pain remain to be established. Although MOR agonist either superfused over the ligature site or injected into the hindpaw ipsilateral to the nerve injury attenuated neuropathic pain in the chronic constriction injury (CCI) model [35,36], intraperitoneal injection of a peripherally restricted MOR-preferring agonist failed to attenuate mechanical allodynia after spinal nerve injury [46]. In addition to differences in route of drug administration and neuropathic pain model, potency of opioids in the treatment of neuropathic pain may also vary with the time after injury. For instance, intra-paw administration of MOR agonists attenuated mechanical allodynia only at an early time point after CCI of sciatic nerve [24]. To our knowledge, no one has systematically examined the post-injury time-dependence of the analgesic effects of peripheral-acting opioids in models of neuropathic pain.
In an effort to expand our knowledge of peripheral opioidergic mechanisms in neuropathic pain, we systematically investigated the dose-response, time-course, post-injury time-dependence, and site of action for loperamide hydrochloride, a peripherally acting MOR-preferring agonist, in modulating the neuropathic pain after L5 spinal nerve injury (SNL) in rats. As a substrate extruded by P-glycoprotein transporter from the brain endothelial cells, loperamide is quickly removed from CNS endothelial cells after systemic administration [10, 34, 52]. Loperamide shows selective binding for MOR over other opioid receptor subtypes, has a long-established safety profile, and should be readily accessible for future clinical trials [10, 34].
2. Materials and methods
2.1. L5 spinal nerve ligation
Ligation of spinal nerve L5 was performed in adult male Sprague-Dawley rats (200–350 g, Harlan Bioproducts for Science, Indianapolis, IN) using a modification of the procedure described previously [25]. The animals were anesthetized with isoflurane (2–3%, Abbott Laboratories, North Chicago, IL) delivered through a nose cone. Under aseptic conditions, the skin was incised at the midline over the lumbar spine, and the L5, L6, and upper sacral vertebrae were exposed. The left transverse process of the L6 vertebra was removed, and the left L5 spinal nerve was exposed and dissected from the underlying tissue with fine forceps. The left L5 spinal nerve was then tightly ligated with a 6-0 silk suture and cut distally, with care being taken not to pull the nerve or touch the L4 spinal nerve. After hemostasis was achieved, the muscle layer was approximated with 4-0 chromic gut suture and the skin closed with metal clips. After the surgery, the rats were returned to their cages, kept warm under a heat lamp, and monitored during recovery. Skin staples were removed approximately 1 week after surgery. All procedures were approved by the Johns Hopkins University Animal Care and Use Committee as consistent with the National Institutes of Health Guide for the Use of Experimental Animals to ensure minimal animal use and discomfort.
2.2. Animal behavioral tests
To minimize experimenter bias, the investigator who performed the behavioral tests was blinded to the drug treatment conditions. Before the behavioral testing, animals were acclimatized to the facilities for 1 week. To minimize variability of the behavioral outcome measures, animals were trained for 3 to 5 days before baseline data were obtained. All experimental conditions (animal age, gender, room temperature, time of the day for behavioral testing, drug preparation, drug injection and animal handling, etc.) were carefully controlled to be consistent across groups. In addition, animals were habituated to the test environment for ≥30 min before testing was begun on a given day. To test for signs of mechanical allodynia, animals were placed under plastic domes on a mesh floor that allowed full access to the plantar surface of the paws. The area tested was the region between the foot-pads in the plantar aspect of the hindpaw. The up-down method was used to quantify the allodynia to mechanical stimuli [4,12]. Mechanical paw-withdrawal thresholds (PWTs) were determined using a series of von Frey filaments that deliver approximately logarithmic incremental forces (0.38, 0.57, 1.23, 1.83, 3.66, 5.93, 9.13, 13.1 g). The von Frey filaments were applied for 4 to 6 sec to the test area between the footpads on the plantar surface of the hindpaw. The 1.83-g stimulus was applied first. If a positive response occurred, the next smaller von Frey hair was used; if a negative response was observed, the next higher force was used. The test was continued until: (1) the responses to five stimuli were assessed after the first crossing of the withdrawal threshold, or (2) the upper/lower end of the von Frey hair set was reached before a positive/negative response had been obtained. Abrupt paw withdrawal, licking, and shaking were regarded as positive responses.
To test for signs of heat hyperalgesia, paw-withdrawal latencies (PWLs) to radiant heat stimuli [19] were measured with a plantar stimulator analgesia meter (IITC model 390, Woodland Hills, CA). Three to five animals were placed under individual plastic boxes on a heated glass floor (30°C) and allowed to habituate for at least 30 min before testing. Radiant heat was applied from below to the plantar surface of each hindpaw, and the withdrawal latency was measured by an electronic timer. The intensity of the stimulus was set to produce a PWL between 10 and 12 sec in a naive rat. Testing was alternated between hindpaws, starting on the unlesioned side. Both hindpaws were tested three times, with at least 2 min between trials; a cutoff time of 20 sec was used to avoid sensitization and damage to the skin. The average PWL of the three trials was used for data analysis.
2.3. Drugs
Loperamide hydrochloride, naloxone hydrochloride (a non-selective opioid receptor antagonist that can have peripheral and central effects), naltrindole hydrochloride (a highly selective delta opioid receptor (DOR) antagonist), CTAP (d-Phe-Cys-Tyr-d-Trp-Arg-Thr-Pen-Thr-NH2, a highly selective MOR antagonist), and CDEX (2-hydroxypropyl-beta-cyclodextrin) were purchased from Sigma-Aldrich, St. Louis, MO. Methyl-naltrexone (a MOR-preferring, peripherally acting opioid receptor antagonist that does not cross the blood-brain barrier) was kindly supplied by Dr. John F. Foss, M.D. (Department of Anesthesiology, University of Chicago, Chicago, IL). Stock solutions were freshly prepared. Loperamide hydrochloride was dissolved in 20% CDEX, a drug carrier system that can increase the water solubility of lipid-soluble drugs and reduce the rate of clearance [21]. All other drugs were dissolved initially in distilled water and then further diluted to the final concentration with saline (0.9%).
2.4. Experimental design
2.4.1. Study 1: Examine the effect of systemic administration of loperamide on neuropathic pain after SNL
First we examined PWTs to punctate mechanical stimulation applied to the hindpaw before injury and from day 4 to day 84 post-SNL injury (n = 8–12 per time point). Then, in a single-drug treatment study, we established the dose-response function for systemically administered loperamide in attenuating mechanical allodynia, a common and characteristic manifestation of neuropathic pain, on day 7 post-SNL. Rats that did not show lowered PWTs on the hindpaw ipsilateral to nerve injury (mechanical allodynia, >50% decrease from the pre-injury baseline) before drug administration were excluded from the study. Baseline PWTs were obtained before injections, and then animals were randomly assigned a drug treatment regimen and given a subcutaneous (s.c.) injection (in the back) of vehicle (20% CDEX, n = 10) or loperamide (0.3 mg/kg, n = 8; 1 mg/kg, n = 8; 3 mg/kg, n = 8; 10 mg/kg, n = 7) in a volume of 1 ml/kg. PWT testing was repeated at 30, 60, 90, 120, and 180 min post-injection. Maximum possible effect (MPE) was calculated at 30 min post-injection to establish the dose-response function, and ED50 (dose estimated to produce 50% MPE) for reversing mechanical allodynia was calculated accordingly.
In a separate group of rats, we examined the effect of systemic administration of loperamide on heat hyperalgesia on day 7 post-SNL. After pre-drug baseline PWL testing, animals were injected s.c. (in the back) with vehicle (20% CDEX, n = 5) or 1.5 mg/kg loperamide (n = 6), a dose two times the ED50 for reversing mechanical allodynia. PWL testing was repeated during the 30 to 60 min after drug injection.
2.4.2. Study 2: Examine the anti-allodynic effect of systemic administration of loperamide at different time points after SNL
To investigate its potential clinical use in chronic pain, we gave the same dose of loperamide systemically as a post-injury treatment at different time points after nerve injury and examined whether the anti-allodynic potency showed time-dependent changes during the progress of neuropathic pain. To avoid any potential tachphylaxis or desensitization of receptors associated with repetitive loperamide treatment, we employed a blinded single-drug treatment experimental design. Specifically, data for each post-SNL time point were derived from one group of animals collected in one day. On the drug-testing day, animals were randomly assigned a drug treatment regimen after pre-drug baseline testing. Loperamide (1.5 mg/kg) was injected s.c. (in the back) in different groups of animals at days 7 (n = 7), 14 (n = 8), 28 (n = 9), or 42 (n = 8) post-SNL. Vehicle-treated animals (n = 4–6 per group) were included in each group to blind the investigator to the drug treatment. PWT testing was repeated at 30, 60, 90, and 120 min post-injection. MPE was calculated at 30 min post-injection for each post-SNL time point.
To determine whether the route of drug administration affects loperamide’s anti-allodynic action, we examined the time course and potency of loperamide in attenuating mechanical allodynia when given intraperitoneally (i.p.). The post-SNL time point and the loperamide dose examined here were based on a previous study to allow comparison [46]. After pre-drug baseline testing, animals were randomly assigned a drug treatment regimen. On day 14 post-SNL, rats were injected i.p. with vehicle (n = 6) or 3.0 mg/kg loperamide (n = 6) in a volume of 1 ml/kg. PWT testing was carried out at 30, 60, and 90 min post-injection.
2.4.3. Study 3: Examine whether systemic pretreatment with a peripherally acting opioid receptor antagonist can block loperamide-induced reduction of allodynia
After pre-drug baseline testing, rats were pretreated with an i.p. injection of saline (n = 7), methyl-naltrexone (5 mg/kg, n = 10), or naloxone hydrochloride (10 mg/kg, n = 6). Loperamide (1.5 mg/kg) was injected s.c. in the back 5 min later. PWTs were obtained at 30, 60, 90, and 120 min following loperamide injection.
2.4.4. Study 4: Examine whether pretreatment with an intraplantar injection of opioid receptor antagonist can attenuate the loperamide-induced reduction in hyperalgesia
Even though loperamide hydrochloride is a peripherally acting MOR-preferring agonist, the anti-allodynic effect could occur anywhere in the peripheral nervous system, including the cutaneous terminals, along the course of the nerve, at the nerve injury site, or at the DRG. We examined whether activation of opioid receptors in the local hindpaw tissue is important for systemic loperamide-induced allodynia reduction at day 7 post-SNL. After pre-drug baseline testing, rats were briefly anesthetized with isoflurane (1.5%) through a nose cone to reduce stress and assure the accuracy of injections. Animals then were pretreated with an intraplantar (i.pl.) injection of methyl-naltrexone (43.5 µg/50 µl, n = 7), CTAP (5.5 µg/50 µl, n = 10), naltrindole hydrochloride (45.1 µg/50 µl, n = 7), or saline (50 µl, n = 7) to the ipsilateral hindpaw. Loperamide (1.5 mg/kg) was injected s.c. in the back 1 min later. PWTs were obtained at 30, 60, 90, and 120 min after loperamide injection.
In a separate cohort of rats, we examined whether the loperamide-induced reduction in allodynia at day 7 post-SNL can be blocked by pretreatment with an injection of CTAP (5.5 µg/50 µl, i.pl.) to the contralateral hindpaw. Rats were separated into two groups (n = 4 per group). The first group was pretreated with an ipsilateral hindpaw injection of CTAP (5.5 µg/50 µl, i.pl.) and a contralateral hindpaw injection of saline; in the second group the drug assignment to each hindpaw was reversed. Loperamide (1.5 mg/kg) was injected s.c. in the back 1 min later. PWTs were obtained at 30, 60, 90, and 120 min after loperamide injection.
2.4.5. Study 5: Examine the effect of intraplantar administration of loperamide on mechanical allodynia after SNL
To further confirm that direct activation of opioid receptors in local tissues is effective at attenuating neuropathic pain, we assessed the effects of i.pl. administration of loperamide into ipsilateral hindpaw on mechanical allodynia in rats. On day 7 after SNL, rats were given pre-drug baseline testing and then injected i.pl. with individual doses of vehicle (n = 8) or loperamide (10 µg/50 µl, n = 6), (50 µg/50 µl, n = 6), or (100 µg/50 µl, n = 10; i.pl.). PWT testing was carried out at 15, 45, and 90 min post-injection. The dose range examined was based on previous studies and was confirmed by our unblinded pilot experiment [10]. MPEs for different doses were calculated at 45 min post-injection to establish the dose-response function.
To exclude potential systemically mediated anti-allodynic effects of ipsilateral i.pl. loperamide injection, we examined the effect of loperamide injected into the contralateral hindpaw. On day 7 post-SNL, one group of rats was injected i.pl. in the contralateral hindpaw with either loperamide (100 µg/50 µl, n = 6) or vehicle (n = 4). A second group received i.pl. injections of loperamide (100µg/50 µl) in either the ipsilateral (n = 4) or contralateral (n = 4) hindpaw and vehicle in the opposite paw. PWT testing was carried out at 15, 45, and 90 min post-injection. Data from these two groups were later combined for analysis.
2.4.6. Study 6: Examine the anti-allodynic effect of intraplantar loperamide at different time points after SNL
In a repetitive-drug treatment study, we examined whether the anti-allodynic effect of i.pl. loperamide showed time-dependent changes during the progress of neuropathic pain. At days 14, 28, 42, and 56 post-SNL, either loperamide (100 µg/50 µl, n = 6) or vehicle (n = 4) was injected i.pl. into the ipsilateral hindpaw after pre-drug baseline testing. The rats were tested for PWT at 15, 45, and 90 min post-injection.
2.5. Statistical analysis
To determine the PWT, the pattern of positive and negative responses to the von Frey filament stimulation was converted to a 50% threshold value using the formula provided by Dixon [12]. Since low and high cut-off values were employed in the measurement of PWTs, these data are not continuous, but rather discrete data points with a ceiling and floor value. Therefore, the PWT data are not normally distributed (especially near the cut-off values). In addition, much of the analysis uses data that are near the cut-off value (e.g., when the PWTs are increased after the loperamide treatment). Accordingly, nonparametric ANOVA (Friedman and Kruskal-Wallis) was used to analyze PWT data which were presented as the median value. Post-hoc tests (Wilcoxon Matched Pairs Test and Mann-Whitney U Test) were used to analyze specific data points.
For establishing the dose-response functions and for comparison of drug effects on mechanical allodynia at different time points after SNL, PWT data were normalized by calculating MPE(%)value. MPE values were calculated with the equation: MPE (%) = [1 – (Cut off PWT – Measured PWT)/(Cut off PWT – Baseline PWT)]x100, where Cut off PWT = 21.5 g. Since two additional factors (Cut off PWT and Baseline PWT) were used in the equation, the resulting MPE (%) data, in contrast to PWT, are continuous with possible values from 0-100%. Although there are ceiling and floor values, most of the analysis occurs near the 50% effective dose where the data are thought to be normally distributed. Thus, parametric statistics were used when analyzing MPE data and establishing a 50% effective dose. Specifically, a one-way ANOVA (Fisher's protected LSD post-hoc test) was used to analyze MPE data which were presented as mean ± standard error of mean (S.E.M). Effect of drug treatment on heat hyperalgesia was determined by measuring PWL during the 30–60 min period after drug administration. Differences in the PWL between pre- and post-drug administration were analyzed by repeated measures analysis of variance with Fisher's protected least significant difference as the post hoc test. PWL data are represented as means ± S.E.M. P < 0.05 was considered statistically significant in all tests.
3. Results
3.1. Systemic administration of loperamide attenuated neuropathic pain after SNL
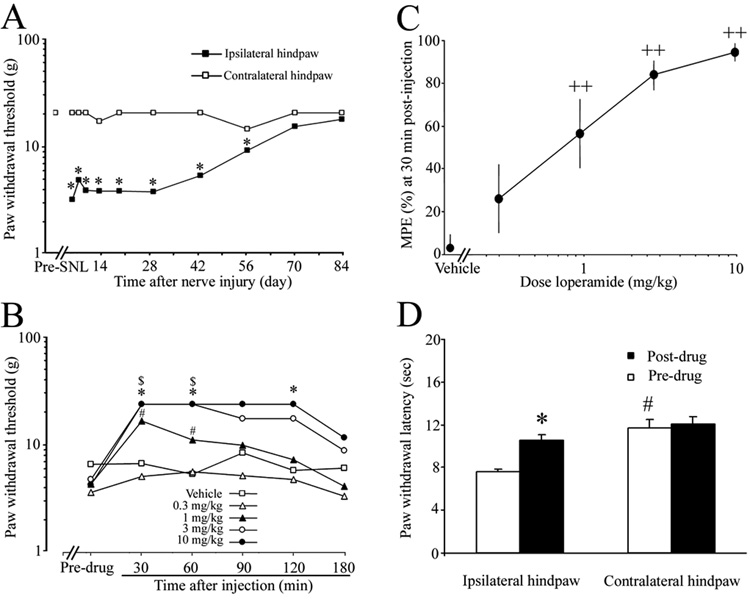
Compared with the pre-injury baseline value, L5 SNL induced a significant decrease in PWT to punctate mechanical stimulation applied to the hindpaw ipsilateral to the injured side from day 4 to day 56 post-SNL (Fig. 1A). PWTs returned to near pre-injury level by day 70 post-SNL. No significant changes in PWTs of the contralateral hindpaws were observed after nerve injury. On day 7 post-SNL, 1, 3, and 10 mg/kg doses of loperamide significantly and dose dependently increased PWTs of the ipsilateral hindpaw at 30 and 60 min post-injection, compared to the respective pre-drug baseline (Fig. 1B). At a dose of 1 mg/kg, loperamide’s anti-allodynic action persisted for at least 60 min. The magnitude and duration of loperamide-induced anti-allodynic effects increased with dose. MPE was calculated at 30 min post-injection to establish the dose-response function and to calculate ED50. MPEs for 1, 3, and10 mg/kg doses were significantly higher than that for vehicle-treated animals (Fig. 1C), and ED50 = 0.78 mg/kg (95% CL: 0.42-1.45). There were no significant changes in PWT in the contralateral hindpaw after loperamide injection (data not shown).
Fig. 1. Systemic loperamide attenuated neuropathic pain after L5 spinal nerve ligation (SNL).
(A) L5 SNL induced a decrease in PWT on the hindpaw ipsilateral to the injured side that lasted for 56 days. *P < 0.05 versus pre-SNL baseline (n = 8–12). (B) Vehicle or different doses of loperamide (n = 7–10) were injected subcutaneously in different groups of rats on day 7 post-SNL. Loperamide dose-dependently reversed mechanical allodynia that developed on the hindpaw ipsilateral to the SNL. *, $, # P < 0.05 versus the respective pre-drug baseline. (C) MPE (%) for loperamide to attenuate mechanical allodynia was calculated at 30 min post-injection for each dose, and a dose-response function was established accordingly. PWT data are presented as median values, and MPE data are expressed as means ± SEM. ++P < 0.01 versus the vehicle-treated group. (D) Systemic loperamide (1.5 mg/kg, n = 6, s.c.) also attenuated heat hyperalgesia that developed in the hindpaw ipsilateral to the SNL in rats on day 7 post-SNL, but did not affect PWL on the contralateral side. *P < 0.05 versus pre-drug baseline, #P < 0.05 versus the ipsilateral hindpaw.
In a separate group of rats, s.c. injection of 1.5 mg/kg loperamide on day 7 post-SNL significantly attenuated heat hyperalgesia that developed in the hindpaw ipsilateral to SNL, but did not affect PWL on the contralateral side (Fig. 1D). Vehicle injection had no effect on PWL of either hindpaw (data not shown).
3.2. The anti-allodynic effect of systemic administration of loperamide varied with time after SNL
At all time points examined (7, 14, 28, and 42 days post-SNL), mechanical allodynia was attenuated 30 min after injection of loperamide (1.5 mg/kg, s.c), as indicated by a significant increase in PWT compared to the respective pre-drug baseline (Fig. 2A). However, the reduction in allodynia at 30 and 60 min post-injection was less potent on day 14 than on day 7 after SNL. Importantly, MPE at 30 min post-injection was also significantly less on day 14 post-SNL than that on day 7, whereas there were no significant differences in MPE among the day 7, 28, and 42 post-SNL groups (Fig. 2C). Systemic loperamide did not change PWT of the contralateral hindpaw at any post-SNL time point examined (Fig. 2B).
Fig. 2. The anti-allodynic effect of systemic loperamide varied with time after L5 SNL.
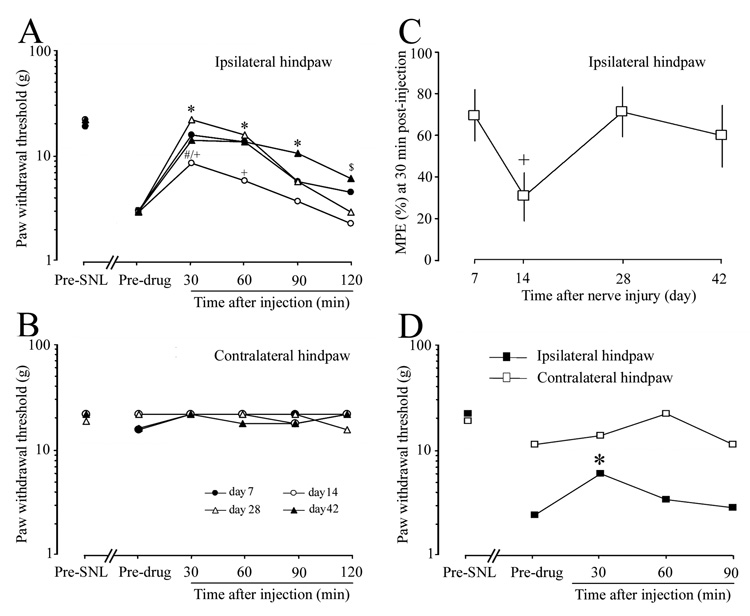
(A) Loperamide (1.5 mg/kg, s.c) was given to different groups of animals on day 7 (n = 7), 14 (n = 8), 28 (n = 9), or 42 (n = 8) post-SNL. Note that the anti-allodynic effect of loperamide at 30 and 60 min after injection was substantially lower in the day-14 post-SNL group than in the day-7 post-SNL group. * (day 7, 28, 42), $ (day 7, 42), # (day 14) P < 0.05 from respective pre-drug baseline; + P < 0.05 from day 7 post-SNL group. (B) Systemic loperamide did not change PWT of the contralateral hindpaw at any post-SNL time point examined. (C) MPE (%) was calculated at 30 min post-injection and plotted against different post-SNL time points. +P < 0.05 versus day 7 post-SNL. (D) Intraperitoneal injection of loperamide (3.0 mg/kg, n = 6) produced a short-term attenuation of mechanical allodynia in the ipsilateral hindpaw on day 14 post-SNL. *P < 0.05 versus pre-drug baseline. PWT data are presented as medians. MPE data are presented as means ± SEM.
On day 14 post-SNL, i.p. injection of 3.0 mg/kg loperamide significantly increased PWT of the ipsilateral hindpaw compared to the pre-drug baseline, but only at 30 min post-injection (Fig. 2D).
3.3. Systemic pretreatment with peripheral-acting opioid receptor antagonists blocked systemic loperamide-induced allodynia reduction
In saline-pretreated animals, s.c. injection of loperamide (1.5 mg/kg) significantly reversed the mechanical allodynia at 30, 60, and 90 min after administration, compared to the pre-drug baseline (Fig. 3A). In contrast, there were no significant differences in PWTs between pre- and post-loperamide conditions in animals pretreated with i.p. injection of methyl-naltrexone or naloxone hydrochloride. Furthermore, PWTs measured at 30 and 60 min after loperamide injection were significantly lower in animals pretreated with either methyl-naltrexone or naloxone hydrochloride than in those pretreated with saline, suggesting that both drugs blocked the anti-allodynic actions of systemic loperamide at day 7 post-SNL. There were no significant changes in the PWTs of the contralateral hindpaw after drug treatments (Fig. 3B).
Fig. 3. Peripherally acting opioid receptor antagonists blocked systemic loperamide-induced reduction in allodynia.
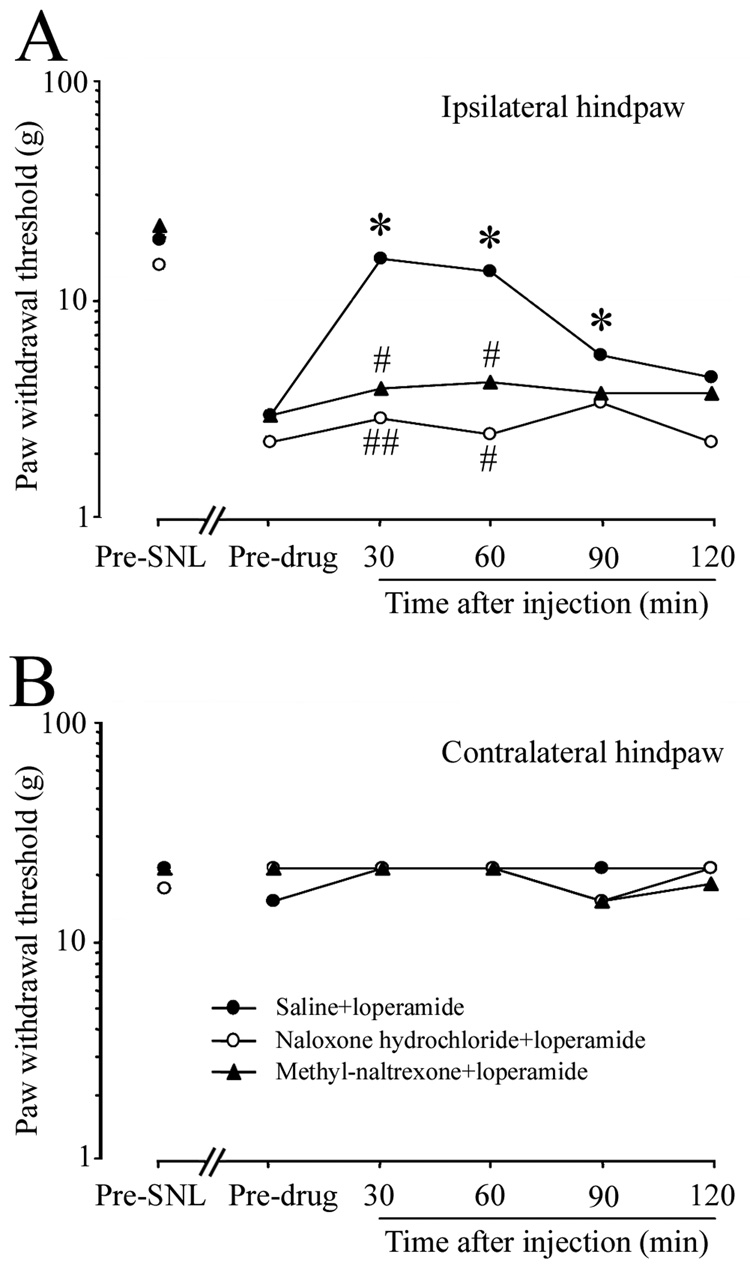
(A) Systemic pretreatment with methyl-naltrexone (5mg/kg, i.p., n = 10) and naloxone hydrochloride (10 mg/kg, i.p., n = 6) each significantly attenuated the anti-allodynic effect of loperamide (1.5 mg/kg, s.c.) on day 7 post-SNL, compared with saline pretreatment (n = 7). (B) PWT on the contralateral side did not change significantly after drug treatment. Data are expressed as medians. *P < 0.05 versus pre-drug baseline; #P < 0.05, ##P < 0.01 versus the saline-pretreated group.
3.4. Pretreatment with ipsilateral i.pl. injection of MOR antagonist attenuated systemic loperamide-induced allodynia reduction
In animals pretreated with ipsilateral i.pl. injection of saline or the DOR antagonist, naltrindole hydrochloride, systemic loperamide remained effective at reversing the mechanical allodynia at 30, 60, and 90 min after administration. In contrast, ipsilateral PWTs at 30, 60, and 90 min post-loperamide injection in animals pretreated with methyl-naltrexone (43.5µg/50 µl, n = 7) were not significantly different from pre-drug baseline values. Importantly, ipsilateral PWTs at 30, 60 and 90 min post-loperamide injection were all significantly lower in animals pretreated with either CTAP (5.5 µg/50 µl, n = 10) or methyl-naltrexone than in saline-pretreated animals, suggesting that the anti-allodynic effect of systemic loperamide was significantly attenuated (Fig. 4A). There were no significant changes in the PWT on the contralateral side after drug treatment (Fig. 4B).
Fig. 4. Intraplantar (i.pl.) injection of a mu-, but not a delta-, opioid receptor antagonist attenuated systemic loperamide-induced allodynia reduction.
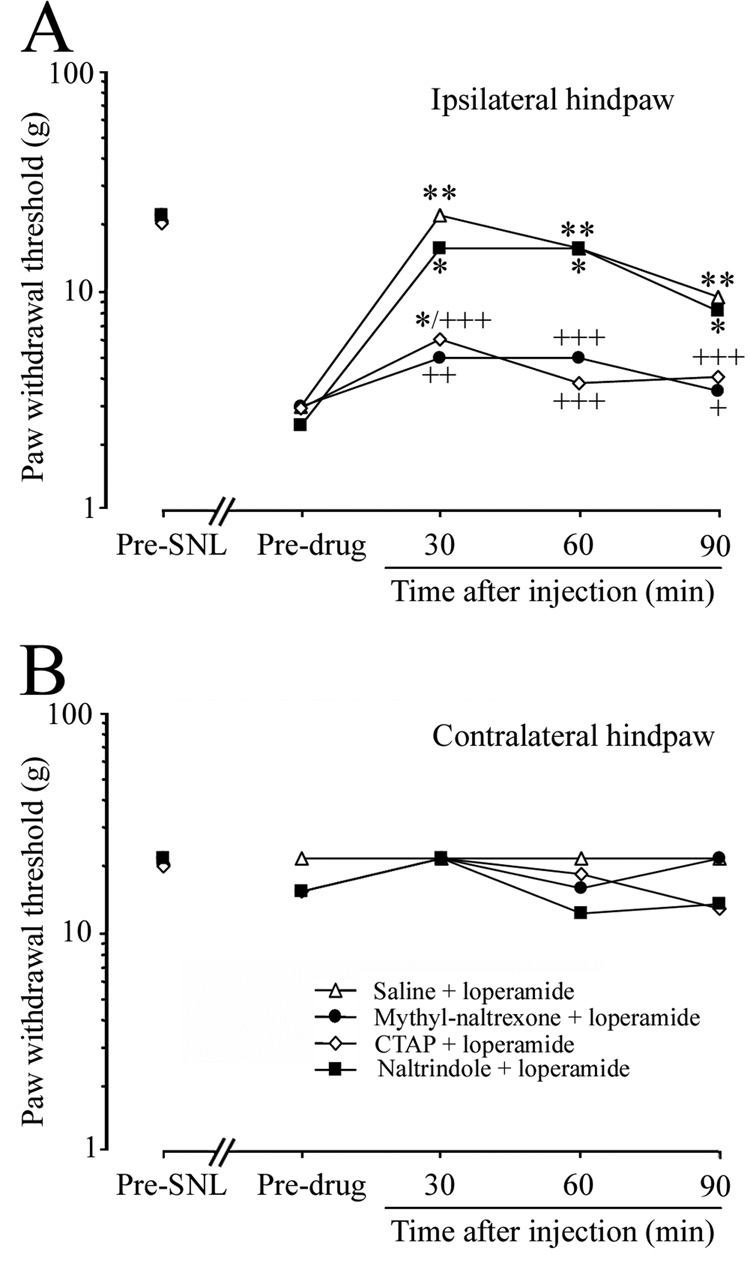
(A) On day 7 post-SNL, ipsilateral i.pl. pretreatment with either methyl-naltrexone (43.5 µg/50 µl, n = 7) or CTAP (5.5 µg/50 µl, n = 10) significantly attenuated systemic loperamide-induced allodynia reduction (1.5 mg/kg, s.c.), compared with saline pretreatment (50 µl, i.pl. n = 7). In contrast, pretreatment with a DOR antagonist, naltrindole hydrochloride (45.1 µg/50 µl, n = 7), was ineffective. (B) PWT on the contralateral side did not change significantly after any drug treatment. Data are expressed as medians. *P < 0.05, **P < 0.01 versus pre-drug baseline, +P < 0.05, ++P < 0.01, +++P < 0.001 versus the saline-pretreated group.
In a separate group of rats, contralateral hindpaw injection of CTAP and ipsilateral hindpaw injection of saline did not block systemic loperamide-induced allodynia reduction on day 7 post-SNL: the median values (range) for PWTs of ipsilateral hindpaw at pre- and 30 min post-loperamide injection were 2.58 g (2.22–4.03 g) and 21.75 g (9.93–21.75 g), respectively. In contrast, ipsilateral hindpaw injection of CTAP (5.5 µg/50 µl, i.pl.) and contralateral hindpaw injection of saline diminished systemic loperamide-induced allodynia reduction: PWTs of ipsilateral hindpaw at pre- and 30 min post-injection of loperamide were 2.14 g (1.87–4.03 g) and 6.14 g (4.03–8.95g).
3.5. Intraplantar administration of loperamide dose-dependently reversed mechanical allodynia after SNL
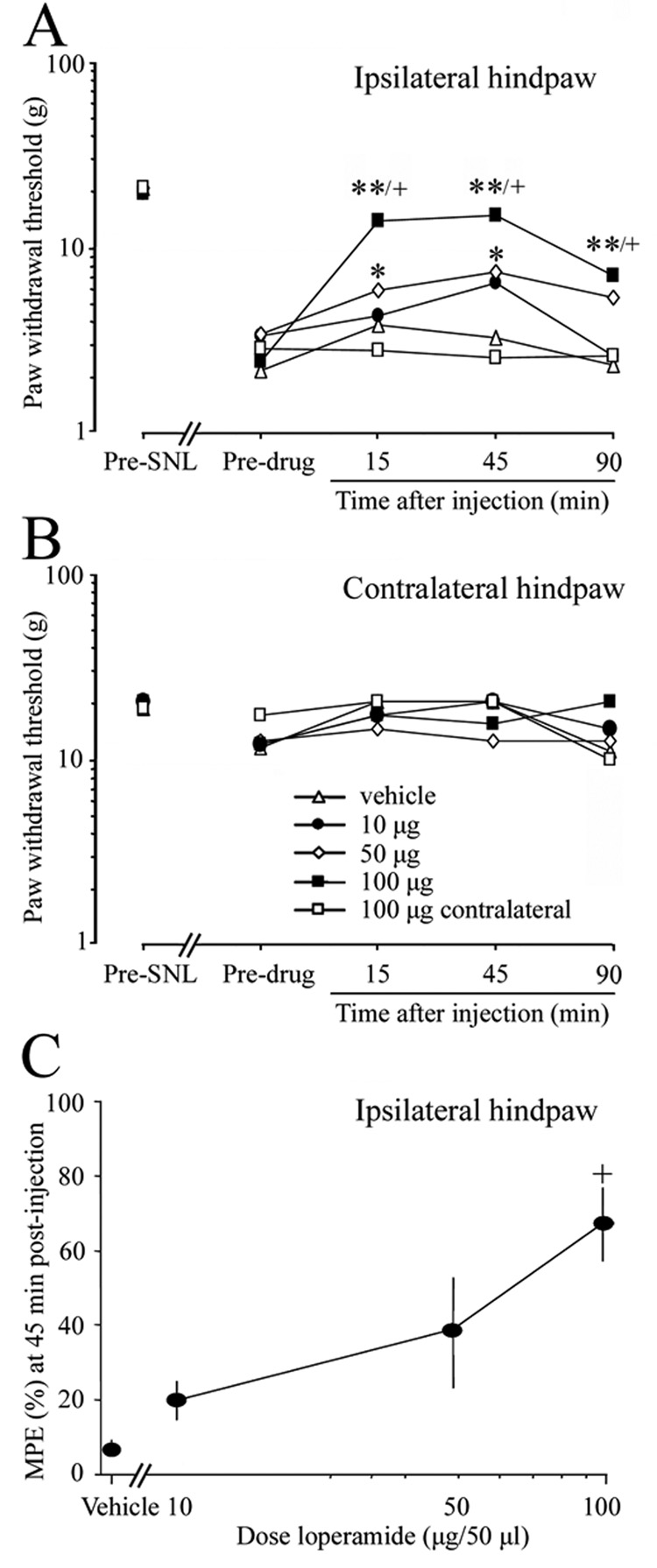
On day 7 post-SNL, i.pl. injection of loperamide into the ipsilateral hindpaw dose-dependently reversed the mechanical allodynia (Fig. 5A). The PWTs were significantly higher than baseline at 15 and 45 min after drug treatment at doses of 50 µg/50 µl and 100 µg/50 µl. Furthermore, PWTs at 15, 45, and 90 min after injection of 100 µg/50 µl loperamide were significantly higher than those of the vehicle-treated group. MPE calculated at 45 min post-loperamide (100 µg/50 µl) injection was significantly higher than that of the vehicle-treated group (Fig. 5C). In contrast, contralateral i.pl. injection of loperamide (100 µg/50 µl) did not reverse mechanical allodynia (open square, Fig. 5A). There were no significant changes in the PWT of the contralateral hindpaw after i.pl. injection of loperamide into either hindpaw (Fig. 5B).
Fig. 5. Intraplantar administration of loperamide dose dependently reversed mechanical allodynia after L5 SNL.
(A) Different groups of rats were given intraplantar injections of vehicle (n = 8) or loperamide (10 µg, n = 6; 50 µg, n = 6; 100 µg, n = 10) in a volume of 50 µl on day 7 post-SNL. Loperamide dose dependently reversed the mechanical allodynia when injected into the ipsilateral but not the contralateral (100 µg, n = 10) hindpaw. (B) No significant changes in the PWT of the contralateral hindpaw were observed in any group. (C) MPE (%) for ipsilateral intraplantar injection of loperamide to attenuate mechanical allodynia was calculated at 45 min post-injection, and dose-response function was established. PWT data are presented as medians, and MPE data are presented as means ± SEM. *P < 0.05, **P < 0.01 versus the respective pre-drug baseline; +P < 0.05 compared with the vehicle-treated group.
3.6. The anti-allodynic effect of i.pl. loperamide did not change significantly at different time points after SNL
In a repetitive-drug treatment study, PWTs at 15 and 45 min after ipsilateral hindpaw injection of loperamide (100 µg/50 µl, i.pl.) were significantly higher than pre-drug baseline values on days 14, 28, 42, and 56 post-SNL (Fig. 6A). The day 7 post-SNL group from a previous single-drug treatment study was included for comparison. Although the MPE on day 14 post-SNL was lower than that on day 7 post-SNL, the difference was not statistically significant (P > 0.05, Fig. 6C). There were no significant changes in the PWT of the contralateral hindpaw after ipsilateral i.pl. loperamide injection at any post-SNL time point (Fig. 6B). In addition, vehicle injection did not affect PWT (data not shown).
Fig. 6. The anti-allodynic effect of intraplantar loperamide did not change significantly at different time points after L5 SNL.
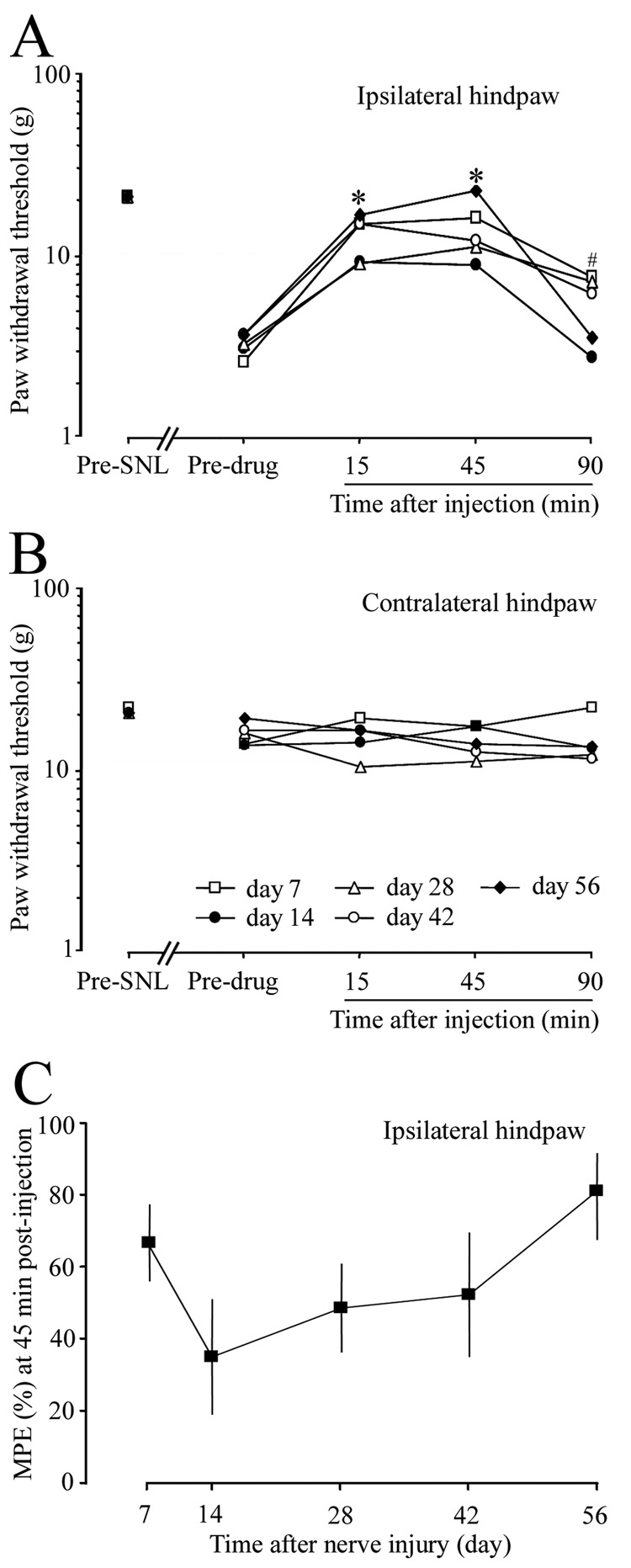
(A) In a repetitive-drug treatment study, loperamide (100 µg/50 µl, n = 6) or vehicle (n = 4) was injected into the ipsilateral hindpaw at days 14, 28, 42, and 56 post-SNL. The day 7 post-SNL group (n = 10) from the single-drug treatment study was included for comparison. On days 7 to 56 post-SNL, mechanical allodynia was significantly attenuated at 15 and 45 min after loperamide administration. * (days 7, 14, 28, 42, 56 at 15 and 45 minutes after drug injection), # (day 7 at 90 minutes after drug injection), P < 0.05 from respective pre-drug baseline. (B) PWT on the contralateral side did not change significantly after loperamide injection. (C) The MPE values on days 14, 28, 42, and 56 post-SNL were not significantly different from that on day 7 post-SNL. PWT data are presented as medians, and MPE data are presented as means ± SEM.
4. Discussion
In addition to an increased central neuronal excitability, peripheral mechanisms are also important to the manifestation of neuropathic pain [5,14,20,28,53,54]. In our study, mechanical allodynia was dose dependently reversed by systemic or local administration of loperamide on day 7 post-SNL. Importantly, loperamide-induced anti-allodynic effects persisted until day 56 post-SNL, suggesting that loperamide is an effective post-injury treatment in the early development and later maintenance phases of neuropathic pain. In line with previous observations done at day 13-15 post-SNL [46], systemic loperamide reversed heat hyperalgesia also at day 7 post-SNL in our study, suggesting that it can attenuate both mechanical and thermal hypersensitivity in neuropathic conditions.
The reduction in allodynia induced by systemic loperamide was blocked by methyl-naltrexone pre-treatment, a peripheral restricted MOR-preferring antagonist. Neither loperamide nor methyl-naltrexone at the doses examined penetrates the CNS in rodents [2,44,56,57], suggesting a predominantly peripheral opioidergic mechanism for systemic loperamide activity. Although spinal nerve lesion might increase local blood-spinal cord barrier permeability [16], there was no evidence that either drug gained access to the CNS after SNL. Importantly, both 1.5 mg/kg (Fig. 1C) and 3.0 mg/kg (unpublished observation) doses of loperamide reversed heat hyperalgesia of the ipsilateral hindpaw without affecting the PWL of the contralateral hindpaw. In naïve rat, systemic loperamide (3.0 mg/kg) did not change the PWL (unpublished observation). Since anti-nociceptive effects of opiates are believed to be mediated by central opioid receptors, the lack of anti-nociceptive effect further supports a peripheral origin of systemic loperamide-induced anti-allodynia after SNL. These findings also suggest that systemic loperamide did not impair motor functions. Because loperamide normalized the exaggerated pain responses toward baseline levels without altering normal nociceptive function, it may represent a valuable peripherally-acting anti-hyperaglesic/anti-allodynic agent for neuropathic pain. We are aware that loperamide may share gastro-intestinal side effects common to all opioids. However, these gastrointestinal side effects are more likely associated with higher doses and/or long term use, and may be minimized by use of appropriate doses of an orally administered low bioavailability peripheral opioid antagonist, or by topical administration.
Intraperitoneal injection of loperamide significantly attenuated allodynia only at 30 min, but not at 60 min post-administration on day 14 post-SNL, similar to a previous observation [46]. The short-lived anti-allodynic effect after i.p. administration may reflect a more prominent first-pass effect and a higher drug clearance/elimination rate than that of a s.c. route. The discrepancy in the anti-allodynic potency of systemic loperamide between the previous study and the current one may also result from differences in post-injury time points for testing. As shown in our study, systemic loperamide-induced anti-allodynia was substantially lower on day 14 post-SNL than on day 7. Therefore, both drug route and post-injury time point are important factors for evaluating the potency of opioids in alleviating neuropathic pain.
Identifying the primary site of action and opioid receptor subtype involved in the anti-allodynic effect of loperamide is essential for future development of peripherally selective opioid therapies. Potential sites of action for systemic loperamide include cutaneous terminals of non-injured nerves, course of peripheral nerve, nerve injury site, and soma of DRG neurons where opioid receptors are located [8,22,49]. In line with previous observations in a CCI model of neuropathic pain [36], direct intra-plantar administration of loperamide dose-dependently attenuated the mechanical allodynia on day 7 post-SNL. Importantly, i.pl. loperamide achieved significant anti-allodynic effect through the full time-course of neuropathic pain, suggesting that local tissue is an attractive target for opioidergic intervention after nerve injury. Although the 100-µg dose of loperamide was effective when given locally, it was ineffective when given systemically: neither contralateral hindpaw injection (100 µg) nor s.c. injection (0.3 mg/kg) attenuated mechanical allodynia. Therefore, loperamide does not require systemic circulation to exert its anti-allodynic effect after SNL. Methyl-naltrexone and CTAP injected i.pl. into the ipsilateral hindpaw each blocked systemic loperamide-induced allodynia reduction. The antagonistic effect of CTAP did not result from access to the systemic circulation, as CTAP administrated into the contralateral hindpaw did not block the effect of loperamide. In contrast to CTAP, naltrindole hydrochloride, at a dose that blocks DOR activation by a local DOR agonist [24], did not block the systemic anti-allodynic effect of loperamide. These findings suggest that local MORs (most likely those located on the terminals of uninjured primary afferent nociceptive neurons innervating the hindpaw) are an essential target for loperamide in alleviating mechanical allodynia. However, activation of other opioid receptor subtypes, particularly DOR, may also contribute to loperamide’s alleviation of thermal hypersensitivity after nerve injury [46]. Since the effective dose for i.pl. loperamide was below that of the systemic route, dose-limiting peripheral side effects known to opioids may also be limited by local administration.
Neuropathic pain often has been reported to be opioid resistant, and in fact, neither systemic nor intrathecal opioid administration have been effective in alleviating neuropathic pain behavior in animal models [3,27,31,37,40]. However, most previous studies have examined drug effects on neuropathic pain only at one post-injury time, usually around 2 weeks [3,27,33,37]. Instead, we examined the anti-allodynic effect of loperamide through the full time-course of neuropathic pain. We found a post-injury time-dependent U-shape-like change in the anti-allodynic potency of systemic loperamide during the progress of neuropathic pain, with a temporary decrease at day 14 post-SNL, compared to that on day 7 post-SNL. However, systemic loperamide’s anti-allodynic potency recovered at later time points. Although not statistically significant, a similar U-shape-like change in the anti-allodynic potency was also observed with intra-plantar loperamide administration after SNL. Furthermore, in a repetitive-drug treatment study in which data for different time points were derived from a single cohort of rats (unpublished observation), anti-allodynic potency of systemic loperamide (1.5 mg/kg) was also significantly reduced at 14 days post-SNL, as compared to that at 7 day post-SNL. Together, these findings demonstrate a time-dependent change in anti-allodynic potency of loperamide after L5 SNL. Accordingly, we suggest that pre-clinical studies of neuropathic pain-alleviating drug candidates should be carried out at multiple post-injury time points. It is not known whether L5 spinal nerve axotomy might decrease MOR levels or down-regulate its function in uninjured L4/L6 DRG neurons, particularly on the peripheral terminals, which might contribute to the reduced peripheral opioid analgesic potency at day 14 post-SNL [41]. The mechanisms that underlie the recovery of loperamide’s anti-allodynic potency are also unclear, but a compensatory mechanism in the peripheral opioidergic system at the later phase of neuropathic pain is likely involved. For example, it has been reported that spinal MOR levels decreased on day 14, but returned to their normal levels by day 31 after peripheral nerve axotomy [9]. Future studies are needed to examine whether a time-dependent reorganization of peripheral opioid receptor expression in the DRG neurons, an increased centrifugal axonal trafficking of MOR to peripheral terminals of intact afferents, and/or upregulation of opioid receptor functions might contribute to the recovery of loperamide’s anti-allodynic potency.
The development of spontaneous activity and peripheral sensitization in neighboring uninjured nociceptive afferent neurons might represent an important mechanism underlying neuropathic pain [45,53,54]. Mechanical allodynia was attenuated by ipsilateral i.pl. loperamide, suggesting a role for uninjured afferents in the maintenance of neuropathic pain after SNL. Although mechanisms underlying loperamide-induced allodynia reduction after SNL remain to be established, they likely involve activation of MOR, which might modulate activity of voltage-gated and ligand-gated ion channels to prevent abnormal spontaneous activity, reduce the excitability and stimulus-evoked noxious afferent inputs carried by uninjured nociceptive afferents, and decrease peripheral sensitization after nerve injury [1,7,13,38]. Loperamide can also exert non-opioid actions, including direct inhibition of L-type calcium channels, functional interaction with N-methyl- d-aspartate receptors, and inhibition of hyperpolarization-activated current after being applied directly to the cell body of DRG neurons [6,7,18,32,42,43,51,55]. However, our findings that systemic loperamide-induced allodynia reduction was nearly completely blocked by local pretreatment with either methyl-naltrexone or CTAP are consistent with agonist activity at local opioid receptors. In addition to neuronal mechanisms, peripheral immune mechanisms may also contribute to neuropathic pain [29,48]. Although the inflammatory reaction in the SNL model of neuropathy is generally less evident than that of the CCI model, especially in the distal part of the affected limb, we do not exclude the possibility that the anti-allodynic actions of loperamide might involve activation of opioid receptors on immune cells. Repeated opioid administration in rodents often induces tolerance to its analgesic effect. A systematic investigation of potential tolerance to repetitive/chronic use of loperamide in neuropathic pain conditions is warranted in future studies to further establish the clinical usefulness of this strategy.
Central opioid analgesia is complicated by undesirable side effects and a diminished analgesic potency after nerve injury [3,26,27], but here we report that loperamide is effective at alleviating neuropathic pain by targeting peripheral MORs. Therefore, attacking pain at its source with loperamide may represent a valid therapeutic strategy for alleviating certain neuropathic pain conditions with the following benefits: a) an absence of the major undesirable CNS side effects known to opioids; b) no potential for addiction and drug abuse; and c) long established drug safety profile.
Acknowledgements
This study was supported by grants from the NIH (NS26363, NS14447) and the Johns Hopkins Blaustein Pain Research Fund. The authors wish to thank Claire Levine, MS, for editing the manuscript and Jasenka Borzan, Ph.D for her advice on statistical analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Andreev N, Urban L, Dray A. Opioids suppress spontaneous activity of polymodal nociceptors in rat paw skin induced by ultraviolet irradiation. Neuroscience. 1994;58:793–798. doi: 10.1016/0306-4522(94)90456-1. [DOI] [PubMed] [Google Scholar]
- 2.Baker AK, Meert TF. Functional effects of systemically administered agonists andantagonists of mu, delta, and kappa opioid receptor subtypes on body temperature in mice. J Pharmacol Exp Ther. 2002;302:1253–1264. doi: 10.1124/jpet.102.037655. [DOI] [PubMed] [Google Scholar]
- 3.Bian D, Nichols ML, Ossipov MH, Lai J, Porreca F. Characterization of the antiallodynic efficacy of morphine in a model of neuropathic pain in rats. Neuroreport. 1995;6:1981–1984. doi: 10.1097/00001756-199510010-00007. [DOI] [PubMed] [Google Scholar]
- 4.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Meth. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 5.Chung JM, Chung K. Importance of hyperexcitability of DRG neurons in neuropathic pain. Pain Pract. 2002;2:87–97. doi: 10.1046/j.1533-2500.2002.02011.x. [DOI] [PubMed] [Google Scholar]
- 6.Church J, Fletcher EJ, Abdel-Hamid K, MacDonald JF. Loperamide blocks high-voltageactivated calcium channels and N-methyl-D-aspartateevoked responses in rat and mouse cultured hippocampal pyramidal neurons. Mol Pharmacol. 1994;45:747–757. [PubMed] [Google Scholar]
- 7.Christensen D, Idänpään-Heikkilä JJ, Guilbaud G, Kayser V. The antinociceptive effect of combined systemic administration of morphine and the glycine/NMDA receptor antagonist, (+)-HA966 in a rat model of peripheral neuropathy. Br J Pharmacol. 1998;125:1641–1650. doi: 10.1038/sj.bjp.0702240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coggeshall RE, Zhou S, Carlton SM. Opioid receptors on peripheral sensory axons. Brain Res. 1997;764:126–132. doi: 10.1016/s0006-8993(97)00446-0. [DOI] [PubMed] [Google Scholar]
- 9.deGroot JF, Coggeshall RE, Carlton SM. The reorganization of mu opioid receptors in the rat dorsal horn following peripheral axotomy. Neurosci Lett. 1997;233:113–116. doi: 10.1016/s0304-3940(97)00642-3. [DOI] [PubMed] [Google Scholar]
- 10.DeHaven-Hudkins DL , Burgos LC, Cassel JA, Daubert JD, DeHaven RN, Mansson E, Nagasaka H, Yu G, Yaksh T. Loperamide (ADL 2-1294), an opioid antihyperalgesic agent with peripheral selectivity. J Pharmacol Exp Ther. 1999;289:494–502. [PubMed] [Google Scholar]
- 11.Dionne RA, Lepinski AM, Gordon SM, Jaber L, Brahim JS, Hargreaves KM. Analgesic effects of peripherally administered opioids in clinical models of acute and chronic inflammation. Clin Pharmacol Ther. 2001;70:66–73. doi: 10.1067/mcp.2001.116443. [DOI] [PubMed] [Google Scholar]
- 12.Dixon WJ. Efficient Analysis of Experimental Observations. Ann Rev Pharmacol Toxicol. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- 13.Endres-Becker J, Heppenstall PA, Mousa SA, Labuz D, Oksche A, Schäfer M, Stein C, Zöllner C. Mu-opioid receptor activation modulates transient receptor potential vanilloid 1 (TRPV1) currents in sensory neurons in a model of inflammatory pain. Mol Pharmacol. 2007;71:12–18. doi: 10.1124/mol.106.026740. [DOI] [PubMed] [Google Scholar]
- 14.Fukuoka T, Tokunaga A, Tachibana T, Dai Y, Yamanaka H, Noguchi K. VR1, but not P2X(3), increases in the spared L4 DRG in rats with L5 spinal nerve ligation. Pain. 2002;99:111–120. doi: 10.1016/s0304-3959(02)00067-2. [DOI] [PubMed] [Google Scholar]
- 15.Gaveriaux-Ruff C, Kieffer BL. Exploring the opioid system by gene knockout. Prog Neurobiol. 2002;66:285–306. doi: 10.1016/s0301-0082(02)00008-4. [DOI] [PubMed] [Google Scholar]
- 16.Gordh T, Chu H, Sharma HS. Spinal nerve lesion alters blood-spinal cord barrier function and activates astrocytes in the rat. Pain. 2006;124:211–221. doi: 10.1016/j.pain.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 17.Hassan AH, Ableitner A, Stein C, Herz A. Inflammation of the rat paw enhances axonal transport of opioid receptors in the sciatic nerve and increases their density in the inflamed tisse. Neuroscience. 1993;55:185–195. doi: 10.1016/0306-4522(93)90465-r. [DOI] [PubMed] [Google Scholar]
- 18.Hagiwara K, Nakagawasai O, Murata A, Yamadera F, Miyoshi I, Tan-No K, Tadano T, Yanagisawa T, Iijima T, Murakami M. Analgesic action of loperamide, an opioid agonist, and its blocking action on voltage-dependent Ca2+ channels. Neurosci Res. 2003;46:493–497. doi: 10.1016/s0168-0102(03)00126-3. [DOI] [PubMed] [Google Scholar]
- 19.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 20.Ibrahim MM, Porreca F, Lai J, Albrecht PJ, Rice FL, Khodorova A, Davar G, Makriyannis A, Vanderah TW, Mata HP, Malan TP. CB2 cannabinoid receptor activation produces antinociception by stimulating peripheral release of endogenous opioids. Proc Natl Acad Sci. 2005;102:3093–3098. doi: 10.1073/pnas.0409888102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jang J, Yaksh TL, Hill HF. Use of 2-hydroxypropyl-beta-cyclodextrin as an intrathecal drug vehicle with opioids. J Pharmacol Exp Ther. 1992;261:592–600. [PubMed] [Google Scholar]
- 22.Ji RR, Zhang Q, Law PY, Low HH, Elde R, Hokfelt T. Expression of mu-, delta-, and kappa-opioid receptor-like immunoreactivities in rat dorsal root ganglia after carrageenan-induced inflammation. J Neurosci. 1995;15:8156–8166. doi: 10.1523/JNEUROSCI.15-12-08156.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Junger H, Moore AC, Sorkin LS. Effects of full-thickness burns on nociceptor sensitization in anesthetized rats. Burns. 2002;28:772–777. doi: 10.1016/s0305-4179(02)00199-7. [DOI] [PubMed] [Google Scholar]
- 24.Kabli N, Cahill CM. Anti-allodynic effects of peripheral delta opioid receptors in neuropathic pain. Pain. 2007;127:84–93. doi: 10.1016/j.pain.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- 26.Kohno T, Ji RR, Ito N, Allchorne AJ, Befort K, Karchewski LA, Woolf CJ. Peripheral axonal injury results in reduced m opioid receptor pre- and post-synaptic action in the spinal cord. Pain. 2005;117:77–87. doi: 10.1016/j.pain.2005.05.035. [DOI] [PubMed] [Google Scholar]
- 27.Lee YW, Chaplan SR, Yaksh TL. Systemic and supraspinal, but not spinal, opiates suppress allodynia in a rat neuropathic pain model. Neurosci Lett. 1995;199:111–114. doi: 10.1016/0304-3940(95)12034-2. [DOI] [PubMed] [Google Scholar]
- 28.Ma C, Shu Y, Zheng Z, Chen Y, Yao H, Greenquist KW, White FA, LaMotte RH. Similar electrophysiological changes in axotomized and neighboring intact dorsal root ganglion neurons. J Neurophysiol. 2003;89:1588–1602. doi: 10.1152/jn.00855.2002. [DOI] [PubMed] [Google Scholar]
- 29.Machelska H, Stein C. Immune mechanisms in pain control. Anesth Anlg. 2002;95:1002–1008. doi: 10.1097/00000539-200210000-00039. [DOI] [PubMed] [Google Scholar]
- 30.MacPherson New directions in pain management. Drugs Today. 2002;38:135–145. doi: 10.1358/dot.2002.38.2.668325. [DOI] [PubMed] [Google Scholar]
- 31.Mao J, Price DD, Mayer DJ. Experimental mononeuropathy reduces the antinociceptive effects of morphine: implications for common intracellular mechanisms involved in morphine tolerance and neuropathic pain. Pain. 1995;61:353–364. doi: 10.1016/0304-3959(95)00022-K. [DOI] [PubMed] [Google Scholar]
- 32.Martinez V, Christensen D, Kayser V. The glycine/NMDA receptor antagonist (+)-HA966 enhances the peripheral effect of morphine in neuropathic rats. Pain. 2002;99:537–545. doi: 10.1016/S0304-3959(02)00270-1. [DOI] [PubMed] [Google Scholar]
- 33.Mazzuca M, Heurteaux C, Alloui A, Diochot S, Baron A, Voilley N, Blondeau N, Escoubas P, Gelot A, Cupo A, Zimmer A, Zimmer AM, Eschalier A, Lazdunski M. A tarantula peptide against pain via ASIC1a channels and opioid mechanisms. Nat Neurosci. 2007 doi: 10.1038/nn1940. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 34.Nozaki-Taguchi N, Yaksh TL. Characterization of the antihyperalgesic action of a novel peripheral mu-opioid receptor agonist—loperamide. Anesthesiology. 1999;90:225–234. doi: 10.1097/00000542-199901000-00029. [DOI] [PubMed] [Google Scholar]
- 35.Obara I, Makuch W, Spetea M, Schütz J, Schmidhammer H, Przewlocki R, Przewlocka B. Local peripheral antinociceptive effects of 14-O-methyloxymorphone derivatives in inflammatory and neuropathic pain in the rat. Eur J Pharmacol. 2007;558:60–67. doi: 10.1016/j.ejphar.2006.11.037. [DOI] [PubMed] [Google Scholar]
- 36.Obara I, Przewlocki R, Przewlocka B. Local peripheral effects of mu-opioid receptor agonists in neuropathic pain in rats. Neurosci Lett. 2004;360:85–89. doi: 10.1016/j.neulet.2004.01.056. [DOI] [PubMed] [Google Scholar]
- 37.Ossipov MH, Lopez Y, Nichols ML, Bian D, Porreca F. The loss of antinociceptive efficacy of spinal morphine in rats with nerve ligation injury is prevented by reducing spinal afferent drive. Neurosci Lett. 1995;199:87–90. doi: 10.1016/0304-3940(95)12022-v. [DOI] [PubMed] [Google Scholar]
- 38.Pertovaara A, Wei H. Peripheral effects of morphine in neuropathic rats: role of sympathetic postganglionic nerve fibers. Eur J Pharmacol. 2001;429:139–145. doi: 10.1016/s0014-2999(01)01315-2. [DOI] [PubMed] [Google Scholar]
- 39.Porreca F, Mosberg HI, Hurst R, Hruby VJ, Burks TF. Roles of mu, delta and kappa opioid receptors in spinal and supraspinal mediation of gastrointestinal transit effects and hot-plate analgesia in the mouse. J Pharmacol Exp Ther. 1984;230:341–348. [PubMed] [Google Scholar]
- 40.Przewlocki R, Przewlocka B. Opioids in chronic pain. Eur J Pharmacol. 2001;429:79–91. doi: 10.1016/s0014-2999(01)01308-5. [DOI] [PubMed] [Google Scholar]
- 41.Rashid MH, Inoue M, Toda K, Ueda H. Loss of peripheral morphine analgesia contributes to the reduced effectiveness of systemic morphine in neuropathic pain. J Pharmacol Exp Ther. 2004;309:380–387. doi: 10.1124/jpet.103.060582. [DOI] [PubMed] [Google Scholar]
- 42.Reynolds IJ, Gould RJ, Snyder SH. Loperamide: blockade of calcium channels as a mechanism for antidiarrheal effects. J Pharmacol Exp Ther. 1984;231:628–632. [PubMed] [Google Scholar]
- 43.Sevostianova N, Danysz W, Bespalov AY. Analgesic effects of morphine and loperamide in the rat formalin test: interactions with NMDA receptor antagonists. Eur J Pharmacol. 2005;525:83–90. doi: 10.1016/j.ejphar.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 44.Shannon HE, Lutz EA. Comparison of the peripheral and central effects of the opioid agonists loperamide and morphine in the formalin test in rats. Neuropharmacology. 2002;42:253–261. doi: 10.1016/s0028-3908(01)00173-3. [DOI] [PubMed] [Google Scholar]
- 45.Shim B, Kim DW, Kim BH, Nam TS, Leem JW, Chung JM. Mechanical and heat sensitization of cutaneous nociceptors in rats with experimental peripheral neuropathy. Neuroscience. 2005;132:193–201. doi: 10.1016/j.neuroscience.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 46.Shinoda K, Hruby VJ, Porreca F. Antihyperalgesic effects of loperamide in a model of rat neuropathic pain are mediated by peripheral delta-opioid receptors. Neuroscience Letter. 2007;411:143–146. doi: 10.1016/j.neulet.2006.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stein C, Schafer M, Machelska H. Attacking pain at its source: new perspectives on opioids. Nat.Med. 2003;9:1003–1008. doi: 10.1038/nm908. [DOI] [PubMed] [Google Scholar]
- 48.Stein C. Neuro-immune interactions in pain. Crit Care Med. 1993;21:S357–S358. doi: 10.1097/00003246-199309001-00034. [DOI] [PubMed] [Google Scholar]
- 49.Stein C, Millan MJ, Shippenberg TS, Peter K, Herz A. Peripheral opioid receptors mediating antinociception in inflammation. Evidence for involvement of mu, delta and kappa receptors. Pharmacol Exp Ther. 1989;248:1269–1275. [PubMed] [Google Scholar]
- 50.Truong W, Cheng C, Xu QG, Li XQ, Zochodne DW. Mu opioid receptors and analgesia at the site of a peripheral nerve injury. Ann Neurol. 2003;53:366–375. doi: 10.1002/ana.10465. [DOI] [PubMed] [Google Scholar]
- 51.Vasilyev DV, Shan Q, Lee Y, Mayer SC, Bowlby MR, Strassle BW, Kaftan EJ, Rogers KE, Dunlop J. Direct inhibition of Ih by analgesic loperamide in rat DRG neurons. J. Neurophysiol. 2007;97:3713–3721. doi: 10.1152/jn.00841.2006. [DOI] [PubMed] [Google Scholar]
- 52.Wandel C, Kim R, Wood M, Wood A. Interaction of morphine, fentanyl, sufentanil, alfentanil, and loperamide with the efflux drug transporter P-glycoprotein. Anesthesiology. 2002;96:913–920. doi: 10.1097/00000542-200204000-00019. [DOI] [PubMed] [Google Scholar]
- 53.Wu G, Ringkamp M, Hartke TV, Murinson BB, Campbell JN, Griffin JW, Meyer RA. Early onset of spontaneous activity in uninjured C-fiber nociceptors after injury to neighboring nerve fibers. J Neurosci. 2001;21:RC140. doi: 10.1523/JNEUROSCI.21-08-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu G, Ringkamp M, Murinson BB, Pogatzki EM, Hartke TV, Weerahandi HM, Campbell JN, Griffin JW, Meyer RA. Degeneration of myelinated efferent fibers induces spontaneous activity in uninjured C-fiber afferents. J Neurosci. 2002;22:7746–7753. doi: 10.1523/JNEUROSCI.22-17-07746.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang SB, Major F, Tietze LF, Rupnik M. Block of delayed-rectifier potassium channels by reduced haloperidol and related compounds in mouse cortical neurons. J Pharmacol Exp Ther. 2005;315:352–362. doi: 10.1124/jpet.105.086561. [DOI] [PubMed] [Google Scholar]
- 56.Yuan CS, Israel RJ. Methylnaltrexone, a novel peripheral opioid receptor antagonist for the treatment of opioid side effects. Expert Opin Investig Drugs. 2006;15:541–552. doi: 10.1517/13543784.15.5.541. [DOI] [PubMed] [Google Scholar]
- 57.Yuan CS, Foss JF, O'Connor M, Osinski J, Roizen MF, Moss J. Effects of intravenous methylnaltrexone on opioid-induced gut motility and transit time changes in subjects receiving chronic methadone therapy: a pilot study. Pain. 1999;83:631–635. doi: 10.1016/S0304-3959(99)00162-1. [DOI] [PubMed] [Google Scholar]