Abstract
The aim of this review is to present age-related changes in the bone marrow and thymus and their effects in later life. Age-related hematologic changes are marked by a decline in marrow cellularity, increased risk of myeloproliferative disorders and anemia, and a decline in adaptive immunity. The exact mechanisms that produce these changes remain undefined. For the most part, the changes in function that are a consequence of aging alone rarely have meaningful clinical consequences. However, in the face of the stresses induced by other illnesses, the decreased physiologic reserve can slow or prevent an appropriate response to the stressors.
Keywords: Aging, bone marrow, bone marrow ontogeny, thymic involution, blood cell changes with age, unexplained anemia, erythropoietin and aging, adaptive immunity
Aging is a universal phenomenon that affects all normal cells, tissues, organ systems and organisms. Accordingly, the bone marrow undergoes changes with age. Age-related hematologic changes are reflected by a decline in bone marrow cellularity, an increased risk of myeloproliferative diseases (1) and anemia (2,3), and a declining adaptive immunity (4–6).
The percentage of marrow space occupied by the hematopoietic tissue declines from 90% at birth to a level of approximately 50% at age 30 and 30% at age 70 (7, 8). A similar change occurs in the thymus, where involution begins at an earlier age and is reflected anatomically by a reduction in lymphoid mass with an increase in fat (9) and functionally by a steady decrease in the production of naïve T cells (10, 11). Fat infiltration into the bone marrow and thymus results in a diminished volume of hematopoietic tissue. As discussed elsewhere in this review, this in turn results in reduction in the number of naïve lymphocytes produced which may diminish adaptive immunity.
Although age-related change in the bone marrow is well described, the exact mechanisms that regulate these changes remains speculative. For example, it remains unclear whether the age-associated expansion of marrow fat is a cause or an effect of aging and whether the changes seen in bone marrow and thymus are intrinsically related to each other. Because of the intricate association of hematologic and immune functions and the common histological patterns of change with age, this review will consider the functional changes of aging in each of the two systems.
BONE MARROW ONTOGENY
Vertebrate hematopoiesis is a complex multitiered process, in which pluripotent stem cells differentiate into functionally distinct mature blood cells that have a limited lifespan but are constantly replenished. In fetal development the manufacture of blood occurs in various organs but after birth this function is subsumed by the bone marrow.
Fetal Hematopoiesis
It had long been believed that blood cell production originated within focal islands of the extraembryonic yolk sac within the first week after fertilization. Current evidence identifies early hematopoietic cells both in the extraembryonic yolk sac as well as an intra-embryonic site within the ventral wall of dorsal aorta-gonad-mesonephros (AGM) (12). At approximately 5–6 weeks of gestation, progenitors derived from the yolk sac colonize the fetal liver and become the major source for blood cells for the remainder of intrauterine life (13). At around 7 weeks of gestation, hematopoietic cells also colonize the spleen (13) and multipotent progenitor cells are seen in the fetal circulation by the end of 12 –14 weeks of gestation (14). Around 8–9 weeks while hematopoiesis within the yolk sac per se is becoming extinct, T-cell production starts in the thymus (15). T-cell precursors derived from the fetal liver colonize the thymus and undergo maturation and differentiation (16–18). Hematopoietic cells first appear in the medullary cavities of bone at around 14 weeks of gestation (19) and by birth the bone marrow has become the primary site of hematopoiesis.
Bone Marrow During Adult Life
The most apparent change seen in the bone marrow with aging is decreased cellularity (7). Under normal circumstances, the bone marrow is the only site of hematopoiesis. Extramedullary hematopoiesis may occur in the liver, spleen and lymph nodes in pathological states when the marrow compensatory mechanisms are outstripped. Until puberty the entire skeleton remains hematopoietically active but by age 18 only the vertebrae, ribs, sternum, skull, pelvis, proximal epiphyseal regions of humerus and femur remain active sites of blood production, with other medullary sites infiltrated with fatty tissue. By age 40, the marrow in sternum, ribs, pelvis and vertebrae is composed of equal amounts of hematopoietic tissue and fat and cellularity declines gradually thereafter. By age 65, bone marrow cellularity has been estimated to be approximately 30% (7, 8) with a corresponding increase in marrow fat. Age-associated imbalanced bone remodeling and osteoporosis results in decreased trabecular bone which itself may contribute to diminished hematopoiesis (20). It has been shown that the presence of fat correlates with the occurrence and severity of osteoporosis, both of which are evident with aging (21).
Growth hormone production declines with age and this, too, has been linked with deposition of fat within the bone marrow (22). Administration of growth hormone to old rats reduces marrow fat and increases hematopoietic tissue (23). Several age-related qualitative changes have been identified in hematopoietic cells including skewed X-chromosome inactivation, telomere shortening (24–26), accumulation of mitochondrial DNA mutations (27, 28) and micronuclei formation (29), any of which could result in cellular dysfunction. Despite the multitude of descriptive age-associated changes, most functional assays do not reveal changes of sufficient magnitude to be clinically important. A similar change happens in the thymus albeit at an earlier age. Once considered the lymphocyte graveyard without functional importance, we now appreciate the role of thymus in normal T-cell development; a concept first espoused in the early 1960s (30). The human thymus is comprised of two compartments, thymic epithelium where thymopoiesis occurs and a non-thymopoietic perivascular space (9). Thymic development occurs around 5–7 weeks of gestation and begins to decline relative to total body mass shortly after birth and decreases approximately 3% per year up to 45 years and continues to decrease by 1% per year throughout the rest of life. By 70 years of age, thymic mass is to less than 10% of its peak. The lymphoid component in the perivascular space of the thymus decreases in size after age 20–25 years but the total perivascular space increases soon after birth by increased deposition of adipose tissue (9, 11, 31, 32).
Thus, age-related changes in both bone marrow and thymus seem related to development and involution of hematopoietic and immune function.
Bone Marrow Function
All blood cells are derived from marrow pluripotent stem cells which comprise 10% of the cellular fraction of cord blood but less than 1% of all adult bone marrow. Hematopoietic stem cells have a unique ability to self renew, proliferate and differentiate into every lineage of mature blood cells. Hematopoietic stem cells then give rise to two distinct multipotent stem cells within the bone marrow. Multipotential myeloid progenitor cells are precursors of granulocytes, monocytes, erythrocytes and platelets and lymphoid stem cells are precursors of lymphocytes and plasma cells. There is always a large pool of maturing progenitors for each lineage within the bone marrow allowing for rapid recruitment and release of cells in times of stress. The factors responsible for the constant turnover of these mature cells both inside the bone marrow and the peripheral blood are poorly understood. However, there is evidence to support a role for both cell-intrinsic genetic programs and several hematopoietic growth factors within the bone marrow microenvironment in the regulation of hematopoiesis. Though a number of measureable changes occur in the stem cell compartment with aging, these changes do not compromise hematopoiesis in the absence of disease. Even when bone marrow is donated from a 65-year-old person to an HLA-matched younger recipient, the transferred marrow supports hematopoiesis for the life of the recipient, although allogeneic marrow from older donors has a greater chance of being associated with graft vs. host disease (33).
Bone marrow also serves as one of the organs of the immune system. B lymphocytes (B-cell) maturation process begins within the bone marrow where precursor cells acqure surface immunoglobulin. In contrast precursor T-cells must travel to the thymus to complete their programmed maturation. Through a remarkable process referred to as thymic education, subsets of T cells, identified by various cell surface proteins are selected, whereas others are eliminated. This process shapes a T cell repertoire that is able to distinguish between self and foreign invaders and prepares the host for defending itself against the world of pathogens. The mature T-cells are then released to the blood stream to populate the secondary lymphoid organs. There is some evidence suggesting bone marrow-derived T-cell precursors are reduced in number with advancing age (6).
Peripheral Blood Cell Changes with Age
Red Blood Cells
Anemia is a significant health problem in the elderly due both to a high prevalence and significant associated morbidity including reduced quality of life, clinical depression, falls functional impairment, slower walking speed, reduced grip strength, loss of mobility, worsening comorbidities, and mortality (34, 35).
The World Health Organization (WHO) defines anemia as a hemoglobin level of less than 13 g/dL in adult males and less than 12 g/dL in adult females (36). In older men and women, anemia by this definition is associated with an increase in mortality (37–42). It has been pointed out the WHO criteria do not take into account inherent ethnic variations particularly with respect to African Americans who may have lower levels of hemoglobin without significant adverse outcomes (43, 44). In a study that analyzed 1018 black and 1583 white adults aged 71 to 82 years, anemia defined by the WHO criteria was associated with increased mortality in whites but not blacks (43, 44). The reasons for these ethnic differences are undefined. The issue of establishing criteria for the diagnosis of anemia is relevant in the context of age, as well. Older women, for example, have better physical performance and function at hemoglobin values between 13–15 g/dL than at 12–12.9 g/dL (45), suggesting perhaps that cut off level of 12 g/dL is too low. Nevertheless, the WHO definition remains the standard utilized in most current epidemiological surveys and many clinical laboratories.
Prevalence of anemia
Guralnik and colleagues examined the third National Health and Nutrition Examination Survey (NHANES III) database, a nationally representative sample of community-dwelling persons and determined age- and sex-specific prevalence rates of anemia in the total US population (4). For those over the age of 65 years, by WHO criteria, approximately 11% were anemic (Table 1). The prevalence of anemia was lowest (1.5%) among males between 17–49 years of age and highest (26.1%) in males over 85 years. Among those 65 years and older, the prevalence rate was notably higher in African Americans as compared to whites and Hispanics. Prevalence rates of anemia in the elderly vary in community dwelling and institutionalized populations. It is also quite clear that anemia is more common among frail elderly. In the nursing home, for example, anemia prevalence approaches 50% or higher (46–49).
Table 1.
Anemia Prevalence in the Elderly using the WHO Criteria
| Study | Age | Population | Prevalence |
|---|---|---|---|
| Guralnik, 2004(3) | ≥ 65 years | Community-dwelling elderly American | 10.6% |
| Ferrucci, 2007(93) | ≥ 70 years | Community-dwelling elderly Italian | 11% |
| Denny, 2006 (94) | ≥ 71 years | Community-dwelling | 24% |
| Joosten, 1992(95) | ≥ 65 years | Hospitalized | 24% (defined as Hb < 11.5 g/dL) |
| Artz, 2004 (46) | Most ≥ 65 years | Nursing-home | 48% |
| Robinson, 2007 (49) | ≥ 65 years | Nursing-home | 59.6% |
Unexplained Anemia
Hematologists are usually successful in uncovering the cause of anemia in young adults. However, this is often not as easy in geriatric populations. Approximately one third of older adults with anemia do not have an obviously discernible cause upon routine evaluation (Table 2). Typically, this anemia is mild (hemoglobin concentration in the 10 −12 g/dL range), normocytic, and hypoproliferative (low reticulocyte count). It has been postulated that the cause relates to a number of factors including declining testosterone level (50), occult inflammation (51), reduced hematopoietic reserve with advancing age (52), inappropriately low serum erythropoietin level (53), and occult myelodysplastic syndromes (54). For elderly patients with unexplained anemia, the presence of macrocytosis, thrombocytopenia, neutropenia, splenomegaly or unexplained constitutional symptoms of fever, chills, or weight loss, or symptoms of early satiety or bone pain should prompt consideration of a bone marrow examination.
Table 2.
| Executive Summary |
|---|
| Introduction |
|
| Bone Marrow Ontogeny |
|
| Bone marrow changes in adult life |
|
| Peripheral blood cell changes with aging |
|
| Clinical Consequences of Age-related Changes in Bone Marrow and Thymus |
|
Serum Erythropoietin and Aging
Data on erythropoietin levels in non-anemic older persons have been inconsistent. Some have suggested that non-anemic older persons have higher erythropoietin levels compared to younger adults (55–57), but other studies failed to confirm these findings (10–12). One longitudinal analysis clearly demonstrated that serum erythropoietin levels rose gradually in healthy individuals who maintained normal hemoglobin levels but the rise was not observed in those who were to develop diabetes or hypertension during the evaluation period (58). An explanation for the rise in serum erythropoietin with age has not been established, but in theory, it could be the result of age-associated shortened red cell survival or reduced sensitivity of erythroid progenitor cells to the erythropoietin signal. Studies in older subjects are ongoing to define the basis for the increasing need for erythropoietin to maintain normal levels of red cells.
White Blood Cells
Although no significant change is seen in the peripheral blood leukocyte count with aging (59, 60), several qualitative neutrophil defects have been described. For example, a decreased respiratory burst response to soluble signals (59), defective phagocytosis (60) and impaired neutrophil migration to sites of stress (61) have been described in accordance with age. Although the exact cause for these functional changes has not been clarified, it may be associated with an age-related alteration in actin cytoskeleton and receptor expression in leukocytes (62). There is a decrease in the peripheral lymphocyte count that is first noticeable in the fourth decade with a gradual progression thereafter throughout the remainder of the lifespan (63). Studies have also demonstrated qualitative alterations in T-lymphocyte function in the elderly (64), as discussed below.
Platelets
At present, knowledge about the influence of age on platelet counts has been limited to cross sectional data derived from selected populations. From that data it appears that there are no, or very limited changes in platelet number with age (65–68). To date, there has not been a longitudinal data set describing alterations in platelet number with advancing age nor conclusive studies describing age-associated changes in platelet function.
Clinical Consequences of Age-related Changes in Bone Marrow and Thymus
To the extent that bone marrow contains continuously repopulating cell lines, it is quite remarkable that changes attributable to aging alone are quite subtle but include a low grade `unexplained' anemia in a small subset of people and a more widely occurring mild immune deficiency. The latter results primarily from qualitative T-cell functional changes and most certainly relate to thymic involution. Cataloguing the myriad of previously described age-related changes is beyond the scope of this review but are detailed nicely elsewhere (69–72). The changes may explain an age-associated predisposition to certain infections (herpes zoster, tuberculosis reactivation) and perhaps a failure to mount a sufficient vaccine response (e.g., to influenza hemagglutinin (73–77)). The more profound immune deficiency commonly observed in older people most often reflects the debilitating effects of concurrent diseases, most of which occur more commonly with age.
Lymphoid progenitors undergo a wide array of changes across the lifespan. Thymus, which is the source for mature T lymphocytes, involutes steadily with increasing age and results in a decreased release of new naïve T cells to the periphery (78) affecting the adaptive immunity. T-cell numbers do not decline to a greater extent because peripheral T cells are capable of expanding to fill the T-cell niche in the absence of generation of new T cells. However, when they do so, the repertoire for antigen recognition becomes less comprehensive. Thymic involution may result due to the aging T-cell progenitor population (79), due to the defects in rearrangement of TCR β genes (80, 81), loss of self-peptide expressing thymic epithelium (82) and/or due to the loss of thymic trophic cytokines (83). Thymic epithelial cells produce a variety of colony stimulating factors and hematopoietic cytokines such as IL-1, IL-3, IL-6, IL-7, transforming growth factor (TGFβ), oncostatin M and leukemia inhibitory factor (LIF) (84–86) which influence the complex process of thymopoiesis. It is proposed that thymic atrophy and decreased thymopoiesis is an active process and mediated by the up-regulation of thymosuppressive cytokines (LIF, IL-6 and OSM) which results in the altered peripheral T lymphocyte function with aging (87).
There is a shift in the T-cell population towards memory T cells (88) and these memory cells attain replicative senescence in responding to repeated antigen exposures (89). With the decreasing numbers of naive T cells in the periphery and increasing memory T cells reaching senescence, elderly persons have difficulties responding to old and new antigens and demonstrate impaired reactions to vaccinations.
With age there is also a shift towards the T- helper 2 (TH2) subset (which supports antibody production) and away from TH1 (which supports cellular cytotoxicity) (90), thereby influencing cytokine production and overall immune response.
There are also age-associated morphological changes within the secondary lymphoid tissues of spleen (91), and lymph nodes (92) which show a decline in the paracortical and medullary zones and increased deposition of fat affecting the germinal centers. Although these changes may affect the immune response, it is unknown to what extent they are contributing factors to immunosenescence.
Summary
Normal aging is associated with anatomic and functional changes in both bone marrow and thymus. These organs, central to the core functions of blood and immunity are intricately linked to each other. That their patterns of development and involution share many features in common is not surprising, but the links have not been sufficiently explored from a gerontological perspective. Interventions aimed at reconstituting age-reduced hematologic or immune function are likely to have consequences in both systems. That stated, from a clinical perspective the changes in bone marrow and thymus function with age in the absence of disease are of only minor consequence. Anemia and immune deficiency are observed in late life in individuals without other recognizable disease, but these are typically mild and associated with minimal morbidity or mortality. However, the alterations are magnified in the presence of chronic or debilitating disease and are more likely then to result in meaningful clinical outcomes, including decline in performance status, predisposition to infection and mortality.
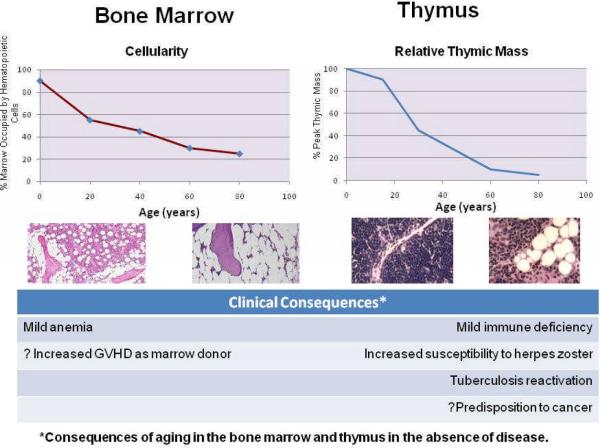
Figure 1.
Aging of bone marrow and thymus. Bone marrow cellularity declines from birth in a manner comparably to thymic mass. This is reflected histologically by the increased presence of fat. The clinical consequences of these age associated changes, in the absence of disease are a mild anemia and immune deficiency. The latter is reflected by an increased predisposition to certain infections (e.g., herpes zoster or reactivation of latent tuberculosis) and possibly to the increased predisposition to cancer.
References
- 1.Lichtman MA, Rowe JM. The relationship of patient age to the pathobiology of the clonal myeloid diseases. Semin Oncol. 2004;31(2):185–97. doi: 10.1053/j.seminoncol.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 2.Beghe C, Wilson A, Ershler WB. Prevalence and outcomes of anemia in geriatrics: a systematic review of the literature. Am J Med. 2004;116(Suppl 7A):3S–10S. doi: 10.1016/j.amjmed.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 3*.Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104(8):2263–8. doi: 10.1182/blood-2004-05-1812. [DOI] [PubMed] [Google Scholar]
- 4.Longo DL. Immunology of aging. In: Paul WE, editor. Fundamental Immunology. 5th edition Williams and Wilkins; Philadelphia, Lippicott: 2003. pp. 1043–1075. [Google Scholar]
- 5.Hakim FT, Gress RE. Immunosenescence: deficits in adaptive immunity in the elderly. Tissue Antigens. 2007;70(3):179–89. doi: 10.1111/j.1399-0039.2007.00891.x. [DOI] [PubMed] [Google Scholar]
- 6.Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5(2):133–9. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- 7.Hartsock RJ, Smith EB, Petty CS. Normal Variations with Aging of the Amount of Hematopoietic Tissue in Bone Marrow from the Anterior Iliac Crest. A Study Made from 177 Cases of Sudden Death Examined by Necropsy. Am J Clin Pathol. 1965;43:326–31. doi: 10.1093/ajcp/43.4.326. [DOI] [PubMed] [Google Scholar]
- 8.Ricci C, Cova M, Kang YS, et al. Normal age-related patterns of cellular and fatty bone marrow distribution in the axial skeleton: MR imaging study. Radiology. 1990;177(1):83–8. doi: 10.1148/radiology.177.1.2399343. [DOI] [PubMed] [Google Scholar]
- 9.Steinmann GG, Klaus B, Muller-Hermelink HK. The involution of the ageing human thymic epithelium is independent of puberty. A morphometric study. Scand J Immunol. 1985;22(5):563–75. doi: 10.1111/j.1365-3083.1985.tb01916.x. [DOI] [PubMed] [Google Scholar]
- 10.Hirokawa K. Understanding the mechanism of the age-related decline in immune function. Nutr Rev. 1992;50(12):361–6. doi: 10.1111/j.1753-4887.1992.tb02481.x. [DOI] [PubMed] [Google Scholar]
- 11.Haynes BF, Sempowski GD, Wells AF, Hale LP. The human thymus during aging. Immunol Res. 2000;22(2–3):253–61. doi: 10.1385/IR:22:2-3:253. [DOI] [PubMed] [Google Scholar]
- 12.Tavian M, Coulombel L, Luton D, Clemente HS, Dieterlen-Lievre F, Peault B. Aorta-associated CD34+ hematopoietic cells in the early human embryo. Blood. 1996;87(1):67–72. [PubMed] [Google Scholar]
- 13.Abe J. Immunocytochemical characterization of lymphocyte development in human embryonic and fetal livers. Clin Immunol Immunopathol. 1989;51(1):13–21. doi: 10.1016/0090-1229(89)90202-x. [DOI] [PubMed] [Google Scholar]
- 14.Campagnoli C, Fisk N, Overton T, Bennett P, Watts T, Roberts I. Circulating hematopoietic progenitor cells in first trimester fetal blood. Blood. 2000;95(6):1967–72. [PubMed] [Google Scholar]
- 15.Kurtzberg J, Denning SM, Nycum LM, Singer KH, Haynes BF. Immature human thymocytes can be driven to differentiate into nonlymphoid lineages by cytokines from thymic epithelial cells. Proc Natl Acad Sci U S A. 1989;86(19):7575–9. doi: 10.1073/pnas.86.19.7575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fowlkes BJ, Pardoll DM. Molecular and cellular events of T cell development. Adv Immunol. 1989;44:207–64. doi: 10.1016/s0065-2776(08)60643-4. [DOI] [PubMed] [Google Scholar]
- 17.Donskoy E, Goldschneider I. Thymocytopoiesis is maintained by blood-borne precursors throughout postnatal life. A study in parabiotic mice. J Immunol. 1992;148(6):1604–12. [PubMed] [Google Scholar]
- 18.Rothenberg EV. The development of functionally responsive T cells. Adv Immunol. 1992;51:85–214. doi: 10.1016/s0065-2776(08)60487-3. [DOI] [PubMed] [Google Scholar]
- 19.Charbord P, Tavian M, Humeau L, Peault B. Early ontogeny of the human marrow from long bones: an immunohistochemical study of hematopoiesis and its microenvironment. Blood. 1996;87(10):4109–19. [PubMed] [Google Scholar]
- 20.Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology. 2001;2(3):165–71. doi: 10.1023/a:1011513223894. [DOI] [PubMed] [Google Scholar]
- 21.Verma S, Rajaratnam JH, Denton J, Hoyland JA, Byers RJ. Adipocytic proportion of bone marrow is inversely related to bone formation in osteoporosis. J Clin Pathol. 2002;55(9):693–8. doi: 10.1136/jcp.55.9.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22*.Lamberts SW, van den Beld AW, van der Lely AJ. The endocrinology of aging. Science. 1997;278(5337):419–24. doi: 10.1126/science.278.5337.419. [DOI] [PubMed] [Google Scholar]; This comprehensive review presents the fact that declining growth hormone production with age is linked to increasing deposition of fat within the bone marrow resulting in dercreased cellularity.
- 23.French RA, Broussard SR, Meier WA, et al. Age-associated loss of bone marrow hematopoietic cells is reversed by GH and accompanies thymic reconstitution. Endocrinology. 2002;143(2):690–9. doi: 10.1210/endo.143.2.8612. [DOI] [PubMed] [Google Scholar]
- 24.De Meyer T, De Buyzere ML, Langlois M, et al. Lower red blood cell counts in middle-aged subjects with shorter peripheral blood leukocyte telomere length. Aging Cell. 2008;7(5):700–5. doi: 10.1111/j.1474-9726.2008.00419.x. [DOI] [PubMed] [Google Scholar]
- 25.Greider CW. Telomeres and senescence: the history, the experiment, the future. Curr Biol. 1998;8(5):R178–81. doi: 10.1016/s0960-9822(98)70105-8. [DOI] [PubMed] [Google Scholar]
- 26.Frenck RW, Jr., Blackburn EH, Shannon KM. The rate of telomere sequence loss in human leukocytes varies with age. Proc Natl Acad Sci U S A. 1998;95(10):5607–10. doi: 10.1073/pnas.95.10.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gattermann N. Mitochondrial DNA mutations in the hematopoietic system. Leukemia. 2004;18(1):18–22. doi: 10.1038/sj.leu.2403209. [DOI] [PubMed] [Google Scholar]
- 28.Kadenbach B, Munscher C, Frank V, Muller-Hocker J, Napiwotzki J. Human aging is associated with stochastic somatic mutations of mitochondrial DNA. Mutat Res. 1995;338(1–6):161–72. doi: 10.1016/0921-8734(95)00021-w. [DOI] [PubMed] [Google Scholar]
- 29.Bolognesi C, Abbondandolo A, Barale R, et al. Age-related increase of baseline frequencies of sister chromatid exchanges, chromosome aberrations, and micronuclei in human lymphocytes. Cancer Epidemiol Biomarkers Prev. 1997;6(4):249–56. [PubMed] [Google Scholar]
- 30.Miller JF. Role of the Thymus in Immunity. Br Med J. 1963;2(5355):459–64. doi: 10.1136/bmj.2.5355.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steinmann GG. Changes in the human thymus during aging. Curr Top Pathol. 1986;75:43–88. doi: 10.1007/978-3-642-82480-7_2. [DOI] [PubMed] [Google Scholar]
- 32.Flores KG, Li J, Sempowski GD, Haynes BF, Hale LP. Analysis of the human thymic perivascular space during aging. J Clin Invest. 1999;104(8):1031–9. doi: 10.1172/JCI7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kollman C, Howe CW, Anasetti C, et al. Donor characteristics as risk factors in recipients after transplantation of bone marrow from unrelated donors: the effect of donor age. Blood. 2001;98(7):2043–51. doi: 10.1182/blood.v98.7.2043. [DOI] [PubMed] [Google Scholar]
- 34.Nissenson AR, Goodnough LT, Dubois RW. Anemia: not just an innocent bystander? Arch Intern Med. 2003;163(12):1400–4. doi: 10.1001/archinte.163.12.1400. [DOI] [PubMed] [Google Scholar]
- 35.Balducci L, Ershler WB, Bennett JM. Anemia in the Elderly. Springer; USA: 2008. pp. 1–145. [Google Scholar]
- 36.Blanc B, Finch CA, Hallberg L. Nutritional anaemias. Report of a WHO Scientific Group. WHO Tech Rep Ser. 1968;405:1–40. [PubMed] [Google Scholar]
- 37.Ania BJ, Suman VJ, Fairbanks VF, Rademacher DM, Melton LJ., 3rd Incidence of anemia in older people: an epidemiologic study in a well defined population. J Am Geriatr Soc. 1997;45(7):825–31. doi: 10.1111/j.1532-5415.1997.tb01509.x. [DOI] [PubMed] [Google Scholar]
- 38.Chaves PH, Ashar B, Guralnik JM, Fried LP. Looking at the relationship between hemoglobin concentration and prevalent mobility difficulty in older women. Should the criteria currently used to define anemia in older people be reevaluated? J Am Geriatr Soc. 2002;50(7):1257–64. doi: 10.1046/j.1532-5415.2002.50313.x. [DOI] [PubMed] [Google Scholar]
- 39.Culleton BF, Manns BJ, Zhang J, Tonelli M, Klarenbach S, Hemmelgarn BR. Impact of anemia on hospitalization and mortality in older adults. Blood. 2006 doi: 10.1182/blood-2005-10-4308. [DOI] [PubMed] [Google Scholar]
- 40.Izaks GJ, Westendorp RG, Knook DL. The definition of anemia in older persons. Jama. 1999;281(18):1714–7. doi: 10.1001/jama.281.18.1714. [DOI] [PubMed] [Google Scholar]
- 41.Zakai NA, Katz R, Hirsch C, et al. A prospective study of anemia status, hemoglobin concentration, and mortality in an elderly cohort: the Cardiovascular Health Study. Arch Intern Med. 2005;165(19):2214–20. doi: 10.1001/archinte.165.19.2214. [DOI] [PubMed] [Google Scholar]
- 42*.Penninx BW, Pahor M, Woodman RC, Guralnik JM. Anemia in old age is associated with increased mortality and hospitalization. J Gerontol A Biol Sci Med Sci. 2006;61(5):474–9. doi: 10.1093/gerona/61.5.474. [DOI] [PubMed] [Google Scholar]
- 43.Beutler E, West C. Hematologic differences between African-Americans and whites: the roles of iron deficiency and alpha-thalassemia on hemoglobin levels and mean corpuscular volume. Blood. 2005;106(2):740–5. doi: 10.1182/blood-2005-02-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patel KV, Harris TB, Faulhaber M, et al. Racial variation in the relationship of anemia with mortality and mobility disability among older adults. Blood. 2007;109(11):4663–70. doi: 10.1182/blood-2006-10-055384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chaves PH, Xue QL, Guralnik JM, Ferrucci L, Volpato S, Fried LP. What constitutes normal hemoglobin concentration in community-dwelling disabled older women? J Am Geriatr Soc. 2004;52(11):1811–6. doi: 10.1111/j.1532-5415.2004.52502.x. [DOI] [PubMed] [Google Scholar]
- 46.Artz AS, Fergusson D, Drinka PJ, et al. Prevalence of anemia in skilled-nursing home residents. Arch Gerontol Geriatr. 2004;39(3):201–6. doi: 10.1016/j.archger.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 47.Gaskell H, Derry S, Andrew Moore R, McQuay HJ. Prevalence of anaemia in older persons: systematic review. BMC Geriatr. 2008;8:1. doi: 10.1186/1471-2318-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pandya N, Bookhart B, Mody SH, Funk Orsini PA, Reardon G. Study of anemia in long-term care (SALT): prevalence of anemia and its relationship with the risk of falls in nursing home residents. Curr Med Res Opin. 2008;24(8):2139–49. doi: 10.1185/03007990802215844. [DOI] [PubMed] [Google Scholar]
- 49.Robinson B, Artz AS, Culleton B, Critchlow C, Sciarra A, Audhya P. Prevalence of anemia in the nursing home: contribution of chronic kidney disease. J Am Geriatr Soc. 2007;55(10):1566–70. doi: 10.1111/j.1532-5415.2007.01389.x. [DOI] [PubMed] [Google Scholar]
- 50.Ferrucci L, Maggio M, Bandinelli S, et al. Low testosterone levels and the risk of anemia in older men and women. Arch Intern Med. 2006;166(13):1380–8. doi: 10.1001/archinte.166.13.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferrucci L, Guralnik JM, Woodman RC, et al. Proinflammatory state and circulating erythropoietin in persons with and without anemia. Am J Med. 2005;118(11):1288. doi: 10.1016/j.amjmed.2005.06.039. [DOI] [PubMed] [Google Scholar]
- 52.Gazit R, Weissman IL, Rossi DJ. Hematopoietic stem cells and the aging hematopoietic system. Semin Hematol. 2008;45(4):218–24. doi: 10.1053/j.seminhematol.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 53.Artz AS, Fergusson D, Drinka PJ, et al. Mechanisms of unexplained anemia in the nursing home. J Am Geriatr Soc. 2004;52(3):423–7. doi: 10.1111/j.1532-5415.2004.52116.x. [DOI] [PubMed] [Google Scholar]
- 54.Strom SS, Velez-Bravo V, Estey EH. Epidemiology of myelodysplastic syndromes. Semin Hematol. 2008;45(1):8–13. doi: 10.1053/j.seminhematol.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 55.Mori M, Murai Y, Hirai M, et al. Serum erythropoietin titers in the aged. Mech Ageing Dev. 1988;46(1–3):105–9. doi: 10.1016/0047-6374(88)90118-2. [DOI] [PubMed] [Google Scholar]
- 56.Kario K, Matsuo T, Nakao K. Serum erythropoietin levels in the elderly. Gerontology. 1991;37(6):345–8. doi: 10.1159/000213283. [DOI] [PubMed] [Google Scholar]
- 57.Kario K, Matsuo T, Kodama K, Nakao K, Asada R. Reduced erythropoietin secretion in senile anemia. Am J Hematol. 1992;41(4):252–7. doi: 10.1002/ajh.2830410406. [DOI] [PubMed] [Google Scholar]
- 58.Ershler WB, Sheng S, McKelvey J, et al. Serum erythropoietin and aging: a longitudinal analysis. J Am Geriatr Soc. 2005;53(8):1360–5. doi: 10.1111/j.1532-5415.2005.53416.x. [DOI] [PubMed] [Google Scholar]
- 59.Lipschitz DA, Udupa KB, Milton KY, Thompson CO. Effect of age on hematopoiesis in man. Blood. 1984;63(3):502–9. [PubMed] [Google Scholar]
- 60.Nagel JE, Pyle RS, Chrest FJ, Adler WH. Oxidative metabolism and bactericidal capacity of polymorphonuclear leukocytes from normal young and aged adults. J Gerontol. 1982;37(5):529–34. doi: 10.1093/geronj/37.5.529. [DOI] [PubMed] [Google Scholar]
- 61.MacGregor RR, Shalit M. Neutrophil function in healthy elderly subjects. J Gerontol. 1990;45(2):M55–60. doi: 10.1093/geronj/45.2.m55. [DOI] [PubMed] [Google Scholar]
- 62.Rao KM, Currie MS, Padmanabhan J, Cohen HJ. Age-related alterations in actin cytoskeleton and receptor expression in human leukocytes. J Gerontol. 1992;47(2):B37–44. doi: 10.1093/geronj/47.2.b37. [DOI] [PubMed] [Google Scholar]
- 63.MacKinney AA., Jr. Effect of aging on the peripheral blood lymphocyte count. J Gerontol. 1978;33(2):213–6. doi: 10.1093/geronj/33.2.213. [DOI] [PubMed] [Google Scholar]
- 64.Pawelec G, Akbar A, Caruso C, Solana R, Grubeck-Loebenstein B, Wikby A. Human immunosenescence: is it infectious? Immunol Rev. 2005;205:257–68. doi: 10.1111/j.0105-2896.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- 65.Lugada ES, Mermin J, Kaharuza F, et al. Population-based hematologic and immunologic reference values for a healthy Ugandan population. Clin Diagn Lab Immunol. 2004;11(1):29–34. doi: 10.1128/CDLI.11.1.29-34.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nilsson-Ehle H, Jagenburg R, Landahl S, Svanborg A, Westin J. Haematological abnormalities and reference intervals in the elderly. A cross-sectional comparative study of three urban Swedish population samples aged 70, 75 and 81 years. Acta Med Scand. 1988;224(6):595–604. [PubMed] [Google Scholar]
- 67.Segal JB, Moliterno AR. Platelet counts differ by sex, ethnicity, and age in the United States. Ann Epidemiol. 2006;16(2):123–30. doi: 10.1016/j.annepidem.2005.06.052. [DOI] [PubMed] [Google Scholar]
- 68.Takubo T, Tatsumi N. Reference values for hematologic laboratory tests and hematologic disorders in the aged. Rinsho Byori. 2000;48(3):207–16. [PubMed] [Google Scholar]
- 69.Effros RB, Cai Z, Linton PJ. CD8 T cells and aging. Crit Rev Immunol. 2003;23(1–2):45–64. doi: 10.1615/critrevimmunol.v23.i12.30. [DOI] [PubMed] [Google Scholar]
- 70.Globerson A, Effros RB. Ageing of lymphocytes and lymphocytes in the aged. Immunol Today. 2000;21(10):515–21. doi: 10.1016/s0167-5699(00)01714-x. [DOI] [PubMed] [Google Scholar]
- 71.Grubeck-Loebenstein B, Wick G. The aging of the immune system. Adv Immunol. 2002;80:243–84. doi: 10.1016/s0065-2776(02)80017-7. [DOI] [PubMed] [Google Scholar]
- 72.Vallejo AN. Age-dependent alterations of the T cell repertoire and functional diversity of T cells of the aged. Immunol Res. 2006;36(1–3):221–8. doi: 10.1385/IR:36:1:221. [DOI] [PubMed] [Google Scholar]; This review helps us understand the biology of NK related receptors on oligoclonal T-cells and their role to ensure host defense despite shrinkage of the T-cell repertoire.
- 73.Bernstein E, Kaye D, Abrutyn E, Gross P, Dorfman M, Murasko DM. Immune response to influenza vaccination in a large healthy elderly population. Vaccine. 1999;17(1):82–94. doi: 10.1016/s0264-410x(98)00117-0. [DOI] [PubMed] [Google Scholar]
- 74.Gross PA, Quinnan GV, Jr., Weksler ME, Setia U, Douglas RG., Jr. Relation of chronic disease and immune response to influenza vaccine in the elderly. Vaccine. 1989;7(4):303–8. doi: 10.1016/0264-410x(89)90190-4. [DOI] [PubMed] [Google Scholar]
- 75.McElhaney JE, Meneilly GS, Lechelt KE, Beattie BL, Bleackley RC. Antibody response to whole-virus and split-virus influenza vaccines in successful ageing. Vaccine. 1993;11(10):1055–60. doi: 10.1016/0264-410x(93)90133-i. [DOI] [PubMed] [Google Scholar]
- 76.Murasko DM, Bernstein ED, Gardner EM, et al. Role of humoral and cell-mediated immunity in protection from influenza disease after immunization of healthy elderly. Exp Gerontol. 2002;37(2–3):427–39. doi: 10.1016/s0531-5565(01)00210-8. [DOI] [PubMed] [Google Scholar]
- 77.Muszkat M, Friedman G, Dannenberg HD, et al. Response to influenza vaccination in community and in nursing home residing elderly: relation to clinical factors. Exp Gerontol. 2003;38(10):1199–203. doi: 10.1016/j.exger.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 78.Aspinall R. Longevity and the immune response. Biogerontology. 2000;1(3):273–8. doi: 10.1023/a:1010046532657. [DOI] [PubMed] [Google Scholar]
- 79.Tyan ML. Age-related decrease in mouse T cell progenitors. J Immunol. 1977;118(3):846–51. [PubMed] [Google Scholar]
- 80.Aspinall R. Age-associated thymic atrophy in the mouse is due to a deficiency affecting rearrangement of the TCR during intrathymic T cell development. J Immunol. 1997;158(7):3037–45. [PubMed] [Google Scholar]
- 81.Lacorazza HD, Guevara Patino JA, Weksler ME, Radu D, Nikolic-Zugic J. Failure of rearranged TCR transgenes to prevent age-associated thymic involution. J Immunol. 1999;163(8):4262–8. [PubMed] [Google Scholar]
- 82.Hartwig M, Steinmann G. On a causal mechanism of chronic thymic involution in man. Mech Ageing Dev. 1994;75(2):151–6. doi: 10.1016/0047-6374(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 83.Plum J, De Smedt M, Leclercq G, Verhasselt B, Vandekerckhove B. Interleukin-7 is a critical growth factor in early human T-cell development. Blood. 1996;88(11):4239–45. [PubMed] [Google Scholar]
- 84.Le PT, Kurtzberg J, Brandt SJ, Niedel JE, Haynes BF, Singer KH. Human thymic epithelial cells produce granulocyte and macrophage colony-stimulating factors. J Immunol. 1988;141(4):1211–7. [PubMed] [Google Scholar]
- 85.Le PT, Lazorick S, Whichard LP, et al. Human thymic epithelial cells produce IL-6, granulocytemonocyte-CSF, and leukemia inhibitory factor. J Immunol. 1990;145(10):3310–5. [PubMed] [Google Scholar]
- 86.Le PT, Tuck DT, Dinarello CA, Haynes BF, Singer KH. Human thymic epithelial cells produce interleukin 1. J Immunol. 1987;138(8):2520–6. [PubMed] [Google Scholar]
- 87.Gruver AL, Hudson LL, Sempowski GD. Immunosenescence of ageing. J Pathol. 2007;211(2):144–56. doi: 10.1002/path.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cakman I, Rohwer J, Schutz RM, Kirchner H, Rink L. Dysregulation between TH1 and TH2 T cell subpopulations in the elderly. Mech Ageing Dev. 1996;87(3):197–209. doi: 10.1016/0047-6374(96)01708-3. [DOI] [PubMed] [Google Scholar]
- 89.Hodes RJ. Aging and the immune system. Immunol Rev. 1997;160:5–8. doi: 10.1111/j.1600-065x.1997.tb01022.x. [DOI] [PubMed] [Google Scholar]
- 90.Perussia B, Kobayashi M, Rossi ME, Anegon I, Trinchieri G. Immune interferon enhances functional properties of human granulocytes: role of Fc receptors and effect of lymphotoxin, tumor necrosis factor, and granulocyte-macrophage colony-stimulating factor. J Immunol. 1987;138(3):765–74. [PubMed] [Google Scholar]
- 91.Sokolov VV, Kaplunova OA, Ovseenko TE. Age factors in architectonics of the splenic arterial vessels. Morfologiia. 2003;124(4):57–60. [PubMed] [Google Scholar]
- 92.Luscieti P, Hubschmid T, Cottier H, Hess MW, Sobin LH. Human lymph node morphology as a function of age and site. J Clin Pathol. 1980;33(5):454–61. doi: 10.1136/jcp.33.5.454. [DOI] [PMC free article] [PubMed] [Google Scholar]