Abstract
Escherichia coli O157:H7 strains fall into three major genetic lineages that differ in their distribution among humans and cattle. Several recent studies have reported differences in the expression of virulence factors between E. coli O157:H7 strains from these two host species. In this study, we wished to determine if important virulence-associated “mobile genetic elements” such as Shiga toxin 2 (Stx2)-encoding prophage are lineage restricted or are host source related and acquired independently of the pathogen genotype. DNA sequencing of the stx2 flanking region from a lineage II (LII) strain, EC970520, revealed that the transcriptional activator gene Q in LI strain EDL933 (upstream of stx2) is replaced by a pphA (serine/threonine phosphatase) homologue and an altered Q gene in this and all other LII strains tested. In addition, nearly all LI strains carried stx2, whereas all LII strains carried variant stx2c and 4 of 14 LI/II strains had copies of both stx2 and variant stx2c. Real-time PCR (RT-PCR) and enzyme-linked immunosorbent assay (ELISA) demonstrated that LI and LI/II strains produce significantly more stx2 mRNA and Stx2 than LII strains. However, among LI strains significantly more Stx2 is also produced by strains from humans than from cattle. Therefore, lineage-associated differences among E. coli O157:H7 strains such as prophage content, toxin type, and toxin expression may contribute to host isolation bias. However, the level of Stx2 production alone may also play an important role in the within-lineage association of E. coli O157:H7 strains with human clinical disease.
Escherichia coli O157:H7 is associated with outbreaks and sporadic cases of hemorrhagic colitis and the hemolytic-uremic syndrome (HUS) in humans (24). Since its isolation in 1982, this organism has become one of the most important food- and waterborne zoonotic pathogens in the world (16, 28). Shiga toxin 1 (Stx1) and Stx2 are two of the most important virulence factors produced by this pathogen and are encoded by lambda-like prophages integrated into the bacterial chromosome.
It is well recognized that E. coli O157:H7 has a bovine reservoir and that the organism is well adapted for life in the ruminant gastrointestinal tract (6, 7, 14, 32). In contrast to humans, cattle colonized by E. coli O157:H7 are asymptomatic.
Several recent studies have reported differences in the phenotype and genotype of E. coli O157:H7 strains from humans and cattle. Baker et al. (2) found that E. coli O157:H7 strains isolated from healthy cattle were less virulent in gnotobiotic piglets compared with strains of the pathogen isolated from human disease outbreaks and that the amount of Stx2 but not Stx1 produced by E. coli O157:H7 strains was correlated with a reduction in piglet survival and signs of central nervous system disease. LeJeune et al. (22) reported that Q-gene allelic variation (upstream of the prophage stx2 region) and Stx2 production differed between E. coli O157:H7 isolates from cattle and humans. Besser et al. (3) also reported a greater diversity of Stx-encoding bacteriophage insertion sites among E. coli O157:H7 isolates from cattle than those from humans. It has also been reported that E. coli O157:H7 strains vary markedly in the levels of locus of enterocyte effacement (LEE) (16, 28) secreted proteins and that a high-level secretion phenotype is more prevalent among strains associated with human disease than strains shed by healthy cattle (30, 31). Bono et al. (5) recently identified five polymorphisms in a 1,627-bp segment of the intimin receptor (tir) and found that alleles of two tir polymorphisms, tir 255 T>A and repeat region 1-repeat unit 3 (RR1-RU3; presence or absence), had significantly different distributions among human and bovine E. coli O157:H7 strains.
Within E. coli O157:H7 populations, significant genomic diversity is evident using high-resolution methods, such as octamer-based genome scanning (OBGS) (17), whole-genome PCR scanning (WGPS) (27), DNA microarray (25, 39, 40, 42), subtractive suppressive hybridization (34), single nucleotide polymorphism analysis (41), and genome nucleotide sequencing. Comparisons of genome sequences of E. coli O157:H7 strains with the laboratory strain K-12 have revealed that prophages are associated with a significant amount of the divergence among E. coli O157:H7 strains (26). In addition, Asadulghani et al. (1) have recently reported that even defective prophages in the E. coli O157:H7 genome are capable of lateral gene transfer and that recombination of DNA segments occurs among prophages present in the genome of this pathogen.
Kim et al. (17) used OBGS to show that E. coli O157:H7 strains can be divided into two major lineages and that these lineages are nonrandomly distributed among human and bovine hosts. E. coli O157:H7 lineage I (LI) strains are isolated from both humans and cattle at approximately equal frequencies while LII isolates are proportionally much more commonly isolated from cattle. In a recent comparative genomic hybridization study (42), we identified a third major cluster of E. coli O157:H7 strains, which we designated LI/II, with genetic characteristics intermediate between LI and LII. Furthermore, in the study 11 different genomic regions were found to be dominant in LI strains whereas the LI/II strains possessed eight of these LI-dominant loci. Several differences in virulence-associated loci were noted between LI and LII strains, including divergence within S-loop 69 (also called Sakai prophage 5 or SP5), which encodes Stx2. In addition, Dowd and Ishizaki (10) have used oligonucleotide miniarrays to compare the expression of a set of 610 genes between three LI and three LII strains, noting differential expression of stx2 when strains were grown under anaerobic conditions. This group has also recently reported that LI strains produce significantly more Stx2 than LII strains (11). Collectively, these studies suggest that E. coli O157:H7 lineages are genetically distinct and that lineage-specific genetic differences may be responsible for the phenotypic differences observed between human and bovine isolates of the pathogen.
In this study, we wished to determine if the differences observed between bovine and human strains in stx2-encoding prophages and stx2 expression could be best explained by host origin and/or the lineage type of the E. coli O157:H7 strain.
MATERIALS AND METHODS
Bacterial strains.
One hundred seventeen E. coli O157:H7 isolates used in this study were obtained from a variety of human and bovine sources across a broad span of time from different geographic origins (see Table S1 in the supplemental material). Lineage I strains included 25 bovine, 3 environmental, and 29 human isolates; lineage I/II strains included 6 bovine and 8 human isolates; lineage II strains included 29 bovine and 15 human isolates and two isolates of unknown source.
PCR analysis.
Primers from previously published papers and those designed in this study are listed in Table 1. Long-template PCR was carried out in a total reaction volume of 25 μl, with each deoxynucleoside triphosphate (dNTP) at 0.2 mM, each primer at 0.25 μM, Advantage 2 PCR buffer at 1×, Advantage 2 polymerase at 0.6 U, and 100 ng of genomic DNA. The reaction mixture was denatured at 95°C for 1 min before being cycled 5 times for 30 s at 95°C, followed by a 10-min extension at 72°C. The reaction mixture was subsequently cycled 25 to 30 times with a 30-s, 95°C denaturation and a 10-min, 68°C extension before being subjected to a final 10-min extension at 72°C. Long-template PCR products were visualized using 0.60% agarose gels containing 0.5 μg/ml of ethidium bromide (EB).
TABLE 1.
The primers used in this study, their sequences, gene targets, and origin
| Name | Sequence | Target gene | Reference |
|---|---|---|---|
| Non-RT-PCR primers | |||
| restx1 | GTGGTATAACTGCTGTCCGTTGTC | stx2 | This study |
| restx2 | GAATACTGGACCAGTCGCTGGAATC | stx2 | This study |
| oli320b | GGTCACTGGTTCGAATCCAGTAC | stx2 and its variants | 9 |
| oli321 | GGGATCCTGAATTGTGACACAGATTACACTTGTTAC | stx2 and its variants | 9 |
| 595 | AAGAAGATGTTTATGGCGGT | stx2 | 22 |
| Q933 | CGGAGGGGATTGTTGAAGGC | Q | 22 |
| P2 | CGACGACGAGAGGAGCAGAA | ninG | This study |
| P4 | GACGGAATCGACGACCTGAG | Q′ | This study |
| RT-PCR primers | |||
| gapA-F | TGGCTCCGCTGGCTAAAGTTATCA | gapA | This study |
| gapA-R | AGTCTTTGTGAGACGGGCCATCAA | gapA | This study |
| gapA-p | 6-FAM-ATCGAAGGTCTGATGACCACCGTTCA-IABFQa | gapA | This study |
| F386 | CCATGACAACGGACAGCA | stx2 | This study |
| R479 | GATGAAACCAGTGAGTGACGA | stx2 | This study |
| P431 | 6-FAM-CGCTGGAACGTTCCGGAATGCAAATCA-IABFQ | stx2 | This study |
IABFQ, Iowa Black FQ (Integrated DNA Technologies).
Amplification of stx2 variants with the oli320b-oli321 primer pair was carried out in a reaction mixture volume of 50 μl, with each deoxynucleoside triphosphate (dNTP; Invitrogen) at 0.2 mM, each primer at 1 μM, Qiagen PCR buffer containing MgCl2 at 1×, and Taq polymerase (Qiagen) at 5 U. Then 200 ng of template DNA was heated at 95°C for 3 min before being cycled 30 times with a 30-s step at 94°C, a 30-s annealing step at 55°C, and a 3-min extension at 72°C before being subjected to a final 5-min extension step at 72°C.
Restriction fragment length polymorphism (RFLP) analysis of stx2 variants.
Genotyping of stx2 variants was conducted as described by De Baets et al. (9) using restriction enzyme digests of oli320b-oli321 amplicons. The four restriction enzymes PvuII (P), HaeIII (H), HincII (I), and AccI (A) were used in separate digestions in a total reaction volume of 20 μl containing 17 μl PCR product, 2 μl of 10× reaction buffer, and 1 μl of each restriction enzyme (Invitrogen) followed by an incubation at 37°C for 3 h. The enzyme digests were subjected to electrophoresis in 2.0% agarose containing 0.5 μg/ml ethidium bromide, and the resulting PHIA patterns were photographed and scored as described previously (9).
Inverse PCR.
Five micrograms of bacterial genomic DNA was digested with the appropriate restriction enzymes (BamHI, EcoRI, HindIII, and SalI), which were selected based on the analysis of the genomic island O island 45 (OI#45) of the published genomic sequence of E. coli O157:H7 strains EDL933 and Sakai (16, 28). After restriction, the DNA was purified with phenol-chloroform-isoamyl alcohol (25:24:1), precipitated with ethanol, and dissolved in 10 μl of Tris-EDTA (TE; 10/1; pH 8.0) buffer. After ligation of the restricted DNA by addition of ligase and ligation buffer (Invitrogen) overnight at 4°C, 1 μl of the ligation product was used as template for PCR amplification performed as described for long-template PCR.
Cloning and sequencing of inverse PCR products.
The inverse PCR products were cloned using the pCRII-Topo cloning system (Invitrogen) or the Expand PCR cloning kit (Roche) depending on the size of the PCR product. The inserts from various strains were used for further subcloning or direct sequencing. DNA sequencing was performed at the DNA sequencing center of the University of Calgary, Alberta, Canada.
Each sequence was divided into a segment based on the EcoRI site.
Quantification of Stx2 production.
The amount of Stx2 produced by each strain was quantified as previously described (43), with the following modification: cells were lysed by incubation with 0.5 mg/ml polymyxin B (Sigma) at 37°C for 60 min, rather than 1.5 mg/ml for 5 min. The values of three independent experiments were used to determine the average toxin production for each strain.
RNA isolation.
An overnight culture at 37°C was diluted in new 10-ml LB broth and cultured at 37°C until it reached an optical density at 600 nm (OD600) of 0.6. One milliliter of this culture was transferred to an RNase-free 1.5-ml tube and spun at 13,000 rpm, after which the supernatant was discarded. The bacterial pellet was dissolved in 1 ml fresh TRIzol (Invitrogen) and incubated for 5 min at room temperature. Afterward, 200 μl of chloroform was added and the suspension was incubated for an additional 5 min at room temperature. The upper phase was subsequently transferred to a fresh RNase-free 1.5-ml tube, and 500 μl of isopropanol was added prior to a 15-min incubation at room temperature. Nucleic acids were collected by centrifugation and washed with 1 ml of 70% ethanol and then dissolved in 50 μl of RNase-free water. The sample was next treated with Turbo DNase (Ambion) to digest the DNA. RNA was precipitated with 1/10 volume of sodium acetate (3 M, pH 5.2), 1 μl of glycogen (Sigma), and 2 volumes of 100% ethanol at −20°C overnight. The isolated RNA was dissolved in a final volume of 50 μl RNase-free water.
cDNA synthesis.
Reverse transcription (RT) was performed in a reaction mixture containing 10 μl of RNA (0.5 μg/μl), 2 μl of random hexamer primer (20 pmol/μl; Invitrogen), 2.5 μl of dNTPs (10 mM; Invitrogen), 5 μl of 5× RT buffer (Invitrogen), 2 μl of dichlorodiphenyltrichloroethane (0.1 M; Invitrogen), and 20 U RNase inhibitor (GE Life Sciences). The mixture was incubated for 10 min at room temperature. Following incubation, 400 U RT Superscript II (Invitrogen) was added to the mixture and incubated in the following stages: 10 min at 25°C, 50 min at 45°C, 15 min at 70°C, and 2 min at 99°C. The second-strand synthesis contained 10 nmol dNTP, 10× Klenow buffer (Invitrogen), and 1 U Klenow polymerase. The reaction mixture was incubated for 30 min at 37°C, followed by the addition of 2 ng RNase A (Sigma). A subsequent incubation of 30 min at 37°C was performed, and the cDNA was purified from solution by use of a Microcon column (Millipore) in 10 mM Tris-HCl (pH 7.5).
Detection of stx2 or stx2c gene expression by RT-PCR using dually labeled probes.
Real-time PCR (RT-PCR) was performed using a Rotorgene 6000 (Corbett Life Science) in a volume of 25 μl containing the following: 12.5 μl of 2× buffer (Platinum SuperMix UDG; Invitrogen), 100 ng of cDNA, and 10 nmol of each primer and probe. As in Table 1, the probes were conjugated with fluorescent reporter dyes 6-carboxyfluorescein (FAM) at the 5′ end and the quencher dye Iowa Black FQ at the 3′ end (Integrated DNA Technologies Inc.). The gapA gene of E. coli was measured as an internal standard to normalize values between RNA samples as previously described (12). For the establishment of a standard curve, standard gene concentrations were required. Three different concentrations of each of the following were prepared: gapA (100 ng/μl, 10 ng/μl, and 1 ng/μl) and the stx2 gene (100 ng/μl, 10 ng/μl, and 1 ng/μl). For each unknown sample, 3 replicates at the same concentration (100 ng/μl) were tested in the same experiment. Reactions were performed with the following cycling conditions: holding at 95°C for 3 min and 30 cycles of 95°C for 10 s followed by 60°C for 45 s. Acquisition was set to the FAM/Sybr channel, and the gain was set to 10.
Statistical analyses.
Student's two-tailed, unpaired t test assuming equal variance was used to analyze Stx2 production data from enzyme-linked immunosorbent assay (ELISA) experiments and mRNA copy number from RT-PCR experiments. Dixon's Q test was used at the 80% confidence limit to remove outliers in the replicate ELISA data prior to further analysis (8).
Nucleotide sequence accession numbers.
The nucleotide sequences have been deposited in GenBank and have the accession numbers EU999145, EU999146, EU999147, EU999148, EU999149, EU999150, EU999151, EU999152, EU999153, and EU999154.
RESULTS
Comparison of the stx2 gene flanking regions in LI, LI/II, and LII E. coli O157:H7 strains.
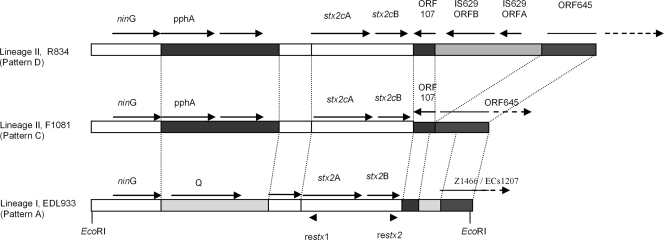
As shown in Table 2, five distinct patterns were obtained following inverse PCR with genomic DNA from 20 strains ligated following EcoRI digestion. Seven LI strains shared the same pattern as EDL933 and Sakai and generated a PCR band of 4.1 kb (pattern A), with one LI strain producing an amplicon of 5.9 kb (pattern B); 10 LII strains gave a 4.9-kb PCR band (pattern C), with one LII strain producing an amplicon of 6.2 kb (pattern D); four lineage I/II strains generated two amplicons, one of 4.1 kb and one of 4.9 kb (pattern E), suggesting that there were two copies of stx2 in these genomes.
TABLE 2.
Inverse PCR investigation using EcoRI-digested sequences of the stx2 flanking region of E. coli O157:H7
| Strain group | Length (kb) | Pattern |
|---|---|---|
| Lineage I (n = 7) | 4.1 | A |
| Lineage I (n = 1) | 5.9 | B |
| Lineage II (n = 10) | 4.9 | C |
| Lineage II (n = 1; strain R834) | 6.2 | D |
| Lineage I/II (n = 4) | 4.1/4.9 | E |
Nucleotide sequence analysis of the stx2 (or stx2c) 5′ flanking segment and the 3′ flanking region surrounding the EcoRI site was carried out. This analysis showed that the 4.1-kb and 4.9-kb amplicons of pattern E had 99% nucleotide sequence identity to the amplicons of LI-associated pattern A and predominantly LII-associated pattern C, respectively (data not shown). The 5′ stx2 flanking sequences of all LII strains shared 99% nucleotide sequence identity with each other. As shown in Fig. 1, LII strains (patterns C and D) shared the same gene organization in the 3.3-kb 5′ stx2 flanking region, which differs from that found in LI strains EDL933 and Sakai (pattern A). In OI#45 of EDL933, the Q gene (38), which encodes a 144-amino-acid-residue antiterminator protein, is located upstream of stx2A and is thought to act as a transcriptional activator. In LII strains (patterns C and pattern D), the Q gene is replaced with the pphA gene (ORF223), which is identical to the gene encoding diadenosine tetraphosphatase and is related to other serine/threonine protein phosphatases (19) (Fig. 1). ORF162 between pphA and stx2A in LII strains showed very low (22%) identity to the LI Q protein at the amino acid sequence level and no identity with Q of 933W at the nucleotide sequence level; despite this low level of identity, it has been designated Q′ (35). The small open reading frame (ORF) Z1460/ECs1204 (in LI strains EDL933 and Sakai, respectively) was not present in any of the LII strain sequences examined. When the LII (pattern C) 3.3-kb 5′ stx2 flanking region was BLASTed against the NCBI database, 99% sequence identity to sequences from E. coli O157:H7 strain Thai-12 (19) (accession no. AB168110.1), phage 2851 in O157:H7 (36) (accession no. AJ605767), and E. coli O157:H7 carrying stx2 vhdA and stx2 vhdB genes (15) (accession no. AB071845.1) was noted. The BLAST results also showed more than 99% sequence identity to the shotgun sequences from E. coli O157:H7 strains EC4113 (accession no. NZ_ABHP01000075) and EC4115 (accession no. NZ_ABHN01000014).
FIG. 1.
Schematic representation of stx2 gene-containing EcoRI fragments in lineage I (EDL933, pattern A) and lineage II (R834, pattern D, and F1081, pattern C) strains. Aligned boxes bearing the same shading show regions of high homology. Solid arrows indicate ORFs and their expression direction in the fragment. The dashed arrows indicate incomplete ORFs and their expression direction. STP, serine/threonine protein phosphatase homologue; Q, transcriptional activator antiterminator Q; Q′, a protein bearing low homology to Q. restx1 and restx2 are two primers used for inverse PCR.
Three of the four sequenced LII strains exhibiting pattern C had the same gene organization in their 3′ stx2 flanking region, which differed from LI strains (Fig. 1). Furthermore, when the 3′ stx2 flanking sequences of these strains were aligned with the stx2 flanking sequence of other E. coli strains from serotypes O26:H11, O145:H−, and E. coli O157:H− (37), high identity (>97% similarity) and the same gene organization were observed among them (Table 3). However, only very low similarity (<60%) was observed between LI and LII strains in the 3′ stx2 flanking sequence (Fig. 1).
TABLE 3.
DNA sequence comparison of the 3′ stx2 flanking region in 11 Shiga toxin-containing E. coli strains, encompassing O157:H7, O26:H11, and O145:H− serotypes, to LII strain EC970520
| Strain | Sequence accession no. | Start nucleotide | End nucleotide | Similarity (%) | Reference |
|---|---|---|---|---|---|
| O157:H7 LII strain EC970520 | EU999153 | 1 | 1629 | This study | |
| O157:H7 LII strain F1081 | EU999151 | 1 | 1629 | 99.9 | This study |
| O157:H7 LII strain LS68 | EU999152 | 1 | 1629 | 99.9 | This study |
| O157:H7 LI/II strain R1388 | EU999145 | 1 | 1629 | 99.9 | This study |
| O157:H− LI/II strain E32511 | AJ251452 | 137 | 1765 | 99.8 | 37 |
| O26:H11 strain 1448/97 | AJ250954 | 137 | 1765 | 97.7 | 37 |
| O26:H11 strain ED-147 | AJ251483 | 137 | 1766 | 97.8 | 37 |
| O145:H− strain 3985/96 | AJ251520 | 137 | 1766 | 97.8 | 37 |
| O145:H− strain 4865/96 | AJ271063 | 137 | 1767 | 97.6 | 37 |
| O157:H7 LI strain EDL933 | 933W | 22568 | 23819 | 59.7 | 28 |
| O157:H7 LI strain Sakai | VT2_sakai | 22059 | 23310 | 59.7 | 16 |
As shown in Fig. 1, ORF107, which is identical to gene B1160 in the K-12 genome (4), is located between stx2B and Z1466/ECs1207 (a 645-amino-acid protein found in both EDL933 and Sakai). In LII strain R834, ORF107 and two genes, orfB and orfA from IS629 (which is located downstream of ECs1207 in the Sakai genome [16, 28]), were inserted between stx2B and Z1466/ECs1207, which corresponds to inverse PCR pattern D in Table 2 and was the only LII strain found to contain this gene arrangement.
Inverse PCR showed that LI/II strain R1388 carried two copies of the Stx2 gene, one with pattern A and another with pattern C. DNA sequence analysis confirmed that one has a flanking region identical to that of LI (pattern A) strains EDL933 and Sakai and the other has a flanking region identical to that of the LII (pattern C) strains (data not shown).
To investigate genetic differences in the stx2 flanking region in a larger set of strains from all three lineages, two primer sets were used to amplify specific targets in the stx2 gene flanking region. The primer set Q933-595 was used to target the flanking region upstream of stx2 in LI strain EDL933 and included the Q antiterminator gene (38). The primer set P2-P4 targeted the region from the lambda ninG gene upstream of stx2c. Results of assays with these two primer sets on DNA from a total of 117 E. coli O157:H7 strains (56 LI, 14 LI/II, and 47 LII strains) are presented in Table 4. All LI strains generated an amplicon of expected size with the primer set Q933-595, while LII strains did not. Forty-six of 47 LII strains (98%) produced an amplicon using the P2-P4 primer set, while no LI strains did. Four of 14 LI/II strains were PCR positive with both primer pairs, while the other 10 were positive only with primer set Q933-595.
TABLE 4.
Genotyping of 117 lineage I, I/II, and II E. coli O157:H7 strains using the primer pairs Q933-595 and P2-P4
| Lineage(s) | Source | n | No. positive by primer set: |
|
|---|---|---|---|---|
| Q933-595 | P2-P4 | |||
| I | Bovine | 25 | 25 | 0 |
| Environment | 3 | 3 | 0 | |
| Human | 28 | 28 | 0 | |
| II | Bovine | 29 | 0 | 28 |
| Human | 16 | 0 | 16 | |
| Unknown | 2 | 0 | 16 | |
| I/II | Bovine | 6 | 6 | 2 |
| Human | 8 | 8 | 2 | |
Analysis of the stx2 (stx2c) gene in LI, LI/II, and LII strains by RFLP.
The coding sequence of the variant stx2c toxin of the E. coli O157:H7 reference strain E32511 differs from that of the prototypical Stx2 toxin found in bacteriophage 933W by three amino acid replacements in the B subunit, whereas the A subunits in the two toxins are identical (33). A comparison of the predicted amino acid sequence of Stx2 from LII strain LS68 to that of Stx2c present in the prototypical strain E32511 revealed 100% identity in the B subunit and one amino acid difference in the A subunit of LII strain LS68.
RFLP analysis of the oli320b-oli321 PCR product using the restriction enzymes PHIA distinguished among the stx2 gene variants from each lineage. As shown in Table 5, the PHIA pattern from LI strains was 1-1-1-1, consistent with stx2; the digestion pattern for lineage II strains was 1-2-2-1, consistent with stx2c; and two lineage I/II strains analyzed produced a PHIA pattern of 1-1 + 2-1 + 2-1, consistent with possession of both the stx2c and stx2 genes.
TABLE 5.
PCR-RFLP typing of stx2 variants in lineage I, I/II, and II E. coli O157:H7 strains using PvuII, HaeIII, HincII, and AccI (PHIA) restriction enzymes
| Lineage(s) (no. of strains) | Pattern according to RFLP analysis of PCR producta |
Stx2 variant type | |||
|---|---|---|---|---|---|
| PvuII (P) | HaeIII (H) | HincII (I) | AccI (A) | ||
| Lineage I (n = 7) | 1 | 1 | 1 | 1 | Stx2 |
| Lineage II (n = 9) | 1 | 2 | 2 | 1 | Stx2c |
| Lineage I/II (n = 6) | 1 | 1 | 1 | 1 | Stx2 |
| Lineage I/II (n = 2) | 1 | 2 | 2 | 1 | Stx2 + Stx2c |
1 and 2 are specific RFLP patterns described by De Baets et al. (9).
Stx2 production by LI, LI/II, and LII strains.
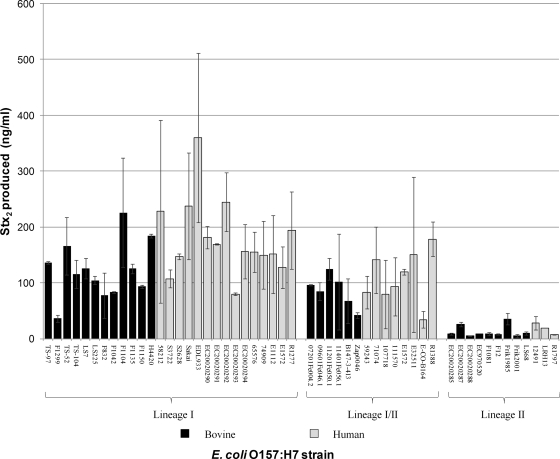
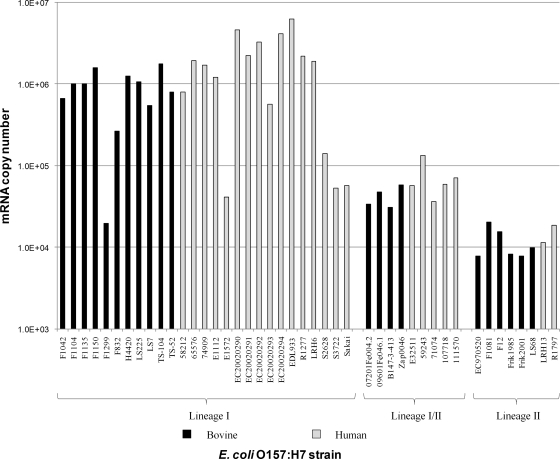
Stx production was measured using an ELISA for 27 LI, 12 LII, and 14 LI/II E. coli O157:H7 strains (Fig. 2). LI strains containing stx2 produced significantly more Stx2 than the Stx2 and/or Stx2c produced by LI/II strains (P = 7.93E−03). LII strains produced significantly less Stx2 and/or Stx2c than LI and LI/II strains (P = 1.43E−08 and P = 2.16E−07, respectively). Stx2 production among LI strains was more variable than among either LI/II or LII strains, with EDL933 producing very high levels of toxin and F1299 producing very low levels of toxin. RT-PCR (Fig. 3) also showed that LI and LI/II strains produced significantly higher levels of stx2 and/or stx2c mRNA than LII strains (P = 8.83E−03 and P = 8.74E−04, respectively) and that LI strains produced significantly higher levels of stx2 and/or stx2c mRNA than LI/II strains (P = 7.11E−03).
FIG. 2.
Comparison of Stx2 production among E. coli O157:H7 LI, LI/II, and LII by using an ELISA. Mean values indicate the average values of three experimental replicates, with outliers removed using Dixon's Q test at an 80% confidence limit, and error bars represent sample standard deviations. Human strains are represented as gray bars and bovine strains as black bars.
FIG. 3.
Comparison of stx2 mRNA production among E. coli O157:H7 LI, LI/II, and LII strains by using RT-PCR. Human strains are represented as gray bars and bovine strains as black bars.
We also compared Stx2 production using an ELISA among LI and LI/II strains isolated from humans and cattle (Fig. 2). Among LI strains, those of human origin produced more of this toxin than those of bovine origin (the means were 179.33 ng/ml and 122.89 ng/ml, respectively) and the difference was statistically significant (P = 2.50E−02). RT-PCR also showed that human strains produced significantly higher levels of stx2 mRNA than bovine strains within LI (Fig. 3) (P = 8.08E−02); however, no significant difference was observed in Stx2 production between bovine and human strains within LI/II or LII using either ELISA or RT-PCR.
DISCUSSION
In this study, we investigated Stx2 production by E. coli O157:H7 strains and found that LI and LI/II strains produce significantly larger amounts of Stx2 than LII strains independently of host origin. However, LI human strains also produced more Stx than LI bovine strains, in agreement with the observations made by other researchers who have studied E. coli O157:H7 strains without consideration of lineage or other genotype (22).
Although the toxin mRNA expression levels differed from the amount of toxin measured in the culture supernatant by ELISA, strains that produced smaller amounts of mRNA tended to produce lower ELISA readings as well. The variation observed is possibly due to differences in mRNA half-life, translation efficiency, amount of cell-associated Stx, the amount of toxin-specific antibody in the ELISA, or differences in experimental conditions used for each of the assays. However, the differences between mRNA and toxin expression levels do not affect the conclusions drawn from these data. While Stx2 production by Stx-producing E. coli (STEC) has been shown to be associated with more-severe clinical disease (13), it is also clear that among E. coli O157:H7 strains, low-level Stx2c-producing strains and strains which produce Stx1 only, as well as LII Stx2c-producing strains, have all been isolated from human patients with clinical disease and therefore should not be considered nonpathogenic.
It was previously reported that basal production of Stx by HUS-associated STEC exceeded that of bovine-associated STEC (29). In addition, following mitomycin C treatment, Stx2 production by HUS-associated STEC was significantly greater than that by bovine-associated STEC. In a separate study, Dowd and Williams (11) recently reported that LI strains produce significantly more Stx2 than LII strains; however, within-lineage production of Stx2 between strains isolated from humans or cattle was not reported nor was Stx2 production by LI/II strains compared with that of LI and LII strains. A recent study by Ziebell et al. also showed on average more Stx2 production among LI strains than strains of LII and found that LII strains of phage type (PT) 23 and PT67 produced less Stx2 than strains of other LII PTs (43). Other studies have also compared Stx production following treatment of cultures with mitomycin C and ciprofloxacin. These agents were found to increase levels of Stx production and exaggerate differences among lineage groups; however, the relevance of studies using phage-inducing agents for in vivo toxin production is not clear.
It has been demonstrated that phage induction results in dramatic increases of Stx production involving two mechanisms. First, there is induction of the Stx-converting phage, which brings about an increase in toxin production due to a concomitant multiplication of toxin gene copies. Second, there is the influence of a phage-encoded regulatory factor, recently characterized as the Q transcription activator protein (23). Our results from RT-PCR assays showed that more stx2/stx2c mRNA is produced by LI and LI/II strains than stx2c mRNA by LII strains. The detailed study of the stx2/stx2c flanking region carried out here showed that LI and LI/II strains carry a different phage Q gene from the Q′ gene found in LII strains. Recent experiments indicate that phage induction may also contribute to the significant difference in Stx2 production observed among LI, LI/II, and LII strains (data not shown).
It has been reported that a bovine isolate, Thai-12, carries an stx2c gene but does not produce Stx, and it has been postulated that modification of the q-stx2 region by homologous recombination may have been responsible for the lack of expression of its stx2c gene (18, 19). Alignment of the 5′ stx2c flanking region of LII strain sequences from the present study showed that all had more than 99% sequence identity to Thai-12 (data not shown). Therefore, LII strains may have lower expression of Stx2c through a mechanism related to that of Thai-12 (19). However, while toxin production measured in this study was low for LII strains, it was nevertheless readily detectable using ELISA and RT-PCR assays. It is therefore possible that the methods used to measure toxin production by the Thai-12 strain were less sensitive than those employed in this study for LII strains; however, distal (trans) factors may also be involved in the regulation of toxin production/phage induction. The precise reason for the lack of toxin production by Thai-12 requires further study.
In this study, we also identified the Q′ gene as a genetic marker specific for the presence of the stx2c-producing phage which could be used in conjunction with the Q gene-specific primers developed by LeJeune et al. (22) to identify phage carrying either or both stx2 and stx2c.
It is well known that Stx2-encoding prophages can integrate into the chromosome of other E. coli strains. Recently, Asadulghani et al. (1) have reported that not only functional but also defective prophage DNA can be transferred to other E. coli strains. They have also shown that recombination of DNA segments between different Stx-encoding prophages occurs in the genome. Based on this information, one would expect to see considerable variation in prophage content and structure in the E. coli O157:H7 genome independent of host bacterial genotype. However, the results of this and other studies suggest that prophage gene content and organization and the phenotypic traits associated with them, such as toxin production, are relatively stable and characteristic of members of specific E. coli O157:H7 genetic lineages. This suggests that selective forces are acting to maintain the prophage content in these lineages and that significant change in these prophages must provide a selective advantage for the bacterium in order for them to be a driving force in the evolution of new genotypes of this human pathogen.
RFLP and DNA sequencing data showed that all of the LII strains examined carried the Stx2c gene. A previous study reported that while stx2c was the only stx2 variant associated with HUS, the frequency of HUS associated with STEC strains harboring the stx2c genotype is significantly lower than that of STEC with the stx2 genotype (13). We noted that there is considerable variation in the levels of toxin production among E. coli O157:H7 strains, and stx2c-bearing LII strains produced the smallest amount of toxin on average, with higher levels of toxin produced by stx2-containing LI and stx2- and stx2c-containing LI/II strains. It has previously been reported that the majority of toxin produced by clade 8 (LI/II) (21) strains containing both stx2 and stx2c is Stx2 (20). It has also been shown that both LI and LI/II strains are more likely to be associated with human illness than LII strains (43). Therefore, the amount of Stx2 toxin produced is likely more important than whether a strain contains only stx2 or both stx2 and stx2c in determining the frequency of association of these lineage types with human illness.
The reason for the difference in Stx2 production among strains within LI was not clear given the high level of similarity in the toxin promoter sequences found within this genotype. This suggests that other factors that affect phage induction, toxin expression, or translation exist elsewhere in the phage or genome. At present, it is not known if high toxin producers cause more frequent and/or more overt human illness or if toxin production is in some way enhanced during the course of illness. Additionally, it is not currently known whether the levels of toxin production observed in vitro correspond to the amount of toxin produced in vivo. It is also possible that other genetic elements contribute to the virulence of these strains and that these elements are only genetically linked to high toxin production. LI and LI/II strains are more frequently associated with human disease than LII strains but do not universally exhibit high toxin production. This suggests that both relatively fixed lineage-specific factors and adaptation may play a role in disease associations and is clearly an area in need of further investigation.
In summary, based on the data from inverse PCR and DNA sequencing, we found the organization of genes flanking the stx2c gene of LII strains differed from that of LI strains. The 5′ stx2 flanking region of LII strains contains a pphA gene (serine/threonine protein phosphatase homologue) integrated in the upstream portion of a gene with very low similarity to the transcriptional activator antiterminator Q of LI strains. The Q and Q′ genes appear to be useful molecular markers to distinguish among Stx2- and Stx2c-encoding phages. PCR-RFLP investigations of Stx2 gene variants showed that all LII strains carried only the Stx2c gene and LI strains carried the Stx2 gene; four LI/II strains carried both stx2c and stx2, while the majority contained only stx2. Both ELISA and RT-PCR revealed that LII strains produce significantly less Stx2 than LI and LI/II strains. Finally, among E. coli O157:H7 LI strains, strains of human origin produced significantly more Stx2 than strains of bovine origin, suggesting that high-level Stx2/Stx2c-producing E. coli O157:H7 strains are likely more virulent (human disease associated) than strains which produce lower levels of Stx2.
Supplementary Material
Acknowledgments
We thank Gary Golds, Thomas Graham, and Fan Mo for their excellent technical assistance. We also thank Irene Yong and Shelly Johnson of the E. coli typing laboratory of the Public Health Agency of Canada and Amanda Mazzocco and Bob Holtslander for assistance with performance of the Stx2-specific ELISA.
Support for this work was provided by the Office of the Chief Scientist, Health Canada; the Safe Food and Water Initiative of the Canadian Institute of Health Research; the Natural Sciences and Engineering Research Council of Canada; and the Public Health Agency of Canada.
Footnotes
Published ahead of print on 30 November 2009.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Asadulghani, M., Y. Ogura, T. Ooka, T. Itoh, A. Sawaguchi, A. Iguchi, K. Nakayama, and T. Hayashi. 2009. The defective prophage pool of Escherichia coli O157: prophage-prophage interactions potentiate horizontal transfer of virulence determinants. PLoS Pathog. 5:e1000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker, D. R., R. A. Moxley, and D. H. Francis. 1997. Variation in virulence in the gnotobiotic pig model of O157:H7 Escherichia coli strains of bovine and human origin. Adv. Exp. Med. Biol. 412:53-58. [DOI] [PubMed] [Google Scholar]
- 3.Besser, T. E., N. Shaikh, N. J. Holt, P. I. Tarr, M. E. Konkel, P. Malik-Kale, C. W. Walsh, T. S. Whittam, and J. L. Bono. 2007. Greater diversity of Shiga toxin-encoding bacteriophage insertion sites among Escherichia coli O157:H7 isolates from cattle than in those from humans. Appl. Environ. Microbiol. 73:671-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. [DOI] [PubMed] [Google Scholar]
- 5.Bono, J. L., J. E. Keen, M. L. Clawson, L. M. Durso, M. P. Heaton, and W. W. Laegreid. 2007. Association of Escherichia coli O157:H7 tir polymorphisms with human infection. BMC Infect. Dis. 7:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chapman, P. A., M. Ellin, and R. Ashton. 2001. A comparison of immunomagnetic separation and culture, Reveal and VIP for the detection of E. coli O157 in enrichment cultures of naturally-contaminated raw beef, lamb and mixed meat products. Lett. Appl. Microbiol. 32:171-175. [DOI] [PubMed] [Google Scholar]
- 7.Chapman, P. A., M. Ellin, R. Ashton, and W. Shafique. 2001. Comparison of culture, PCR and immunoassays for detecting Escherichia coli O157 following enrichment culture and immunomagnetic separation performed on naturally contaminated raw meat products. Int. J. Food Microbiol. 68:11-20. [DOI] [PubMed] [Google Scholar]
- 8.Dean, R. B., and W. J. Dixon. 1951. Simplified statistics for small numbers of observations. Anal. Chem. 23:636-638. [Google Scholar]
- 9.De Baets, L., I. Van der Taelen, M. De Filette, D. Pierard, L. Allison, H. De Greve, J. P. Hernalsteens, and H. Imberechts. 2004. Genetic typing of Shiga toxin 2 variants of Escherichia coli by PCR-restriction fragment length polymorphism analysis. Appl. Environ. Microbiol. 70:6309-6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dowd, S. E., and H. Ishizaki. 2006. Microarray based comparison of two Escherichia coli O157:H7 lineages. BMC Microbiol. 6:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dowd, S. E., and J. B. Williams. 2008. Comparison of Shiga-like toxin II expression between two genetically diverse lineages of Escherichia coli O157:H7. J. Food Prot. 71:1673-1678. [DOI] [PubMed] [Google Scholar]
- 12.Fitzmaurice, J., M. Glennon, G. Duffy, J. J. Sheridan, C. Carroll, and M. Maher. 2004. Application of real-time PCR and RT-PCR assays for the detection and quantitation of VT 1 and VT 2 toxin genes in E. coli O157:H7. Mol. Cell. Probes 18:123-132. [DOI] [PubMed] [Google Scholar]
- 13.Friedrich, A. W., M. Bielaszewska, W. L. Zhang, M. Pulz, T. Kuczius, A. Ammon, and H. Karch. 2002. Escherichia coli harboring Shiga toxin 2 gene variants: frequency and association with clinical symptoms. J. Infect. Dis. 185:74-84. [DOI] [PubMed] [Google Scholar]
- 14.Gannon, V. P., T. A. Graham, R. King, P. Michel, S. Read, K. Ziebell, and R. P. Johnson. 2002. Escherichia coli O157:H7 infection in cows and calves in a beef cattle herd in Alberta, Canada. Epidemiol. Infect. 129:163-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamabata, T., T. Tanaka, A. Ozawa, T. Shima, T. Sato, and Y. Takeda. 2002. Genetic variation in the flanking regions of Shiga toxin 2 gene in Shiga toxin-producing Escherichia coli O157:H7 isolated in Japan. FEMS Microbiol. Lett. 215:229-236. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi, T., K. Makino, M. Ohnishi, K. Kurokawa, K. Ishii, K. Yokoyama, C. G. Han, E. Ohtsubo, K. Nakayama, T. Murata, M. Tanaka, T. Tobe, T. Iida, H. Takami, T. Honda, C. Sasakawa, N. Ogasawara, T. Yasunaga, S. Kuhara, T. Shiba, M. Hattori, and H. Shinagawa. 2001. Complete genome sequence of enterohemorrhagic Escherichia coli O157:H7 and genomic comparison with a laboratory strain K-12. DNA Res. 8:11-22. [DOI] [PubMed] [Google Scholar]
- 17.Kim, J., J. Nietfeldt, and A. K. Benson. 1999. Octamer-based genome scanning distinguishes a unique subpopulation of Escherichia coli O157:H7 strains in cattle. Proc. Natl. Acad. Sci. U. S. A. 96:13288-13293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koitabashi, T., V. Vuddhakul, T. Morigaki, N. Asai, and M. Nishibuchi. 2003. Stx2-positive but Stx2-negative Escherichia coli O157:H7/− strains isolated in Thailand and Japan, abstr. 038. 10th Asian Conf. Diarrhoeal Dis. Nutr. (ASCODD).
- 19.Koitabashi, T., V. Vuddhakul, S. Radu, T. Morigaki, N. Asai, Y. Nakaguchi, and M. Nishibuchi. 2006. Genetic characterization of Escherichia coli O157:H7/− strains carrying the stx2 gene but not producing Shiga toxin 2. Microbiol. Immunol. 50:135-148. [DOI] [PubMed] [Google Scholar]
- 20.Kulasekara, B. R., M. Jacobs, Y. Zhou, Z. Wu, E. Sims, C. Saenphimmachak, L. Rohmer, J. M. Ritchie, M. Radey, M. McKevitt, T. L. Freeman, H. Hayden, E. Haugen, W. Gillett, C. Fong, J. Chang, V. Beskhlebnaya, M. K. Waldor, M. Samadpour, T. S. Whittam, R. Kaul, M. Brittnacher, and S. I. Miller. 2009. Analysis of the genome of the Escherichia coli O157:H7 2006 spinach-associated outbreak isolate indicates candidate genes that may enhance virulence. Infect. Immun. 77:3713-3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laing, C. R., C. Buchanan, E. N. Taboada, Y. Zhang, M. A. Karmali, J. E. Thomas, and V. P. Gannon. 2009. In silico genomic analyses reveal three distinct lineages of Escherichia coli O157:H7, one of which is associated with hyper-virulence. BMC Genomics 10:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LeJeune, J. T., S. T. Abedon, K. Takemura, N. P. Christie, and S. Sreevatsan. 2004. Human Escherichia coli O157:H7 genetic marker in isolates of bovine origin. Emerg. Infect. Dis. 10:1482-1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muhldorfer, I., J. Hacker, G. T. Keusch, D. W. Acheson, H. Tschape, A. V. Kane, A. Ritter, T. Olschlager, and A. Donohue-Rolfe. 1996. Regulation of the Shiga-like toxin II operon in Escherichia coli. Infect. Immun. 64:495-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogura, Y., K. Kurokawa, T. Ooka, K. Tashiro, T. Tobe, M. Ohnishi, K. Nakayama, T. Morimoto, J. Terajima, H. Watanabe, S. Kuhara, and T. Hayashi. 2006. Complexity of the genomic diversity in enterohemorrhagic Escherichia coli O157 revealed by the combinational use of the O157 Sakai OligoDNA microarray and the Whole Genome PCR scanning. DNA Res. 13:3-14. [DOI] [PubMed] [Google Scholar]
- 26.Ohnishi, M., K. Kurokawa, and T. Hayashi. 2001. Diversification of Escherichia coli genomes: are bacteriophages the major contributors? Trends Microbiol. 9:481-485. [DOI] [PubMed] [Google Scholar]
- 27.Ohnishi, M., J. Terajima, K. Kurokawa, K. Nakayama, T. Murata, K. Tamura, Y. Ogura, H. Watanabe, and T. Hayashi. 2002. Genomic diversity of enterohemorrhagic Escherichia coli O157 revealed by whole genome PCR scanning. Proc. Natl. Acad. Sci. U. S. A. 99:17043-17048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perna, N. T., G. Plunkett III, V. Burland, B. Mau, J. D. Glasner, D. J. Rose, G. F. Mayhew, P. S. Evans, J. Gregor, H. A. Kirkpatrick, G. Posfai, J. Hackett, S. Klink, A. Boutin, Y. Shao, L. Miller, E. J. Grotbeck, N. W. Davis, A. Lim, E. T. Dimalanta, K. D. Potamousis, J. Apodaca, T. S. Anantharaman, J. Lin, G. Yen, D. C. Schwartz, R. A. Welch, and F. R. Blattner. 2001. Genome sequence of enterohaemorrhagic Escherichia coli O157:H7. Nature 409:529-533. [DOI] [PubMed] [Google Scholar]
- 29.Ritchie, J. M., P. L. Wagner, D. W. Acheson, and M. K. Waldor. 2003. Comparison of Shiga toxin production by hemolytic-uremic syndrome-associated and bovine-associated Shiga toxin-producing Escherichia coli isolates. Appl. Environ. Microbiol. 69:1059-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roe, A. J., S. W. Naylor, K. J. Spears, H. M. Yull, T. A. Dransfield, M. Oxford, I. J. McKendrick, M. Porter, M. J. Woodward, D. G. Smith, and D. L. Gally. 2004. Co-ordinate single-cell expression of LEE4- and LEE5-encoded proteins of Escherichia coli O157:H7. Mol. Microbiol. 54:337-352. [DOI] [PubMed] [Google Scholar]
- 31.Roe, A. J., H. Yull, S. W. Naylor, M. J. Woodward, D. G. Smith, and D. L. Gally. 2003. Heterogeneous surface expression of EspA translocon filaments by Escherichia coli O157:H7 is controlled at the posttranscriptional level. Infect. Immun. 71:5900-5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sargeant, J. M., M. W. Sanderson, R. A. Smith, and D. D. Griffin. 2003. Escherichia coli O157 in feedlot cattle feces and water in four major feeder-cattle states in the U. S. A. Prev. Vet. Med. 61:127-135. [DOI] [PubMed] [Google Scholar]
- 33.Schmitt, C. K., M. L. McKee, and A. D. O'Brien. 1991. Two copies of Shiga-like toxin II-related genes common in enterohemorrhagic Escherichia coli strains are responsible for the antigenic heterogeneity of the O157:H− strain E32511. Infect. Immun. 59:1065-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steele, M., K. Ziebell, Y. Zhang, A. Benson, P. Konczy, R. Johnson, and V. Gannon. 2007. Identification of Escherichia coli O157:H7 genomic regions conserved in strains with a genotype associated with human infection. Appl. Environ. Microbiol. 73:22-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strauch, E., J. A. Hammerl, A. Konietzny, S. Schneiker-Bekel, W. Arnold, A. Goesmann, A. Puhler, and L. Beutin. 2008. Bacteriophage 2851 is a prototype phage for dissemination of the Shiga toxin variant gene 2c in Escherichia coli O157:H7. Infect. Immun. 76:5466-5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strauch, E., C. Schaudinn, and L. Beutin. 2004. First-time isolation and characterization of a bacteriophage encoding the Shiga toxin 2c variant, which is globally spread in strains of Escherichia coli O157. Infect. Immun. 72:7030-7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Unkmeir, A., and H. Schmidt. 2000. Structural analysis of phage-borne stx genes and their flanking sequences in Shiga toxin-producing Escherichia coli and Shigella dysenteriae type 1 strains. Infect. Immun. 68:4856-4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wagner, P. L., M. N. Neely, X. Zhang, D. W. Acheson, M. K. Waldor, and D. I. Friedman. 2001. Role for a phage promoter in Shiga toxin 2 expression from a pathogenic Escherichia coli strain. J. Bacteriol. 183:2081-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wick, L. M., W. Qi, D. W. Lacher, and T. S. Whittam. 2005. Evolution of genomic content in the stepwise emergence of Escherichia coli O157:H7. J. Bacteriol. 187:1783-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu, G., B. Carter, M. Mafura, E. Liebana, M. J. Woodward, and M. F. Anjum. 2008. Genetic diversity among Escherichia coli O157:H7 isolates and identification of genes linked to human infections. Infect. Immun. 76:845-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang, W., W. Qi, T. J. Albert, A. S. Motiwala, D. Alland, E. K. Hyytia-Trees, E. M. Ribot, P. I. Fields, T. S. Whittam, and B. Swaminathan. 2006. Probing genomic diversity and evolution of Escherichia coli O157 by single nucleotide polymorphisms. Genome Res. 16:757-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang, Y., C. Laing, M. Steele, K. Ziebell, R. Johnson, A. K. Benson, E. Taboada, and V. P. Gannon. 2007. Genome evolution in major Escherichia coli O157:H7 lineages. BMC Genomics 8:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ziebell, K., M. Steele, Y. Zhang, A. Benson, E. N. Taboada, C. Laing, S. McEwen, B. Ciebin, R. Johnson, and V. Gannon. 2008. Genotypic characterization and prevalence of virulence factors among Canadian Escherichia coli O157:H7 strains. Appl. Environ. Microbiol. 74:4314-4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.