Abstract
The attachment of murine norovirus 1 (MNV) in biosolids, swine manure, and dairy manure to Romaine lettuce and internalization of this virus were evaluated. The MNV in animal manures had behavior similar to that of pure MNV; however, MNV in biosolids had significantly higher levels of attachment and internalization than pure MNV or MNV in manures. The incubation time did not affect the attachment of MNV in biosolids or manure. Confocal microscopy was used to observe MNV on lettuce after SYBR gold-labeled MNV was added directly to lettuce or after lettuce was submersed in labeled virus. MNV was observed on the lettuce surface, inside open cuts, and occasionally within stomata. In general, lettuce pieces with a long cut on the edge and short cuts on the stem was more likely to contain internalized MNV than intact lettuce pieces, as observed by confocal microscopy; however, while the difference was visible, it was not statistically significant. This study showed that the presence of MNV in biosolids may increase the risk of fresh produce contamination and that the MNV in open cuts and stomata is likely to be protected from sanitization.
Noroviruses (NoVs) are leading food-borne pathogens, accounting for over 60% of food-borne disease in the United States (15). They are the most common cause of nonbacterial gastroenteritis, and an estimated 23 million cases occur annually in the United States. NoVs are prevalent in the environment and can be found in waste treatment plant influent and effluent (7), biosolids (4), and animal feces (14). Due to these facts, the use of biosolids and animal manure on agricultural land may disseminate human pathogens in the environment and subsequently increase the chance of crop contamination (22). Recently, increasing outbreaks of NoV infection have been associated with salads and vegetables (9, 11, 13). Fresh produce could be contaminated from preharvest to postharvest at any point in the chain of production, and one of the major routes with a high likelihood of contamination is the use of contaminated water for irrigation and washing (12). Water could be contaminated by the use of biosolids and manure as organic fertilizer on United States farms or by runoff from animal production zones close to produce fields. Changes in processing, including more cutting and coring performed in the field during harvest, also increase the potential risk of microbial contamination. Furthermore, postharvest sanitizing regimens used by industry have a limited effect on the removal or inactivation of enteric viruses on lettuce (1), and since it only takes a few infectious particles to cause an infection, consumption of fresh produce continues to be a public health risk.
Intensive studies of the behavior of bacteria such as Escherichia coli, Pseudomonas, and Salmonella on fresh produce have been conducted (2, 3, 16, 17), but little work has been conducted with viruses. It has been reported that E. coli and Pseudomonas can grow on lettuce surfaces (17). While Pseudomonas tended to adhere to intact leaf surfaces, E. coli cells were entrapped in stomata and preferentially penetrated through the cut edge, which protected them from disinfection by chlorine treatment. For Salmonella enterica serovar Typhimurium, attachment preferentially occurred at the plant cell wall junction, suggesting that there might be a receptor site at this location for bacterial attachment (16). Virus adsorption to lettuce has also been found to vary depending on the strain and surface properties of the virus. Feline calicivirus (FCV) had a higher level of attachment to lettuce when the pH was above its isoelectric point (pI), while for bacteriophage MS2, strong adsorption to lettuce was observed at a value below its pI (21). As viruses are small particles that most likely are associated with feces when they are present in biosolids or animal manure, it is important to understand the mechanism of their attachment to and internalization by leafy greens if biosolids or manure is used in vegetable production. The objective of this study was to evaluate murine norovirus 1 (MNV), a widely used surrogate for human NoV, to determine its adsorption to and internalization by lettuce after the virus was stored in manure or biosolids for up to 30 days, and confocal microscopy was also used to observe virus on lettuce.
MATERIALS AND METHODS
Viruses.
MNV-1.CW1 was propagated in the RAW 264.7 cell line cultured in Dulbecco's modified Eagle's medium (DMEM) (Gibco-Invitrogen, CA) supplemented with 10% fetal bovine serum (FBS), 1% penicillin/streptomycin, 1% glutamine, 1% HEPES buffer, and 1% glutamate (25). Virus-infected cell lysates were purified by three freeze-thaw cycles, and the supernatant was recovered after centrifugation at 2,500 × g for 15 min and stored at −80°C for further study. A plaque assay was conducted as described previously (25). In brief, virus was diluted and inoculated onto confluent monolayers of RAW 264.7 cells grown in 12-well plates, and after 2 h of agitation at 37°C, the inoculum was aspirated and the cells were overlaid with 1 ml of 1.5% SeaPlaque agarose in 2× DMEM containing 2% FBS. The plates were incubated at 37°C with 5% CO2 for 48 h, and plaques were visualized by staining with 0.5 ml complete minimum essential medium Eagle containing 0.5% neutral red per well for 6 to 8 h.
Biosolids and animal manure.
Biosolids were obtained from the Back River Wastewater Treatment Plant (WWTP) (Baltimore). Solid swine manure (SM) was collected from a local farm in Kent County, DE. Liquid dairy manure (DM) was collected from the University of Delaware College of Agriculture and Natural Resources farm. Manure and biosolids were autoclaved and stored at 4°C before use.
Attachment of MNV in biosolids or animal manure to lettuce.
Romaine lettuce was purchased from a local supermarket (Newark, DE) and cut into pieces that were 1 by 1 cm. Four pieces were submerged in 5 ml of a suspension containing pure virus (∼2 × 105 PFU/ml) and agitated for 5 min. The lettuce pieces were then removed from the viral suspension and incubated at 4°C for 0.5, 6, 12, and 24 h. To analyze attachment, lettuce pieces were vortexed with 5 ml Hanks’ balanced salt solution (HBSS) for 10 min, and the concentration of attached MNV was determined by the plaque assay as described above. For liquid DM, MNV was inoculated into 5 ml of a manure suspension containing ∼2 × 105 PFU/ml (final concentration), and the MNV-contaminated DM suspension was then agitated with lettuce pieces. For biosolids and SM, 1 ml of an MNV suspension was added to 2 g biosolids or manure, dried for 15 min in a biosafety cabinet, vortexed for 30 min with 10 ml Na2HPO4 (0.15 M, pH 9.5), and diluted with HBSS to obtain a final concentration of ∼2 × 105 PFU/ml. Lettuce pieces were submerged in diluted biosolids or SM samples and incubated as described above. To study whether the survival of virus in manure or biosolids affected their attachment to lettuce, MNV was also incubated in biosolids, SM, and DM for 10, 20, and 30 days at 20°C, after which solid manure and biosolids were diluted as described above for the attachment study.
Internalization of MNV in biosolids or manure by lettuce.
Intact whole pieces of Romaine lettuce were dipped into MNV (∼25 cm2 of leaf was submerged in a virus solution) and incubated for 5 min. Each lettuce piece was removed and incubated at 4°C for 30 min before it was analyzed for virus attachment as described above. Similar lettuce pieces were cut so that they had an approximately 10-cm cut at the leaf edge and two 2-cm cuts at the stem, and they were analyzed in the same manner. To differentiate possible internalization from attachment, lettuce pieces were wiped with 1% Virkon for 3 min using a Q-tip to inactivate attached viruses but not internalized viruses. The internalized viruses were then recovered by vortexing in HBSS for 10 min, and the virus titer was determined by the plaque assay. The internalization ratio was calculated as follows: (quantity of MNV recovered from Virkon-wiped lettuce)/(quantity of MNV recovered after lettuce was removed from virus suspension) × 100. MNV in biosolids, SM, and DM on day zero were also used in the viral internalization study.
Virus particle purification.
Cesium chloride (CsCl)-purified MNV was used for confocal microscopy analysis of virus on lettuce. Purification of virus particles was performed as described previously (25), with little modification. RAW 264.7 cells were infected with MNV and incubated for 48 h; next, cellular debris was removed by three cycles of freezing and thawing and centrifugation at 2,500 × g for 15 min. The supernatant was layered on top of 3 ml 30% sucrose and centrifuged at 90,000 × g for 3 h using a Sorvall WX ultracentrifuge (Thermo Scientific, NC). The debris was then washed with phosphate-buffered saline (PBS), mixed with CsCl to obtain a final density of ∼1.336 g/cm3, and centrifuged at 115,000 × g for 22 h using the Sorvall WX ultracentrifuge (Thermo Scientific, NC). The gradient was fractionated, and the density of each fraction was determined to locate the virus particles. The density of MNV was 1.36 ± 0.04 g/cm3 (25). RAW 264.7 cells not infected with MNV were purified in the same way and used as a control.
Virus staining with SYBR gold.
The original SYBR gold stock solution (Invitrogen, CA) was diluted 1:1,250 with PBS, mixed with CsCl-purified MNV (∼1.5 × 108 PFU/ml) or a control solution at a 1:1 ratio, and agitated in the dark for 30 min. The SYBR gold-labeled MNV was then transferred to 100,000-molecular-weight Microcon centrifugal filter devices (Millipore, MA) and washed with PBS three times using centrifugation at 10,000 × g for ∼5 min. The MNV was then recovered from the membrane in PBS. Lettuce was cut into pieces that were 1 by 1 cm or 1 by 0.2 cm, added to 0.5 ml of a SYBR gold-labeled MNV suspension or a control solution, and agitated in the dark for 5 min, or 100 μl of an MNV suspension or a control solution was directly pipetted onto a lettuce piece that was 1 by 1 cm. The lettuce samples were analyzed by confocal light microscopy as described below.
Confocal light microscopy.
Confocal images were acquired with a Zeiss LSM 510 NLO laser scanning microscope (Carl Zeiss, Inc., Germany) using a Zeiss 40× C-Apochromat (1.2NA) water immersion objective lens. Multichannel images of SYBR gold fluorescence and autofluorescence were acquired in fastline-switch mode using the 488-nm laser line of a 25-mW argon laser (LASOS, Ebersberg, Germany) and 543-nm helium neon laser lines (LASOS) with a 560 long-pass emission filter. The SYBR gold fluorescence was green, and the plant autofluorescence was red. The confocal images were captured either as two-dimensional single optical sections or as three-dimensional Z stack optical sections.
Statistical analysis.
All experiments were performed with three replicates. The statistical analysis was conducted using an analysis of variance single-factor test with Office 2007 software to assess the significance of variations. Data were considered to be statistically significantly different if the P value was <0.05.
RESULTS AND DISCUSSION
Attachment of MNV in biosolids or animal manure to lettuce.
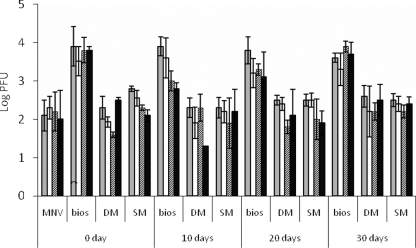
For pure virus or MNV in animal manure at day zero, ∼2 to 2.5 log PFU MNV attached to the lettuce pieces, while for MNV in biosolids, ∼4 log PFU virus attached, a value that was significantly higher than the values for the other three samples (Fig. 1). For storage in manure or biosolids, MNV was stable in the biosolids and SM, as there was no loss of infectious virus after 30 days of incubation at 20°C. For DM, there was a 1-log loss at 10 days, and then the infectivity titer remained at ∼4 log PFU/ml at 20 and 30 days. For MNV in SM and DM stored for 10, 20, and 30 days, ∼2 log PFU MNV was attached to the lettuce in all samples. For MNV in biosolids at all incubation times, ∼4 log PFU MNV was attached, and there was no significant difference from attachment at day zero (Fig. 1). These findings provide evidence that the length of incubation of biosolids or manure does not affect the attachment of virus to lettuce, as long as the virus remains infective during storage. After attachment to the lettuce, MNV was quite stable for both pure virus samples and all of the biosolids and manure samples, and no significant loss of infectious virus was observed after incubation for 24 h at 4°C (Fig. 1). While it is not known exactly why the biosolids enhanced MNV attachment to lettuce, FeCl3 is added to the Back River WWTP biosolids for phosphate control and the biosolids contain a significantly larger amount of iron than DM and SM (19). The presence of iron oxides has been shown to improve adsorption of virus (MS2 and φX174) to sand particles (26), and it was possible that more MNV particles aggregated on biosolids than on DM and SM and thus were concentrated on biosolids particles, which led to increasing virus attachment to lettuce.
FIG. 1.
Quantity of virus on lettuce after lettuce pieces were agitated in a pure MNV solution or a biosolids, SM, or DM suspension (after manure or biosolids were incubated for 0, 10, 20, and 30 days at 20°C) and virus stability after lettuce pieces were removed from the virus solution or manure suspension and incubated at 4°C for up to 24 h. The incubation times were 0.5 h (gray bars), 6 h (open bars), 12 h (bars with diagonal lines), and 24 h (black bars). bios, biosolids.
Internalization of MNV in biosolids or manure by lettuce.
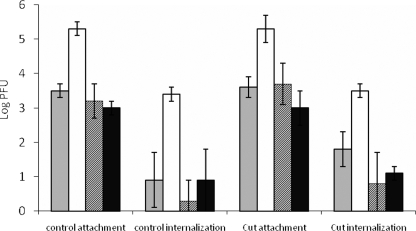
Virkon was used to inactivate attached MNV but not internalized MNV, and our preliminary study showed that wiping lettuce with 1% Vikon for 3 min could inactivate >4 log MNV/cm2 (23). For both control (noncut) and cut lettuce samples, ∼3 log PFU of virus was attached for pure MNV or MNV in SM and DM, while for biosolids, ∼5 log PFU was attached (Fig. 2). For MNV in biosolids ∼3 log PFU of virus was internalized by both control and cut lettuce pieces, a value which was significantly higher than the value for pure MNV or MNV in DM or SM (∼1 log PFU). However, there was no significant difference in the internalization ratio between intact and cut lettuce pieces dipped in the same MNV sample (P < 0.05); also, there was no significant difference in the internalization ratios for all four MNV samples (Table 1). This indicates that the significantly higher level of internalization of MNV in biosolids resulted from the large number of attached virus particles. There were no significant differences in either attachment or internalization between control and cut lettuce pieces for all four MNV samples, implying that MNV may be internalized by lettuce through some mechanism other than entry through open cuts. However, the level of internalization of pure MNV was a bit higher for cut lettuce based on both raw data and confocal microscopy, and the lack of statistical differences between cut and noncut samples could also have resulted from variations due to virus behavior (8).
FIG. 2.
Internalization of pure MNV or MNV in biosolids, DM, or SM by lettuce. Intact lettuce pieces or lettuce pieces with a long cut on the edge and short cuts on the stem were submerged in a manure suspension for 5 min. Each lettuce piece was then wiped with 1% Virkon to eliminate the attached viruses but not the internalized viruses. Gray bars, pure virus; open bars, MNV in biosolids; bars with diagonal lines, MNV in DM; black bars, MNV in SM.
TABLE 1.
Internalization ratios for pure MNV and MNV in biosolids or manure for lettuce pieces
| Virus | Internalization ratioa |
|
|---|---|---|
| Control (intact lettuce) | Lettuce with cuts | |
| Pure virus | 0.5 ± 0.5 | 3.0 ± 2.9 |
| MNV in biosolids | 0.1 ± 0.1 | 0.65 ± 1.0 |
| MNV in SM | 1.7 ± 1.8 | 1.5 ± 1.2 |
| MNV in DM | 1.7 ± 1.8 | 1.9 ± 1.1 |
For each sample, the mean and standard deviation were calculated based on the results for three replicates. The internalization ratio was calculated as follows: (quantity of MNV recovered from Virkon-wiped lettuce)/(quantity of MNV recovered after lettuce was removed from virus suspension) × 100.
Observation of virus on lettuce by confocal microscopy.
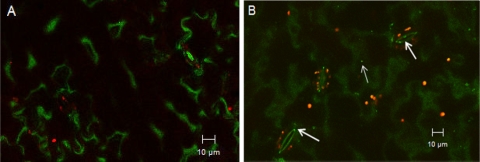
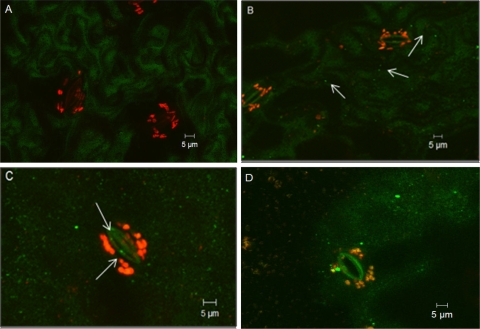
SYBR gold is a sensitive fluorescent dye for detecting double- or single-stranded DNA or RNA, and it has been widely used to enumerate viral particles collected from natural seawater with epifluorescence microscopy (6, 18, 24). However, since our virus samples were obtained from cell lysates, cell debris could also bind to the SYBR gold dye and emit fluorescent signals. Purification of the virus with CsCl greatly reduced the contamination from cellular debris, as few fluorescent dots were observed with the control, and the control sample was significantly different from purified virus samples (data not shown). We observed that SYBR gold-labeled viruses were attached to lettuce surfaces after virus was pipetted directly onto lettuce or after lettuce was agitated in virus suspensions (Fig. 3 and 4). Furthermore, with both treatments, viral particles were occasionally seen inside stomata (∼1 to 2 μm inside stomata), suggesting that viruses could internalize through the guard cells in lettuce. Viruses were also observed on the cut edges of lettuce pieces; however, from the front view of a lettuce surface, it was difficult to differentiate whether the viruses were on the surface or inside the cut edge (Fig. 4D). Front views of the cut edges showed that viruses were inside the cut (under the epidermis) about 3.5 μm from the cut edge (Fig. 5), which could be protected from washing and sanitization. With control samples, no viral particles were observed on lettuce surfaces or inside the cut edges. As viruses could internalize in lettuce through both the stomata and the cut edges, these observations may explain why cut samples had more MNV particles but there was no statistical difference in internalization compared to noncut lettuce, as shown in Fig. 2.
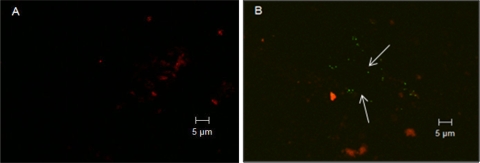
FIG. 3.
Confocal microscopy images of control (A) and MNV attached to Romaine lettuce (B) after 100-μl drops were pipetted on lettuce leaves. The arrows indicate MNV on the lettuce surface or inside stomata. Green indicates plant cell walls, and red indicates autofluorescence from plant chlorophyll.
FIG. 4.
Confocal three-dimensional stack images of lettuce pieces agitated in a control solution (A) and an MNV suspension (B) for 5 min. (C and D) Virus in stoma (C) and attached to a cut edge (D) after 5 min of agitation. In panel D, the green fluorescence in the middle indicates the cut edge, and the red fluorescence on the left indicates cellular leakage. The arrows indicate MNV on lettuce surfaces (B) or in stomata (C).
FIG. 5.
Confocal microscopy images of front views of cut edges after lettuce was agitated in a control solution (A) and in an MNV suspension (B). MNV is indicated by arrows. The virus in panel B was ∼3.5 μm inside the cut edge.
It was reported previously that stomata and damaged areas or cuts were important in protecting food-borne microorganisms such as E. coli from different sanitizers (10, 17, 20). After submersion of a leaf in an E. coli suspension, E. coli cells were found in most stomata without penetration (10, 17). Compared with intact surfaces, E. coli also seemed to preferentially attach to cut edges, and during a 24-h incubation period, E. coli was found to penetrate cut edges, while little penetration was observed for bacteria on intact surfaces (17). Since viruses are different from bacteria and are considered nonliving when they are outside their hosts, the attachment of viruses to stomata or to a cut edge is a matter of probability rather than preference. As shown in our study, viruses were found on lettuce surfaces but only occasionally in stomata, while E. coli preferred to gather inside the stomata (17). The major driving force behind virus attachment should be physicochemical forces that control the interactions between viruses and plant surfaces. But little is known about such interactions, and different viruses and phages have exhibited viable attachment patterns (21). Since enteric viruses are frequently associated with feces and biosolids in the environment, information on interactions between solids, viruses, and leaf surfaces can contribute to reliable methods that prevent attachment or remove attached viral particles.
In conclusion, this study showed that biosolids could promote the attachment of MNV to lettuce and resulted in an increased number of virus internalized in lettuce, which may pose a food safety risk. Also, it was found that MNV, like bacteria (17), could internalize in lettuce through cut edges as well as stomata. Since the infectious dose of human norovirus is as low as <100 particles (5), the viruses that escape from sanitization during washing due to protection by stomata or cut edges could pose a threat to food safety as well as to human health.
Acknowledgments
We thank Kirk J. Czymmek and Deborah H. Powell (Delaware Biotechnology Institute, University of Delaware) for help with the confocal microscopy. We also thank Christiane Wobus (Department of Microbiology and Immunology, University of Michigan Medical School) for help with MNV purification.
This project was funded in part by USDA CSREES NRI Water Shed Project grant 2006-35102-17405.
Footnotes
Published ahead of print on 20 November 2009.
REFERENCES
- 1.Allwood, P. B., Y. S. Malik, C. W. Hedberg, and S. M. Goyal. 2004. Effect of temperature and sanitizers on the survival of feline calicivirus, Escherichia coli, and F-specific coliphage MS2 on leafy salad vegetables. J. Food Prot. 67:1451-1456. [DOI] [PubMed] [Google Scholar]
- 2.Boyer, R. R., S. S. Sumner, R. C. Williams, M. D. Pierson, D. L. Popham, and K. E. Kniel. 2007. Influence of curli expression by Escherichia coli O157:H7 on the cell's overall hydrophobicity charge and ability to attach to lettuce. J. Food Prot. 70:1339-1345. [DOI] [PubMed] [Google Scholar]
- 3.Brandl, M. T. 2008. Plant lesions promote the rapid multiplication of Escherichia coli O157:H7 on postharvest lettuce. Appl. Environ. Microbiol. 74:5285-5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brooks, J. P., B. D. Tanner, K. L. Josephson, C. P. Gerba, C. N. Haas, and I. L. Pepper. 2005. A national study on the residential impact of biological aerosols from the land application of biosolids. J. Appl. Microbiol. 99:310-322. [DOI] [PubMed] [Google Scholar]
- 5.Caul, E. O. 1994. Small round structured viruses: airborne transmission and hospital control. Lancet 343:1240-1242. [DOI] [PubMed] [Google Scholar]
- 6.Chen, F., J. Lu, B. J. Binder, Y. Liu, and R. E. Hodson. 2001. Application of digital image analysis and flow cytometry to enumerate marine viruses stained with SYBR gold. Appl. Environ. Microbiol. 67:539-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.da Silva, A. K., J. L. Saux, S. Parnaudeau, M. Pommepuy, M. Elimelech, and F. S. Le Guyader. 2007. Evaluation of removal of noroviruses during wastewater treatment, using real-time reverse transcription-PCR: different behaviors of genogroups I and II. Appl. Environ. Microbiol. 73:7891-7897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D'Souza, D. H., A. Sair, K. Williams, E. Papafragkou, J. Jean, C. Moore, and L. Jaykus. 2006. Persistence of caliciviruses on environmental surfaces and their transfer to food. Int. J. Food Microbiol. 108:84-91. [DOI] [PubMed] [Google Scholar]
- 9.Grotto, I., M. Huerta, R. D. Balicer, T. Halperin, D. Cohen, N. Orr, and M. Gdalevich. 2004. An outbreak of norovirus gastroenteritis on an Israeli military base. Infection 32:339-343. [DOI] [PubMed] [Google Scholar]
- 10.Hassan, A. N., and J. F. Frank. 2003. Influence of surfactant hydrophobicity on the detachment of Escherichia coli O157:H7 from lettuce. Int. J. Food Microbiol. 87:145-152. [DOI] [PubMed] [Google Scholar]
- 11.Holtby, I., G. M. Tebbutt, J. Green, J. Hedgeley, G. Weeks, and V. Ashton. 2001. Outbreak of Norwalk-like virus infection associated with salad provided in restaurant. Commun. Dis. Public Health 4:305-310. [PubMed] [Google Scholar]
- 12.Lynch, M. F., R. V. Tauxe, and C. W. Hedberg. 2009. The growing burden of foodborne outbreaks due to contaminated fresh produce: risks and opportunities. Epidemiol. Infect. 137:307-315. [DOI] [PubMed] [Google Scholar]
- 13.Makary, P., L. Maunula, T. Niskanen, M. Kuusi, M. Virtanen, S. Pajunen, J. Ollgren, and N. N. Tran Minh. 2009. Multiple norovirus outbreaks among workplace canteen users in Finland, July 2006. Epidemiol. Infect. 137:402-407. [DOI] [PubMed] [Google Scholar]
- 14.Mattison, K., A. Shukla, A. Cook, F. Pollari, R. Friendship, D. Kelton, S. Bidawid, and J. M. Farber. 2007. Human noroviruses in swine and cattle. Emerg. Infect. Dis. 13:1184-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saggers, E. J., C. R. Waspe, M. L. Parker, K. W. Waldron, and T. F. Brocklehurst. 2008. Salmonella must be viable in order to attach to the surface of prepared vegetable tissues. J. Appl. Microbiol. 105:1239- 1245. [DOI] [PubMed] [Google Scholar]
- 17.Seo, K. H., and J. F. Frank. 1999. Attachment of Escherichia coli O157:H7 to lettuce leaf surface and bacterial viability in response to chlorine treatment as demonstrated by using confocal scanning laser microscopy. J. Food Prot. 62:3-9. [DOI] [PubMed] [Google Scholar]
- 18.Shibata, A., Y. Goto, H. Saito, T. Kikuchi, T. Toda, and S. Taguchi. 2006. Comparison of SYBR Green I and SYBR Gold stains for enumerating bacteria and viruses by epifluorescence microscopy. Aquat. Microb. Ecol. 43:223-231. [Google Scholar]
- 19.Shober, A. L., and J. T. Sims. 2009. Evaluating phosphorus release from biosolids and manure-amended soils under anoxic conditions. J. Environ. Qual. 38:309-318. [DOI] [PubMed] [Google Scholar]
- 20.Takeuchi, K., and J. F. Frank. 2001. Quantitative determination of the role of lettuce leaf structures in protecting Escherichia coli O157:H7 from chlorine disinfection. J. Food Prot. 64:147-151. [DOI] [PubMed] [Google Scholar]
- 21.Vega, E., J. Smith, J. Garland, A. Matos, and S. D. Pillai. 2005. Variability of virus attachment patterns to butterhead lettuce. J. Food Prot. 68:2112-2117. [DOI] [PubMed] [Google Scholar]
- 22.Venglovsky, J., J. Martinez, and I. Placha. 2006. Hygienic and ecological risks connected with utilization of animal manures and biosolids in agriculture. Livest. Sci. 102:197-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei, J., K. E. Kniel, Y. Jin, and T. Sims. 2008. Survival of norovirus in biosolids, T106. Int. Assoc. Food Prot. Annu. Meeting, Columbus, OH, 3 to 6 August 2008.
- 24.Wen, K., A. C. Ortmann, and C. A. Suttle. 2004. Accurate estimation of viral abundance by epifluorescence microscopy. Appl. Environ. Microbiol. 70:3862-3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wobus, C. E., S. M. Karst, L. B. Thackray, K. Chang, S. V. Sosnovtsev, G. Belliot, A. Krug, J. M. Mackenzie, K. Y. Green, and H. W. Virgin IV. 2004. Replication of Norovirus in cell culture reveals a tropism for dendritic cells and macrophages. PLoS Biol. 2:2076-2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.You, Y., J. Han, P. C. Chiu, and Y. Jin. 2005. Removal and inactivation of waterborne viruses using zerovalent iron. Environ. Sci. Technol. 39:9263-9269. [DOI] [PubMed] [Google Scholar]