Abstract
Hydrogen peroxide production by vaginal lactobacilli represents one of the most important defense mechanisms against vaginal colonization by undesirable microorganisms. To quantify the ability of a collection of 45 vaginal Lactobacillus strains to generate H2O2, we first compared three published colorimetric methods. It was found that the use of DA-64 as a substrate rendered the highest sensitivity, while tetramethyl-benzidine (TMB) maintained its linearity from nanomolar to millimolar H2O2 concentrations. Generation of H2O2 was found to be especially common and strong for L. jensenii strains, while it was variable among L. crispatus and L. gasseri strains. Biosynthesis of H2O2 only occurred upon agitation of the cultures, but the H2O2-producing machinery was already present in them before aeration started. Calcium, magnesium, manganese, and zinc ions did not affect H2O2 production, while Cu2+ inhibited the growth of Lactobacillus jensenii CECT 4306, which was chosen as a model strain. Cultures with Fe3+, hemin, and hemoglobin did not accumulate H2O2. Fe3+ activated an extracellular peroxidase that destroyed the H2O2 being produced by the cultures. This protected the lactobacilli against its antimicrobial effect. The production of the enzyme appears to be constitutive, the Fe3+ ions being a necessary cofactor of the reaction.
The vaginal microbiota of healthy, fertile women is dominated by lactobacilli. Their proportion in this habitat is consistently higher than 70%, in some cases being practically exclusive (8, 20, 26). This abundance contrasts with their scarcity on other mucosae; for example, they never account for more than 1% of the intestinal microbiota and, in many cases, they are not found in feces (35).
Upon first isolation, the vaginal lactobacilli behave as fastidious microorganisms, whose recovery and growth are improved by the addition of a heme source, such as hemin or hemoglobin, to the medium and by incubation under an atmosphere enriched to 10% CO2 or even under anaerobic conditions. The identification of newly isolated strains leads to their ascription to Lactobacillus acidophilus or L. fermentum when culture-dependent methods are used (1, 30). However, genotyping reveals a predominance of L. crispatus, L. gasseri, and L. jensenii, with L. iners and L. vaginalis also being found (5, 6, 34).
The dominance of the lactobacilli in the vagina suggests that they play a fundamental role in the protection of the cavity against the generation of pathological conditions. Among them are those caused by the excessive proliferation of indigenous microorganisms, such as Gardnerella vaginalis, whose dominance may lead to bacterial vaginosis, and colonization by pathogens, such as Candida spp. and Trichomonas vaginalis, which may induce vaginitis or, less frequently, cervicitis and other regional and systemic problems (15, 24, 28).
The antagonistic properties of the vaginal microbiota have two complementary aims: promotion of specific adherence to the epithelial cells and production of antimicrobial substances. Of these, the generation of organic acids and hydrogen peroxide is of paramount importance. Lactobacilli present a strictly fermentative metabolism of sugars, with production of mainly lactic acid (4). As a consequence, the physiological pH of the vagina is around 4, which is inhibitory to the growth of many potential colonizers. In fact, a differential symptom of bacterial vaginosis is an exudate with a pH close to neutral (24, 28).
Hydrogen peroxide generation appears to be common among vaginal lactobacilli, while it is exceptional in strains that usually live in the intestines or in the external environment, such as L. plantarum and L. paracasei, even if they are isolated from the vagina (29). Hawes et al. (13) have shown that the frequency of vaginosis is lower among women that harbor H2O2-producing lactobacilli (3%) than among those colonized by H2O2-negative strains (25%) or by other bacterial species (46%). However, not all H2O2-positive lactobacilli appear to be equally protective, with the most efficient strains belonging to the species L. crispatus and L. jensenii (2).
The bactericidal effect of H2O2 operates through the generation of oxidizing metabolites, such as the radical OH− that introduces breaks in the DNA of the cell. This effect is notably enhanced in the acidic vaginal environment by the presence of myeloperoxidase and halides, which are very abundant in the uterine mucus, especially during ovulation (17, 18).
Given the apparent importance of hydrogen peroxide in the maintenance of vaginal homeostasis, we wanted to know the capacity for generation of this compound of a collection of vaginal lactobacilli, as part of selecting those with the best probiotic properties (19). Previously, we compared the sensitivity and range of measurable concentrations provided by three published H2O2 quantification methods (11, 22, 25). We have also studied the influence of a series of environmental conditions on the production and persistence of H2O2 in the cultures and how H2O2 affects the viability of the lactobacilli producing it.
MATERIALS AND METHODS
Microorganisms and culture conditions.
In this study, 45 vaginal isolates of Lactobacillus, obtained from healthy premenopausal women, were used. They were previously classified as L. jensenii (17 strains), L. crispatus (21 strains), L. gasseri (6 strains), and L. plantarum (one strain) (19). L. jensenii CECT 4306 was used as a positive control for H2O2 generation. The bacteria were grown on MRS agar (Oxoid) supplemented with 1% hemoglobin (Sigma) and incubated at 37°C under a 10% CO2 atmosphere. Liquid cultures were performed in MRS medium and incubated under the same conditions without agitation. Inhibition of protein synthesis was performed with 100 μg/ml chloramphenicol (it was previously determined that the MIC was 1.5 μg/ml).
Quantitative hydrogen peroxide determination tests.
The concentration of H2O2 was determined colorimetrically through detection of the absorbance induced with the following chromophores: N-(carboxymethylaminocarbonyl)-4,4′-bis(dimethylamino)-diphenylamine sodium salt (DA-64) (Wako), 3,3′,5,5′-tetramethylbenzidine (TMB) (Sigma), and o-dianisidine (Sigma) (11, 22, 25). The reaction mixtures were set up as follows: a commercial H2O2 stock solution (30%, corresponding to 9.812 M) was diluted with 100 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES) buffer (pH 6.5) or MRS broth to 1 M. The working concentrations were prepared by diluting this solution with PIPES buffer by a factor of at least 10 (to avoid any color interference by the MRS broth), and 100-μl amounts of the resulting solutions were mixed with 100 μl of the corresponding chromophore (10 mM, final concentration) and 2 μl of a horseradish peroxidase solution (1 mg/ml). After incubation for 10 min at 16°C, the absorbance was measured at 405, 620, and 727 nm for o-dianisidine, tetramethylbenzidine, and DA-64, respectively.
H2O2 generation by the Lactobacillus cultures.
Cultures were set up in 10 ml of liquid MRS medium by inoculation with 1 ml of a 48-h-old culture. Incubation proceeded for several time periods (usually 8 or 16 h) at 37°C under a 10% CO2 atmosphere or anaerobically, without agitation. The following cofactors (2 mM, final concentration) were added to the cultures to determine their influence on H2O2 production: MgCl2, MnSO4, FeCl3, CaCl2, ZnCl2, and CuSO4 (Sigma). Hemoglobin and hemin (1% and 30 mM final concentration, respectively) (Sigma) were also tested. At the end of the incubation period, H2O2 generation was induced by placing the cultures in 100-ml Erlenmeyer flasks, which were subjected to agitation at 300 rpm for up to 3 h at 37°C. Samples (220 μl) were taken every 30 min and centrifuged for 2 min at 12,000 rpm, and the supernatants stored at 4°C until the end of the experiment. The concentration of H2O2 was immediately determined as described in the previous paragraph, using at least three replicates from each sample.
H2O2 degradation measurements.
Cultures of L. jensenii CECT 4306 were set in liquid MRS medium with or without 2 mM FeCl3. After incubation for 16 h at 37°C, the culture grown without added Fe cations was divided into two aliquots and one of them was adjusted to 2 mM Fe3+. Fifteen minutes later, H2O2 (2.5 mM final concentration) was added to the three cultures and incubation continued at 37°C for 3 h. Samples were taken every 15 min, and the residual H2O2 concentration was quantified with tetramethylbenzidine.
RESULTS
Comparison of H2O2 determination tests.
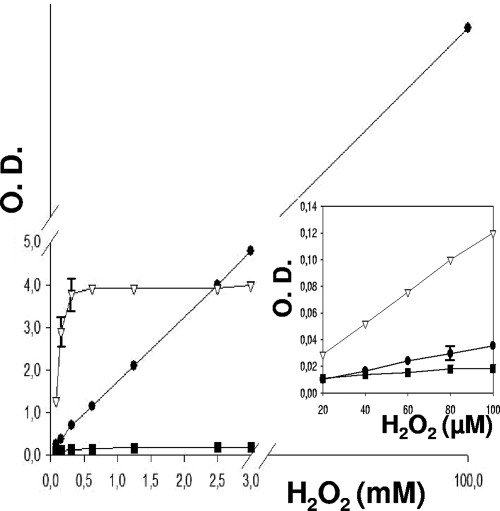
H2O2 in solution was quantified by measuring the degree of color generation by oxidation of 3,3′,5,5′-tetramethylbenzidine (TMB), o-dianisidine, and DA-64 in the presence of horseradish peroxidase (Fig. 1).
FIG. 1.
Standard curves of the relation between the concentration of hydrogen peroxide and the intensity of color produced by its reaction with the chromophores tetramethylbenzidine (•), o-dianisidine (▪), and DA-64 (▿), catalyzed by horseradish peroxidase (the wavelengths used were 620, 405, and 727 nm, respectively). DA-64 was the most sensitive at low concentrations of hydrogen peroxide (inset). Error bars show standard deviations. O.D., optical density.
The procedure using DA-64 turned out to be the most sensitive at low concentrations of hydrogen peroxide (Fig. 1, inset). However, the system became saturated at relatively low H2O2 concentrations. In contrast, the reaction mixtures set with TMB kept the linearity of the determination up to at least 100 mM, irrespective of whether the determination was made in PIPES or in 1/10 volume of MRS (diluted in PIPES).
H2O2 generation in response to aeration by vaginal lactobacilli.
As part of a previous communication, the isolation of 45 strains of lactobacilli from the vaginas of an equal number of healthy, fertile women was reported (19). Liquid cultures of all these strains were taken during the exponential and stationary phases and subjected to strong agitation under a normal aerobic atmosphere for 3 h, and the generation of H2O2 was quantified at 30-min intervals, using DA-64 and TMB to cover the whole range of production shown by the strains.
The kinetics of production was similar for all producing strains, i.e., H2O2 started to accumulate after 30 min of aeration, with no production in static cultures (Fig. 2).
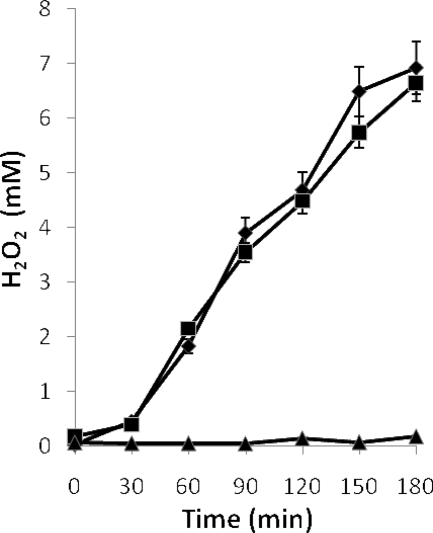
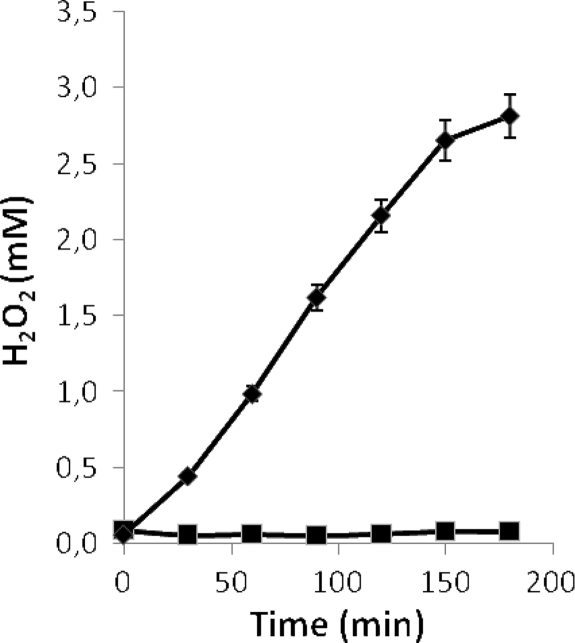
FIG. 2.
Production of hydrogen peroxide by exponential-phase cultures of L. jensenii CECT 4306 (8 h of incubation) subjected to aeration (▪) or maintained in static conditions (▴). The same culture was treated with chloramphenicol 30 min before the start of aeration (♦). Error bars show standard deviations.
The data obtained indicate that the majority of strong producers were L. jensenii strains, followed by L. crispatus isolates (Table 1). The cultures of strains that achieved a high H2O2 concentration at 8 h of incubation (exponential phase) remained so or with slightly lower values at 16 h (stationary phase). Some others reached levels of 0.5 mM or more only after 16 h of incubation.
TABLE 1.
Classification of the 45 strains of vaginal lactobacilli studied as a function of their H2O2 generation ability
| Species | Amt of H2O2 (mM) generated at indicated timea |
Total no. of strains | |||||||
|---|---|---|---|---|---|---|---|---|---|
| <0.3 |
0.3 to 1 |
1 to 2 |
>2 |
||||||
| 8 h | 16 h | 8 h | 16 h | 8 h | 16 h | 8 h | 16 h | ||
| L. crispatus | 18 | 16 | 3 | 5 | 0 | 0 | 0 | 0 | 21 |
| L. gasseri | 5 | 4 | 0 | 2 | 1 | 0 | 0 | 0 | 6 |
| L. jensenii | 6 | 5 | 6 | 7 | 4 | 2 | 1 | 3 | 17 |
| L. plantarum | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Samples were taken from liquid MRS cultures incubated for 8 and 16 h at 37°C under a 10% CO2 atmosphere.
Cultures of L. jensenii CECT 4306 (a strong H2O2 producer, which was chosen for further work) treated with chloramphenicol prior to the agitation period produced concentrations of H2O2 similar to those in the untreated cultures (Fig. 2), indicating that the specific H2O2-generating system was already present in the cells before the culture was subjected to aeration and, consequently, that this treatment did not act as an inducer of the system.
Effect of cofactors on H2O2 biosynthesis.
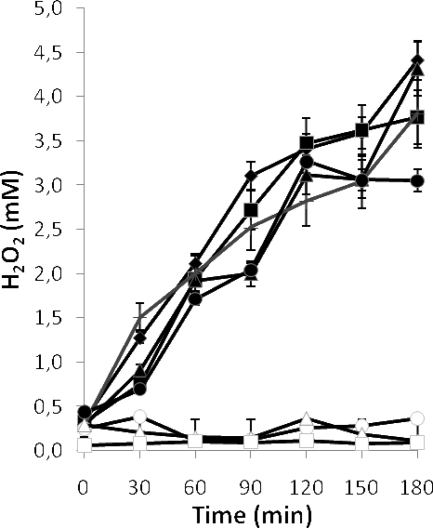
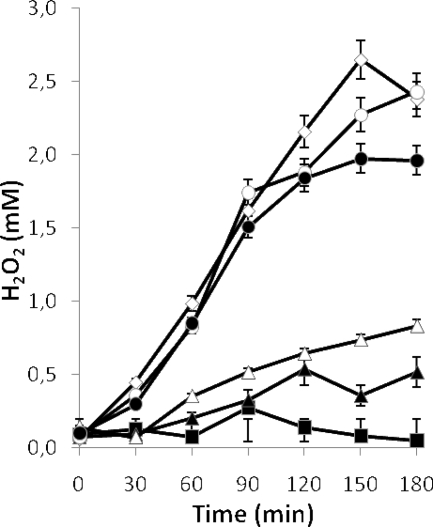
To determine whether H2O2 generation could be improved, different cofactors were added to cultures of L. jensenii CECT 4306 at the beginning of the incubation. Supplementation with Mg2+, Mn2+, Ca2+, and Zn2+ affected the production of H2O2 only slightly with respect to the levels in cultures grown in MRS without additions (Fig. 3). Cu2+ inhibited bacterial growth. Surprisingly, cultures containing Fe3+, hemin, or hemoglobin did not accumulate H2O2 upon agitation (Fig. 3), while the growth of the strain was generally slightly improved (data not shown, but see Fig. 7).
FIG. 3.
Effect of the addition of cations (2 mM) and other cofactors on H2O2 generation by cultures of L. jensenii CECT 4306 grown at 37°C for 16 h and subjected afterwards to aeration. ♦, No additions; ▴, Mg3+; ▪, Ca3+; •, Zn3+; + (gray line), Mn3+; ○, Fe3+; □, 30 mM hemin; ▵, 1% hemoglobin. Error bars show standard deviations.
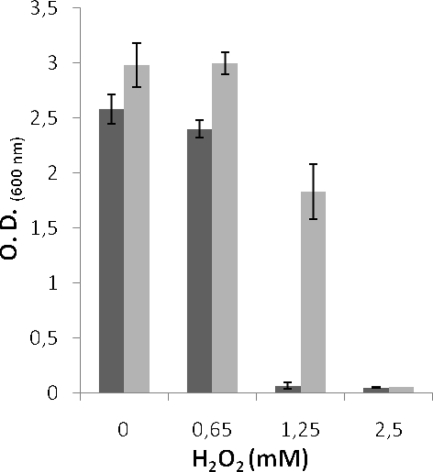
FIG. 7.
Effect of Fe3+ on the growth of L. jensenii CECT 4306 cultures in the presence of increasing H2O2 concentrations. The absorbance of the cultures after 6 h of incubation is represented. Light gray bars, cultures with added Fe3+; dark gray bars, cultures without added Fe3+. Error bars show standard deviations. O.D., optical density.
Fe3+ induces degradation of H2O2 by lactobacilli.
The lack of H2O2 in the supernatants of cultures grown in the presence of Fe3+ might be due to inability to generate the compound or to its subsequent destruction. To discriminate between these possibilities, two complementary experiments were performed. In the first, cultures were set up with increasing iron concentrations (from 25 nM to 2 mM), and their capacity to generate H2O2 after 16 h of incubation was tested. An inverse relationship was found between the concentrations of Fe3+ added to the cultures and those of H2O2 recovered (Fig. 4). In the second, cultures were set up and H2O2 was added to them after growth for 16 h. The culture medium with or without Fe3+ and the cultures grown without an excess of the cation did not destroy H2O2. Conversely, Fe3+-containing cultures eliminated the exogenously added H2O2, as did the cultures grown in the absence of added Fe3+ upon addition of the cation (Fig. 5).
FIG. 4.
Effects of different concentrations of Fe3+ added to cultures of L. jensenii CECT 4306 on H2O2 generation upon aeration. ⋄, Control culture without added Fe3+; ○, 25 nM; •, 50 nM; ▵, 0.1 mM; ▴, 0.2 mM; ▪, 2 mM. Error bars show standard deviations.
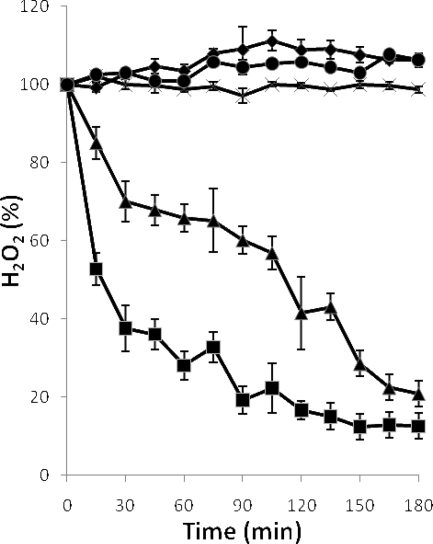
FIG. 5.
Degradation of H2O2 by cultures of L. jensenii CECT 4306. Cultures were grown for 16 h in MRS medium prior to the addition of H2O2 (2.5 mM). Results shown are for replicate cultures that were supplemented with 2 mM Fe3+ (▪) or grown without supplements (×), the same unsupplemented cultures to which 2 mM Fe3+ and H2O2 were added with a 30-min interval (▴), and uninoculated MRS medium with (♦) and without (•) added Fe3+. Error bars show standard deviations.
To determine whether Fe3+ was an inducer of the synthesis of a peroxidase or simply acted as a cofactor for a preformed enzyme, chloramphenicol was added to aliquots of 16-h-old cultures grown in MRS medium with or without Fe3+, to inhibit their protein synthesis. After 30 min, Fe3+ was added to the latter cultures and incubation continued for 30 min before the cells were subjected to forced aeration. The cultures with Fe3+ did not accumulate H2O2, irrespective of the moment of its addition and whether chloramphenicol was present or not, while parallel controls without Fe3+ did. This indicates that iron was a necessary cofactor for the enzymatic activity of a constitutively synthesized peroxidase (Fig. 6).
FIG. 6.
Effect of inhibition of protein synthesis on H2O2 accumulation by aerated cultures of L. jensenii CECT 4306. Results shown are for replicate control cultures grown without Fe3+ (♦) and replicate cultures grown without Fe3+ and subjected successively to 100 μg/ml chloramphenicol, 2 mM Fe3+, and aeration at 30-min intervals (▪). Similar data for lack of H2O2 accumulation were obtained from cultures grown in the presence of Fe3+ and the same cultures treated with chloramphenicol. Error bars show standard deviations.
Protective role of Fe3+ against H2O2-driven growth inhibition of lactobacilli.
Cultures of L. jensenii were inoculated into liquid MRS medium with or without Fe3+, containing increasing concentrations of H2O2. Incubation proceeded for 6 h at 37°C. The data obtained (Fig. 7) indicate that Fe3+ slightly improved the growth of the strain (cultures without H2O2) and that this compound, at concentrations equal to or greater than 2.5 mM, completely inhibited the development of L. jensenii. However, complete inhibition of growth was observed at 1.25 mM H2O2 in the cultures devoid of Fe3+, while those containing the cation were only marginally inhibited.
DISCUSSION
Usually, determination of the ability of vaginal lactobacilli to generate H2O2 is performed by semiquantitative methods based mainly on the growth of the strains under anaerobiosis on the surface of solid medium containing a chromophore and a peroxidase (8, 25). The resulting colonies are exposed to air to allow color development by the positive strains. The intensity of the color is the clue for classification of the lactobacilli as non-, weak, or strong H2O2 producers. However, a more precise determination is desirable to allow for the selection of probiotic precursor strains with the most significant properties for protection of the vaginal environment. In this respect, it must be taken into account that H2O2-producing strains appear to be more stable in the vaginal environment (32) and to effectively antagonize undesirable microorganisms (3, 8, 17, 32), including those that produce sexually transmitted infections, such as Neisseria gonorrhoeae (31).
We chose to test the three selected H2O2 quantification methods because they use similar technologies, thus allowing an easy comparison. Other methods, such as iodination (10) and oxidation of 4-amino-antipyrine (12), were discarded because they were considered to have a low sensitivity (11) or because there was some interference with components of MRS medium (21), the medium used almost universally to grow lactobacilli. The data obtained make the use of both the DA-64- and the TMB-based procedures advisable. The first will allow a precise quantification at low H2O2 concentrations due to its extreme sensitivity, while the second maintains its usefulness through a wide range of concentrations, allowing discrimination between high producers.
The data obtained by applying these procedures to our 45 strains were only partially coincident with those reported after testing H2O2 production on TMB-plus solid medium (19, 25). In both cases, L. jensenii accounted for the highest proportion of producing strains, but some that were considered to be strong producers on TMB-plus medium behaved differently after growth in liquid medium and vice versa.
The application of these methods also enabled the study of the characteristics of H2O2 generation and degradation by L. jensenii CECT 4306, a vaginal isolate that was chosen as a model.
Hydrogen peroxide starts to be produced as a consequence of the aeration of the cultures, presumably because the static liquid cultures are virtually anaerobic, i.e., the limiting factor is the absence of O2 rather than the lack of the responsible enzymes. In fact, the H2O2 generation system was already present in the cultures prior to aeration, as was determined with cultures whose protein synthesis was inhibited before aeration started. This suggests that the production of H2O2 is constitutive upon exposure of the cells to oxygen. Synthesis of H2O2 in response to aeration is common not only among vaginal lactobacilli but in other lactic acid bacteria as well. However, instead of being considered a positive trait, it is perceived as detrimental for starters of food fermentation because it may inhibit their growth (21, 27, 33) and/or affect the sensory quality of the products; for example, H2O2 induces fat rancidity and discoloration of cured meats (23, 33).
Most probably, H2O2 generation proceeds through oxidation of NADH and/or pyruvate by the corresponding dehydrogenases, as happens in Lactobacillus delbrueckii (21) and Bifidobacterium bifidum (16). Furthermore, in the sequenced genomes of two strains of Lactobacillus acidophilus and L. gasseri, two species closely related to L. jensenii, there are genes that encode putative pyruvate dehydrogenases, and the first harbors an NADH oxidase as well. In addition, neither encodes sequence-recognizable superoxide dismutase determinants (GenBank accession numbers NC_006814 and NC_008530).
H2O2, either produced by L. jensenii or incorporated into the medium, was degraded when the cultures contained Fe3+ ions, added as part of an inorganic salt or as an iron-containing metabolite, such as hemin or hemoglobin. Even cultures grown without added Fe3+ showed this ability upon addition of the cation. This Fe3+-dependent, H2O2-degrading activity could be a consequence of a Fenton reaction (9) or an enzymatic process catalyzed by a peroxidase. We favor the second hypothesis because liquid MRS medium with Fe3+ did not destroy H2O2 (Fig. 5), indicating that the reaction is a consequence of the growth of the lactobacilli. Besides, when grown cultures were diluted and Fe3+ was subsequently added to them, the ability to destroy H2O2 decreased as a function of the dilution factor (data not shown). Finally, the activity became completely lost upon heating of the cultures to 60°C for 10 min. These findings suggest that the reaction is catalyzed by a heat-susceptible peroxidase that uses Fe3+ as a cofactor. Heme-dependent peroxidases have been found previously in Lactobacillus plantarum (14), Lactobacillus sakei (7), and other environmental, aerotolerant lactobacilli (33) but not in species that are normal inhabitants of the vagina, although it is known that their growth is improved by the addition of a heme source to the medium under partially aerobic conditions (19). However, a second, heme-independent catalase activity that is present in L. plantarum but not in other environmental species (14, 27) appears to also be absent in vaginal lactobacilli.
The addition of an iron source to the cultures did not induce the synthesis of the peroxidase, because cultures grown without added Fe3+, in which the protein synthesis was inhibited, destroyed exogenously added H2O2 upon addition of the cation. However, it cannot be ruled out that the iron included in the culture medium per se might induce the synthesis of the apoenzyme. In a previous study, it was found that Lactobacillus pentosus cells suspended in phosphate buffer degraded H2O2 following the addition of hematin (33). Our data indicate that under these nonproliferating conditions, hematin acted as an iron source to be used as a cofactor by a preexisting peroxidase.
The results obtained indicate that the processes of H2O2 generation and degradation may be part of the same detoxification mechanism and that this might occur on the vaginal mucosa. On one hand, the cavity is exposed to atmospheric oxygen, which might mediate deleterious oxidation of essential cell components, possibly explaining why most of the lactobacilli used in this work do not grow on the surface of solid media under aerobiosis (19). Besides that, the NADH oxidase activity postulated as the H2O2-generating system is energetically neutral (21), leaving detoxification as the only benefit of the reaction to the cell. On the other hand, the vaginal surface presumably contains an elevated concentration of Fe3+, which would be provided, among other sources, by menstruation. The benefit to the cell of the resulting H2O2-degrading activity is quite clear after having observed that the growth of the producing Lactobacillus is inhibited by H2O2. Thus, the MIC for L. jensenii became doubled in the presence of added Fe3+ with respect to MICs of cultures without any additions. It appears, then, that the protection provided by H2O2 against colonization by undesirable microorganisms would result from the equilibrium between its generation and destruction by the resident lactobacilli, to avoid the toxicity of O2 and of H2O2 in excess.
Acknowledgments
This work was supported by CICYT grants SAF2004-0033 and BFU2007-65781 from the Ministry of Science and Technology (Spain) and the FEDER Plan. R.M. is the holder of a scholarship from FICYT (Principado de Asturias).
Footnotes
Published ahead of print on 30 November 2009.
REFERENCES
- 1.Álvarez-Olmos, M. I., M. M. Barousse, L. Rajan, B. J. Van Der Pol, D. Fortenberry, D. Orr, and P. L. Fidel. 2004. Vaginal lactobacilli in adolescents: presence and relationship to local and systemic immunity, and to bacterial vaginosis. Sex. Transm. Dis. 31:393-400. [DOI] [PubMed] [Google Scholar]
- 2.Antonio, M. A., L. K. Rabe, and S. L. Hillier. 2005. Colonization of the rectum by Lactobacillus species and decreased risk of bacterial vaginosis. J. Infect. Dis. 192:394-398. [DOI] [PubMed] [Google Scholar]
- 3.Boris, S., J. E. Suarez, F. Vazquez, and C. Barbes. 1998. Adherence of human vaginal lactobacilli to vaginal epithelial cells and interaction with uropathogens. Infect. Immun. 66:1985-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boskey, E. R., R. A. Cone, K. J. Whaley, and T. R. Moench. 2001. Origins of vaginal acidity: high D/L lactate ratio is consistent with bacteria being the primary source. Hum. Reprod. 16:1809-1813. [DOI] [PubMed] [Google Scholar]
- 5.Boyd, M. A., M. A. Antonio, and S. L. Hillier. 2005. Comparison of API 50 CH strips to whole-chromosomal DNA probes for identification of Lactobacillus species. J. Clin. Microbiol. 43:5309-5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burton, J. P., P. A. Cadieux, and G. Reid. 2003. Improved understanding of the bacterial vaginal microbiota of women before and after probiotic instillation. Appl. Environ. Microbiol. 69:97-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaillou, S., M. C. Champomier-Vergès, A. M. Cornet, M. Crutz-Le Coq, A. M. Dudez, V. Martin, S. Beaufils, E. Darbon-Rongère, R. Bossy, V. Loux, and M. Zagorec. 2005. The complete genome sequence of the meat-borne lactic acid bacterium Lactobacillus sakei 23K. Nat. Biotechnol. 23:1527-1533. [DOI] [PubMed] [Google Scholar]
- 8.Eschenbach, D. A., P. R. Davick, B. L. Williams, S. J. Klebanoff, K. Young-Smith, and C. M. Critchlow. 1989. Prevalence of hydrogen peroxide-producing Lactobacillus species in normal women and women with bacterial vaginosis. J. Clin. Microbiol. 27:251-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fenton, H. J. H. 1894. Oxidation of tartaric acid in presence of iron. J. Chem. Soc. Trans. 65:899-911. [Google Scholar]
- 10.Fontaine, E. A., and D. Taylor-Robinson. 1990. Comparison of quantitative and qualitative methods of detecting hydrogen peroxide produced by human vaginal strains of lactobacilli. J. Appl. Bacteriol. 69:326-331. [DOI] [PubMed] [Google Scholar]
- 11.Gilliland, S. E. 1968. Enzymatic determination of residual hydrogen peroxide in milk. J. Dairy Sci. 52:321-324. [Google Scholar]
- 12.Green, M. J., and H. A. O. Hill. 1984. Chemistry of dioxygen. Meth. Enzymol. 105:3-22. [DOI] [PubMed] [Google Scholar]
- 13.Hawes, S. E., S. L. Hillier, J. Benedetti, C. E. Stevens, L. A. Koutsky, P. Wolner-Hanssen, and K. K. Holmes. 1996. Hydrogen peroxide-producing lactobacilli and acquisition of vaginal infections. J. Infect. Dis. 174:1058-1063. [DOI] [PubMed] [Google Scholar]
- 14.Johnston, M. A., and E. A. Delwiche. 1965. Distribution and characteristics of the catalases of Lactobacillaceae. J. Bacteriol. 90:347-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnston, V. J., and D. C. Mabey. 2008. Global epidemiology and control of Trichomonas vaginalis. Curr. Opin. Infect. Dis. 21:56-64. [DOI] [PubMed] [Google Scholar]
- 16.Kawasaki, S., T. Satoh, M. Todoroki, and Y. Niimura. 2009. b-Type dihydroorotate dehydrogenase is purified as a H2O2-forming NADH oxidase from Bifidobacterium bifidum. Appl. Environ. Microbiol. 75:629-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klebanoff, S., and M. Belding. 1974. Virucidal activity of H2O2-generating bacteria: requirement for peroxidase and halide. J. Infect. Dis. 129:345-348. [DOI] [PubMed] [Google Scholar]
- 18.Lepargneur, J. P., and V. Rousseau. 2002. Protective role of the Doderleïn flora. J. Gynecol. Obstet. Biol. Reprod. 31:485-494. [PubMed] [Google Scholar]
- 19.Martin, R., N. Soberon, M. Vaneechoutte, A. B. Florez, F. Vazquez, and J. E. Suarez. 2008. Characterization of indigenous vaginal lactobacilli from healthy women as probiotic candidates. Int. Microbiol. 11:261-266. [DOI] [PubMed] [Google Scholar]
- 20.Martin, R., N. Soberon, F. Vazquez, and J. E. Suarez. 2008. Vaginal microbiota: composition, protective role, associated pathologies, and therapeutic perspectives. Enferm. Infecc. Microbiol. Clin. 26:160-167. (In Spanish.) [DOI] [PubMed] [Google Scholar]
- 21.Marty-Teysset, C., F. de la Torre, and J. Garel. 2000. Increased production of hydrogen peroxide by Lactobacillus delbrueckii subsp. bulgaricus upon aeration: involvement of an NADH oxidase in oxidative stress. Appl. Environ. Microbiol. 66:262-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyata, K., M. Miyashita, R. Nose, Y. Otake, and H. Miyagawa. 2006. Development of a colorimetric assay for determining the amount of H2O2 generated in tobacco cells in response to elicitors and its application to study of the structure-activity relationship of flagellin-derived peptides. Biosci. Biotechnol. Biochem. 70:2138-2144. [DOI] [PubMed] [Google Scholar]
- 23.Niven, C. F., and J. B. Evans. 1957. Lactobacillus viridescens nov. spec., a heterofermentative species that produces a green discoloration of cured meat pigments. J. Bacteriol. 73:758-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nyirjesy, P. 2008. Vulvovaginal candidiasis and bacterial vaginosis. Infect. Dis. Clin. North Am. 22:637-652. [DOI] [PubMed] [Google Scholar]
- 25.Rabe, L. K., and S. L. Hillier. 2003. Optimization of media for detection of hydrogen peroxide production by Lactobacillus species. J. Clin. Microbiol. 41:3260-3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Redondo-Lopez, V., R. L. Cook, and J. D. Sobel. 1990. Emerging role of lactobacilli in the control and maintenance of the vaginal bacterial microflora. Rev. Infect. Dis. 12:856-872. [DOI] [PubMed] [Google Scholar]
- 27.Rochat, T., J. J. Gratadoux, A. Gruss, G. Corthier, E. Maguin, P. Langella, and M. van de Guchte. 2006. Production of a heterologous nonheme catalase by Lactobacillus casei: an efficient tool for removal of H2O2 and protection of Lactobacillus bulgaricus from oxidative stress in milk. Appl. Environ. Microbiol. 72:5143-5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sobel, J. D. 2000. Bacterial vaginosis. Annu. Rev. Med. 51:349-356. [DOI] [PubMed] [Google Scholar]
- 29.Song, Y. L., N. Kato, Y. Matsumiya, C. X. Liu, H. Kato, and K. Watanabe. 1999. Identification of and hydrogen peroxide production by fecal and vaginal lactobacilli isolated from Japanese women and newborn infants. J. Clin. Microbiol. 37:3062-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song, Y. L., N. Kato, Y. Matsumiya, C. X. Liu, H. Kato, and K. Watanabe. 1999. Identification of Lactobacillus species of human origin by a commercial kit, API50CHL. Rinsho Biseibutshu Jinsoku Shindan Kenkyukai Shi 10:77-82. [PubMed] [Google Scholar]
- 31.St. Amant, D. C., I. E. Valentin-Bon, and A. E. Jerse. 2002. Inhibition of Neisseria gonorrhoeae by Lactobacillus species that are commonly isolated from the female genital tract. Infect. Immun. 70:7169-7171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vallor, A. C., M. A. Antonio, S. E. Hawes, and S. L. Hillier. 2001. Factors associated with acquisition of, or persistent colonization by, vaginal lactobacilli: role of hydrogen peroxide production. J. Infect. Dis. 184:1431-1436. [DOI] [PubMed] [Google Scholar]
- 33.Wolf, G., A. Strahl, J. Meisel, and W. P. Hammes. 1991. Heme-dependent catalase activity of lactobacilli. Int. J. Food Microbiol. 12:133-140. [DOI] [PubMed] [Google Scholar]
- 34.Zhou, X., S. Bent, M. Schneider, C. Davis, M. Islam, and L. Forney. 2004. Characterization of vaginal microbial communities in adult healthy women using cultivation-independent methods. Microbiology 150:2565-2573. [DOI] [PubMed] [Google Scholar]
- 35.Zoetendal, E. G., E. E. Vaughan, and W. M. De Vos. 2006. A microbial world within us. Mol. Microbiol. 59:1639-1650. [DOI] [PubMed] [Google Scholar]