Abstract
Glycolic acid was detected as an exudate in actively growing cultures of three chemolithotrophic acidophiles that are important in biomining operations, Leptospirillum ferriphilum, Acidithiobacillus (At.) ferrooxidans, and At. caldus. Although similar concentrations of glycolic acid were found in all cases, the concentrations corresponded to ca. 24% of the total dissolved organic carbon (DOC) in cultures of L. ferriphilum but only ca. 5% of the total DOC in cultures of the two Acidithiobacillus spp. Rapid acidification (to pH 1.0) of the culture medium of At. caldus resulted in a large increase in the level of DOC, although the concentration of glycolic acid did not change in proportion. The archaeon Ferroplasma acidiphilum grew in the cell-free spent medium of At. caldus; glycolic acid was not metabolized, although other unidentified compounds in the DOC pool were metabolized. Glycolic acid exhibited levels of toxicity with 21 strains of acidophiles screened similar to those of acetic acid. The most sensitive species were chemolithotrophs (L. ferriphilum and At. ferrivorans), while the most tolerant species were chemoorganotrophs (Acidocella, Acidobacterium, and Ferroplasma species), and the ability to metabolize glycolic acid appeared to be restricted (among acidophiles) to Firmicutes (chiefly Sulfobacillus spp.). Results of this study help explain why Sulfobacillus spp. rather than other acidophiles are the main organic carbon-degrading bacteria in continuously fed stirred tanks used to bioprocess sulfide mineral concentrates and also why temporary cessation of pH control in these systems, resulting in rapid acidification, often results in a plume of the archaeon Ferroplasma.
Extremely acidic environments (generally considered environments that have a pH of <3) are unusual in that chemoautotrophs rather than photoautotrophs are often the dominant (and sometimes exclusive) primary production agents (15). There are two major reasons for this: (i) inorganic electron donors (ferrous iron and reduced forms of sulfur) are often very abundant, as many of the most extremely acidic environments are in sulfur-rich (e.g., solfatara springs) or sulfide mineral-rich (e.g., metal mine wastes) locations, and (ii) in general, cyanobacteria, algae, and higher plants are more sensitive than chemotrophic bacteria and archaea to the elevated concentrations of soluble transition metals and other solutes often present in acidic waters. Lithotrophy-based primary production may be evident in subterranean chambers (caves and worked-out mines) in the form of slimes and massive “acid streamer” growths (2, 22, 30). In lower-temperature (<40°C) environments, the microorganisms that use energy derived from the oxidation of ferrous iron and/or reduced sulfur to fix CO2 are predominantly bacteria and include a number of species (e.g., bacteria belonging to the genera Acidithiobacillus and Leptospirillum, as well as the archaeon Ferroplasma) that are known to be fundamentally important in biomining operations (35) and in the generation of acidic, metal-rich mine effluents (31).
Commercial mineral bioprocessing operations that use large (often >1,000-m3) stirred tanks operate under of fixed temperature (generally 35 to 45°C) and pH (often pH ∼1.5) conditions. The main primary producers identified in these systems are the iron oxidizer Leptospirillum ferriphilum and the sulfur oxidizer Acidithiobacillus (At.) caldus. The chemomixotrophic bacterium Sulfobacillus and the chemoheterotrophic archaeon Ferroplasma are also often present, although the numbers of these organisms are smaller (35). The acid-generating nature of pyrite oxidation means that continuous addition of an alkaline material (such as lime) is necessary to maintain the required pH of the leach liquor. When, on occasion, the pHs of the liquors have decreased due to temporary failure of the control mechanisms, the microbial communities in the tanks have been observed to change markedly, and the numbers of Ferroplasma cells have increased greatly relative to the numbers of cells of iron- and sulfur-oxidizing autotrophic bacteria (D. E. Rawlings, Stellenbosch University, personal communication). The same trends have been observed in pilot-scale (34) and laboratory-scale (33) systems.
Acidophilic chemolithotrophic bacteria, like other autotrophs, lose significant amounts of the carbon that they fix as low-molecular-weight compounds during active growth (exudates), as well as due to lysis of dead and dying cells. Schnaitman and Lundgren (36) estimated that 9.6% of labeled carbon (14CO2) was leaked by Acidithiobacillus ferrooxidans into its growth medium, and they identified pyruvic acid as one of the low-molecular-weight exudates. Borichewski (3) reported that in cultures of Acidithiobacillus thiooxidans grown on elemental sulfur, keto acids accumulated to concentrations which inhibited growth of the bacterium. Oxaloacetic acid and, again, pyruvic acid were identified in cell-free culture media, and removal of these organic acids by dialysis eliminated growth inhibition. Okibe and Johnson (33) found that dissolved organic carbon (DOC) accumulated to concentrations of 88 ± 17 mg liter−1 in cultures of the thermotolerant iron oxidizer L. ferriphilum grown on pyrite and to concentrations of >100 mg liter−1 when this acidophile was grown in coculture with the sulfur oxidizer At. caldus. Inclusion of organic carbon-degrading acidophiles (Acidimicrobium [Am.] ferrooxidans or a Ferroplasma sp.) resulted not only in detection of far lower concentrations of DOC in cultures but also, in most cases, in more rapid dissolution of pyrite. Heterotrophic and/or mixotrophic acidophiles are inevitably found growing alongside chemoautotrophic primary producers in natural (22) and anthropogenic (34, 35) environments.
Chemoautotrophic acidophilic bacteria use different pathways for assimilating carbon dioxide (17, 28). At. ferrooxidans and At. thiooxidans (both mesophiles) use the Calvin-Benson-Bassham (CBB) pathway, while L. ferriphilum is thought to utilize the reductive tricarboxylic acid (rTCA) cycle. Genes coding for enzymes involved in the CBB cycle have also been found in the moderate thermophile At. caldus (39). A key enzyme in the CBB cycle is ribulose bisphosphate carboxylase oxygenase (RuBisCO). Besides combining carbon dioxide and ribulose bisphosphate (RUBP), RuBisCO also oxidizes RUBP to phosphoglyceric acid and phosphoglycolate. Enzymatic hydrolysis of the latter compound produces glycolate, much of which is exported out of actively growing cells. Glycolate has been detected as an exudate in cultures of marine and freshwater algae (41), photosynthetic bacteria such as Rhodospirillum rubrum (37), the facultative chemolithotroph Alcaligenes eutrophus (8), and the neutrophilic sulfur-oxidizing bacterium Thiobacillus neapolitanus (9). Glycolic acid (CH2OHCOOH) has not been reported previously to be an exudate in cultures of acidophilic chemoautotrophic bacteria, yet it could be of considerable significance not only in sustaining organic carbon-utilizing acidophiles but also because of its potential biotoxic effect. The pKa of glycolic acid is 3.83, which means that at the pHs of extremely acidic environments it exists almost exclusively as the more toxic undissociated form rather than the glycolate anion.
Here we report the production and excretion of glycolic acid by three species of chemoautotrophic acidophiles that are important in biomining operations, the relative sensitivities of acidophilic microorganisms to this acid, and the different abilities of mixotrophic and heterotrophic acidophiles to metabolize this compound.
MATERIALS AND METHODS
Microorganisms and growth media.
Twenty-one strains (belonging to 19 species) of acidophilic microorganisms, including chemoautotrophic bacteria, mixotrophic bacteria, and obligately heterotrophic acidophiles, and one acidophilic archaeon (Ferroplasma acidiphilum [Fp. acidiphilum]) were used in the present study (Table 1). The growth media used varied in composition depending on the nutritional requirements of the different acidophiles, and a common basal salts and trace element mixture (40) was used throughout the study (trace elements were not included when yeast extract was added to the culture media). Iron-oxidizing chemoautotrophs were grown routinely in media containing 20 mM ferrous iron (pH 1.7 to 2.2, depending on the optimum pH for growth), and sulfur oxidizers that do not oxidize iron (At. thiooxidans and At. caldus) were grown in a similar medium in which elemental sulfur (5%, wt/vol) replaced ferrous sulfate. Mixotrophic and heterotrophic iron oxidizers were grown routinely in a liquid medium containing 10 to 20 mM ferrous iron supplemented with 0.02% (wt/vol) yeast extract (at pH 1.8 to 2.0). Heterotrophic iron-reducing acidophiles were grown in basal salts-trace element medium supplemented with 5 mM glucose plus 0.001% yeast extract (Acidiphilum SJH and Acidobacterium capsulatum [Ab. capsulatum]) or 5 mM fructose (Acidocella PFBC). The pHs of these media were adjusted to either 2.5 (Acidiphilium SJH and Acidocella PFBC) or 3.0 (Ab. capsulatum). Finally, the archaeon Fp. acidiphilum, which does not possess a cell wall, was grown in a higher-osmotic-potential growth medium containing 50 mM ferrous sulfate, 50 mM potassium sulfate, 0.02% (wt/vol) yeast extract, and basal salts (pH 1.5).
TABLE 1.
Acidophilic bacteria and archaea used in the present studya
| Taxon | Strain | Reference |
|---|---|---|
| Iron-oxidizing autotrophic bacteria | ||
| Leptospirillum ferrooxidans | Type | 14 |
| Leptospirillum ferriphilumb | Type | 10 |
| “Leptospirillum ferrodiazotrophum”b | UBA1 | 38 |
| Iron- and sulfur-oxidizing autotrophic bacteria | ||
| Acidithobacillus ferrooxidans | Type | 24 |
| Acidithiobacillus ferrivorans | Type | 13 |
| Sulfur-oxidizing autotrophic bacteria | ||
| Acidithiobacillus caldusb | Type | 12 |
| Acidithiobacillus thiooxidans | Type | 24 |
| Iron-oxidizing mixotrophic bacterium | ||
| Acidimicrobium ferrooxidansb | TH3 | 7 |
| Iron- and sulfur-oxidizing mixotrophic bacteria | ||
| Sulfobacillus thermosulfidooxidansb | Type | 23 |
| Sulfobacillus thermosulfidooxidansb | TH1 | 5 |
| Sulfobacillus acidophilusb | ALV | 32 |
| Sulfobacillus acidophilusb | YTF1 | 21 |
| Sulfobacillus benefaciensb | Type | 19 |
| Sulfobacillus thermotoleransb | L15 | 21 |
| Firmicutesb | G1 | 20 |
| Iron-oxidizing heterotrophic bacteria | ||
| Ferrimicrobium acidiphilum | Type | 18 |
| Firmicutes | SLC1 | 21 |
| Iron-reducing heterotrophic bacteria | ||
| Acidiphilium sp. | SJH | 4 |
| Acidocella sp. | PFBC | 25 |
| Acidobacterium capsulatum | Type | 26 |
| Iron-oxidizing archaeon | ||
| Ferroplasma acidiphilumb | BRGM4 | D. B. Johnson et al., unpublished data |
Unless indicated otherwise, cultures were incubated at 30°C.
Culture incubated at 37°C.
Bioreactor cultures.
L. ferriphilum, At. ferrooxidans, and At. caldus were each grown in 2-liter bioreactors with 1.0-liter working volumes (Electrolab Ltd., United Kingdom) in which the pH and temperature were maintained at predetermined levels. In the case of L. ferriphilum, the growth medium contained 5% (wt/vol) pyrite (Strem Chemicals, Newburyport, MA), and the pH and temperature were maintained at 1.7 and 37°C, respectively. At. ferrooxidans and At. caldus were grown on 5% (wt/vol) elemental sulfur (VWR, United Kingdom) at pH 2.5 and 30°C (At. ferrooxidans) or 45°C (At. caldus). All bioreactors were aerated (1 liter min−1) and stirred at 150 rpm. Samples were withdrawn at regular intervals to measure the concentrations of ferrous and total iron (pyrite culture) or sulfate (sulfur cultures), dissolved organic carbon (DOC), and glycolic acid and the numbers of planktonic-phase cells. After the bioreactor cultures had been running for between 10 and 13 days, they were subjected to a “pH shock,” which involved rapidly decreasing the culture pH (to pH 1.0, completed within 10 min) by automated addition of 1 M sulfuric acid. This treatment was designed to mimic the occasional failure of pH control in stirred tank biomining operations. Samples were withdrawn both before and after cultures were subjected to this pH shock to measure the parameters listed above and also the numbers of total and viable bacteria.
Growth of Fp. acidiphilum on At. caldus cell-free medium.
Four days after the pH of the At. caldus bioreactor culture was lowered to 1.0, the reactor was drained, and the bacterial cells and residual sulfur were removed by centrifugation (15,000 relative centrifugal force, 15 min), followed by filtration through 0.2-μm-pore-size cellulose nitrate membrane filters (Whatman, United Kingdom). Four-hundred-milliliter aliquots were dispensed into three sterile 1-liter conical flasks, and filter-sterilized ferrous sulfate (1 M, pH 2.0) was added to a final concentration of 20 mM. Two of the flasks were inoculated with 10 ml of an actively growing culture of Fp. acidiphilum (strain BRGM4), and the third flask was used as a noninoculated control. The cultures were incubated with shaking (150 rpm) at 37°C, and samples were withdrawn every 2 days to determine the concentrations of DOC, glycolic acid, and ferrous iron and to determine the total numbers of cells.
Screening acidophilic prokaryotes for sensitivity to glycolic acid.
The acidophilic prokaryotes listed in Table 1 were tested for growth in the presence of different concentrations of glycolic acid. Five-milliliter cultures were prepared in 20-ml universal bottles using the liquid media described above. Glycolic acid was added to these cultures to obtain final concentrations of 0.1, 0.5, 1.0, 2.5, and 5.0 mM. In order to assess the relative sensitivities of these acidophiles to acetic acid, cultures containing the same concentrations of acetic acid were prepared simultaneously, and organic acid-free cultures were prepared as controls. These cultures were incubated at 30 or 37°C for up to 10 days, and positive or negative growth was determined on the basis of changes in cell numbers and, in the case of iron-oxidizing prokaryotes, on the basis of the production of ferric iron.
Metabolism of glycolic acid by acidophilic prokaryotes.
The mixotrophic and heterotrophic acidophiles listed in Table 1 were assessed to determine their abilities to metabolize glycolic acid. Positive cultures from the toxicity screening experiment that contained 0.1 mM glycolic acid were used as inocula in all cases. Mixotrophic acidophiles were subcultured in a liquid medium (10-ml aliquots in universal bottles) containing 0.1 mM glycolic acid and 10 mM ferrous iron (plus basal salts and trace elements) at pH 1.9, and the iron-reducing heterotrophic bacteria were subcultured in the same medium but with no added iron (pH 2.5 for Acidocella PFBC and Acidiphilium SJH and pH 3.0 for Ab. capsulatum). The cultures were incubated at 30 or 37°C for up to 20 days, and samples were removed at regular intervals to measure the concentrations of glycolic acid. Noninoculated controls were also prepared in order to account for any glycolic acid lost via evaporation or by abiotic means.
For the acidophiles that showed an apparent ability to metabolize glycolic acid, a second experiment was performed, in which the time course of glycolic acid removal was recorded. Cultures were inoculated into 20 ml of growth medium (as described above) in duplicate 100-ml conical flasks, which were incubated (at 30 or 37°C) and shaken at 150 rpm. Samples were removed every 2 days to measure the concentration of glycolic acid.
Analytical techniques.
The glycolic acid concentration was measured by ion chromatography (IC) using a Dionex IC25 ion chromatograph fitted with an IonPac AS-11 column equipped with a conductivity detector (Dionex Inc., United States) and occasionally by a colorimetric technique (6). The IC protocol used enabled separation of glycolic and pyruvic acids and was used routinely except when excessively high concentrations of sulfate (from microbial oxidation of elemental sulfur in bioreactors) precluded adequate separation of glycolate and sulfate. Dissolved organic carbon was measured using a LABTOC DOC analyzer (Pollution and Process Monitoring, United Kingdom). Concentrations of ferrous iron were measured using the ferrozine colorimetric assay (29), and sulfate concentrations were measured using a turbidometric technique (27). The amount of total iron was determined by ion chromatography using a Dionex 320 ion chromatograph fitted with an IonPac CS5A column and an AD25 absorbance detector. pH was measured using a pHase combined electrode (VWR, United Kingdom) coupled to an Accumet 50 pH meter (Cole-Palmer, Vernon Hills, IL). Microbial cells were enumerated using a Helber counting chamber marked with Thoma ruling (Hawksley, United Kingdom) and viewed with a Leitz Labolux phase-contrast microscope (at a magnification of ×400). Viable bacteria were enumerated by plating cells onto selective solid media (16).
RESULTS
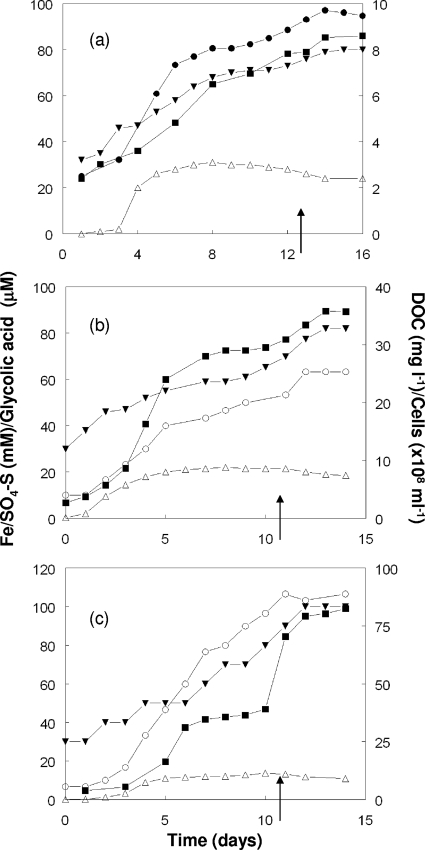
In bioreactor cultures of L. ferriphilum, At. ferrooxidans, and At. caldus, there were general increases in the concentrations of both total DOC and glycolic acid as oxidation of pyrite or elemental sulfur progressed, although the concentrations of DOC were much greater in the bioreactor containing At. caldus than in the other two bioreactors (Fig. 1). Pyruvic acid was not detected by IC analysis. The numbers of planktonic cells did not parallel the DOC and glycolic acid concentrations, presumably because of the attachment of the bacteria to the solid substrates present. There was no obvious formation of biofilms on the bioreactor vessel walls. The pH shock induced by rapid acidification of the bioreactors caused different perturbations in the three bacterial cultures. With L. ferriphilum and At. ferrooxidans, the concentrations of both DOC and glycolic acid increased after the pH was lowered to 1.0, but they increased at a rate similar to the rate before the change was made. The pH shock caused the oxidation of pyrite by L. ferriphilum and the oxidation of sulfur by At. ferrooxidans to come to a halt, as shown by no net increases in the concentrations of soluble iron and sulfate for the following 2 days. Sulfur oxidation was also arrested by the pH shock in the At. caldus bioreactor culture, although in the culture with this acidophile the sudden decrease in the pH also resulted in a large (80%) increase in the total DOC concentration within 48 h; however, there was only a 25% increase in the concentration of glycolic acid in the same time period.
FIG. 1.
Changes in concentrations of dissolved organic carbon (DOC) and glycolic acid in bioreactor cultures of (a) L. ferriphilum, (b) At. ferrooxidans, and (c) At. caldus. Symbols: •, total soluble iron concentration (L. ferriphilum); ○, sulfate S concentration (At. ferrooxidans and At. caldus); ▪, DOC concentration; ⧫, glycolic acid concentration (colorimetric assay); ▵, total number of cells. The arrows indicate the time at which the “pH shock” (rapid lowering of the bioreactor pH to 1.0) was imposed.
In order to estimate the net contribution of glycolic acid to the total DOC pool in the bioreactor cultures, concentrations of glycolic acid were converted to carbon equivalents (expressed in mg liter−1). Table 2 shows that, although the concentrations of glycolic acid were similar in all three cultures prior to the acidification of the bioreactors, this aliphatic acid accounted for a far higher percentage of the total DOC in the L. ferriphilum culture than in either of the Acidithiobacillus cultures. It was also found that with both L. ferriphilum and At. ferrooxidans there were only minor changes in the glycolic acid/DOC ratios immediately before and after the pH shock, whereas in the case of At. caldus the percentage of the total DOC that was glycolic acid fell by 41% following acidification of the reactor.
TABLE 2.
Percentages of total dissolved organic carbon present as glycolic acid in bioreactor cultures of L. ferriphilum, At. ferrooxidans, and At. caldus before and after the “pH shock”
| Bacterium | Bioreactor pH | Glycolic acid concn (mg C liter−1) | DOC concn (mg liter−1) | % of total DOC present as glycolic acid |
|---|---|---|---|---|
| L. ferriphilum | 1.7 | 1.70 | 7.0 | 24 |
| 1.0 | 1.92 | 8.6 | 22 | |
| At. ferrooxidans | 2.5 | 1.46 | 29 | 5.0 |
| 1.0 | 1.97 | 36 | 5.5 | |
| At. caldus | 2.5 | 1.92 | 39 | 4.9 |
| 1.0 | 2.40 | 82.5 | 2.9 |
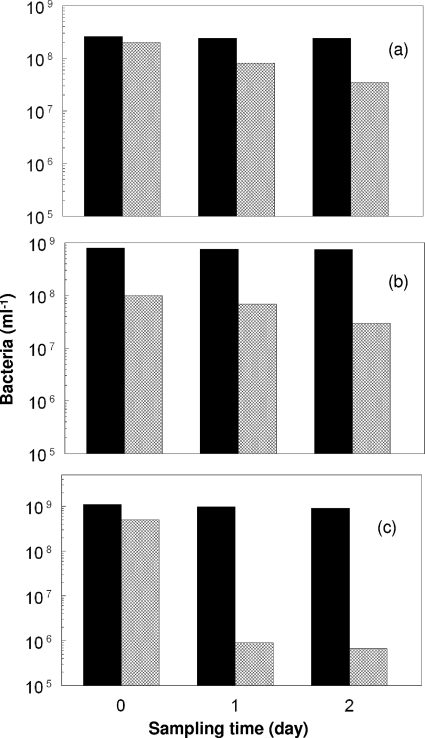
Rapid acidification of the bioreactors also affected the viabilities of the three chemoautotrophic bacteria to different extents, as shown by their abilities to grow on solid media. While the total numbers of the three bacterial species remained fairly similar for up to 2 days following adjustment of the pH of the bioreactors to 1.0, the plate counts declined far more for At. caldus than for L. ferriphilum or At. ferrooxidans (Fig. 2). The numbers of cultivatable At. caldus cells fell by >99.8% within 1 day after culture acidification and continued to decline subsequently, although at a lower rate (data not shown).
FIG. 2.
Changes in total numbers (solid bars) and viable counts (cross-hatched bars) of (a) L. ferriphilum, (b) At. ferrooxidans, and (c) At. caldus immediately before (day 0) and 1 and 2 days following acidification of the bioreactors to pH 1.0.
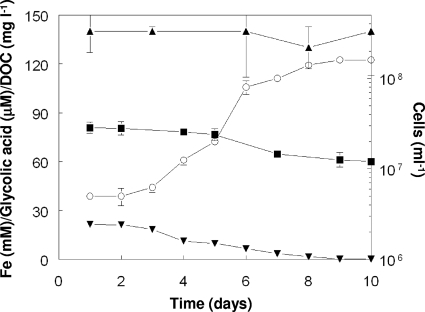
The archaeon Fp. acidiphilum was able to grow on the spent At. caldus cell-free bioreactor medium. Figure 3 shows that the numbers of Fp. acidiphilum cells increased and all of the available ferrous iron was oxidized within 9 days in amended spent medium from the At. caldus bioreactor. However, only about 25% of the total DOC was metabolized over this period, and there was no significant change in the concentration of glycolic acid. The cessation of growth of Fp. acidiphilum after day 9 suggests that the residual DOC (including glycolic acid) could not be metabolized by this archaeon. No changes in the concentrations of DOC, glycolic acid, and ferrous iron were observed in control cultures containing sterile spent At. caldus medium.
FIG. 3.
Growth of Fp. acidiphilum on spent At. caldus medium. Symbols: ▾, Fe2+ concentration; ▪, DOC concentration; ▴, glycolic acid concentration; ○, number of cells. The symbols indicate means for duplicate cultures, and the error bars indicate ranges.
Glycolic acid displayed levels of toxicity for the 21 strains of acidophilic prokaryotes tested that were similar to the levels of toxicity of acetic acid (data not shown). While all of the acidophiles tested could grow in the presence of 100 μM glycolic acid, progressively fewer species were able to grow at higher concentrations (Table 3). The most sensitive acidophiles tested with both acids were the obligate chemoautotrophs L. ferriphilum and At. ferrivorans, while the least sensitive acidophiles tested were all chemoorganotrophs (Fp. acidiphilum, Acidocella PFBC, and Ab. capsulatum).
TABLE 3.
Comparison of the relative toxicities of glycolic acid to 21 strains (belonging to 19 species) of acidophilic microorganisms
| Maximum glycolic acid concn permitting growth (mM) | Acidophile strains |
|---|---|
| 0.1 | L. ferriphilum type strain, At. ferrivorans type strain |
| 0.5 | L. ferrooxidans type strain, At. caldus type strain |
| 1.0 | “L. ferrodiazotrophum” UBA1, Am. ferrooxidans type strain, S. thermosulfidooxidans type strain, S. thermosulfidooxidans TH1, S. acidophilus ALV, S. acidophilus YTF1, S. benefaciens type strain, S. thermotolerans L15, Firmicutes strain G1, Ferrimicrobium acidiphilum type strain, Firmicutes strain SLC1 |
| 2.5 | At. ferrooxidans type strain, At. thiooxidans type strain, Acidiphilium SJH |
| 5.0 | Acidocella PFBC, Ab. capsulatum, Fp. acidiphilum BRGM4 |
The sensitivities of At. ferrooxidans (which can grow in both sulfur-containing and ferrous iron-containing media) to glycolic and acetic acids were similar regardless of the energy source (data not shown), indicating that the trends observed were not related to the different energy sources used.
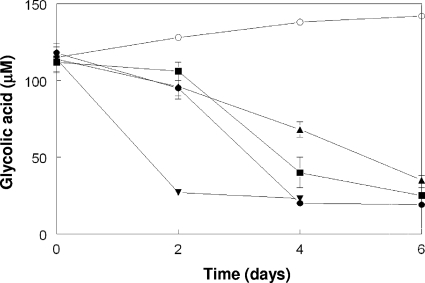
Of the 14 mixotrophic and heterotrophic acidophiles tested for the ability to metabolize glycolic acid, 5 were confirmed to be positive, and all of these organisms were Firmicutes, including the Sulfobacillus benefaciens type strain, Sulfobacillus acidophilus (both strains), Sulfobacillus thermosulfidooxidans strain TH1, and unclassified isolate SLC1 (Fig. 4). The type strain of S. thermosulfidooxidans showed only limited (30%) degradation of glycolic acid over 14 days, which was similar to the results obtained for unclassified Firmicutes isolate G1.
FIG. 4.
Degradation of glycolic acid by acidophilic Firmicutes. Symbols: ▴, S. thermosulfidooxidans strain TH1; ▪, S. acidophilus strain YTF1; ▾, S. benefaciens type strain; •, isolate SLC1; ○, noninoculated control. The symbols indicate the mean glycolic acid concentrations for duplicate cultures, and the error bars (where visible) indicate the ranges.
DISCUSSION
Glycolic acid was detected in cell-free culture liquors of three chemolithotrophic bacteria (L. ferriphilum, At. ferrooxidans, and At. caldus) that are considered to have major roles in the oxidative dissolution of sulfide minerals in acidic environments and biomining operations (35). The identity of glycolic acid was confirmed using both ion chromatography and a colorimetric technique. The latter technique was claimed by Calkins (6) to be free of interference from commonly encountered low-molecular-weight organic acids, as found (with acetic and pyruvic acids) in the present study. In contrast to previous reports, we did not detect pyruvic acid as a microbial exudate. The fact that the concentrations of glycolic acid paralleled those of DOC during culture growth indicated that this organic acid was an exudate of actively growing cells rather than a lysate product of dead bacteria. This was confirmed in the case of At. caldus, where the pH shock led to mass mortality of the cells and a rapid and large increase in the DOC concentration but only a relatively small increase in the glycolic acid concentration. While the DOC values (prior to the pH shock) varied between 7 and 39 mg liter−1 for the three bioreactor cultures, this finding was related mostly to differences in cell numbers, and plots of total numbers of bacteria versus DOC concentrations for the three cultures showed a linear fit with a regression coefficient of 0.999 (data not shown).
The presumed origin of the glycolic acid in cultures of the two Acidithiobacillus spp. is thought to be activity of RuBisCO as an oxygenase, and both of these bacteria use the CBB pathway for carbon assimilation. In the case of L. ferriphilum, which is thought (based on genomic analysis) to use the rTCA cycle, the biochemical pathway which might have overproduced glycolic acid, resulting in its excretion from viable cells, is unknown. A gene encoding phosphoglycolate phosphatase, an enzyme that produces glycolate from phosphoglycolate, has been annotated in the genome of “Leptospirillum rubarum,” a proposed novel species that is closely related to L. ferriphilum (1). The origin of the phosphoglycolate as an intermediate in the central metabolism of Leptospirillum spp. is currently unclear. This apparent conundrum is accentuated by the fact that the percentage of DOC present as glycolic acid was far greater (24%) in the L. ferriphilum culture than in the cultures of the Acidithiobacillus spp., where it accounted for about 5% of the total DOC.
The effect of rapid acidification of the bioreactor cultures (“pH shock”) was far more lethal to At. caldus than to the other two bacteria investigated. The percentage of viable At. caldus cells (determined by comparing plate counts to total counts) fell from 45% to <1% within 1 h after the bioreactor pH was lowered to 1.0; the corresponding values for the L. ferriphillum and At. ferrooxidans cultures were decreases from 77 to 33% and from 13 to 9%, respectively. These different responses do not directly correlate with the pH optima and minima for growth described for these acidophiles (pH 2.0 to 2.5 and ∼1.0 for the At. caldus type strain, pH 1.5 and 0.8 for L. ferriphilum strain MT6, and pH 2.0 to 2.5 and ∼1.4 for the At. ferrooxidans type strain) and are probably more related to the different degrees of stress induced by the rapidity of the acidification of the cultures.
In general, the toxicity of glycolic acid to the acidophiles tested was similar to the toxicity of acetic acid, although both of these acids appear to be less toxic to these extremophiles than pyruvic acid, which was reported previously to completely inhibit the growth of At. thiooxidans when it was added at concentrations between 20 and 40 μM (3). The most glycolic acid-tolerant acidophiles were all heterotrophic. Chemoautotrophic acidophiles in general and Leptospirillum spp. in particular tend to be more sensitive to low-molecular-weight organic compounds than their mixotrophic and heterotrophic counterparts (16). The concentration of glycolic acid required to inhibit the growth of L. ferriphilum (the most important primary mineral oxidizer in stirred tank biomining operations) was 500 μM, which was five to six times greater than the concentrations of this acid recorded for the three bioreactor cultures. However, concentrations of DOC have been reported to increase to 88 ± 17 mg liter−1 in pure cultures of L. ferriphilum (33). Assuming that, as in the present study, about 24% of DOC was present as glycolic acid carbon, this gives an equivalent concentration of about 870 μM glycolic acid, a concentration that would be predicted to be inhibitory to the iron oxidizer.
One of the most intriguing findings was that, for the organic carbon-degrading acidophiles investigated, the ability to metabolize glycolic acid was observed only for Firmicutes, and this ability appeared to be widespread in the eight species and strains (mostly Sulfobacillus) tested. It was particularly surprising to find that Acidocella PFBC, a strain of the proposed species “Acidocella aromatica,” which has been found to be adept at growing on low-molecular-weight organic acids, including acetic acid (11), was not able to metabolize glycolic acid.
The results of this study help explain the compositions of microbial consortia in biomining operations, particularly those using continuously fed stirred tanks (35). In the majority of these consortia, the numerically dominant acidophiles are the iron oxidizer L. ferriphilum and the sulfur oxidizer At. caldus, both of which fix inorganic carbon. Other bacteria are also found, but they tend to be exclusively Sulfobacillus spp. (often S. benefaciens, although S. thermosulfidooxidans is more common when copper-containing mineral concentrates are bioleached). Sulfobacillus spp. are iron- and sulfur-oxidizing mixotrophs (they preferentially utilize organic carbon, although they are able to fix CO2), but they probably have only a minor impact on net mineral oxidation, and their role in degrading organic carbon compounds, thereby preventing accumulation and potential toxicity to the “organic compound-sensitive” chemoautotrophs, is probably more important for efficient operation of stirred tank systems. Other mixotrophic or heterotrophic bacteria, such as the iron oxidizer Am. ferrooxidans, have not been detected in commercial stirred tank operations, even though physicochemical conditions in the tanks are conducive to growth of these bacteria. One reason for this might be that the ability of Sulfobacillus spp. to metabolize glycolic acid, which is provided continuously by the primary producers in the tanks, gives the Firmicutes a competitive advantage. On the other hand, iron-oxidizing heterotrophic Ferroplasma spp. are often also detected in stirred tanks, even though (on the basis of the single isolate used in the current study, which was isolated from a tank system bioleaching cobaltiferous pyrite) these archaea appear not to be able to metabolize glycolic acid. Ferroplasma appears to be adept at scavenging organic carbon released from dead and dying cells, a conclusion supported here by the growth of Ferroplasma in cell-free medium from the At. caldus bioreactor collected after the pH shock. In a previous study (34) of a stirred tank bioleaching pilot-scale system comprising three in-line tanks, Ferroplasma was not detected in the first tank, although it outnumbered the bacteria (L. ferriphilum, At. caldus. and a Sulfobacillus sp.) in the second and third tanks. Its emergence in the second tank and its dominance in the third tank coincided with the demise and death of a large proportion of the bacterial population. Data obtained in the present study strongly suggest that this was due to rapid input of DOC into the mineral leachate from killed At. caldus rather than from L. ferriphilum.
This study highlighted the importance of glycolic acid in acidophile microbiology. While excretion of this acid by chemoautotrophic iron- and sulfur-oxidizing bacteria has the potential to inhibit the bacteria that are key players in commercial bioprocessing of minerals, glycolic acid-metabolizing Sulfobacillus spp. can eliminate this problem, further highlighting the importance of microbial consortia in biomining operations.
Acknowledgments
We are grateful to Kevin Hallberg for his ideas and constructive advice.
I.Ñ. is grateful to the Mecesup Programme of the Chilean Government, and Barrie Johnson is grateful to the Royal Society for provision of an industrial fellowship.
Footnotes
Published ahead of print on 20 November 2009.
REFERENCES
- 1.Aliaga Goltsman, D. S., V. J. Denef, S. W. Singer, N. C. VerBerkmoes, M. Lefsrud, R. S. Mueller, G. J. Dick, C. L. Sun, K. E. Wheeler, A. Zemla, B. J. Baker, L. Hauser, M. Land, M. B. Shah, M. P. Thelen, R. L. Hettich, and J. F. Banfield. 2009. Community genomic and proteomic analysis of chemoautotrophic iron-oxidizing “Leptospirillum rubarum” (group II) and “Leptospirillum ferrodiazotrophum (group III) bacteria in acid mine drainage biofilms. Appl. Environ. Microbiol. 75:4599-4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bond, P. L., S. P. Smriga, and J. F. Banfield. 2000. Phylogeny of microorganisms populating a thick, subaerial, predominantly lithotrophic biofilm at an extreme acid mine drainage site. Appl. Environ. Microbiol. 66:3842-3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borichewski, R. M. 1967. Keto acids as growth-limiting factors in autotrophic growth of Thiobacillus thiooxidans. J. Bacteriol. 93:597-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bridge, T. A. M., and D. B. Johnson. 2000. Reductive dissolution of ferric iron minerals by Acidiphilium SJH. Geomicrobiol. J. 17:193-206. [Google Scholar]
- 5.Brierley, J. A., P. R. Norris, D. P. Kelly, and N. W. Le Roux. 1978. Characteristics of a moderately thermophilic and acidophilic iron-oxidizing Thiobacillus. Eur. J. Appl. Microbiol. Biotechnol. 5:291-299. [Google Scholar]
- 6.Calkins, V. P. 1943. Microdetermination of glycolic and oxalic acids. Ind. Eng. Chem. 15:762-763. [Google Scholar]
- 7.Clark, D. A., and P. R. Norris. 1996. Acidimicrobium ferrooxidans gen. nov., sp. nov.: mixed culture ferrous iron oxidation with Sulfobacillus species. Microbiology 142:785-790. [DOI] [PubMed] [Google Scholar]
- 8.Codd, G. A., B. Bowien, and H. G. Schlegel. 1976. Glycollate production and excretion by Alcaligenes eutrophus. Arch. Microbiol. 110:167-171. [DOI] [PubMed] [Google Scholar]
- 9.Cohen, Y., I. de Jonge, and J. G. Kuenen. 1979. Excretion of glycolate by Thiobacillus neapolitanus grown in continuous culture. Arch. Microbiol. 122:189-194. [Google Scholar]
- 10.Coram, N. J., and D. E. Rawlings. 2002. Molecular relationship between two groups of the genus Leptospirillum and the finding that Leptospirillum ferriphilum sp. nov. dominates South African commercial biooxidation tanks that operate at 40°C. Appl. Environ. Microbiol. 68:838-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gemmell, R. T., and C. J. Knowles. 2000. Utilisation of aliphatic compounds by acidophilic heterotrophic bacteria. The potential for bioremediation of acidic wastewaters contaminated with toxic organic compounds and heavy metals. FEMS Microbiol. Lett. 192:185-190. [DOI] [PubMed] [Google Scholar]
- 12.Hallberg, K. B., and E. B. Lindstrom. 1994. Characterization of Thiobacillus caldus sp. nov., a moderately thermophilic acidophile. Microbiology 140:3451-3456. [DOI] [PubMed] [Google Scholar]
- 13.Hallberg, K. B., E. González-Toril, and D. B. Johnson. 29 September 2009, posting date. Acidithiobacillus ferrivorans, sp. nov., facultatively anaerobic, psychrotolerant iron- and sulfur-oxidizing acidophiles isolated from metal mine-impacted environments. Extremophiles. doi: 10.1007/s00792-009-0282-y. [DOI] [PubMed]
- 14.Hippe, H. 2000. Leptospirillum gen. nov. (ex Markosyan 1972), nom. rev., including Leptospirillum ferrooxidans sp. nov. (ex Markosyan 1972), nom. rev. and Leptospirillum thermoferrooxidans sp. nov. (Golovacheva et al. 1992). Int. J. Syst. Evol. Microbiol. 50:501-503. [DOI] [PubMed] [Google Scholar]
- 15.Johnson, D. B. 2009. Extremophiles: acid environments, p. 107-126. In M. Schaechter (ed.), Encyclopaedia of microbiology. Elsevier, Oxford, United Kingdom.
- 16.Johnson, D. B. 1995. Selective solid media for isolating and enumerating acidophilic bacteria. J. Microbiol. Methods 23:205-218. [Google Scholar]
- 17.Johnson, D. B., and K. B. Hallberg. 2008. Carbon, iron and sulfur metabolism in acidophilic micro-organisms. Adv. Microb. Physiol. 54:202-256. [DOI] [PubMed] [Google Scholar]
- 18.Johnson, D. B., P. Bacelar-Nicolau, N. Okibe, A. Thomas, and K. B. Hallberg. 2009. Characteristics of Ferrimicrobium acidiphilum gen. nov., sp. nov., and Ferrithrix thermotolerans gen. nov., sp. nov.: heterotrophic iron-oxidizing, extremely acidophilic Actinobacteria. Int. J. Syst. Evol. Microbiol. 59:1082-1089. [DOI] [PubMed] [Google Scholar]
- 19.Johnson, D. B., C. Joulian, P. d'Hugues, and K. B. Hallberg. 2008. Sulfobacillus benefaciens, sp. nov., an acidophilic facultative anaerobic Firmicute isolated from mineral bioleaching operations. Extremophiles 12:789-798. [DOI] [PubMed] [Google Scholar]
- 20.Johnson, D. B., N. Okibe, and K. B. Hallberg. 2005. Differentiation and identification of iron-oxidizing acidophilic bacteria using cultivation techniques and amplified ribosomal DNA restriction enzyme analysis (ARDREA). J. Microbiol. Methods 60:299-313. [DOI] [PubMed] [Google Scholar]
- 21.Johnson, D. B., P. Bacelar-Nicolau, N. Okibe, A. Yahya, and K. B. Hallberg. 2001. Role of pure and mixed cultures of Gram-positive eubacteria in mineral leaching, p. 461-470. In V. S. T. Ciminelli and O. Garcia, Jr. (ed.), Biohydrometallurgy: fundamentals, technology and sustainable development. Process metallurgy, vol. 11A. Elsevier, Amsterdam, the Netherlands. [Google Scholar]
- 22.Johnson, D. B., W. I. Kelso, and D. A. Jenkins. 1979. Bacterial streamer growth in a disused pyrite mine. Environ. Pollut. 18:107-118. [Google Scholar]
- 23.Karavaiko, G. I., T. P. Tourova, I. A. Tsaplina, and T. I. Bogdanova. 2000. Investigation of the phylogenetic position of aerobic, moderately thermophilic bacteria oxidizing Fe2+, S0, and sulfide minerals and affiliated to the genus Sulfobacillus. Microbiology 69:857-860. [PubMed] [Google Scholar]
- 24.Kelly, D. P., and A. P. Wood. 2000. Reclassification of some species of Thiobacillus to the newly designated genera Acidithiobacillus gen. nov., Halothiobacillus gen. nov., and Thermothiobacillus gen. nov. Int. J. Syst. Evol. Microbiol. 50:511-516. [DOI] [PubMed] [Google Scholar]
- 25.Kimura, S., K. B. Hallberg, and D. B. Johnson. 2006. Sulfidogenesis in low pH (3.8-4.2) media by a mixed population of acidophilic bacteria. Biodegradation 17:159-167. [DOI] [PubMed] [Google Scholar]
- 26.Kishimoto, N., Y. Kosako, and T. Tano. 1991. Acidobacterium capsulatum gen. nov., sp. nov.: an acidophilic chemoorganotrophic bacterium containing menaquinone from acidic mineral environment. Curr. Microbiol. 22:1-7. [DOI] [PubMed] [Google Scholar]
- 27.Kolmert, A., P. Wikstrom, and K. B. Hallberg. 2000. A fast and simple turbidometric method for the determination of sulfate in sulfate-reducing bacterial cultures. J. Microbiol. Methods 41:179-184. [DOI] [PubMed] [Google Scholar]
- 28.Levican, G., J. A. Ugalde, N. Ehrenfeld, A. Maass, and P. Parada. 2008. Comparative genomic analysis of carbon and nitrogen assimilation mechanisms in three indigenous bioleaching bacteria: predictions and validations. BMC Genomics 9:581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lovley, D. R., and E. J. P. Phillips. 1987. Rapid assay for microbially reducible ferric iron in aquatic sediments. Appl. Environ. Microbiol. 53:1536-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macalady, J. L., D. S. Jones, and E. H. Lyon. 2007. Extremely acidic, pendulous cave wall biofilms from the Frasassi cave system, Italy. Environ. Microbiol. 9:1402-1414. [DOI] [PubMed] [Google Scholar]
- 31.Nordstrom, D. K. 2000. Advances in the hydrogeochemistry and microbiology of acid mine waters. Int. Geol. Rev. 42:499-515. [Google Scholar]
- 32.Norris, P. R., D. A. Clark, J. P. Owen, and S. Waterhouse. 1996. Characteristics of Sulfobacillus acidophilus sp. nov. and other moderately thermophilic mineral sulphide-oxidizing bacteria. Microbiology 142:775-783. [DOI] [PubMed] [Google Scholar]
- 33.Okibe, N., and D. B. Johnson. 2004. Biooxidation of pyrite by defined mixed cultures of moderately thermophilic acidophiles in pH-controlled bioreactors: the significance of microbial interactions. Biotechnol. Bioeng. 87:574-583. [DOI] [PubMed] [Google Scholar]
- 34.Okibe, N., M. Gericke, K. B. Hallberg, and D. B. Johnson. 2003. Enumeration and characterization of acidophilic microorganisms isolated from a pilot plant stirred tank bioleaching operation. Appl. Environ. Microbiol. 69:1936-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rawlings, D. E., and D. B. Johnson. 2007. The microbiology of biomining: development and optimization of mineral-oxidizing microbial consortia. Microbiology 153:315-324. [DOI] [PubMed] [Google Scholar]
- 36.Schnaitman, C., and D. G. Lundgren. 1965. Organic compounds in the spent medium of Ferrobacillus ferrooxidans. Can. J. Microbiol. 11:23-27. [DOI] [PubMed] [Google Scholar]
- 37.Storro, I., and B. A. McFadden. 1981. Glycolate excretion by Rhodospirillum rubrum. Arch. Microbiol. 129:317-320. [Google Scholar]
- 38.Tyson, G. W., I. Lo, B. J. Baker, E. E. Allen, P. Hugenholtz, and J. F. Banfield. 2005. Genome-directed isolation of the key nitrogen fixer Leptospirillum ferrodiazotrophum sp. nov. from an acidophilic microbial community. Appl. Environ. Microbiol. 71:6319-6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valdes, J., I. Pedroso, R. Quatrini, and D. S. Holmes. 2008. Comparative genome analysis of Acidithiobacillus ferrooxidans, A. thiooxidans and A. caldus: insights into their metabolism and ecophysiology. Hydrometallurgy 94:180-184. [Google Scholar]
- 40.Wakeman, K., H. Auvinen, and D. B. Johnson. 2008. Microbiological and geochemical dynamics in simulated heap leaching of a polymetallic sulfide ore. Biotechnol. Bioeng. 101:739-750. [DOI] [PubMed] [Google Scholar]
- 41.Wright, T. R., and N. M. Shah. 1975. The trophic role of glycolic acid in coastal seawater. I. Heterotrophic metabolism in seawater and bacterial cultures. Mar. Biol. 33:175-183. [Google Scholar]