Abstract
Background
Ganciclovir protects against hearing deterioration in infants with symptomatic congenital cytomegalovirus (CMV) disease involving the central nervous system (CNS).
Objectives
To assess the neurodevelopmental impact of ganciclovir therapy in this population.
Study Design
100 neonates were enrolled into a controlled Phase III study of symptomatic congenital CMV involving the CNS, and were randomized to either 6 weeks of intravenous ganciclovir or no treatment. Denver developmental tests were performed at 6 weeks, 6 months, and 12 months. For each age, developmental milestones that ≥90% of normal children would be expected to have achieved were identified. The numbers of milestones not met (“delays”) were determined for each subject. The average number of delays per subject was compared for each treatment group.
Results
At 6 months, the average number of delays was 4.46 and 7.51, respectively, for ganciclovir recipients and “no treatment” subjects (p=0.02). At 12 months, the average number of delays was 10.06 and 17.14, respectively (p=0.007). In a multivariate regression model, the effect of ganciclovir therapy remained statistically significant at 12 months (p=0.007).
Conclusions
Infants with symptomatic congenital CMV involving the CNS receiving intravenous ganciclovir therapy have fewer developmental delays at 6 and 12 months compared with untreated infants. Based on these data as well as the previously published data regarding ganciclovir treatment and hearing outcomes, six weeks of intravenous ganciclovir therapy can be considered in the management of babies with symptomatic congenital CMV disease involving the CNS. If treatment is initiated, it should be started within the first month of life and patients should be monitored closely for toxicity, especially neutropenia. Since existing data only address the treatment of symptomatic congenital CMV disease involving the CNS, these data cannot be extrapolated to neonates with other manifestations of CMV disease, including asymptomatic babies and symptomatic babies who do not have CNS involvement.
Keywords: Ganciclovir, antiviral treatment, congenital CMV, cytomegalovirus, neurologic outcomes, developmental outcomes
INTRODUCTION
Congenital cytomegalovirus (CMV) infection is the most frequent known viral cause of mental retardation,1, 2 and is the leading non-genetic cause of sensorineural hearing loss in many countries including the United States.3–6 Approximately 1% of all live births in the United States are infected with CMV (~ 40,000 babies per year).7 Of those fetuses infected, approximately 10% are symptomatic at birth, and a majority of these patients subsequently experience significant neurological sequelae, including sensorineural hearing loss, mental retardation, microcephaly, seizures, or paresis/paralysis.8–14 The overall societal costs of providing specialized services for surviving infants and children with congenital CMV infections are in the billions of dollars annually.15
The National Institute of Allergy and Infectious Diseases (NIAID) Collaborative Antiviral Study Group (CASG) completed a Phase III randomized controlled investigation of intravenous ganciclovir for the treatment of symptomatic congenital CMV disease involving the central nervous system (CNS).16 Results from this study indicate that six weeks of intravenous (IV) ganciclovir therapy decreases the likelihood that hearing loss will worsen over at least the first two years of life. The impact of antiviral therapy on neurodevelopmental outcomes is unknown. To evaluate this important question, we analyzed in a blinded fashion the Denver II developmental assessments of babies previously enrolled in the Phase III randomized, controlled trial.
METHODS
Study Population
From 1991 to 1999, 100 neonates were enrolled in a Phase III controlled trial and randomized to 6 weeks of intravenous ganciclovir at 12 mg/kg/day delivered in two divided doses (n=48) or to no antiviral treatment (n=52).16 Block randomization by center was utilized. A placebo was not used in the study due to ethical concerns over maintaining intravenous access for six weeks in order to administer a placebo. All study subjects had confirmed isolation of CMV from a urine specimen obtained prior to study enrollment and within the first month of life, and all had evidence of CNS disease such as: 1) microcephaly; 2) intracranial calcifications; 3) abnormal cerebrospinal fluid (CSF) for age; 4) chorioretinitis; or 5) hearing loss. Infants ≤1 month of age, ≥32 weeks gestation, and ≥1200 grams at birth were eligible for study participation. The analysis of Denver developmental data presented herein were secondary analyses and followed the publication of the impact of antiviral therapy on hearing loss.16 Prior to participation in the treatment study, informed consent was obtained from the parent(s) or guardian(s). The analysis of the Denver developmental data stored in the Phase III dataset was approved by the University of Alabama at Birmingham’s Institutional Review Board.
Denver II Developmental Assessment
During the course of the clinical trial, Denver II developmental tests were performed on subjects at 6 weeks, 6 months, and 12 months of age by study personnel at each site, who were not able to be blinded due to the lack of a placebo. The Denver II is used routinely in pediatric care to assess developmental milestones, and has high inter-rater reliability. It consists of four objectively-assessed and -defined categories which evaluate different aspects of a child’s neurological development: Personal/Social, Fine Motor, Language, and Gross Motor. Each category in turn consists of many elements that a child achieves as they attain neurodevelopmental milestones. The Denver II categorizes a child’s performance as “Caution” (a child failing an element which between 75% and 90% of children who are his/her age would pass) or as “Delay” (a child failing an element which ≥90% of children who are his/her age would pass).17
Utilizing the Denver II, ≥90% of 6 week old babies would be expected to have achieved 6 total elements in the four categories. By 6 months of age, 21 total elements should be achieved by ≥ 90% of children. By 12 months of age, 38 total elements from the four Denver II categories should be achieved by ≥90% of children. The increasing number of elements expected to be achieved with increasing age is a reflection of developmental milestones that are met as a child grows. In this study, the total number of delays was determined for each subject at each testing age by an investigator at the CASG Central Unit who was blinded to their treatment randomization. These were then split into treatment versus no treatment categories, and the average for each group was determined. Since one of the most important outcomes of congenital CMV infection is hearing loss, and because the language category of the Denver II involves the subject’s ability to hear and respond to certain stimuli, total delays also were calculated for the Personal/Social, Fine Motor, and Gross Motor categories but excluding the Language category.
Statistical Analyses
Univariate descriptive statistics summarized each of the four Denver II categories, as well as the total number of delays. The Student’s t-test was utilized to compare each separate Denver II component and total delays with regard to therapy assignment and other prognostic indicators. Multivariate regression models were utilized to test independent factors known to be related to poor developmental outcome in congenital CMV infection.18, 19 Treatment interactions with developmental outcomes were also explored using regression models. A mixed model was utilized to analyze the Denver II test deficits longitudinally by using each subject as a random effect. Each subject’s individual scores were assessed across their follow-up visits, and a slope for that subject’s score changes was included in the final model.
RESULTS
Of the 100 subjects enrolled in the Phase III randomized controlled study, 74 had a Denver II developmental test performed at 6 weeks of age (34 ganciclovir, 40 no treatment), 74 had a 6 month evaluation (35 ganciclovir, 39 no treatment), and 71 had a 12 month evaluation (35 ganciclovir, 36 no treatment). Demographic data are presented in Table 1 by treatment category for those subjects with a 12 month Denver II assessment. Demographic and baseline characteristics for all three measurement times were similar between the two treatment regimens. Eighty-four of the 100 subjects had at least one Denver II assessment by 12 months of age, and 60 had Denver II tests during all three follow-up intervals (29 in the ganciclovir-treated group and 31 in the no treatment group). There were no significant differences in demographic and baseline characteristics between the 71 subjects with Denver II assessments at 12 months and the 29 subjects who did not have a Denver II developmental test and thus were unevaluable for this study (Table 2). The average subject age at the 6 week assessment was 8.05 weeks (range: 5 weeks to 11 weeks); at the 6 month (26 week) assessment was 27.42 weeks (range: 22 weeks to 34 weeks); and at the 12 month (52 week) assessment was 54.21 weeks (range: 41 weeks to 68 weeks).
Table 1.
Demographic and Baseline Characteristics for Subjects with a Denver II Developmental Assessment at 12 Months
| Characteristics | Treatment (n=35) | No Treatment (n=36) | P-Value |
|---|---|---|---|
| Age at enrollment (days) | |||
| Median | 12 | 12 | 0.77 |
| Range | 3–33 | 2–33 | |
| Gender | |||
| Female | 16 ( 46%) | 21 ( 58%) | 0.29 |
| Male | 19 ( 54%) | 15 ( 42%) | |
| Race | |||
| White | 23 ( 66%) | 22 ( 61%) | 0.77 |
| Black | 8 ( 23%) | 8 ( 22%) | |
| Hispanic | 4 ( 11%) | 5 ( 14%) | |
| Other | 0 ( 0%) | 1 ( 3%) | |
| Prematurity (≤37 weeks) | 14 ( 40%) | 11 ( 31%) | 0.40 |
| Gestational Age (weeks) | |||
| Median | 37 | 38 | 0.56 |
| Range | 32–41 | 29–41 | |
| Birth weight (grams) | |||
| Median | 2307 | 2410 | 0.96 |
| Range | 1335–3730 | 1012–3425 | |
| Head Circumference (cm) | |||
| Median | 30 | 30 | 0.15 |
| Range | 27–37 | 24–36 | |
| Abnormal computed tomography (CT) (calcifications) | 9 ( 26%) | 11 ( 31%) | 0.60 |
| Abnormal cerebrospinal fluid indices | 5 ( 14%) | 10 ( 28%) | 0.18 |
| Chorioretinitis | 2 ( 6%) | 5 ( 14%) | 0.23 |
| Alanine aminotransferase (ALT) ≥ 100 IU/L | 6 ( 17%) | 6 ( 17%) | 0.89 |
| Platelet count ≤100,000/mm3 | 12 (34%) | 14 (39%) | 0.68 |
| Abnormal bilirubin | 5 (14%) | 6 (17%) | 0.63 |
| Splenomegaly | 24 (69%) | 25 (69%) | 0.79 |
| Hepatomegaly | 24 (69%) | 25 (69%) | 0.79 |
| Absolute Neutrophil Count (ANC) | 7 (20%) | 4 (11%) | 0.30 |
| Grade 3–4 | |||
| Baseline Brainstem Evoked Response (BSER) (Best Ear) | |||
| Normal | 17 (49%) | 16 (44%) | 0.12 |
| Mild | 5 (14%) | 7 (19%) | |
| Moderate | 1 (3%) | 5 (14%) | |
| Severe | 7 (20%) | 2 (6%) |
Table 2.
Demographic and Baseline Characteristics for Subjects with a Denver II Developmental Assessment at 12 Months versus No Assessment
| Characteristics | Evaluable (Denver II Assessment) n=71 | Unevaluable (No Denver II Assessment) n=29 | P-Value |
|---|---|---|---|
| Age at enrollment (days) Median Range |
12 2–33 |
12.5 3–33 |
0.50 |
| Gender Female Male Unknown |
37 (52%) 34 (48%) 0 ( 0%) |
10 (34%) 18 (62%) 1 ( 3%) |
0.14 |
| Race White Black Hispanic Other Unknown |
45 (63%) 16 (23%) 9 (13%) 1 ( 1%) 0 ( 0%) |
12 (41%) 11 (38%) 4 (14%) 1 ( 3%) 1 ( 3%) |
0.26 |
| Prematurity (≤37 weeks) | 25 (35%) | 13 (45%) | 0.24 |
| Gestational Age (weeks) Median Range |
38.0 29–41 |
37.0 32–40 |
0.82 |
| Birth weight (grams) Median Range |
2383 1012–3730 |
2314 1256–3795 |
0.92 |
| Head Circumference (cm) Median Range |
30 24.3–37.0 |
30.5 25.5–34.9 |
0.81 |
| Abnormal computed tomography (CT) (calcifications) | 20 (28%) | 8 (28%) | 0.92 |
| Abnormal cerebrospinal fluid indices | 15 (21%) | 8 (28%) | 0.22 |
| Alanine aminotransferase(ALT) ≥100 IU/L | 12 (17%) | 5 (17%) | 0.58 |
| Platelet count ≤100,000/mm3 | 26 (37%) | 8 (28%) | 0.63 |
| Elevated bilirubin | 11 (15%) | 4 (14%) | 0.87 |
| Splenomegaly | 40 (56%) | 12 (41%) | 0.18 |
| Hepatomegaly | 41 (58%) | 15 (52%) | 0.86 |
| Absolute Neutrophil Count(ANC) Grade 3–4 | 11 (15%) | 2 (7%) | 0.25 |
| Randomized Therapy Drug No treatment |
35 (49%) 36 (51%) |
13 (45%) 16 (55%) |
0.68 |
| Baseline Brainstem Evoked Response (BSER) (Best Ear) Normal Mild Moderate Severe |
33 (46%) 12 (17%) 6 ( 8%) 9 (13%) |
11 (38%) 2 (7%) 0 (0%) 5 (17%) |
0.29 |
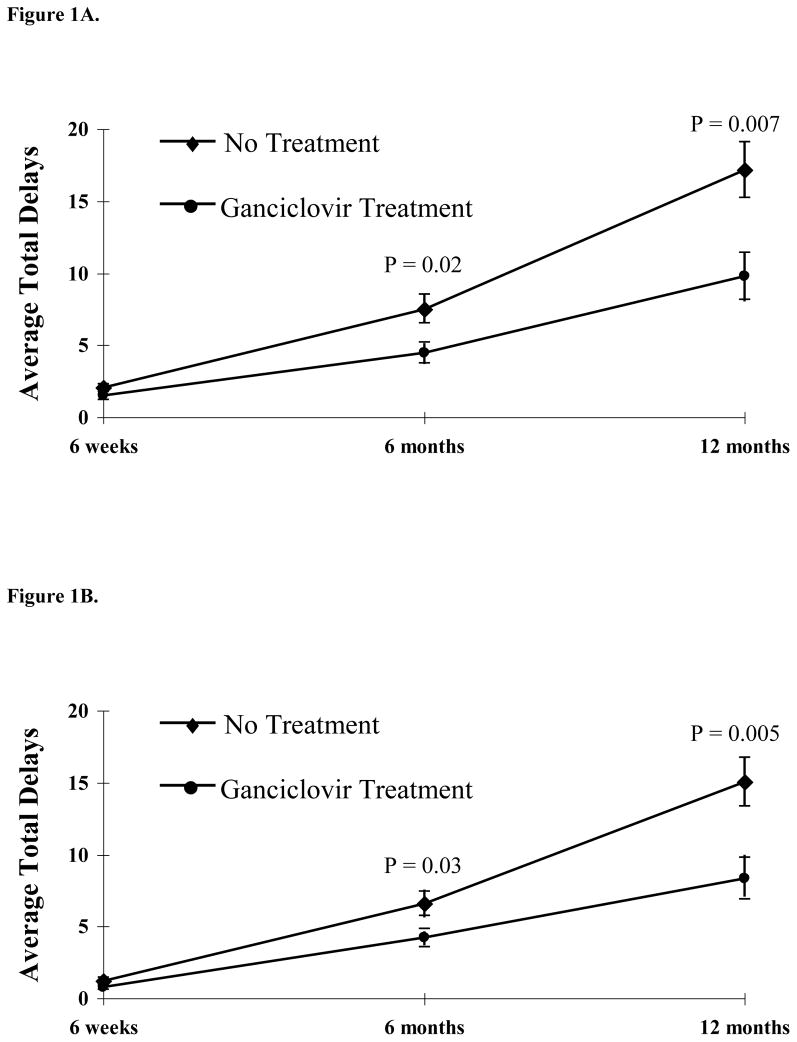
With increasing age, subjects who received ganciclovir therapy experienced fewer developmental delays compared with subjects receiving no treatment (Table 3 and Figure 1A). At 6 months and 12 months, the numbers of delays in ganciclovir treated subjects were significantly lower compared with the number of delays among untreated controls (p=0.02 and p=0.007, respectively). Fewer delays were seen in each of the four components of the Denver developmental test for ganciclovir recipients compared with subjects receiving no treatment. Eliminating the language component from the analyses in order to minimize an association between developmental outcome and hearing status, ganciclovir-treated subjects continued to have fewer developmental delays at 6 months and 12 months (p=0.03 and p=0.005, respectively) (Table 3 and Figure 1B).
Table 3.
Average Delays Per Subject by Denver II Category (mean ± SE)
| Treatment | No Treatment | P-value | |
|---|---|---|---|
| 6 weeks | |||
| Personal/Social (2 total*) | 0.5 ± .12 | 0.78 ± .12 | 0.11 |
| Fine Motor (1) | 0.21 ± .07 | 0.28 ± .07 | 0.50 |
| Gross Motor (1) | 0.09 ± .05 | 0.18 ± .06 | 0.29 |
| Language (2) | 0.71 ± .14 | 0.83 ± .13 | 0.54 |
| Total Delays (6) | 1.5 ± .27 | 2.05 ± .27 | 0.15 |
| Total Delays without Language (4) | 0.79 ± .18 | 1.23 ± .19 | 0.11 |
| 6 months | |||
| Personal/Social (4 total) | 0.77 ± .16 | 1.21 ± .20 | 0.10 |
| Fine Motor (6) | 1.31 ± .29 | 2.46 ± .37 | 0.02 |
| Gross Motor (7) | 2.11 ± .32 | 2.90 ± .42 | 0.15 |
| Language (4) | 0.26 ± .13 | 0.95 ± .22 | 0.009 |
| Total Delays (21) | 4.46 ± .74 | 7.51 ± 1.00 | 0.02 |
| Total Delays without Language (17) | 4.20 ± .65 | 6.56 ± .85 | 0.03 |
| 12 months | |||
| Personal/Social (6 total) | 1.28 ± .23 | 2.22 ± .28 | 0.01 |
| Fine Motor (12) | 3.31 ± .66 | 6.19 ± .72 | 0.004 |
| Gross Motor (13) | 4.00 ± .69 | 6.61 ± .75 | 0.01 |
| Language (7) | 1.23 ± .26 | 2.11 ± .31 | 0.03 |
| Total Delays (38) | 10.06 ± 1.67 | 17.14 ± 1.93 | 0.007 |
| Total Delays without Language (31) | 8.58 ± 1.49 | 15.03 ± 1.68 | 0.005 |
Total potential number of delays for each category listed in parenthesis
Figure 1.
Figure 1A. Total Delays, including Personal/Social, Fine Motor, Language, and Gross Motor Components of the Denver II Developmental Test (mean ± SE)
Figure 1B. Total Delays, including Personal/Social, Fine Motor, and Gross Motor Components of the Denver II Developmental Test but Excluding the Language Component (mean ± SE)
Of the factors explored in univariate analysis (abnormal CNS imaging, abnormal CSF protein concentration, premature birth, microcephaly, calcifications, and abnormal hearing at the given time period), intracranial calcification, abnormal computed tomography (CT) imaging of the head, and microcephaly at birth were significantly associated with developmental outcome and so were then controlled for in a cross-sectional regression model. The effect of ganciclovir therapy in this model remained statistically significant at 12 months for the 71 subjects with 12 month evaluations (P = 0.007). While we were not able to control for length of disease, timing of infection in utero, or primary vs. recurrent maternal infection, we were able to control for extent of disease, which has been shown to correlate with timing of the infection in utero as well as type of maternal infection.
All 84 subjects with at least one Denver II assessment were included in a longitudinal regression model to evaluate developmental delays across the year of evaluation. After adjusting for abnormal CT imaging of the head, microcephaly at birth, and intracranial calcifications, the beneficial effect of ganciclovir therapy on neurodevelopmental outcomes across the 12 months of testing trended toward significance (p= 0.07).
DISCUSSION
These data suggest that 6 weeks of intravenous ganciclovir treatment for infants with symptomatic congenital CMV disease involving the CNS may improve neurodevelopmental outcomes at 6 months and 12 months of age compared with babies who receive no antiviral therapy. Treated subjects had fewer neurodevelopmental delays compared with subjects who did not receive antiviral therapy, even when the language component of the Denver II developmental assessment was eliminated from the analysis. The 12 month assessment individually showed the largest impact on developmental outcomes, and therefore the trend toward significance in the longitudinal regression model, in which not all subjects had 12 month data, further supports a beneficial impact of antiviral therapy on neurodevelopment. These results, however, do not suggest that treatment with ganciclovir can prevent all neurodevelopmental delays from occurring. While the ganciclovir recipients had fewer delays and appear to have more normal neurologic outcomes, most were still behind what would be considered “normal development” for 6 weeks, 6 months, or 12 months of age.
Prior to this study, no controlled data existed regarding the effect of antiviral treatment on neurological development in babies with congenital CMV disease. This study provides preliminary data suggesting that treatment with ganciclovir may improve the likelihood of affected children reaching age-appropriate milestones. Importantly, these results were demonstrable in the most severely affected group of infants with congenital CMV disease.
A weakness of this trial relates to the “screening” aspect of the Denver II developmental test.17 However, a recent comparison of the Denver II with the Wechsler Intelligence Scale for Children third edition (WISC-III), a more sophisticated assessment tool for cognitive function, in children with CNS injury due to viral disease found strong concordance between the two tests.20 Additionally, performance of the Denver II developmental test at six months of age has proven to be a good predictor of severe neurologic outcomes at two years of age in babies with neurologic injury due to hypoxic-ischemic encephalopathy, with a sensitivity of 100% and a specificity of 95%.21 In order to be as conservative as possible with our analyses, we considered a subject to have a “delay” for a given element only if they have failed to achieve a milestone that ≥90% of children at that age are able to do.
Based on these data as well as the previously published data regarding ganciclovir treatment and hearing outcomes,16 ganciclovir therapy can be considered in the management of babies with symptomatic congenital CMV disease involving the CNS.22 Approximately two-thirds of neonates and infants receiving intravenous ganciclovir will develop significant neutropenia, and the requirement of long-term intravenous access can allow for intravascular bacterial superinfections.16 Both family and physician should carefully consider the potential benefits of ganciclovir therapy versus the risks associated with treatment. If treatment is initiated, it should be started within the first month of life and patients should be monitored closely for toxicity, especially neutropenia. Since existing data only address the treatment of symptomatic congenital CMV disease involving the CNS, these data cannot be extrapolated to neonates with other manifestations of CMV disease, including asymptomatic babies and symptomatic babies who do not have CNS involvement. At this time, ganciclovir should not be used in infants with asymptomatic congenital CMV infection.
While not utilized during this study, the Bayley Scales of Infant Development – Revised are being utilized in a newly initiated study of oral valganciclovir being conducted by the NIAID Collaborative Antiviral Study Group (www.casg.uab.edu). It is anticipated that this will allow for more precise assessment of the impact of antiviral treatment on neurodevelopmental outcomes of babies with symptomatic congenital CMV disease.
Acknowledgments
This work was supported under contract with the Division of Microbiology and Infectious Diseases of the National Institute of Allergy and Infectious Diseases (NIAID) (N01-AI-30025, N01-AI -65306, N01-AI -15113, N01-AI-62554), and by grants from the General Clinical Research Center Program (M01-RR00032) and the State of Alabama.
List of Abbreviations
- ALT
Alanine Aminotransferase
- ANC
Absolute Neutrophil Count
- BSER
Brainstem Evoked Response
- CASG
Collaborative Antiviral Study Group
- CSF
Cerebrospinal Fluid
- CMV
Cytomegalovirus
- CNS
Central Nervous System
- CT
Computed Tomography
- IV
Intravenous
- NIAID
National Institute of Allergy and Infectious Diseases
- WISC-III
Wechsler Intelligence Scale for Children third edition
Footnotes
Presented at the 2006 Pediatric Academic Societies/Society for Pediatric Research Annual Meeting, San Francisco, California, April 29, 2006; Abstract # 752908.
CONFLICT OF INTEREST DECLARATION
No authors have a commercial or other association which may pose a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Elek SD, Stern H. Development of a vaccine against mental retardation caused by cytomegalovirus infection in utero. Lancet. 1974;1:1–5. doi: 10.1016/s0140-6736(74)92997-3. [DOI] [PubMed] [Google Scholar]
- 2.Arvin AM, Fast P, Myers M, Plotkin S, Rabinovich R. Vaccine development to prevent cytomegalovirus disease: report from the National Vaccine Advisory Committee. Clin Infect Dis. 2004;39:233–9. doi: 10.1086/421999. [DOI] [PubMed] [Google Scholar]
- 3.Fowler KB, McCollister FP, Dahle AJ, et al. Progressive and fluctuating sensorineural hearing loss in children with asymptomatic congenital cytomegalovirus infection. J Pediatr. 1997;130:624–30. doi: 10.1016/s0022-3476(97)70248-8. [DOI] [PubMed] [Google Scholar]
- 4.Harris S, Ahlfors K, Ivarsson S, Lernmark B, Svanberg L. Congenital cytomegalovirus infection and sensorineural hearing loss. Ear & Hearing. 1984;5:352–5. doi: 10.1097/00003446-198411000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Fowler KB, Dahle AJ, Boppana SB, Pass RF. Newborn hearing screening: will children with hearing loss caused by congenital cytomegalovirus infection be missed? J Pediatr. 1999;135:60–4. doi: 10.1016/s0022-3476(99)70328-8. [DOI] [PubMed] [Google Scholar]
- 6.Grosse SD, Ross DS, Dollard SC. Congenital cytomegalovirus (CMV) infection as a cause of permanent bilateral hearing loss: a quantitative assessment. J Clin Virol. 2008;41:57–62. doi: 10.1016/j.jcv.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Demmler GJ. Infectious Diseases Society of America and Centers for Disease Control. Summary of a workshop on surveillance for congenital cytomegalovirus disease. Rev Infect Dis. 1991;13:315–29. doi: 10.1093/clinids/13.2.315. [DOI] [PubMed] [Google Scholar]
- 8.Stagno S, Whitley RJ. Herpesvirus infections of pregnancy. Part I: Cytomegalovirus and Epstein-Barr virus infections. N Engl J Med. 1985;313:1270–4. doi: 10.1056/NEJM198511143132006. [DOI] [PubMed] [Google Scholar]
- 9.McCracken GH, Jr, Shinefield HM, Cobb K, et al. Congenital cytomegalic inclusion disease. A longitudinal study of 20 patients. Am J Dis Child. 1969;117:522–39. doi: 10.1001/archpedi.1969.02100030524005. [DOI] [PubMed] [Google Scholar]
- 10.Pass RF, Stagno S, Myers GJ, Alford CA. Outcome of symptomatic congenital cytomegalovirus infection: results of long-term longitudinal follow-up. Pediatrics. 1980;66:758–62. [PubMed] [Google Scholar]
- 11.Weller TH. The cytomegaloviruses: ubiquitous agents with protean clinical manifestations. I N Engl J Med. 1971;285:203–14. doi: 10.1056/NEJM197107222850406. [DOI] [PubMed] [Google Scholar]
- 12.Weller TH, Hanshaw JB. Virologic and clinical observations on cytomegalic inclusion disease. N Engl J Med. 1962;266:1233–44. doi: 10.1056/NEJM196206142662401. [DOI] [PubMed] [Google Scholar]
- 13.Conboy TJ, Pass RF, Stagno S, et al. Early clinical manifestations and intellectual outcome in children with symptomatic congenital cytomegalovirus infection. J Pediatr. 1987;111:343–8. doi: 10.1016/s0022-3476(87)80451-1. [DOI] [PubMed] [Google Scholar]
- 14.Dollard SC, Grosse SD, Ross DS. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol. 2007 doi: 10.1002/rmv.544. [DOI] [PubMed] [Google Scholar]
- 15.Yow MD, Demmler GJ. Congenital cytomegalovirus disease--20 years is long enough. N Engl J Med. 1992;326:702–3. doi: 10.1056/NEJM199203053261010. [DOI] [PubMed] [Google Scholar]
- 16.Kimberlin DW, Lin CY, Sanchez PJ, et al. Effect of ganciclovir therapy on hearing in symptomatic congenital cytomegalovirus disease involving the central nervous system: a randomized, controlled trial. J Pediatr. 2003;143:16–25. doi: 10.1016/s0022-3476(03)00192-6. [DOI] [PubMed] [Google Scholar]
- 17.Frankenburg WK, Dodds J, Archer P, Shapiro H, Bresnick B. The Denver II: a major revision and restandardization of the Denver Developmental Screening Test. Pediatrics. 1992;89:91–7. [PubMed] [Google Scholar]
- 18.Boppana SB, Fowler KB, Vaid Y, et al. Neuroradiographic findings in the newborn period and long-term outcome in children with symptomatic congenital cytomegalovirus infection. Pediatrics. 1997;99:409–14. doi: 10.1542/peds.99.3.409. [DOI] [PubMed] [Google Scholar]
- 19.Noyola DE, Demmler GJ, Nelson CT, et al. Early predictors of neurodevelopmental outcome in symptomatic congenital cytomegalovirus infection. J Pediatr. 2001;138:325–31. doi: 10.1067/mpd.2001.112061. [DOI] [PubMed] [Google Scholar]
- 20.Chang LY, Huang LM, Gau SS, et al. Neurodevelopment and cognition in children after enterovirus 71 infection. N Engl J Med. 2007;356:1226–34. doi: 10.1056/NEJMoa065954. [DOI] [PubMed] [Google Scholar]
- 21.Hallioglu O, Topaloglu AK, Zenciroglu A, et al. Denver developmental screening test II for early identification of the infants who will develop major neurological deficit as a sequalea of hypoxic-ischemic encephalopathy. Pediatr Int. 2001;43:400–4. doi: 10.1046/j.1442-200x.2001.01418.x. [DOI] [PubMed] [Google Scholar]
- 22.American Academy of Pediatrics. Cytomegalovirus infection. In: Pickering LK, editor. 2006 Red Book: Report of the Committee on Infectious Diseases. 27. Elk Grove Village, IL: American Academy of Pediatrics; 2006. pp. 273–7. [Google Scholar]