Abstract
Background
It is unclear whether coinfection with HCV increases mortality in HIV patients in the HAART era. Using a meta-analysis, we estimated the effect of HCV on HIV disease progression and overall mortality in the pre- and HAART era.
Method
PubMed and EMBASE were searched for studies published until April 30, 2008. Additional studies were identified from cited references. Studies reporting disease progression or mortality in HCV/HIV coinfected patients were selected. Cross sectional studies, studies without HCV negative controls and studies among children and/or post-liver transplant were excluded. Two authors reviewed articles and extracted data on demographics of study populations and risk estimates. Meta-regression was used to explore heterogeneity.
Results
10 pre-HAART studies and 27 HAART era studies were selected. In the pre-HAART era, the risk ratio for overall mortality among HCV/HIV coinfected patients was 0.68 (95% CI: 0.53~0.87) compared to patients with HIV infection alone. In the HAART era, the risk ratio was 1.12 (95% CI: 0.82~1.51) for AIDS-defining events and 1.35 (95% CI: 1.11~1.63) for overall mortality in coinfected patients compared to HIV monoinfection.
Conclusions
HCV coinfection did not increase mortality in HIV patients before the introduction of HAART. In contrast, HCV coinfection increases the risk of mortality but not AIDS-defining events compared to HIV infection alone in the HAART era. Future studies should determine whether successfully treated HCV could reduce this excess risk of mortality in coinfected patients.
Keywords: HCV/HIV coinfection, mortality, meta-analysis
Introduction
Hepatitis C virus (HCV) and human immunodeficiency virus (HIV) share routes of transmission, and thus the prevalence of HCV infection is approximately 15-30% in HIV infected patients. Due to enhanced transmissibility of HCV by percutaneous injection compared to HIV, HCV prevalence can exceed 85% in HIV-infected injection drug users [1, 2]. Before the introduction of highly active antiretroviral therapy (HAART), mortality related to HIV overwhelmed that related to HCV. By contrast, while HIV patients live longer in the HAART era, chronic diseases such as viral hepatitis have emerged as important causes of morbidity and mortality [3, 4]. It has been strongly suggested that HIV infection accelerates HCV-related disease progression and mortality [5-7] but the reciprocal effect of HCV on the rate of progression of HIV remains muddled due to the heterogeneity of study results. Understanding whether coinfection with HCV affects progression and mortality related to HIV may elucidate challenging issues for providers treating HIV patients with HCV coinfection and shed insight into the complex interactions between these two persistent viruses.
Some studies have reported a strong association between HCV/HIV coinfection and increased risk of HIV disease progression [8, 9], while other studies have not confirmed this result after the widespread use of HAART [10, 11]. This systematic review estimates the effect of HCV on both mortality and HIV progression in HCV/HIV coinfected patients in the era of HAART. We compared studies from both the pre-HAART and the HAART era, focusing on the latter as these are most likely to inform future practice.
Methods
Data Sources
A literature search was conducted to identify publications reporting disease progression or survival of HIV among HIV and HCV coinfected cohorts using PubMed and EMBASE without language restriction until April 30, 2008. Titles and/or abstract were screened to determine the relevance of studies. Full texts of selected studies were reviewed. Additional studies were identified from cited references.
Study Selection
Publications that reported on disease progression, mortality and/or survival of HIV among adult HIV and HCV coinfected adult cohorts were selected for inclusion, while studies among nonadolescent children were excluded. Studies were classified in the pre-HAART era if occurring entirely in the period before January 1996 and in the HAART era if half or greater than half of the study period was after January 1996, unless specifically split into two categories by the author. Publications on liver disease-related mortality were not included unless overall mortality was also reported due to potential misclassification. Cross-sectional studies, studies on survival after liver transplant, and studies without HIV-monoinfected control groups were excluded.
Data Extraction
One author (TC) completed the search and extracted data from the studies on two occasions. Study design, period and population, number of subjects, median/mean follow up, treatments for HIV and HCV, outcome measures, percentage of intravenous drug users (IDU) and adjustments for potential confounders were extracted by two authors (TC and AK). For studies reporting only incidence rates or cumulative incidence rates of death or AIDS-defining events, risk ratio and 95% confidence interval were calculated from the available data provided. If studies presented several risk estimates, the final adjusted risk estimate was recorded.
Study Quality Assessment
Study quality was assessed for studies in the era of HAART with primary outcomes of overall mortality and/or AIDS-defining diseases. Quality of these studies was evaluated by the following: 1) study design: prospective studies were considered to have higher quality than retrospective; 2) median follow-up time: studies with a longer duration of follow-up were considered more likely to reflect the true effect of HCV on HIV-related disease progression.
Statistical Analysis
Quantitative analyses were conducted for overall mortality in the pre-HAART era and three different outcome measures: overall mortality, AIDS-defining events and the combination of AIDS-defining events and mortality in the HAART era. Conventional random effect models were used to estimate summary risks ratios and 95% confidence intervals given the variability inherent among observational studies [12]. Heterogeneity across studies was evaluated by I2 test [13]. I2 statistic greater than 20% suggests heterogeneity and an I2 statistic greater than 50% indicates substantial heterogeneity. Publication bias was assessed with funnel plots, Begg's and Egger's tests [14, 15]. Sensitivity analysis was conducted by omitting one study at a time to examine the influence of individual studies for overall mortality. Univariate random-effect meta-regressions using aggregate-level data including percentage of patients on HAART, percentage of IDU patients in the study population, study duration and gender were performed for overall mortality in HAART era to assess potential effect modification [16]. The effect of study quality was assessed by subgroup analysis. STATA v. 9 was used for all analysis.
Results
Study Selection
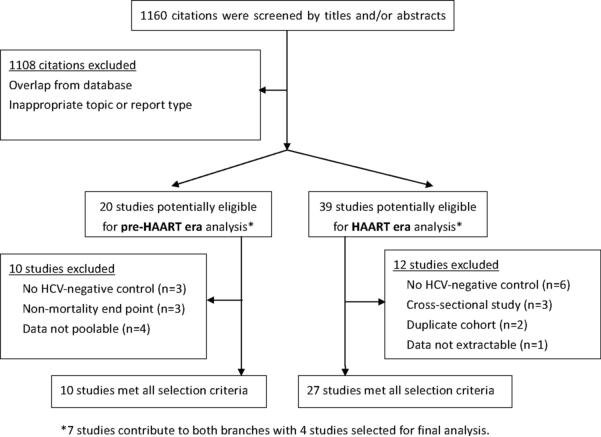
Figure 1 summarizes the study selection process. The electronic search yielded 537 citations in PubMed and 623 in EMBASE. Among the total of 1160 titles/abstracts screened, 52 papers were selected for in-depth review. Seven papers provided data from eras pre- and post- HAART introduction [11, 17-22]. For pre-HAART analysis, 10 studies were excluded for one or more of the following reasons: 1) AIDS and overall mortality were combined as a single endpoint [17, 23, 24], 2) lack of HCV-negative control [21, 25, 26] or 3) data were not able to be pooled [22, 27-29]. As a result, data were extracted for 10 studies [11, 18-20, 30-35]. For analysis of studies conducted in the HAART era, 12 studies were excluded for one or more of the following: 1) they lacked an HCV-negative control group [5, 21, 36-39], 2) used a cross-sectional design [40-42], 3) data were not extractable [22], or 4) the cohort was duplicated [43, 44]. Thus, data were extracted from 33 studies, 10 pre-HAART and 27 HAART era with 4 contributing to both eras.
Figure 1.
Selection process for study inclusion in the meta-analysis of HCV on overall mortality and/or HIV-related progression
Study Characteristics
Ten studies, involving 4413 HCV/HIV coinfected patients and 10213 solely HIV infected patients were included for the pre-HAART era, and 27 HAART era studies including 25,319 HCV/HIV coinfected patients and 61,697 HIV-monoinfected patients were included (Table 1). For the pre-HAART era, study sample sizes ranged from 95 [11] to 10,896 [18]. In the HAART era, study sample sizes ranged from 330 [10] to 23,441 [4]. Study outcomes included: overall mortality in 20 studies [4, 10, 11, 18-20, 45-58], AIDS-defining events in 7 studies [11, 19, 54-57, 59]; and combined AIDS-defining events and mortality in 7 studies [8, 9, 17, 54, 60-62]. One study reported all 3 outcome measures and 5 studies reported both mortality and AIDS-defining events. HAART use was defined as a regimen including at least three antiretroviral agents. Nine studies recruited only patients on HAART [8, 9, 46, 48-50, 58, 60, 61] and 2 studies did not report percentage of patients on HAART [18, 20]. The percentage of patients on HAART in the remaining studies ranged from 35 to 89 %. Most studies either did not report treatment of HCV or reported only small percentage (range: 0~7 %) of coinfected patients on treatment. Percentage of IDU in study populations varied from 0 to 64%. The study without IDU was selected for a subgroup analysis of non-IDU patients [20]. Mean/median follow-up time ranged from 1.1 to 5.9 years for studies conducted only in the HAART era.
Table 1.
Studies included in meta-analysis of HIV and HCV coinfection
| Author (published year) |
Country | Study design |
Study year | Outcome measures |
Study population |
% of male |
Mean/ median age |
No. of HCV+/HCV− |
% on HAART |
HCV Tx | % of IDUΔ |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Pre-HAART era | |||||||||||
| Diamondstone (1995) | USA | RS | 1984~1993 | M | Hemophilia | 100 | 25 | 565 / 16 | - | - | NR |
| El-Serag (2005) | USA | RS | 1991~1996 | M | Hospitalized Veterans | 98 | 45 | 3048 / 7848 | - | - | NR |
| Haydon (1998) | UK | PC+RS | 1985~1996 | M | IDU | 68 | 23* | 202 / 38 | - | - | 100 |
| Hung (2005) | Taiwan | PC | 1994~1997 | M | Adults | 92 | 36 | 11 / 84 | - | - | 3 |
| Klein (2003) | Canada | PC+RS | 1989~1996 | M | Adults | 86 | 36 | 42 / 620 | - | - | 5 |
| Macias (1998) | Spain | RS | 1989~1996 | M | Adults | 77 | 34 | 214 / 106 | - | - | 61 |
| Ockenga (1997) | Germany | PC | 1993~1996 | M | Adults | 78 | 38 | 56 / 154 | - | - | 28 |
| Staples (1999) | USA | RS | 1992~1997 | M | Veterans | 99 | 38 | 115 / 235 | - | - | 20 |
| Voirin (2004) | France | PC | 1992~1996 | M | Adults | 81 | 34 | 121 / 980 | - | - | 0 |
| Wright (1994) | USA | RS | 1984~1989 | M | Adults | 98 | NR | 39 / 132 | - | - | 40 |
|
HAART era | |||||||||||
| Anderson (2004) | USA | RS | 1997~2001 | M | Veterans | 99 | 42 | 306 / 664 | 73 | NR | 22 |
| Backus (2005) | USA | PC | 1997~2003 | M | Veterans | 98 | 46 | 4668 / 7548 | 100 | 3% on Tx | 37‡ |
| Bonacini (2004) | USA | PC | 1993~2001 | M | Adults | 84 | 41 | 256 / 126 | 52 | NR | 21 |
| Braitstein (2005) | Canada | PC | 1996~2003 | M | Adults | 85 | 37 | 606 / 580 | 100 | NR | 27 |
| Crane (2007) | USA | RS | 1995~2005 | M | Adults | 87 | 37 | 144 / 550 | 100 | NR | NR |
| De Luca (2002) | Italy | PC | 1997~2001 | C | Adults | 75 | 33 | 600 / 720 | 100 | No Tx | 39 |
| Dorrucci (2004) | Italy | PC | 1996~2001 | C | Adults | 66 | 27* | 458 / 337 | 35 | NR | 56 |
| El-Serag (2005) | USA | RS | 1996~2001 | M | Hospitalized Veterans | 98 | 45 | 2272 / 4913 | NR | NR | NR |
| Greub (2000) | Switzerland | PC | 1996~2000 | C | Age~16 | 71 | 36 | 1157 / 1954 | 100 | NR | 36 |
| Hung (2005) | Taiwan | PC | 1997~2002 | M, A | Adults | 92 | 36 | 42 / 303 | 85 | No Tx | 3 |
| Jaen (2008) | Spain | PC+RS | 1998~2004 | C | Age~16 | 75 | 36 | 642 / 1135 | 100 | NR | 33 |
| Jaggy (2003)§ | Switzerland | PC | 1997~2001 | M | Adults | 71 | 36 | 1645 / 2318 | 100 | NR | 36 |
| Klein (2003) | Canada | PC+RS | 1996~1999 | M, A | Adults | 77 | 38 | 83 / 456 | 58 | NR | 17 |
| Lincoln (2003) | Australia | PC | 1999~2002 | C | Adults | 94 | NR | 223 / 1481 | 61 | NR | 8 |
| Marins (2005) | Brazil | RS | 1995~2002 | M | Adults | 72 | 30 | 279 / 554 | 50 | NR | 34 |
| Mayor (2006) | Puerto Rico | PC | 1998~2003 | M | Adults | 70 | 38 | 193 / 163 | 45 | NR | 58 |
| Monga (2001) | USA | RS | 1994~1998 | M | Veterans | 99# | 46 | 166 / 263 | 42 | NR | 30 |
| Riley (2005) | USA | PC | 1996~2002 | M | Homeless | 84 | 40 | 212 / 118 | 75 | NR | 64 |
| Rockstroh (2005) | Europe | PC | 1994~2004 | M, A, C | Adults | 75 | 36 | 1960 / 3997 | 51 | 2% on Tx | 28 |
| Stebbing (2005) | UK | PC | 1996~ NR | A | Adults | 87 | 34 | 85 / 1382 | < 49% | NR | NR |
| Sulkowski (2002) | USA | PC | 1995~2001 | M, A | Adults | 70 | 37 | 873 / 1082 | 61 | <2% on Tx | 45 |
| Sullivan (2006) | USA | RS | 1998~2004 | M, A | Age ≥13 | 72 | 35 | 2024 / 8457 | 70 | NR | 21 |
| Tedaldi (2003) | USA | PC | 1996~2001 | M, A | Adults | NR | 37 | 267 / 556 | 84 | 7% on Tx | 26 |
| Torti (2007) | Italy | PC | 1996~2002 | C | Adults | 78 | 38 | 334 / 417 | 100 | NR | 41 |
| Voirin (2004) | France | PC | 1996~2002 | M | Adults | 79 | 35 | 107 / 1273 | NR | NR | 0 |
| Weber (2006) | International | PC | 1999~2004 | M | Adults | 76 | 39 | 5274 /18167 | 89 | NR | NR |
| Weis (2006) | Denmark | PC | 1995~2004 | M | Age > 15 | 75 | 39 | 443 / 2183 | 100 | <1% on Tx | 10 |
IDU: injection drug use, Tx: therapy, HAART: highly-active antiretroviral therapy, PC: prospective cohort, RS: retrospective, NR: not reported. M: overall mortality, A: AIDS, C: combination of AIDS and mortality.
Calculated according to the proportion reported in HCV positive and negative groups if not reported directly.
Hard drug use.
Age at HIV seroconversion.
Assumption made according to the study population.
Demographic data derived from Greub et al.
Study Quality
Of 27 study designs in the era of HAART, 4 studies reported overall mortality or AIDS-defining diseases as secondary outcomes and hence were excluded from quality assessment [4, 49, 50, 61]. Of 23 studies with a primary outcome of interest, 5 were retrospective designs [18, 45, 51, 53, 56] and 1 collected data both retrospectively and prospectively [19]. There are 8 studies with mean/median follow-up time between 1 to 3 years [8, 11, 19, 47, 53, 55-57] and 10 studies have mean/median follow-up time over 3 years [9, 10, 17, 18, 46, 48, 54, 58-60]. Five studies did not report mean/median follow-up time [20, 45, 51, 52, 62].
Measured Outcomes
Pre-HAART
Overall mortality
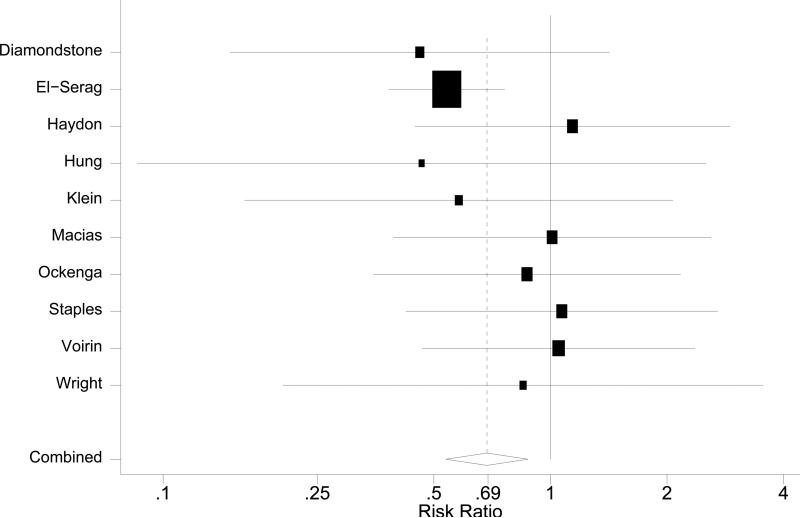
The risk ratio for overall mortality among HCV/HIV coinfected patients was 0.69 (95% CI: 0.54~0.88, Figure 2) in comparison to patients with HIV-monoinfection. The chi-square test for heterogeneity was not significant (p=0.67) with I2 statistic of 0 (95% CI: 0~62). Sensitivity tests on the influence of individual studies showed pooled risk ratios ranged from 0.66 to 0.87. The study by El-Serag et al. [18] showed a dominant influence on the pooled risk ratio: when removed, the protective effect of HCV coinfection became statistically insignificant with a risk ratio of 0.87 (95% CI: 0.62~1.23). Only one of nine studies from this era included use of antiretroviral therapy as a covariate, precluding analysis on the impact of treatment [34].
Figure 2.
Forest plots of overall mortality in pre-HAART era.
HAART era
Overall mortality
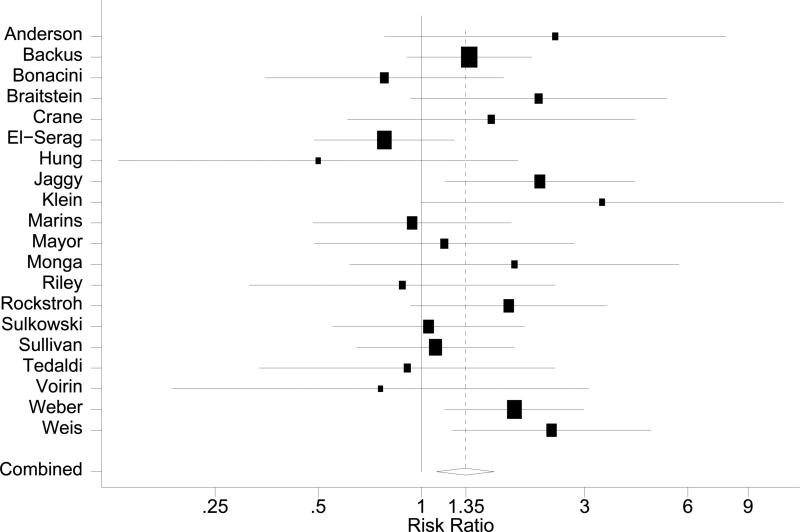
Compared to patients with HIV-monoinfection, the pooled risk ratio for overall mortality among HCV/HIV coinfected patients was 1.35 (95% CI: 1.11~1.63, Figure 3 A). The chi-square test for heterogeneity was not significant (p=0.13) with an I2 statistic of 26 (95% CI: 0~58). A sensitivity test on the influence of individual studies showed pooled risk ratios ranging from 1.30 to 1.43, all statistically significant. Evaluation of publication bias by funnel plot showed a symmetric distribution of studies (data not shown). Neither Begg's test (p=0.72) nor Egger's test (p=0.95) suggested publication bias.
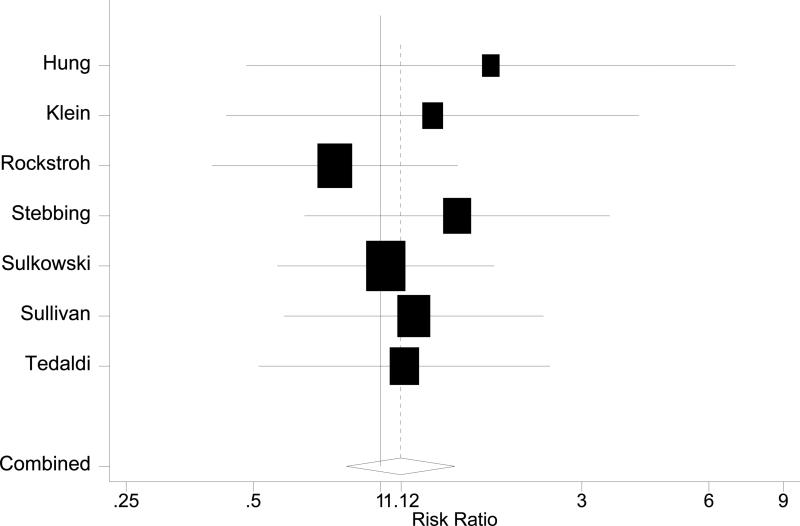
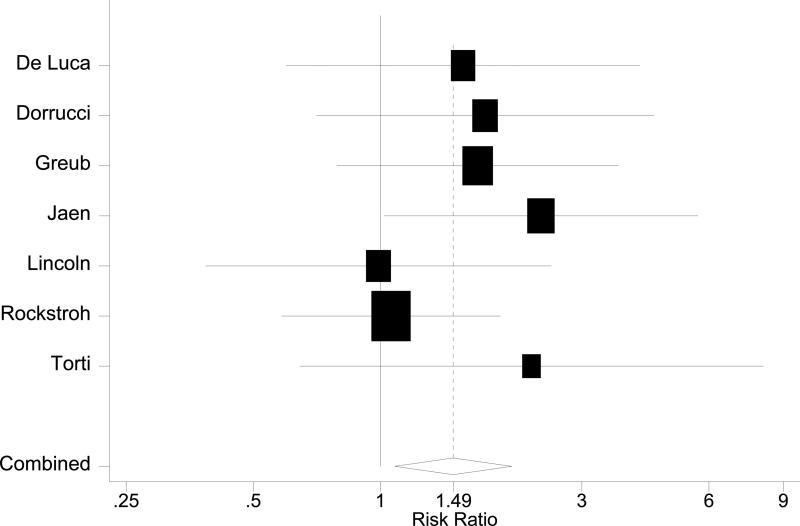
Figure 3.
Forest plots in HAART era. A: Overall mortality. B: AIDS-defining events. C: AIDS plus mortality
Potential sources of between-studies heterogeneity were explored by meta-regression stratified analyses and subgroup analyses. Stratified results are summarized in Table 2. Although stratification for sex, age, follow-up duration and treatment did not reveal significant effect modifications, the result suggested that adverse effects of HCV on overall mortality may be stronger among older patients and among patients on HAART. The adverse effect of HCV/HIV coinfection was also more apparent with longer follow-up. Subgroup analyses according to quality of studies yielded pooled estimates 1.22 (95% CI: 0.82~1.81) for 6 retrospective studies [18, 19, 45, 51, 53, 56] and 1.30 (95% CI: 1.02~1.66) for 11 prospective studies [10, 11, 20, 46-48, 52, 54, 55, 57, 58].
Table 2.
Stratified analysis of HCV coinfection and total mortality among HIV positive patients in HAART era
| Variable | RR (95% CI) | P for interaction |
|---|---|---|
|
Sex (n=19)* | ||
| Women | 2.49 (0.57~11.00) | 0.420 |
| Men | 1.19 (0.81~1.75) | |
|
Age (n=20), year | ||
| <38 | 1.28 (0.97~1.69) | 0.611 |
| ≥ 38 | 1.42 (1.07~1.89) | |
|
Follow up duration (n=17) | ||
| < 3 years | 1.11 (0.79~1.55) | 0.120 |
| ≥ 3 years | 1.54 (1.20~1.98) | |
|
IDU (n=17)* | ||
| Non-IDU | 1.57 (0.98~2.51) | 0.533 |
| IDU | 1.00 (0.37~2.73) | |
|
Concurrent Treatment (n=18)‡ | ||
| Not on HAART | 0.81 (0.42~1.56) | 0.074 |
| On HAART | 1.70 (1.33~2.17) | |
IDU: injection drug use, HAART: highly-active antiretroviral therapy
Sex-specific and IDU-specific results estimated from meta-regression of sex-and IDU-proportion in each trial.
HAART-specific results estimated from meta-regression of proportion of patients ever on HAART in each trial
AIDS-defining events
Seven studies reported data on the risk of developing AIDS-defining events among HIV patients with and without HCV infection (Table 1) [11, 19, 54-57, 59]. The pooled risk ratio was 1.12 (95% CI 0.82~1.51, Figure 3 B), indicating similar risk of developing AIDS-defining events between HIV infected patients with and without HCV infection. The chi-square for heterogeneity was not significant (p=0.88). The funnel plot did not show asymmetry, and neither Begg's test (p=0.13) nor Egger's test (p=0.11) supported publication bias.
Overall mortality and AIDS-defining events
Seven other post-HAART studies reported on the effect of HCV infection on HIV disease progression, defined as either the development of an AIDS-defining disease or overall mortality (Table 1) [8, 9, 17, 54, 60-62], with a pooled risk ratio of 1.49 (95%CI 1.08~2.05, Figure 3 C). No publication bias was observed.
Discussion
Our meta-analysis summarized 10 studies with 14626 patients in the pre-HAART era and 20 studies with 113073 patients in the HAART era. The study demonstrated a 32% reduction of overall mortality risk in HCV/HIV coinfected patient in the pre-HAART era and a 35% elevation of overall mortality risk in the HAART era.
The potentially protective role of HCV in mortality during the pre-HAART is dominantly influenced by El-Serag's study, which included many patients but also unique in its methodology, including restriction to an inpatient population and a retrospective design compared to other studies [19, 20, 34]. Recruiting patients through hospitalization is prone to survival bias. If patient died of AIDS-events prior to diagnosis of HCV, the study would not identify them especially considering that HCV was not routinely screened for in the pre-HAART era. Excluding this study, the remaining of selected studies did not show a protective effect individually or in aggregate.
By contrast, our meta-analysis demonstrates that HCV coinfection is associated with increased mortality in the HAART era. Subgroup analyses showed that: 1) a longer duration of follow-up is needed to observe a significant difference in mortality between HCV/HIV coinfected patients and HIV-monoinfected patients; and 2) the adverse effect of coinfection was more apparent in patients on HAART compared to those not on HAART. The latter finding parallels the observation that HIV-related mortality dominated the pre-HAART era [63]. Patients not on HAART are likely to die of AIDS-related complications regardless of HCV status and increased risk of mortality attributable to HCV coinfection is less likely to be observed.
The meta-analysis showed no statistically significant increased risk of developing AIDS-defining events among HIV patients with HCV coinfection in the HAART era, despite studies that suggested this possibility [25, 59]. There might be some effect of HCV on HIV disease progression that is masked because death from hepatic complications is a competing risk of AIDS-defining events. However, considering the prolonged time period required to develop HCV-related complications and that death due to HIV-related complications remains the leading cause of mortality in HIV-infected individuals [4, 64], competing risk and insufficient end-point events are unlikely to confound our results. When examining the few studies with combined AIDS-defining events and mortality as their outcome, we found increased (~50% higher) risk of HCV/HIV coinfected patients; however determining the relative contribution of each outcome was not possible. Therefore, potential mechanisms by which HCV may accelerate progression to AIDS, (i.e. a blunted immunologic response to HAART), may not carry significant clinical impact [65].
Given the lack of association of progression to AIDS and HCV coinfection, the major contributor to mortality among coinfected subjects during the HAART era is likely liver disease, based on an expanding body of supporting data [39, 66, 67]. Generally, AIDS-related mortality would be heralded by AIDS-related events. As AIDS-related events were not affected by coinfection status, excess mortality during the HAART era in coinfected patients compared to HIV-monoinfected patients was unmasked, coincident with the acceleration of liver disease present in almost exclusively the coinfected group. Unfortunately we did not observe that greater duration of HAART availability positively impacts coinfected patients, but rather the opposite effect—a trend towards a greater effect of HCV on mortality with length of study. While HAART may have benefits for liver disease progression and related-mortality [36, 68] it does not reverse it; implying that broader application of more effective anti-HCV therapies are needed to reduce this excess mortality. Moreover, if HCV is the major direct contributor to increased mortality, death rates between coinfected patients with HCV viremia versus those with spontaneous clearance (and reduced risk of liver-disease progression) should differ. Unfortunately, this parameter was not controlled for in past studies as ascertainment of HCV status was largely determined by antibody.
Independent of HCV pathogenesis, HCV coinfected patients may have increased mortality if they are less likely to be prescribed HAART and/or have poorer adherence to their regimens. Several studies within this meta-analysis observed shorter duration of exposure to HAART for HCV coinfected patients compared to HIV-monoinfection [17, 45, 46, 55, 57, 58]. In one study, coinfected patients were significantly less likely to initiate HAART than HIV-monoinfected patients even after adjusting for IDU [58]. Moreover, HCV coinfection is also independently associated with decreased adherence to therapy [69].
Since HCV is highly correlated with IDU, the increased risk of coinfection on mortality might arguably be confounded by IDU who have less access to HAART [70]. Despite an expectation that IDU would be an effect modifier, coinfection with HCV increased risk of death in both non-IDU and IDU patients in our subgroup analysis. The studies by Weis et al. and Klein et al. demonstrated increased risk of mortality after excluding/stratifying patients with IDU in their studies [19, 58], suggesting that the negative effect of HCV on mortality is independent of IDU. The non-significant interaction could be partially explained by lack of power due to the small numbers of coinfected patients without IDU (reported number ranging from 29 (15% of coinfected patients) [52] to 1774 (38% of coinfected patients) [46]) and misclassification of IDU to non-IDU. Our findings are nevertheless consistent with a recent study that did not find a significant association between IDU and increased mortality in HIV in those initiating HAART [71]. Mortality attributable to IDU is further confounded by the timing of HIV introduction into various populations and whether IDU is historical or current. HIV may have been acquired later in IDU populations compared to MSM [72] and thus HIV-related mortality may have been relatively delayed, resulting in a form of lead-time bias. Current IDU has additional risks from complications such as infections, drug overdose and homicide; however, these covariates were not reported in most studies. Finally, active IDU with the highest mortality rates may not be fully represented in these cohorts due to poor access to care and/or follow-up.
Our study has potential limitations. Although tests for publication bias were negative, studies presented solely in conferences or local journals may have been overlooked. In addition, each outcome analyses in HAART era may not be entirely comparable as studies differed regarding the data provided. Nevertheless, all analyses included more than 15000 HIV patients and sensitivity analysis revealed no significant change when subtracting any single study from the HAART era, suggesting that these limitations are unlikely to weaken the validity of our results. The observational nature of selected studies limits our ability to overcome residual confounding from individual studies, especially time from seroconversion to the initiation of HAART, alcohol consumption and GBV-C coinfection, factors which were analyzed only in a small minority or none of studies. However, twenty out of twenty-seven studies adjusted for important factors such as CD4 counts and HIV therapy. Finally, we could not examine the role of interferon-based treatments, but the lack of their widespread application in coinfected individuals suggests that HCV treatment would not be a major confounder during the era studied [55, 58, 73].
To conclude, this study synthesizes over thirty studies including over 100,000 patients and indicates increased risk of overall mortality in HIV patients with HCV coinfection in the HAART era. The meta-analysis did not demonstrate increased risk of developing AIDS-defining events in coinfected patients. Future studies examining mortality among coinfected subjects should attempt to dissect out the relative contributions of 1) HCV viremia as a surrogate for liver disease risk, 2) whether IDU is current or active and 3) whether broader application of HCV treatment positively impacts mortality of coinfected individuals.
Grant Support
Dr. Ding is supported by a fellowship from the Paul and Daisy Soros Foundation. Dr. Kim is supported by the National Institutes of Health / National Institute of Allergy and Infectious Diseases (U19 AI066345, K23 AI054379 to AYK). The funding sources had no role in the study conduct, analysis, or interpretation of results.
Footnotes
Conception and design: Chen, Seage, Kim
Acquisition of data: Chen, Kim
Analysis and interpretation of data: Chen, Ding, Kim
Drafting of manuscript: Chen, Kim
Critical revision for intellectual content: Chen, Ding, Seage, Kim
Statistical expertise: Chen, Ding
Obtained funding: none
Administrative, technical, or material support: none
Study supervision: none
Conflict of Interest: None.
Reference
- 1.Sherman KE, Rouster SD, Chung RT, Rajicic N. Hepatitis C Virus prevalence among patients infected with Human Immunodeficiency Virus: a cross-sectional analysis of the US adult AIDS Clinical Trials Group. Clin Infect Dis. 2002 Mar 15;34(6):831–7. doi: 10.1086/339042. [DOI] [PubMed] [Google Scholar]
- 2.Sulkowski MS, Thomas DL. Hepatitis C in the HIV-infected person. Annals of Internal Medicine. 2003;138(3):197–207. doi: 10.7326/0003-4819-138-3-200302040-00012. [DOI] [PubMed] [Google Scholar]
- 3.Bica I, McGovern B, Dhar R, et al. Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clin Infect Dis. 2001 Feb 1;32(3):492–7. doi: 10.1086/318501. [DOI] [PubMed] [Google Scholar]
- 4.Weber R, Sabin CA, Friis-Moller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006 Aug 14-28;166(15):1632–41. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 5.Smit C, van den Berg C, Geskus R, Berkhout B, Coutinho R, Prins M. Risk of hepatitis-related mortality increased among hepatitis C virus/HIV-coinfected drug users compared with drug users infected only with hepatitis C virus: a 20-year prospective study. J Acquir Immune Defic Syndr. 2008 Feb 1;47(2):221–5. doi: 10.1097/QAI.0b013e31815d2f59. [DOI] [PubMed] [Google Scholar]
- 6.Mohsen AH, Easterbrook PJ, Taylor C, et al. Impact of human immunodeficiency virus (HIV) infection on the progression of liver fibrosis in hepatitis C virus infected patients. Gut. 2003 Jul;52(7):1035–40. doi: 10.1136/gut.52.7.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benhamou Y, Bochet M, Di Martino V, et al. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology. 1999 Oct;30(4):1054–8. doi: 10.1002/hep.510300409. [DOI] [PubMed] [Google Scholar]
- 8.Greub G, Ledergerber B, Battegay M, et al. Clinical progression, survival, and immune recovery during antiretroviral therapy in patients with HIV-1 and hepatitis C virus coinfection: the Swiss HIV Cohort Study. Lancet. 2000 Nov 25;356(9244):1800–5. doi: 10.1016/s0140-6736(00)03232-3. [DOI] [PubMed] [Google Scholar]
- 9.De Luca A, Bugarini R, Lepri AC, et al. Coinfection with hepatitis viruses and outcome of initial antiretroviral regimens in previously naive HIV-infected subjects. Arch Intern Med. 2002 Oct 14;162(18):2125–32. doi: 10.1001/archinte.162.18.2125. [DOI] [PubMed] [Google Scholar]
- 10.Riley ED, Bangsberg DR, Guzman D, Perry S, Moss AR. Antiretroviral therapy, hepatitis C virus, and AIDS mortality among San Francisco's homeless and marginally housed. J Acquir Immune Defic Syndr. 2005 Feb 1;38(2):191–5. doi: 10.1097/00126334-200502010-00010. [DOI] [PubMed] [Google Scholar]
- 11.Hung CC, Chen MY, Hsieh SM, Hsiao CF, Sheng WH, Chang SC. Impact of chronic hepatitis C infection on outcomes of patients with an advanced stage of HIV-1 infection in an area of low prevalence of co-infection. Int J STD AIDS. 2005 Jan;16(1):42–8. doi: 10.1258/0956462052932629. [DOI] [PubMed] [Google Scholar]
- 12.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986 Sep;7(3):177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 13.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002 Jun 15;21(11):1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 14.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994 Dec;50(4):1088–101. [PubMed] [Google Scholar]
- 15.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997 Sep 13;315(7109):629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med. 2003 Sep 15;22(17):2693–710. doi: 10.1002/sim.1482. [DOI] [PubMed] [Google Scholar]
- 17.Dorrucci M, Valdarchi C, Suligoi B, et al. The effect of hepatitis C on progression to AIDS before and after highly active antiretroviral therapy. AIDS. 2004 Nov 19;18(17):2313–8. doi: 10.1097/00002030-200411190-00012. [DOI] [PubMed] [Google Scholar]
- 18.El-Serag HB, Giordano TP, Kramer J, Richardson P, Souchek J. Survival in hepatitis C and HIV co-infection: a cohort study of hospitalized veterans. Clin Gastroenterol Hepatol. 2005 Feb;3(2):175–83. doi: 10.1016/s1542-3565(04)00620-2. [DOI] [PubMed] [Google Scholar]
- 19.Klein MB, Lalonde RG, Suissa S. The impact of hepatitis C virus coinfection on HIV progression before and after highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2003 Jul 1;33(3):365–72. doi: 10.1097/00126334-200307010-00011. [DOI] [PubMed] [Google Scholar]
- 20.Voirin N, Trepo C, Miailhes P, et al. Survival in HIV-infected patients is associated with hepatitis C virus infection and injecting drug use since the use of highly active antiretroviral therapy in the Lyon observational database. J Viral Hepat. 2004 Nov;11(6):559–62. doi: 10.1111/j.1365-2893.2004.00544.x. [DOI] [PubMed] [Google Scholar]
- 21.Lumbreras B, Jarrin I, Del Amo J, et al. Impact of hepatitis C infection on long-term mortality of injecting drug users from 1990 to 2002: Differences before and after HAART. AIDS. 2006;20(1):111–6. doi: 10.1097/01.aids.0000196164.71388.3b. [DOI] [PubMed] [Google Scholar]
- 22.Macias J, Melguizo I. Mortality due to Liver Failure and Impact on Survival of Hepatitis Virus Infections in HIV-infected Patients Receiving Potent antiretroviral Therapy. Eur J Clin Microbiol Infect Dis. 2002;21:775–81. doi: 10.1007/s10096-002-0823-0. 2002. [DOI] [PubMed] [Google Scholar]
- 23.Hershow RC, O'Driscoll PT, Handelsman E, et al. Hepatitis C virus coinfection and HIV load, CD4+ cell percentage, and clinical progression to AIDS or death among HIV-infected women: Women and Infants Transmission Study. Clin Infect Dis. 2005 Mar 15;40(6):859–67. doi: 10.1086/428121. [DOI] [PubMed] [Google Scholar]
- 24.Amin J, Kaye M, Skidmore S, Pillay D, Cooper DA, Dore GJ. HIV and hepatitis C coinfection within the CAESAR study. HIV Med. 2004 May;5(3):174–9. doi: 10.1111/j.1468-1293.2004.00207.x. [DOI] [PubMed] [Google Scholar]
- 25.Yoo TW, Donfield S, Lail A, Lynn HS, Daar ES. Effect of hepatitis C virus (HCV) genotype on HCV and HIV-1 disease. Journal of Infectious Diseases. 2005;191(1):4–10. doi: 10.1086/426513. [DOI] [PubMed] [Google Scholar]
- 26.Daar ES, Lynn H, Donfield S, et al. Hepatitis C virus load is associated with human immunodeficiency virus type 1 disease progression in hemophiliacs. J Infect Dis. 2001 Feb 15;183(4):589–95. doi: 10.1086/318539. [DOI] [PubMed] [Google Scholar]
- 27.Soriano V, Garcia-Samaniego J, Gutierrez M, Bravo R, Gonzalez-Lahoz J. High morbidity and mortality of chronic viral liver disease in HIV-infected individuals in Spain. J Infect. 1994 Jan;28(1):100–2. doi: 10.1016/s0163-4453(94)94440-7. [DOI] [PubMed] [Google Scholar]
- 28.Piroth L, Grappin M, Cuzin L, et al. Hepatitis C virus co-infection is a negative prognostic factor for clinical evolution in human immunodeficiency virus-positive patients. J Viral Hepat. 2000 Jul;7(4):302–8. doi: 10.1046/j.1365-2893.2000.00227.x. [DOI] [PubMed] [Google Scholar]
- 29.Llibre JM, Garcia E, Aloy A, Valls J. Hepatitis C virus infection and progression of infection due to human immunodeficiency virus. Clin Infect Dis. 1993 Jan;16(1):182. doi: 10.1093/clinids/16.1.182. [DOI] [PubMed] [Google Scholar]
- 30.Wright TL, Hollander H, Pu X, et al. Hepatitis C in HIV-infected patients with and without AIDS: prevalence and relationship to patient survival. Hepatology. 1994 Nov;20(5):1152–5. [PubMed] [Google Scholar]
- 31.Macias J, Pineda JA, Leal M, et al. Influence of hepatitis C virus infection on the mortality of antiretroviral-treated patients with HIV disease. Eur J Clin Microbiol Infect Dis. 1998 Mar;17(3):167–70. doi: 10.1007/BF01691112. [DOI] [PubMed] [Google Scholar]
- 32.Ockenga J, Tillmann HL, Trautwein C, Stoll M, Manns MP, Schmidt RE. Hepatitis B and C in HIV-infected patients. Prevalence and prognostic value. J Hepatol. 1997 Jul;27(1):18–24. doi: 10.1016/s0168-8278(97)80274-7. [DOI] [PubMed] [Google Scholar]
- 33.Haydon GH, Flegg PJ, Blair CS, Brettle RP, Burns SM, Hayes PC. The impact of chronic hepatitis C virus infection on HIV disease and progression in intravenous drug users. Eur J Gastroenterol Hepatol. 1998 Jun;10(6):485–9. doi: 10.1097/00042737-199806000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Staples CT, Jr., Rimland D, Dudas D. Hepatitis C in the HIV (human immunodeficiency virus) Atlanta V.A. (Veterans Affairs Medical Center) Cohort Study (HAVACS): the effect of coinfection on survival. Clin Infect Dis. 1999 Jul;29(1):150–4. doi: 10.1086/520144. [DOI] [PubMed] [Google Scholar]
- 35.Diamondstone LS, Blakley SA, Rice JC, Clark RA, Goedert JJ. Prognostic factors for all-cause mortality among hemophiliacs infected with human immunodeficiency virus. Am J Epidemiol. 1995 Aug 1;142(3):304–13. doi: 10.1093/oxfordjournals.aje.a117636. [DOI] [PubMed] [Google Scholar]
- 36.Qurishi N, Kreuzberg C, Luchters G, et al. Effect of antiretroviral therapy on liver-related mortality in patients with HIV and hepatitis C virus coinfection. Lancet. 2003 Nov 22;362(9397):1708–13. doi: 10.1016/S0140-6736(03)14844-1. [DOI] [PubMed] [Google Scholar]
- 37.Sabin CA, Griffioen A, Yee TT, et al. Markers of HIV-1 disease progression in individuals with haemophilia coinfected with hepatitis C virus: a longitudinal study. Lancet. 2002 Nov 16;360(9345):1546–51. doi: 10.1016/S0140-6736(02)11519-4. [DOI] [PubMed] [Google Scholar]
- 38.Torre D, Tambini R, Cadario F, Barbarini G, Moroni M, Basilico C. Evolution of coinfection with human immunodeficiency virus and hepatitis C virus in patients treated with highly active antiretroviral therapy. Clin Infect Dis. 2001 Nov 1;33(9):1579–85. doi: 10.1086/322611. [DOI] [PubMed] [Google Scholar]
- 39.Pineda JA, Garcia-Garcia JA, Aguilar-Guisado M, et al. Clinical progression of hepatitis C virus-related chronic liver disease in human immunodeficiency virus-infected patients undergoing highly active antiretroviral therapy. Hepatology. 2007 Sep;46(3):622–30. doi: 10.1002/hep.21757. [DOI] [PubMed] [Google Scholar]
- 40.Merriman NA, Porter SB, Brensinger CM, Reddy KR, Chang KM. Racial difference in mortality among U.S. veterans with HCV/HIV coinfection. Am J Gastroenterol. 2006 Apr;101(4):760–7. doi: 10.1111/j.1572-0241.2006.00531.x. [DOI] [PubMed] [Google Scholar]
- 41.Salmon-Ceron D, Lewden C, Morlat P, et al. Liver disease as a major cause of death among HIV infected patients: role of hepatitis C and B viruses and alcohol. J Hepatol. 2005 Jun;42(6):799–805. doi: 10.1016/j.jhep.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 42.Reiche EM, Bonametti AM, Morimoto HK, et al. Epidemiological, immunological and virological characteristics, and disease progression of HIV-1/HCV-co-infected patients from a southern Brazilian population. Int J Mol Med. 2008 Mar;21(3):387–95. [PubMed] [Google Scholar]
- 43.Phillips E, Gutierrez S, Jahnke N, et al. Determinants of nevirapine hypersensitivity and its effect on the association between hepatitis C status and mortality in antiretroviral drug-naive HIV-positive patients. AIDS. 2007 Jul 31;21(12):1561–8. doi: 10.1097/QAD.0b013e3282170a9d. [DOI] [PubMed] [Google Scholar]
- 44.Moore DM, Hogg RS, Braitstein P, Wood E, Yip B, Montaner JS. Risks of non-accidental mortality by baseline CD4+ T-cell strata in hepatitis-C-positive and -negative individuals initiating highly active antiretroviral therapy. Antivir Ther. 2006;11(1):125–9. [PubMed] [Google Scholar]
- 45.Anderson KB, Guest JL, Rimland D. Hepatitis C virus coinfection increases mortality in HIV-infected patients in the highly active antiretroviral therapy era: data from the HIV Atlanta VA Cohort Study. Clin Infect Dis. 2004 Nov 15;39(10):1507–13. doi: 10.1086/425360. [DOI] [PubMed] [Google Scholar]
- 46.Backus LI, Phillips BR, Boothroyd DB, et al. Effects of hepatitis C virus coinfection on survival in veterans with HIV treated with highly active antiretroviral therapy. Journal of Acquired Immune Deficiency Syndromes. 2005;39(5):613–9. [PubMed] [Google Scholar]
- 47.Bonacini M, Louie S, Bzowej N, Wohl AR. Survival in patients with HIV infection and viral hepatitis B or C: a cohort study. AIDS. 2004 Oct 21;18(15):2039–45. doi: 10.1097/00002030-200410210-00008. [DOI] [PubMed] [Google Scholar]
- 48.Braitstein P, Yip B, Montessori V, Moore D, Montaner JS, Hogg RS. Effect of serostatus for hepatitis C virus on mortality among antiretrovirally naive HIV-positive patients. CMAJ. 2005 Jul 19;173(2):160–4. doi: 10.1503/cmaj.045202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crane HM, Van Rompaey SE, Kitahata MM. Initiating highly active antiretroviral therapy with newer protease inhibitors is associated with better survival compared to first-generation protease inhibitors or nevirapine. AIDS Patient Care STDS. 2007 Dec;21(12):920–9. doi: 10.1089/apc.2007.0020. [DOI] [PubMed] [Google Scholar]
- 50.Jaggy C, von Overbeck J, Ledergerber B, et al. Mortality in the Swiss HIV Cohort Study (SHCS) and the Swiss general population. Lancet. 2003 Sep 13;362(9387):877–8. doi: 10.1016/S0140-6736(03)14307-3. [DOI] [PubMed] [Google Scholar]
- 51.Marins JR, Barros MB, Machado H, Chen S, Jamal LF, Hearst N. Characteristics and survival of AIDS patients with hepatitis C: the Brazilian National Cohort of 1995-1996. AIDS. 2005 Oct;19(Suppl 4):S27–30. doi: 10.1097/01.aids.0000191487.69414.88. [DOI] [PubMed] [Google Scholar]
- 52.Mayor AM, Gomez MA, Fernandez DM, Rios-Olivares E, Thomas JC, Hunter RF. Morbidity and mortality profile of human immunodeficiency virus-infected patients with and without hepatitis C co-infection. Am J Trop Med Hyg. 2006 Feb;74(2):239–45. [PMC free article] [PubMed] [Google Scholar]
- 53.Monga HK, Rodriguez-Barradas MC, Breaux K, et al. Hepatitis C virus infection-related morbidity and mortality among patients with human immunodeficiency virus infection. Clin Infect Dis. 2001 Jul 15;33(2):240–7. doi: 10.1086/321819. [DOI] [PubMed] [Google Scholar]
- 54.Rockstroh JK, Mocroft A, Soriano V, et al. Influence of hepatitis C virus infection on HIV-1 disease progression and response to highly active antiretroviral therapy. J Infect Dis. 2005 Sep 15;192(6):992–1002. doi: 10.1086/432762. [DOI] [PubMed] [Google Scholar]
- 55.Sulkowski MS, Moore RD, Mehta SH, Chaisson RE, Thomas DL. Hepatitis C and progression of HIV disease. JAMA. 2002 Jul 10;288(2):199–206. doi: 10.1001/jama.288.2.199. [DOI] [PubMed] [Google Scholar]
- 56.Sullivan PS, Hanson DL, Teshale EH, Wotring LL, Brooks JT. Effect of hepatitis C infection on progression of HIV disease and early response to initial antiretroviral therapy. AIDS. 2006 May 12;20(8):1171–9. doi: 10.1097/01.aids.0000226958.87471.48. [DOI] [PubMed] [Google Scholar]
- 57.Tedaldi EM, Baker RK, Moorman AC, et al. Influence of coinfection with hepatitis C virus on morbidity and mortality due to human immunodeficiency virus infection in the era of highly active antiretroviral therapy. Clin Infect Dis. 2003 Feb 1;36(3):363–7. doi: 10.1086/345953. [DOI] [PubMed] [Google Scholar]
- 58.Weis N, Lindhardt BO, Kronborg G, et al. Impact of hepatitis C virus coinfection on response to highly active antiretroviral therapy and outcome in HIV-infected individuals: A nationwide cohort study. Clinical Infectious Diseases. 2006;42(10):1481–7. doi: 10.1086/503569. [DOI] [PubMed] [Google Scholar]
- 59.Stebbing J, Waters L, Mandalia S, Bower M, Nelson M, Gazzard B. Hepatitis C virus infection in HIV type 1-infected individuals does not accelerate a decrease in the CD4+ cell count but does increase the likelihood of AIDS-defining events. Clin Infect Dis. 2005 Sep 15;41(6):906–11. doi: 10.1086/432885. [DOI] [PubMed] [Google Scholar]
- 60.Torti C, Lapadula G, Maggiolo F, et al. Predictors of AIDS-defining events among advanced naive patients after HAART. HIV Clin Trials. 2007 May-Jun;8(3):112–20. doi: 10.1310/hct0803-112. [DOI] [PubMed] [Google Scholar]
- 61.Jaen A, Esteve A, Miro JM, et al. Determinants of HIV progression and assessment of the optimal time to initiate highly active antiretroviral therapy: PISCIS Cohort (Spain). J Acquir Immune Defic Syndr. 2008 Feb 1;47(2):212–20. doi: 10.1097/qai.0b013e31815ee282. [DOI] [PubMed] [Google Scholar]
- 62.Lincoln D, Petoumenos K, Dore GJ. HIV/HBV and HIV/HCV coinfection, and outcomes following highly active antiretroviral therapy. HIV Med. 2003 Jul;4(3):241–9. doi: 10.1046/j.1468-1293.2003.00152.x. [DOI] [PubMed] [Google Scholar]
- 63.Karon JM, Fleming PL, Steketee RW, De Cock KM. HIV in the United States at the turn of the century: an epidemic in transition. Am J Public Health. 2001 Jul;91(7):1060–8. doi: 10.2105/ajph.91.7.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bonnet F, Morlat P, Chene G, et al. Causes of death among HIV-infected patients in the era of highly active antiretroviral therapy, Bordeaux, France, 1998-1999. HIV Med. 2002 Jul;3(3):195–9. doi: 10.1046/j.1468-1293.2002.00117.x. [DOI] [PubMed] [Google Scholar]
- 65.Miller MF, Haley C, Koziel MJ, Rowley CF. Impact of hepatitis C virus on immune restoration in HIV-infected patients who start highly active antiretroviral therapy: a meta-analysis. Clin Infect Dis. 2005 Sep 1;41(5):713–20. doi: 10.1086/432618. [DOI] [PubMed] [Google Scholar]
- 66.Thein HH, Yi Q, Dore GJ, Krahn MD. Natural history of hepatitis C virus infection in HIV-infected individuals and the impact of HIV in the era of highly active antiretroviral therapy: a meta-analysis. AIDS. 2008 Oct 1;22(15):1979–91. doi: 10.1097/QAD.0b013e32830e6d51. [DOI] [PubMed] [Google Scholar]
- 67.Rosenthal E, Poiree M, Pradier C, et al. Mortality due to hepatitis C-related liver disease in HIV-infected patients in France (Mortavic 2001 study). AIDS. 2003 Aug 15;17(12):1803–9. doi: 10.1097/00002030-200308150-00009. [DOI] [PubMed] [Google Scholar]
- 68.Brau N, Salvatore M, Rios-Bedoya CF, et al. Slower fibrosis progression in HIV/HCV-coinfected patients with successful HIV suppression using antiretroviral therapy. J Hepatol. 2006 Jan;44(1):47–55. doi: 10.1016/j.jhep.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 69.Braitstein P, Justice A, Bangsberg DR, et al. Hepatitis C coinfection is independently associated with decreased adherence to antiretroviral therapy in a population-based HIV cohort. AIDS. 2006 Feb 14;20(3):323–31. doi: 10.1097/01.aids.0000198091.70325.f4. [DOI] [PubMed] [Google Scholar]
- 70.Porter K, Babiker A, Bhaskaran K, Darbyshire J, Pezzotti P, Walker AS. Determinants of survival following HIV-1 seroconversion after the introduction of HAART. Lancet. 2003 Oct 18;362(9392):1267–74. doi: 10.1016/s0140-6736(03)14570-9. [DOI] [PubMed] [Google Scholar]
- 71.Wood E, Hogg RS, Lima VD, et al. Highly active antiretroviral therapy and survival in HIV-infected injection drug users. JAMA. 2008 Aug 6;300(5):550–4. doi: 10.1001/jama.300.5.550. [DOI] [PubMed] [Google Scholar]
- 72.Bureau of HIV/AIDS SaT, Centre for Infectious Disease Prevention and Control, Health Canada HIV/AIDS cases among injecting drug users in Canada. HIV/AIDS Epi Update 2003. 2003 [Google Scholar]
- 73.Mehta SH, Thomas DL, Sulkowski MS, Safaein M, Vlahov D, Strathdee SA. A framework for understanding factors that affect access and utilization of treatment for hepatitis C virus infection among HCV-mono-infected and HIV/HCV-co-infected injection drug users. AIDS. 2005 Oct;19(Suppl 3):S179–89. doi: 10.1097/01.aids.0000192088.72055.90. [DOI] [PubMed] [Google Scholar]