Abstract
Persistence of T cells engineered with chimeric antigen receptors (CARs) has been a major barrier to use of these cells for molecularly targeted adoptive immunotherapy. To address this issue, we created a series of CARs that contain the T cell receptor-ζ (TCR-ζ) signal transduction domain with the CD28 and/or CD137 (4-1BB) intracellular domains in tandem. After short-term expansion, primary human T cells were subjected to lentiviral gene transfer, resulting in large numbers of cells with >85% CAR expression. In an immunodeficient mouse xenograft model of primary human pre-B-cell acute lymphoblastic leukemia, human T cells expressing anti-CD19 CARs containing CD137 exhibited the greatest antileukemic efficacy and prolonged (>6 months) survival in vivo, and were significantly more effective than cells expressing CARs containing TCR-ζ alone or CD28-ζ signaling receptors. We uncovered a previously unrecognized, antigen-independent effect of CARs expressing the CD137 cytoplasmic domain that likely contributes to the enhanced antileukemic efficacy and survival in tumor bearing mice. Furthermore, our studies revealed significant discrepancies between in vitro and in vivo surrogate measures of CAR efficacy. Together these results suggest that incorporation of the CD137 signaling domain in CARs should improve the persistence of CARs in the hematologic malignancies and hence maximize their antitumor activity.
Introduction
With the advent of efficient gene transfer technologies, such as murine retroviral and HIV-derived lentiviral vectors, it has become feasible to confer novel antigenic specificity to T cells by transfer of chimeric antigen receptors (CARs) with stable, long-term expression. This technology has been used to generate T cells specific for HIV and several human tumor antigens, and some of these engineered T cells have been tested in Phase I/II studies in humans demonstrating the feasibility and relative safety of this approach.1,2,3 One study has demonstrated antitumor activity in patients with neuroblastoma given a single CAR infusion.4
CARs combine the antigen recognition domain of antibody with the intracellular domain of the T cell receptor-ζ (TCR-ζ) chain or FcγRI protein into a single chimeric protein that are capable of triggering T-cell activation in a manner very similar to that of the endogenous TCR.5,6 Several studies demonstrate that the addition of costimulatory domains, particularly the intracellular domain of CD28 can significantly augment the ability of these receptors to stimulate cytokine secretion and enhance antitumor efficacy in preclinical animal models using both solid tumors and leukemia that lack the expression of the CD28 receptor ligands CD80 and CD86.7,8,9 Inclusion of domains from receptors such as the tumor necrosis factor receptor family members, CD134 (OX-40) and CD137 (4-1BB) into CARs has also been shown to augment CAR-mediated T-cell responses.10,11 Gene transfer approaches using these engineered CARs may therefore provide significant improvements over current adoptive immunotherapy strategies that must rely on the endogenous TCR specificities, for which significant issues of TCR repertoire limitation and impaired tumor major histocompatibility complex class I expression may exist.
In this study, we have addressed the issue of limited in vivo persistence of CARs by defining the relative contributions of TCR-ζ, CD137 and CD28 signaling domains in mice engrafted with hematopoietic malignancies. We chose the human CD19 antigen as our initial target for several reasons: (i) CD19 displays a pattern of expression that is highly restricted to B cells and B-cell progenitor cells,12 (ii) CD19 does not appear to be expressed by hematopoietic stem cells permitting the targeting of the B-cell lineage without affecting other hematopoietic lineages,13 and (iii) CD19 is widely expressed by malignant cells that are derived from the B-cell lineage including most lymphomas and lymphocytic leukemias.14 After optimizing the generation of CARs with an efficient T-cell culture process, in vitro studies indicate that incorporation of either CD28 or 4-1BB signaling domains enhances activity over TCR–ζ, confirming previous studies. In contrast, compared to CARs that contain CD28, our in vivo studies indicate that CARs containing CD137 have superior antileukemic efficacy and improved persistence in a primary human acute lymphoblastic leukemia xenograft model. Furthermore, we also find that CARs expressing CD137 signaling domains can provide significant activity that appears to be antigen independent and may contribute to the efficacy of CARs in vivo.
Results
Efficient generation of CAR+ T cells using artificial bead-based antigen-presenting cells and lentiviral gene transfer
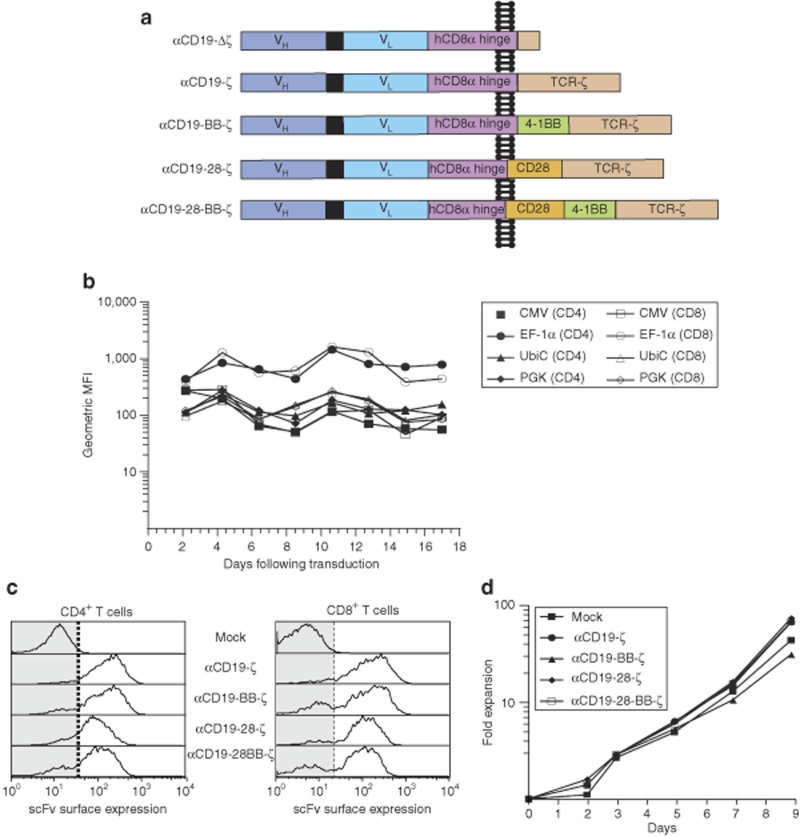
Lentiviral vectors can transfer genes into activated CD4+ and CD8+ human T cells with high efficiency but expression of the vector-encoded transgene depends on the internal promoter that drives its transcription. Therefore, successful CAR expression and gene therapies with CAR-expressing T cells rely on the ability of T cells to maintain adequate receptor expression over long periods of time. We tested several promoters to identify the one with the highest stable expression in both primary CD4+ and CD8+ T cells. Transduction was performed at limiting dilution to ensure that the cells have a single integrated vector per cell (data not shown). Although the cytomegalovirus (CMV) promoter exhibited high levels of expression of a green fluorescent protein (GFP) transgene early after transduction, expression decreased to <25% of the initial expression after 10 days of culture (Figure 1b). The distribution of CMV-driven GFP expression was also quite variable compared with the other promoters tested (Supplementary Figure S1). In contrast, the elongation factor-1α (EF-1α) promoter not only induced the highest level of GFP expression but also optimally maintained it in both CD4 cells and CD8 cells (Figure 1b). These findings confirmed and extended other studies in primary human T cells.15 The EF-1α promoter was therefore selected for all future studies using CARs. By using lentiviral vectors and transductions at a multiplicity of infection of 5, the different CARs could be expressed with high expression in >85% primary human T cells (Figure 1c). Western blotting under both reducing and nonreducing conditions demonstrated that the CARs are present as both covalent dimers and monomers within T cells (Supplementary Figure S2). Using the artificial bead-based antigen-presenting cell system previously described by our laboratory,16 >50-fold expansion of CAR+ T cells could be achieved over the course of transduction and growth in ~10 days (Figure 1d).
Figure 1.
Lentiviral gene transfer combined with αCD3/αCD28 coated magnetic bead activation of T cells permits generation of large numbers of CD19-specific chimeric antigen receptor (CAR+) T cells. (a) A schematic diagram showing the CD19-specific CAR used in this study. (b) Comparison of green fluorescent protein (GFP) expression under the control of different eukaryotic promoters in primary human CD4+ and CD8+ T cells over time. GFP fluorescence was compared in the indicated T cell subset in cells that were stimulated with αCD3/αCD28 coated beads followed by lentiviral transduction at an multiplicity of infection (MOI) of 0.2 on day 1 with vector expressing enhanced GFP under the control of the promoter indicated. Flow cytometric detection of GFP fluorescence was calibrated using Rainbow Calibration Particles (Spherotech, Lake Forest, IL) to correct for day-to-day variation. (c) αCD19-specific CAR surface expression in primary human CD4+ and CD8+ T cells. Expression was examined 6 days following transduction with the indicated CAR-encoding lentiviral vector at a MOI of ~8. (d) In vitro expansion of CD4+ and CD8+ T cells following activation with αCD3/αCD28 coated magnetic beads and transduction of the indicated CAR on day 1. Data are representative of >3 independent experiments.
Functional characterization of anti-CD19 CAR expressing primary human T cells
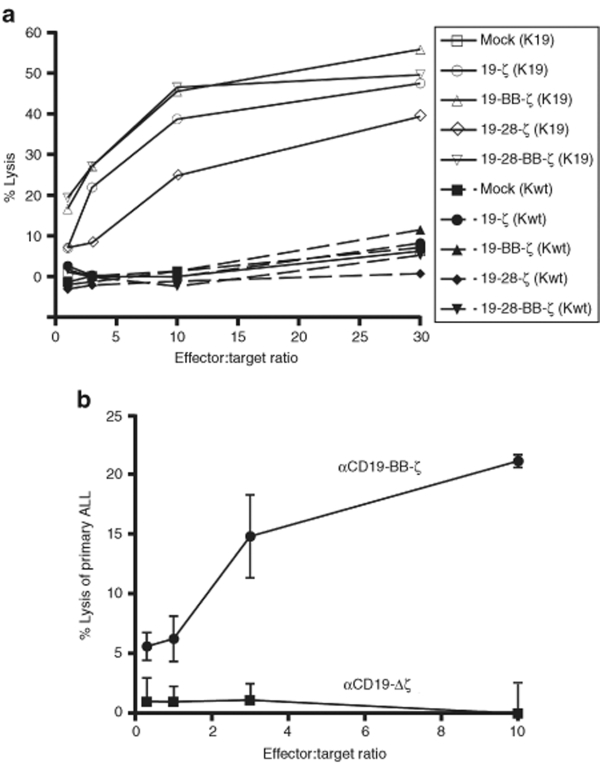
To enhance the functionality of the immunoreceptor, we introduce the signal transduction domains of CD28 or CD137 in the TCR-ζ containing CAR (Figure 1a). Similar to data reported by other groups,11,17 the introduction of costimulatory domains into CARs does not improve the antigen-specific cytotoxicity triggered by these receptors (Figure 2b). Lytic activity of transduced T cells against K562 target cells expressing CD19 correlated with the transduction efficiency of the T cells (data not shown). CAR-triggered cytotoxicity is antigen-specific with only negligible lysis of wild-type K562 cells that lack expression of the CD19 antigen (Figure 2a). CAR+ T cells are also able to efficiently kill primary pre-B acute lymphoblastic leukemia (ALL) cells that express physiologic levels of CD19 (Figure 2b). Of note, these primary ALL cells lack expression of endogenous CD80 or CD86 (Figure S3).
Figure 2.
CD19-specific CAR+ T cells demonstrate antigen-specific killing of CD19+ tumor cells. (a) CAR+ T cell cytotoxic activity towards K562 cells that are engineered to express human CD19 (K19) or target antigen negative wild-type K562 cells (K wild type). Following >10 days expansion, CAR+ T cells (Effector cells) were mixed with the K562 cells (Target cells) at the indicated ratios. Results represent the mean percent of target cell lysis as described in materials. Results are representative of three-independent experiments. (b) Cytotoxic activity of T cells expressing either the aCD19-BB-ζ or the aCD19-Δζ control receptor towards primary human ALL target cells using the same method described in (a). Error bars represent the standard error of the mean for three replicates.
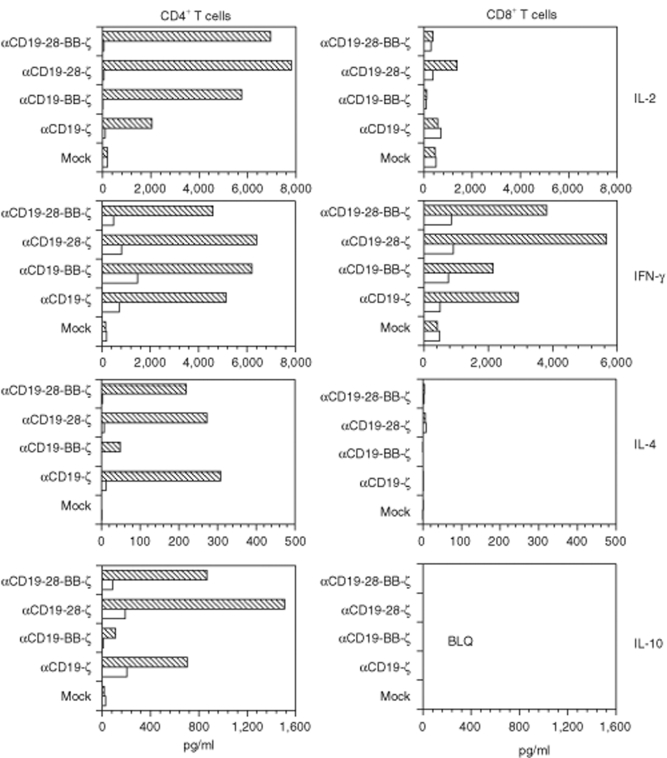
Following CAR activation with CD19+ K562 cells, CD4+ T cells expressing CARs produced abundant quantities of interleukin-2 (IL-2) and interferon-γ (Figure 3) comparable to cells stimulated via the endogenous TCR and CD28 receptors (data not shown). T cells expressing CD28 and CD137 domain-containing CARs produced greater quantities of IL-2 when compared with cells expressing the αCD19-ζ receptor (Figure 3). The production of the type 2 cytokines, IL-4 and IL-10, by CD4+ T cells was also stimulated by all of the CARs tested; however, the levels of these cytokines were much lower, consistent with the Th1-like phenotype of T cells generated by anti-CD3 and CD28 stimulatory beads.16 It was notable that the incorporation of the CD137 domain into CARs decreased the production of these type 2 cytokines, consistent with previous reports of the 4-1BB signaling pathway in natural T cells.18 All CARs stimulated interferon-γ production by CD8+ T cells. These findings confirm that the addition of costimulatory domains into CARs modulates cytokine secretion in a manner that is dependent on the type of costimulatory domain.7,8,17,19 However, it is less well appreciated that the pattern of cytokine expression is altered by incorporation of different signal transduction domains into the CARs. These differences may have important consequences for the functionality of T cells engineered to express CARs.
Figure 3.
The cytokines produced by CD19-specific CAR+ T cells are dependent on the presence of costimulatory domains within the CAR. Following >10 days of expansion, 1 × 106 CAR+ T cells, as indicated, were stimulated with K562 cells expressing either human CD19 (hatched bars) or antigen negative wild-type K562 cells (open bars). Supernatant was harvested after 24 hours of incubation, and the indicated cytokines were measured by cytokine bead array (BD Biosciences, San Jose, CA). Results are representative of two-independent experiments; BLQ, below the limit of quantification.
The effects of costimulatory domains on CAR-driven T cell proliferation
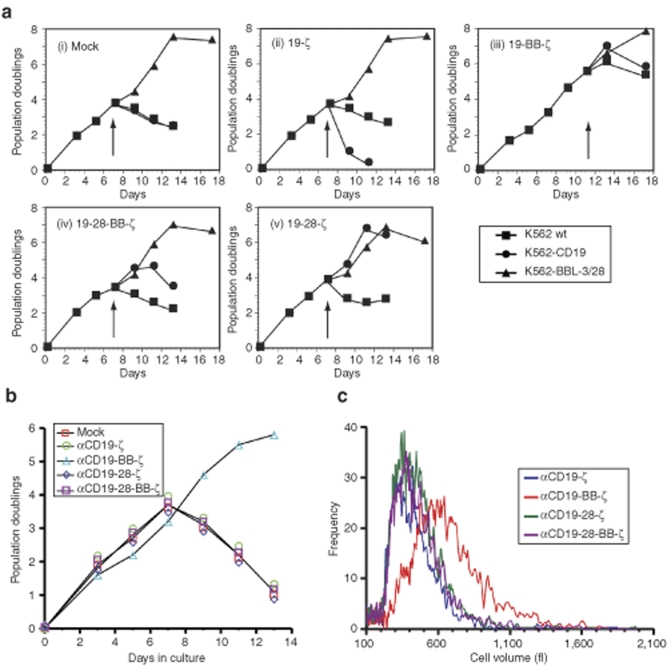
The generation of a robust and sustained antitumor immune response requires not only triggering of cytotoxicity and cytokine production but also stimulation of T cell proliferation. To assess the relative contribution of different costimulatory domains to proliferative signals delivered by CARs, we engineered primary human T cells to express CARs in conjunction with GFP to permit evaluation of both CAR+ and CAR− T cells in the same culture. Following T-cell restimulation with CD19+ K562 (K562-CD19 cells), T cells expressing the αCD19-28-ζ receptor exhibited proliferation comparable to that obtained with full stimulation of the endogenous TCR complex with K562 cells loaded with anti-CD3 and CD28 antibodies, a condition shown previously to support long-term expansion of primary human T cells (KT32-BBL)20 (Figure 4a[v]). The αCD19-28-BB-ζ triple receptor also stimulated CD19 driven proliferation (Figure 4a[iv]), but to a lesser extent than that observed with the αCD19-28-ζ double costimulatory receptor. No significant proliferation was observed when these same T cells were stimulated with wild-type K562 cells lacking the CD19 antigen (K562 wild type). As previously shown by other investigators,17,19,21,22 T cells expressing the αCD19-ζ receptor showed little proliferation on exposure to the surrogate CD19 antigen (Figure 4a[ii]), demonstrating the dependence of CAR-driven proliferation on costimulatory signals.
Figure 4.
CD28 and 4-1BB costimulatory domains enhance αCD19 CAR-induced T cell proliferation in vitro with both antigen-dependent and antigen-independent effects. (a) In vitro expansion of CAR+ T cells following antigen stimulation. T cells were stimulated with αCD3/αCD28 coated magnetic beads on day 0, and transduced with the indicated CAR on day 1 using a bicistronic lentiviral vector expressing CAR along with eGFP using the 2A ribosomal skipping sequence as described in Materials. Cultures were restimulated (arrow) with either CD19+ K562 cells (K562-CD19), wild-type K562 cells (K562 wild type) or K562 cells expressing hCD32 and 4-1BBL in the presence of aCD3 and aCD28 antibody (K562-BBL-3/28) following washing. Exogenous IL-2 was added to the cultures every other day at 100 IU/ml. GFP+ T cells were enumerated by flow cytometry using bead-based counting. Results are reported as the number of population doublings, and they are representative of four-independent experiments. (b) Sustained CAR+ T cell expansion in the absence of restimulation. CD4+ and CD8+ T cells were engineered with the indicated CAR, expanded and enumerated as in panel (a) In the absence of any K562 stimulator cells. Results are representative of at least three-independent experiments. (c) Histogram of mean T cell volume (fl) on day 8 of culture measured using a Coulter Multisizer III particle counter following stimulation with αCD3/αCD28 coated magnetic beads on day 0, and transduction with the indicated CAR on day 1. Results are representative of at least three-independent experiments.
Unexpectedly, T cells containing the αCD19-BB-ζ double costimulatory domain CAR had significantly increased proliferative capacity during in vitro expansion independently of receptor ligation with the surrogate CD19 antigen (Figure 4a[iii],b). This increased proliferation was observed in both CD4+ and CD8+ T cells (data not shown), and it was associated with a prolonged blast phase after the initial stimulation and transduction, as revealed by a longer maintenance of an elevated mean cellular volume (Figure 4c), a parameter that correlates well with log phase proliferation of T cells.16 These findings suggest that incorporation of the CD137 intracellular domain mediates antigen-independent activity that is similar to that provided by the natural 4-1BB receptor in T cells following ligation.23 As a result of the enhanced proliferation observed following the initial activation of T cells via αCD3/αCD28-coated beads used to enhance T-cell transduction24,25 (Figure 4b), CAR+ T cells expressing the αCD19-BB-ζ receptor had relatively low CAR-driven proliferation (Figure 4a[iii]).
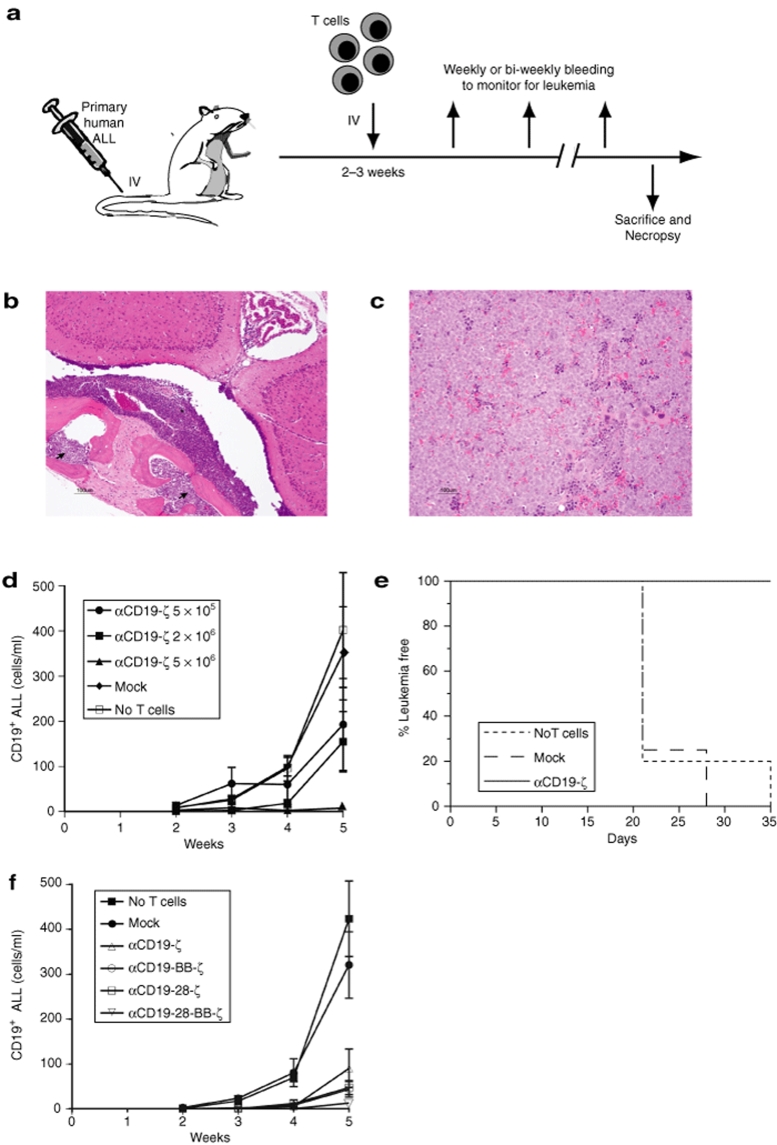
Evaluating antitumor responses of CAR+ human primary T cells in vivo
Other than the antigen-independent proliferation of the 4-1BB containing CAR, the above in vitro findings, in aggregate, suggested that the αCD19-28-ζ CAR would be the most effective receptor for generating a sustained antileukemic T cell response in vivo. We evaluated the in vivo efficacy of αCD19 CARs using an in vivo model of ALL (Figure 5a) in which primary human pre-B ALL cells are engrafted into immunodeficient mice. In this model system, intravenous injection of primary ALL cells leads to development of progressive leukemia with significant involvement of the bone marrow, spleen and blood including leptomeningeal involvement (Figure 5b,c) that eventually leads to the death of the animal.26,27 Primary human T cells also readily engraft within these animals, and engraftment of mock-transduced human CD4+ and CD8+ T cells in leukemia-bearing animals has little or no impact on the development of leukemia (Figure 5d,e). As little as 5 × 106 CAR+ T cells can significantly delay leukemia in most mice injected with ALL 2 weeks prior (Figure 5d,e, P = 0.008) compared with mock-transduced T cells. A dose-dependent affect is also apparent with as little as 5 × 105 CAR+ cells showing an effect on development of leukemia (Figure 5d). All of the CARs demonstrated potent antileukemic activity when 2 × 106 CAR+ T cells were injected 2 weeks after establishing leukemia in the mice (Figure 5f). The treatment effect was significant for the αCD19-ζ CAR (P < 0.05) and for CARs that expressed costimulatory domains (P < 0.01). The αCD19-28-BB-ζ triple CAR was the most potent of the CARs tested; however, this difference was not significant (Figure 5f). We confirmed this result in another experiment using a more aggressive ALL (patient 96). This experiment used 10 mice per group, and was powered to detect smaller treatment effects than the experiment shown in Figure 5f. Again, the αCD19-28-BB-ζ triple CAR was the most potent of the CARs tested, however, the triple CAR could not be demonstrated to be superior to the αCD19-BB-ζ CAR (data not shown).
Figure 5.
CD19-specific CAR+ human T cells display significant antileukemic activity in an immunodeficient mouse model of human pre-B ALL. (a) Xenograft model using human CD19-specific CAR+ T cells to treat a primary human pre-B ALL in immunodeficient mice. After establishment of ALL, mice were randomized to treatment with the indicated T cell populations. (b and c) Hematoxylin & Eosin stained section of a NOD-SCID-β2−/− mouse calvarium (b) and spleen (c) 7–8 weeks following injection of pre-B ALL. Arrows indicate areas of bone marrow infiltrated by malignant ALL cells. The asterisk indicates leukemic cells that have invaded the leptomeningeal membranes. (d) Dose dependent antileukemic effect of CAR+ T cells given 2 weeks after establishing leukemia. Peripheral blood CD19+ B-ALL blast cell counts were measured at weekly intervals in mice (>4 mice/group) that were injected with the indicated numbers of αCD19-ζ CAR+ T cells or mock-transduced T cells The blast count in the 5 × 106 CAR+ T cell group is significantly lower than the count in the Mock and no T cell groups (ANOVA on the log-transformed blast counts, F-test P = 0.008) (e) Leukemia-free survival over time in animals described in panel D receiving no T cells, mock-transduced T cells, or αCD19 -ζ CAR+ T cells (5 × 106). Animals were assessed for leukemia at weekly intervals. Survival curves for the indicated groups were compared using the log-rank test. The group receiving αCD19 -ζ CAR+ T cells show a significantly increased median survival (log-rank test, P < 0.001) compared to animals receiving mock-transduced or no T cells. (f) Increased antileukemic effect of CAR+ T cells expressing costimulatory domains given 2 weeks after establishing leukemia. Peripheral blood CD19+ ALL blast cell counts were measured in leukemic mice (≥7 mice/group) that were injected with 2 × 106 CAR T cells or mock-transduced T cells. At week 5, animals in all CAR+ T cell groups expressing costimulatory domains have significantly lower blast counts than Mock and no T cell groups by ANOVA (using Scheffe, P < 0.01). Results represent mean and standard error of mean in d and f.
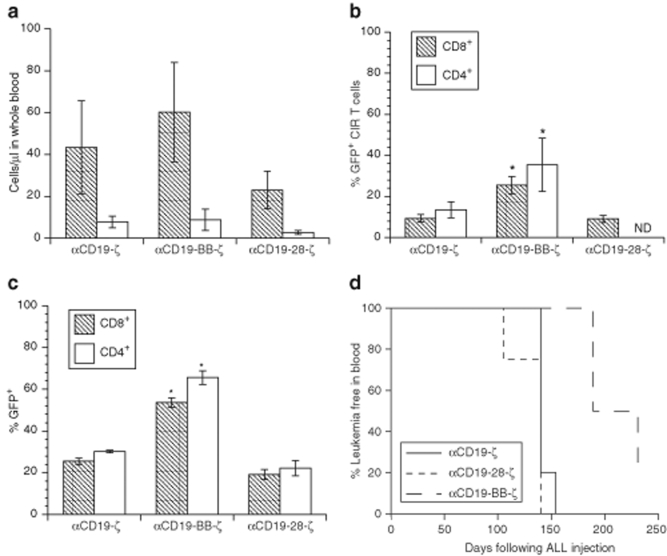
In vitro observations suggested that the CD28 intracellular domain should permit CAR+ T cell proliferation in vivo when transduced with CARs that contain this domain. We therefore compared the in vivo efficacy of T cells expressing the αCD19-ζ, αCD19-28-ζ, and αCD19-BB-ζ CARs by injecting 10 million bulk T cells (adjusted to 50% CAR+ T cells in order to follow the fate of CAR+ versus CAR− cells) 3 weeks after establishment of leukemia in nonobese diabetic–severe combined immunodeficiency (NOD-SCID)-γ−/− mice. The T cells were engineered to express GFP as well as the CAR by using a vector that encodes these two genes separated by the 2A ribosomal skipping sequence to allow monitoring of CAR+ T cells. All CAR+ T cells retain significant antileukemic efficacy compared with mock-transduced T cells when limiting numbers of T cells necessary for engraftment are transferred (Figure 5f); however, studies using higher numbers of CAR+ T cells reveal significant differences in the engraftment and persistence of the CAR+ T cells bearing different costimulatory domains (Figure 6a–d). The total T cell counts were highest in mice after injection with αCD19-BB-ζ CAR+ T cells (Figure 6a), and the T cells were comprised of CD4+ and CD8+ CAR+ T cells (Figure 6b). After injection into leukemic animals, the proportion of αCD19-BB-ζ CAR+ T cells was significantly higher than αCD19-ζ CARs+ T cells (P < 0.01), whereas it was notable that the proportion of αCD19-28-ζ CAR+ T cells were not higher than the αCD19-ζ only CARs. Interestingly, the enhanced engraftment and/or persistence of the αCD19-BB-ζ CAR+ CD4 and CD8 T cells was CD19 antigen independent, because it was also observed in animals that were not injected with ALL cells (Figure 6c, P < 0.05).
Figure 6.
The 4-1BB costimulatory domain enhances CAR+ T cell survival and antileukemic efficacy in vivo. (a) Absolute peripheral blood CD4+ and CD8+ T cell counts 4 weeks following T cell injection in NOD-SCID-γ−/− mice. 8 × 106 T cells engineered to express the indicated CAR by a bicistronic lentiviral vector that encodes the CAR linked to eGFP were injected 3 weeks after injection of 2 × 106 leukemic cells. T cells were normalized to 45–50% input GFP+ T cells by mixing with mock-transduced cells prior to injection, and confirmed by flow cytometry (data not shown). Results represent the mean and SEM of the absolute number of cells per ml of whole blood (measured by TruCount assay) in at least 4 mice/group. (b) The mean and SEM of the % of CAR+GFP+CD4+ and CD8+ T cells in the same samples shown in a. The overall F-test in a one-way ANOVA comparing the mean across groups was significant (P < 0.01). The asterisk indicates results that are significantly different from the other two groups by a post hoc pairwise comparison of the means (Scheffe F-test at P = 0.05). (c) Enhanced antigen-independent survival of 4-1BB CARs. The mean and SEM of the % of CAR+GFP+ CD4+ and CD8+ T cells in animals injected with T cells is shown at the same time as in a, but in nonleukemic NOD-SCID-γ−/− mice. The overall F-test in a one-way ANOVA comparing the mean across groups was significant (P < 0.01). The asterisk indicates results that are significantly different from the other two groups by a post hoc pairwise comparison of means (Scheffe F-test at P = 0.05). (d) Leukemia-free survival over time in animals described in a and b. Animals were assessed for leukemia at 1-week intervals. Survival curves for the indicated CAR+ T cell groups were compared using the log-rank test. The αCD19-BB-ζ group shows a significantly increased median survival (log-rank test, P = 0.009) compared to either the αCD19-ζ or αCD19-28-ζ groups.
It is notable that the T cells expressing the αCD19-28-ζ receptor also did not exhibit greater antitumor efficacy compared with T cells expressing αCD19-ζ only (Figures 5f and 6d). It is possible that this could relate to low level expression of CD86 on the pre-B ALL cells (Supplementary Figure S3). In contrast, αCD19-BB-ζ expressing T cells demonstrated a significant enhancement in antileukemic efficacy compared with T cells expressing either the αCD19-ζ or αCD19-28-ζ receptors. Median leukemia-free survival was increased by 7 weeks (Figure 6d, P = 0.009). Based on an approximate doubling time of 2.7 days for pre-B ALL cells (derived by fitting the leukemic blast counts in untreated animals to an exponential growth model), this 7-week delay in onset of leukemia corresponds to a reduction in leukemia burden of >105-fold following T cell injection when compared with the burden present in animals receiving either the αCD19-ζ or αCD19-28-ζ modified T cells.
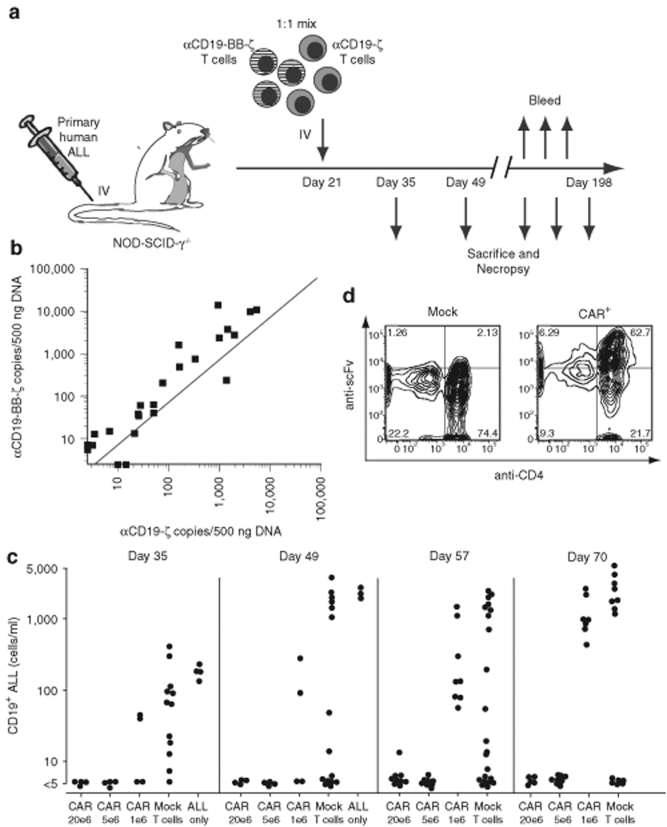
The above experiments suggested that the CD137 signaling domain confers an antigen-independent effect to enhance the survival and/or proliferation of CAR T+ cells in vivo, and the results are consistent with the in vitro effects shown in Figure 4b. To further characterize this effect, a long-term competitive engraftment experiment was carried out as shown in Figure 7a and described in detail in the Supplementary Materials and Methods and Supplementary Table S1. After establishing leukemia in the mice, a 1:1 mixture of T cells expressing either the αCD19-ζ or αCD19-BB-ζ CAR was injected 3 weeks later. Mice were injected with CAR T cells at 1, 5, and 20 × 106 cells per mouse; and the mice were bled and/or killed at intervals between 5 weeks and 6 months after establishment of leukemia. There was a consistent enrichment for αCD19-BB-ζ in the spleens of the mice (Figure 7b, P = 0.0001) and other organs (data not shown). The enrichment was independent of the level of engraftment in that the bias of the log αCD19-BB-ζ to αCD19-ζ was consistent throughout a 3log10 range of engraftment (Figure 7b).
Figure 7.
Long-term expression and survival of T cells engineered to express a CD19-specific CAR with the 4-1BB costimulatory domain in vivo. (a) Schematic diagram of a competitive experiment in which different numbers of αCD19-ζ and αCD19-BB-ζ engineered T cells are coinjected at a 1:1 ratio into NOD-SCID-γ−/− mice bearing B-ALL; see Supplementary Materials and Methods for detailed description of experimental design. (b) The number of copies of αCD19-ζ and αCD19-BB-ζ vector in spleen DNA from 27 mice evaluated at various times following T cell injection as described in a and Supplementary Table S2. The dotted line represents the expected copy number for each receptor based on the injected T cells containing an average of 2.4 copies of αCD19-ζ and 1.7 copies of αCD19-BB-ζ per cell. The mean of the ratio of αCD19-BB-ζ: αCD19-ζ following injection was compared to the ratio in injected T cells following log transformation, and was found to be significantly different (Student's t-test, P = 0.0001). The ratio of αCD19-BB-ζ: αCD19-ζ at baseline (0.708) was significantly different from the geometric mean ratio of 1.756 after engraftment with 95% confidence interval (1.178, 3.696). (c) Dose dependent CAR treatment response. Peripheral blood was obtained 35–70 days after establishing leukemia in mice injected on day 21 with either 1, 5 or 20 × 106 CAR T cells, an equivalent number of mock-transduced T cells, or no T cells. There were 16 mice per T cell group and four animals in the no T cell group; four mice from each group were randomly bled for determination of peripheral blood CD19+ ALL blast counts and then killed on days 35 and 49. The remainder of the animals were evaluated on days 57 and 70. The ALL blast counts were significantly lower on days 57 and 70 in the CAR mice compared to mice engrafted with mock-transduced T cells (P < 0.01 by Kruskal-Wallis test). (d) Surface expression of the CD19-specific CAR on T cells isolated from the spleen of a mouse in the mock-transduced T cell group and the CAR+ T cell group 198 days following injection of the T cells into NOD-SCID-γ−/− mice bearing B-ALL.
A robust, dose-dependent antileukemic treatment effect was observed in the mice given the mixture of T cells expressing the αCD19-ζ or αCD19-BB-ζ CARs (Figure 7c). Peripheral blood analyzed between 35 and 70 days after establishment of leukemia showed that the mice treated at the 5 and 20 × 106 dose levels controlled the leukemia, whereas there was only partial control in the mice given 1 × 106 CAR T cells. However, even animals given the low dose of CARs had a significant treatment effects on days 57 and 70, comparing blast counts in mice engrafted with the CAR+ T cell mixture to mice engrafted with equivalent number of mock-transduced T cells (P < 0.01). Long-term engraftment of the CAR+ T cells was observed in the animals with controlled leukemia, as 3 of 7 mice examined >6 months after T cell transfer were still engrafted in the spleen (Supplementary Table S2) and other organs (data not shown). There was no evidence of a change in the ratio of αCD19-ζ to αCD19-BB-ζ over time, or as a function of T cell dose (Supplementary Table S2). Finally, animal 245 (Supplementary Table S2) was necropsied on day 198, and found to be free of leukemia and to harbor CAR+ T cells in the spleen that had constitutive surface expression of the single-chain variable fragment at readily detectable levels (Figure 7d).
Together, these results demonstrate that the αCD19-BB-ζ modified T cells persisted longer and had more vigorous antileukemic effects than CAR+ T cells that expressed CD28 signaling domains. In addition, although T cells expressing CARs that contain the TCR-ζ only or the CD28 costimulatory domain along with TCR-ζ are capable of killing ALL cells in vitro, their survival is significantly shorter than that of T cells with CARs expressing the CD137 signaling domain. Finally, there was no evidence for transformation of the CAR+ T cells that expressed the CD137 signaling domains over the course of the 6 month experiment shown in Figure 7 and Supplementary Table S2.
Discussion
Artificial chimeric immunoreceptors offer the possibility of reprogramming T cells for efficient targeting of tumors in an human leukocyte antigen-independent fashion. However, although initial clinical studies demonstrate feasibility with the retargeted T cells, poor in vivo persistence and low expression of the transgene have been documented, and these limitations have reduced potential clinical activity.2,3,28 To address these issues, our studies have used a robust preclinical model, and we demonstrate that a single infusion of as few as 2 million engineered T cells could control and in some cases, eliminate preestablished disseminated leukemia. Surprisingly, expression of the CD137 signaling domain rather than the CD28 domain was most correlated with reprogramming T cells for persistence in vivo.
Previous in vitro studies have characterized the incorporation of CD137 domains into CARs.10,11,29 Our results represent the first in vivo characterization of these CARs and uncover several important advantages of CARs that express CD137 that were not revealed by the previous in vitro studies. We demonstrated that CARs expressing the CD137 signaling domain could survive for at least 6 months in mice bearing tumor xenografts. This may have significant implications for immunosurveillance, as well as for tumor eradication. For example, in a mouse prostate cancer xenograft model, survival of CAR+ T cells for at least a week was required for tumor eradication.30
Long-term survival of the CARs did not require administration of exogenous cytokines, and these results significantly extend the duration of survival of human T cells expressing CARs shown in previous studies.17,31 To our knowledge, this is the first report demonstrating elimination of primary leukemia xenografts in a preclinical model using CAR+ T cells. Furthermore, complete eradication was achieved in some animals in the absence of further in vivo therapy, including prior chemotherapy or subsequent cytokine support.
The long-term control of well-established tumors by immunotherapy has rarely been reported. Most preclinical models in a therapeutic setting have tested tumors that have been implanted for a week or less before initiation of therapy.32 After establishing leukemia 2–3 weeks before T cell transfer, we found that many animals had long-term control of leukemia for at least 6 months. The efficacy of targeted, adoptive immunotherapy in this xenograft model of primary human ALL compares favorably to our prior experience testing the antileukemic efficacy of single cytotoxic (ref. 27 and data not shown) or targeted agents,26 where we have observed extension of survival but not cure of disease. Additionally, we have not previously observed the ability to control xenografted ALL for a period of as long as 6 months.
It is likely that several mechanisms account for the enhanced efficiency of the redirected T cells observed in the present report. First, previous studies have generally used T cells after a culture for a month or longer.2,3,28 In the present work we have used an efficient bead-based artificial antigen-presenting cell, which shortens the culture to ~10 days, and permits the use of the T cells early at a time when we have shown previously that the average telomere length of the cultured T cells is actually longer than at the start of culture.33 We attribute this to the previous demonstration that the anti-CD28 driven culture system induces telomerase activity.34 Furthermore, the addition of anti-CD28 antibody to culture conditions promotes transduction of central memory T cells.35 CD28 bead-based cell expansion has the capacity to routinely generate >1010 CAR+ T cells in ~10 days using Food and Drug Administration compliant manufacturing procedures already in use for clinical trials in humans.36
Second, previous studies have generally used murine retroviruses or electroporation to introduce the chimeric receptor.2,3,28 We have used lentiviral gene transfer which permits highly efficient engineering of T cells with >85% successful gene transfer.24,36 As shown in our study, CAR expression was maintained for at least 6 months in vivo with no evidence of silencing using the EF-1α promoter. In comparison, murine retroviral vectors have been shown to exhibit significant silencing of gene expression over time, despite the incorporation of elements such as chromatin-insulator sequences.
Similar to other groups who have evaluated CARs incorporating costimulatory domains,9,10,17,37,38,39,40,41 we confirmed that the addition of the CD28 intracellular domain into CARs enhances the in vitro proliferation and cytokine production of T cells stimulated through these receptors. Interestingly, the αCD19-BB-ζ CAR appears to antagonize the production of the type 2 cytokines, IL-4 and IL-10, but it remains unclear whether this was due to direct signaling or to selective outgrowth of Th1-like cells.
In other studies using CAR+ T cells and trans costimulation of CD28 and CD137 through genetic expression of CD80 and 4-1BBL, redirected T cells were also found to have potent antitumor effects.31 The significantly enhanced antileukemic activity in vivo is associated with the improved persistence of the CAR+ T cells. Our results suggest that enhanced survival and/or proliferation of CAR+ T cells contribute to the increased antitumor effects.
CD137 plays an important role in T cell proliferation and survival, particularly for T cells within the memory T cell pool.42 CD137 mediates its effects on T cell survival and proliferation through activation of the AKT/mammalian target of rapamycin pathway43 and the upregulation of the antiapoptotic genes, Bcl-xL and BFL-1.44
Surprisingly, the αCD19-28-ζ modified T cells failed to show a significant improvement in antileukemic efficacy using our primary pre-B ALL model compared with the αCD19-ζ modified T cells. In addition, we were unable to demonstrate superior therapeutic efficacy of αCD19-28-BB-ζ over αCD19-BB-ζ. Low level expression of CD86 on the ALL cells may in part account for this. Most other reports, including a recent study by our laboratory using a mesothelin-directed CAR find that CD28 domain-containing CARs show enhanced antitumor efficacy.45 In that study we found that CARs containing either 4-1BB or CD28 endomains were equivalently active at controlling large tumors, that the combination of CD28 and 4-1BB cytosolic domains resulted in the best persistence of CAR T cells in the tumor bearing mice and that 4-1BB endodomains tended to keep CAR T cells in a central memory state. Together, these studies suggest that the optimal signals required by CARs may be dependent on the particular tumor being targeted and/or the nature of the particular single-chain variable fragment antibody.
Our studies are the first to reveal antigen-independent effects of the αCD19-BB-ζ receptor on T cells. This receptor, although capable of triggering cytotoxicity in an antigen-dependent fashion, also significantly prolonged the initial blast-phase of T cell activation. There are several possible mechanisms by which the CARs could deliver antigen-independent signals. CARs, like some natural receptors, may deliver tonic ligand-independent signals. Impairment of the regulatory mechanisms that normally extinguish receptor signaling such as the SHP-1 and PTPH1 phosphatase that dephosphorylate the TCR-ζ immunoreceptor tyrosine–based activation motifs might be impaired, leading to the antigen-independent effects observed in this study. Although CARs appear to exist predominantly as homodimers, these artificially-constructed receptors might also spontaneously aggregate into oligomers, especially at the high levels of expression possible with the EF-1α promoter.
The enhanced growth effects of the αCD19-BB-ζ receptor are consistent with the antigen-independent growth effects that are observed in T cells stimulated through the natural CD137 receptor by agonist monoclonal antibody.23,46 Normally, CD137 expression is tightly regulated on T cells with expression limited to a window of a few days following T cell activation or following IL-15 treatment.47 The 4-1BB/4-1BBL interaction has been proposed as one mechanism by which IL-15 mediates its effect on memory T cells under limiting CD137 expression.42 The antigen-independent signals derived from the CD137 domain within the CAR may be critical to the antileukemic effects observed in our study, analogous to the continued presence of a 4-1BB agonist antibody, and ectopic trans expression of 4-1BBL, both of which have been shown to promote antitumor effects in vivo.48 Although previously unreported, these antigen-independent effects of CARs have important implications for the clinical use of these receptors.
Materials and Methods
Construction of lentiviral vectors with different eukaryotic promoters and CARs. Lentiviral vectors that encode a mouse CD8-human CD28 chimeric protein and enhanced GFP (eGFP) separated by the encephalomyocarditis virus internal ribosomal entry sequence under the transcriptional control of either the CMV IE gene, EF-1α, ubiquitin C, or phosphoglycerokinase (PGK) promoter were generated by replacing the CMV promoter within the third generation self-inactivating lentiviral vector plasmid, pRRL-SIN-CMV-eGFP-WPRE (Cell Genesys, San Francisco, CA).49 The EF-1α promoter (derived from pTracer-CMV2; Invitrogen, Carlsbad, CA), the ubiquitin C promoter (derived from pHUG-1 lentiviral vector, a kind gift of Eric Brown, University of Pennsylvania) and the PGK promoter (derived from pRRLsin.sppt.PGK.GFP.pre, Cell Genesys) were all cloned into pRRL-SIN-CMV-eGFP-WPRE using PCR and standard molecular biology techniques.
Figure1a shows schematic diagrams of the CARs used in this study. All CARs contain an single-chain variable fragment that recognizes the human CD19 antigen. The cDNA for the CARs that contain a truncated form of the TCR-ζ intracellular domain (αCD19-Δζ), a full-length TCR-ζ domain (αCD19-ζ) or a TCR-ζ domain in cis with the intracellular domain of the 4-1BB receptor (αCD19-BB-ζ) were generated at St Jude's Childrens Research Hospital.11 These complete CAR sequences were amplified directly from the provided plasmids by PCR. Constructs containing the CD28 transmembrane and intracellular domain alone (αCD19-28-ζ) or in combination with the 4-1BB intracellular domain (αCD19-28-BB-ζ) were generated by the procedure of splicing by overlap extension (Supplementary Table S3). A plasmid encoding a mCD28-huCD28 chimeric protein50 and the above constructs were used as templates for PCR. The resulting PCR fragments containing the complete CARs were then cloned into pELPS 3′ of the promoter using standard molecular biology techniques. pELPS is a derivative of the third-generation lentiviral vector pRRL-SIN-CMV-eGFP-WPRE in which the CMV promoter was replaced with the EF-1α promoter as described above and the central polypurine tract of HIV was inserted 5′ of the promoter. CAR-expressing lentiviral vectors in which the CAR sequences were preceded in frame by an eGFP sequence followed by the 2A ribosomal skipping sequence from FMDV were also generated. These vectors permit dual expression of GFP and the CARs from a single RNA transcript. All constructs were verified by sequencing.
Mouse xenograft studies. Xenograft studies were performed as previously described.26,27 Briefly, 6–12 week old mice NOD-SCID-γc−/− or NOD-SCID-β2−/− mice were obtained from JAX (Bar Harbor, ME) or bred in-house under an approved IACUC protocol and maintained under pathogen-free conditions. Animals were injected with 5 × 105 to 2 × 106 viable human ALL cells via the tail vein in a volume of 0.3 ml. T cells were injected into animals 9–21 days following ALL injection as indicated via the tail vein. Animals were closely monitored for signs of graft-versus-host disease as evidenced by >10% weight loss, loss of fur, and/or diarrhea. Peripheral blood was obtained by retro-orbital bleeding, and ALL and T cell engraftment were determined by flow cytometry using BD TruCount tubes as described in the manufacturer's instructions. CD19, CD3, CD4, and/or CD8 expression was detected by staining with fluorescently-conjugated monoclonal antibodies. CAR+ T cells were identified by GFP expression using lentiviral vectors in which the CAR was linked to eGFP-2A as described above.
Detection of integrated CAR-expressing vectors by quantitative PCR. DNA was extracted from either cultured T cells or 1 mm3 of spleen tissue using a QiAmp or PureGene kit (Qiagen, Valencia, CA), respectively, according to the manufacturer. Quantitative real-time PCR was performed on 500 ng of tissue-derived DNA with four replicates for each DNA sample using the ABI 2X Taqman Universal Master Mix with AmpErase UNG (Applied Biosystems, Foster City, CA). The αCD19-ζ specific primers and probe were designed to amplify the junction region between the CD8 transmembrane region and the ζ signaling chain:
CD19 Zeta F primer: 5′-TCC TTC TCC TGT CAC TGG TTA TCA A-3′
CD19 Zeta R primer: 5′-G GTT CTG GCC CTG CTG GTA-3′
CD19 Zeta MGB probe: 5′-FAM CTT TAC TGC AGA GTG AAG T-3′
and the αCD19-BB-ζ specific primers and probe were designed to amplify the junction region between the 4-1BB and ζ signaling chains:
CD19 4-1BB F primer: 5′-TGC CGA TTT CCA GAA GAA GAA GAA G-3′
CD19 4-1BB R primer: 5′-GCG CTC CTG CTG AAC TTC-3′
CD19 4-1BB MGB probe: 5′-VIC ACT CTC AGT TCA CAT CCT C-3′
PCR with real-time fluorescence detection was performed on a 384 well HT7900 real-time PCR thermocycler (Applied Biosystems). The numbers of copies for each vector, expressed as copies/500 ng DNA, was determined by comparison of the measured cycle threshold for each well to the cycle threshold of a standard curve prepared by dilution of a receptor-encoding plasmid in 500 ng pooled genomic DNA (Bioline USA, Taunton, MA) per well. Water added to 500 ng genomic DNA was used as the negative control, which was run in 2–3 wells per plate. Each sample was also evaluated in duplicate following the spiking of 20 copies of plasmid DNA for each CAR receptor into 500 ng of sample to evaluate for the presence of a PCR inhibitor. The assay was qualified to a limit of quantitation of five copies per 500 ng genomic DNA, which correlates to five copies in ~75,000 cells; the precision of values below five copies per well was not evaluated. Variation between runs during assay qualification was minimal and operator independent, with a coefficient of variation among two operators and four runs ranging from 5.31 to 1.47, an R2 value of >0.995 and a slope ranging from −3.05 to −3.65. Known spike controls ranging from 5 × 103 to 1 × 106 copies per well were also included in validation runs and were typically within 90% of the expected value.
Statistical analysis. Statistical analyses were performed as indicated using STATA version 10 (StataCorp, College Station, TX). In analysis where multiple groups were compared, a one-way analysis of variance was performed with a threshold F-test P value of 0.05 prior to performance of post hoc analysis by the Scheffe F-test. Absolute peripheral blood T cell counts, ALL blast counts and αCD19-ζ:αCD19-BB-ζ copy number ratios were log-transformed prior to analysis. Survival curves were compared using the log-rank test.
SUPPLEMENTARY MATERIALFigure S1. Fluorescence of T cells expressing GFP under the control of different eukaryotic promoters. A 1:1 mixture of CD4+ and CD8+ T cells were stimulated with αCD3/αCD28 aAPCs followed by transduction with lentiviral vectors expressing GFP under the control of the indicated promoters. GFP fluorescence was evaluated on day 6 of culture in the indicated T cell subsets by flow cytometry. Data shown in representative of 3 independent experiments.Figure S2. Western blot analysis of CAR expression in primary T cells demonstrates presence of monomers and dimers. a) T cells (1:1 mixture of CD4 + and CD8+ T cells) expressing the indicated CARs were expanded in vitro for >10 days followed by lysis and SDS-PAGE under reducing conditions. CARs containing the full length TCR-ζ cytoplasmic domain and the endogenous TCR-ζ chain were detected by western blotting using an antibody to the TCR-ζ chain. b) Same T cells as shown in panel A except SDS-PAGE was performed under non-reducing conditions to permit evaluation of covalent dimer formation. Results are representative of 2 independent experiments.Figure S3. Primary patient 240 ALL cells show negligible surface expression of ligands for CD28 and CD137. 1x106 primary patient 240 ALL cells or Ramos cells (a well-established EBV-associated Burkitt's lymphoma cell line with a more mature B cell immunophenotype) were stained with antibodies to CD19, CD20, CD45, CD80, CD86 and CD137L as indicated in the figure. Quadrant gating was established by evaluation of cells stained with the appropriate isotype control antibodies.Table S1. Treatment Groups for Figure 7.Table S2. In vivo comparison of αCD19-BB-ζ and αCD19-ζ persistence in spleen from day 35 to day 198 post injection.Table S3. Primer sequences used for construction of the different CARs using the splicing by overlap extension technique.Materials and Methods.
Supplementary Material
Fluorescence of T cells expressing GFP under the control of different eukaryotic promoters. A 1:1 mixture of CD4+ and CD8+ T cells were stimulated with αCD3/αCD28 aAPCs followed by transduction with lentiviral vectors expressing GFP under the control of the indicated promoters. GFP fluorescence was evaluated on day 6 of culture in the indicated T cell subsets by flow cytometry. Data shown in representative of 3 independent experiments.
Western blot analysis of CAR expression in primary T cells demonstrates presence of monomers and dimers. a) T cells (1:1 mixture of CD4 + and CD8+ T cells) expressing the indicated CARs were expanded in vitro for >10 days followed by lysis and SDS-PAGE under reducing conditions. CARs containing the full length TCR-ζ cytoplasmic domain and the endogenous TCR-ζ chain were detected by western blotting using an antibody to the TCR-ζ chain. b) Same T cells as shown in panel A except SDS-PAGE was performed under non-reducing conditions to permit evaluation of covalent dimer formation. Results are representative of 2 independent experiments.
Primary patient 240 ALL cells show negligible surface expression of ligands for CD28 and CD137. 1x106 primary patient 240 ALL cells or Ramos cells (a well-established EBV-associated Burkitt's lymphoma cell line with a more mature B cell immunophenotype) were stained with antibodies to CD19, CD20, CD45, CD80, CD86 and CD137L as indicated in the figure. Quadrant gating was established by evaluation of cells stained with the appropriate isotype control antibodies.
Treatment Groups for Figure 7.
In vivo comparison of αCD19-BB-ζ and αCD19-ζ persistence in spleen from day 35 to day 198 post injection.
Primer sequences used for construction of the different CARs using the splicing by overlap extension technique.
Acknowledgments
We thank Wei-Ting Hwang for statistical analysis, Bruce Levine for helpful discussions, the Human Immunology Core for obtaining and purifying the primary T cells used in this study, Martin Carroll for providing the leukemia samples, and John Scholler, Treasa Smith, Ronghua Liu, Xiaochuan Shan, Junior Hall and Anthony Secreto for expert technical assistance. This work was supported by NIH grants 1R01CA105216, 1R01CA120409, RO1AI057838, and R01113482, the Alliance for Cancer Gene Therapy, the Weinberg and Foerderer-Murray Funds at Children's Hospital, and the Leukemia & Lymphoma Society. J.L.R. and C.H.J. have patent applications in some of the technology described in the manuscript.
REFERENCES
- Deeks SG, Wagner B, Anton PA, Mitsuyasu RT, Scadden DT, Huang C, et al. A phase II randomized study of HIV-specific T-cell gene therapy in subjects with undetectable plasma viremia on combination antiretroviral therapy. Mol Ther. 2002;5:788–797. doi: 10.1006/mthe.2002.0611. [DOI] [PubMed] [Google Scholar]
- Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z, Mavroukakis SA, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer Clin Cancer Res 2006126106–6115.20 Pt 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till BG, Jensen MC, Wang J, Chen EY, Wood BL, Greisman HA, et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112:2261–2271. doi: 10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14:1264–1270. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving BA., and , Weiss A. The cytoplasmic domain of the T cell receptor zeta chain is sufficient to couple to receptor-associated signal transduction pathways. Cell. 1991;64:891–901. doi: 10.1016/0092-8674(91)90314-o. [DOI] [PubMed] [Google Scholar]
- Eshhar Z, Waks T, Gross G., and , Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci USA. 1993;90:720–724. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney HM, Lawson AD, Bebbington CR., and , Weir AN. Chimeric receptors providing both primary and costimulatory signaling in T cells from a single gene product. J Immunol. 1998;161:2791–2797. [PubMed] [Google Scholar]
- Maher J, Brentjens RJ, Gunset G, Rivière I., and , Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta /CD28 receptor. Nat Biotechnol. 2002;20:70–75. doi: 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- Friedmann-Morvinski D, Bendavid A, Waks T, Schindler D., and , Eshhar Z. Redirected primary T cells harboring a chimeric receptor require costimulation for their antigen-specific activation. Blood. 2005;105:3087–3093. doi: 10.1182/blood-2004-09-3737. [DOI] [PubMed] [Google Scholar]
- Finney HM, Akbar AN., and , Lawson AD. Activation of resting human primary T cells with chimeric receptors: costimulation from CD28, inducible costimulator, CD134, and CD137 in series with signals from the TCR zeta chain. J Immunol. 2004;172:104–113. doi: 10.4049/jimmunol.172.1.104. [DOI] [PubMed] [Google Scholar]
- Imai C, Mihara K, Andreansky M, Nicholson IC, Pui CH, Geiger TL, et al. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia. 2004;18:676–684. doi: 10.1038/sj.leu.2403302. [DOI] [PubMed] [Google Scholar]
- Stamenkovic I., and , Seed B. CD19, the earliest differentiation antigen of the B cell lineage, bears three extracellular immunoglobulin-like domains and an Epstein-Barr virus-related cytoplasmic tail. J Exp Med. 1988;168:1205–1210. doi: 10.1084/jem.168.3.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müschen M, Lee S, Zhou G, Feldhahn N, Barath VS, Chen J, et al. Molecular portraits of B cell lineage commitment. Proc Natl Acad Sci USA. 2002;99:10014–10019. doi: 10.1073/pnas.152327399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe ES, Harris NL, Stein H., and , Vardiman JW. IARC Press: Lyon, France; 2001. Pathology and Genetics: Tumours of Haematopoietic and Lymphoid Tissues (World Health Organization Classification of Tumours) [Google Scholar]
- Cooper LJ, Topp MS, Pinzon C, Plavec I, Jensen MC, Riddell SR, et al. Enhanced transgene expression in quiescent and activated human CD8+ T cells. Hum Gene Ther. 2004;15:648–658. doi: 10.1089/1043034041361217. [DOI] [PubMed] [Google Scholar]
- Levine BL, Bernstein WB, Connors M, Craighead N, Lindsten T, Thompson CB, et al. Effects of CD28 costimulation on long-term proliferation of CD4+ T cells in the absence of exogenous feeder cells. J Immunol. 1997;159:5921–5930. [PubMed] [Google Scholar]
- Kowolik CM, Topp MS, Gonzalez S, Pfeiffer T, Olivares S, Gonzalez N, et al. CD28 costimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T cells. Cancer Res. 2006;66:10995–11004. doi: 10.1158/0008-5472.CAN-06-0160. [DOI] [PubMed] [Google Scholar]
- Shuford WW, Klussman K, Tritchler DD, Loo DT, Chalupny J, Siadak AW, et al. 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J Exp Med. 1997;186:47–55. doi: 10.1084/jem.186.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulè MA, Straathof KC, Dotti G, Heslop HE, Rooney CM., and , Brenner MK. A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Mol Ther. 2005;12:933–941. doi: 10.1016/j.ymthe.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Suhoski MM, Golovina TN, Aqui NA, Tai VC, Varela-Rohena A, Milone MC, et al. Engineering artificial antigen-presenting cells to express a diverse array of co-stimulatory molecules. Mol Ther. 2007;15:981–988. doi: 10.1038/mt.sj.6300134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brentjens RJ, Latouche JB, Santos E, Marti F, Gong MC, Lyddane C, et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat Med. 2003;9:279–286. doi: 10.1038/nm827. [DOI] [PubMed] [Google Scholar]
- Friedmann-Morvinski D, Bendavid A, Waks T, Schindler D., and , Eshhar Z. Redirected primary T cells harboring a chimeric receptor require costimulation for their antigen-specific activation. Blood. 2005;105:3087–3093. doi: 10.1182/blood-2004-09-3737. [DOI] [PubMed] [Google Scholar]
- Pulle G, Vidric M., and , Watts TH. IL-15-dependent induction of 4-1BB promotes antigen-independent CD8 memory T cell survival. J Immunol. 2006;176:2739–2748. doi: 10.4049/jimmunol.176.5.2739. [DOI] [PubMed] [Google Scholar]
- Humeau LM, Binder GK, Lu X, Slepushkin V, Merling R, Echeagaray P, et al. Efficient lentiviral vector-mediated control of HIV-1 replication in CD4 lymphocytes from diverse HIV+ infected patients grouped according to CD4 count and viral load. Mol Ther. 2004;9:902–913. doi: 10.1016/j.ymthe.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Hollatz G, Grez M, Mastaglio S, Quaritsch R, Huenecke S, Ciceri F, et al. T cells for suicide gene therapy: activation, functionality and clinical relevance. J Immunol Methods. 2008;331:69–81. doi: 10.1016/j.jim.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Teachey DT, Obzut DA, Cooperman J, Fang J, Carroll M, Choi JK, et al. The mTOR inhibitor CCI-779 induces apoptosis and inhibits growth in preclinical models of primary adult human ALL. Blood. 2006;107:1149–1155. doi: 10.1182/blood-2005-05-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teachey DT, Sheen C, Hall J, Ryan T, Brown VI, Fish J, et al. mTOR inhibitors are synergistic with methotrexate: an effective combination to treat acute lymphoblastic leukemia. Blood. 2008;112:2020–2023. doi: 10.1182/blood-2008-02-137141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JR, Digiusto DL, Slovak M, Wright C, Naranjo A, Wagner J, et al. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol Ther. 2007;15:825–833. doi: 10.1038/sj.mt.6300104. [DOI] [PubMed] [Google Scholar]
- Wang J, Jensen M, Lin Y, Sui X, Chen E, Lindgren CG, et al. Optimizing adoptive polyclonal T cell immunotherapy of lymphomas, using a chimeric T cell receptor possessing CD28 and CD137 costimulatory domains. Hum Gene Ther. 2007;18:712–725. doi: 10.1089/hum.2007.028. [DOI] [PubMed] [Google Scholar]
- Gade TP, Hassen W, Santos E, Gunset G, Saudemont A, Gong MC, et al. Targeted elimination of prostate cancer by genetically directed human T lymphocytes. Cancer Res. 2005;65:9080–9088. doi: 10.1158/0008-5472.CAN-05-0436. [DOI] [PubMed] [Google Scholar]
- Stephan MT, Ponomarev V, Brentjens RJ, Chang AH, Dobrenkov KV, Heller G, et al. T cell-encoded CD80 and 4-1BBL induce auto- and transcostimulation, resulting in potent tumor rejection. Nat Med. 2007;13:1440–1449. doi: 10.1038/nm1676. [DOI] [PubMed] [Google Scholar]
- Yu P, Rowley DA, Fu YX., and , Schreiber H. The role of stroma in immune recognition and destruction of well-established solid tumors. Curr Opin Immunol. 2006;18:226–231. doi: 10.1016/j.coi.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Weng NP, Palmer LD, Levine BL, Lane HC, June CH., and , Hodes RJ. Tales of tails: regulation of telomere length and telomerase activity during lymphocyte development, differentiation, activation, and aging. Immunol Rev. 1997;160:43–54. doi: 10.1111/j.1600-065x.1997.tb01026.x. [DOI] [PubMed] [Google Scholar]
- Weng NP, Levine BL, June CH., and , Hodes RJ. Regulated expression of telomerase activity in human T lymphocyte development and activation. J Exp Med. 1996;183:2471–2479. doi: 10.1084/jem.183.6.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondanza A, Valtolina V, Magnani Z, Ponzoni M, Fleischhauer K, Bonyhadi M, et al. Suicide gene therapy of graft-versus-host disease induced by central memory human T lymphocytes. Blood. 2006;107:1828–1836. doi: 10.1182/blood-2005-09-3716. [DOI] [PubMed] [Google Scholar]
- Levine BL, Humeau LM, Boyer J, MacGregor RR, Rebello T, Lu X, et al. Gene transfer in humans using a conditionally replicating lentiviral vector. Proc Natl Acad Sci USA. 2006;103:17372–17377. doi: 10.1073/pnas.0608138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger TL, Nguyen P, Leitenberg D., and , Flavell RA. Integrated src kinase and costimulatory activity enhances signal transduction through single-chain chimeric receptors in T lymphocytes. Blood. 2001;98:2364–2371. doi: 10.1182/blood.v98.8.2364. [DOI] [PubMed] [Google Scholar]
- Brentjens RJ, Santos E, Nikhamin Y, Yeh R, Matsushita M, La Perle K, et al. Genetically targeted T cells eradicate systemic acute lymphoblastic leukemia xenografts Clin Cancer Res 2007135426–5435.18 Pt 1 [DOI] [PubMed] [Google Scholar]
- Haynes NM, Trapani JA, Teng MW, Jackson JT, Cerruti L, Jane SM, et al. Single-chain antigen recognition receptors that costimulate potent rejection of established experimental tumors. Blood. 2002;100:3155–3163. doi: 10.1182/blood-2002-04-1041. [DOI] [PubMed] [Google Scholar]
- Hombach A, Wieczarkowiecz A, Marquardt T, Heuser C, Usai L, Pohl C, et al. Tumor-specific T cell activation by recombinant immunoreceptors: CD3 zeta signaling and CD28 costimulation are simultaneously required for efficient IL-2 secretion and can be integrated into one combined CD28/CD3 zeta signaling receptor molecule. J Immunol. 2001;167:6123–6131. doi: 10.4049/jimmunol.167.11.6123. [DOI] [PubMed] [Google Scholar]
- Shibaguchi H, Luo NX, Kuroki M, Zhao J, Huang J, Hachimine K, et al. A fully human chimeric immune receptor for retargeting T-cells to CEA-expressing tumor cells Anticancer Res 2006264067–4072.6A [PubMed] [Google Scholar]
- Sabbagh L, Snell LM., and , Watts TH. TNF family ligands define niches for T cell memory. Trends Immunol. 2007;28:333–339. doi: 10.1016/j.it.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Lee HW, Nam KO, Park SJ., and , Kwon BS. 4-1BB enhances CD8+ T cell expansion by regulating cell cycle progression through changes in expression of cyclins D and E and cyclin-dependent kinase inhibitor p27kip1. Eur J Immunol. 2003;33:2133–2141. doi: 10.1002/eji.200323996. [DOI] [PubMed] [Google Scholar]
- Lee HW, Park SJ, Choi BK, Kim HH, Nam KO., and , Kwon BS. 4-1BB promotes the survival of CD8+ T lymphocytes by increasing expression of Bcl-xL and Bfl-1. J Immunol. 2002;169:4882–4888. doi: 10.4049/jimmunol.169.9.4882. [DOI] [PubMed] [Google Scholar]
- Carpenito C, Milone MC, Hassan R, Simonet JC, Lakhal M, Suhoski MM, et al. Eradication of large established tumor xenografts with genetically re-targeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci USA. 2009;106:3360–3365. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Zhu G, Luo L, Flies AS., and , Chen L. CD137 stimulation delivers an antigen-independent growth signal for T lymphocytes with memory phenotype. Blood. 2007;109:4882–4889. doi: 10.1182/blood-2006-10-043463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol. 2003;3:609–620. doi: 10.1038/nri1148. [DOI] [PubMed] [Google Scholar]
- Melero I, Shuford WW, Newby SA, Aruffo A, Ledbetter JA, Hellström KE, et al. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat Med. 1997;3:682–685. doi: 10.1038/nm0697-682. [DOI] [PubMed] [Google Scholar]
- Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D, et al. A third-generation lentivirus vector with a conditional packaging system. J Virol. 1998;72:8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry RV, Rumbley CA, Vandenberghe LH, June CH., and , Riley JL. CD28 and inducible costimulatory protein Src homology 2 binding domains show distinct regulation of phosphatidylinositol 3-kinase, Bcl-xL, and IL-2 expression in primary human CD4 T lymphocytes. J Immunol. 2003;171:166–174. doi: 10.4049/jimmunol.171.1.166. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fluorescence of T cells expressing GFP under the control of different eukaryotic promoters. A 1:1 mixture of CD4+ and CD8+ T cells were stimulated with αCD3/αCD28 aAPCs followed by transduction with lentiviral vectors expressing GFP under the control of the indicated promoters. GFP fluorescence was evaluated on day 6 of culture in the indicated T cell subsets by flow cytometry. Data shown in representative of 3 independent experiments.
Western blot analysis of CAR expression in primary T cells demonstrates presence of monomers and dimers. a) T cells (1:1 mixture of CD4 + and CD8+ T cells) expressing the indicated CARs were expanded in vitro for >10 days followed by lysis and SDS-PAGE under reducing conditions. CARs containing the full length TCR-ζ cytoplasmic domain and the endogenous TCR-ζ chain were detected by western blotting using an antibody to the TCR-ζ chain. b) Same T cells as shown in panel A except SDS-PAGE was performed under non-reducing conditions to permit evaluation of covalent dimer formation. Results are representative of 2 independent experiments.
Primary patient 240 ALL cells show negligible surface expression of ligands for CD28 and CD137. 1x106 primary patient 240 ALL cells or Ramos cells (a well-established EBV-associated Burkitt's lymphoma cell line with a more mature B cell immunophenotype) were stained with antibodies to CD19, CD20, CD45, CD80, CD86 and CD137L as indicated in the figure. Quadrant gating was established by evaluation of cells stained with the appropriate isotype control antibodies.
Treatment Groups for Figure 7.
In vivo comparison of αCD19-BB-ζ and αCD19-ζ persistence in spleen from day 35 to day 198 post injection.
Primer sequences used for construction of the different CARs using the splicing by overlap extension technique.