Abstract
Osteoporosis is a systemic disease that is associated with increased morbidity, mortality and health care costs. Whereas osteoclasts and osteoblasts are the main regulators of bone homeostasis, recent studies underscore a key role for the immune system, particularly via activation-induced T lymphocyte production of Receptor Activator of NFκB Ligand (RANKL). Well-documented as a mediator of T lymphocyte/dendritic cell interactions, RANKL also stimulates the maturation and activation of bone-resorbing osteoclasts. Given that lipid oxidation products mediate inflammatory and metabolic disorders such as osteoporosis and atherosclerosis, and since oxidized lipids affect several T lymphocyte functions, we hypothesized that RANKL production might also be subject to modulation by oxidized lipids. Here, we show that short term exposure of both unstimulated and activated human T lymphocytes to minimally oxidized low density lipoprotein (LDL), but not native LDL, significantly enhances RANKL production and promotes expression of the lectin-like oxidized LDL receptor-1 (LOX-1). The effect, which is also observed with 8-iso-Prostaglandin E2, an inflammatory isoprostane produced by lipid peroxidation, is mediated via the NFκB pathway, and involves increased RANKL mRNA expression. The link between oxidized lipids and T lymphocytes is further reinforced by analysis of hyperlipidemic mice, in which bone loss is associated with increased RANKL mRNA in T lymphocytes and elevated RANKL serum levels. Our results suggest a novel pathway by which T lymphocytes contribute to bone changes, namely, via oxidized lipid enhancement of RANKL production. These findings may help elucidate clinical associations between cardiovascular disease and decreased bone mass, and may also lead to new immune-based approaches to osteoporosis.
Keywords: T lymphocytes, RANKL, lipids, bone, osteoporosis, human, mouse
INTRODUCTION
Osteoporosis and osteopenia are asymptomatic systemic pathologies that are characterized by decreased bone mass and diminished skeletal integrity. Reduced bone mass is associated with greater risk of fracture, as well as increased morbidity and mortality and elevated health care expenditures [1; 2]. The dynamic process of bone remodeling had heretofore been assumed to mainly involve osteoblasts, bone forming cells that secrete organic matrix molecules, and bone-resorbing osteoclasts, which are derived from hematopoietic precursors. However, it is becoming increasingly evident that the immune system also plays a significant role in bone homeostasis [3]. Indeed, the term “osteoimmunology” was coined to reflect the intricate bidirectional communication between the immune and skeletal systems [4].
A central component of the interaction between the immune and skeletal systems is the tumor necrosis factor (TNF) receptor family molecule, receptor activator of NFκB ligand (RANKL). This protein (also known as TNF-related activation cytokine, TRANCE) was actually first identified as a T lymphocyte surface marker that had regulatory effects on immune cell function [5]; only later was it shown to also be essential for the differentiation, activation, and survival of osteoclasts [6; 7; 8]. RANKL, which is now known to be secreted by activated T lymphocytes, has been studied extensively in the context of bone loss associated with rheumatoid arthritis [9; 10], periodontal disease [11; 12], and osteoporosis [3], where it has been shown to bind to RANK on osteoclasts, and induce these bone-resorbing cells to mature and become active. The regulation of T lymphocyte RANKL production in response to physiologic stimuli that are distinct from those that mediate activation via the antigen receptor has not been studied.
The notion that products of lipid and lipoprotein oxidation may contribute to the pathophysiology of osteoporosis is suggested by a variety of studies [13; 14; 15]. Epidemiological analyses have repeatedly shown associations between osteoporosis and hyperlipidemia [16; 17; 18; 19; 20; 21; 22], and patients with lower bone density and osteoporosis have higher lipid levels, more severe coronary atherosclerosis and increased risk of stroke [18; 20; 23; 24; 25]. Conversely, lipid-lowering treatments are associated with both reduced coronary vascular calcification [26] and fewer osteoporotic fractures [27; 28; 29; 30]. In vitro studies demonstrate that minimally oxidized LDL (MM-LDL) inhibits the differentiation of osteoblasts [31] and can induce differentiation of osteoclasts [32]. Within the immune system, oxidized LDL is an inducer of apoptosis in T lymphocytes [33], and mildly oxidized LDL decreases early production of IL2 by T lymphocytes [34]. In addition, dendritic cell function is inhibited in a mouse model of high fat diet induced dyslipidemia [35].
Since bone development and repair require adequate vascularization [36], it is likely that both circulating lipoproteins and immune cells are in close contact with cortical and trabecular bone. Therefore, in the present study, we sought to examine whether oxidized lipids, and particularly, MM-LDL, which is highly inflammatory, might have a direct effect on T lymphocyte RANKL production. Our results show that exposure of both ex vivo, unmanipulated human T lymphocytes, as well as in vitro-activated T lymphocytes to MM-LDL, results in enhanced RANKL secretion, involving the NFκB pathway and increased RANKL transcripts. Oxidized lipids also induced expression of the lectin-like oxidized LDL receptor-1 (LOX-1) on T lymphocytes. Complementary in vivo data from a mouse model of high fat diet-induced osteopenia show that these mice have elevated serum levels of RANKL as well as increased T lymphocyte RANKL mRNA. These observations support a role for oxidized lipids in immune-mediated bone loss, and provide a possible mechanistic link for the well documented association between dyslipidemia and osteoporosis.
MATERIALS AND METHODS
Cell isolation and culture
Human peripheral blood mononuclear cells (PBMCs) from healthy donors with normal lipid levels, as documented by the UCLA Clinical Labs, were isolated by density gradient centrifugation on Ficoll-Hypaque (Cellgro). T lymphocytes were purified by immunomagnetic depletion using a mixture of biotinylated mAbs against CD14, CD16, CD19, CD56, CD36, CD123, CD235a, which were subsequently labeled with anti-biotin microbeads (Miltenyi Biotec). Purity of T lymphocytes (>98%) was confirmed by flow cytometry (Becton Dickson) using anti-CD3 antibody (BD Pharmingen). Cell viability following purification was consistently >90%, as assessed by trypan blue exclusion. Cells were plated at a concentration of 1 × 106/ml in AIM-V serum-free medium (Gibco) for cell culture experiments. For experiments testing activated cells, the T lymphocytes were stimulated with either CD2/CD3/CD28-antibody coated beads (Miltenyi Biotec) at a bead:cell ratio of 1:1 or 6 ug/ml PHA (Sigma).
Lipoprotein preparation and oxidation
Human LDL was isolated by density-gradient centrifugation of serum, and stored in phosphate-buffered 0.15M NaCl containing 0.01% EDTA [37]. Minimally oxidized LDL was prepared by iron oxidation of human LDL, as described in Parhami et al. [31]. Minimal oxidation of LDL resulted in a 3- to 4-fold increase in conjugated dienes. The lipoproteins were tested pre- and post-oxidation for lipopolysaccharide levels and found to have <30 pg of lipopolysaccharide/ml of medium. In some experiments, components of MM-LDL were tested, including 8-iso-Prostaglandin E2 (iso-PGE2; Cayman Chemical) and oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine (Ox-PAPC), which was a generous gift of Dr. Judy Berliner, UCLA. The concentrations of lipoproteins used in this study are reported in micrograms of protein.
Cytokine measurement
Supernatants from cultured cells were harvested after 72 hours of incubation with 25ug/ml nLDL, MM-LDL, 25uM iso-PGE2 or control buffer, centrifuged, and immediately frozen at −80°C until further analysis. All samples were thawed immediately prior to ELISA evaluation. The following ELISA kits were used: RANKL, (Peprotech), IFN γ (Biotrack ELISA System, Amersham), IL-6, TGF-β and TNFα (Quantikine, R&D Systems). All measurements were performed in triplicate wells, and in accordance to manufacturer’s instructions.
Flow cytometry
Cell activation status and viability were assessed by flow cytometry. Cells were harvested after 72 hours of incubation with 25ug/ml nLDL, MM-LDL or buffer control, centrifuged and washed twice with 1X PBS, then resuspended in 1X binding buffer (BD Biosciences) prior to the staining. Briefly, cells were incubated with Annexin V–FITC, propidium iodide (PI), CD38 PerCp-Cy5.5, CD25 APC-Cy7, HLA-DR APC, CD8 PE-Cy7 (BD Biosciences) and CD3 Pacific Blue (Invitrogen) for 15 minutes at room temperature in the dark, washed and resupended in 1X binding buffer. The 7-color staining flow cytometry data was acquired immediately by BD LSRII and FACS Diva software (BD Biosciences). Data analysis was performed using FlowJo (Tree Star). Cells that were negative for Annexin V and PI were considered as viable; apoptotic cells (Annexin V+) and necrotic cells (both Annexin V+/PI+ or PI+ alone) were detected and quantified as a percentage of the entire population [38]. Data from 4 independent experiments, each performed on triplicate samples, were pooled to determine the mean cell viability. For LOX-1 measurement, cells were harvested after 72 hours of incubation with 25ug/ml nLDL, MM-LDL, 25uM iso-PGE2, or control buffer, centrifuged and washed twice with 1X PBS, then resuspended with staining buffer (BD Biosciences). Cells were incubated with biotinylated LOX-1 (Hycult Biotechnology), CD8 PerCp, and CD3 APC (BD Biosciences) for 30 minutes at 4°C in the dark, washed and resuspended in staining buffer for secondary staining for 30 minutes at 4°C in the dark. Cell acquisition and data analysis were performed as described above.
Mice and diets
Male C57Bl/6 (atherosclerosis-susceptible strain) mice (Jackson Laboratory) were placed on either a control chow diet (National Institute of Health [NIH]-31 Mouse/Rat Diet 7013 containing 6% fat) or high-fat (atherogenic) diet (Teklad TD90221; Harlan Tekland; containing 1.25% cholesterol, 15.8% fat, and 0.5% cholate) starting at 1 month of age. This diet causes significant hypercholesterolemia [39; 40] and osteopenia [41] in C57Bl/6 mice. Spleen and serum were collected from control and high-fat diet fed animals at 11 months of age. T lymphocytes were isolated from splenocytes by immunomagnetic negative selection, using a mixture of mAbs of biotinylated CD14, CD16, CD19, CD56, CD36, CD123, CD235a and subsequently labeled with anti-biotin microbeads (Miltenyi Biotec). Purity of T lymphocytes (>98%) was confirmed by flow cytometry (Becton Dickson) using anti-CD3 antibody (BD Pharmingen).
Quantitative computed tomographic scanning
Femurs were carefully cleaned of soft tissue and fixed in 95% ethanol. Peripheral quantitative computed tomographic (pQCT) scans were performed on the left femur from each mouse. Scanning was done with a Stratec XCT-Research M (Norland Medical Systems) and associated software (version 5.4; Stratec Medizintechnik), and Bone Mineral Content (BMC) analysis was performed as previously described [42]. The mean of 3 evenly-spaced slices located in the mid-diaphysis was selected for data analysis.
Pathway inhibitors
The following pathway inhibitors were used: Akt inhibitors (124005 or 124009 – Calbiochem; both at 25 uM), MAPK inhibitor (PD98059 – Calbiochem at 10 uM), NFκB inhibitors (sc3060 – Santa Cruz Biotechnology at 75 ug/ml (inhibitor 1) or Bay 11–7082 – BioMol at 2 uM (inhibitor 2)), PKA inhibitor (H89 – Calbiochem at 5 uM), PKC inhibitor (RO-31-7549 – Calbiochem at 1 uM). All inhibitors were dissolved in DMSO, according to manufacturer’s instruction, except for inhibitor 1 (sc-3060), which was dissolved in 1X PBS supplemented with 0.2% BSA and 0.1% NaN3. Prior to treatment with oxidized lipids or control buffer, T lymphocytes, at a concentration of 1 × 106/ml, were pre-incubated with inhibitors or appropriate vehicle for 2 hours at 37°C.
NFκB activity assay
Purified T lymphocytes were incubated with 25ug/ml MM-LDL for 1 hour or up to 2 hours, then harvested, and the pellet washed with ice-cold PBS/Phosphatase inhibitors. Nuclear extraction and analysis were performed according to manufacturer's instructions (Active Motif). The nuclear fraction was analyzed using a TransAM NFκB family transcription factor assay kit. Briefly, in a 96-well plate, the active form of NF-κB in the nuclear extract binds specifically to the oligonucleotide containing the NFκB consensus binding site (GGGACTTTCC) that has been immobilized on the plate. To monitor the specificity of the assay, the wild-type consensus oligonucleotide competitor for NFκB binding is added to the well prior to addition of cell extract. Activated NFκB was detected by the addition of primary antibody for the p65 subunit, and measurement was achieved with a HRP-conjugated secondary antibody, providing a colorimetric readout at 450 nm with a reference wavelength of 655 nm.
Quantitative real-time PCR (QT-PCR) for RANKL expression
Purified T lymphocytes were incubated with 25 ug/ml of nLDL or MM-LDL, or 25 uM iso-PGE2 for 24 hours. Total RNA was extracted using RNeasy Mini Kit (Qiagen), and RNA concentrations were determined using the Quant-iT Ribogreen RNA Assay Kit (Molecular Probes). Template cDNAs were synthesized with the iScript cDNA synthesis kit (Bio-Rad) using 500 ng of RNA. The QT-PCR assays were performed using the iQ SYBR Green SuperMix and IQCycler (Bio-Rad). As an internal control, QT-PCR for the housekeeping gene, GAPDH, was performed. The sequences of the primers were designed with the aid from the Beacon software. hRANKL – 3’- TATCGTTGGATCACAGCACATCAGAG; 5’- GAGGACAGACTCACTTTATGGGAACC. hOPG – 3’- TGTGAGGAGGCATTCTTCAGGTTTG; 5’- TCTCTACACTCTCTGCGTTTACTTTGG. hLOX- 1 – 3’- TGGGAAAAGAGCCAAGAGAA; 5’- TAAGTGGGGCATCAAAGGAG. hGAPDH – 3’- CCTCAAGATCATCAGCAATGCCTCCT; 5’-GGTCATGAGTCCTTCCACGATACCAA. mRANKL – 3’- GCCTCCCGCTCCATGTTCC; 5’- TGAGTGCTGTCTTCTGATATTCTGTTAG. mGAPDH – 3’- ATTGTCAGCAATGCATCCTG; 5’- ATGGACTGTGGTCATGAGCC. Samples were run in triplicate in a 96-well plate using the settings of 95°C for 15 seconds, 61°C for 30 seconds and 72°C for 30 seconds (single fluorescence measurement).
Statistical Analysis
Data in the graphs indicate the mean ± S.D. calculated from independent experiments. The Wilcoxon rank sum test, a non-parametric test that is powerful in analyzing small sample sizes [43], was used to compare groups. Statistical significance was defined as p ≤ 0.05.
RESULTS
RANKL production is induced in human T lymphocytes by oxidized lipids
Consistent with previous reports [44], we observed that T lymphocytes stimulated with CD2/CD3/CD28 antibodies produce high levels of RANKL, which can be detected by ELISA (data not shown). To address the potential effect of oxidized lipids on T lymphocyte RANKL production, we selected MM-LDL for these experiments since it is known to be highly inflammatory, yet does not induce the cytotoxicity associated with highly oxidized LDL. Preliminary titration experiments were performed to identify the optimum concentration of MM-LDL required to induce maximum RANKL production without compromising cell viability. Highly purified human T lymphocytes were exposed to various concentrations of MM-LDL (0, 1, 10, 25, 50, 100 ug/ml) for 72 hours. As shown in Figure 1A, MM-LDL concentrations up to 25 ug/ml had no effect on cell viability, whereas the higher concentrations were toxic, as determined by trypan blue dye exclusion. Flow cytometric Annexin V and PI staining confirmed that exposure of T lymphocytes to 25 ug/ml of MM-LDL did not cause significant cell death (data not shown). Thus, although 50 ug/ml MM-LDL induced the highest level of RANKL production, due to the dramatic loss in cell viability at this concentration, all subsequent experiments were performed using 25 ug/ml (Fig 1B). Nevertheless, to further control for any minor differences in cell viability over the 72-hour incubation period, RANKL production by T lymphocytes was also normalized based on the production per million viable cells; these values were similar to those displayed in Figure 1C, 1D, 1E, 1F, Figure 2A and 2B below.
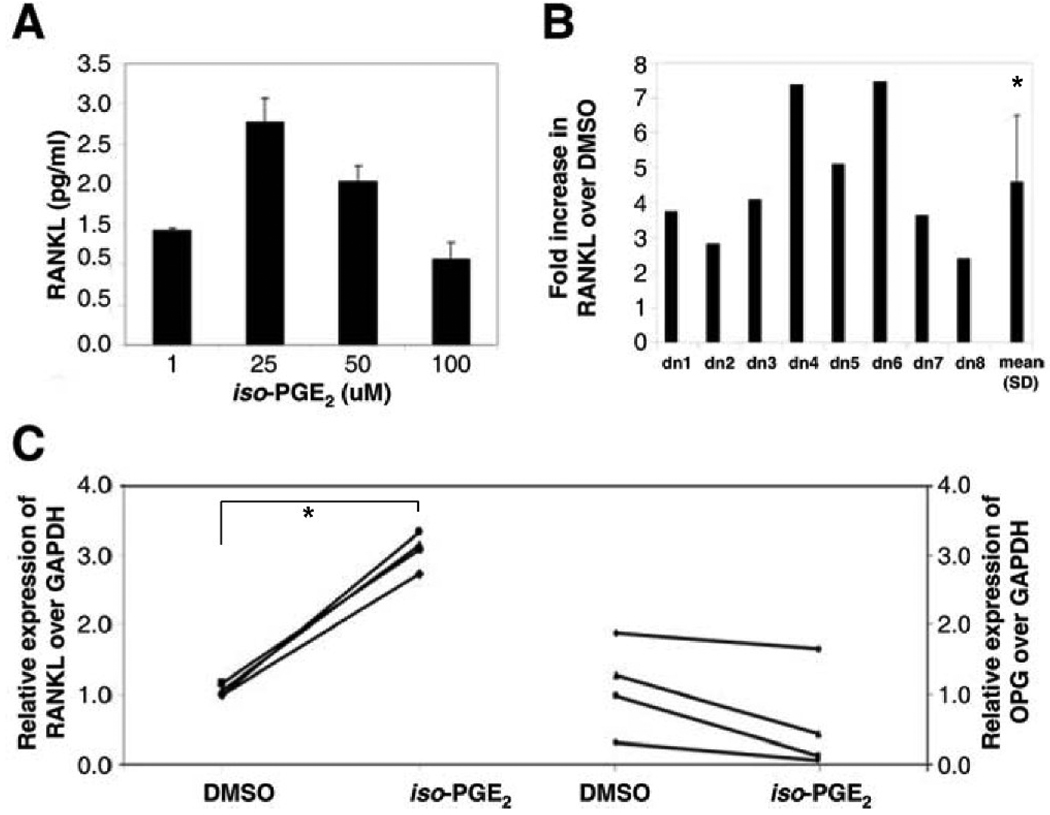
Figure 1. RANKL production by T lymphocytes exposed to oxidized lipids.
A. Unstimulated T lymphocytes were plated in culture medium with various concentrations of MM-LDL (0, 1, 10, 25, 50, 100ug/ml), and incubated for 72 hours. Cell viability was determined by counting the number of live cells under light microscope analysis and trypan blue exclusion (n = 8). B. Measurement of RANKL production by ELISA of unstimulated T lymphocytes exposed to various concentrations of MM-LDL (0, 1, 25, 50, 100ug/ml) (n = 4). Error bars indicate SD. C. T lymphocytes were stimulated with CD2/CD3/CD28 antibody-coated beads in the presence of 25 ug/ml of either nLDL or MM-LDL, and after 72 hours, RANKL (pg/ml) in the culture supernatant was evaluated by ELISA. Representative ELISA results from one donor. D. Fold-increase (compared to buffer control) of RANKL production by activated T lymphocytes treated with oxidized lipids (n = 18). Error bars indicate SD. E. Representative ELISA results for RANKL (pg/ml) production by unstimulated T lymphocytes cultured for 72 hours in the presence of 25 ug/ml of either nLDL or MM-LDL. F. Fold-increase (compared to buffer control) in RANKL production by unstimulated T lymphocytes treated with oxidized lipids (n = 15). Error bars indicate SD. G. Relative expression (compared to GAPDH) of RANKL (left) and OPG (right) transcript in unstimulated T lymphocytes treated with 25 ug/ml nLDL or MM-LDL for 24 hours, as detected by QT- PCR (n = 4). Error bars indicate SD. H. Relative expression (compared to GAPDH) of RANKL transcript in T lymphocytes from mice fed either chow (n = 4) or high fat diet (n = 4) for 11 months as detected by QT- PCR. Error bars indicate SD. I. Serum levels of RANKL (pg/ml), detected by ELISA from mice fed either chow (n = 4) or high fat (n = 4) for 11 months. Error bars indicate SD. * – p ≤ 0.05. ** – p ≤ 0.01.
Figure 2. iso-PGE2 induces RANKL production by unstimulated T lymphocytes.
A. T lymphocytes were exposed to various concentrations of iso-PGE2 (0, 10, 25, 50 uM) and incubated for 72 hours. RANKL production in culture supernatant was measured by ELISA. Graph represents fold increase over DMSO control (n = 3). Error bars indicate SD. B. T lymphocytes were cultured with 25 uM iso-PGE2 for 72 hours and RANKL measurement in culture supernatant was evaluated by ELISA. Graph represents fold-increase over DMSO control (n = 8). Error bar indicate SD. C. Relative expression (compared to GAPDH) of RANKL (left) and OPG (right) transcript in unstimulated T lymphocytes treated with 25 uM iso-PGE2 for 24 hours, as detected by QT- PCR (n = 4). Error bar indicate SD. * – p ≤ 0.01.
The first set of experiments evaluated whether MM-LDL had an additive effect on the already robust levels of activation-induced T lymphocyte RANKL production. We observed that the concentration of RANKL in the culture supernatants of purified T lymphocytes stimulated with CD2/CD3/CD28 antibodies was significantly increased in the presence of MM-LDL, as compared to buffer or native LDL (nLDL). Figure 1C shows representative ELISA data for one donor, and in Figure 1D, the results of experiments on purified T lymphocytes from 18 individual donors are presented. Unexpectedly, even in the absence of an activation stimulus, exposure of T lymphocytes to MM-LDL resulted in a significant increase in RANKL production (Fig 1E). Indeed, this pattern was seen in T lymphocytes from all 15 healthy individuals tested, with fold-increases (over buffer alone) ranging from 4 to 55, and a mean fold-increase of 16 (Fig. 1F). The effect of MM-LDL on unstimulated T lymphocyte RANKL production was significantly greater than that of nLDL, which had only a modest effect (Fig 1F). Flow cytometry evaluation of the CD38, CD25 and HLA-DR activation markers confirmed that the RANKL production by unstimulated T lymphocytes was not due to possible MM-LDL induced activation induced (data not shown). QT-PCR experiments showed that the increased RANKL protein induced in unstimulated T lymphocytes exposed to MM-LDL was associated with a significant increase in RANKL mRNA (Fig 1G). Osteoprotegerin (OPG) is a natural decoy receptor that blocks RANKL from binding to its receptor (RANK) and the ratio of RANKL to OPG is considered a key parameter in regulating bone metabolism. Unstimulated T lymphocytes exposed to MM-LDL had lower OPG expression compared to nLDL (Fig 1G), and the RANKL: OPG ratio for nLDL and MM-LDL is 0.9 and 3.4, respectively. To determine whether MM-LDL exposure induced unstimulated T lymphocytes to produce osteoclastogenic factors in addition to RANKL, ELISA assays were performed. These assays, which detect concentrations as low as 0.7 pg/ml, 1.6 pg/ml, and 4.6 pg/ml of IL-6, TNFα, and TGFβ, respectively, did not show any increases in these cytokines (data not shown).
In parallel with our in vitro experiments on human T lymphocytes, we evaluated an in vivo model of bone loss induced in C57BL/6 mice that become hyperlipidemic when treated with a high-fat diet [41]. Consistent with our earlier studies [41], pQCT analysis of femoral bones from high-fat fed mice showed a significant reduction in bone mineral content compared to their chow-fed counterparts (Table 1). Importantly, T lymphocytes from these mice, tested immediately ex vivo, in the absence of any stimulation, expressed RANKL mRNA that was significantly greater than that of T lymphocytes from the chow-fed mice (Fig 1H). The hyperlipidemic mice also had significant increases in serum levels of RANKL protein (Fig 1I). Overall, the in vitro data on human T lymphocytes, and the murine in vivo model of hyperlipidemia-induced bone loss indicate that exposure to oxidized lipids enhances T lymphocyte RANKL production.
Table 1.
Bone mineral content (BMC) of femur from C57Bl/6 mice after 11 months on a control chow or high fat diet
| Slice | Chow | BMC (mg) | Mean ± SD | High Fat | BMC (mg) | Mean ± SD | Percent change |
p-value |
|---|---|---|---|---|---|---|---|---|
| 6 | 1-Chow | 1.33 | 5-High Fat | 1.09 | ||||
| 6 | 2-Chow | 1.05 | 6-High Fat | 0.86 | ||||
| 6 | 3-Chow | 1.42 | 7-High Fat | 0.98 | ||||
| 6 | 4-Chow | 1.24 | 1.3 ± 0.16 | 8-High Fat | 0.99 | 1.0 ± 0.09 | 22.2 | 0.03 |
| 7 | 1-Chow | 1.48 | 5-High Fat | 1.21 | ||||
| 7 | 2-Chow | 1.24 | 6-High Fat | 0.97 | ||||
| 7 | 3-Chow | 1.61 | 7-High Fat | 1.00 | ||||
| 7 | 4-Chow | 1.32 | 1.4 ± 0.17 | 8-High Fat | 1.10 | 1.1 ± 0.11 | 23.7 | 0.02 |
| 8 | 1-Chow | 1.75 | 5-High Fat | 1.47 | ||||
| 8 | 2-Chow | 1.42 | 6-High Fat | 1.13 | ||||
| 8 | 3-Chow | 1.82 | 7-High Fat | 1.25 | ||||
| 8 | 4-Chow | 1.54 | 1.6 ± 0.19 | 8-High Fat | 1.36 | 1.3 ± 0.15 | 20.2 | 0.03 |
iso-PGE2 also induces RANKL production by unstimulated humanT lymphocytes
The prostaglandin-like isoprostane, 8-iso-prostaglandin E2 (iso-PGE2), is produced in vivo as one of the by-products of free radical-catalyzed lipid peroxidation, and may contribute to oxidative injury [45]. Previous studies have shown that oxidized lipids, including iso-PGE2, inhibit differentiation of preosteoblasts [31; 32; 46; 47]. Titration experiments on unstimulated T lymphocytes showed the maximum RANKL production was induced in the presence of 25 uM iso-PGE2 (Fig 2A). We found that unstimulated T lymphocytes exposed to iso-PGE2 produced a mean increase in RANKL protein that was 4-fold greater than that of control cultures exposed to the DMSO diluent (Fig 2B). QT-PCR experiments showed that the increased RANKL protein induced in unstimulated T lymphocytes exposed to iso-PGE2 was preceded by increased RANKL mRNA (Fig 2C). We also found that T lymphocytes exposed to iso-PGE2 had lower OPG expression as compared to DMSO, with RANKL:OPG ratios of 0.9 and 5.3 for DMSO and iso-PGE2, respectively. Ox-PAPC (oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine), a biologically active component of MM-LDL, which is known to have pleiotropic effects on human endothelial cells [46] and also affects T lymphocyte activation [48], was found to be toxic to unstimulated human T lymphocytes, even at concentrations as low as 0.5 ug/ml (data not shown).
MM-LDL induction of T lymphocyte RANKL requires NFκB signaling
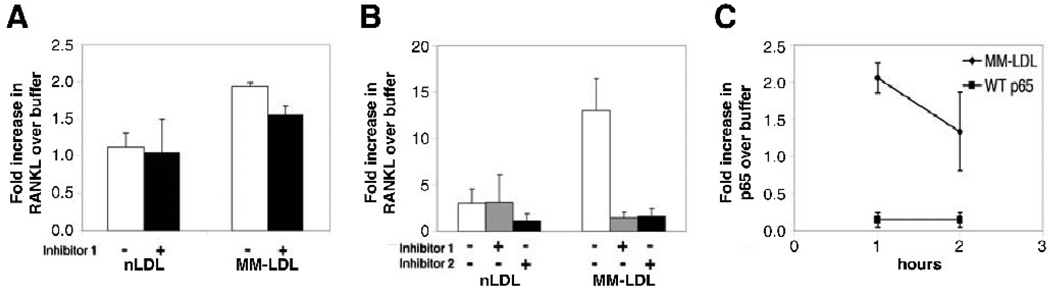
To elucidate the mechanism involved in the oxidized lipid-induced RANKL production in T lymphocytes, we tested a panel of pathway inhibitors, using concentrations that did not affect cell viability. We first investigated the NFκB pathway, based on previous reports showing that this pathway is central to the maturation and activation of osteoclast precursor cells induced by RANK/RANKL binding [44]. Furthermore, the biological activity of MM-LDL and oxidized lipids in several other cell types is mediated, at least in part, through the NFκB pathway [49]. Two different NFκB inhibitors were used, one that blocks the translocation of the active NFκB complex from the cytoplasm into the nucleus (inhibitor 1), and one that prevents the phosphorylation of IκBα (inhibitor 2). We found that the MM-LDL-induced RANKL production by the stimulated T lymphocytes was only modestly reduced when the NFκB pathway was inhibited. (Fig 3A). By contrast, in the unstimulated T lymphocytes, RANKL production induced by MM-LDL was nearly completely abolished in the presence of the NFκB inhibitors (Fig 3B). The NFκB pathway role in this effect was further confirmed using an assay that measures the presence of the NFκB p65 subunit in the nucleus, a downstream effect of NFκB activation. A one-hour incubation of unstimulated T lymphocytes with MM-LDL resulted in a 2-fold increase in nuclear p65; this activity decreased after 2 hours (Fig. 3C). No inhibition of the MM-LDL induction of RANKL production by unstimulated T lymphocytes was observed in the presence of specific MAPK (10 uM), PKA (5 uM) and PKC (1 uM) pathway inhibitors (data not shown). Thus, our data indicate that unstimulated T lymphocytes exposed to MM-LDL upregulate RANKL message and protein via an NFκB-mediated pathway. The very modest reduction in RANKL seen in the stimulated T lymphocytes exposed to MM-LDL (Fig 3A) suggests that RANKL induction in activated T lymphocytes may be mediated by both NFκB-dependent and -independent pathways. Other reports on oxidized lipid effects in several cell types suggest varying degrees of NFκB pathway involvement in response to oxidized lipids, ranging from total absence of NFκB signaling [46; 47] to inhibitory effects on the NFκB pathway [35; 50], depending on cell type, function being assessed and the type of oxidized lipids tested.
Figure 3. Mechanism of oxidized lipid-induced RANKL production in T lymphocytes.
A. Activated T lymphocytes: T lymphocytes were pre-incubated with NFκB inhibitor 1 for one hour prior to stimulation with CD2/CD3/CD28 antibody-coated beads in the presence of 25 ug/ml nLDL or MM-LDL. Cells were harvested after 72 hours of treatment, and RANKL in culture supernatant was evaluated by ELISA. Graph represents fold-increase over buffer (n = 4). Error bars indicate SD.; p = 0.052 for MM-LDL no inhibitor vs MM-LDL with inhibitor 1. B. Unstimulated T lymphocytes: T lymphocytes were preincubated with either NFκB inhibitor 1 or inhibitor 2 prior to treatment with oxidized lipids. RANKL (pg/ml) in culture supernatant was evaluated by ELISA after 72 hours of treatment. Data is presented as fold-increase over buffer (n = 4). Error bars indicate SD. p ≤ 0.05 for MM-LDL no inhibitor vs MM-LDL with inhibitor 1 and MM-LDL no inhibitor vs MM-LDL with inhibitor 2. C. Nuclear localization of activated NFκB: Unstimulated T lymphocytes were incubated with 25 ug/ml of MM-LDL for 1 or up to 2 hours, and nuclear extracts were tested in wells containing the NFκB consensus binding site. Assay specificity was determined by competition with wild-type NFκB oligonucleotide (WT p65). Data is presented from 4 independent experiments. Error bars indicate SD. p ≤ 0.05 for buffer vs. MM-LDL.
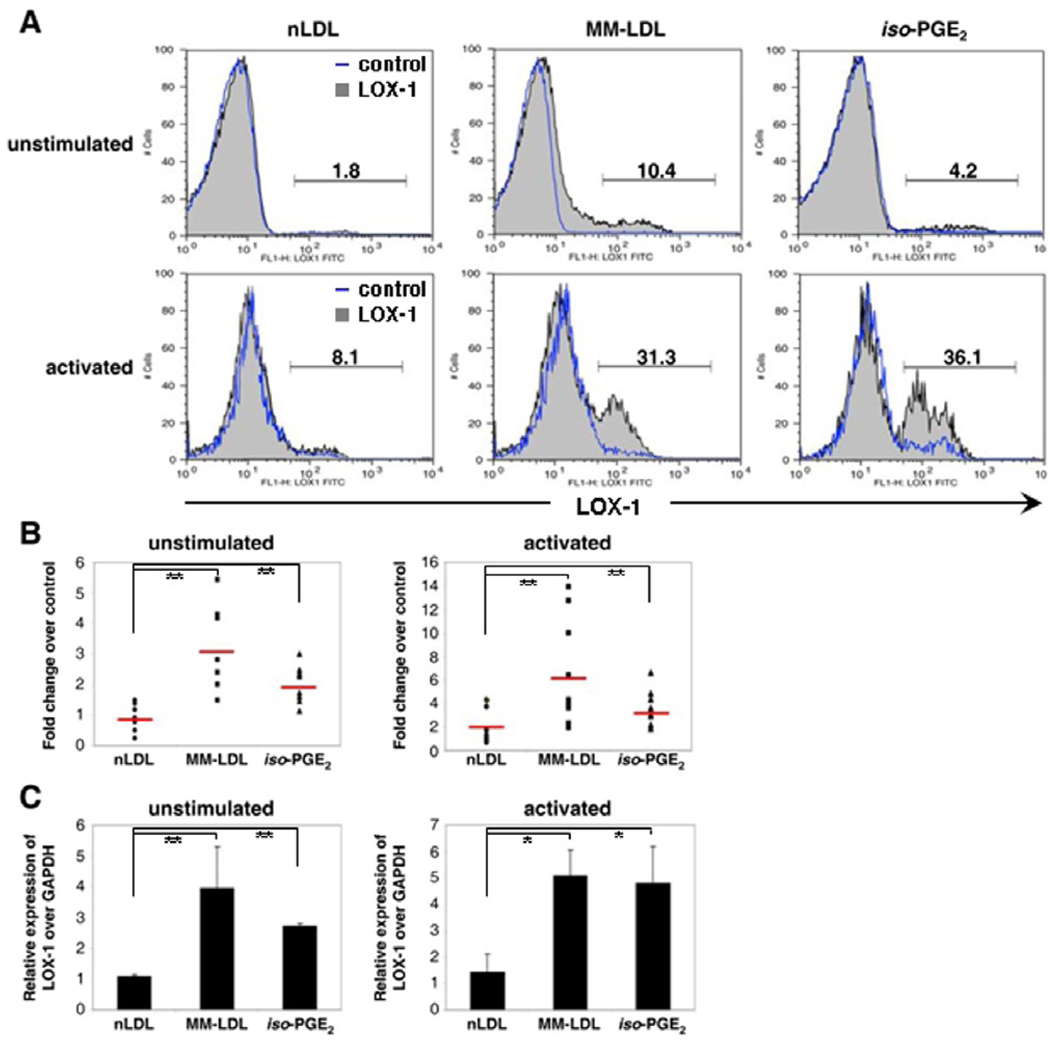
Oxidized lipids induce T lymphocyte LOX-1 expression
The lectin-like oxidized LDL receptor-1 (LOX-1) has been identified as the primary Ox-LDL receptor on endothelial cells [51] and is associated with cardiovascular disease [52; 53]. Macrophages also express LOX-1 when exposed to TNF-α [54]. A recent report documented LOX-1 mRNA in the Jurkat T cell tumor line following exposure to a component of oxidized lipids [55], suggesting that normal T lymphocytes, which infiltrate and accumulate in the vascular wall during the development of atherosclerosis [56], and are therefore exposed to Ox-LDL in vivo might also express LOX-1. Purified T lymphocytes were exposed to 25 ug/ml MM-LDL, 25 ug/ml nLDL, 25 uM iso-PGE2 or control buffers for 72 hours and then analyzed by flow cytometry. Figure 4A shows a flow cytometry histogram for a representative donor, and in Figure 4B, the results of experiments on purified T lymphocytes from 9 unstimulated and 10 stimulated samples. Our data show that the percentage of LOX-1 positive T lymphocytes treated with oxidized lipids ranged from 1 to 10% for unstimulated and 10 – 39% for activated cells (Figure 4B), and both CD4 and CD8 T lymphocytes were found to express LOX-1(data not shown). The effect of oxidized lipids on LOX-1 expression was significantly higher than that of nLDL on both unstimulated and PHA-activated T lymphocytes. These data show that unstimulated T lymphocytes exposed to either MM-LDL or iso-PGE2 had fold-changes in surface LOX-1 expression (over control buffers) of 6.1 and 3.5, respectively; the fold-changes for the activated T lymphocytes were 3.0 and 2.0, respectively. QT- PCR experiments showed that increased LOX-1 expression on cell surface of T lymphocytes exposed to oxidized lipids was associated with increased LOX-1 mRNA (Fig 4C).
Figure 4. LOX-1 expression on T lymphocytes treated with oxidized lipids.
A. T lymphocytes, unstimulated or activated with 6 ug/ml PHA, were exposed to 25 ug/ml nLDL, 25 ug/ml MM-LDL or 25 uM iso-PGE2 for 72 hours. Representative histogram analysis of LOX-1 expression from one donor. B. Fold increase (compared to buffer control) of LOX-1 expression by T lymphocytes, unstimulated (n = 9) and activated (n = 10), treated with oxidized lipids. Error bars indicate SD. C. Relative expression (compared to GAPDH) of LOX-1 transcripts in unstimulated (n = 5) and activated (n = 4) T lymphocytes treated with 25 ug/ml nLDL, 25 ug/ml MM-LDL or 25 uM iso-PGE2 for 24 hours as detected by QT- PCR. Error bars indicate SD. * – p ≤ 0.05. ** – p ≤ 0.01.
DISCUSSION
The present study provides the first demonstration that oxidized lipids can induce human T lymphocytes to secrete RANKL, a cytokine that is a key mediator of osteoclast differentiation and bone loss. We show that exposure of human T lymphocytes to either the lipoprotein oxidation product, MM-LDL, or to iso-PGE2, a by-product of the free radical-catalyzed peroxidation of essential fatty acids, significantly increases RANKL production. The effect is observed in unstimulated T lymphocytes tested immediately ex vivo, as well as in T lymphocytes stimulated via activatory antibodies. The MM-LDL induction of RANKL protein is preceded by upregulation of RANKL mRNA, and the effect on the unstimulated T lymphocytes is mediated through the NFκB pathway. Additionally, T lymphocytes exposed to oxidized lipids had higher RANKL:OPG ratios, which might further disrupt bone homeostasis in vivo. Our study also provides the first evidence that LOX-1, previously documented on endothelial cells and macrophages, is also expressed on primary T lymphocytes. Consistent with our in vitro observations on human T lymphocytes, splenic T lymphocytes from hyperlipidemic mice, which show both bone loss and increased levels of oxidized lipids [13; 57], also express significantly greater RANKL mRNA. Thus T lymphocytes may be one of the sources of the significantly higher serum levels of RANKL protein in these mice. The results of our study provide a novel pathway by which T lymphocytes may contribute to bone loss, and suggest that the well-established association between cardiovascular disease and bone loss [13; 16; 58; 59] may involve T lymphocytes, along with other known sources of RANKL.
Our results expand upon the growing repertoire of diverse biological effects mediated by oxidized lipids on cells of the immune system. For example, human peripheral blood T lymphocytes are strongly inhibited in anti-CD3/CD28-induced proliferation and de novo synthesis of activation markers by certain concentrations of Ox-PAPC, an oxidized phosopholipid [48], consistent with the proposed link between sterol metabolism and T cell proliferative responses to antigens [60]. Oxidized lipids have also been shown to negatively regulate dendritic cell maturation. Human dendritic cells that are pretreated with Ox-PAPC are inhibited in LPS-induced upregulation of a variety of surface markers, including the CD40 and CD80 costimulatory molecules, the ICAM-1 cell adhesion marker, and MHC Class I and II transplantation antigens [50]. Finally, in macrophages, MM-LDL is able to offset the apoptosis induction by more extensively oxidized LDL [61]. Thus, oxidized lipids have diverse and important regulatory functions within both the innate and adaptive immune systems.
LOX-1, the specific receptor for oxidized forms of lipids [62] has been studied in a variety of non-immune cell types, such as cells within the placenta [63], chondrocytes, [64] breast cancer cells [65] and endothelial cells, in which it not only mediates uptake of Ox-LDL, but also functions as a scavenger receptor that interacts with a variety of distinct ligands such as platelets, apoptotic cells, heat shock proteins, and phosphatidylserine [51; 54; 66]. To our knowledge, our study is the first to demonstrate that LOX-1 is induced on primary T lymphotyes. Interestingly, C-reactive protein (CRP), an acute phase serum marker that is a strong predictor of cardiovascular disease, hypertension and diabetes [67; 68] also binds to LOX-1. Ongoing studies in our laboratory are, therefore, examining the potential involvement of CRP in the MM-LDL induction of T lymphocyte-mediated bone loss.
Our study provides another example of the interplay of immune cells and oxidized lipids in a disease that has major implications for human health. This interaction has already been documented for atherosclerosis, in which one of the initiating events is the uptake of oxidized lipids by macrophages, leading to the formation of the foam cells that are involved in the actual plaque formation [69; 70; 71; 72]. Oxidized lipids may also stimulate autoreactive T lymphocytes, which could further contribute to atherosclerosis. Indeed, adoptive transfer of Ox-LDL-reactive CD4 T lymphocytes into mice that are genetically engineered to develop atherosclerosis leads to accelerated lesion progression [73]. Moreover, human CD8 T cells can mediate Ox-LDL-specific cytotoxicity, thus contributing to the inflammatory process associated with atherosclerosis [74]. A role for oxidized phospholipids has recently been documented in human leprosy, where host-derived oxidized phospholipids accumulate in macrophages within lepromatous lesions, a process that is strikingly similar to observations in atherosclerotic lesions [75]. Finally, recent research has also suggested an association between lipid levels and both telomerase activity and telomere length in PBMC [76; 77], findings which have important implications for immunosenescence. Thus, immune-mediated pathogenic changes in a variety of diseases have been documented to be modulated by oxidized lipids. Our ongoing in vitro studies on the effects of long-term exposure of T lymphocytes to oxidized lipids may identify additional effects that relate to age-related pathologies in addition to bone loss.
It has been well-established that chronic immune activation plays a key role in bone homeostasis. Indeed, skeletal changes are observed in a variety of clinical situations involving chronic, long-term immune activation. For example, reduced bone mass is being increasingly reported in HIV/AIDS, even in the absence of anti-retroviral drugs [78]. RANKL production by activated T lymphocytes has also been shown to play a central role in inflammatory bone loss, particularly in rheumatoid arthritis. The current study provides the first demonstration that not only activated, but also resting, unstimulated T lymphocytes are potential sources of RANKL, if they are exposed to oxidized lipids. In summary, our results underscore the interaction between T lymphocytes and oxidized lipids in osteoporosis and osteopenia, and provide a possible pathway for the documented improved bone mass seen in patients who are treated with lipid-lowering medications [26; 28].
Acknowledgments
This study was supported by NIH AG023720, AG024134 and AI 056945 (RBE) as well as HL081202 (LLD) and by USPHS Fellowship F31AG023838 (LSG). Lipoprotein isolation and preparation of MMLDL was performed by the UCLA Atherosclerosis Unit Core Facility supported by NIH/NHLBI HL30568 (Dr. Alan Fogelman). We also thank Vicente Meliton for his technical support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22:465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 2.Kanis JA. Diagnosis of osteoporosis and assessment of fracture risk. Lancet. 2002;359:1929–1936. doi: 10.1016/S0140-6736(02)08761-5. [DOI] [PubMed] [Google Scholar]
- 3.Walsh MC, Kim N, Kadono Y, Rho J, Lee SY, Lorenzo J, Choi Y. Osteoimmunology: interplay between the immune system and bone metabolism. Annu Rev Immunol. 2006;24:33–63. doi: 10.1146/annurev.immunol.24.021605.090646. [DOI] [PubMed] [Google Scholar]
- 4.Arron JR, Choi Y. Bone versus immune system. Nature. 2000;408:535–536. doi: 10.1038/35046196. [DOI] [PubMed] [Google Scholar]
- 5.Wong BR, Rho J, Arron J, Robinson E, Orlinick J, Chao M, Kalachikov S, Cayani E, Bartlett FS, 3rd, Frankel WN, Lee SY, Choi Y. TRANCE is a novel ligand of the tumor necrosis factor receptor family that activates c-Jun N-terminal kinase in T cells. J Biol Chem. 1997;272:25190–25194. doi: 10.1074/jbc.272.40.25190. [DOI] [PubMed] [Google Scholar]
- 6.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 7.Wong BR, Josien R, Choi Y. TRANCE is a TNF family member that regulates dendritic cell and osteoclast function. J Leukoc Biol. 1999;65:715–724. doi: 10.1002/jlb.65.6.715. [DOI] [PubMed] [Google Scholar]
- 8.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, Capparelli C, Li J, Elliott R, McCabe S, Wong T, Campagnuolo G, Moran E, Bogoch ER, Van G, Nguyen LT, Ohashi PS, Lacey DL, Fish E, Boyle WJ, Penninger JM. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999;402:304–309. doi: 10.1038/46303. [DOI] [PubMed] [Google Scholar]
- 10.Vanderborght A, Linsen L, Thewissen M, Geusens P, Raus J, Stinissen P. Osteoprotegerin and receptor activator of nuclear factor-kappaB ligand mRNA expression in patients with rheumatoid arthritis and healthy controls. J Rheumatol. 2004;31:1483–1490. [PubMed] [Google Scholar]
- 11.Liu D, Xu J, Figliomeni L, Huang L, Pavlos N, Rogers M, Tan A, Price P, Zheng M. Expression of RANKL and OPG mRNA in periodontal disease: possible involvement in bone destruction. Int J Mol Med. 2003;11:17–21. doi: 10.3892/ijmm.11.1.17. [DOI] [PubMed] [Google Scholar]
- 12.Baker PJ, Howe L, Garneau J, Roopenian DC. T cell knockout mice have diminished alveolar bone loss after oral infection with Porphyromonas gingivalis. FEMS Immunol Med Microbiol. 2002;34:45–50. doi: 10.1111/j.1574-695X.2002.tb00601.x. [DOI] [PubMed] [Google Scholar]
- 13.Parhami F. Possible role of oxidized lipids in osteoporosis: could hyperlipidemia be a risk factor? Prostaglandins Leukot Essent Fatty Acids. 2003;68:373–378. doi: 10.1016/s0952-3278(03)00061-9. [DOI] [PubMed] [Google Scholar]
- 14.Rajamannan NM. Low-density lipoprotein and aortic stenosis. Heart. 2008;94:1111–1112. doi: 10.1136/hrt.2007.130971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rajamannan NM, Subramaniam M, Rickard D. Human aortic valve calcification is associated with osteoblast phenotype. Circulation. 2003;107:2181–2184. doi: 10.1161/01.CIR.0000070591.21548.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Banks LM, Lees B, MacSweeney JE, Stevenson JC. Effect of degenerative spinal and aortic calcification on bone density measurements in post-menopausal women: links between osteoporosis and cardiovascular disease? Eur J Clin Invest. 1994;24:813–817. doi: 10.1111/j.1365-2362.1994.tb02024.x. [DOI] [PubMed] [Google Scholar]
- 17.Tanko LB, Bagger YZ, Christiansen C. Low bone mineral density in the hip as a marker of advanced atherosclerosis in elderly women. Calcif Tissue Int. 2003;73:15–20. doi: 10.1007/s00223-002-2070-x. [DOI] [PubMed] [Google Scholar]
- 18.Broulik PD, Kapitola J. Interrelations between body weight, cigarette smoking and spine mineral density in osteoporotic Czech women. Endocr Regul. 1993;27:57–60. [PubMed] [Google Scholar]
- 19.Jie KG, Bots ML, Vermeer C, Witteman JC, Grobbee DE. Vitamin K status and bone mass in women with and without aortic atherosclerosis: a population-based study. Calcif Tissue Int. 1996;59:352–356. doi: 10.1007/s002239900139. [DOI] [PubMed] [Google Scholar]
- 20.Laroche M, Pouilles JM, Ribot C, Bendayan P, Bernard J, Boccalon H, Mazieres B. Comparison of the bone mineral content of the lower limbs in men with ischaemic atherosclerotic disease. Clin Rheumatol. 1994;13:611–614. doi: 10.1007/BF02243003. [DOI] [PubMed] [Google Scholar]
- 21.Ouchi Y, Akishita M, de Souza AC, Nakamura T, Orimo H. Age-related loss of bone mass and aortic/aortic valve calcification--reevaluation of recommended dietary allowance of calcium in the elderly. Ann N Y Acad Sci. 1993;676:297–307. doi: 10.1111/j.1749-6632.1993.tb38743.x. [DOI] [PubMed] [Google Scholar]
- 22.Tintut Y, Morony S, Demer LL. Hyperlipidemia promotes osteoclastic potential of bone marrow cells ex vivo. Arterioscler Thromb Vasc Biol. 2004;24:e6–e10. doi: 10.1161/01.ATV.0000112023.62695.7f. [DOI] [PubMed] [Google Scholar]
- 23.Barengolts EI, Berman M, Kukreja SC, Kouznetsova T, Lin C, Chomka EV. Osteoporosis and coronary atherosclerosis in asymptomatic postmenopausal women. Calcif Tissue Int. 1998;62:209–213. doi: 10.1007/s002239900419. [DOI] [PubMed] [Google Scholar]
- 24.Browner WS, Seeley DG, Vogt TM, Cummings SR Study of Osteoporotic Fractures Research Group. Non-trauma mortality in elderly women with low bone mineral density. Lancet. 1991;338:355–358. doi: 10.1016/0140-6736(91)90489-c. [DOI] [PubMed] [Google Scholar]
- 25.Uyama O, Yoshimoto Y, Yamamoto Y, Kawai A. Bone changes and carotid atherosclerosis in postmenopausal women. Stroke. 1997;28:1730–1732. doi: 10.1161/01.str.28.9.1730. [DOI] [PubMed] [Google Scholar]
- 26.Edwards CJ, Hart DJ, Spector TD. Oral statins and increased bone-mineral density in postmenopausal women. Lancet. 2000;355:2218–2219. doi: 10.1016/s0140-6736(00)02408-9. [DOI] [PubMed] [Google Scholar]
- 27.Callister TQ, Raggi P, Cooil B, Lippolis NJ, Russo DJ. Effect of HMG-CoA reductase inhibitors on coronary artery disease as assessed by electron-beam computed tomography. N Engl J Med. 1998;339:1972–1978. doi: 10.1056/NEJM199812313392703. [DOI] [PubMed] [Google Scholar]
- 28.Meier CR, Schlienger RG, Kraenzlin ME, Schlegel B, Jick H. Statins and fracture risk. JAMA. 2001;286:669–670. doi: 10.1001/jama.286.6.669. [DOI] [PubMed] [Google Scholar]
- 29.Meier CR, Schlienger RG, Kraenzlin ME, Schlegel B, Jick H. HMG-CoA reductase inhibitors and the risk of fractures. JAMA. 2000;283:3205–3210. doi: 10.1001/jama.283.24.3205. [DOI] [PubMed] [Google Scholar]
- 30.Schlienger RG, Kraenzlin ME, Jick SS, Meier CR. Use of beta-blockers and risk of fractures. JAMA. 2004;292:1326–1332. doi: 10.1001/jama.292.11.1326. [DOI] [PubMed] [Google Scholar]
- 31.Parhami F, Morrow AD, Balucan J, Leitinger N, Watson AD, Tintut Y, Berliner JA, Demer LL. Lipid oxidation products have opposite effects on calcifying vascular cell and bone cell differentiation. A possible explanation for the paradox of arterial calcification in osteoporotic patients. Arterioscler Thromb Vasc Biol. 1997;17:680–687. doi: 10.1161/01.atv.17.4.680. [DOI] [PubMed] [Google Scholar]
- 32.Tintut Y, Parhami F, Tsingotjidou A, Tetradis S, Territo M, Demer LL. 8-Isoprostaglandin E2 enhances receptor-activated NFkappa B ligand (RANKL)-dependent osteoclastic potential of marrow hematopoietic precursors via the cAMP pathway. J Biol Chem. 2002;277:14221–14226. doi: 10.1074/jbc.M111551200. [DOI] [PubMed] [Google Scholar]
- 33.Bustamante M, Diaz F, Munoz M, Gross HJ, Rivas CI, Llancaqueo A, Nunez L, Campos L, Kirsten L, Grandon J, Gonzalez M, Barra V, Vera JC, Bachem MG. Oxidized low density lipoproteins induce apoptosis in human lymphocytes: involvement of mitogen-activated protein kinases. Cell Mol Biol (Noisy-le-grand) 2007;53 Suppl:OL954–OL964. [PubMed] [Google Scholar]
- 34.Caspar-Bauguil S, Tkaczuk J, Haure MJ, Durand M, Alcouffe J, Thomsen M, Salvayre R, Benoist H. Mildly oxidized low-density lipoproteins decrease early production of interleukin 2 and nuclear factor kappaB binding to DNA in activated T-lymphocytes. Biochem J. 1999;337(Pt 2):269–274. [PMC free article] [PubMed] [Google Scholar]
- 35.Shamshiev AT, Ampenberger F, Ernst B, Rohrer L, Marsland BJ, Kopf M. Dyslipidemia inhibits Toll-like receptor-induced activation of CD8alpha-negative dendritic cells and protective Th1 type immunity. J Exp Med. 2007;204:441–452. doi: 10.1084/jem.20061737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trueta J, Buhr AJ. The Vascular Contribution to Osteogenesis. V. The Vasculature Supplying the Epiphysial Cartilage in Rachitic Rats. J Bone Joint Surg Br. 1963;45:572–581. [PubMed] [Google Scholar]
- 37.Havel R, Eder H, Bradgon J. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955;34:1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 39.Paigen B, Mitchell D, Reue K, Morrow A, Lusis AJ, LeBoeuf RC. Ath-1, a gene determining atherosclerosis susceptibility and high density lipoprotein levels in mice. Proc Natl Acad Sci U S A. 1987;84:3763–3767. doi: 10.1073/pnas.84.11.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paigen B, Morrow A, Brandon C, Mitchell D, Holmes P. Variation in susceptibility to atherosclerosis among inbred strains of mice. Atherosclerosis. 1985;57:65–73. doi: 10.1016/0021-9150(85)90138-8. [DOI] [PubMed] [Google Scholar]
- 41.Parhami F, Tintut Y, Beamer WG, Gharavi N, Goodman W, Demer LL. Atherogenic high-fat diet reduces bone mineralization in mice. J Bone Miner Res. 2001;16:182–188. doi: 10.1359/jbmr.2001.16.1.182. [DOI] [PubMed] [Google Scholar]
- 42.Huang MS, Lu J, Ivanov Y, Sage AP, Tseng W, Demer LL, Tintut Y. Hyperlipidemia impairs osteoanabolic effects of PTH. J Bone Miner Res. 2008;23:1672–1679. doi: 10.1359/JBMR.080513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kitchen CM. Nonparametric vs parametric tests of location in biomedical research. Am J Ophthalmol. 2009;147:571–572. doi: 10.1016/j.ajo.2008.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Theill LE, Boyle WJ, Penninger JM. RANK-L and RANK: T cells, bone loss, and mammalian evolution. Annu Rev Immunol. 2002;20:795–823. doi: 10.1146/annurev.immunol.20.100301.064753. [DOI] [PubMed] [Google Scholar]
- 45.Morrow JD, Roberts LJ., 2nd The isoprostanes. Current knowledge and directions for future research. Biochem Pharmacol. 1996;51:1–9. doi: 10.1016/0006-2952(95)02072-1. [DOI] [PubMed] [Google Scholar]
- 46.Bochkov VN, Mechtcheriakova D, Lucerna M, Huber J, Malli R, Graier WF, Hofer E, Binder BR, Leitinger N. Oxidized phospholipids stimulate tissue factor expression in human endothelial cells via activation of ERK/EGR-1 and Ca(++)/NFAT. Blood. 2002;99:199–206. doi: 10.1182/blood.v99.1.199. [DOI] [PubMed] [Google Scholar]
- 47.Huber J, Bochkov VN, Binder BR, Leitinger N. The isoprostane 8-iso-PGE2 stimulates endothelial cells to bind monocytes via cyclic AMP- and p38 MAP kinase-dependent signaling pathways. Antioxid Redox Signal. 2003;5:163–169. doi: 10.1089/152308603764816523. [DOI] [PubMed] [Google Scholar]
- 48.Seyerl M, Bluml S, Kirchberger S, Bochkov VN, Oskolkova O, Majdic O, Stockl J. Oxidized phospholipids induce anergy in human peripheral blood T cells. Eur J Immunol. 2008;38:778–787. doi: 10.1002/eji.200737619. [DOI] [PubMed] [Google Scholar]
- 49.Foncea R, Carvajal C, Almarza C, Leighton F. Endothelial cell oxidative stress and signal transduction. Biol Res. 2000;33:89–96. doi: 10.4067/s0716-97602000000200008. [DOI] [PubMed] [Google Scholar]
- 50.Bluml S, Kirchberger S, Bochkov VN, Kronke G, Stuhlmeier K, Majdic O, Zlabinger GJ, Knapp W, Binder BR, Stockl J, Leitinger N. Oxidized phospholipids negatively regulate dendritic cell maturation induced by TLRs and CD40. J Immunol. 2005;175:501–508. doi: 10.4049/jimmunol.175.1.501. [DOI] [PubMed] [Google Scholar]
- 51.Sawamura T, Kume N, Aoyama T, Moriwaki H, Hoshikawa H, Aiba Y, Tanaka T, Miwa S, Katsura Y, Kita T, Masaki T. An endothelial receptor for oxidized low-density lipoprotein. Nature. 1997;386:73–77. doi: 10.1038/386073a0. [DOI] [PubMed] [Google Scholar]
- 52.Ohmori R, Momiyama Y, Nagano M, Taniguchi H, Egashira T, Yonemura A, Nakamura H, Kondo K, Ohsuzu F. An oxidized low-density lipoprotein receptor gene variant is inversely associated with the severity of coronary artery disease. Clin Cardiol. 2004;27:641–644. doi: 10.1002/clc.4960271112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tatsuguchi M, Furutani M, Hinagata J, Tanaka T, Furutani Y, Imamura S, Kawana M, Masaki T, Kasanuki H, Sawamura T, Matsuoka R. Oxidized LDL receptor gene (OLR1) is associated with the risk of myocardial infarction. Biochem Biophys Res Commun. 2003;303:247–250. doi: 10.1016/s0006-291x(03)00326-7. [DOI] [PubMed] [Google Scholar]
- 54.Moriwaki H, Kume N, Kataoka H, Murase T, Nishi E, Sawamura T, Masaki T, Kita T. Expression of lectin-like oxidized low density lipoprotein receptor-1 in human and murine macrophages: upregulated expression by TNF-alpha. FEBS Lett. 1998;440:29–32. doi: 10.1016/s0014-5793(98)01414-8. [DOI] [PubMed] [Google Scholar]
- 55.Hara Y, Kusumi Y, Mitsumata M, Li XK, Fujino M. Lysophosphatidylcholine upregulates LOX-1, chemokine receptors, and activation-related transcription factors in human T-cell line Jurkat. J Thromb Thrombolysis. 2007 doi: 10.1007/s11239-007-0158-x. [DOI] [PubMed] [Google Scholar]
- 56.Hansson GK, Libby P, Schonbeck U, Yan ZQ. Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ Res. 2002;91:281–291. doi: 10.1161/01.res.0000029784.15893.10. [DOI] [PubMed] [Google Scholar]
- 57.Parhami F, Jackson S, Tintut Y, Le V, Balucan J, Territo M, Demer L. Atherogenic diet and minimally oxidized low density lipoprotein inhibit osteogenic and promote adipogenic differentiation of marrow stromal cells. J Bone Miner Res. 1999;14:2067–2078. doi: 10.1359/jbmr.1999.14.12.2067. [DOI] [PubMed] [Google Scholar]
- 58.von der Recke P, Hansen MA, Hassager C. The association between low bone mass at the menopause and cardiovascular mortality. Am J Med. 1999;106:273–278. doi: 10.1016/s0002-9343(99)00028-5. [DOI] [PubMed] [Google Scholar]
- 59.Towler DA. The osteogenic-angiogenic interface: novel insights into the biology of bone formation and fracture repair. Curr Osteoporos Rep. 2008;6:67–71. doi: 10.1007/s11914-008-0012-x. [DOI] [PubMed] [Google Scholar]
- 60.Bensinger SJ, Bradley MN, Joseph SB, Zelcer N, Janssen EM, Hausner MA, Shih R, Parks JS, Edwards PA, Jamieson BD, Tontonoz P. LXR signaling couples sterol metabolism to proliferation in the acquired immune response. Cell. 2008;134:97–111. doi: 10.1016/j.cell.2008.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boullier A, Li Y, Quehenberger O, Palinski W, Tabas I, Witztum JL, Miller YI. Minimally oxidized LDL offsets the apoptotic effects of extensively oxidized LDL and free cholesterol in macrophages. Arterioscler Thromb Vasc Biol. 2006;26:1169–1176. doi: 10.1161/01.ATV.0000210279.97308.9a. [DOI] [PubMed] [Google Scholar]
- 62.Kuge Y, Kume N, Ishino S, Takai N, Ogawa Y, Mukai T, Minami M, Shiomi M, Saji H. Prominent lectin-like oxidized low density lipoprotein (LDL) receptor-1 (LOX-1) expression in atherosclerotic lesions is associated with tissue factor expression and apoptosis in hypercholesterolemic rabbits. Biol Pharm Bull. 2008;31:1475–1482. doi: 10.1248/bpb.31.1475. [DOI] [PubMed] [Google Scholar]
- 63.Satoh H, Kiyota E, Terasaki Y, Sawamura T, Takagi K, Mizuta H, Takeya M. Expression and localization of lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) in murine and human placentas. J Histochem Cytochem. 2008;56:773–784. doi: 10.1369/jhc.2008.950543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Simopoulou T, Malizos KN, Tsezou A. Lectin-like oxidized low density lipoprotein receptor 1 (LOX-1) expression in human articular chondrocytes. Clin Exp Rheumatol. 2007;25:605–612. [PubMed] [Google Scholar]
- 65.Liang M, Zhang P, Fu J. Up-regulation of LOX-1 expression by TNF-alpha promotes trans-endothelial migration of MDA-MB-231 breast cancer cells. Cancer Lett. 2007;258:31–37. doi: 10.1016/j.canlet.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 66.Chen XP, Zhang TT, Du GH. Lectin-like oxidized low-density lipoprotein receptor-1, a new promising target for the therapy of atherosclerosis? Cardiovasc Drug Rev. 2007;25:146–161. doi: 10.1111/j.1527-3466.2007.00009.x. [DOI] [PubMed] [Google Scholar]
- 67.Blake GJ, Rifai N, Buring JE, Ridker PM. Blood pressure, C-reactive protein, and risk of future cardiovascular events. Circulation. 2003;108:2993–2999. doi: 10.1161/01.CIR.0000104566.10178.AF. [DOI] [PubMed] [Google Scholar]
- 68.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 69.Kita T, Kume N, Minami M, Hayashida K, Murayama T, Sano H, Moriwaki H, Kataoka H, Nishi E, Horiuchi H, Arai H, Yokode M. Role of oxidized LDL in atherosclerosis. Ann N Y Acad Sci. 2001;947:199–205. doi: 10.1111/j.1749-6632.2001.tb03941.x. discussion 205–6. [DOI] [PubMed] [Google Scholar]
- 70.Steinberg D. Low density lipoprotein oxidation and its pathobiological significance. J Biol Chem. 1997;272:20963–20966. doi: 10.1074/jbc.272.34.20963. [DOI] [PubMed] [Google Scholar]
- 71.Yla-Herttuala S, Palinski W, Rosenfeld ME, Parthasarathy S, Carew TE, Butler S, Witztum JL, Steinberg D. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest. 1989;84:1086–1095. doi: 10.1172/JCI114271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Young IS, McEneny J. Lipoprotein oxidation and atherosclerosis. Biochem Soc Trans. 2001;29:358–362. doi: 10.1042/0300-5127:0290358. [DOI] [PubMed] [Google Scholar]
- 73.Zhou X, Robertson AK, Hjerpe C, Hansson GK. Adoptive transfer of CD4+ T cells reactive to modified low-density lipoprotein aggravates atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:864–870. doi: 10.1161/01.ATV.0000206122.61591.ff. [DOI] [PubMed] [Google Scholar]
- 74.Wu R, Giscombe R, Holm G, Lefvert AK. Induction of human cytotoxic T lymphocytes by oxidized low density lipoproteins. Scand J Immunol. 1996;43:381–384. doi: 10.1046/j.1365-3083.1996.d01-51.x. [DOI] [PubMed] [Google Scholar]
- 75.Cruz D, Watson AD, Miller CS, Montoya D, Ochoa MT, Sieling PA, Gutierrez MA, Navab M, Reddy ST, Witztum JL, Fogelman AM, Rea TH, Eisenberg D, Berliner J, Modlin RL. Host-derived oxidized phospholipids and HDL regulate innate immunity in human leprosy. J Clin Invest. 2008;118:2917–2928. doi: 10.1172/JCI34189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen W, Gardner JP, Kimura M, Brimacombe M, Cao X, Srinivasan SR, Berenson GS, Aviv A. Leukocyte telomere length is associated with HDL cholesterol levels: The Bogalusa heart study. Atherosclerosis. 2009 doi: 10.1016/j.atherosclerosis.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 77.Tsirpanlis G, Chatzipanagiotou S, Boufidou F, Kordinas V, Zoga M, Alevyzaki F, Stamatelou K, Frangou E, Savva L, Nicolaou C. Serum oxidized low-density lipoprotein is inversely correlated to telomerase activity in peripheral blood mononuclear cells of haemodialysis patients. Nephrology (Carlton) 2006;11:506–509. doi: 10.1111/j.1440-1797.2006.00697.x. [DOI] [PubMed] [Google Scholar]
- 78.Delpierre C, Bonnet E, Marion-Latard F, Aquilina C, Obadia M, Marchou B, Massip P, Perret B, Bernard J. Impact of HIV infection on total body composition in treatment-naive men evaluated by dual-energy X-ray absorptiometry comparison of 90 untreated HIV-infected men to 241 controls. J Clin Densitom. 2007;10:376–380. doi: 10.1016/j.jocd.2007.07.006. [DOI] [PubMed] [Google Scholar]