Abstract
A role for the Plasmodium falciparum cyclic GMP (cGMP)-dependent protein kinase (PfPKG) in gametogenesis in the malaria parasite was elucidated previously. In the present study we examined the role of PfPKG in the asexual blood-stage of the parasite life cycle, the stage that causes malaria pathology. A specific PKG inhibitor (compound 1, a trisubstituted pyrrole) prevented the progression of P. falciparum schizonts through to ring stages in erythrocyte invasion assays. Addition of compound 1 to ring-stage parasites allowed normal development up to 30 h postinvasion, and segmented schizonts were able to form. However, synchronized schizonts treated with compound 1 for ≥6 h became large and dysmorphic and were unable to rupture or liberate merozoites. To conclusively demonstrate that the effect of compound 1 on schizogony was due to its selective action on PfPKG, we utilized genetically manipulated P. falciparum parasites expressing a compound 1-insensitive PfPKG. The mutant parasites were able to complete schizogony in the presence of compound 1 but not in the presence of the broad-spectrum protein kinase inhibitor staurosporine. This shows that PfPKG is the primary target of compound 1 during schizogony and provides direct evidence of a role for PfPKG in this process. Discovery of essential roles for the P. falciparum PKG in both asexual and sexual development demonstrates that cGMP signaling is a key regulator of both of these crucial life cycle phases and defines this molecule as an exciting potential drug target for both therapeutic and transmission blocking action against malaria.
Cyclic GMP (cGMP)-dependent protein kinases (PKGs) are the major intracellular mediators of cGMP signal transduction in eukaryotic cells. Mammalian PKGs regulate a number of physiological processes including smooth muscle relaxation, platelet aggregation, intestinal secretion and hippocampal and cerebellar learning (reviewed in reference 27). Unlike cAMP-dependent protein kinase (PKA), PKG comprises both a catalytic domain and a regulatory domain in a single polypeptide. In the inactivated state, part of the regulatory domain (a substrate-like sequence known as the autoinhibitory domain) is thought to be bound to the catalytic domain. This binding is released and the enzyme activated upon binding of cGMP to the regulatory domain and opening of the substrate-binding region within the catalytic domain (1, 14). The PKG of the malaria parasite Plasmodium falciparum (PfPKG) and orthologues from the related coccidian parasites Toxoplasma and Eimeria are encoded by a single-copy gene and have been shown to differ from the mammalian enzymes in several respects. These include a larger number of cGMP binding sites (three functional plus one degenerate) in the regulatory domain of the apicomplexan enzymes (6, 7, 17), highly cooperative stimulation by cGMP (8, 17), and relative insensitivity to 8-substituted cGMP analogues (6, 7, 26). The parasite isoforms also lack an N-terminal leucine zipper motif that mediates homodimerization in mammalian isoforms, and evidence suggests that they are monomeric (17). Interestingly, it has been shown that the coccidian PKGs exist as both cytosolic and membrane-associated (mediated by N-terminal myristoylation and palmitoylation) isoforms (12), but the amino acid motifs required for these modifications are absent in the PfPKG sequence, and it seems to lack acylated forms (8).
The coccidian PKGs have been shown to be the target of a potent anticoccidial agent, 4-[2-(4-fluorphenyl)-5-(1-methylpiperidine-4-yl)-1H pyrrol-3-yl]pyridine (compound 1), which is a competitive inhibitor of ATP binding (17). The selectivity of the compound for apicomplexan enzymes is attributed to the relative accessibility of a hydrophobic pocket that overlaps with the ATP binding site conferred by a critical threonine residue in the key “gatekeeper” position (T761 in Toxoplasma gondii; T770 in Eimeria tenella) (11). Access is thought to be prevented by the relatively bulky side chain of the residue that occupies the corresponding position in mammalian PKGs. Expression of compound 1-insensitive coccidian PKGs (harboring T/Q or T/M gatekeeper residue substitutions) in Toxoplasma abolished the effects of the inhibitor on the parasite, thus establishing that PKG is the primary target of compound 1 in T. gondii. These compound 1-insensitive mutants were used to determine a role for coccidian PKG in secretion of micronemal adhesive proteins, attachment to and invasion of host cells and gliding motility of E. tenella sporozoites and T. gondii tachyzoites. Compound 1 has also been shown to be a potent inhibitor of the native P. falciparum PKG enzyme (50% inhibitory concentration [IC50] of 8.53 nM on cGMP-dependent kinase activity in purified fractions using a fixed ATP concentration of 5 μM in the assay) but has limited in vivo activity in the rodent malaria model P. berghei (8).
P. falciparum causes the most serious form of malaria in humans and is responsible for the deaths of approximately one million people each year. The parasite life cycle is complex consisting of distinct phases in the mosquito vector and the human host. Pathogenesis is caused by asexual parasites which proliferate within red blood cells. The invasive form (merozoite) enters a red blood cell and forms a ring-stage parasite. This develops into a trophozoite which feeds on hemoglobin, and the resulting schizont releases up to 32 daughter merozoites in a 48-h cycle. A small proportion of the schizonts release merozoites that differentiate into male or female gamete precursors (gametocytes). These sexual cells are essential for malaria transmission and must be taken up by an Anopheles mosquito to continue the life cycle. Upon entering the insect midgut, environmental cues trigger gametogenesis. Recently, we reported that PfPKG plays an essential role in initiating this process. The initial morphological change after activation of P. falciparum gametocytes, from crescent-shaped to spherical (known as rounding up), is inhibited by compound 1. Genetically manipulated parasites expressing a mutant (T618Q gatekeeper substitution) compound 1-insensitive but fully active PKG, introduced by allelic replacement, were resistant to the effects of compound 1 on gametogenesis, demonstrating a direct role for the enzyme in the initiation of this process (23).
There is also evidence suggesting a role for PKG in P. falciparum erythrocytic stages. It has been reported that PfPKG is expressed at both the mRNA and the protein level in asexual blood stages, as well as the sexual phase of the life cycle (6, 8). Although the PfPKG gene could be targeted for allelic replacement, we were unable to disrupt it, suggesting an essential role for PKG in P. falciparum asexual blood stage development since it is this life cycle stage on which transfection and drug selection of genetically modified parasites are performed. Furthermore, we and others (8, 23) have observed that compound 1 inhibits in vitro-cultured P. falciparum (IC50s in the sub-low-μM range depending on the isolate). Although the role of PKG in the initiation of gametogenesis has been elucidated, its role in erythrocytic stages was unknown prior to the present study.
We demonstrate here that PfPKG has a central role in the late stages of erythrocytic schizogony. Compound 1-treated P. falciparum parasites develop normally through the trophozoite stage but arrest late in schizont development, forming large dysmorphic schizonts. Genetically manipulated P. falciparum parasites expressing a compound 1-insensitive PfPKG are resistant to the effects of both compound 1 and (a second imidazopyridine PKG inhibitor) compound 2 on schizogony but not to the broad-specificity serine/threonine kinase inhibitor staurosporine. This demonstrates that PfPKG is the primary target of the inhibitors and provides direct evidence that this enzyme is essential for progression of the asexual erythrocytic stage of the malaria parasite life cycle.
MATERIALS AND METHODS
Inhibitors.
4-[2-(4-Fluorphenyl)-5-(1-methylpiperidine-4-yl)-1H pyrrol-3-yl] pyridine (compound 1) and 4-[7-[(dimethylamino)methyl]-2-(4-fluorphenyl)imidazo[1,2-a]pyridin-3-yl]pyrimidin-2-amine (compound 2) were gifts from Merck & Co., Inc. Compound 1 and staurosporine (Sigma) were diluted to 10 mM in dimethyl sulfoxide (DMSO; Sigma) and further diluted in RPMI 1640/albumax (Invitrogen) to working dilutions.
P. falciparum cultivation.
P. falciparum (clone 3D7) was grown in vitro using human O+ erythrocytes in RPMI 1640-0.5% Albumax as previously described (2). Synchronization was carried out by treatment with sorbitol according to standard procedures (21). Mutant parasites (PfPKGT618Q harboring a human DHFR [methotrexate resistance] allele) were maintained under selection with 5 nM WR99210 (an antifolate) and were generated as described previously (23).
Erythrocyte invasion assays and statistical analysis.
For erythrocyte invasion assays, schizonts were produced by magnetic purification and cultured in 96-well plates at 1 to 2% parasitemia and 2% hematocrit as previously described (28). Inhibitors (dissolved in 10% DMSO to give a final concentration of 0.5%) were added to schizonts (wild-type 3D7 or PfPKGT618Q mutants derived from 3D7) at a starting parasitemia of 0.5% and 1% hematocrit in 24-well plates in a humidified chamber supplied with 7% CO2-7% O2 in N2. The proportion of ring-stage parasitized red blood cells (rPRBCs) was measured by counting Giemsa-stained thin blood films after approximately 24 h of treatment.
Samples were counted blind in triplicate (minimum 2,000 cells per slide) or in replicates of six (1,000 per slide) and analyzed as proportions. Invasion assay data were analyzed in SPSS using nested logistic regression analysis with a generalized linear model (the factors included treatment and sample [treatment]). Nested analysis was performed to detect variations in between treatments above the variation between the replicates for an individual treatment. The data are presented as the exponential of the B statistic, with the 95% confidence interval (CI) for Exp(B). The results are considered significantly different from the reference group if the 95% CI for Exp(B) does not include 1. Graphs show proportions of ring-infected parasitized erythrocytes. Error bars represent the mean binomial error of the counts of replicate samples. The protocol used for treating parasites for different periods of time is shown in Fig. S1 in the supplemental material.
IFA and Western blotting.
For immunofluorescence assay (IFA) with the Pf225 antibody, compound 1-treated (2 μM) and untreated schizonts were smeared and fixed with 1% paraformaldehyde as previously described (28). For localization of Pf225 (PfRon4), the monoclonal antibody 24C6 4F12 (25), kindly provided by Jean Francois Dubremetz, was diluted 1:100 and visualized with a fluorescein isothiocyanate-labeled anti-mouse IgG antibody (Sigma). Nuclei were stained with 0.5 μg of DAPI (4′,6′-diamidino-2-phenylindole)/ml. Microscopy was performed by using a Deltavision cooled charged-coupled device imaging system. Images from the fluorescence microscope were collected and deconvolved in Softworx and prepared in Adobe Photoshop with no further manipulation. For IFA with the PKG and MSP1 antibodies, parasites were fixed for 20 min with 4% paraformaldehyde, samples were permeabilized in 0.1% Triton X-100 for 10 min and blocked overnight in 3% (wt/vol) bovine serum albumin. Fixed samples were incubated with primary antibody (1:100), followed by incubation with Alexa Fluor-coupled anti-rabbit or anti-mouse antibody (1:5,000), respectively. Nuclei were stained with 0.5 μg of DAPI/ml. Anti-human PKGIα antibody (PK10; Calbiochem) was raised in rabbit to a C-terminal peptide (amino acids 657 to 671) and cross-reacts with PfPKG due to a high degree of identity in this region, as previously noted (8, 23). Western blotting was performed as previously described (23).
Electron microscopy (EM).
For morphology, cells were fixed in 2.5% (vol/vol) glutaraldehyde-0.75% (wt/vol) tannic acid in 0.1 M sodium cacodylate buffer (pH 7.0) for 3 h at 4°C. They were osmicated (1% in cacodylate buffer), followed by uranyl acetate (1%) staining, both for 1 h at 4°C, and then dehydrated in an acetone series before being embedded in agar resin (Agar Scientific, Ltd., United Kingdom). Epoxy resin sections were stained with uranyl acetate and lead citrate and then examined in JEOL 1200EX and Hitachi 7600 electron microscopes.
RESULTS
Compound 1 prevents the progression of schizonts through to rings but does not interfere with host cell kinases required for invasion.
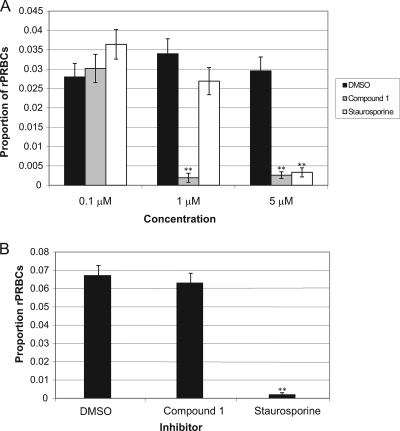
The effect of compound 1 on asexual blood stage development was firstly investigated using a simple invasion assay. This involved adding the inhibitor to purified, synchronized P. falciparum schizonts and then measuring parasitemia on Giemsa-stained blood films after 24 h of treatment. Schizonts treated with either compound 1, staurosporine, or a DMSO control (at 0.1, 1, or 5 μM) showed that compound 1 (at 1 and 5 μM) and staurosporine (at 5 μM) inhibited progression from schizonts to ring stages significantly (Fig. 1A [see the figure legend for statistical analysis]). This could be due to an effect on one or more events occurring between schizont development and invasion. However, the persistence of schizonts in the treated wells suggested that both compounds have some effect on schizogony.
FIG. 1.
Compound 1 inhibits progression of P. falciparum schizonts to ring stages in erythrocyte invasion assays. (A) Proportion of rPRbCs after 24 h treatment of schizonts with inhibitor or control. Bars represent the mean proportion of triplicate samples. Error bars represent the mean binomial error of the proportions. The data were further analyzed in SPSS by using nested logistic regression analysis with a generalized linear model (factors included treatment and sample [treatment]). Counts were performed in triplicate or replicates of six. Control versus compound 1: 1 μM, Exp(B) = 0.125; 95% CI = 0.039 to 0.397. Control versus compound 1: 5 μM, Exp(B) = 0.206; 95% CI = 0.113 to 0.376. Control versus staurosporine: 5 μM, Exp(B) = 0.234; 95% CI = 0.130 to 0.421. The results marked with asterisks (**) showed a statistically significant difference in rPRBCs from the DMSO control group [the 95% CI for Exp(B) did not include 1]. (B) Proportion of ring stage-parasitized RBCs 24 h after the start of the experiment using erythrocytes that had been treated (for 1.5 h) with inhibitor or control. Bars represent the mean proportion of triplicate samples. Error bars represent the mean binomial error of the proportions. The data were further analyzed in SPSS as described above.
As an initial step to examine the specificity of compound 1 on P. falciparum asexual blood-stage development, uninfected erythrocytes were pretreated with 10 μM compound 1 or staurosporine (for 1.5 h), followed by removal of the inhibitor by washing before the addition of schizonts. This led to a significant reduction in parasitemia for staurosporine compared to DMSO-treated control cells [Exp(B) = 0.032; 95% CI = 0.013 to 0.077] but not for compound 1 [Exp(B) = 0.878; 95% CI = 0.694 to 1.110] (Fig. 1B). Merozoites could not invade erythrocytes pretreated with staurosporine but could invade those pretreated with compound 1. One possible interpretation is that the effects of compound 1 are likely to be specific to a parasite kinase, whereas the effects of staurosporine are likely to be mediated in part against host cell kinases.
The effects of compound 1 on schizonts are reversible after periods of treatment of <6 h.
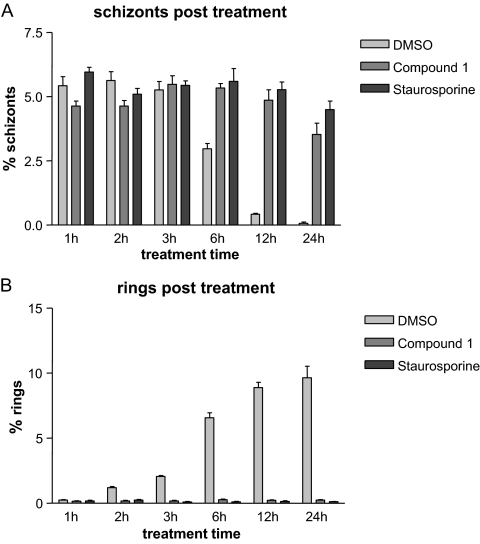
The results presented described above (Fig. 1A) suggested that both compounds have an inhibitory effect on P. falciparum schizogony. We therefore examined the effects on schizogony of treatment with 2 μM compound 1 or 2 μM staurosporine for increasing lengths of time (1, 2, 3, 6, 12, and 24 h). Starting schizonts were between 38 and 42 h postinvasion. After treatment, the parasites were washed and returned to culture and were observed again after 24 and 72 h (see Fig. S1 in the supplemental material for experimental strategy). Giemsa-stained smears were prepared from all cultures immediately after treatment. Figure 2 shows a summary of the cell counts taken from these smears and indicates that schizonts persist in both compound 1- and staurosporine-treated cultures. A very low level of ring-stage parasites was observed at each treatment length, showing that the majority of schizonts could not release invasive merozoites. Control treatment of schizonts with DMSO shows the expected gradual decrease in schizonts (Fig. 2A) and consequent increase in rings (Fig. 2B) over time after successful schizont rupture and red cell invasion.
FIG. 2.
Schizonts persist and do not lead to normal levels of ring-stage parasite formation after inhibitor treatment for 1 to 24 h. Starting schizonts (38 to 42 h postinvasion) were treated with inhibitor or control for increasing lengths of time. Immediately after treatment, the parasites were smeared, and parasitemia measured after Giemsa staining. This figure shows the counts taken from these smears. (A) Bars represent the percentage of schizonts remaining in cultures treated with inhibitor or control for periods of time between 1 and 24 h. (B) Bars represent the percentage of resulting ring-stage parasites in cultures treated with inhibitor or control for periods of time between 1 and 24 h.
The proportion of rPRBCs for all treatment lengths was measured 24 h after starting treatment (see Fig. S2A in the supplemental material). A significant reduction in rPRBCs was seen in cultures treated with staurosporine for 1 h or more, showing that schizonts could not recover from even a short period of treatment with staurosporine (see Fig. S2A in the supplemental material). In contrast, treatment with compound 1 is reversible to some extent. Although parasites treated with compound 1 were developmentally delayed for a period corresponding to the length of the treatment time, they were able to recover fully if treatment was for 1 or 2 h. Analysis of the data for parasites treated for 3 h suggests a very slight reduction in the proportion of rPRBCs if a nested model taking into account the variation between the replicate samples is used [Exp(B) = 0.761; 95% CI = 0.590 to 0.981)], but if a simpler model is used that only compares the treatment this result is not significant [Exp(B) = 0.761; 95% CI = 0.908 to 1.113]. More obvious differences occur after 6 h treatment, where the proportion of rPRBCs at 24 h drops significantly [nested model: Exp(B) = 0.619; 95% CI = 0.477 to 0.802] and more so after 12 h and 24 h (see Fig. S2A in the supplemental material).
Parasitemia was checked 24 h after starting (see Fig. S2A in the supplemental material) and again after 72 h (see Fig. S2B in the supplemental material). For the 72-h measurements, cultures were diluted 1/10 at 24 h to ensure parasitemia levels did not rise too high in the second cycle (see Fig. S1 in the supplemental material). Parasites treated with compound 1 for up to 6 h showed the same level of parasitemia as the control treated cultures after 24 and 72 h (with a difference in age corresponding to the developmental delay). However, after 12 h of treatment, the parasitemia levels at 24 h were ∼3-fold lower than those of the control (3.1% rings compared to 10%) and, after 24 h of treatment, the mean percentage of the rings was 0.36% compared to 9.69% in the control. After 72 h, the mean percentage of the rings had fallen further to 0.14%, indicating that although compound 1 is a reversible inhibitor, irreversible damage to the schizont occurs after prolonged treatment (see Fig. S2 in the supplemental material).
Schizonts treated with compound 1 for prolonged periods show aberrant morphology.
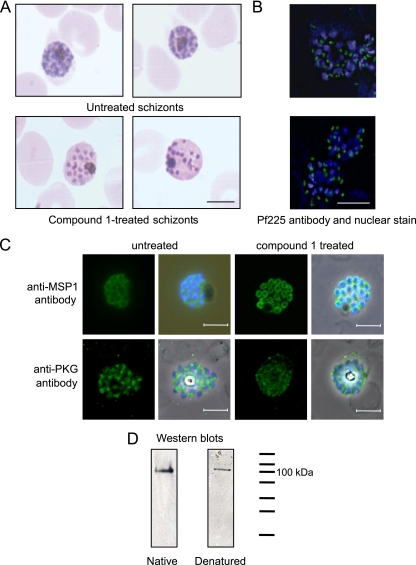
Development was slowed in the compound 1-treated schizonts and distinct morphological abnormalities visible by light microscopy were apparent after between 6 and 12 h of treatment (compared to 3 h for staurosporine) and more strikingly obvious after 24 h of treatment. Compound 1-treated schizonts stained less well with Giemsa, and the intracellular merozoites looked more separated and irregularly spaced than in untreated schizonts (Fig. 3A). To study these morphological abnormalities in more detail, we took purified schizonts and treated them with 2 μM compound 1 for 24 h for IFA and EM analysis. Localization of the rhoptry neck protein Pf225 (PfRON4) and MSP1 were apparently normal in the inhibitor-treated parasites (Fig. 3B and C), as were those of the microneme proteins AMA1 and EBA175, the apical protein RH2b and the calcium-dependent protein kinase CDPK1 (data not shown). A polyclonal PKG anti-peptide antibody that reacts with an ∼100 kDa band on Western blots of mixed stage P. falciparum proteins (denatured and nondenatured, Fig. 3D) was also tested on compound 1-treated schizonts (Fig. 3C, lower panels). The staining pattern observed throughout the merozoites suggests a predominantly cytosolic localization. The pattern was similar in both treated and untreated schizonts and was not observed in earlier asexual blood-stage parasites.
FIG. 3.
Treatment of schizonts with compound 1 causes morphological abnormalities, but merozoite proteins localize normally. (A) Giemsa-stained smears of untreated or schizonts treated with 2 μM compound 1 for 24 h. Scale bar, 5 μm. (B) Fluorescence micrographs of untreated schizonts or schizonts treated with 2 μM compound 1. The primary antibody was a monoclonal antibody that reacts with the P. falciparum rhoptry neck protein Pf225 (green). Nuclei were counterstained with 0.5 g/ml DAPI (blue). Scale bar, 5 μm. (C) Fluorescence micrographs of schizonts treated with 2 μM compound 1. The primary antibodies were a monoclonal antibody that reacts with P. falciparum MSP1 (top row) and a polyclonal antibody that reacts with PfPKG (bottom row) (both green). Nuclei were counterstained with 0.5 g/ml DAPI (blue). (D) Western blots containing mixed-stage P. falciparum (clone 3D7) parasites separated on both native and denaturing polyacrylamide gels. The PKG antibody was raised to the C-terminal peptide of human PKGI and cross-reacts with PfPKG due to the high degree of sequence identity at the C terminus.
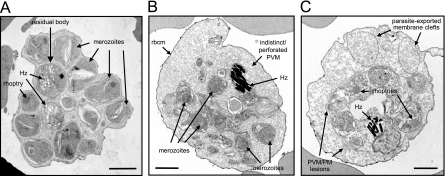
Schizonts treated with 2 μM compound 1 for 24 h were also examined by transmission EM. Consistent with the light microscopy results, aberrant morphology was clearly observed when the ultrastructure of schizont stages, treated with a 24-h exposure of compound 1 (Fig. 4B and C), is compared to untreated controls (Fig. 4A). Under these conditions schizont development was arrested, with the majority being unable to reach the point where they can rupture. The population of treated schizonts comprised parasites with 8 to 16 nuclei stages, segmented schizonts that are still attached to the residual body, as well as fully segmented schizonts. Thus, compound 1 did not appear to synchronize development to an obvious morphological/time point. In treated schizonts (Fig. 4B and C) merozoite morphology is abnormal, with many of the major organelles ill defined or “leached out,” there is an increase in the amount of membranous material, as well as evidence of membrane fragmentation (notably the parasitophorous vacuolar membrane [PVM] and possibly the parasite plasma membrane [PM]). In some schizonts the red blood cell cytosol appears to be partially digested (loss of dense staining and a mottled appearance, Fig. 4B and C); this would be consistent with PVM/PM perforation and the leakage of proteases into the red blood cell.
FIG. 4.
EM morphology of untreated and compound 1-treated schizonts. (A) Untreated control P. falciparum segmented schizont. Scale bar, 1 μm. (B and C) Electron micrographs showing schizonts after treatment with 2 μM compound 1 for 24 h. Hz, haemozoin; rbcm, red blood cell membrane; PVM, parasitophorous vacuolar membrane; PM, plasma membrane. Scale bar, 1 μm.
PfPKG is central to schizogony.
To establish whether the effect of compound 1 on P. falciparum schizont development was due to the action of the inhibitor on PfPKG or on an alternative target, we used genetically manipulated P. falciparum parasites expressing a compound 1-insensitive PfPKG introduced into the haploid parasite genome by allelic replacement. This mutant line is also insensitive to a distinct anticoccidian PKG inhibitor (4-[7-[(dimethylamino)methyl]-2-(4-fluorphenyl)imidazo[1,2-a]pyridin-3-yl]pyrimidin-2-amine, compound 2). Compound 2 has been shown to have very similar actions to compound 1, but additional parasite protein kinase targets (casein kinase 1 and calcium-dependent protein kinase 1) have been identified in T. gondii (13). The inhibitor-insensitive P. falciparum line expressing a mutant PfPKG enzyme (T618Q) was generated by allelic replacement. No differences in the growth rates of either sexual or asexual parasites were noted between mutant and wild-type parasites in the absence of compound 1. These observations confirm that the mutant kinase is fully active and that the genetic manipulation procedure had had no detectable effects on parasite development (23).
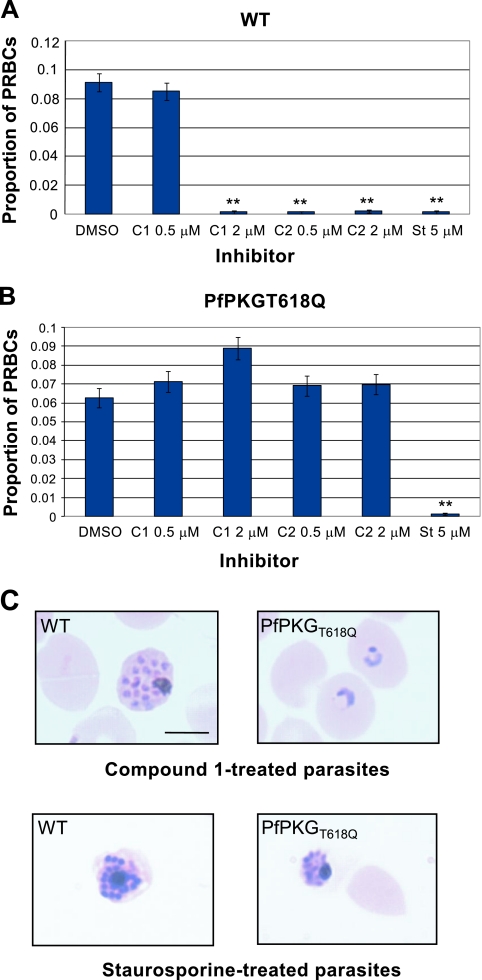
Treatment of purified wild-type schizonts with either 2 μM compound 1, 0.5 to 2 μM compound 2, or 5 μM staurosporine dramatically reduced the formation of ring-stage parasites in invasion assays (Fig. 5A). In contrast, schizogony was able to proceed, and ring-stage parasites developed at normal levels after treatment of PfPKGT618Q mutant schizonts with compounds 1 and 2 at these concentrations (Fig. 5B and C). There was no difference between the parasitemia levels or developmental stage of compound 1- or compound 2-treated versus control DMSO-treated PfPKGT618Q parasites. Ring-stage parasite formation in staurosporine-treated mutant schizonts was fully inhibited, as observed with wild-type parasites (Fig. 5B and C). Wild-type schizonts treated with compound 1 or staurosporine did not rupture and showed aberrant morphology (Fig. 5C, left panels). In contrast, compound 1-treated schizonts expressing PfPKGT618Q were able to rupture and to form ring-stage parasites after reinvasion (Fig. 5C, upper right panel). The presence of the mutant PKG did not reverse the inhibitory effects of staurosporine on schizonts since it is likely to target numerous protein kinases in Plasmodium. Together, these data demonstrate conclusively that the effects of compounds 1 and 2 on schizont development are due primarily to the action of the inhibitors on PfPKG and provide direct evidence for a key role for PKG in schizogony.
FIG. 5.
Mutant schizonts expressing a compound 1-insensitive PfPKG progress through to ring stage normally in the presence of compound 1 in erythrocyte invasion assays. The proportions of ring parasitized RBCs in wild-type (WT) (A) and compound 1-insensitive (PfPKGT618Q) (B) parasite lines measured after treatment for 24 h with either compound 1, compound 2, staurosporine, or DMSO alone. Bars represent the mean proportion of triplicate samples. Error bars represent the mean binomial error of the proportions. The data were further analyzed in SPSS as for Fig. 1. The results marked with asterisks (**) showed a statistically significant reduction in rPRBCs from the DMSO control group [the 95% CI for Exp(B) did not include 1]. (C) Giemsa-stained smears of compound 1 and staurosporine-treated wild-type and PfPKGT618Q parasites. Scale bar, 5 μm.
DISCUSSION
When a P. falciparum merozoite enters the human bloodstream after release from a schizont, it must quickly bind to and invade a red blood cell to escape the lethal environment. In the present study, we have utilized highly specific inhibitors in conjunction with inhibitor-insensitive transgenic parasites to demonstrate a central role for the parasite cGMP-dependent protein kinase in regulating the progression of P. falciparum schizogony.
This study was initiated based on previous evidence that cGMP and PfPKG are involved in P. falciparum asexual replication, but it was not clear at which phase of the cycle PfPKG may be involved or what its precise role might be. Previous work using the potent nonselective protein kinase inhibitor staurosporine suggested a role for protein kinases in invasion of erythrocytes by Plasmodium (9, 29) and host cell invasion by Toxoplasma (4). We therefore began by testing the PKG inhibitor compound 1 in erythrocyte invasion assays. Our results showed that compound 1 is a more potent inhibitor of ring-stage formation than staurosporine in these assays. The results also show that, unlike staurosporine, compound 1 has no additional effects on host red cell kinases, suggesting that its inhibitory effects are specific to parasite molecules. The previous study which investigated the effects of staurosporine on P. knowlesi merozoite invasion concluded that the inhibitor had little or no effect on rhesus monkey red blood cells (29); the different conclusion may be due in part to the shorter pretreatment period used in the earlier study.
Inhibition of ring-stage formation by a test compound in an invasion assay does not necessarily indicate direct interference with the invasion process itself. We therefore looked more closely at the treatment of schizonts with compound 1 and staurosporine. The treatment of schizonts for increasing lengths of time showed clearly that they were unable to rupture and were irreversibly damaged after prolonged treatment. It was possible to reverse these effects using shorter treatment periods (1 to 3 h), indicating that compound 1 is a reversible inhibitor. This contrasted with staurosporine treatment, where even short periods of treatment led to irreversible inhibition of schizogony. This probably reflects the likelihood that staurosporine, a broad-range ATP-competitive kinase inhibitor, has numerous targets in the parasite.
Although schizonts treated with compound 1 for 6 to 24 h exhibited aberrant morphology by Giemsa staining, immunostaining with antibodies raised to specific merozoite proteins showed apparently normal localization, indicating limited disruption of schizont formation. It is possible that the appearance of Giemsa-stained schizonts after compound 1 treatment may be due to destabilizing effects on the PVM and PM that do not adversely affect expression and trafficking of the merozoite membrane proteins tested. The pattern obtained by EM analysis was consistent with Giemsa-staining of schizonts treated with compound 1. There was clear evidence of membrane disruption, but it is not clear whether or not these morphological abnormalities are a primary effect of the inhibitor or secondary effects after arrest.
The arrest of schizogony prior to rupture by compound 1 suggests a potential role for PKG at this stage of the life cycle. This idea is consistent with genome-wide microarray data that measured peak expression of PfPKG mRNA levels in late schizonts (22) (PlasmoDB [http://plasmodb.org]). Furthermore, IFA with the anti-PfPKG antibody suggests that the protein is expressed in late trophozoites but peaks in schizonts. However, earlier work reported peak expression of PfPKG and native cGMP-dependent kinase activity in P. falciparum ring-stage parasites and gametocytes (6, 8). The discrepancy in the asexual stage specificity of activity might be partly explained by the relative purity of parasite preparations between studies or the possibility that native PfPKG present in schizont lysates may already be fully activated and therefore not stimulated further by cGMP addition in kinase assays. Genes responsible for the synthesis (encoding guanylyl cyclase PfGCα [PF11_0395]) and hydrolysis (encoding phosphodiesterases PfPDEα [PFL0475w], a cGMP-PDE [30, 32], and PfPDEβ [MAL13P1.118], a PDE with potential for dual substrate specificity [30]) of cGMP also have peak mRNA expression in late schizonts (PlasmoDB). This further suggests a role for the cGMP signaling pathway at this stage of parasite development.
It is clear that all protein kinase inhibitors have the potential to target more than one enzyme in the cell, and it is necessary to generate an inhibitor-insensitive line to demonstrate specificity. We previously reported that in a compound 1-insensitive P. falciparum line (expressing a mutant PKG with its capacity to bind to compound 1 reduced >3,000-fold), the effects of the inhibitor on the initiation of gametogenesis were abolished and thereby established specificity for PfPKG (23). In the present study we showed that the effects of the PKG inhibitors compound 1 and compound 2 on schizogony in this P. falciparum line were abolished, demonstrating that PfPKG is the primary target of these inhibitors during this process and highlighting an essential role for this kinase in schizogony.
To determine whether the inhibitors affect P. falciparum throughout the erythrocytic cycle or specifically schizont stages, a tritiated hypoxanthine incorporation assay was performed on synchronized ring stage parasites (starting treatment approximately 2 to 6 h after invasion). Interestingly, ring-stage parasites treated with either compound 1 or compound 2 (4 μM) showed a reduced level (ca. 25%) of incorporation of tritiated hypoxanthine over the subsequent 56-h assay period compared to DMSO-treated controls (data not shown). This suggests that there is reduced nucleic acid synthesis in the presence of either compound even though the morphology of trophozoites and developing schizonts is normal. However, when this experiment was repeated on both the wild type (clone 3D7) and the inhibitor-insensitive line, the slight decrease in hypoxanthine incorporation was seen in both parasite lines in the presence of either compound 1 or compound 2 (data not shown). This suggests that (in contrast to the effects on schizont rupture) the effects of both compounds on hypoxanthine incorporation are not (primarily) due to inhibition of PfPKG but rather are a result of inhibition of other P. falciparum kinases as secondary targets of compounds 1 and 2 during the period of DNA synthesis. We previously reported a similar phenomenon during sexual development, where the effects of the compounds on the first step of gametogenesis were specific to inhibition of PKG, but not the later events and that secondary targets were likely involved during the subsequent steps of exflagellation (23).
Compound 1 and inhibitor-insensitive parasites were used previously to elucidate a role for PKG in secretion of the micronemal adhesive proteins (TgMIC2 and EtMIC1 and -2), gliding motility, and attachment to and invasion of host cells of E. tenella sporozoites and T. gondii tachyzoites (31). Interestingly, staurosporine also inhibits release of the Toxoplasma MIC2 (4) that mediates host cell attachment and invasion (3, 18). In Toxoplasma it is known that increases in intracellular calcium can trigger microneme discharge (5). Inhibitor studies have implicated a calcium-dependent protein kinase (TgCDPK1; not the orthologue of the Plasmodium CDPK1) in TgMIC2 secretion, gliding motility, and host cell attachment and invasion (10, 20). Evidence thus points to a role for both PKG and a calcium-dependent kinase in these processes. Purfalcamine, an inhibitor of the calcium-dependent protein kinase PfCDPK1, was also reported to cause the arrest of P. falciparum schizont development. Microarray analysis has shown that the PfCDPK1 mRNA clusters with other mRNAs encoding a number of motor complex proteins associated with parasite motility (which may indicate a shared function), and the enzyme has been shown to phosphorylate one of these (myosin A tail domain interacting protein [MTIP]). It was concluded that PfCDPK1 could play a role in motor-dependent parasite processes such as cytokinesis and merozoite egress that occur in late schizonts (19). Independent confirmation that PfCDPK1 can phosphorylate MTIP, as well as glideosome-associated protein 45 (GAP45), has been obtained (16). It was concluded that the localization of CDPK1 at the periphery of the merozoite was consistent with its interaction with motor complex proteins during erythrocyte invasion. Interestingly, phosphorylation of GAP45 controls the assembly of the Toxoplasma myosin XIV motor complex (15). Use of a potent inhibitor of CDPK1 (the bisindolocarbazole, K252a) prevented merozoite egress (16), which is reminiscent of the effects of PfPKG inhibition by compounds 1 and 2. Although the specificity of these CDPK1 inhibitors requires confirmation, the investigation of how calcium and cGMP signaling might coregulate schizogony and parasite egress will be a direction of future work.
In the present study we demonstrated that PfPKG has a central role in P. falciparum schizogony and that it can be targeted specifically in this life cycle stage by the trisubsubstituted pyrrole, compound 1, and the imidazopyridine, compound 2—both of which are potent anticoccidial PKG inhibitors. Previously, we demonstrated a crucial role for PfPKG in the initiation of gametogenesis (23), and our recent data indicate an additional role in ookinete motility (24). This establishes PfPKG as a potentially exciting drug target in the malaria parasite for the following reasons. The anticoccidial agents compounds 1 and 2 are able to inhibit P. falciparum development, and their effects are both specific to PKG and selective for the parasite isoform. However, it is clear from the strategy used here that resistance to these particular compounds can be conferred by substitution of a single residue and also that the activity of these compounds against P. berghei in vivo is limited (8). PfPKG is the first example of a validated P. falciparum drug target whose inhibition could have both therapeutic and transmission blocking effects.
Supplementary Material
Acknowledgments
This study was supported by Wellcome Trust grant 066742 (to D.A.B. and A.A.H.), a Wellcome Trust Junior Research Fellowship in Biodiversity (to H.M.T.), MRC and European Commission grants FP6 SIGMAL (012174 [to D.A.B.]) and FP7 MALSIG (223044 partly funding work in the D.A.B., A.A.H., and INSERM U609 Laboratories).
We thank Christian Doerig for generous guidance with kinase work, Judith Green for help with IFA analysis and helpful discussions, Lawrie Bannister for advice on interpretation of EM images, and Liz Hirst (Electron Microscope Lab/Developmental Neurobiology, NIMR) for sectioning and EM assistance. We also thank The Centre for Ultrastructural Imaging, King's College London, for EM assistance; Eloise Thompson for assistance with hypoxanthine incorporation assays; David Jacobus (Jacobus Pharmaceutical Company, Princeton, NJ) for providing WR99210; Merck & Co., Inc., for kindly providing compounds 1 and 2; and Katy Kettleborough, Simon Osborne, and coworkers at MRC Technology for the generous provision of compounds.
Footnotes
Published ahead of print on 13 November 2009.
Supplemental material for this article may be found at http://ec.asm.org/.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1.Alverdi, V., H. Mazon, C. Versluis, W. Hemrika, G. Esposito, R. van den Heuvel, A. Scholten, and A. J. Heck. 2008. cGMP-binding prepares PKG for substrate binding by disclosing the C-terminal domain. J. Mol. Biol. 375:1380-1393. [DOI] [PubMed] [Google Scholar]
- 2.Blackman, M. J., and A. A. Holder. 1992. Secondary processing of the Plasmodium falciparum merozoite surface protein-1 (MSP1) by a calcium-dependent membrane-bound serine protease: shedding of MSP133 as a noncovalently associated complex with other fragments of the MSP1. Mol. Biochem. Parasitol. 50:307-315. [DOI] [PubMed] [Google Scholar]
- 3.Brossier, F., T. J. Jewett, J. L. Lovett, and L. D. Sibley. 2003. C-terminal processing of the toxoplasma protein MIC2 is essential for invasion into host cells. J. Biol. Chem. 278:6229-6234. [DOI] [PubMed] [Google Scholar]
- 4.Carruthers, V. B., O. K. Giddings, and L. D. Sibley. 1999. Secretion of micronemal proteins is associated with toxoplasma invasion of host cells. Cell Microbiol. 1:225-235. [DOI] [PubMed] [Google Scholar]
- 5.Carruthers, V. B., and L. D. Sibley. 1999. Mobilization of intracellular calcium stimulates microneme discharge in Toxoplasma gondii. Mol. Microbiol. 31:421-428. [DOI] [PubMed] [Google Scholar]
- 6.Deng, W., and D. A. Baker. 2002. A novel cyclic GMP-dependent protein kinase is expressed in the ring stage of the Plasmodium falciparum life cycle. Mol. Microbiol. 44:1141-1151. [DOI] [PubMed] [Google Scholar]
- 7.Deng, W., A. Parbhu-Patel, D. J. Meyer, and D. A. Baker. 2003. The role of two novel regulatory sites in the activation of the cGMP-dependent protein kinase from Plasmodium falciparum. Biochem. J. 374:559-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diaz, C. A., J. Allocco, M. A. Powles, L. Yeung, R. G. Donald, J. W. Anderson, and P. A. Liberator. 2006. Characterization of Plasmodium falciparum cGMP-dependent protein kinase (PfPKG): antiparasitic activity of a PKG inhibitor. Mol. Biochem. Parasitol. 146:78-88. [DOI] [PubMed] [Google Scholar]
- 9.Dluzewski, A. R., and C. R. Garcia. 1996. Inhibition of invasion and intraerythrocytic development of Plasmodium falciparum by kinase inhibitors. Experientia 52:621-623. [DOI] [PubMed] [Google Scholar]
- 10.Dobrowolski, J. M., V. B. Carruthers, and L. D. Sibley. 1997. Participation of myosin in gliding motility and host cell invasion by Toxoplasma gondii. Mol. Microbiol. 26:163-173. [DOI] [PubMed] [Google Scholar]
- 11.Donald, R. G., J. Allocco, S. B. Singh, B. Nare, S. P. Salowe, J. Wiltsie, and P. A. Liberator. 2002. Toxoplasma gondii cyclic GMP-dependent kinase: chemotherapeutic targeting of an essential parasite protein kinase. Eukaryot. Cell 1:317-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donald, R. G., and P. A. Liberator. 2002. Molecular characterization of a coccidian parasite cGMP-dependent protein kinase. Mol. Biochem. Parasitol. 120:165-175. [DOI] [PubMed] [Google Scholar]
- 13.Donald, R. G., T. Zhong, H. Wiersma, B. Nare, D. Yao, A. Lee, J. Allocco, and P. A. Liberator. 2006. Anticoccidial kinase inhibitors: identification of protein kinase targets secondary to cGMP-dependent protein kinase. Mol. Biochem. Parasitol. 149:86-98. [DOI] [PubMed] [Google Scholar]
- 14.Francis, S. H., J. A. Smith, J. L. Colbran, K. Grimes, K. A. Walsh, S. Kumar, and J. D. Corbin. 1996. Arginine 75 in the pseudosubstrate sequence of type Iβ cGMP-dependent protein kinase is critical for autoinhibition, although autophosphorylated serine 63 is outside this sequence. J. Biol. Chem. 271:20748-20755. [PubMed] [Google Scholar]
- 15.Gilk, S. D., E. Gaskins, G. E. Ward, and C. J. Beckers. 2009. GAP45 phosphorylation controls assembly of the Toxoplasma myosin XIV complex. Eukaryot. Cell 8:190-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green, J. L., R. R. Rees-Channer, S. A. Howell, S. R. Martin, E. Knuepfer, H. M. Taylor, M. Grainger, and A. A. Holder. 2008. The motor complex of Plasmodium falciparum: phosphorylation by a calcium-dependent protein kinase. J. Biol. Chem. 283:30980-30989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gurnett, A. M., P. A. Liberator, P. M. Dulski, S. P. Salowe, R. G. Donald, J. W. Anderson, J. Wiltsie, C. A. Diaz, G. Harris, B. Chang, S. J. Darkin-Rattray, B. Nare, T. Crumley, P. S. Blum, A. S. Misura, T. Tamas, M. K. Sardana, J. Yuan, T. Biftu, and D. M. Schmatz. 2002. Purification and molecular characterization of cGMP-dependent protein kinase from Apicomplexan parasites. A novel chemotherapeutic target. J. Biol. Chem. 277:15913-15922. [DOI] [PubMed] [Google Scholar]
- 18.Huynh, M. H., K. E. Rabenau, J. M. Harper, W. L. Beatty, L. D. Sibley, and V. B. Carruthers. 2003. Rapid invasion of host cells by Toxoplasma requires secretion of the MIC2-M2AP adhesive protein complex. EMBO J. 22:2082-2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kato, N., T. Sakata, G. Breton, K. G. Le Roch, A. Nagle, C. Andersen, B. Bursulaya, K. Henson, J. Johnson, K. A. Kumar, F. Marr, D. Mason, C. McNamara, D. Plouffe, V. Ramachandran, M. Spooner, T. Tuntland, Y. Zhou, E. C. Peters, A. Chatterjee, P. G. Schultz, G. E. Ward, N. Gray, J. Harper, and E. A. Winzeler. 2008. Gene expression signatures and small-molecule compounds link a protein kinase to Plasmodium falciparum motility. Nat. Chem. Biol. 4:347-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kieschnick, H., T. Wakefield, C. A. Narducci, and C. Beckers. 2001. Toxoplasma gondii attachment to host cells is regulated by a calmodulin-like domain protein kinase. J. Biol. Chem. 276:12369-12377. [DOI] [PubMed] [Google Scholar]
- 21.Lambros, C., and J. P. Vanderberg. 1979. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 65:418-420. [PubMed] [Google Scholar]
- 22.Le Roch, K. G., Y. Zhou, P. L. Blair, M. Grainger, J. K. Moch, J. D. Haynes, P. De La Vega, A. A. Holder, S. Batalov, D. J. Carucci, and E. A. Winzeler. 2003. Discovery of gene function by expression profiling of the malaria parasite life cycle. Science 301:1503-1508. [DOI] [PubMed] [Google Scholar]
- 23.McRobert, L., C. J. Taylor, W. Deng, Q. L. Fivelman, R. M. Cummings, S. D. Polley, O. Billker, and D. A. Baker. 2008. Gametogenesis in malaria parasites is mediated by the cGMP-dependent protein kinase. PLoS Biol. 6:e139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moon, R. W., C. J. Taylor, C. Bex, R. Schepers, D. Goulding, C. J. Janse, A. P. Waters, D. A. Baker, and O. Billker. 2009. A cyclic GMP signalling module that regulates gliding motility in a malaria parasite. PLoS Pathog. 5:e1000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roger, N., J. F. Dubremetz, P. Delplace, B. Fortier, G. Tronchin, and A. Vernes. 1988. Characterization of a 225 kilodalton rhoptry protein of Plasmodium falciparum. Mol. Biochem. Parasitol. 27:135-141. [DOI] [PubMed] [Google Scholar]
- 26.Salowe, S. P., J. Wiltsie, P. A. Liberator, and R. G. Donald. 2002. The role of a parasite-specific allosteric site in the distinctive activation behavior of Eimeria tenella cGMP-dependent protein kinase. Biochemistry 41:4385-4391. [DOI] [PubMed] [Google Scholar]
- 27.Schlossmann, J., and F. Hofmann. 2005. cGMP-dependent protein kinases in drug discovery. Drug Discov. Today 10:627-634. [DOI] [PubMed] [Google Scholar]
- 28.Taylor, H. M., M. Grainger, and A. A. Holder. 2002. Variation in the expression of a Plasmodium falciparum protein family implicated in erythrocyte invasion. Infect. Immun. 70:5779-5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ward, G. E., H. Fujioka, M. Aikawa, and L. H. Miller. 1994. Staurosporine inhibits invasion of erythrocytes by malarial merozoites. Exp. Parasitol. 79:480-487. [DOI] [PubMed] [Google Scholar]
- 30.Wentzinger, L., S. Bopp, H. Tenor, J. Klar, R. Brun, H. P. Beck, and T. Seebeck. 2008. Cyclic nucleotide-specific phosphodiesterases of Plasmodium falciparum: PfPDEα, a nonessential cGMP-specific PDE that is an integral membrane protein. Int. J. Parasitol. 38:1625-1637. [DOI] [PubMed] [Google Scholar]
- 31.Wiersma, H. I., S. E. Galuska, F. M. Tomley, L. D. Sibley, P. A. Liberator, and R. G. Donald. 2004. A role for coccidian cGMP-dependent protein kinase in motility and invasion. Int. J. Parasitol. 34:369-380. [DOI] [PubMed] [Google Scholar]
- 32.Yuasa, K., F. Mi-Ichi, T. Kobayashi, M. Yamanouchi, J. Kotera, K. Kita, and K. Omori. 2005. PfPDE1, a novel cGMP-specific phosphodiesterase from the human malaria parasite Plasmodium falciparum. Biochem. J. 392:221-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.