Abstract
In most eukaryotic cells, tubulin is subjected to posttranslational glutamylation, a conserved modification of unclear function. The glutamyl side chains form as branches of the primary sequence glutamic acids in two biochemically distinct steps: initiation and elongation. The length of the glutamyl side chain is spatially controlled and microtubule type specific. Here, we probe the significance of the glutamyl side chain length regulation in vivo by overexpressing a potent side chain elongase enzyme, Ttll6Ap, in Tetrahymena. Overexpression of Ttll6Ap caused hyperelongation of glutamyl side chains on the tubulin of axonemal, cortical, and cytoplasmic microtubules. Strikingly, in the same cell, hyperelongation of glutamyl side chains stabilized cytoplasmic microtubules and destabilized axonemal microtubules. Our observations suggest that the cellular outcomes of glutamylation are mediated by spatially restricted tubulin interactors of diverse nature.
Microtubules are dynamic elements of the cytoskeleton that are assembled from heterodimers of α- and β-tubulin. Once assembled, tubulin subunits undergo several conserved posttranslational modifications (PTMs) that diversify the external and luminal surfaces of microtubules (51). Two tubulin PTMs, glycylation and glutamylation, collectively known as polymodifications, form peptide side chains that are attached to the γ-carboxyl groups of glutamic acids in the primary sequence of the C-terminal tails (CTTs) of α- and β-tubulin (14, 36). Glutamylated microtubules are abundant in projections of neurons (14), axonemes (8, 15, 17), and centrioles/basal bodies (5, 31) and are detectable in the mitotic spindle and on a subset of cytoplasmic network microtubules (1, 5). The modifying enzymes, tubulin glutamic acid ligases (tubulin E-ligases), belong to the family of proteins related to the tubulin tyrosine ligase (TTL), known as TTL-like (TTLL) proteins (22, 50, 53). Tubulin glutamylation appears to be important in vivo. A knockdown of the TTLL7 E-ligase mRNA in cultured neurons inhibits the outgrowth of neurites (20). A loss of PGs1, a protein associated with TTLL1 E-ligase (22, 37), disorganizes sperm axonemes in the mouse (11), and a morpholino knockdown of TTLL6 E-ligase expression in zebrafish inhibits the assembly of olfactory cilia (33). The biochemical consequences of tubulin glutamylation in vivo are poorly understood, but the emerging model is that this PTM regulates interactions between microtubules and microtubule-associated proteins (MAPs) (6, 7, 19, 27).
The ciliate Tetrahymena thermophila has 18 types of diverse microtubules that are all assembled in a single cell. Although most, if not all, of these microtubules are glutamylated, the length of glutamyl side chains is spatially regulated (8, 53). Minimal side chains composed of a single glutamic acid (monoglutamylation) are present on the cytoplasmic and nuclear microtubules, whereas elongated side chains are present on the basal bodies and axonemes (53). In Tetrahymena, Ttll6Ap is a β-tubulin-preferring E-ligase (22), with a strong if not exclusive, side chain elongating activity (50). Here, by overproducing Ttll6Ap in vivo, we explore the consequences of glutamyl side chain hyper-elongation. Unexpectedly, we show that in the same cells, hyperelongation of glutamyl side chains stabilizes cell body and destabilizes axonemal microtubules. The simplest explanation of these data is that, in vivo, the cellular outcomes of tubulin glutamylation are mediated by diverse microtubule type-specific MAPs. To our knowledge, we are first to report that excessive tubulin glutamylation can either stabilize or destabilize microtubules in the same cell.
MATERIALS AND METHODS
Strains, culture, and green fluorescent protein (GFP) tagging.
Tetrahymena cells were grown in the SPP medium (18) supplied with the antibiotic-antimycotic mix (Invitrogen, Carlsbad, CA). To overexpress Ttll6Ap variants tagged at the N terminus with GFP, fragments of the coding region of TTLL6A were amplified with addition of MluI and BamHI sites at the 5′ and 3′ ends, respectively, and cloned into pMTT1-GFP plasmid (52). The primers are listed in Table S1 in the supplemental material. The transgenic strains were constructed and induced as described previously (22, 53).
Immunofluorescence and electron microscopy.
GFP-Ttll6Ap-expressing cells were grown in SPP with 0.5 to 2.5 μg of CdCl2/ml for 2 to 4 h. For GFP-Ttll6Ap localization, a 10-μl drop of cells was placed on a coverslip, followed by the addition of 20 μl of 2% paraformaldehyde in PHEM buffer (43) and, after 20 s, the addition of 10 μl of 0.5% Triton X-100 in PHEM. The cells were subjected to immunofluorescence (22) with the following primary antibodies: 12G10, an anti-α-tubulin monoclonal antibody (MAb) (23) at 1:50; MAb ID5 (40), which in Tetrahymena is specific to polyglutamylated tubulin (53), at 1:50; poly(E) anti-polyglutamic acid antibodies (1:100) (44); TAP952, an anti-monoglycylated tubulin MAb (1:5,000) (9, 10); and 6-11 B-1, an anti-acetyl-K40 α-tubulin MAb (1:200) (28). The secondary goat antibodies were as follows: anti-mouse-FITC, anti-mouse-Cy3, anti-rabbit-Cy3, and anti-rabbit-Cy5 (Zymed) at 1:200 dilutions. Cells were viewed under either Leica TCS SP or Zeiss LSM 510 VIS/META confocal microscopes. To measure the length of the cilia, the cells were labeled with MAb 12G10, [and, in some experiments, double labeled with poly(E) antibodies]. Confocal images were recorded with a 0.8-μm distance between z-sections, and sets of two to four z-sections were merged. The lengths of the cilia were measured by using ImageJ 1.37. For transmission electron microscopy (TEM), the cells were prepared as described previously (24).
Western blots and 2D gels.
For a two-dimensional (2D) separation of tubulin isoforms, cytoskeletons were prepared as described previously (22) from uninduced or induced GFP-Ttll6Ap-expressing cells and washed with the lysis buffer without Triton X-100. A 100-μg portion of the cytoskeletons (15 μl) was separated by isoelectric focusing on 18-cm Immobiline dry strips (4.5-5.5), followed by SDS-PAGE (10%) and silver staining. For Western blots, total extracts from 5 × 103 cells (22) were separated by SDS-8% PAGE (22). The primary antibodies were used as follows at the indicated concentrations: 12G10 (1:10,000), poly(E) (1:2,000), GT335 anti-glutamylation MAb (1:1,000) (55), TAP952 (1:10,000), and 6-11 B-1 (1:10,000).
RESULTS
Overproduction of Ttll6Ap hyperelongates glutamyl side chains on axonemal and cell body microtubules.
Ttll6Ap of Tetrahymena is a potent E-ligase (22) with strong side chain-elongating activity on β-tubulin (50). Here, we explore the consequences of deregulation of the glutamyl side chain length by overexpression of Ttll6Ap in Tetrahymena. We overexpressed Ttll6Ap as an N-terminal GFP fusion using the strong cadmium-dependent MTT1 promoter (22, 45). A Western blot with the anti-polyglutamic acid antibody, poly(E), which recognizes elongated glutamyl side chains (>= 3E), showed that overexpression of GFP-Ttll6Ap increased the levels of polyglutamylation of proteins in the tubulin size range, whereas the levels of other proteins recognized by the same antibody (likely glutamylated nontubulin proteins), remained unchanged (Fig. 1A). 2D SDS-PAGE showed that overproduction of GFP-Ttll6Ap led to appearance of strings of highly acidic isoforms migrating near the main spots of α- and β-tubulin (Fig. 1B and C, arrows). The levels of tubulin glutamylation detected by GT335, an antibody that recognizes a glutamyl side chain of any length (55), were unchanged or slightly lower in overexpressing cells (Fig. 1A). Thus, in vivo, overexpressed Ttll6Ap appears to have primarily a side chain-elongating activity on tubulin.
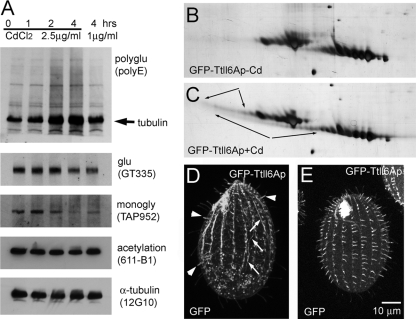
FIG. 1.
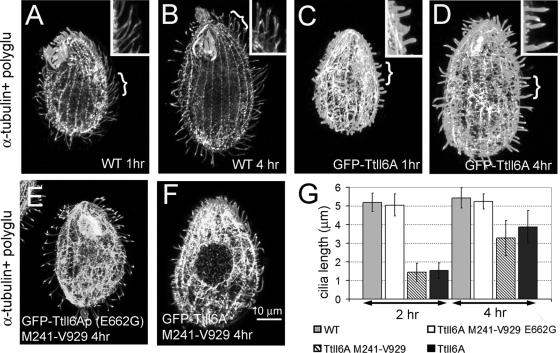
Overproduction of GFP-Ttll6Ap causes elongation of glutamyl side chains on tubulin in vivo. (A) Western blots of total proteins from GFP-Ttll6Ap-expressing cells that are either uninduced (0 h) or induced for 1, 2, or 4 h with either 1 or 2.5 μg of CdCl2/ml. (B and C) 2D silver-stained protein gels containing cytoskeletons of uninduced (B) or induced (C) GFP-Ttll6Ap cells. The arrows point at strings of highly acidic protein isoforms that appear at the mass level of the main spots of α- and β-tubulin upon induction of GFP-Ttll6Ap. (D to E) Confocal fluorescence images of the GFP signal in growing (D) and cilia regenerating (E) GFP-Ttll6Ap cells induced with 2.5 μg of CdCl2/ml for 3 h. Note the increase of the signal of GFP-Ttll6Ap in short growing cilia (arrowheads) and on subcortical microtubules (arrows). Bar, 10 μm.
GFP-Ttll6Ap localized mainly to a subset of short, most likely assembling cilia (see Fig. S1A and B [arrows] in the supplemental material). Consistently, when GFP-Ttll6Ap was overproduced in cilium-regenerating cells, the transgenic protein was targeted to most if not all cilia (Fig. 1E). Overproduction of increased duration (or increased strength) resulted in colocalization of GFP-Ttll6Ap to subcortical and cytoplasmic microtubules (Fig. 1D, arrows), in addition to cilia (Fig. 1D, arrowheads) (see also reference 22).
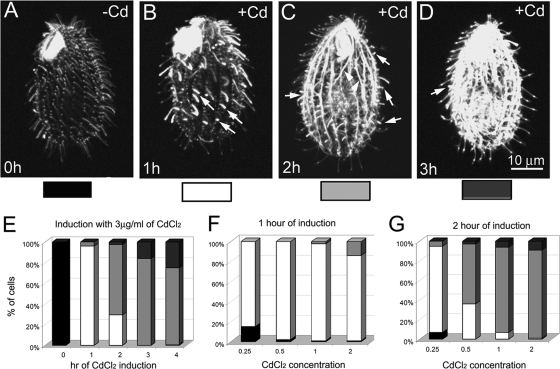
Overproduction of GFP-Ttll6Ap strongly increased the levels of tubulin polyglutamylation on ciliary, cell body, and nuclear microtubules (Fig. 2 and see Fig. S2 in the supplemental material). The pattern of accumulation of tubulin polyglutamylation was dependent on the time and strength of overexpression of GFP-Ttll6Ap (Fig. 1A and 2). A 1-h induction of GFP-Ttll6Ap with 2.5 μg of CdCl2/ml or induction for a longer time by a lower cadmium concentration increased the level of tubulin polyglutamylation mainly in assembling axonemes (Fig. 2A and B). This indicates that cilia are the primary site of the native Ttll6Ap activity. Functional studies agree with this hypothesis: deletion of TTLL6A and a closely related TTLL6F led to a loss of ciliary motility and deletion of additional paralogs (TTLL6B and 6D) led to shortening of cilia (S. Suryavanshi and J. Gaertig, unpublished data). The hyperglutamylated axonemes in GFP-Ttll6Ap cells were shorter than axonemes in untreated cells (Fig. 2B and see Fig. S2B1 to B3 in the supplemental material). With longer induction period or increased strength of induction (cadmium concentration), tubulin polyglutamylation accumulated on cortical and cytoplasmic microtubules (Fig. 2C and D [arrowheads] and Fig. 2E to G).
FIG. 2.
Overproduced GFP-Ttll6Ap increases the levels of tubulin polyglutamylation on ciliary and cell-body microtubules in a time- and overexpression strength-dependent manner. (A to D) Confocal fluorescence images of GFP-Ttll6Ap cells that are untreated (A) or induced with 2.5 μg of CdCl2/ml for 1 (B), 2 (C), or 3 h (D) and subjected to immunofluorescence with a MAb that recognized polyglutamylation (ID5). We reduced the gain levels in B to D to avoid overexposure. Arrows point to short hyperglutamylated cilia, arrowheads points to hyperglutamylated cell body microtubules. Bar, 10 μm. (E to G) Graphs that document the distribution of cells with distinct pattern of tubulin hyperglutamylation as shown in panels A to D, as a function of either cadmium concentration or duration of induction with cadmium.
To determine which parts of Tll6Ap are required for ciliary localization and enzymatic activity, we overexpressed truncated variants of Ttll6Ap as GFP fusions (see Fig. S1and S3 in the supplemental material). The predicted Ttll6Ap is composed of 1,217 amino acids with the conserved TTL-like domain located between V395 and N703 (based on SMART prediction [29]). Truncations of the C-terminal portion of the protein beyond Q828 residue resulted in increased retention of the fusion protein in the cell body (see Fig. S1 in the supplemental material). On the other hand, the fragment R712-L1217 lacking the TTL-like domain was sufficient to target GFP to cilia (see Fig. S1H in the supplemental material). Thus, the R712-L1217 region contains determinants involved in targeting of Ttl6Ap to cilia.
Truncation of 240 amino acids on the N-terminal side and 390 amino acids on the C-terminal side of the TTL-like domain (GFP-Ttll6Ap-M241-Q828 variant) had an E-ligase activity in vivo (see Fig. S3A to D in the supplemental material). Further deletions on either the N- or C-terminal side (resulting in fragments E337-Q828, M241-E725, and A326-V929) abolished the E-ligase activity in vivo (see Fig. S3E in the supplemental material and data not shown). Therefore, among the tested variants, the M241-Q828 fragment is the smallest enzymatically active protein. The TTL homology domain is contained between V395 and N703. Thus, less conserved amino acids adjacent to the TTL-like homology domain contribute to the enzymatic activity, as seen earlier for mammalian E-ligases (50). The majority of GFP-Ttll6Ap-M241-Q828 was associated with cytoplasmic microtubules, and only weak signal was observed in growing cilia during the formation of new oral apparatus prior to cell division (see Fig. S1E [arrowhead] in the supplemental material). In vegetatively growing GFP-Ttll6Ap-M241-Q828-overexpressing cells, short hyperglutamylated cilia were rarely observed but bundles of hyperglutamylated cell body microtubules were abundant (see Fig. S3D in the supplemental material). In contrast to GFP-Ttll6Ap and GFP-Ttll6Ap-M241-V929-overexpressing cells that gradually loose motility (22), GFP-Ttll6Ap-M241-Q828-overexpressing cells remained motile (data not shown). Thus, GFP-Ttll6Ap M241-Q828 acts primarily in the cell body. We used truncated variants of GFP-Ttll6Ap to selectively drive tubulin hyperglutamylation in the cell body (see below). The growth rate of cells overexpressing either full-length or enzymatically active fragments of GFP-Ttll6Ap was reduced compared to wild-type cells treated with the same cadmium concentration (see Fig. S3G in the supplemental material), suggesting that hyperglutamylation of cell body microtubules is deleterious for cell growth.
Tubulin hyperglutamylation increases the abundance and stability of cell body microtubules.
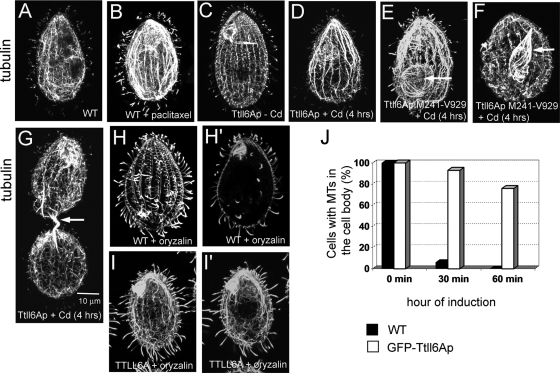
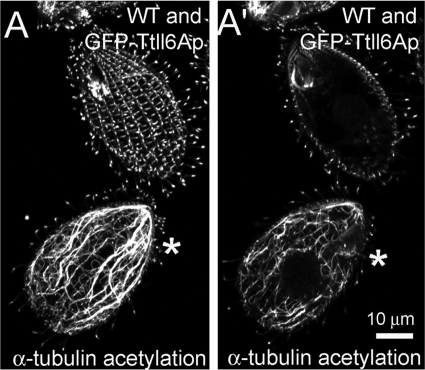
In wild-type Tetrahymena cells, the length of the glutamyl side chains on tubulin is spatially regulated. The cell body microtubules (cytoplasmic, subcortical, and nuclear) have tubulin subunits with side chains limited to a single E (monoglutamylated) except for the postoral fiber microtubules that carry biglutamylated side chains (53). Within the cell cortex, axonemes and basal bodies contain mono- and polyglutamylated microtubules (with biglutamylated or longer side chains), whereas cortical bundles have side chains limited to a single E (53). Overproduction of GFP-Ttll6Ap resulted in the polyglutamylation of diverse microtubules in the cell body, which in wild-type cells are only monoglutamylated, including cytoplasmic network and nuclear microtubules (see Fig. S2C to L in the supplemental material). Moreover, in GFP-Ttll6Ap-overexpressing cells, anti-α-tubulin antibodies revealed abnormally thick bundles of subcortical and nuclear microtubules (Fig. 3C to G and see Fig. S2C to L in the supplemental material). In dividing cells, abnormally thick bundles of intramacronuclear microtubules were present around and within the cytoplasmic bridge connecting the future daughter cells (Fig. 3G, arrow). Bundling of nuclear microtubules was especially apparent in the GFP-Ttll6Ap-M241-V929 cells, and this was likely due to the increased presence of this variant in the cell body (see Fig. S1D in the supplemental material; Fig. 3E and F, arrows). The increased abundance, bundling, and curvature of microtubules indicated that hyperglutamylated microtubules are excessively stable. For example, similar curved bundles of microtubules appear in Tetrahymena cells treated with the microtubule stabilizing drug, paclitaxel (16) (Fig. 3A and B) and in cells with a K350M mutation in β-tubulin that confers paclitaxel sensitivity (47). To probe the stability of cell body microtubules, we treated the wild-type and GFP-Ttll6Ap-overexpressing cells with the microtubule-destabilizing compounds, nocodazole (40 μM) and oryzalin (10 μM). Although these drugs caused rapid depolymerization of cytoplasmic microtubules in wild-type cells (Fig. 3H, H′, and J and data not shown for nocodazole), similarly treated GFP-Ttll6Ap-overexpressing cells retained abundant cell body microtubules (Fig. 3I, I′, and J). Next, we investigated the levels of α-tubulin K40 acetylation in GFP-Ttll6Ap-overproducing cells, since this PTM accumulates on long-lived microtubules (34). Although wild-type cells had a strong K40 acetylation signal that was limited to the stable microtubules of cell cortex and cilia, the GFP-Ttll6Ap-overproducing cells showed abundant K40 acetylation on cytoplasmic microtubules, a finding consistent with increased stability of these microtubules (Fig. 4). Cells overexpressing a variant of GFP-Ttll6Ap that lacks enzymatic activity due to a mutation in the catalytic domain (22) had a wild-type organization of cell body and cortical microtubules and a normal the pattern of K40 acetylation (data not shown). Thus, hyperelongation of glutamyl side chains stabilizes at least a subset of cell body microtubules.
FIG. 3.
Tubulin hyperglutamylation stabilizes cell body microtubules. (A to I′) Confocal microscopic images of cells stained with anti-α-tubulin MAb 12G10. (A and B) Wild-type cells grown without (A) or with (B) 40 μM paclitaxel. Note the appearance of thick bundles of subcortical microtubules in the drug-treated cells. (C to G). Cells expressing GFP-Ttll6Ap or its truncated variant M241-V929 that are uninduced (C) and induced with cadmium (D to G). Note the appearance of bundles of microtubules in the cell body and macronuclei (arrow) in the overproducing cell. (H to I′) Overexpression of GFP-Ttll6Ap protects subcortical and cytoplasmic microtubules against oryzalin-induced depolymerization. Wild-type (H and H′) and GFP-Ttll6Ap-overexpressing (I and I′) cells grown for 4 h in the presence of 2.5 μg of CdCl2/ml were treated for 30 min with 10 μM oryzalin. Panels H′ and I′ show the internal sections of cells shown in panels H and I, respectively. Note the nearly complete depolymerization of cell body microtubules in the wild-type cells but not in Ttll6Ap-overproducing cells. Bar, 10 μm. (J) A graph documents the percentages of wild-type and GFP-Ttll6Ap cells with cytoplasmic microtubules during oryzalin treatment.
FIG. 4.
Hyperelongation of glutamyl side chains in the cell body causes accumulation of α-tubulin K40 acetylation. (A) Wild-type and GFP-Ttll6Ap cells (indicated by the asterisk) were grown for 4 h in the presence of 2.5 μg of CdCl2/ml and labeled side-by-side with antiacetylated tubulin antibodies (6-11 B-1). (A) Projection image of z-section from the top half of the cell; (A′) section showing the middle part of the cell. Bar, 10 μm.
GFP-Ttll6Ap-overproducing cells also had reduced levels of tubulin monoglycylation on stabilized cytoplasmic and cortical microtubules (Fig. 1A and see Fig. S4 in the supplemental material). Similar observations were made for tubulin polyglycylation (data not shown). We have previously documented that the two tubulin polymodifications, glutamylation and glycylation, inhibit each other, and we suggested that this mutual inhibition can be explained either by competition for the same modification sites or steric inhibition of adjacent sites (54). Overproduction of GFP-Ttll6Ap does not lead to an increase in the total number of glutamyl side chains on tubulin (based on Western blots with GT335 MAb that recognizes a side chain of any length [Fig. 1A]). Thus, it is more likely that elongation of glutamyl side chains sterically inhibits the activity of tubulin G-ligases (TTLL3 [21, 38, 54]) on adjacent polymodification sites.
Hyperglutamylation destabilizes microtubules in axonemes.
GFP-Ttll6Ap-overexpressing cells contained both excessively short hyperglutamylated cilia (in which GFP-Ttll6Ap accumulates) and unaffected cilia (with a low GFP-Ttll6Ap signal; Fig. 1D and Fig. S1A and B and S2B in the supplemental material). The short, hyperglutamylated cilia observed within 1 to 4 h after induction of GFP-Ttll6Ap overexpression could be assembling cilia that had failed to elongate or preexisting cilia that had undergone shortening (or both). Since mildly overproduced GFP-Ttll6Ap is preferentially targeted to assembling cilia (Fig. 1E), hyperglutamylation could primarily affect axonemes during their assembly. To test this hypothesis, we deciliated wild-type and GFP-Ttll6Ap-overproducing cells and examined the lengths of the cilia during regeneration. While at 1 h after deciliation wild-type cells had regenerated cilia to 85% of the original length (4.32 ± 0.57 μm, n = 30), during the same period the GFP-Ttll6Ap-overexpressing cells regenerated significantly shorter cilia (1.07 ± 0.35 μm, n = 50 [Fig. 5A and C]). The GFP-Ttll6Ap-overexpressing cells maintained short hyperglutamylated cilia even 4 h after deciliation (3.88 ± 0.89 μm, n = 82; wild-type cells 5.43 ± 0.59 μm, n = 60 [Fig. 5B, D, and G]).
FIG. 5.
Hyperelongation of glutamyl side chains delays cilium regeneration. (A to F) Confocal immunofluorescence images of wild-type (A and B) and GFP-Ttll6Ap (C and D)-, GFP-Ttll6Ap-M241-V929-E662G (E)-, and GFP-Ttll6Ap-M241-V929 (F)-overexpressing cells that regenerated cilia for 1 (A and C), 2 (E and F), or 4 h (B and D) after deciliation. Cells were costained with anti-α-tubulin 12G10 MAb and the anti-polyglutamylation antibody poly(E). Note the shorter size of cilia of GFP-Ttll6Ap cells. Insets show cilia at higher magnifications from the areas indicated by a bracket. (G) A graph shows the average lengths of cilia as a function of time of cilium regeneration. Bar, 10 μm.
The failed elongation of regenerating cilia in cells overproducing GFP-Ttll6Ap could be caused either by the physical presence of GFP-Ttll6Ap or by tubulin hyperglutamylation. To distinguish between these two effects, we compared the lengths of cilia in Tetrahymena cells overexpressing either an enzymatically active and cilium-targeted enzyme (GFP-Ttll6Ap-M241-V929) or an inactive enzyme with an amino acid substitution in the ATP-binding site (GFP-Ttll6Ap-M241-V929-E662G [22]). Whereas cells overproducing an active enzyme regenerated excessively short axonemes (1.44 ± 0.49, n = 62, after 2 h [Fig. 5F and G]), cells overexpressing an inactive enzyme assembled normal length axonemes (5.05 ± 0.59 μm, n = 40, after 2 h [Fig. 5E and G]) despite the fact that the inactive enzyme is targeted to cilia (22). Cells overexpressing GFP-M241-Q828 that localizes mainly to cell bodies, regenerated cilia at the rate similar to that of wild-type cells (see Fig. S5 in the supplemental material). Thus, most likely, the inhibitory effect of GFP-Ttll6Ap on the elongation of axonemes is mediated by hyperelongation of glutamyl side chains on axonemal tubulin.
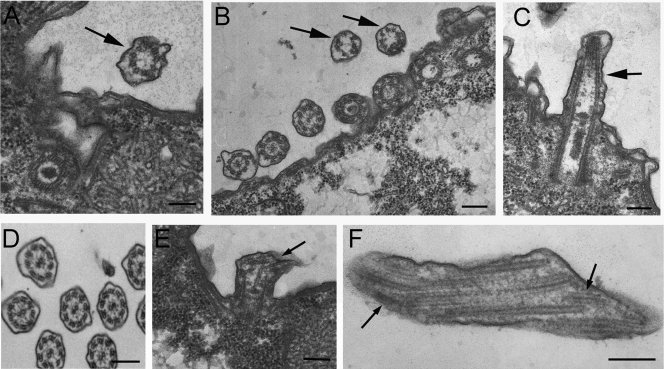
The TEM analysis of vegetatively growing cells overproducing GFP-Ttll6Ap (Fig. 6) revealed two types of axonemes: unaffected 9+2 axonemes and structurally defective mostly 9+0 axonemes. The intact 9+2 axonemes are likely present in the nonassembling cilia that do not accumulate GFP-Ttll6Ap. The defective axonemes frequently lacked a central pair (Fig. 6A to C) and had broken outer doublets (Fig. 6E and F). These observations indicate that, in GFP-Ttll6Ap-overproducing cells, assembling axonemes are unstable.
FIG. 6.
Hyperglutamylation of ciliary microtubules results in structural defects in axonemes. TEM cross-sections (A, B, and D) and longitudinal sections (C, E, and F) of cilia in wild-type (D) and GFP-Ttll6Ap-overproducing (A to C, E, and F) cells. Note that short cilia (A to C) lack a central pair (9+0, arrows) and that broken microtubules are visible (E and F, arrows). Bar, 200 nm.
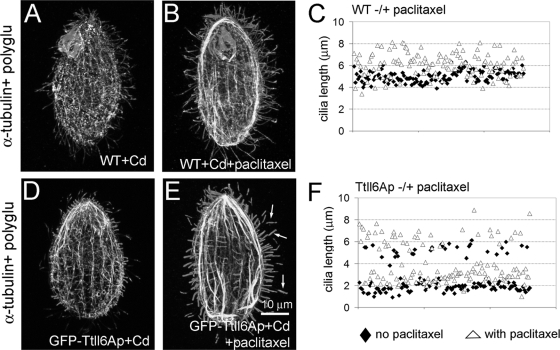
In an attempt to probe how the dynamic properties of axonemal microtubules change as a function of glutamyl side chain hyper-elongation, we treated control and GFP-Ttll6Ap-overproducing cells with 40 μM paclitaxel for 3 h. As previously described in wild-type cells (54), paclitaxel increased the average axoneme length by 20% (length before treatment, 5.02 ± 0.52 μm, n = 129; length after treatment, 6.05 ± 1.12 μm, n = 173 [Fig. 7A to C]). In GFP-overproducing cells, the assembling cilia were 44% longer in the presence of paclitaxel compared to non-drug-treated GFP-Ttll6Ap-overproducing cells (no drug, 1.87 ± 0.36 μm; paclitaxel treated, 2.7 ± 0.6 μm [Fig. 7D to F]). Furthermore, the assembling cilia in GFP-Ttll6Ap-overproducing, paclitaxel-treated cells had elevated levels of tubulin polyglutamylation comparable to those in axonemes of the untreated GFP-Ttll6Ap-overproducing cells. The simplest explanation of these observations is that hyperelongation of glutamyl side chains on the tubulin of assembling axonemal microtubules makes them unstable and that this effect can be counteracted by paclitaxel. Interestingly, in GFP-Ttll6Ap-overexpressing paclitaxel-treated cells, preexisting cilia underwent elongation by 22% by forming a hyperglutamylated distal segment (before treatment, 5.25 ± 0.5 μm; paclitaxel treated, 6.39 ± 0.82 μm [Fig. 7E and F, arrows]). In wild-type cells treated with paclitaxel the distal segments of preexisting elongated axonemes had lower levels of tubulin polyglutamylation compared to the preexisting proximal axoneme segment (see Fig. S6 in the supplemental material). This argues again that the destabilizing effects of hyperglutamylation in the axoneme are counteracted by paclitaxel.
FIG. 7.
Paclitaxel partly rescues the destabilizing effect of hyperglutamylation on elongation of assembling cilia in vegetatively growing Tetrahymena. Confocal immunofluorescence images of wild-type (A and B) and GFP-Ttll6Ap-overexpressing (D and E) cells grown without (A and D) or with (B and E) 40 μM paclitaxel. Cells were costained with anti-α-tubulin antibodies and anti-poly(E) antibodies. The arrows in panel E point to hyperglutamylated distal segments. Bar, 10 μm. (C and F) A graph documents the distribution of cilium length in wild-type (C) and GFP-Ttll6Ap-overexpressing (F) cells. Black diamonds indicate the cilium lengths in cells grown without drug, and open triangles represent the cilium lengths in cells grown in the presence of paclitaxel.
DISCUSSION
The polymeric character of glycylation and glutamylation, as well as the fact that these PTMs affect multiple modification sites within the tubulin CTTs and can coexist on the same tubulin proteins, generates an exceptionally large number of tubulin isoforms. The polymodification sites on β-tubulin are required for axoneme assembly (49). Functional studies on the E- and G-ligases indicate that both glutamylation and glycylation on tubulin are important and contribute to either the assembly or the stability of microtubules, including those present in neural extensions (20), and axonemes (33, 38, 54). However, it is not known what the structural consequences of tubulin polymodifications on microtubules are.
The length of the polymodification side chain is spatially regulated and dependent on the microtubule type and possibly on the position of tubulin subunits within the microtubule (8, 53). Moreover, in multicellular organisms, the glutamyl side chain length changes during organismal development (3, 20). Among the mechanisms that regulate the side chain length could be (i) temporal and spatial regulation of the initiation and elongation steps performed by E-ligases and (ii) selective shortening of glutamyl side chain by deglutamylases (3). Whereas some E-ligases, such as the murine TTLL7, can both initiate and elongate the side chains (32), the majority of studied E-ligases have a bias for either chain elongation or initiation (22, 50, 53). Ttll6Ap is a strong elongase for β-tubulin (50; the present study). We have studied here the consequences of hyperelongation of glutamyl side chain in vivo by overexpressing Ttll6Ap. Although the activity mediated by GFP-Ttll6Ap on the nonciliary microtubules could be nonphysiological, this ectopic activity gave us a tool to investigate the importance of the side chain length regulation on multiple types of microtubules within the same cell.
We show that the consequences of overexpression of Ttll6Ap depend on the cellular context. Hyperelongation of glutamyl side chains on cell body microtubules increased the density and bundling of microtubules, resistance to depolymerizing drugs, and α-tubulin K40 acetylation. These effects are consistent with an increased stability of hyperglutamylated cell body microtubules. In wild-type Tetrahymena cells, the most dynamic microtubules (e.g., the cytoplasmic network, micronuclear spindle, and macronuclear and longitudinal cortical microtubules) have tubulin subunits with side chains limited to monoglutamylation (8, 53). In contrast, basal bodies and axonemes have elongated glutamyl side chains, and these microtubules are known to be extremely stable (turnover slowly and resist standard depolymerizing treatments) (48). We speculate that the physiological elongation of glutamyl side chains on tubulin of basal body and axonemal microtubules contributes to their increased stability. Indeed, deletion of some TTLL6 genes led to shortening of axonemes (Suryavanshi and Gaertig, unpublished). In GFP-Ttll6Ap-overproducing cells, hyperelongation of the cell body microtubules could lead to capture of axoneme-stabilizing MAPs that are in transit to cilia. A model that elongation of the glutamyl side chains stabilizes microtubules by recruiting MAPs likely applies to other contexts. In neurons, the accumulation of tubulin polyglutamylation during differentiation correlates with increased stability of microtubules and the accumulation of MAP2 in dendrites and the cell body (20). Moreover, knockdown of TTLL7 E-ligase inhibited the formation of MAP2-positive neurites in PC-12 cells (20). In vitro studies show that the levels of tubulin glutamylation affect the binding of certain structural MAPs and motor proteins to microtubules (6, 7, 19, 27). Future studies in vitro based on microtubule polyglutamylation with purified E-ligases should shed light on the mechanism of polyglutamylation-induced microtubule stabilization and, in particular, should reveal whether polyglutamylation has a direct effect on the microtubule dynamics or acts via MAPs.
Given the apparent stabilizing effect of the hyperelongation of glutamyl side chains on the cell body microtubules and the fact that native axonemes have relatively long glutamyl side chains, it was surprising that we observed a seemingly opposite effect of hyperglutamylation on axonemes. It appears that the destabilizing effects of overexpressed Ttll6Ap on axonemes are largely autonomous and cannot be explained by the retention of stabilizing axoneme-destined MAPs in the cell body. The shortening of axonemes could be explained by inhibition of the intraflagellar transport (IFT) pathway, a motility mechanism that moves precursors required for cilia assembly along growing outer doublet microtubules (26). However, the defect in the elongation of axonemes in GFP-Ttll6Ap cells is partly rescuable by paclitaxel, suggesting that the elongation of glutamyl side chains destabilizes axonemal microtubules. Moreover, paclitaxel failed to rescue an axoneme assembly defect caused by a loss of function of IFT in the DYF1 knockout strain of Tetrahymena (12; unpublished data).
Hyperelongation of glutamyl side chains on tubulin could affect the dynamics of microtubules. This model agrees with the observation that the removal of CTTs by proteolysis with subtilisin increases the resistance of microtubules to depolymerization by high salt and cold (4, 41), although other studies disagree with this conclusion (25, 42). CTTs are highly negatively charged and could interact with the positively charged surface of the tubulin dimer (35). Glutamylation further increases the negative charge of CTTs. The bonds between the dimers could be weakened by charge repulsion, especially if CTTs of neighboring tubulin subunits can interact with each other. However, we would need to assume that in GFP-Ttll6Ap-overproducing cells, and specifically in the cell body, the lattice-weakening effects of glutamylation are counteracted by stabilizing MAPs that preferentially bind to hyperglutamylated microtubules.
Alternatively, the restriction of the destabilizing effect of hyperglutamylation to the axoneme could result from differential utilization of tubulin subunits. In vitro studies showed that Ttll6Ap prefers β-tubulin (22), but the same enzyme, when overexpressed in vivo, modified both α- and β-tubulin (Fig. 1C). Thus, some differences in the consequences of excessive activity of Ttll6Ap could result from the differential utilization of α- and β-tubulin subunits in different microtubules, which in turn could be caused by competing MAPs that selectively hinder one of the two tubulin subunits.
However, another explanation of the restriction of destabilizing influence of hyperglutamylation to axonemes is that this effect is mediated by axoneme-restricted factors that are regulated by polyglutamylation. Specifically, in assembling axoneme, hyperelongation of glutamyl side chains could increase the activity of factors that promote microtubule depolymerization. A microtubule-severing protein, katanin, plays a prominent role in the axoneme assembly. Katanin localizes to cilia in Tetrahymena and Chlamydomonas (13, 46). The presence of CTTs is required for the katanin-mediated microtubule-severing activity in vitro (30). Knockouts of katanin subunit genes in Tetrahymena phenocopy the substitutions of glutamic acids that undergo polymodifications in the CTT of β-tubulin (46). The activity of spastin, another microtubule-severing protein, is blocked by an antibody that recognized a terminal glutamic acid, a finding consistent with a requirement of either detyrosination or polyglutamylation (or both) for severing activity (39). In the axoneme, tubulin hyperglutamylation could cause an excessive activity of severing factors such as katanin, specifically during axoneme assembly, and this could prevent axoneme elongation and assembly of central microtubules. Interestingly, the levels of tubulin glutamylation appear to change during axoneme assembly in wild-type cells. The short assembling cilia label more strongly with antibodies that recognize elongated glutamyl side chains. The signal of polyglutamylation decreases as cilia mature, while the levels of polyglycylation increase in these cilia (46). Thus, axonemal microtubules undergo remodeling of the PTM composition as part of the polymer maturation. It is possible that some glutamyl side chains are trimmed down or completely removed by deglutamylating enzymes (2) and are replaced by glycyl side chains. Thus, tubulin glutamylation could play distinct roles during and after assembly of the axoneme. In a growing axoneme, tubulin polyglutamylation could promote polymer turnover, whereas in the mature axoneme the modification could contribute to increased stability of the polymer.
To summarize, we show that the effects of tubulin hyperglutamylation are subcellular context specific. In the same cells, hyperglutamylation stabilizes cell body microtubules and destabilizes axonemes. We propose that the differential effects of hyperglutamylation are mediated by nonuniformly distributed MAPs.
Supplementary Material
Acknowledgments
This study was supported by the National Science Foundation (MBC-033965 to J.G.).
We are grateful to the following researchers for providing reagents: Martin A. Gorovsky (University of Rochester) for poly(E) antibodies, Joseph Frankel (University of Iowa) for MAb 12G10 (available from the Developmental Studies Hybridoma Bank), Marie-Helene Bré for TAP952 MAb, and Klaus Weber (Max Planck Institute, Goettingen, Germany) for MAb ID5.
Footnotes
Published ahead of print on 21 August 2009.
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1.Abal, M., G. Keryer, and M. Bornens. 2005. Centrioles resist forces applied on centrosomes during G2/M transition. Biol. Cell 97:425-434. [DOI] [PubMed] [Google Scholar]
- 2.Audebert, S., E. Desbruyères, C. Gruszczynski, A. Koulakoff, F. Gros, P. Denoulet, and B. Eddé. 1993. Reversible polyglutamylation of alpha- and beta-tubulin and microtubule dynamics in mouse brain neurons. Mol. Biol. Cell 4:615-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Audebert, S., A. Koulakoff, Y. Berwald-Netter, F. Gros, P. Denoulet, and B. Eddé. 1994. Developmental regulation of polyglutamylated alpha- and beta-tubulin in mouse brain neurons. J. Cell Sci. 107(Pt. 8):2313-2322. [DOI] [PubMed] [Google Scholar]
- 4.Bhattacharyya, B., D. L. Sackett, and J. Wolff. 1985. Tubulin, hybrid dimers, and tubulin S: stepwise charge reduction and polymerization. J. Biol. Chem. 260:10208-10216. [PubMed] [Google Scholar]
- 5.Bobinnec, Y., M. Moudjou, J. P. Fouquet, E. Desbruyères, B. Eddé, and M. Bornens. 1998. Glutamylation of centriole and cytoplasmic tubulin in proliferating non-neuronal cells. Cell Motil. Cytoskeleton 39:223-232. [DOI] [PubMed] [Google Scholar]
- 6.Bonnet, C., D. Boucher, S. Lazereg, B. Pedrotti, K. Islam, P. Denoulet, and J. C. Larcher. 2001. Differential binding regulation of microtubule-associated proteins MAP1A, MAP1B, and MAP2 by tubulin polyglutamylation. J. Biol. Chem. 276:12839-12848. [DOI] [PubMed] [Google Scholar]
- 7.Boucher, D., J. C. Larcher, F. Gros, and P. Denoulet. 1994. Polyglutamylation of tubulin as a progressive regulator of in vitro interactions between the microtubule-associated protein Tau and tubulin. Biochemistry 33:12471-12477. [DOI] [PubMed] [Google Scholar]
- 8.Bré, M. H., B. de Nechaud, A. Wolff, and A. Fleury. 1994. Glutamylated tubulin probed in ciliates with the monoclonal antibody GT335. Cell Motil. Cytoskeleton 27:337-349. [DOI] [PubMed] [Google Scholar]
- 9.Bré, M. H., V. Redeker, J. Vinh, J. Rossier, and N. Levilliers. 1998. Tubulin polyglycylation: differential posttranslational modification of dynamic cytoplasmic and stable axonemal microtubules in paramecium. Mol. Biol. Cell 9:2655-2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Callen, A. M., A. Adoutte, J. M. Andrew, A. Baroin-Tourancheau, M. H. Bré, P. C. Ruiz, J. C. Clerot, P. Delgado, A. Fleury, R. Jeanmaire-Wolf, et al. 1994. Isolation and characterization of libraries of monoclonal antibodies directed against various forms of tubulin in paramecium. Biol. Cell 81:95-119. [DOI] [PubMed] [Google Scholar]
- 11.Campbell, P. K., K. G. Waymire, R. L. Heier, C. Sharer, D. E. Day, H. Reimann, J. M. Jaje, G. A. Friedrich, M. Burmeister, T. J. Bartness, L. D. Russell, L. J. Young, M. Zimmer, D. E. Jenne, and G. R. MacGregor. 2002. Mutation of a novel gene results in abnormal development of spermatid flagella, loss of intermale aggression and reduced body fat in mice. Genetics 162:307-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dave, D., D. Wloga, N. Sharma, and J. Gaertig. 2009. DYF-1 is required for assembly of the axoneme in Tetrahymena thermophila. Eukaryot. Cell 8:1397-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dymek, E. E., P. A. Lefebvre, and E. F. Smith. 2004. PF15p is the Chlamydomonas homologue of the Katanin p80 subunit and is required for assembly of flagellar central microtubules. Eukaryot. Cell 3:870-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eddé, B., J. Rossier, J. P. Le Caer, E. Desbruyères, F. Gros, and P. Denoulet. 1990. Posttranslational glutamylation of alpha-tubulin. Science 247:83-85. [DOI] [PubMed] [Google Scholar]
- 15.Fouquet, J. P., B. Eddé, M. L. Kann, A. Wolff, E. Desbruyères, and P. Denoulet. 1994. Differential distribution of glutamylated tubulin during spermatogenesis in mammalian testis. Cell Motil. Cytoskeleton 27:49-58. [DOI] [PubMed] [Google Scholar]
- 16.Fujiu, K., and O. Numata. 2000. Reorganization of microtubules in the amitotically dividing macronucleus of Tetrahymena. Cell Motil. Cytoskeleton 46:17-27. [DOI] [PubMed] [Google Scholar]
- 17.Gagnon, C., D. White, J. Cosson, P. Huitorel, B. Eddé, E. Desbruyères, L. Paturle-Lafanechere, L. Multigner, D. Job, and C. Cibert. 1996. The polyglutamylated lateral chain of alpha-tubulin plays a key role in flagellar motility. J. Cell Sci. 109(Pt. 6):1545-1553. [DOI] [PubMed] [Google Scholar]
- 18.Gorovsky, M. A., M. C. Yao, J. B. Keevert, and G. L. Pleger. 1975. Isolation of micro- and macronuclei of Tetrahymena pyriformis. Methods Cell Biol. 9:311-327. [DOI] [PubMed] [Google Scholar]
- 19.Ikegami, K., R. L. Heier, M. Taruishi, H. Takagi, M. Mukai, S. Shimma, S. Taira, K. Hatanaka, N. Morone, I. Yao, P. K. Campbell, S. Yuasa, C. Janke, G. R. Macgregor, and M. Setou. 2007. Loss of alpha-tubulin polyglutamylation in ROSA22 mice is associated with abnormal targeting of KIF1A and modulated synaptic function. Proc. Natl. Acad. Sci. U. S. A. 104:3213-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ikegami, K., M. Mukai, J. Tsuchida, R. L. Heier, G. R. Macgregor, and M. Setou. 2006. TTLL7 is a mammalian beta-tubulin polyglutamylase required for growth of MAP2-positive neurites. J. Biol. Chem. 281:30707-30716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ikegami, K., and M. Setou. 2009. TTLL10 can perform tubulin glycylation when coexpressed with TTLL8. FEBS Lett. 583:1957-1963. [DOI] [PubMed] [Google Scholar]
- 22.Janke, C., K. Rogowski, D. Wloga, C. Regnard, A. V. Kajava, J. M. Strub, N. Temurak, J. van Dijk, D. Boucher, A. van Dorsselaer, S. Suryavanshi, J. Gaertig, and B. Eddé. 2005. Tubulin polyglutamylase enzymes are members of the TTL domain protein family. Science 308:1758-1762. [DOI] [PubMed] [Google Scholar]
- 23.Jerka-Dziadosz, M., L. M. Jenkins, E. M. Nelsen, N. E. Williams, R. Jaeckel-Williams, and J. Frankel. 1995. Cellular polarity in ciliates: persistence of global polarity in a disorganized mutant of Tetrahymena thermophila that disrupts cytoskeletal organization. Dev. Biol. 169:644-661. [DOI] [PubMed] [Google Scholar]
- 24.Jerka-Dziadosz, M., I. Strzyzewska-Jowko, U. Wojsa-Lugowska, W. Krawczynska, and A. Krzywicka. 2001. The dynamics of filamentous structures in the apical band, oral crescent, fission line and the postoral meridional filament in Tetrahymena thermophila revealed by monoclonal antibody 12G9. Protist 152:53-67. [DOI] [PubMed] [Google Scholar]
- 25.Knipling, L., J. Hwang, and J. Wolff. 1999. Preparation and properties of pure tubulin S. Cell Motil. Cytoskeleton 43:63-71. [DOI] [PubMed] [Google Scholar]
- 26.Kozminski, K. G., K. A. Johnson, P. Forscher, and J. L. Rosenbaum. 1993. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc. Natl. Acad. Sci. U. S. A. 90:5519-5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larcher, J. C., D. Boucher, S. Lazereg, F. Gros, and P. Denoulet. 1996. Interaction of kinesin motor domains with alpha- and beta-tubulin subunits at a tau-independent binding site: regulation by polyglutamylation. J. Biol. Chem. 271:22117-22124. [DOI] [PubMed] [Google Scholar]
- 28.LeDizet, M., and G. Piperno. 1991. Detection of acetylated alpha-tubulin by specific antibodies. Methods Enzymol. 196:264-274. [DOI] [PubMed] [Google Scholar]
- 29.Letunic, I., T. Doerks, and P. Bork. 2009. SMART 6: recent updates and new developments. Nucleic Acids Res. 37:D229-D232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McNally, F. J., and R. D. Vale. 1993. Identification of katanin, an ATPase that severs and disassembles stable microtubules. Cell 75:419-429. [DOI] [PubMed] [Google Scholar]
- 31.Million, K., J. Larcher, J. Laoukili, D. Bourguignon, F. Marano, and F. Tournier. 1999. Polyglutamylation and polyglycylation of alpha- and beta-tubulins during in vitro ciliated cell differentiation of human respiratory epithelial cells. J. Cell Sci. 112(Pt. 23):4357-4366. [DOI] [PubMed] [Google Scholar]
- 32.Mukai, M., K. Ikegami, Y. Sugiura, K. Takeshita, A. Nakagawa, and M. Setou. 2009. Recombinant mammalian tubulin polyglutamylase TTLL7 performs both initiation and elongation of polyglutamylation on beta-tubulin through a random sequential pathway. Biochemistry 48:1084-1093. [DOI] [PubMed] [Google Scholar]
- 33.Pathak, N., T. Obara, S. Mangos, Y. Liu, and I. A. Drummond. 2007. The zebrafish fleer gene encodes an essential regulator of cilia tubulin polyglutamylation. Mol. Biol. Cell 18:4353-4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piperno, G., M. LeDizet, and X. J. Chang. 1987. Microtubules containing acetylated alpha-tubulin in mammalian cells in culture. J. Cell Biol. 104:289-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Priel, A., J. A. Tuszynski, and N. J. Woolf. 2005. Transitions in microtubule C termini conformations as a possible dendritic signaling phenomenon. Eur. Biophys. J. 35:40-52. [DOI] [PubMed] [Google Scholar]
- 36.Redeker, V., N. Levilliers, J. M. Schmitter, J. P. Le Caer, J. Rossier, A. Adoutte, and M. H. Bré. 1994. Polyglycylation of tubulin: a posttranslational modification in axonemal microtubules. Science 266:1688-1691. [DOI] [PubMed] [Google Scholar]
- 37.Regnard, C., D. Fesquet, C. Janke, D. Boucher, E. Desbruyères, A. Koulakoff, C. Insina, P. Travo, and B. Eddé. 2003. Characterisation of PGs1, a subunit of a protein complex co-purifying with tubulin polyglutamylase. J. Cell Sci. 116:4181-4190. [DOI] [PubMed] [Google Scholar]
- 38.Rogowski, K., F. Juge, J. van Dijk, D. Wloga, J. M. Strub, N. Levilliers, D. Thomas, M. H. Bré, A. Van Dorsselaer, J. Gaertig, and C. Janke. 2009. Evolutionary divergence of enzymatic mechanisms for posttranslational polyglycylation. Cell 137:1076-1087. [DOI] [PubMed] [Google Scholar]
- 39.Roll-Mecak, A., and R. D. Vale. 2008. Structural basis of microtubule severing by the hereditary spastic paraplegia protein spastin. Nature 451:363-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rudiger, A. H., M. Rudiger, J. Wehland, and K. Weber. 1999. Monoclonal antibody ID5: epitope characterization and minimal requirements for the recognition of polyglutamylated alpha- and beta-tubulin. Eur. J. Cell Biol. 78:15-20. [DOI] [PubMed] [Google Scholar]
- 41.Sackett, D. L., B. Bhattacharyya, and J. Wolff. 1985. Tubulin subunit carboxyl termini determine polymerization efficiency. J. Biol. Chem. 260:43-45. [PubMed] [Google Scholar]
- 42.Saoudi, Y., I. Paintrand, L. Multigner, and D. Job. 1995. Stabilization and bundling of subtilisin-treated microtubules induced by microtubule associated proteins. J. Cell Sci. 108(Pt. 1):357-367. [DOI] [PubMed] [Google Scholar]
- 43.Schliwa, M., and J. van Blerkom. 1981. Structural interaction of cytoskeletal components. J. Cell Biol. 90:222-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shang, Y., B. Li, and M. A. Gorovsky. 2002. Tetrahymena thermophila contains a conventional gamma-tubulin that is differentially required for the maintenance of different microtubule-organizing centers. J. Cell Biol. 158:1195-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shang, Y., X. Song, J. Bowen, R. Corstanje, Y. Gao, J. Gaertig, and M. A. Gorovsky. 2002. A robust inducible-repressible promoter greatly facilitates gene knockouts, conditional expression, and overexpression of homologous and heterologous genes in Tetrahymena thermophila. Proc. Natl. Acad. Sci. U. S. A. 99:3734-3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharma, N., J. Bryant, D. Wloga, R. Donaldson, R. C. Davis, M. Jerka-Dziadosz, and J. Gaertig. 2007. Katanin regulates dynamics of microtubules and biogenesis of motile cilia. J. Cell Biol. 178:1065-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith, J. J., J. S. Yakisich, G. M. Kapler, E. S. Cole, and D. P. Romero. 2004. A beta-tubulin mutation selectively uncouples nuclear division and cytokinesis in Tetrahymena thermophila. Eukaryot. Cell 3:1217-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thazhath, R., M. Jerka-Dziadosz, J. Duan, D. Wloga, M. A. Gorovsky, J. Frankel, and J. Gaertig. 2004. Cell context-specific effects of the beta-tubulin glycylation domain on assembly and size of microtubular organelles. Mol. Biol. Cell 15:4136-4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thazhath, R., C. Liu, and J. Gaertig. 2002. Polyglycylation domain of beta-tubulin maintains axonemal architecture and affects cytokinesis in Tetrahymena. Nat. Cell Biol. 4:256-259. [DOI] [PubMed] [Google Scholar]
- 50.van Dijk, J., K. Rogowski, J. Miro, B. Lacroix, B. Eddé, and C. Janke. 2007. A targeted multienzyme mechanism for selective microtubule polyglutamylation. Mol. Cell 26:437-448. [DOI] [PubMed] [Google Scholar]
- 51.Verhey, K. J., and J. Gaertig. 2007. The tubulin code. Cell Cycle 6:2152-2160. [DOI] [PubMed] [Google Scholar]
- 52.Wloga, D., A. Camba, K. Rogowski, G. Manning, M. Jerka-Dziadosz, and J. Gaertig. 2006. Members of the NIMA-related kinase family promote disassembly of cilia by multiple mechanisms. Mol. Biol. Cell 17:2799-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wloga, D., K. Rogowski, N. Sharma, J. Van Dijk, C. Janke, B. Eddé, M. H. Bré, N. Levilliers, V. Redeker, J. Duan, M. A. Gorovsky, M. Jerka-Dziadosz, and J. Gaertig. 2008. Glutamylation on alpha-tubulin is not essential but affects the assembly and functions of a subset of microtubules in Tetrahymena thermophila. Eukaryot. Cell 7:1362-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wloga, D., D. M. Webster, K. Rogowski, M. H. Bré, N. Levilliers, M. Jerka-Dziadosz, C. Janke, S. T. Dougan, and J. Gaertig. 2009. TTLL3 is a tubulin glycine ligase that regulates the assembly of cilia. Dev. Cell 16:867-876. [DOI] [PubMed] [Google Scholar]
- 55.Wolff, A., B. de Nechaud, D. Chillet, H. Mazarguil, E. Desbruyères, S. Audebert, B. Eddé, F. Gros, and P. Denoulet. 1992. Distribution of glutamylated alpha and beta-tubulin in mouse tissues using a specific monoclonal antibody, GT335. Eur. J. Cell Biol. 59:425-432. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.