Abstract
The export of virulence factors, such as the capsule polysaccharide, to the cell surface is a critical aspect of the pathogenicity of Cryptococcus neoformans. A view of capsule export via exocytosis and extracellular vesicles is emerging, but the molecular mechanisms underlying virulence factor transport pathways remain to be established. In this study, we characterized the APT1 gene, which encodes a predicted integral membrane P-type ATPase belonging to the type IV, Drs2 family of aminophospholipid translocases (flippases) (APTs). APTs maintain the phospholipid asymmetry that is critical in membrane fusion events for trafficking and in establishing cell polarity. Deletion of the APT1 gene resulted in phenotypes consistent with similar roles in C. neoformans. These included altered actin distribution, increased sensitivity to stress conditions (oxidative and nitrosative stress) and to trafficking inhibitors, such as brefeldin A and monensin, a reduction in exported acid phosphatase activity, and hypersensitivity to the antifungal drugs amphotericin B, fluconazole, and cinnamycin. However, there was no difference in growth, capsule size, or melanin production between the wild type and the apt1 mutant strains at either 30°C or 37°C. Despite the absence of an influence on these major virulence factors, Apt1 was required for survival during interactions with macrophages, and apt1 mutants exhibited attenuated virulence in a mouse inhalation model of cryptococcosis. Therefore, Apt1 contributes to virulence and the stress response in C. neoformans through apparent functions in membrane fusion and trafficking that do not influence the deposition of major virulence factors, such as capsule and melanin, outside the cell.
The opportunistic fungal pathogen Cryptococcus neoformans causes life-threatening meningoencephalitis in immunocompromised individuals (44). One million cases of cryptococcosis are estimated to occur each year, and approximately two-thirds of these are fatal (43). Key virulence traits for the fungus include growth at the mammalian host temperature, production of a polysaccharide capsule, deposition of laccase-synthesized melanin in the cell wall, secretion of enzymes, and resistance to host defenses, such as oxidative and nitrosative killing (44).
The polysaccharide capsule is a key virulence factor and is both cell associated and released during infection (4). The two species of polysaccharide in the capsule, an abundant glucuronoxylomannan (GXM) and a minor galactoxylomannan (GalXM), cause a number of deleterious effects in mammalian hosts (4, 44). Extracellular vesicles (exosomes) containing capsule polysaccharide are present in culture supernatants, in lysates of macrophages containing C. neoformans, and in association with fungal cells during murine infection (41, 49, 50, 54). These so-called “virulence factor delivery bags” are thought to pass through the cell wall to deliver material outside the cell (50). Proteomic analysis of the vesicles identified 76 proteins, and many of these are associated with virulence, including urease, laccase, heat shock proteins, superoxide dismutase, thiol-specific antioxidants, and catalases (49).
The mechanisms of trafficking of capsule polysaccharide and laccase are being actively pursued. For example, analysis of a mutant with a defect in the exocyst GTPase Sec4/Rab8 (designated Sav1) revealed the accumulation of intracellular vesicles containing capsule polysaccharide, thus providing support for intracellular synthesis and secretion via exocytosis (60). In addition, reduced expression of the exocyst protein Sec6 due to RNA interference (RNAi) resulted in partial attenuation of virulence as well as defects in melanin production and the export of urease and soluble capsule polysaccharide (42). The RNAi strains were also completely defective in the production of extracellular exosomes but retained wild-type (WT) levels of cell-associated capsule. Trafficking of the laccase required for melanin production and virulence has also been examined. Hu et al. (25) showed that C. neoformans lacking Vps34 (vacuolar protein sorting 34) had a marked reduction in melanin formation, suggesting that laccase-containing vesicles are derived from the endocytic pathway. Overall, the current evidence suggests that exocytic, endocytic, and specialized extracellular vesicles mediate the export of capsule and other virulence factors in C. neoformans (42, 49, 60).
We demonstrated previously that vesicle trafficking functions in C. neoformans are regulated by the cAMP signal transduction pathway, which also controls the elaboration of both the capsule and melanin (28). We found that treatment of C. neoformans with inhibitors of Golgi apparatus-mediated transport (e.g., brefeldin A or monensin) or with lithium chloride results in inhibition of capsule expression (28). In addition, we found that cAMP-dependent protein kinase regulated the expression of a predicted phospatidylethanolamine binding protein, Ova1, which negatively influences capsule and melanin formation. These findings focused our attention on the roles of intracellular trafficking functions and phospholipids in virulence factor expression.
In the context of phospholipid trafficking, some aminophospholipid translocases within the P-type ATPases are known to play roles in fungal virulence. For example, the aminophospholipid translocase MgApt2 is required for exocytosis during plant infection by the rice blast pathogen Magnaporthe grisea (18). P-type ATPases are a large family of multitransmembrane domain, ATP-dependent transporters, and three subfamilies are found in eukaryotes (29): (i) heavy metal ion ATPases (e.g., copper transporters), (ii) non-heavy-metal ion ATPases (e.g., Ca2+, H+, Na+, and K+ ATPases), and (iii) aminophospholipid translocases (APTs/flippases of the type IV or Drs2 family). APTs maintain the asymmetrical distribution of aminophospholipids in membranes by translocating phosphatidylserine (PS) and/or phosphatidylethanolamine (PE) from one leaflet of the bilayer to the other. Phospholipid asymmetry is important in membrane fusion events (vesicle budding and docking) at the plasma membrane and in the trans-Golgi network (3). Thus, APTs are required for efficient Golgi function and play roles in both endocytosis and exocytosis. Some disorders in humans have been linked or attributed to genes from the APT subfamily, including familial intrahepatic cholestasis and Angelman syndrome (32, 55).
Previously, we constructed a deletion of the APT1 gene, encoding a putative aminophospholipid translocase, as part of a study to examine disomy at chromosome 13 in C. neoformans (27). Our preliminary phenotypic analysis suggested a connection to nitrosative stress and prompted further investigation of virulence-related functions. In the present study, we show that Apt1 is functionally related to Drs2 in Saccharomyces cerevisiae and has roles in membrane trafficking and sensitivity to stress (oxidative and nitrosative) and drugs targeting ergosterol biosynthesis and secretion. Importantly, loss of Apt1 does not influence capsule and melanin formation, but the protein is required for intracellular growth in macrophages and for full virulence in mice.
MATERIALS AND METHODS
Fungal strains, plasmids, and media.
Serotype A strains H99 and CBS7779 (C. neoformans var. grubii) and S. cerevisiae strains BY4742 (MATα his3 ura3 leu2 lys2), ZHY615M2D (MATα his3 ura3 leu2 lys2 drs2Δ), and PFY3273A (MATα his3 ura3 leu2 met15 dnf1Δ dnf2Δ dnf3Δ) (29) were employed. The strains were maintained on YPD medium (1% yeast extract, 2% peptone, 2% dextrose, and 2% agar). Plasmid pCH233 was the source of a nourseothricin resistance cassette, and pJAF1 was the source of a neomycin resistance cassette. Plasmid pBPH618 was used for yeast transformation. YPD plates containing neomycin (200 μg/ml) were used to select C. neoformans apt1 deletion transformants. YNB agar without uracil (yeast nitrogen base without amino acids; 6.7% g/liter) supplemented with 2% glucose and other nutrients as needed was used to select the S. cerevisiae transformants. YPD and/or YNB plates (YNB with amino acids) supplemented with different inhibitors or chemicals were used for phenotypic characterization. Escherichia coli was grown at 37°C in LB broth or on agar supplemented with 100 μg/ml of ampicillin.
Complementation of S. cerevisiae drs2 mutation with C. neoformans APT1 gene.
To obtain an APT1 cDNA from C. neoformans, total RNA was isolated and cDNA was synthesized using random hexamers and Superscript transcriptase II (Invitrogen Canada). A 4,686-bp PCR product was obtained using primers Apt1-cDNA-5 and Apt1-cDNA-3 (see Table S1 in the supplemental material) and cloned into pBPH618 to create pCnAPT1c. To complement the yeast drs2Δ single mutant and the dnf1Δ dnf2Δ dnf3Δ triple mutant with the C. neoformans APT1 gene, pCnAPT1c and pBPH618 (empty vector) were transformed into yeast strains (ZHY615M2D and PFY3273A) by polyethylene glycol (PEG) and lithium acetate treatment. Transformants were selected on synthetic complete (SC) dextrose-URA medium and 5-fluoroorotic acid (5-FOA) plates for uracil prototrophy and on SC medium supplemented with calcofluor white (0.5 mg/ml) for growth assays.
Deletion of APT1 in C. neoformans.
The APT1 gene in strains H99 and CBS7779 was replaced with a neomycin resistance cassette to examine the disomy of chromosome 13 in C. neoformans serotype A strains, as previously described (27). In the present study, two additional biolistic transformations were performed to obtain a larger number of independent deletion mutants. An apt1::NEO disruption allele was constructed using a modified overlap PCR procedure (10, 61) and the primers listed in Table S1 in the supplemental material. Briefly, the primers apt1-1/apt1-3 and apt1-4/apt1-6 were used with genomic DNA to obtain the left and right arms for the deletion construct. The selectable marker NEO was amplified from the plasmid pJAF1 using the primers apt1-2/apt1-5. Overlap PCR was performed using the primers apt1-1/apt1-6 to yield an apt1::NEO allele that lacks the entire open reading frame of APT1 (4,888 bp). The resulting PCR product (3.9 kb) was used to transform strain H99 by biolistic transformation (11). Transformants were screened by colony PCR with Extaq polymerase (Takara) using the primers apt1-7/apt1-8 (negative screen) and apt1-9/hug-Neo (positive screen). Primer apt1-9 was designed from the region upstream of APT1, and hug-Neo was designed for the NEO gene. Transformants in which the wild-type (WT) allele was replaced were confirmed by genomic hybridization analysis as described previously (26). Three independent mutants in the H99 background were designated the apt1-3, apt1-22, and apt1-40 strains and were studied further. In addition, two apt1 mutants, the apt1-6 and apt1-16 strains, from the CBS7779 strain (27) were included in some phenotypic assays.
Complementation of C. neoformans apt1 mutation.
The APT1 gene for complementation was amplified from strain H99 with the primers Apt1-rec-NheI-5 and Apt1-rec-NheI-3 (see Table S1 in the supplemental material). The ∼6-kb product was digested with NheI and cloned into the SpeI site of pCH233, creating the plasmid pAPT1rec. The apt1-3 strain was transformed with pAPT1rec by biolistic transformation with selection on nourseothricin (100 μg/ml). Reintroduction of APT1 was confirmed by colony PCR and genomic hybridization. The two complemented strains are designated the apt1+APT1-1 and apt1+APT1-25 strains.
Stress and drug response assays.
Exponentially growing cultures of wild-type, apt1 mutant, and reconstituted strains were washed, resuspended in H2O, and adjusted to 2 × 104 cells/μl. The cell suspensions were serially diluted 10-fold, and 5 μl of each dilution was spotted onto YPD and/or YNB plates supplemented with or without 1.5 M KCl, 1.5 M NaCl, 0.1% SDS, 0.5 mg/ml Congo Red, 0.5 mg/ml calcofluor white, 0.5 mg/ml caffeine, 5 mM H2O2, 50 μM or 100 μM tBOOH (tert-butylhydroperoxide), 2 mg/ml or 4 mg/ml sodium nitrite (NaNO2) (pH 4), 20 μg/ml or 40 μg/ml brefeldin A, 0.5 mg/ml monensin, 5 μg/ml fluconazole, or 0.2 μg/ml amphotericin B. Plates were incubated for 2 to 5 days at 30°C or 37°C and photographed. To assess the sensitivity of WT and apt1 mutant strains to cinnamycin (Ro09-0198), the fungal cells were seeded in microtiter wells in YNB at 1 × 106 cells/ml (∼0.08 optical density at 600 nm [OD600]) in triplicate with or without the drug and grown for 72 h at 30°C. For each concentration of the drug and for each strain, the increase in OD600 for treated samples was divided by that for the untreated control to determine the percent growth (6).
Capsule formation and melanin production.
Capsule formation was examined by differential interference microscopy (DIC) after incubation for 24 h at 30°C in low-iron medium (LIM) and staining with India ink. Melanin production was examined on l-3,4-dihydroxyphenylalanine (L-DOPA) plates containing 0.1% glucose.
Visualization of endocytosis and the vacuolar membrane.
An FM4-64 internalization assay was performed to assess endocytosis. The lipophilic dye FM4-64 [N-(3-triethylammoniumpropyl)-4-(6-(4-(diethylamino) phenyl) hexatrienyl) pyridinium dibromide] (T-3166; Invitrogen Canada) was used at a final concentration of 20 μM. Cells were harvested after overnight growth, stained with FM4-64 for 2 or 30 min, washed, and transferred to fresh medium without stain. The cells were viewed at 5 and 30 min.
F-actin and DNA staining.
The WT and apt1 mutant cells were cultured overnight in YPD at 30°C. The cells were then fixed with 4% paraformaldehyde for 20 min, washed twice with phosphate-buffered saline (PBS) (pH 7.4), and permeabilized with 1% Triton X-100 for 5 min. The F-actin in the cells was labeled with Alexa Fluor 568 phalloidin (Invitrogen), and 1 μl of stained cells was mixed with 1 μl of mounting medium (Prolong Gold Antifade reagent with 4′,6′-diamidino-2-phenylindole [DAPI]) on a slide. The cells were visualized using an Axioplan 2 imaging microscope (Zeiss) with magnification ×1,000. The software program Metamorph, version 6.1r6 (Universal Imaging Corp.), was used to process images.
Enzyme assays.
Urease activity was measured with the BBL Taxo urease differentiation disk (BD Bioscience, Sparks, MD) as described previously (47). Briefly, one colony of WT or apt1 mutant strains was added to sterile, deionized H2O in an Eppendorf tube and vortexed vigorously. One disk was added to each tube, incubated at 30°C for 30 min, and then checked for urease activity according to the manufacturer's instructions. To measure superoxide dismutase (SOD) activity, cells of WT and apt1 mutant strains were cultured in YPD at 30°C overnight and washed with PBS (7). Subsequently, 2 × 107 cells were tested for SOD activity using the SOD determination kit (S7571; Sigma-Aldrich, St. Louis, MO) according to the manufacturer's instructions. To measure acid phosphatase activity, cells of WT and apt1 mutant strains were cultured in YPD at 30°C overnight and washed with PBS. A total of 2 × 107 fungal cells were used in each sample, and acid phosphatase activity was measured using an acid phosphatase assay kit (CS0740; Sigma-Aldrich, St. Louis, MO) according to the manufacturer's instructions. Acid phosphatase was used previously as a marker of exocytosis in C. neoformans (60).
Macrophage assay.
The effect of APT1 deletion on fungal survival during incubation with macrophages was assessed as previously described (7, 8). Briefly, the murine macrophage-like cell line J774A.1 was maintained at 37°C in 10% CO2 in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated fetal calf serum (FBS), 1% nonessential amino acids, 100 μg/ml penicillin-streptomycin, and 4 mM l-glutamine (Invitrogen). The cell line was used between passages 5 and 10. Cells of the WT, two apt1 mutants, and a complemented mutant were opsonized with monoclonal antibody 18B7 against capsule (1 μg/ml) (a generous gift from Arturo Casadevall), and macrophages were treated with recombinant mouse gamma interferon (IFN-γ) (50 U/ml) and lipopolysaccharide (LPS) (0.3 μg/ml) prior to coincubation at a multiplicity of infection (MOI) of 2:1. The pka1 mutant (9, 15) was also included in the tests. Macrophages were inoculated at 1 × 105 cells and washed after 1 h of inoculation to remove unattached, extracellular fungal cells. After 24 h of incubation, sterile, ice-cold distilled H2O was applied to each well to lyse the macrophages (confirmed microscopically). Fungal growth was measured by plating on YPD and determining CFU. The assay was performed in triplicate wells for each strain, and the experiment was repeated three times with consistent results. Student's t test was used to determine the statistical significance of differences in fungal survival.
Assessment of virulence in a murine model.
For an initial virulence assay, three female A/Jcr mice obtained from Jackson Laboratories (Maine) were used for each cryptococcal strain. Fungal cells were cultured in 5 ml YPD at 30°C overnight, washed twice with PBS, and resuspended in PBS (pH 7.4). A cell suspension of 5 × 104 cells in 50 μl was intranasally instilled. The virulence of the strains was also tested with female BALB/c mice. Female BALB/c mice, 4 to 6 weeks old, were obtained from Charles Rivers Laboratories (Pointe-Claire, Quebec, Canada). The fungal cells were cultured and washed as described. A cell suspension of 1 × 106 cells in a 50-μl volume was used for intranasal instillation. The status of the mice was monitored twice per day postinoculation. Differences in virulence were statistically assessed by log rank tests using the GraphPad Prism 4 for Mac software program (GraphPad Software, San Diego, CA). The protocols for the virulence assays (protocol A99-0252) were approved by the University of British Columbia Committee on Animal Care.
RESULTS
Apt1 in C. neoformans is functionally related to Drs2 in S. cerevisiae.
Five genes encoding APTs (DRS2, DNF1, DNF2, DNF3, and NEO1) have been characterized for S. cerevisiae (29). BLASTp searches of the genome of C. neoformans strain H99 (www.broad.mit.edu/annotation/genome/cryptococcus_neoformans/Info.html) with the yeast sequences identified four putative APTs. These were designated APT1 (CNAG_06469.2), APT2 (CNAG_01278.2), APT3 (CNAG_00383.2), and APT4 (CNAG_05282.2). Phylogenetic analysis revealed that Apt1 from C. neoformans is closely related to P-type ATPase of the Drs2 family of APTs (P4 type) but clearly distinct from the other main classes of P-type ATPases identified so far in C. neoformans. These include the P2 types, such as the sarcoplasmic/ER Ca2+ ATPase EcaI (14), the sodium/potassium P-type ATPase Ena1 (30), the H+ ATPases Pma1 and Pma2 (21, 53), the Ca2+/Mg2+-transporting ATPase Pmr1, and a P1-type copper-exporting ATPase, Atu2/Ccc2 (57) (see Fig. S1 in the supplemental material). Alignments of the Apt1 amino acid sequence with the Apts of S. cerevisiae revealed similar degrees of sequence similarity (47 to 55%) and identity (27 to 36%) with the closest matches to Dnf1 (see Table S2 in the supplemental material). We focused on the characterization of Apt1 because our previous study suggested a role in the response to nitrosative stress (27).
We initially determined whether the C. neoformans APT1 gene could functionally complement S. cerevisiae strains with defects in the Drs2 family of APTs. In yeast, the dnf1Δ dnf2Δ dnf3Δ and drs2Δ mutants exhibit hypersensitivity to calcofluor white and growth defects at lower temperatures (<18°C) (16, 29). A C. neoformans cDNA for APT1 (CnAPT1) was expressed in the drs2Δ and dnf1Δ dnf2Δ dnf3Δ mutants under the control of the MET1 promoter (40). Expression of CnAPT1 in two independent transformants partially restored the growth of the drs2Δ strain, ZHY615M2D, at 18°C, as well as growth in the presence of 0.5 mg/ml calcofluor white at both 18°C and 30°C (although complementation was more pronounced at 30°C) (Fig. 1). In contrast, expression of C. neoformans APT1 from the same plasmid in three independent transformants failed to restore the growth phenotype of the dnf1Δ dnf2Δ dnf3Δ triple mutant (strain PFY3273A) at both 18°C and 30°C or in the presence of calcofluor white (see Fig. S2 in the supplemental material). Overall, our data suggest that C. neoformans can partially fulfill the functions of Drs2 in S. cerevisiae with respect to growth.
FIG. 1.
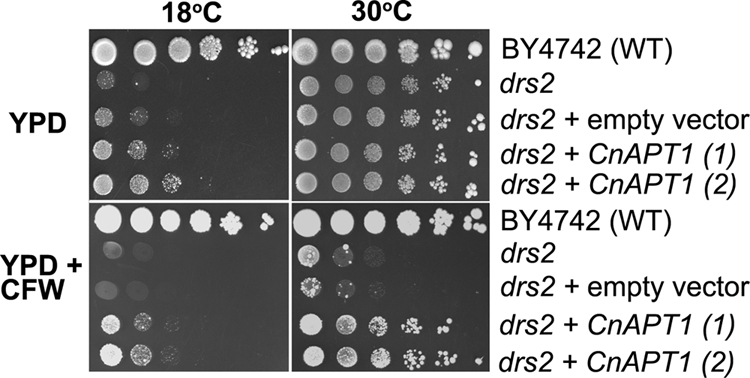
C. neoformans APT1 restores the growth of an S. cerevisiae drs2 mutant. The C. neoformans APT1 (CnAPT1) gene was expressed in the drs2 mutant of S. cerevisiae (drs2 + CnAPT1). The drs2 mutant is able grow at 30°C but not at 18°C. Tenfold serial dilutions of yeast cells were plated on YPD or YPD with calcofluor white (CFW) and cultured at 18°C or 30°C. The plates were incubated for 3 days.
Loss of APT1 does not influence major virulence traits or cell wall integrity but increases sensitivity to nitrosative and oxidative stress.
Given the complementation of the drs2 deletion in yeast, we proceeded to construct additional apt1 mutants in C. neoformans in order to assess the role of the gene in virulence. Previously, we deleted the APT1 gene in strains H99 and CBS7779 to examine chromosome copy number variation (27). To obtain additional independent mutants, we performed biolistic transformations and generated eight additional deletion strains in the H99 background. Deletion of the APT1 gene was monitored by colony PCR and confirmed by Southern blot analysis (27). We also constructed reconstituted strains in which the apt1 mutation was complemented with the wild-type gene. Initially, we tested the mutants for their ability to grow at 37°C, for capsule size, and for melanin production because these traits are the major virulence factors in C. neoformans. No significant defects were observed for these factors or for two other virulence-associated activities, superoxide dismutase and urease (Fig. 2 and data not shown). Deletion of APT1 also did not cause growth defects on a range of carbon sources, including acetate, sucrose, ethanol, glycerol, and raffinose (data not shown). In contrast to the temperature sensitivity of the drs2 mutant in yeast, we did not observe growth differences between the WT strain and the apt1 mutants on YPD or YNB at 15°C, 20°C, 25°C, 30°C, or 37°C (data not shown). Furthermore, the apt1 mutants grew as well as the WT strains on YPD plates supplemented with agents that perturb cell wall integrity (calcofluor white, caffeine, SDS, and Congo Red) (data not shown). The same results were found with the apt1 mutants (apt1-6 and apt1-16) of strain CBS7779 (data not shown). These results indicated that Apt1 does not have a major role in the production of the known virulence factors in C. neoformans and that loss of the gene does not influence sensitivity to growth temperature or agents that influence the cell wall.
FIG. 2.
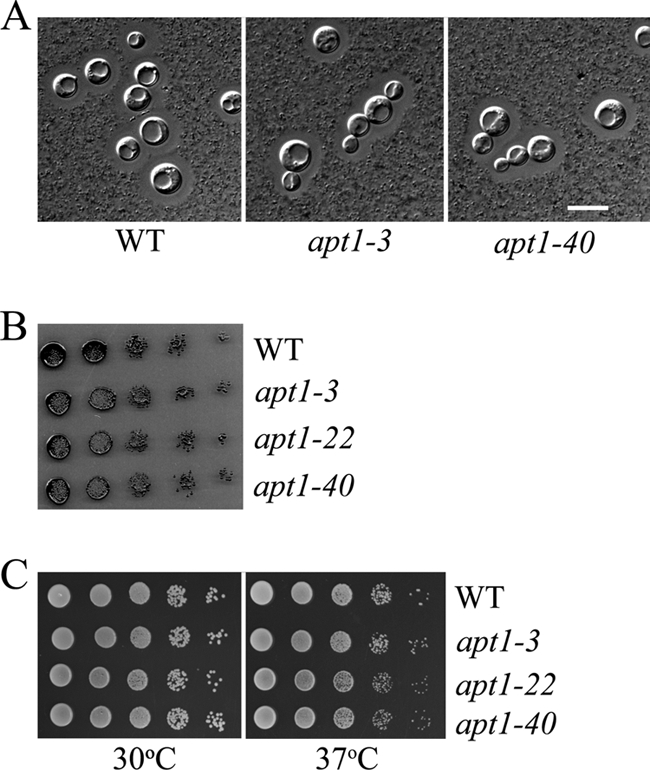
Deletion of APT1 in C. neoformans does not influence the three major virulence factors. The apt1 mutants did not shown differences in capsule size (A), melanin production (B), or high-temperature growth (C) compared to the WT strain. Capsule size was evaluated for cells grown overnight in low-iron medium at 30°C. To examine melanin production, serial 10-fold dilutions of each strain were spotted on L-DOPA plates and incubated for 2 days at 30°C before being photographed. The sensitivity of WT and apt1 mutant strains to temperatures (30°C and 37°C) was tested on both YPD and YNB plates. The bar in panel A represents 10 μM. For the evaluation of capsule size, the same results were found for the apt1-22 mutant (data not shown).
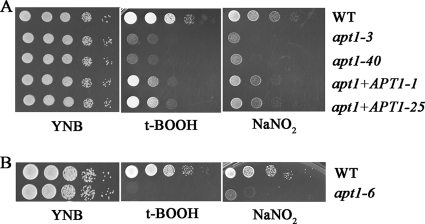
We next examined the response of the apt1 mutants to nitrosative stress by growing the strains on YNB medium in the presence of 2 mM and 4 mM sodium nitrite (NaNO2, pH 4). As found previously (27), the apt1 mutants exhibited increased sensitivity to sodium nitrite (Fig. 3A). We established that the phenotype resulted from the deletion of APT1 by demonstrating that reintroduction of APT1 restored the wild-type level of sensitivity. We extended the characterization to include oxidative stress by growing the cells on YNB medium supplemented with either 50 or 100 μM tBOOH. Again, deletion of APT1 resulted in increased sensitivity, and complementation restored the growth to wild-type levels. Similarly, loss of Apt1 also increased sensitivity to hydrogen peroxide and diamide (see Fig. S3 in the supplemental material). We also found that two independent apt1 mutants of strain CBS7779 displayed similar responses to oxidative and nitrosative stresses (Fig. 3B). The apt1 mutants in either strain background did not show increased sensitivity to osmotic and salt stress, as determined by growth in the presence of 1.5 M KCl, 1.5 M NaCl, or 1.2 M sorbitol. Overall, we conclude that Apt1 is required for C. neoformans to respond to oxidative and nitrosative stress.
FIG. 3.
Deletion of APT1 caused hypersensitivity to oxidative and nitrosative stress. (A) Serial 10-fold dilutions of the WT (H99), two independent apt1 mutants, and two reconstituted strains were spotted on YNB or YNB supplemented with tBOOH (100 μM) or NaNO2 (2 mM, pH 4.0). The apt1+APT1-1 and apt1+APT1-25 strains contain the WT gene in the apt1-3 mutant background. (B) Serial 10-fold dilutions of the WT (CBS7779) and the apt1-6 apt1 mutant strain in the CBS7779 background were spotted onto YNB or YNB supplemented with tBOOH (100 μM) or NaNO2 (2 mM, pH 4.0). All of the plates were incubated at 30°C for 3 days.
APT1 is required for survival during interactions with macrophages.
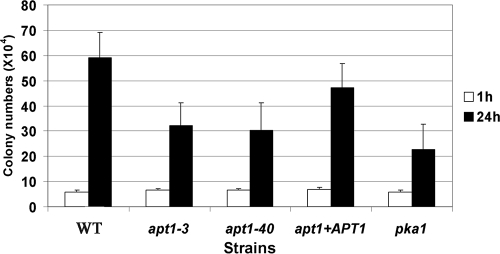
The ability of C. neoformans to withstand oxidative and nitrosative stress upon phagocytosis is an important virulence attribute (12, 38). We therefore examined the survival of the apt1 mutant during interactions with a murine macrophage-like cell line. Cells of the WT (H99), two independent apt1 mutants (apt1-3 and apt1-22 strains), and a complemented mutant were coincubated with the J774A.1 cell line. The pka1 mutant was included as a control because PKA1 was shown previously to play a role in intracellular growth in macrophages (15). As shown in Fig. 4, the CFU numbers of the apt1 and pka1 deletion mutants were lower than those of the WT strain, indicating a reduced ability to survive and proliferate during interactions with macrophages. A control inoculation of the strains into macrophage-free DMEM precluded the possibility that the reduced number of apt1 cells was due to poor growth in the medium (data not shown).
FIG. 4.
Apt1 is required for survival during interactions with macrophages. Cells of the WT strain H99, two apt1 mutants, and the complemented strain were incubated with macrophage J774A.1 cells at an MOI of 2:1. The pka1 mutant was also compared in the tests because of its known growth defect in macrophages (15). C. neoformans was inoculated at 1 × 105 cells, and the wells were washed after 1 h of incubation to remove extracellular fungal cells. After 24 h of incubation, growth was measured by plating on YPD and counting CFU. The experiment was repeated three times, and similar results were also obtained with a third independent apt1 mutant.
Loss of APT1 results in attenuated virulence.
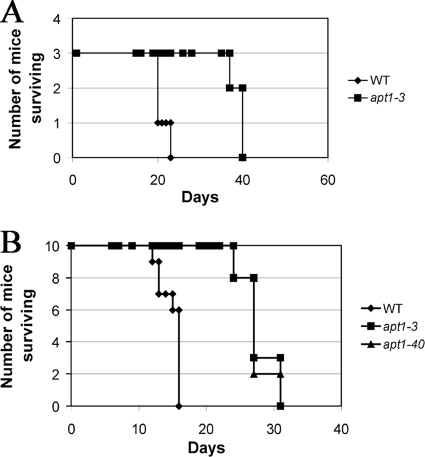
The in vitro phenotypes of the apt1 mutants prompted us to assay their virulence in a mouse inhalation model of cryptococcosis. In an initial experiment, three AJ/cr mice were employed for each strain (WT and the apt1-3 mutant), and we found that deletion of APT1 resulted in attenuation of virulence (Fig. 5A). In the second experiment, 10 BALB/c mice were challenged with the WT strain (H99) and two independent apt1 mutant strains (apt1-3 and apt1-40 strains). All mice infected with the WT strain succumbed by days 12 to 15, while the mice infected with the apt1 mutant strains survived to day 31 after inoculation (Fig. 5B). The virulence assays with the two different mouse strains therefore produced similar results, and we concluded that APT1 is required for full virulence in C. neoformans.
FIG. 5.
Apt1 is required for virulence in a mouse inhalation model. (A) Three female A/Jcr mice were inoculated intranasally with each of the strains indicated, and the survival of the mice was monitored twice per day. The apt1 mutants showed a difference in virulence from the WT strain, H99 (P = 0.0246), by the log rank test. (B) Ten female BALB/c mice were challenged by intranasal inoculation with 106 cells of either the WT strain, H99, or two independent apt1 mutant strains. The survival of the mice was monitored twice per day. The apt1 mutants showed a difference in virulence from the WT strain (P < 0.001) by the log rank test.
Deletion of APT1 influences actin polarization, endocytosis, and acid phosphatase activity.
In S. cerevisiae, the Drs2 protein is localized to the trans-Golgi network and loss of the protein results in a decrease in clathrin-coated vesicle budding (5, 16, 29). The protein appears to play a role in the transport of dense vesicles containing both invertase and acid phosphatase and in exocytosis (16, 22). Moreover, deletion of DRS2 results in endocytosis defects, in particular, in endosome-vacuole docking (5, 6, 16, 22, 29, 35). Finally, we noted that the S. cerevisiae drs2 mutant also exhibits defects in the establishment of cell polarity (52). In this context, we examined the phenotypes of apt1 mutants of C. neoformans with regard to actin polarization, endocytosis, and sensitivity to trafficking inhibitors.
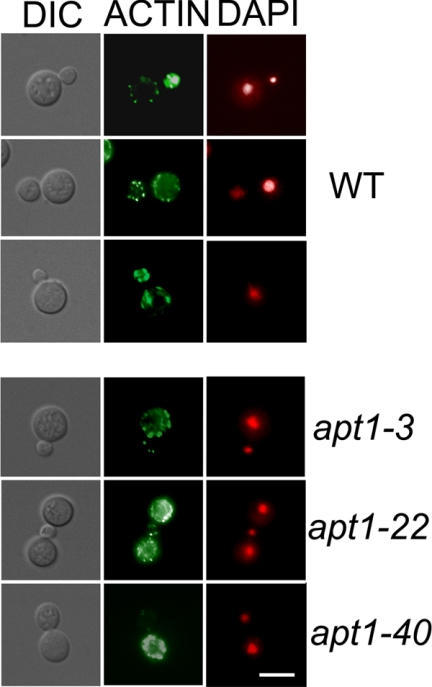
To examine actin polarization, we stained cells of the WT and apt1 mutant strains with phalloidin and compared cellular morphology and the distribution of F-actin. We did not observe notable morphological differences between the strains, but we did find reduced accumulation of actin patches in the buds of apt1 mutant cells compared to WT cells (Fig. 6). Specifically, ∼60% of the mutant buds did not contain an accumulation of actin patches, compared with ∼7% of the WT buds. Staining DNA with DAPI revealed that the cells of the WT strain and the apt1 mutants contained a single nucleus (Fig. 6).
FIG. 6.
F-actin distribution is altered in apt1 mutant cells. Cells of the WT (H99) and apt1 mutant strains were grown to mid-log phase in YPD medium at 30°C. After harvest, the cells were fixed and stained with Alexa Fluor 568 phalloidin and DAPI. One hundred budded cells for each strain were examined using fluorescence microscopy and scored for actin distribution in buds. Bar = 10 μM.
To examine endocytosis, we tested the uptake of the lipophilic styryl dye FM4-64, a fluorescent endocytic marker (56), in both WT and apt1 mutant strains at different times after exposure to the dye. The apt1 mutants exhibited less-apparent uptake and a more diffuse staining of punctate structures in the cytoplasm upon a short (2-min) exposure to the dye (Fig. 7). That is, the punctate pattern of staining in the wild-type cells (presumably endosomal vesicles) was not as readily apparent in the mutant (Fig. 7). Upon longer exposure to the dye (30 min), the apt1 mutant cells displayed a more obvious staining of internal punctate structures while the WT cells continued to exhibit pronounced staining of internal vesicles and the presumed vacuolar membrane (Fig. 7). In yeast, the defect in exocytosis in a drs2 mutant occurred at a lower temperature (15°C). In light of this observation, we compared FM4-64 uptake by the strains at two temperatures: 15°C and 25°C. Interestingly, we did not observe the effect of temperature on FM4-64 uptake in both the WT and the apt1 mutants (data not shown). Overall, these experiments suggested that APT1 in C. neoformans is required for endocytosis without the temperature influence seen in S. cerevisiae.
FIG. 7.
Deletion of APT1 results in altered staining with FM4-64. Cells of the WT (H99) strain and the apt1-3 mutant were grown to mid-log phase in YPD medium at 30°C. As indicated, the cells were stained with FM4-64 for 2 min or 30 min, washed with water, and viewed by fluorescence microscopy (magnification, ×100) after 5 and 30 min. Bar = 10 μM.
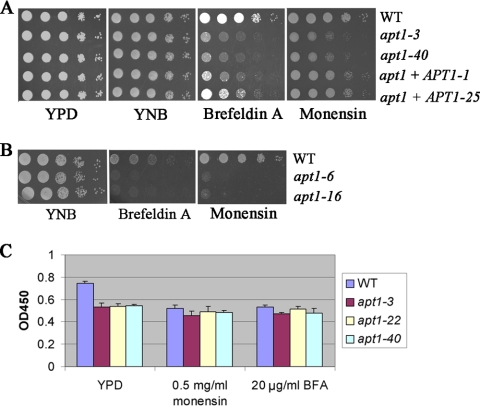
We next examined the sensitivity of apt1 mutants to drugs that interfere with vesicle trafficking (28). These drugs included brefeldin A, which arrests the anterograde transport of proteins between the endoplasmic reticulum (ER) and the Golgi apparatus, and monensin, a Na+/H+ ionophore that blocks intracellular transport in both trans-Golgi and Golgi compartments. We previously found that these drugs reduced capsule size in C. neoformans (28). As shown in Fig. 8A, deletion of APT1 caused an increased sensitivity to these two drugs, thus indicating a role for Apt1 in ER-Golgi trafficking and in both trans-Golgi and post-Golgi complexes. Reintroduction of APT1 in the reconstituted strain restored growth, thus confirming that the phenotype was due to deletion of APT1. We also confirmed that the two independent apt1 mutants from strain CBS7779 (27) showed the increased sensitivity to the drugs (Fig. 8B).
FIG. 8.
The apt1 mutants are sensitive to secretion inhibitors and have reduced acid phosphatase activity. (A) Serial 10-fold dilutions of the WT (H99), two independent apt1 mutants, and two reconstituted strains were spotted on YNB or YNB supplemented with the drugs brefeldin A (25 or 40 μg/ml) or monensin (0.5 μg/ml). The apt1+APT1-1 and apt1+APT1-25 strains contain the WT gene in the apt1-3 mutant background. (B) Serial 10-fold dilutions of the WT (CBS7779) and two independent apt1 mutants were spotted on YNB or YNB supplemented with the inhibitors. Plates were incubated at 30°C for 3 days. (C) Acid phosphatase activity was measured for 2 × 107 intact cells of each strain after growth to mid-log phase in YPD medium. As indicated, the concentrations of monensin and brefeldin A used in the acid phosphatase assays were 0.5 mg/ml and 20 μg/ml, respectively. The inhibitors were added during the growth of the cells.
Finally, we measured the acid phosphatase activity of the WT and apt1 mutant strains to examine the influence of the APT1 deletion on exocytosis. We postulated that, like Drs2 in S. cerevisiae (16), Apt1 may play a role in the trans-Golgi network to produce exocytic vesicles for the delivery of enzymes to the cell surface. Acid phosphatase activity has previously been shown to be dependent on exocytosis in C. neoformans (60). As predicted, loss of APT1 resulted in reduced acid phosphatase activity at the cell surface (Fig. 8C), thus supporting the hypothesis of Apt1 involvement in exocytosis. In further support, we found that addition of vesicle trafficking inhibitors (brefeldin A and monensin) in the growth medium also reduced acid phosphatase activity to the level seen in the apt1 mutant. The inhibitors provided a means to identify the proportion of activity that was dependent on vesicle trafficking relative to the overall background level of activity (Fig. 8C).
Deletion of APT1 caused increased sensitivity to cinnamycin, fluconazole, and amphotericin B.
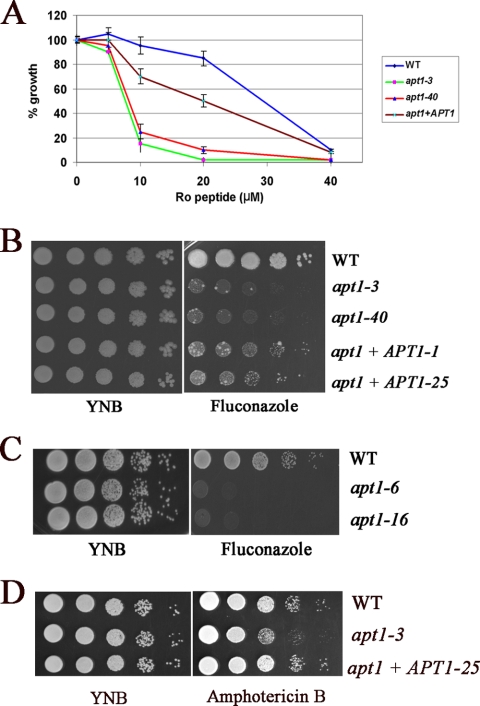
The transbilayer distribution of lipids across biological membranes is asymmetric, and both phosphatidylethanolamine (PE) and phosphatidylserine (PS) are located preferentially on the cytoplasmic leaflet (36). Disruption of endocytosis in S. cerevisiae causes a loss of plasma membrane asymmetry and exposure of PS and PE on the outer leaflet (6). To probe changes in phospholipid asymmetry, we examined the sensitivity of WT and apt1 mutant strains to cinnamycin (previously named Ro09-0198), a cyclic antifungal peptide that targets PE exposed on the outer leaflet of the plasma membrane. The toxicity of this peptide is thought be mediated by its influence on transbilayer lipid movement. Similar to the yeast drs2 mutant (6, 46, 52), apt1 mutants (apt1-3 and apt1-40 strains) displayed increased sensitivity to cinnamycin, suggesting that deletion of APT1 resulted in a loss of membrane asymmetry and exposure of PE on the outer leaflet. A reconstituted strain with the APT1 gene partially restored the growth defects of the mutants on the peptide, indicating that the phenotype was due to deletion of APT1 (Fig. 9A).
FIG. 9.
Deletion of APT1 causes increased sensitivity to the antifungal peptide cinnamycin (Ro09-0198) and the antifungal drugs fluconazole and amphotericin B. (A) The sensitivities of WT (H99), apt1 mutant, and reconstituted strains to the PE-binding peptide cinnamycin are shown at a range of indicated concentrations. After 72 h of incubation at 30°C, OD600s were measured and the values of cultures incubated without the peptide were normalized to 100% growth. (B) Serial 10-fold dilutions of the WT (H99), two independent apt1 mutant strains, and two reconstituted strains were spotted on YNB or YNB supplemented with fluconazole. (C) Serial 10-fold dilutions of the WT (CBS7779) and the apt1 mutant strains were spotted on YNB or YNB supplemented with fluconazole. (D) Serial 10-fold dilutions of the WT (H99), two independent apt1 mutant strains, and two reconstituted strains were spotted on YNB or YNB supplemented with amphotericin B. The apt1+APT1-1 and apt1+APT1-25 strains contain the WT gene in the apt1-3 mutant background. For all assays, the plates were incubated at 30°C for 3 days.
Drs2 in yeast also plays a role in vesicle budding from the trans-Golgi network (35). Furthermore, although transport vesicles are moving between various compartments, sterols are heterogeneously distributed across the secretory and endocytic pathways; for example, cholesterol in mammalian cells is relatively low in the ER and higher in the trans-Golgi network (48). We hypothesized that loss of Apt1 might perturb these processes in C. neoformans, leading to changes in sterol functions or distribution. As an indirect measure of these changes, we examined the sensitivity of WT and apt1 mutant strains to fluconazole, a drug that targets the Erg11 enzyme in ergosterol biosynthesis, and to amphotericin B, a drug that binds irreversibly to ergosterol, resulting in disruption of membrane integrity. Both of these drugs are currently used to treat cryptococcosis. The apt1 mutants displayed a pronounced growth defect on the plates supplemented with fluconazole, and reintroduction of APT1 to the mutant strain restored growth (Fig. 9B). Moreover, the apt1 mutants from the CBS7779 background also exhibited increased sensitivity to fluconazole, further indicating a role for Apt1 (Fig. 9C). The apt1 mutant exhibited increased sensitivity to amphotericin B, and reintroduction of APT1 to the mutant restored WT growth (Fig. 9D). Taken together, the phenotypes of the apt1 mutant on cinnamycin, fluconazole, and amphotericin B are consistent with a role for Apt1 in lipid trafficking and membrane composition.
DISCUSSION
Apt1 plays a role in virulence that is distinct from capsule- and melanin-related transport.
Aminophospholipid translocases play important roles in maintaining phospholipid asymmetry in membranes and in vesicle-mediated transport. In this study, we characterized the gene APT1, which encodes a putative APT in C. neoformans, to further investigate trafficking pathways for virulence factor elaboration. We found that APT1 is required for membrane trafficking during endocytosis, for exocytic export of acid phosphatase, for the response to oxidative and nitrosative stresses, and for full virulence. Interestingly, APT1 was not required for melanin deposition in the cell wall, for the production of cell-associated capsule, or for growth at the host temperature. In addition, the apt1 mutant still produced extracellular vesicles (data not shown), and these vesicles are known to be associated with capsule transport (42, 49, 50, 60). It is possible that a more detailed examination of phospholipid composition and content in the vesicles may reveal an influence of Apt1. Overall, these results suggest that Apt1 acts in a distinct trafficking pathway, one that may contribute to the function of stress response factors needed to protect C. neoformans from host defense mechanisms.
Apt1 from C. neoformans is functionally related to the Drs2 family of APTs in S. cerevisiae based on sequence analysis and complementation of an S. cerevisiae drs2 mutation. We found that Apt1 shares similar levels of sequence identity with Drs2 and other APTs in yeast (Dnf1, Dnf2, and Dnf3), but Apt1 was unable to complement the growth defects of a dnf1Δ dnf2Δ dnf3Δ triple mutant. This suggests that Apt1 only partially fulfils the functions of the Drs2 family of APTs, although it is possible that poor heterologous expression interfered with full complementation. Moreover, the drs2 mutant in S. cerevisiae exhibited temperature-dependent growth defects and increased sensitivity to calcofluor white. However, deletion of APT1 in C. neoformans caused neither temperature-dependent growth defects nor increased sensitivity to calcofluor white, although APT1 successfully restored those phenotypes in the yeast drs2 mutant. It is possible that redundancy with other APT genes (APT2-APT4) contributed to the absence of temperature or calcofluor sensitivity phenotypes in C. neoformans. Partial complementation has also been observed for another fungal APT gene (18). Specifically, the MgAPT2 gene from the rice blast fungus M. grisea restored the temperature-sensitive growth phenotype of the S. cerevisiae dnf1Δ dnf2Δ dnf3Δ triple mutant but not that of a drs2Δ mutant (18).
Role for Apt1 in transport and membrane function.
APT1 complementation of the yeast drs2 mutation is interesting given the role of Drs2 in protein transport. Drs2 was originally thought to encode a plasma membrane ATPase, but the protein was subsequently found to reside in the trans-Golgi network and to participate in multiple vesicle transport pathways (5). Moreover, loss of Drs2 is synthetically lethal with mutations in the genes for clathrin heavy chain (Chc1) and the ADP ribosylation factor (Arf1) (5). Arf1 is a small GTPase involved in formation of COPI-coated and clathrin-coated vesicles in S. cerevisiae. Several lines of evidence support a role for Apt1 in similar functions in C. neoformans. First, the increased sensitivity of apt1 mutants to brefeldin A and monensin indicated that Apt1 is involved in the secretion pathway. More specifically, Apt1 may have a role in late Golgi function and/or ER-Golgi trafficking, since these drugs target such machinery. Of course, it is also possible that Apt1 functions in another pathway that is sensitive to the drugs. Second, the apt1 mutant was defective in cell-associated acid phosphatase activity, suggesting a defect in exocytosis. In S. cerevisiae, at least two types of vesicles (light and dense) are involved in protein trafficking from the ER to the Golgi apparatus and subsequently to the cell surface; some of the vesicles that bud from the Golgi apparatus require Drs2 (5, 16, 29). Deletion of APT1 also caused defects in the normal distribution of actin patches in the developing buds of cells, although growth arrest was not observed. Actin patches and filaments and associated proteins participate in the establishment of regions of polarized growth and are also required during endocytosis for membrane internalization (17, 59).
The increased sensitivity of apt1 mutant strains to cinnamycin (Ro09-0198), a tetracyclic peptide that specifically binds to phosphatidylethanolamine (PE) (13, 31, 52), suggested that loss of Apt1 affects the distribution of PE across the plasma membrane. This is consistent with Apt1 playing a role in maintaining the lipid asymmetry in C. neoformans. Similar defects are found in drs2 and dnf1 dnf2 mutants in yeast (46, 52). In S. cerevisiae, deletion of DRS2 caused increased amount of surface-exposed PE, which likely reflects an altered aminophospholipid distribution across the bilayer of the plasma membrane (45, 46). A breakdown of lipid asymmetry in the plasma membrane could result from the incoming flow of secretory vesicles with aberrant lipid orientation from the Golgi apparatus in the drs2 mutant (46). Furthermore, a defect in lipid asymmetry in the plasma membrane may conceivably affect the membrane features, for example, the binding properties and sensitivities to drugs. In this regard, the apt1 mutants displayed increased sensitivity to fluconazole and amphotericin B, two drugs that act on ergosterol biosynthesis and function in the plasma membrane, respectively. Defects in iron uptake functions also increase fluconazole sensitivity in C. neoformans (33, 34). In this case, we found that heme and siderophores restore the wild-type level of fluconazole sensitivity by providing iron for enzymes in the ergosterol biosynthetic pathway (33). In the current study, heme did not influence the fluconazole sensitivity of the apt1 mutant (see Fig. S4 in the supplemental material), suggesting that Apt1 has a distinct role, perhaps by influencing membrane permeability to the drug or membrane function.
Virulence defect in apt1 mutants.
In pathogenic fungi such as C. neoformans, tolerance to nitrosative and oxidative stress is critical to survival in the presence of phagocytic cells. Mutants defective in the response to the oxidative and nitrosative stresses show attenuated virulence. Examples include mutants lacking superoxide dismutase Sod1 or Sod2 (7, 19), alternative oxidase Aox1 (1), flavohemoglobin denitrosylase Fhb1 (12), thiol peroxidase Tsa1 (39), thioredoxin Trx1 (37), or the nutrient/stress response protein kinase Snf1 (24). Moreover, transcriptome analysis revealed a connection between oxidative stress and the mechanisms of action of fluconazole (51). The increased stress sensitivity of the apt1 mutant suggests an explanation for the attenuated macrophage survival and virulence of the mutant. In terms of the other stresses, such as thermotolerance, the apt1 mutant grew at the level of the wild type at all temperatures that we examined (Fig. 2 and data not shown). As mentioned above, APT mutants in S. cerevisiae are cold sensitive, and a connection between APTs and low temperature acclimation has been described for plants (20).
Roles for APTs in other pathogenic fungi.
Interestingly, MgAPT2 and MgPDE1, two putative APTs, are implicated in virulence in the plant pathogen M. grisea. The Drs2 homologue MgApt2 is needed for exocytosis to secrete a set of extracellular enzymes that enable the fungus to grow on a range of substrates when provided as sole carbon sources (18). Furthermore, MgApt2 is also required for secretion of fungal proteins perceived by the host rice plant during a resistance response. A gene-for-gene interaction is present between rice and M. grisea, in which single major resistance genes are required for the recognition of pathogen-derived molecules encoded by fungal avirulence genes (18). The MgApt2 gene therefore influences the rapid induction of host defense responses in an incompatible reaction (18). Disruption of MgPDE1 resulted in impaired penetration by hyphae and the proliferation of the fungus beyond colonization of the first epidermal cells of the host (2). It is likely that similar functions for exocytosis and endocytosis contribute to virulence in other plant pathogens, such as Ustilago maydis (58) and Uromyces fabae (23).
In conclusion, we have shown that Apt1, a predicted APT in C. neoformans, is critical for survival during interactions with macrophages and for virulence, although it is not required for the production of the major virulence factors. It is likely that Apt1 functions in the maintenance of phospholipid asymmetry and in endocytosis and exocytosis; the latter functions may contribute to the export of factors to protect the fungus from oxidative and nitrosative challenges in mammalian hosts.
Supplementary Material
Acknowledgments
We thank Joseph Heitman, Todd Graham, Teun Boekhout, and Philip Hieter for strains and plasmids.
This work was supported by the National Institutes of Health (R01 AI053721) and the Canadian Institutes of Health Research. J.W.K. is a Burroughs Wellcome Fund Scholar in molecular pathogenic mycology.
Footnotes
Published ahead of print on 30 November 2009.
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1.Akhter, S., H. C. McDade, J. M. Gorlach, G. Heinrich, G. M. Cox, and J. R. Perfect. 2003. Role of alternative oxidase gene in pathogenesis of Cryptococcus neoformans. Infect. Immun. 71:5794-5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balhadere, P. V., and N. J. Talbot. 2001. PDE1 encodes a P-type ATPase involved in appressorium-mediated plant infection by the rice blast fungus Magnaporthe grisea. Plant Cell 13:1987-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bevers, E. M., P. Comfurius, D. W. Dekkers, M. Harmsma, and R. F. Zwaal. 1998. Regulatory mechanisms of transmembrane phospholipid distributions and pathophysiological implications of transbilayer lipid scrambling. Lupus 7(Suppl. 2):S126-S131. [DOI] [PubMed] [Google Scholar]
- 4.Bose, I., A. J. Reese, J. J. Ory, G. Janbon, and T. L. Doering. 2003. A yeast under cover: the capsule of Cryptococcus neoformans. Eukaryot. Cell 2:655-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, C. Y., M. F. Ingram, P. H. Rosal, and T. R. Graham. 1999. Role for Drs2p, a P-type ATPase and potential aminophospholipid translocase, in yeast late Golgi function. J. Cell Biol. 147:1223-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, S., J. Wang, B. P. Muthusamy, K. Liu, S. Zare, R. J. Andersen, and T. R. Graham. 2006. Roles for the Drs2p-Cdc50p complex in protein transport and phosphatidylserine asymmetry of the yeast plasma membrane. Traffic 7:1503-1517. [DOI] [PubMed] [Google Scholar]
- 7.Cox, G. M., T. S. Harrison, H. C. McDade, C. P. Taborda, G. Heinrich, A. Casadevall, and J. R. Perfect. 2003. Superoxide dismutase influences the virulence of Cryptococcus neoformans by affecting growth within macrophages. Infect. Immun. 71:173-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cox, G. M., J. Mukherjee, G. T. Cole, A. Casadevall, and J. R. Perfect. 2000. Urease as a virulence factor in experimental cryptococcosis. Infect. Immun. 68:443-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Souza, C. A., J. A. Alspaugh, C. Yue, T. Harashima, G. M. Cox, J. R. Perfect, and J. Heitman. 2001. Cyclic AMP-dependent protein kinase controls virulence of the fungal pathogen Cryptococcus neoformans. Mol. Cell. Biol. 21:3179-3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davidson, R. C., J. R. Blankenship, P. R. Kraus, M. de Jesus Berrios, C. M. Hull, C. D'Souza, P. Wang, and J. Heitman. 2002. A PCR-based strategy to generate integrative targeting alleles with large regions of homology. Microbiology 148:2607-2615. [DOI] [PubMed] [Google Scholar]
- 11.Davidson, R. C., M. C. Cruz, R. A. Sia, B. Allen, J. A. Alspaugh, and J. Heitman. 2000. Gene disruption by biolistic transformation in serotype D strains of Cryptococcus neoformans. Fungal Genet. Biol. 29:38-48. [DOI] [PubMed] [Google Scholar]
- 12.de Jesus-Berrios, M., L. Liu, J. C. Nussbaum, G. M. Cox, J. S. Stamler, and J. Heitman. 2003. Enzymes that counteract nitrosative stress promote fungal virulence. Curr. Biol. 13:1963-1968. [DOI] [PubMed] [Google Scholar]
- 13.Emoto, K., N. Toyama-Sorimachi, H. Karasuyama, K. Inoue, and M. Umeda. 1997. Exposure of phosphatidylethanolamine on the surface of apoptotic cells. Exp. Cell Res. 232:430-434. [DOI] [PubMed] [Google Scholar]
- 14.Fan, W., A. Idnurm, J. Breger, E. Mylonakis, and J. Heitman. 2007. EcaI, a sarcoplasmic/endoplasmic reticulum Ca2+-ATPase, is involved in stress tolerance and virulence in Cryptococcus neoformans. Infect. Immun. 75:3394-3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan, W., P. R. Kraus, M. J. Boily, and J. Heitman. 2005. Cryptococcus neoformans gene expression during murine macrophage infection. Eukaryot. Cell 4:1420-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gall, W. E., N. C. Geething, Z. Hua, M. F. Ingram, K. Liu, S. I. Chen, and T. R. Graham. 2002. Drs2p-dependent formation of exocytic clathrin-coated vesicles in vivo. Curr. Biol. 12:1623-1627. [DOI] [PubMed] [Google Scholar]
- 17.Geli, M. I., and H. Riezman. 1998. Endocytic internalization in yeast and animal cells: similar and different. J. Cell Sci. 111(Part 8):1031-1037. [DOI] [PubMed] [Google Scholar]
- 18.Gilbert, M. J., C. R. Thornton, G. E. Wakley, and N. J. Talbot. 2006. A P-type ATPase required for rice blast disease and induction of host resistance. Nature 440:535-539. [DOI] [PubMed] [Google Scholar]
- 19.Giles, S. S., I. Batinic-Haberle, J. R. Perfect, and G. M. Cox. 2005. Cryptococcus neoformans mitochondrial superoxide dismutase: an essential link between antioxidant function and high-temperature growth. Eukaryot. Cell 4:46-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gomes, E., M. K. Jakobsen, K. B. Axelsen, M. Geisler, and M. G. Palmgren. 2000. Chilling tolerance in Arabidopsis involves ALA1, a member of a new family of putative aminophospholipid translocases. Plant Cell 12:2441-2454. [PMC free article] [PubMed] [Google Scholar]
- 21.Gorgojo, B., F. Portillo, and J. V. Martinez-Suarez. 2000. Sequencing and heterologous expression in Saccharomyces cerevisiae of a Cryptococcus neoformans cDNA encoding a plasma membrane H(+)-ATPase. Biochim. Biophys. Acta 1509:103-110. [DOI] [PubMed] [Google Scholar]
- 22.Graham, T. R. 2004. Flippases and vesicle-mediated protein transport. Trends Cell Biol. 14:670-677. [DOI] [PubMed] [Google Scholar]
- 23.Hoffmann, J., and K. Mendgen. 1998. Endocytosis and membrane turnover in the germ tube of Uromyces fabae. Fungal Genet. Biol. 24:77-85. [DOI] [PubMed] [Google Scholar]
- 24.Hu, G., P. Y. Cheng, A. Sham, J. R. Perfect, and J. W. Kronstad. 2008. Metabolic adaptation in Cryptococcus neoformans during early murine pulmonary infection. Mol. Microbiol. 69:1456-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu, G., M. Hacham, S. R. Waterman, J. Panepinto, S. Shin, X. Liu, J. Gibbons, T. Valyi-Nagy, K. Obara, H. A. Jaffe, Y. Ohsumi, and P. R. Williamson. 2008. PI3K signaling of autophagy is required for starvation tolerance and virulence of Cryptococcus neoformans. J. Clin. Investig. 118:1186-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu, G., and J. W. Kronstad. 2006. Gene disruption in Cryptococcus neoformans and Cryptococcus gattii by in vitro transposition. Curr. Genet. 49:341-350. [DOI] [PubMed] [Google Scholar]
- 27.Hu, G., I. Liu, A. Sham, J. E. Stajich, F. S. Dietrich, and J. W. Kronstad. 2008. Comparative hybridization reveals extensive genome variation in the AIDS-associated pathogen Cryptococcus neoformans. Genome Biol. 9:R41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu, G., B. R. Steen, T. Lian, A. P. Sham, N. Tam, K. L. Tangen, and J. W. Kronstad. 2007. Transcriptional regulation by protein kinase A in Cryptococcus neoformans. PLoS Pathog. 3:e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hua, Z., P. Fatheddin, and T. R. Graham. 2002. An essential subfamily of Drs2p-related P-type ATPases is required for protein trafficking between Golgi complex and endosomal/vacuolar system. Mol. Biol. Cell 13:3162-3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Idnurm, A., F. J. Walton, A. Floyd, J. L. Reedy, and J. Heitman. 2009. Identification of ENA1 as a virulence gene of the human pathogenic fungus Cryptococcus neoformans through signature-tagged insertional mutagenesis. Eukaryot. Cell 8:315-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iwamoto, K., S. Kobayashi, R. Fukuda, M. Umeda, T. Kobayashi, and A. Ohta. 2004. Local exposure of phosphatidylethanolamine on the yeast plasma membrane is implicated in cell polarity. Genes Cells 9:891-903. [DOI] [PubMed] [Google Scholar]
- 32.Jansen, P. L., S. S. Strautnieks, E. Jacquemin, M. Hadchouel, E. M. Sokal, G. J. Hooiveld, J. H. Koning, A. De Jager-Krikken, F. Kuipers, F. Stellaard, C. M. Bijleveld, A. Gouw, H. Van Goor, R. J. Thompson, and M. Muller. 1999. Hepatocanalicular bile salt export pump deficiency in patients with progressive familial intrahepatic cholestasis. Gastroenterology 117:1370-1379. [DOI] [PubMed] [Google Scholar]
- 33.Jung, W. H., G. Hu, W. Kuo, and J. W. Kronstad. 2009. The role of ferroxidases in iron uptake and virulence for Cryptococcus neoformans. Eukaryot. Cell 8:1511-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jung, W. H., A. Sham, T. Lian, A. Singh, D. J. Kosman, and J. W. Kronstad. 2008. Iron source preference and regulation of iron uptake in Cryptococcus neoformans. PLoS Pathog. 4:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu, K., K. Surendhran, S. F. Nothwehr, and T. R. Graham. 2008. P4-ATPase requirement for AP-1/clathrin function in protein transport from the trans-Golgi network and early endosomes. Mol. Biol. Cell 19:3526-3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loffler, J., H. Einsele, H. Hebart, U. Schumacher, C. Hrastnik, and G. Daum. 2000. Phospholipid and sterol analysis of plasma membranes of azole-resistant Candida albicans strains. FEMS Microbiol. Lett. 185:59-63. [DOI] [PubMed] [Google Scholar]
- 37.Missall, T. A., and J. K. Lodge. 2005. Thioredoxin reductase is essential for viability in the fungal pathogen Cryptococcus neoformans. Eukaryot. Cell 4:487-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Missall, T. A., M. E. Pusateri, M. J. Donlin, K. T. Chambers, J. A. Corbett, and J. K. Lodge. 2006. Posttranslational, translational, and transcriptional responses to nitric oxide stress in Cryptococcus neoformans: implications for virulence. Eukaryot. Cell 5:518-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Missall, T. A., M. E. Pusateri, and J. K. Lodge. 2004. Thiol peroxidase is critical for virulence and resistance to nitric oxide and peroxide in the fungal pathogen, Cryptococcus neoformans. Mol. Microbiol. 51:1447-1458. [DOI] [PubMed] [Google Scholar]
- 40.Mumberg, D., R. Muller, and M. Funk. 1994. Regulatable promoters of Saccharomyces cerevisiae: comparison of transcriptional activity and their use for heterologous expression. Nucleic Acids Res. 22:5767-5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nosanchuk, J. D., L. Nimrichter, A. Casadevall, and M. L. Rodrigues. 2008. A role for vesicular transport of macromolecules across cell walls in fungal pathogenesis. Commun. Integr. Biol. 1:37-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Panepinto, J., K. Komperda, S. Frases, Y. D. Park, J. T. Djordjevic, A. Casadevall, and P. R. Williamson. 2009. Sec6-dependent sorting of fungal extracellular exosomes and laccase of Cryptococcus neoformans. Mol. Microbiol. 71:1165-1176. [DOI] [PubMed] [Google Scholar]
- 43.Park, B. J., K. A. Wannemuehler, B. J. Marston, N. Govender, P. G. Pappas, and T. M. Chiller. 2009. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS (London) 23:525-530. [DOI] [PubMed] [Google Scholar]
- 44.Perfect, J. R., B. Wong, Y. C. Chang, K. J. Kwon-Chung, and P. R. Williamson. 1998. Cryptococcus neoformans: virulence and host defences. Med. Mycol. 36(Suppl. 1):79-86. [PubMed] [Google Scholar]
- 45.Pomorski, T., J. C. Holthuis, A. Herrmann, and G. van Meer. 2004. Tracking down lipid flippases and their biological functions. J. Cell Sci. 117:805-813. [DOI] [PubMed] [Google Scholar]
- 46.Pomorski, T., R. Lombardi, H. Riezman, P. F. Devaux, G. van Meer, and J. C. Holthuis. 2003. Drs2p-related P-type ATPases Dnf1p and Dnf2p are required for phospholipid translocation across the yeast plasma membrane and serve a role in endocytosis. Mol. Biol. Cell 14:1240-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Price, M. S., C. B. Nichols, and J. A. Alspaugh. 2008. The Cryptococcus neoformans Rho-GDP dissociation inhibitor mediates intracellular survival and virulence. Infect. Immun. 76:5729-5737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prinz, W. 2002. Cholesterol trafficking in the secretory and endocytic systems. Semin. Cell Dev. Biol. 13:197-203. [DOI] [PubMed] [Google Scholar]
- 49.Rodrigues, M. L., E. S. Nakayasu, D. L. Oliveira, L. Nimrichter, J. D. Nosanchuk, I. C. Almeida, and A. Casadevall. 2008. Extracellular vesicles produced by Cryptococcus neoformans contain protein components associated with virulence. Eukaryot. Cell 7:58-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodrigues, M. L., L. Nimrichter, D. L. Oliveira, S. Frases, K. Miranda, O. Zaragoza, M. Alvarez, A. Nakouzi, M. Feldmesser, and A. Casadevall. 2007. Vesicular polysaccharide export in Cryptococcus neoformans is a eukaryotic solution to the problem of fungal trans-cell wall transport. Eukaryot. Cell 6:48-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rogers, P. D., and K. S. Barker. 2003. Genome-wide expression profile analysis reveals coordinately regulated genes associated with stepwise acquisition of azole resistance in Candida albicans clinical isolates. Antimicrob. Agents Chemother. 47:1220-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saito, K., K. Fujimura-Kamada, N. Furuta, U. Kato, M. Umeda, and K. Tanaka. 2004. Cdc50p, a protein required for polarized growth, associates with the Drs2p P-type ATPase implicated in phospholipid translocation in Saccharomyces cerevisiae. Mol. Biol. Cell 15:3418-3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Soteropoulos, P., T. Vaz, R. Santangelo, P. Paderu, D. Y. Huang, M. J. Tamas, and D. S. Perlin. 2000. Molecular characterization of the plasma membrane H(+)-ATPase, an antifungal target in Cryptococcus neoformans. Antimicrob. Agents Chemother. 44:2349-2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tucker, S. C., and A. Casadevall. 2002. Replication of Cryptococcus neoformans in macrophages is accompanied by phagosomal permeabilization and accumulation of vesicles containing polysaccharide in the cytoplasm. Proc. Natl. Acad. Sci. U. S. A. 99:3165-3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ujhazy, P., D. Ortiz, S. Misra, S. Li, J. Moseley, H. Jones, and I. M. Arias. 2001. Familial intrahepatic cholestasis 1: studies of localization and function. Hepatology (Baltimore) 34:768-775. [DOI] [PubMed] [Google Scholar]
- 56.Vida, T. A., and S. D. Emr. 1995. A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J. Cell Biol. 128:779-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walton, F. J., A. Idnurm, and J. Heitman. 2005. Novel gene functions required for melanization of the human pathogen Cryptococcus neoformans. Mol. Microbiol. 57:1381-1396. [DOI] [PubMed] [Google Scholar]
- 58.Wedlich-Soldner, R., M. Bolker, R. Kahmann, and G. Steinberg. 2000. A putative endosomal t-SNARE links exo- and endocytosis in the phytopathogenic fungus Ustilago maydis. EMBO J. 19:1974-1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wendland, B., S. D. Emr, and H. Riezman. 1998. Protein traffic in the yeast endocytic and vacuolar protein sorting pathways. Curr. Opin. Cell Biol. 10:513-522. [DOI] [PubMed] [Google Scholar]
- 60.Yoneda, A., and T. L. Doering. 2006. A eukaryotic capsular polysaccharide is synthesized intracellularly and secreted via exocytosis. Mol. Biol. Cell 17:5131-5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu, J. H., Z. Hamari, K. H. Han, J. A. Seo, Y. Reyes-Dominguez, and C. Scazzocchio. 2004. Double-joint PCR: a PCR-based molecular tool for gene manipulations in filamentous fungi. Fungal Genet. Biol. 41:973-981. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.