Abstract
The mitogen-activated protein kinase (MAPK) Sty1 is essential for the regulation of transcriptional responses that promote cell survival in response to different types of environmental stimuli in Schizosaccharomyces pombe. In fission yeast, three distinct eukaryotic initiation factor 2α (eIF2α) kinases, two mammalian HRI-related protein kinases (Hri1 and Hri2) and the Gcn2 ortholog, regulate protein synthesis in response to cellular stress conditions. In this study, we demonstrate that both Hri1 and Hri2 exhibited an autokinase activity, specifically phosphorylated eIF2α, and functionally replaced the endogenous Saccharomyces cerevisiae Gcn2. We further show that Gcn2, but not Hri1 or Hri2, is activated early after exposure to hydrogen peroxide and methyl methanesulfonate (MMS). Cells lacking Gcn2 exhibit a later activation of Hri2. The activated MAPK Sty1 negatively regulates Gcn2 and Hri2 activities under oxidative stress but not in response to MMS. In contrast, Hri2 is the primary activated eIF2α kinase in response to heat shock. In this case, the activation of Sty1 appears to be transitory and does not contribute to the modulation of the eIF2α kinase stress pathway. In strains lacking Hri2, a type 2A protein phosphatase is activated soon after heat shock to reduce eIF2α phosphorylation. Finally, the MAPK Sty1, but not the eIF2α kinases, is essential for survival upon oxidative stress or heat shock, but not upon MMS treatment. These findings point to a regulatory coordination between the Sty1 MAPK and eIF2α kinase pathways for a particular range of stress responses.
Global inhibition of protein synthesis is widely considered as a response of biological systems to stress conditions. However, it is becoming increasingly recognized that translation is not completely inhibited and that translational control of specific mRNAs is required for survival during growth under stress conditions (38, 47). In eukaryotic cells, the reversible phosphorylation of the α-subunit of eukaryotic translation initiation factor 2 (eIF2α) is a well-characterized mechanism of translational control in response to a wide variety of cellular stresses (7, 12).
Four mammalian protein kinases that inhibit translation initiation by phosphorylating eIF2α on Ser-51 have been identified. They are regulated independently in response to various different cellular stresses (12, 39). Thus, heme-regulated inhibitor (HRI) is activated both by heme deficiency and under conditions of heat shock and oxidative stress (28). Double-stranded RNA-dependent protein kinase (PKR) is induced by interferon and activated by double-stranded RNA during viral infection (23). General control nonderepressible-2 (GCN2) is an eIF2α kinase that is activated by amino acid or serum deprivation, UV irradiation, and viral RNAs (3, 4, 10, 19). PKR-like endoplasmic reticulum (ER) kinase (PERK, also known as PEK) is activated by unfolded proteins in the ER (16, 44).
In the yeast Saccharomyces cerevisiae, Gcn2 is the sole eIF2α kinase (12). It is activated in response to a variety of conditions, including nutrient starvation (amino acids, purines, and glucose) and exposure to hydroperoxides, sodium chloride, and rapamycin (20, 29). In the fission yeast Schizosaccharomyces pombe, besides Gcn2, two additional eIF2α kinases related to mammalian HRI, called Hri1 and Hri2, phosphorylate eIF2α at serine 51 (serine 52 in S. pombe). Hri1 and Hri2 showed a differential activation pattern in response to cellular stresses (52).
It is well known that eIF2α phosphorylation can regulate both gene-specific and general translation. Thus, the eIF2α kinases phosphorylate eIF2α, leading to the inhibition of eIF2B activity. This fact produces a marked decrease in the levels of ternary complex eIF2-GTP-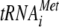 , resulting in reduced general translation and increased translation of the transcription factors, Gcn4 in S. cerevisiae and ATF4 in mammals, which activate expression of their target genes involved in the stress response (15, 18). Paradoxically, S. pombe does not have a Gcn4 homologue. However, other related transcription factors may facilitate its eIF2α kinase-mediated stress response.
, resulting in reduced general translation and increased translation of the transcription factors, Gcn4 in S. cerevisiae and ATF4 in mammals, which activate expression of their target genes involved in the stress response (15, 18). Paradoxically, S. pombe does not have a Gcn4 homologue. However, other related transcription factors may facilitate its eIF2α kinase-mediated stress response.
Despite the finding that phosphorylation of eIF2α is a general response to cellular stress, it is well known that, in S. pombe, cellular responses to various environmental stresses are regulated primarily through the stress-activated mitogen-activated protein kinase (MAPK) Sty1. The Sty1 protein (also known as Spc1 or Phh1) is a member of the stress-activated protein kinase (SAPK) family, which includes Hog1 from S. cerevisiae and the mammalian SAPKs, p38 and c-Jun N-terminal kinase (JNK), and can be activated by heat shock, high osmolarity stress, nutrient depletion, and oxidative stress (22, 50). Upon stress activation, Sty1 reversibly accumulates in the nucleus, where it stimulates gene expression via the Atf1 transcription factor. Thus, in response to the stress stimuli, Sty1 is required for the transcriptional regulation of a large set of genes that constitute the core environmental stress response (CESR) (5). For the majority of these genes, regulation is also dependent on Atf1 (46, 48, 51). Phosphorylation of Atf1 by Sty1 upon stress activation has been demonstrated both in vitro (51) and in vivo (46).
Previous studies showed that Sty1 is important for survival when cells are exposed to hydrogen peroxide (6, 13, 40, 41). Also, it was previously observed that the growth of Sty1 mutant cells was defective at high temperatures (32). On the other hand, more recently it has been reported that in S. cerevisiae, whereas mutants lacking Gcn4 were sensitive to H2O2, the Gcn2 mutant was no more sensitive than the wild-type to peroxides in spot tests (29).
In a previous report, it was suggested that downstream components of the SAPK pathway can contribute both positively and negatively to the modulation of the eIF2α kinase stress pathway (13). However, the relationship between the highly conserved SAPK pathway and the translational machinery in fission yeast remains to be elucidated. Here, we further investigate the participation of each eIF2α kinase in the fission yeast translational program in response to stress. Our data show that, upon distinct cellular stresses, phosphorylation of eIF2α constitutes a very early response. Interestingly, a particular eIF2α kinase is activated in response to each stress stimuli. Thus, Hri2 responds to heat shock, whereas Gcn2 is activated early after exposure to hydrogen peroxide and methyl methanesulfonate (MMS) in S. pombe. In addition, some analyses reveal a strong correlation between changes in mRNA and protein levels of the distinct eIF2α kinases in response to oxidative stress and heat shock. Finally, we found a striking relationship between the MAPK Sty1 and the eIF2α kinase family under some, but not all, of the stress conditions in fission yeast.
MATERIALS AND METHODS
Yeast strains and growth conditions.
The S. pombe strains used in the present study are listed in Table 1. Deletion of hri1+ and hri2+ from the S. pombe strains was generated by homologous recombination transforming linear DNA containing ura4+ replacing the entire coding region of hri1+ and hri2+. Transformants were selected for growth in minimal medium lacking uracil. The hri1 and hri2 knockouts were confirmed by Southern blotting. The opposing mating types of Δhri1 and Δhri2 strains were mated to each other and with Δgcn2, Δspc1, Δpyp1, Δdis2, and Δppa2. The double- and triple-mutant strains were obtained after sporulation and tetrad analysis, and genotypes were confirmed by PCR analysis. The S. pombe strains Hri1-HA, Hri2-HA, and Gcn2-HA, expressing endogenous Hri1-, Hri2-, and Gcn2-tagged proteins bearing 3 copies of the influenza virus hemagglutinin (HA) epitope, were constructed as previously described (1). S. pombe strains were routinely grown with shaking at 32°C in yeast extract plus supplements medium (YES; Bio 101) with all 20 amino acids (225 g/ml) or on agar plates at the same temperature. The addition of the amino acids to the YES medium assures minimal phosphorylation of eIF2α in nonstressed conditions. Sporulation tests were performed using solid malt extract agar medium (MEA; Difco).
TABLE 1.
S. pombe strains
| Strain | Genotype | Source |
|---|---|---|
| PN1 | 972 h− | P. Nurse |
| PN22 | leu1-32 h− | P. Nurse |
| Δhri1 | hri1::ura4+ ura4-d18 h+ | This study |
| Δhri2 | hri2::ura4+ ura4-d18 h+ | This study |
| Δgcn2 | gcn2::ura4+ ura4-d18 h− | This study |
| IH2194 | dis2::ura4+ ura4-d18 leu1-32 h− | I. Álvarez |
| IH1233 | ppa2::ura4+ ura4-d18 leu1-32 h− | I. Álvarez |
| S1017 | rad3::ura4+ ade6 leu h− | T. Carr |
| S652 | spc1::HA6His [ura4+] ura4-d18 leu1-32 h− | S. Moreno |
| Δsty1 | spc1::ura4+ ura4-d18 leu1-32 h− | E. Hidalgo |
| Δpyp1 | pyp1::ura4+ ura4-d18 leu1-32 h− | E. Hidalgo |
| Δhri1 Δhri2 | hri1::ura4+ hri2::ura4+ ura4-d18 h− | This study |
| Δhri1 Δ gcn2 | hri1::ura4+ gcn2::ura4+ ura4-d18 h− | This study |
| Δhri2 Δ gcn2 | hri2::ura4+ gcn2::ura4+ ura4-d18 h+ | This study |
| Δhri2 Δ dis2 | hri2::ura4+ dis2::ura4+ ura4-d18 h+ | This study |
| Δhri2 Δ ppa2 | hri2::ura4+ ppa2::ura4+ ura4-d18 h+ | This study |
| Δhri1 Δhri2 Δ gcn2 | hri1::ura4+ hri2::ura4+ gcn2::ura4+ ura4-d18 h+ | This study |
| Δhri1 Δhri2 Δsty1 | spc1::ura4+ hri1::ura4+ hri2::ura4+ ura4-d18 | This study |
| Δhri1 Δ gcn2 Δsty1 | spc1::ura4+ hri1::ura4+ gcn2::ura4+ ura4-d18 | This study |
| Δhri2 Δ gcn2 Δsty1 | spc1::ura4+ hri2::ura4+ gcn2::ura4+ ura4-d18 | This study |
| Δhri1 Δhri2 Δpyp1 | pyp1::ura4+ hri1::ura4+ hri2::ura4+ ura4-d18 | This study |
| Δhri1 Δ gcn2 Δpyp1 | pyp1::ura4+ hri1::ura4+ gcn2::ura4+ ura4-d18 | This study |
| Δhri2 Δ gcn2 Δpyp1 | pyp1::ura4+ hri2::ura4+ gcn2::ura4+ ura4-d18 | This study |
| Hri1-HA | hri1::hri1-HA/Kanr | This study |
| Hri2-HA | hri2::hri2-HA/Kanr | This study |
| Gcn2-HA | gcn2::gcn2-HA/Kanr | This study |
Prokaryotic expression and affinity purification of Hri1 and Hri2 proteins.
Plasmids pRSETB-Hri1 and the pRSETB-Hri2 encoding His-tagged Hri1 and Hri2 proteins were used to transform a competent BL21(DE3)/pLys S strain of E. coli. Protein expression, bacterial lysis, and His-tagged protein purification were carried out as previously described (2).
Growth assay in S. cerevisiae.
Strains J80 (MATa gcn2Δ ura3-52 trp1-Δ63 Δsui2 [SUI2-LEU2]) and J82 (MATa gcn2Δ ura3-52 trp1-Δ63 Δsui2 [SUI2-S51A LEU2]) were used. Cells were transformed with empty pEMBLyex4 and PYX212 vectors or with the same plasmids encoding S. cerevisiae Gcn2 or S. pombe Hri1 and Hri2. The transformation of cells was carried out by using an improved lithium acetate procedure (14). Transformant cells were grown on plates containing SD medium complemented with amino acids (SD+aa) or SD medium plus 3-aminotriazole (SD+3-AT) to elicit histidine starvation conditions. Plates were incubated at 30°C for 3 days before being photographed.
In vitro phosphorylation assays.
The eluted proteins from the metal affinity resin or immune complexes were assayed for their ability to phosphorylate eIF2α as reported previously (30, 42), with modifications as described. In a total volume of 20 μl, 5-μl portions of kinase fractions were incubated for 30 min at 30°C in kinase buffer (20 mM Tris-HCl [pH 7.6], 2.5 mM MgCl2, 2.5 mM magnesium acetate, 0.25 mg of bovine serum albumin/ml, 50 μM ATP) containing purified His-tagged human eIF2α (0.5 μg) and 3 μCi of [γ-32P]ATP (3,000 Ci/mmol). Incubations were terminated by the addition of sodium dodecyl sulfate (SDS) sample buffer, and phosphoproteins were analyzed by SDS-PAGE on a 10% polyacrylamide gel (28.5:1 [wt/wt] acrylamide-bisacrylamide) (26), followed by autoradiography using Agfa Curix RP2 film and an Amersham-Pharmacia Biotech intensifying screen.
Stress assays.
The yeast cells were grown to an optical density at 595 nm (OD595) of 0.7 and subjected to stress. Oxidative stress was produced by adding 1.5 mM hydrogen peroxide (H2O2) to the medium for different time periods. DNA damage-induced stress was produced by treatment with the DNA alkylating agent MMS at a concentration of 0.02% for times ranging from 15 to 60 min. For heat shock experiments, cells were collected by centrifugation, resuspended in medium previously warmed at 40 or 48°C, and incubated for different time periods at the same temperature; when indicated, cells were incubated in the presence of 20 μM okadaic acid for 6 h before the stress treatment. At the end of the stress treatment the cells were collected by centrifugation at 3,000 × g for 3 min and frozen immediately on dry ice. The pellets were resuspended in lysis buffer (20 mM Tris-HCl [pH 7.6], 500 mM NaCl, 10% glycerol, 1 mM phenylmethylsulfonyl fluoride, 40 mM glycerol 2-phosphate, 1 mM EDTA, 1 mM dithiothreitol, 50 mM sodium fluoride, 1% Triton X-100, 25 μg of DNase/ml, and a protease inhibitor cocktail [Complete EDTA-free; Roche]) and broken in the presence of acid-washed glass beads (425 to 600 μm; Sigma) by using a FastPrep FP120 homogenizer (Bio 101), in four cycles of 40 s to a power of 6. The lysates were clarified by centrifugation at 12,000 × g at 4°C for 15 min and stored at −70°C. Protein determination was performed by using a Bio-Rad protein assay according to the manufacturer's instructions. For the stress experiments on plates, cells were grown as described above and diluted in order to plate different amounts of cells ranging from 10 to 106. For oxidative stress, cells were grown at 32°C for 48 h in the absence or the presence of 0.2, 0.75, or 1.5 mM H2O2. For heat shock, cells were incubated the first 24 h at 40°C and then at 32°C for an additional 48 h.
Immunoprecipitation, SDS-PAGE, and immunoblotting.
For immunoprecipitation, cell lysates containing 10 mg of total protein were incubated for 3 h at 4°C with 10 μg of anti-HA monoclonal antibody (Covance) and protein G-Sepharose (Amersham Pharmacia Biotech). After extensive washing with lysis buffer, the immune complexes were equilibrated in kinase buffer and assayed for their ability to phosphorylate eIF2α. For the analysis of the presence of phosphoproteins in cell lysates, an aliquot of 50 or 100 μg of each protein sample was separated by electrophoresis on SDS-PAGE (10% acrylamide, 0.26% bisacrylamide). The proteins were transferred to polyvinylidene difluoride (PVDF) membranes (Immobilon; Millipore) and sequentially probed with rabbit anti-eIF2α (Santa Cruz), rabbit anti-eIF2α-P (Cell Signaling), and rabbit anti-p38-P (Cell Signaling), followed by rabbit secondary antibody conjugated with horseradish peroxidase (Promega). After extensive washing, the immunoreactive bands were detected by enhanced chemiluminescence (Amersham Pharmacia Biotech). The relative intensities of bands were quantified by using a GS-710 densitometer (Bio-Rad) with the QuantityOne program. In order to determine the ratio between the values obtained in the treated samples with respect to the control, both values were divided by the control, assuming that the control has a value of 1.
Sty1 overexpression in different S. pombe strains.
Wild-type (PN22) and Δsty1 strains were transformed by using the lithium acetate procedure (33) with plasmid pSty1.41x (a gift from Jonathan Millar), encoding a HA- and His6-tagged version of the Sty1 protein under the control of the nmt1 (41x) promoter. For Sty1-HA-His6 expression, cells were grown in Edinburgh minimal medium (EMM) alone or EMM supplemented with 0.5 μM thiamine to repress the expression from the nmt1 promoter.
RNA extraction, RT, and real-time PCR.
Cells were grown to an OD595 of 0.7 and either subjected or not subjected to stress conditions, as described above. A total of 5 × 107 cells were collected by centrifugation, and the total RNA was extracted by using an RNeasy minikit (Qiagen) according to the manufacturer's instructions. To avoid genomic DNA contamination, RNA samples were treated with RQ1 RNase-Free DNase (Promega) and precipitated in the presence of 3 M LiCl and 20 mM EDTA. For the determination of hri1, hri2, and gcn2 mRNA expression, 1 μg of total RNA was reverse transcribed in a 20-μl reaction mixture by using a reverse transcription (RT) system (Promega) and oligo(dT) primers. The reaction mixture was incubated at 42°C for 15 min, followed by enzyme inactivation at 95°C for 5 min. The LightCycler FastStart DNA MasterPLUS SYBR green I system (Roche) was used for real-time PCR amplification and quantification using 2 μl of first-strand cDNA reaction as a template. The level of actin mRNA in each sample was determined in order to normalize for differences in the total amount of RNA. The data were derived from at least three independent RT reactions, and real-time PCR was performed in duplicate. The data analysis to determine relative hri1, hri2, and gcn2 mRNA expression was performed according to the 2−ΔΔCT method (27). Primers were designed to amplify fragments less than 300 bp long in the open reading frame of hri1 (nucleotides 1026 to 1203), hri2 (nucleotides 1016 to 1194), gcn2 (nucleotides 1261 to 11517), and actin (nucleotides 169 to 363).
RESULTS
Hri1 and Hri2 show both autokinase and eIF2α kinase activities in vitro and mediate translational control in S. cerevisiae.
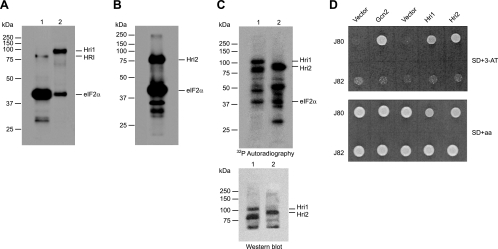
In the yeast S. cerevisiae, phosphorylation of eIF2α is carried out only by the Gcn2 protein kinase (18). In S. pombe, besides Gcn2, two additional eIF2α kinases, Hri1 and Hri2, have been characterized, both of which show higher homology with mammalian HRI than with other eIF2α kinases (53). It should be noted that these two novel eIF2α kinases lack the two putative heme regulatory motifs (HRMs) present in the mammalian HRI, although they are also sensitive to heme regulation in vitro (17, 53). To further characterize Hri1 and Hri2 as eIF2α kinases, we expressed them in Escherichia coli cells, and the recombinant proteins were affinity purified and subjected to eIF2α kinase assays as described in Materials and Methods. As a positive control, the reticulocyte heme-reversible HRI (31) was assayed under the same conditions (Fig. 1A, lane 1). Both Hri1 and Hri2 underwent autophosphorylation and were fully active in phosphorylating mammalian eIF2α (Fig. 1A, lane 2, and Fig. 1B). Moreover, the immunoprecipitated endogenous HA-tagged Hri1 and Hri2 exhibited autokinase activity and phosphorylated mammalian eIF2α (Fig. 1C, upper panel). These phosphorylated kinases were detected by using immunoblot analysis (Fig. 1C, lower panel). As a negative control, neither wild-type cell extracts immunoprecipitated with anti-HA antibodies nor HA-tagged Hri2 cell extracts immunoprecipitated with anti-FLAG antibodies showed eIF2α kinase activity (data not shown). We therefore conclude that endogenous HA-tagged Hri1 and Hri2 show both autokinase and eIF2α kinase activities in vitro.
FIG. 1.
In vitro and in vivo activity of S. pombe eIF2α kinases Hri1 and Hri2. (A) His-tagged purified Hri1 (lane 2) and purified rabbit HRI (as a control, lane 1) were assayed for their ability to phosphorylate eIF2α, as described in Materials and Methods. Proteins were resolved in SDS-PAGE, and phosphoproteins were visualized by autoradiography of the dried gel. The position of Hri1, HRI, and eIF2α are indicated on the right, and the positions of molecular weight markers are indicated on the left. (B) His-tagged purified Hri2 was assayed for its ability to phosphorylate eIF2α and phosphoproteins were resolved and visualized as described in panel A. The position of Hri2 and eIF2α are indicated on the right, and positions of molecular weight markers are indicated on the left. (C) Cell extracts from S. pombe strains modified to express HA-tagged Hri1 or Hri2 were subjected to immunoprecipitation with anti-HA monoclonal antibody. The immune complexes were assayed for their ability to phosphorylate eIF2α, and proteins were resolved in SDS-PAGE and transferred to a PVDF membrane. Phosphoproteins were visualized by autoradiography of the dried membrane (upper panel), and HA-tagged proteins were detected by Western blotting with anti-HA antibody (lower panel). The position of HA-tagged Hri1 and Hri2 and eIF2α are indicated on the right, and positions of molecular weight markers are indicated on the left. (D) J80 and J82 strains of S. cerevisiae transformed with empty pEMBLyex4 and pYX212 vectors or with plasmids encoding S. pombe Hri1 and Hri2 or S. cerevisiae GCN2 were grown on SD+aa (lower panel) or SD+3-AT (upper panel) plates at 30°C for 3 days. Shown are the results of representative experiments of at least three independent experiments with similar results.
It is well known that S. cerevisiae is a useful model system for studying the in vivo role of eIF2α kinases in translational control (11). To address whether Hri1 and Hri2 can functionally replace S. cerevisiae GCN2, plasmids encoding kinases or the vector alone were introduced into two isogenic yeast strains lacking the Gcn2 kinase. Yeast cells expressing these S. pombe eIF2α kinases were compared to cells containing plasmid-encoded S. cerevisiae GCN2 as a positive control. As expected, all transformants grew to similar levels in synthetic medium (Fig. 1D, lower panel). Under amino acid starvation produced by supplementing the medium with 3-aminotriazole (3-AT), only the cells expressing an active eIF2α kinase, either S. cerevisiae Gcn2 or both S. pombe Hri1 and Hri2, were able to grow. Moreover, the use of strain J82 (Δgcn2 SUI2-S51A), isogenic to J80 (Δgcn2), with an alanine substitution for serine 51 in eIF2α, revealed that phosphorylation of this site was required for the cells to grow in the presence of 3-AT (Fig. 1D, upper panel). Therefore, these S. pombe eIF2α kinases also require the regulatory phosphorylation site in eIF2α for translational control.
Gcn2, but not Hri1 or Hri2, is activated early after oxidative stress.
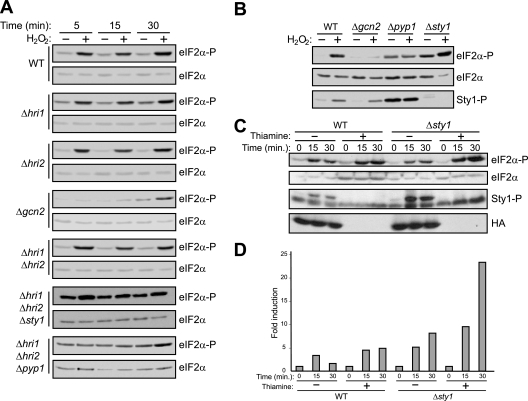
Differential activation of eIF2α kinases in response to various cellular stresses in S. pombe was previously suggested (52). However, this particular work concluded that there was early tandem activation of both Hri2 and Gcn2 after exposure to H2O2 (52). We wanted to further investigate the contributions of the individual Hri2 and Gcn2 activities during the course of oxidative stress. We observed that the level of eIF2α phosphorylation increased very rapidly after the exposure of cells to hydrogen peroxide (Fig. 2A). To test whether Hri2 and Gcn2 are both equally implicated in this early response, we next compared the behavior of cells lacking (or not) each of the eIF2α kinases under oxidative stress. Compared to wild-type cells, cells devoid of Hri1, Hri2, or both showed a very similar behavior in response to oxidative stress (Fig. 2A). However, deletion of Gcn2 significantly reduced the early activation of eIF2α phosphorylation after oxidative stress (Fig. 2A). At longer times, there was an increase of eIF2α phosphorylation in cells lacking Gcn2, probably due to the activation of the other eIF2α kinase. These data demonstrate that soon after H2O2 treatment of S. pombe cells Gcn2, but not Hri1 or Hri2, is activated, leading to phosphorylation of eIF2α.
FIG. 2.
Effect of oxidative stress on eIF2α phosphorylation by distinct eIF2α kinases. Role of the MAPK Sty1. (A and B) Gcn2 is the primary eIF2α kinase activated in response to oxidative stress; the MAPK Sty1 negatively regulates Gcn2 activity during oxidative stress. (C) Hyperactivation of Sty1 reduces the eIF2α phosphorylation induced in response to oxidative stress. (D) Quantification of the levels of phosphorylated eIF2α (ratio eIF2α-P/eIF2α referring to untreated cells, whose value was set as 1) in panel C. Wild-type (WT) S. pombe cells or cells of strains lacking the different eIF2α kinases and/or the MAPK Sty1 (Δsty1) and its negative regulator Pyp1 (Δpyp1) were subjected to oxidative stress (1.5 mM H2O2), as indicated. Phosphorylation of eIF2α and Sty1 were analyzed in the cell extracts by Western blotting with phospho-specific antibodies, as described in Materials and Methods. Shown are the durations of the stress, the names of the strains, and the antibody used for the Western blot (right). In panel B, the length of treatment with H2O2 was 15 min. In panel C, WT and Δsty1 strains transformed to overexpress a recombinant Sty1-HA-His6 protein were used; experiments were performed in EMM alone or EMM supplemented with 0.5 μM thiamine to repress recombinant protein expression. Shown are the results of representative experiments of at least three independent experiments with similar results.
Sty1 negatively regulates Gcn2 activity during oxidative stress.
A well-studied stress-activated MAPK pathway in S. pombe has Sty1 as its central component (32, 45). Sty1 is activated by phosphorylation of two residues: Thr171 and Tyr173 (45). Furthermore, inactivation of Sty1 is carried out by two tyrosine phosphatases, Pyp1 and Pyp2, which directly dephosphorylate Tyr173 of Sty1, both in vivo and in vitro (9, 45). Whereas pyp1 is constitutively expressed, pyp2 expression is induced in response to stress by a process that requires the Sty1 kinase (51). To investigate the possible role of the SAPK pathway in modulating eIF2α kinase activation in response to oxidative stress, further experiments were performed in cells lacking sty1, pyp1, or the hri genes. As shown before, eIF2α phosphorylation after oxidative stress in the Δhri1 Δhri2 mutant was similar to that of wild-type cells (Fig. 2A). However, no significant induction of eIF2α phosphorylation occurred in Δhri1 Δhri2 Δpyp1 cells. Moreover, in Δhri1 Δhri2 Δsty1 cells, eIF2α phosphorylation levels were high in comparison to those of Δhri1 Δhri2 cells at all time points examined under stress conditions (Fig. 2A). At early time points, we found no significant induction of eIF2α phosphorylation in the Δgcn2 mutant after exposure to H2O2 compared to that of the wild-type cells; however, the level of Sty1 phosphorylation after oxidative stress was similar to that found in wild-type cells (Fig. 2B). Interestingly, no induction of eIF2α phosphorylation was observed in the Δpyp1 mutant after exposure to oxidative stress (Fig. 2B). In such a mutant, as expected, markedly increased Sty1 phosphorylation levels were detected even under unstressed conditions (Fig. 2B). In good agreement with these results, the analysis of Δsty1 cells overexpressing Sty1 revealed a significant decrease in eIF2α phosphorylation levels compared to those of Δsty1 cells upon oxidative stress (Fig. 2C and D). We conclude that the H2O2-induced phosphorylation of eIF2α, at early time points, is dependent on Gcn2 and that Sty1 negatively regulates Gcn2 activity during oxidative stress.
Role of each eIF2α kinase and the MAPK Sty1 on eIF2α phosphorylation and cell viability during oxidative stress.
Multiple pathways differentially regulate global oxidative stress responses in fission yeast. The activation of the complex MAPK Sty1 pathway is critical for the response to hydrogen peroxide (6). The work of several laboratories has determined that oxidative stress induces a strong activation of the Wis1 MAPK kinase (MEK), which activates Sty1 through Thr171/Tyr173 phosphorylation, resulting in the phosphorylation of its substrates, such as the transcription factor Atf1 (46). Moreover, Sty1 is negatively regulated by two tyrosine phosphatases, Pyp1 and Pyp2. Since the Sty1 pathway is required for induction of Pyp2 mRNA in response to oxidative stress (34), presumably Pyp1 is the major tyrosine phosphatase that dephosphorylates and inactivates Sty1 at early times of H2O2 exposure. On the other hand, as shown before (13) and in Fig. 2A, phosphorylation of eIF2α is an early response to oxidative stress.
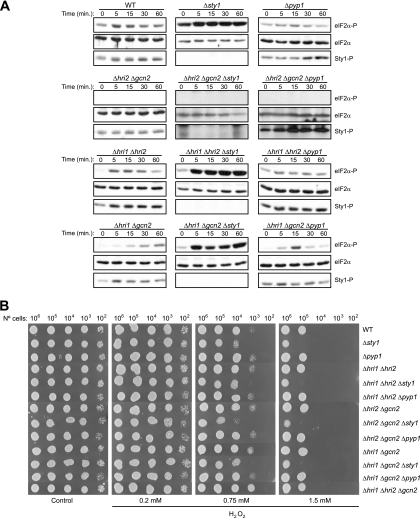
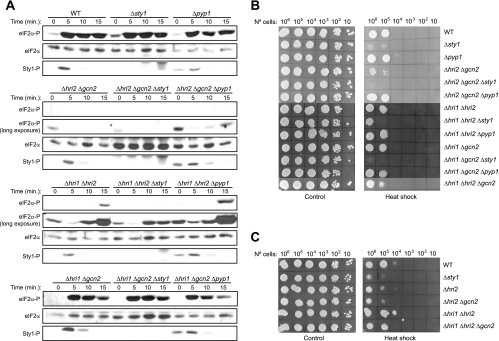
We decided to study which elements of the SAPK and eIF2α kinase stress pathways are required for the response to hydrogen peroxide and for a possible coordination between these two pathways in S. pombe, using mutants in different components of the pathways. Thus, to characterize the role of each eIF2α kinase and of the MAPK Sty1 in response to oxidative stress, we studied different strains, including a wild-type strain, strains devoid of two of the three eIF2α kinase genes (thereby expressing only Hri1, Hri2, or Gcn2), and the triple mutants in which there were additional disruptions in sty1 or pyp1 genes. We examined eIF2α phosphorylation levels in response to oxidative stress in both wild-type cells and in mutants in which either the SAPK pathway was disrupted (Δsty1) or hyperactivated (Δpyp1). As described before, exposure of wild-type cells to 1.5 mM H2O2 resulted in enhanced eIF2α phosphorylation levels within 5 min. A similar pattern of eIF2α phosphorylation was detected in the strain containing only Gcn2. However, there was a significant induction of eIF2α phosphorylation in cells expressing only Hri2 under longer periods of stress treatment. No eIF2α phosphorylation was detected in the strains expressing only Hri1 (Fig. 3A, left panels). Moreover, in response to hydrogen peroxide, both wild-type cells and mutants showed an increased level of Sty1 phosphorylation within 5 min, which was sustained for at least 60 min (Fig. 3A, left panels). Interestingly, when we eliminated Sty1 in these wild-type and mutant backgrounds, the analysis revealed a marked increase in the eIF2α phosphorylation levels in comparison to those of the Sty1-expressing cells, at all time points examined after exposure to oxidative stress. A small increase in eIF2α phosphorylation levels was also observed in nonstressed cells (Fig. 3A, middle panels). Moreover, when we eliminated Sty1 in the strain expressing only Hri2, we observed that a significant induction of eIF2α phosphorylation took place earlier than it did for Sty1-expressing cells (Fig. 3A, bottom panels). In contrast, eIF2α phosphorylation was strongly decreased in the pyp1-disrupted cells either in the absence or in the presence of oxidative stress (Fig. 3A, right panels). These results indicate that, at early time points, Gcn2 is responsible for the induction of eIF2α phosphorylation occurring in wild-type cells after exposure to hydrogen peroxide. However, Gcn2 and Hri2 are both implicated in the eIF2α phosphorylation enhancement in the sty1-disrupted cells under conditions of oxidative stress. Our findings suggest a model in which the SAPK pathway functions to negatively regulate both Gcn2 and Hri2, thus maintaining eIF2 activity under conditions of oxidative stress in fission yeast.
FIG. 3.
Effect of the deletion of the different eIF2α kinases and Sty1 on eIF2α phosphorylation and cell viability during oxidative stress. (A) Differential activation of eIF2α kinases in response to oxidative stress. Role of the MAPK Sty1. Wild-type (WT) S. pombe cells or cells of strains lacking the different eIF2α kinases and/or the MAPK Sty1 and its negative regulator Pyp1, were subjected to oxidative stress (1.5 mM H2O2), as indicated. Phosphorylation of eIF2α and Sty1 were analyzed in the cell extracts by Western blotting with phospho-specific antibodies, as described in Materials and Methods. Shown are the length of the stress and the name of the strains (top) and the antibody used for the Western blot (right). (B) Cell viability during oxidative stress in the absence of the eIF2α kinases and/or Sty1. The same cell strains as in panel A were grown on YES-agar plates either containing or not containing H2O2 0.2, 0.75 or 1.5 mM, as indicated, and incubated for 48 h at 32°C. Shown are the results of representative experiments of at least three independent experiments with similar results.
We further wanted to investigate whether eIF2α phosphorylation contributed to the loss of viability observed in sty1-disrupted cells when grown under oxidative stress conditions at different doses of the oxidant. As shown previously (6) and in the cell plating assays of Fig. 3B, elimination of the MAPK Sty1 causes sensitivity to hydrogen peroxide. Notably, all sty1-disrupted strains were hypersensitive to high (0.75 and 1.5 mM), but not to low (0.2 mM), concentrations of hydrogen peroxide. Previous studies have reported that the MAPK Sty1 pathway is only activated by high levels of H2O2 (50). However, none of the sty1-expressing mutants exhibited any difference in viability compared to wild-type cells, irrespective of the eIF2α phosphorylation level under these conditions. There was a significant induction of eIF2α phosphorylation in Δhri1 Δhri2 and Δhri1 Δgcn2 mutants, whereas eIF2α phosphorylation was not observed in Δhri2 Δgcn2 (Fig. 3A) and Δhri1 Δhri2 Δgcn2 (not shown) mutants. We conclude that Sty1, but not the eIF2α kinases, is essential for survival of cells under oxidative stress only at high levels of H2O2. Sty1 is not required for viability in response to low concentrations of H2O2.
Role of each eIF2α kinase and the MAPK Sty1 on eIF2α phosphorylation and cell viability during exposure to MMS.
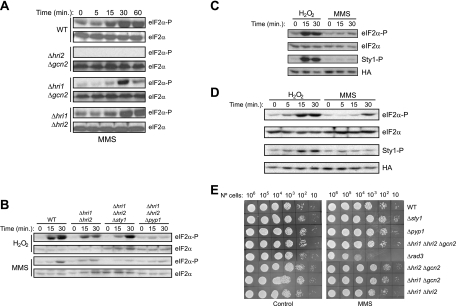
Recently, it has been reported that exposure of S. pombe cells to the alkylating agent MMS resulted in the phosphorylation of eIF2α in a concentration-dependent manner (25). In order to investigate the contribution of each eIF2α kinase and the role of the Sty1 in response to genotoxic stress by MMS, wild-type S. pombe cells or mutant cells devoid of two of the three eIF2α kinase genes were subjected to DNA damage-induced stress for different times. As described before, exposure of wild-type cells to 0.02% MMS resulted in enhanced eIF2α phosphorylation levels within 15 min (Fig. 4A). As in oxidative stress, Gcn2 is the primary eIF2α kinase activated in response to MMS, and Hri2 is only activated at longer times of stress treatment. Again, no eIF2α phosphorylation was detected in the strains expressing only Hri1 (Fig. 4A).
FIG. 4.
Effect of the deletion of the different eIF2α kinases and Sty1 on eIF2α phosphorylation and cell viability during genotoxic stress by MMS. (A) Differential activation of eIF2α kinases in response to genotoxic stress by MMS. (B) Gcn2 is the primary eIF2α kinase activated in response to DNA damage-induced stress caused by MMS, without any effect due to the absence or the overactivation of the MAPK Sty1. (C and D) Sty1 is not activated in response to DNA damage-induced stress caused by MMS. Wild-type (WT) S. pombe cells or cells of strains lacking the different eIF2α kinases and/or the MAPK Sty1 and its negative regulator Pyp1, were subjected to oxidative stress (1.5 mM H2O2) or DNA damage-induced stress (0.02% MMS), as indicated. Phosphorylation of eIF2α and Sty1 were analyzed in the cell extracts by Western blotting with phospho-specific antibodies, as described in Materials and Methods. Shown are the durations of the stress and the names of the strains and the antibody used for the Western blot (right). In panel C, the Δsty1 strain transformed to overexpress a recombinant Sty1-HA-His6 protein was used; experiments were performed in EMM alone or EMM supplemented with 0.5 μM thiamine to repress recombinant protein expression. In panel D, cells expressing endogenous HA-tagged Sty1 protein were used. (E) Cell viability during genotoxic stress in the absence of the eIF2α kinases and/or Sty1. Cell strains were grown on YES-agar plates either containing or not containing 0.02% MMS, as indicated, and incubated for 48 h at 32°C. Shown are the results of representative experiments of at least three independent experiments with similar results.
To explore the possible role of Sty1 in modulating eIF2α kinase activation in response to DNA damage caused by MMS, we performed an experiment by using cells in which sty1 or pyp1 was deleted, along with the two hri genes. These cells were subjected to oxidative or to DNA damage-induced stresses. Interestingly, while the absence of the MAPK Sty1 markedly increased the levels of eIF2α phosphorylation in response to H2O2, it had no effect on eIF2α phosphorylation after MMS treatment (Fig. 4B). Furthermore, by using the Δsty1 cells overexpressing Sty1, we obtained an increased level of Sty1 phosphorylation in response to hydrogen peroxide, as expected, but not after MMS treatment (Fig. 4C). It had previously been reported that UV irradiation induced activation of Sty1 and that the sty1 mutant was hypersensitive to this stress (8). Therefore, we performed an additional control experiment by using cells expressing endogenous HA-tagged Sty1 protein (Fig. 4D). We conclude that, in contrast to oxidative stress and UV radiation, the DNA damage caused by MMS activates Gcn2 and Hri2 without the activation of the SAPK pathway.
We showed previously that Sty1, but not the eIF2α kinases, is essential for survival under oxidative stress. Therefore, we wanted to investigate the effect of Sty1 and the three eIF2α kinases on cell viability during genotoxic stress. We used the Δrad3 mutant, which is sensitive to MMS (37), as a control. In this case, we found that, unlike Δrad3, the Δsty1 mutant was not sensitive to the DNA-damaging agent MMS. In fact, all of the mutants exhibited no difference in viability compared to wild-type cells (Fig. 4E). We conclude that, in contrast to oxidative stress, neither Sty1 nor the eIF2α kinases are essential for survival under MMS-induced DNA damage stress conditions. Moreover, the SAPK pathway does not appear to play any role in regulating the genotoxic stress response caused by MMS in S. pombe.
Hri2, but not Hri1 or GCN2, is activated early after heat shock.
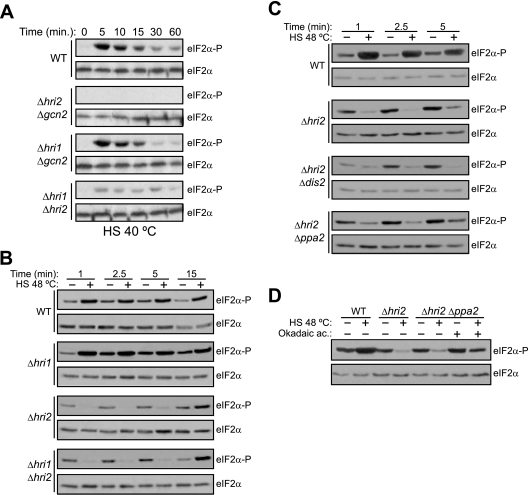
To examine the role of each eIF2α kinase in response to heat shock at 40°C, wild-type S. pombe cells and the mutant strains used in Fig. 4A, grown at 30°C, were exposed to heat shock at 40°C for different periods of time. A marked increase on eIF2α phosphorylation levels was observed in wild-type cells within 5 min. However, in contrast to oxidative or genotoxic stress exposure, a similar pattern of eIF2α phosphorylation was only detected in the strain expressing Hri2, whereas the Gcn2-expressing cells showed a modest effect (Fig. 5A). Therefore, it is clear that Hri2 is the primary eIF2α kinase activated in response to heat shock at 40°C.
FIG. 5.
Effect of heat shock on eIF2α phosphorylation by distinct eIF2α kinases. Role of the activation of the type 2A protein phosphatases in the dephosphorylation of eIF2α. (A and B) Hri2 is the primary eIF2α kinase activated in response to heat shock at 40 and 48°C. (C and D) The type 2A protein phosphatases are activated during heat shock at 48°C and dephosphorylate eIF2α. Wild-type (WT) S. pombe cells or cells of strains lacking the different eIF2α kinases and/or the protein phosphatases 1-1 (dis2) and 2A (ppa2) were subjected to heat shock (48°C), as indicated. Phosphorylation of eIF2α was analyzed in the cell extracts by Western blotting with a phospho-specific antibody, as described in Materials and Methods. Shown are the durations of the stress (in minutes), the names of the strains, and the antibody used for the Western blot (right). In panel D, the length of stress was 5 min; also indicated is the name of the strain and the pretreatment with okadaic acid (top) and the antibody used for the Western blot (right). Shown are the results of representative experiments of at least three independent experiments with similar results.
It was previously reported that Hri2 is the primary activated eIF2α kinase in response to exposure to heat shock and that Gcn2 has a compensatory role at longer periods of treatment (52). In these experiments, different S. pombe strains were exposed to heat shock at 48°C for 10, 20, and 30 min (52). To further investigate the role of each eIF2α kinase in response to heat shock at earlier times, we studied mutant strains in which either hri1 or hri2 genes or else both were deleted. Phosphorylation of eIF2α in the wild-type cells was significantly enhanced in response to heat shock after 1 min of heat exposure (Fig. 5B). Although Δhri1 cells showed a minimal effect on eIF2α phosphorylation, deletion of the hri2 gene reduced the levels of eIF2α phosphorylation in response to heat shock compared to that found in the wild-type cells (Fig. 5B). Interestingly, at early times, these reduced levels of eIF2α phosphorylation were in fact much lower than those found in the corresponding unstressed cells (Fig. 5B), suggesting the activation of an eIF2α phosphatase very early during heat treatment. It is well known that protein serine/threonine phosphatases type I and 2A promote the dephosphorylation of mammalian eIF2α in vitro (35). Two PP1-related genes (dis2 and sds21) and two PP2A-related genes (ppa1 and ppa2) are present in fission yeast (24). To test the possible effect of these phosphatases on the Δhri2 mutant in response to heat shock, we analyzed strains in which the hri2+ gene was deleted in combination with one of these phosphatases. Although deletion of either dis2 (Fig. 5C) or sds21 (data not shown) had no effect on eIF2α phosphorylation, the deletion of ppa2 markedly increased the levels of eIF2α phosphorylation in response to heat exposure compared to that found in Δhri2 cells (Fig. 5C). Since the Δhri2 Δppa2 mutant still contains the other PP2A-related gene ppa1, we repeated the experiment in the presence of okadaic acid (Fig. 5D), a highly effective inhibitor for mammalian type 2A phosphatases. In this case, the recovery of eIF2α phosphorylation observed after heat shock was fully dependent on type 2A-like protein phosphatase inhibition. All of these data indicate that Hri2, but not Gcn2, and the type 2A protein phosphatases are activated early during the heat shock exposure at 48°C.
Role of each eIF2α kinase and Sty1 on eIF2α phosphorylation and cell viability during heat shock.
In heat-shocked cells, Pyp1 is inhibited for its interaction with Sty1, which leads to strong activation of Sty1. Subsequently, Sty1 activity is rapidly attenuated by Thr171 dephosphorylation, whereas Tyr173 remains phosphorylated. Previous in vivo and in vitro data strongly suggested that PP2C enzymes Ptc1 and Ptc3 dephosphorylate Sty1 Thr171 (34). On the other hand, a differential activation of eIF2α kinases in response to heat shock in S. pombe was reported earlier (16). To further characterize the roles of each eIF2α kinase and of the MAPK Sty1 in the high level of eIF2α phosphorylation observed early during heat stress (Fig. 5B), the strain collection was next exposed to heat shock at 48°C for 5, 10, or 15 min. As shown before, after 5 min of heat shock the strain expressing only Hri2 exhibited levels of phosphorylated eIF2α similar to those of the wild-type cells (Fig. 6A). At this time, no eIF2α phosphorylation was detected in the strains expressing only Hri1 or Gcn2 (Fig. 6A) or the mutant cells devoid of all three eIF2α kinases (not shown). At 15 min after the heat shock, significant levels of eIF2α phosphorylation were observed in cells expressing only Gcn2. Interestingly, there were modest amounts of eIF2α phosphorylation in cells expressing either Hri1 or Gcn2 under unstressed conditions, but the level of eIF2α phosphorylation was significantly reduced just after 5 min of heat shock (Fig. 6A, long exposure). The observed early activation of PP2A during the heat shock exposure (Fig. 5C) may explain this effect.
FIG. 6.
Effect of the deletion of the different eIF2α kinases and Sty1 on eIF2α phosphorylation and cell viability during heat shock. (A) Differential activation of eIF2α kinases in response to heat shock. Role of the MAPK Sty1. Wild-type (WT) S. pombe cells or cells of strains lacking the different eIF2α kinases and/or the MAPK Sty1 and its negative regulator Pyp1 were subjected to heat shock (48°C), as indicated. Phosphorylation of eIF2α and Sty1 were analyzed in the cell extracts by Western blotting with phospho-specific antibodies, as described in Materials and Methods. Shown are the durations of the stress, the names of the strains (top), and the antibody used for the Western blot (right). (B and C) Cell viability during heat shock in the absence of the eIF2α kinases and/or Sty1. The same cell strains as in panel A plus a strain lacking the hri2 gene (Δhri2) were grown on YES-agar plates; for the heat shock, plates were incubated at 40°C for 24 h and then incubated for 48 additional hours at 32°C. Shown are the results of representative experiments of at least three independent experiments with similar results.
On the other hand, in good agreement with a previous report (34), only transient induction of the catalytic activity of Sty1 was observed in response to heat shock. Thus, the peak of Sty1 phosphorylation observed 5 min after the heat shock decreased rapidly at subsequent time points (Fig. 6A), which correlates with the reported kinetics of Sty1 activation after heat shock (34). Interestingly, all of the sty1-disrupted strains exhibited no difference in the eIF2α phosphorylation levels in comparison to those of the Sty1-expressing cells at all time points examined after exposure to heat shock (Fig. 6A, middle panels). All of these results indicate that Hri2, but not Gcn2, is responsible for the induction of eIF2α phosphorylation which occurred following exposure to heat shock. Moreover, Sty1 is activated soon after heat shock, but in this case does not regulate the eIF2α kinase activity.
To test whether Sty1 and the eIF2α kinases are both required for stress survival, we compared the phenotypes of cells lacking (or not) each kinase under heat stress conditions. As previously reported with the hydrogen peroxide treatment, Sty1, but not the eIF2α kinases, is essential to survive heat stress. Thus, whereas all cells lacking Sty1 showed severely impaired survival, the inactivation of all three eIF2α kinases completely eliminates eIF2α phosphorylation, and yet these cells showed an intermediate behavior, with survival efficiencies closer to those of the wild-type rather than the Δsty1 cells (Fig. 6B). Surprisingly, a Δhri2 Δgcn2 strain was markedly more sensitive to heat shock than wild-type cells but not nearly as sensitive as Δsty1 mutants. Moreover, when Pyp1 was further eliminated in this mutant, the resistance to heat shock was restored (Fig. 6B). In addition, the same behavior was observed particularly with the Δhri2 strain (Fig. 6C). These results support the notion that in the S. pombe strains containing Hri1, the presence of Hri2 is essential for cell survival under heat stress conditions. We do not yet know the molecular mechanism.
Regulation of the eIF2α kinase gene expression during oxidative stress and heat shock.
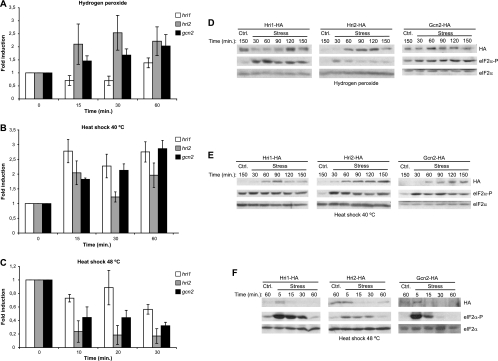
We next examined whether the induction of eIF2α phosphorylation observed in response to oxidative stress and heat shock is accompanied by changes at the level of transcription and translation of the distinct eIF2α kinases in S. pombe. To assess whether the response to these stress conditions is coordinated in terms of transcriptional and translational levels, further experiments were performed using wild-type S. pombe cells or cells of strains expressing the different HA-tagged eIF2α kinases. We studied the expression of the distinct mRNAs by using quantitative real-time RT-PCR, and we analyzed the protein levels in the cell extracts by Western blotting with anti-HA antibodies. As shown in Fig. 7, the expression of the hri2 and gcn2 genes was induced at the transcriptional level (1.5- to 3-fold) by 1.5 mM H2O2 (Fig. 7A), as well as after heat shock treatment at 40°C (Fig. 7B). In contrast, a significant decrease in transcript levels of hri2 and gcn2 mRNAs was observed after heat shock exposure at 48°C (0.2- to 0.4-fold compared to that of unstressed cells) (Fig. 7C). It is of interest that the expression of hri1 was moderately downregulated in response to hydrogen peroxide at early time points, and it was strongly upregulated after heat shock treatment at 40°C, but it showed little change in gene expression after heat shock treatment at 48°C (Fig. 7A to C). Interestingly, the changes in protein levels of the distinct eIF2α kinases were coordinated with the mRNA levels shown for each stress condition. Thus, mRNAs that were transcriptionally upregulated also changed in protein production with similar values (a 1.5- to 4-fold increase) (Fig. 7D and E). Moreover, mRNAs that were transcriptionally downregulated also changed in the protein levels accordingly (0.15- to 0.4-fold compared to that of unstressed cells) (Fig. 7F). We conclude that a significant correlation exists between changes in mRNA and protein levels of the distinct eIF2α kinases in S. pombe in response to oxidative stress and heat shock and that the significant induction of eIF2α phosphorylation observed in response to each stress condition represents an early event that is not related to these changes in gene expression.
FIG. 7.
Changes in mRNA and protein levels of the distinct eIF2α kinases in response to oxidative stress and heat shock. (A, B, and C) Levels of hr1, hri2, and gcn2 mRNAs increase after oxidative stress and 40°C heat shock, but decrease after 48°C heat shock. (D, E, and F) Levels of Hri1, Hri2, and Gcn2 proteins increase after oxidative stress and 40°C heat shock but decrease after 48°C heat shock. Wild-type (WT) S. pombe cells or cells of strains expressing the different HA-tagged eIF2α kinases were subjected to oxidative stress (1.5 mM H2O2) or heat shock (40 and 48°C). The levels of the distinct mRNAs were quantified by quantitative real-time RT-PCR, as described in Materials and Methods. Values show the fold induction compared to untreated control cells, whose level of mRNA was set as 1. The results show the means of three to five independent experiments plus the standard deviations. HA-tagged proteins levels and phosphorylation of eIF2α were analyzed in the cell extracts by Western blotting with anti-HA or phospho-specific antibodies, as described in Materials and Methods. Shown are the durations of the stress (minutes) and the names of the antibody used for the Western blot (right). Shown are the results of representative experiments of at least three independent experiments with similar results.
DISCUSSION
The results presented here provide important insights into the translational responses of fission yeast to oxidative stress and heat shock. After exposure to both stresses, elevated levels of eIF2α phosphorylation were rapidly observed. Our results fully agree with previous reports concluding that both Gcn2 and Hri2 are mainly involved in these responses (13, 52). However, in contrast to these findings, we found important differences between these two eIF2α kinases. Thus, Gcn2, but not Hri2, is activated early in response to H2O2, and conversely, Hri2, but not Gcn2, is stimulated early under exposure to high temperatures. The apparent discrepancy between our data and previous results is that previous experiments were performed for longer periods of exposure to stress. Indeed, our studies also demonstrate that the levels of eIF2α phosphorylation in the cells that only express Hri2 or Gcn2 were significantly elevated after longer exposures to oxidative stress or heat shock, respectively.
It is well known that eIF2α phosphorylation can promote changes in gene expression through preferential translation of stress response genes. Thus, in S. cerevisiae, Gcn4, a transcriptional activator of genes involved in the biosynthesis of amino acids, was found to be translationally induced by stress signals such as amino acid or glucose deprivation, allowing the transcriptional activation of stress response mRNAs (18, 19). In mammalian cells, under stress, the eIF2α kinases stimulate translation of ATF4, which activates the transcription of CHOP, a downstream target that is itself a transcription factor that controls expression of a set of stress-induced target genes (15). Such conservation of translational control of transcriptional regulators from yeast to mammals suggests that a related transcriptional activator is also controlled by eIF2α kinases in S. pombe. However, a Gcn4 ortholog is not present in S. pombe, despite the conservation of this transcriptional activator among a range of fungi, including S. cerevisiae, Neurospora crassa, Candida albicans, and Aspergillus nidulans (21, 36, 49). Since both GCN4 and ATF4 mRNAs are preferentially translated in response to phosphorylation of eIF2α by a mechanism involving upstream open reading frames (uORFs) (15, 19), we have used the presence of these uORFs as a query to search for related transcriptional activators in S. pombe by using the S. pombe Genome Project. During the course of our analysis of the S. pombe genome, we found various putative candidates. Nevertheless, the identification of potential regulatory targets for eIF2α phosphorylation by the eIF2α kinases in S. pombe awaits further biochemical studies.
Studies performed over the last few years have highlighted the importance of a SAPK pathway in regulating a variety of stress responses in S. pombe. Activation of Sty1 is required for the transcription of genes involved in stress responses. In S. pombe, the transcriptional response to oxidative stress elicited by H2O2 is mediated by at least two transcription factors, Pap1 and Atf1. Pap1 is important for the response to low levels of H2O2, whereas Atf1 is more important for the response to high levels of H2O2 (50). Heat shock-induced activation of Sty1 is regulated via inhibition of the Pyp1 tyrosine phosphatase, which dephosphorylates and inactivates Sty1 (34). Previous findings, together with some data of our study, suggest a possible relationship between SAPK and eIF2α kinase stress pathways. Our results demonstrate for the first time that the MAPK Sty1 negatively regulates Gcn2 and Hri2 kinases under conditions of oxidative stress, but not upon heat shock, in fission yeast. Interestingly, in response to heat shock, a functional type 2A serine/threonine phosphatase was found to maintain eIF2α activity. The overall conclusion from these studies is that the responses to heat shock and hydrogen peroxide are quite different, with distinct requirements for signaling but quite similar ones for survival. Thus, our studies confirm that the presence of Sty1 is essential for cell survival in both heat shock and hydrogen peroxide stresses. In contrast, the absence of all three eIF2α kinases leads to very similar viabilities of S. pombe cells compared to wild-type cells under both types of stress. Interestingly, both Δhri2 and Δhri2 Δgcn2 mutants display an unexpected behavior upon exposure to high temperatures. Thus, when exposed to heat shock, these cells are more sensitive than Δhri1 Δhri2 Δgcn2 cells, indicating that Hri1 could function as a negative factor in the response to high temperatures. Note that its survival was reproducibly restored when Sty1 activity was increased in the Δhri2 Δgcn2 Δpyp1 mutant (Fig. 6B). Most important, our data strongly suggest that the presence of Hri2 is essential for cell survival under heat stress conditions for the S. pombe strains containing Hri1. The mechanistic insights of such an observation remain to be clarified.
To our surprise, Sty1 does not play any role in response to the genotoxic stress induced by MMS in S. pombe. Thus, exposure to MMS induces Gcn2-dependent phosphorylation of eIF2α but does not affect Sty1 phosphorylation. Moreover, Sty1 is not essential for survival under exposure to MMS. Therefore, we believe that the regulatory coordination between the SAPK and eIF2α kinase pathways is a stress-specific phenomenon.
Previous studies, using microarray analysis, explored the transcriptional responses of S. pombe to various environmental stresses, including oxidative stress caused by hydrogen peroxide and heat shock caused by temperature increase to 39°C (5). Thus, a core environmental stress response (CESR) common to all, or most, stresses was defined. The CESR genes were controlled primarily by Sty1 and the transcription factor Atf1 (5). Further investigations by the same researchers into gene expression responses to different doses of hydrogen peroxide provided an overview of genes with changes in transcriptional levels. Some genes were induced, whereas another cluster of genes was specifically repressed in a dose-dependent manner (6). On the other hand, earlier analysis did not reveal any strong correlation between changes in transcript level and the translational response to H2O2 (43). Here, we found important differences in the gene expression response of the fission yeast eIF2α kinases to different environmental stresses. Thus, both hri2 and gcn2 genes were induced, whereas the hri1 gene was repressed in response to H2O2. Our results demonstrate for the first time that the eIF2α kinase genes were repressed by heat shock caused by a temperature increase to 48°C and that the hri2 transcript appears to be preferentially degraded. Interestingly, our results reveal a coregulation at the transcription and translation levels in all of the stress conditions studied. Our data further indicate that the stimulation of a particular eIF2α kinase activity observed in response to each stress condition is unrelated with these changes in gene expression.
Taken together, these results indicate that hydrogen peroxide and heat shock have different effects on fission yeast physiology, even though some of their effects seem to be similar (e.g., Δsty1 mutant sensitivities). On the other hand, it seems that the transcriptional program displayed to respond to different types of stress requires several transcription factors from the SAPK and eIF2α kinase pathways, with a different degree of importance and functional dependence for each type of stress. The requirement for the eIF2α kinases is apparent early during adaptive conditions, when it is needed to inhibit translation initiation and to induce the expression of unknown target genes in response to both types of stress in actively growing cells. Our current studies are focused on identifying these regulatory genes.
Acknowledgments
We thank Mónica Elías for useful experimental work during the initial stage of this study. We thank Elena Hidalgo (University Pompeu Fabra, Barcelona, Spain), Isabel Álvarez (Paterson Institute for Cancer Research, Manchester, United Kingdom), and Antony Carr (University of Sussex, Sussex, United Kingdom) for providing reagents and fission yeast strains. We also thank José Alcalde, Carlos Chillón, and Ana Elisa Rozalén for excellent technical assistance.
This study was supported in part by grants BMC2002-03933, BFU2005-07627, and BFU2007-62987 from the DGICYT (to C.D.H.), BFU2008-01808 (to S.M.), and an institutional grant from the Fundación Ramón Areces to the Centro de Biología Molecular Severo Ochoa. J.J.B. is a researcher of the Ramón y Cajal Program of the Ministry of Science and Innovation of Spain.
Footnotes
Published ahead of print on 30 October 2009.
REFERENCES
- 1.Bahler, J., J. Q. Wu, M. S. Longtine, N. G. Shah, A. McKenzie III, A. B. Steever, A. Wach, P. Philippsen, and J. R. Pringle. 1998. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14:943-951. [DOI] [PubMed] [Google Scholar]
- 2.Berlanga, J. J., S. Herrero, and C. de Haro. 1998. Characterization of the hemin-sensitive eukaryotic initiation factor 2α kinase from mouse nonerythroid cells. J. Biol. Chem. 273:32340-32346. [DOI] [PubMed] [Google Scholar]
- 3.Berlanga, J. J., J. Santoyo, and C. De Haro. 1999. Characterization of a mammalian homolog of the GCN2 eukaryotic initiation factor 2α kinase. Eur. J. Biochem. 265:754-762. [DOI] [PubMed] [Google Scholar]
- 4.Berlanga, J. J., I. Ventoso, H. P. Harding, J. Deng, D. Ron, N. Sonenberg, L. Carrasco, and C. de Haro. 2006. Antiviral effect of the mammalian translation initiation factor 2α kinase GCN2 against RNA viruses. EMBO J. 25:1730-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, D., W. M. Toone, J. Mata, R. Lyne, G. Burns, K. Kivinen, A. Brazma, N. Jones, and J. Bahler. 2003. Global transcriptional responses of fission yeast to environmental stress. Mol. Biol. Cell 14:214-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, D., C. R. Wilkinson, S. Watt, C. J. Penkett, W. M. Toone, N. Jones, and J. Bahler. 2008. Multiple pathways differentially regulate global oxidative stress responses in fission yeast. Mol. Biol. Cell 19:308-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Haro, C., R. Mendez, and J. Santoyo. 1996. The eIF-2α kinases and the control of protein synthesis. FASEB J. 10:1378-1387. [DOI] [PubMed] [Google Scholar]
- 8.Degols, G., and P. Russell. 1997. Discrete roles of the Spc1 kinase and the Atf1 transcription factor in the UV response of Schizosaccharomyces pombe. Mol. Cell. Biol. 17:3356-3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Degols, G., K. Shiozaki, and P. Russell. 1996. Activation and regulation of the Spc1 stress-activated protein kinase in Schizosaccharomyces pombe. Mol. Cell. Biol. 16:2870-2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng, J., H. P. Harding, B. Raught, A. C. Gingras, J. J. Berlanga, D. Scheuner, R. J. Kaufman, D. Ron, and N. Sonenberg. 2002. Activation of GCN2 in UV-irradiated cells inhibits translation. Curr. Biol. 12:1279-1286. [DOI] [PubMed] [Google Scholar]
- 11.Dever, T. E. 1997. Using GCN4 as a reporter of eIF2 alpha phosphorylation and translational regulation in yeast. Methods 11:403-417. [DOI] [PubMed] [Google Scholar]
- 12.Dever, T. E. 2002. Gene-specific regulation by general translation factors. Cell 108:545-556. [DOI] [PubMed] [Google Scholar]
- 13.Dunand-Sauthier, I., C. A. Walker, J. Narasimhan, A. K. Pearce, R. C. Wek, and T. C. Humphrey. 2005. Stress-activated protein kinase pathway functions to support protein synthesis and translational adaptation in response to environmental stress in fission yeast. Eukaryot. Cell 4:1785-1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gietz, R. D., and R. A. Woods. 2001. Genetic transformation of yeast. Biotechniques 30:816-828. [DOI] [PubMed] [Google Scholar]
- 15.Harding, H. P., I. Novoa, Y. Zhang, H. Zeng, R. Wek, M. Schapira, and D. Ron. 2000. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell 6:1099-1108. [DOI] [PubMed] [Google Scholar]
- 16.Harding, H. P., Y. Zhang, and D. Ron. 1999. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397:271-274. [DOI] [PubMed] [Google Scholar]
- 17.Herrero, S., J. J. Berlanga, I. Alonso, and C. de Haro. 2006. Heme responsiveness in vitro is a common feature shared by the eukaryotic factor 2α kinase family. An. R. Acad. Nac. Farm. 72:611-627. [Google Scholar]
- 18.Hinnebusch, A. G. 1997. Translational regulation of yeast GCN4. A window on factors that control initiator-tRNA binding to the ribosome. J. Biol. Chem. 272:21661-21664. [DOI] [PubMed] [Google Scholar]
- 19.Hinnebusch, A. G. 2000. Mechanism and regulation of initiator methionyl-tRNA binding to ribosome, p. 185-243. In N. Sonenberg, J. W. B. Hershey, and M. B. Mathews (ed.), Translational control of gene expression. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 20.Hinnebusch, A. G. 2005. Translational regulation of GCN4 and the general amino acid control of yeast. Annu. Rev. Microbiol. 59:407-450. [DOI] [PubMed] [Google Scholar]
- 21.Hoffmann, B., O. Valerius, M. Andermann, and G. H. Braus. 2001. Transcriptional autoregulation and inhibition of mRNA translation of amino acid regulator gene cpcA of filamentous fungus Aspergillus nidulans. Mol. Biol. Cell 12:2846-2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ikner, A., and K. Shiozaki. 2005. Yeast signaling pathways in the oxidative stress response. Mutat. Res. 569:13-27. [DOI] [PubMed] [Google Scholar]
- 23.Kaufman, R. J. 2000. The double-stranded RNA-activated protein kinase PKR, p. 503-527. In N. Sonenberg, J. W. B. Hershey, and M. B. Mathews (ed.), Translational control of gene expression. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 24.Kinoshita, N., H. Yamano, H. Niwa, T. Yoshida, and M. Yanagida. 1993. Negative regulation of mitosis by the fission yeast protein phosphatase ppa2. Genes Dev. 7:1059-1071. [DOI] [PubMed] [Google Scholar]
- 25.Krohn, M., H. C. Skjolberg, H. Soltani, B. Grallert, and E. Boye. 2008. The G1-S checkpoint in fission yeast is not a general DNA damage checkpoint. J. Cell Sci. 121:4047-4054. [DOI] [PubMed] [Google Scholar]
- 26.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 27.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar]
- 28.Lu, L., A. P. Han, and J. J. Chen. 2001. Translation initiation control by heme-regulated eukaryotic initiation factor 2α kinase in erythroid cells under cytoplasmic stresses. Mol. Cell. Biol. 21:7971-7980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mascarenhas, C., L. C. Edwards-Ingram, L. Zeef, D. Shenton, M. P. Ashe, and C. M. Grant. 2008. Gcn4 is required for the response to peroxide stress in the yeast Saccharomyces cerevisiae. Mol. Biol. Cell 19:2995-3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mendez, R., and C. de Haro. 1994. Casein kinase II is implicated in the regulation of heme-controlled translational inhibitor of reticulocyte lysates. J. Biol. Chem. 269:6170-6176. [PubMed] [Google Scholar]
- 31.Mendez, R., A. Moreno, and C. de Haro. 1992. Regulation of heme-controlled eukaryotic polypeptide chain initiation factor 2α-subunit kinase of reticulocyte lysates. J. Biol. Chem. 267:11500-11507. [PubMed] [Google Scholar]
- 32.Millar, J. B., V. Buck, and M. G. Wilkinson. 1995. Pyp1 and Pyp2 PTPases dephosphorylate an osmosensing MAP kinase controlling cell size at division in fission yeast. Genes Dev. 9:2117-2130. [DOI] [PubMed] [Google Scholar]
- 33.Moreno, S., A. Klar, and P. Nurse. 1991. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194:795-823. [DOI] [PubMed] [Google Scholar]
- 34.Nguyen, A. N., and K. Shiozaki. 1999. Heat-shock-induced activation of stress MAP kinase is regulated by threonine- and tyrosine-specific phosphatases. Genes Dev. 13:1653-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Novoa, I., H. Zeng, H. P. Harding, and D. Ron. 2001. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2α. J. Cell Biol. 153:1011-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paluh, J. L., M. J. Orbach, T. L. Legerton, and C. Yanofsky. 1988. The cross-pathway control gene of Neurospora crassa, cpc-1, encodes a protein similar to GCN4 of yeast and the DNA-binding domain of the oncogene v-Jun-encoded protein. Proc. Natl. Acad. Sci. U.S.A. 85:3728-3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perez-Hidalgo, L., S. Moreno, and P. A. San-Segundo. 2008. The fission yeast meiotic checkpoint kinase Mek1 regulates nuclear localization of Cdc25 by phosphorylation. Cell Cycle 7:3720-3730. [DOI] [PubMed] [Google Scholar]
- 38.Preiss, T., J. Baron-Benhamou, W. Ansorge, and M. W. Hentze. 2003. Homodirectional changes in transcriptome composition and mRNA translation induced by rapamycin and heat shock. Nat. Struct. Biol. 10:1039-1047. [DOI] [PubMed] [Google Scholar]
- 39.Proud, C. G. 2005. eIF2 and the control of cell physiology. Semin. Cell Dev. Biol. 16:3-12. [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez-Gabriel, M. A., and P. Russell. 2005. Distinct signaling pathways respond to arsenite and reactive oxygen species in Schizosaccharomyces pombe. Eukaryot. Cell 4:1396-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanso, M., M. Gogol, J. Ayte, C. Seidel, and E. Hidalgo. 2008. Transcription factors Pcr1 and Atf1 have distinct roles in stress- and Sty1-dependent gene regulation. Eukaryot. Cell 7:826-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Santoyo, J., J. Alcalde, R. Mendez, D. Pulido, and C. de Haro. 1997. Cloning and characterization of a cDNA encoding a protein synthesis initiation factor-2α (eIF-2α) kinase from Drosophila melanogaster: homology to yeast GCN2 protein kinase. J. Biol. Chem. 272:12544-12550. [DOI] [PubMed] [Google Scholar]
- 43.Shenton, D., J. B. Smirnova, J. N. Selley, K. Carroll, S. J. Hubbard, G. D. Pavitt, M. P. Ashe, and C. M. Grant. 2006. Global translational responses to oxidative stress impact upon multiple levels of protein synthesis. J. Biol. Chem. 281:29011-29021. [DOI] [PubMed] [Google Scholar]
- 44.Shi, Y., K. M. Vattem, R. Sood, J. An, J. Liang, L. Stramm, and R. C. Wek. 1998. Identification and characterization of pancreatic eukaryotic initiation factor 2 alpha-subunit kinase, PEK, involved in translational control. Mol. Cell. Biol. 18:7499-7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shiozaki, K., and P. Russell. 1995. Cell-cycle control linked to extracellular environment by MAP kinase pathway in fission yeast. Nature 378:739-743. [DOI] [PubMed] [Google Scholar]
- 46.Shiozaki, K., and P. Russell. 1996. Conjugation, meiosis, and the osmotic stress response are regulated by Spc1 kinase through Atf1 transcription factor in fission yeast. Genes Dev. 10:2276-2288. [DOI] [PubMed] [Google Scholar]
- 47.Smirnova, J. B., J. N. Selley, F. Sanchez-Cabo, K. Carroll, A. A. Eddy, J. E. McCarthy, S. J. Hubbard, G. D. Pavitt, C. M. Grant, and M. P. Ashe. 2005. Global gene expression profiling reveals widespread yet distinctive translational responses to different eukaryotic translation initiation factor 2B-targeting stress pathways. Mol. Cell. Biol. 25:9340-9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takeda, T., T. Toda, K. Kominami, A. Kohnosu, M. Yanagida, and N. Jones. 1995. Schizosaccharomyces pombe atf1+ encodes a transcription factor required for sexual development and entry into stationary phase. EMBO J. 14:6193-6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tripathi, G., C. Wiltshire, S. Macaskill, H. Tournu, S. Budge, and A. J. Brown. 2002. Gcn4 co-ordinates morphogenetic and metabolic responses to amino acid starvation in Candida albicans. EMBO J. 21:5448-5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vivancos, A. P., M. Jara, A. Zuin, M. Sanso, and E. Hidalgo. 2006. Oxidative stress in Schizosaccharomyces pombe: different H2O2 levels, different response pathways. Mol. Genet. Genomics 276:495-502. [DOI] [PubMed] [Google Scholar]
- 51.Wilkinson, M. G., M. Samuels, T. Takeda, W. M. Toone, J. C. Shieh, T. Toda, J. B. Millar, and N. Jones. 1996. The Atf1 transcription factor is a target for the Sty1 stress-activated MAP kinase pathway in fission yeast. Genes Dev. 10:2289-2301. [DOI] [PubMed] [Google Scholar]
- 52.Zhan, K., J. Narasimhan, and R. C. Wek. 2004. Differential activation of eIF2 kinases in response to cellular stresses in Schizosaccharomyces pombe. Genetics 168:1867-1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhan, K., K. M. Vattem, B. N. Bauer, T. E. Dever, J. J. Chen, and R. C. Wek. 2002. Phosphorylation of eukaryotic initiation factor 2 by heme-regulated inhibitor kinase-related protein kinases in Schizosaccharomyces pombe is important for resistance to environmental stresses. Mol. Cell. Biol. 22:7134-7146. [DOI] [PMC free article] [PubMed] [Google Scholar]