Abstract
Clostridium sordellii is a spore-forming, obligately anaerobic, Gram-positive bacterium that can cause toxic shock syndrome after gynecological procedures. Although the incidence of C. sordellii infection is low, it is fatal in most cases. Since spore germination is believed to be the first step in the establishment of Bacilli and Clostridia infections, we analyzed the requirements for C. sordellii spore germination in vitro. Our data showed that C. sordellii spores require three structurally different amino acids and bicarbonate for maximum germination. Unlike the case for Bacilli species, d-alanine had no effect on C. sordellii spore germination. C. sordellii spores germinated only in a narrow pH range between 5.7 and 6.5. In contrast, C. sordellii spore germination was significantly less sensitive to temperature changes than that of the Bacilli. The analysis of the kinetics of C. sordellii spore germination showed strong allosteric behavior in the binding of l-phenylalanine and l-alanine but not in that of bicarbonate or l-arginine. By comparing germinant apparent binding affinities to their known in vivo concentrations, we postulated a mechanism for differential C. sordellii spore activation in the female reproductive tract.
Clostridium sordellii is an anaerobic, Gram-positive, spore-forming bacterium that is commonly found in soil and in the intestines of animals (4). Many C. sordellii strains are nonpathogenic; however, virulent strains cause lethal infections in several animal species, such as hemorrhagic enteritis in foals, sheep, and cattle (5, 10, 16, 28), omphalitis in foals (43), and wound infection in humans (4, 35).
C. sordellii also can cause life-threatening necrotizing infections after gynecological procedures (4). In addition, fatal cases of C. sordellii endometritis following medical abortion with a mifepristone-misoprostol combination have been reported recently (13, 19, 56). The increased use of mifepristone-misoprostol for medical abortion may result in larger numbers of C. sordellii infections (38, 40).
Although C. sordellii rarely has been identified in the genital tract, a correlation between gynecological procedures and C. sordellii-mediated toxic shock syndrome is apparent (19). Pregnancy, childbirth, or abortion may predispose some women to acquire C. sordellii in the vaginal tract (19). Under these conditions, C. sordellii infections result in an almost 100% mortality rate.
Since there is no national system for tracking and reporting complications associated with gynecological procedures, the identification of the true rates of reproductive tract infections in women is not readily available (8). Therefore, the number of known C. sordellii-associated infections, although low, may be underreported (19, 29). Furthermore, unsafe abortion practices in developing countries cause large mortality rates due to complicating infections (24, 34). In many cases, however, the causative agent of the abortion-associated sepsis have not been characterized (24). Thus, the worldwide morbidity and mortality associated with C. sordellii infections is not currently known.
C. sordellii produces several virulence factors. The two major toxins are the lethal toxin (TcsL) and the hemorrhagic toxin (37, 46). The lethal toxin produced by C. sordellii is causally involved in enteritis of domestic animals and in systemic toxicity following infections of humans (46). Furthermore, TcsL is associated with rapid mortality in C. sordellii endometritis rodent models (26). Interestingly, TcsL cytopathic effects are increased at low pH, a characteristic found in the vaginal tract (48). The hemorrhagic toxin is not well characterized, but it has been reported to cause dermal and intestinal necrosis in guinea pigs (6, 52).
C. sordellii, like other Bacilli and Clostridia species, has the ability to form metabolically dormant spores that are extremely resistant to environmental stresses, such as heat, radiation, and toxic chemicals (42, 55). Upon encountering a suitable environment, spores germinate into vegetative cells, the form that is responsible for toxin production and disease onset (39, 54).
In most cases, the germination process initially is triggered by the detection of low-molecular-weight germinants by a sensitive biosensor (39, 54). This sensor consists of a proteinaceous germination (Ger) receptor encoded, in general, by a tricistronic operon. Spore germination requirements have been studied most extensively for Bacilli and can be initiated by a variety of factors, including amino acids, sugars, and nucleosides (20, 30).
Spore germination in the Clostridia generally requires combinations of multiple germinants. The germination of spores of proteolytic Clostridium botulinum types A and B was triggered by a defined three-component mixture comprised of l-alanine (or l-cysteine), l-lactate (or sodium thioglycolate), and sodium bicarbonate (3). In contrast, the optimum germination of spores of nonproteolytic C. botulinum types B, E, and F required binary combinations of l-alanine-l-lactate, l-cysteine-l-lactate, and l-serine-l-lactate (45).
Clostridium difficile is a human pathogen that can cause fulminant colitis (11). Interestingly, C. difficile does not encode any known Ger receptors (53). However, it is likely that germination receptors exist, because C. difficile spores must germinate in order to complete their life cycle. While C. difficile germination receptors remain elusive, the spores of C. difficile germinate in rich medium supplemented with bile salts (62). More recently, taurocholate (a bile salt) and glycine (an amino acid) were shown to act as cogerminants for C. difficile spore germination (57, 61).
Clostridium bifermentans is a close relative of C. sordellii (14). The minimum requirement for C. bifermentans spore germination was the presence of l-alanine, l-phenylalanine, and l-lactate (59). In addition, an unknown factor present in yeast extract was suggested to enhance germination (59). However, the Ger receptors involved in C. bifermentans spore germination are not known.
Even though many Bacilli and Clostridia species use similar metabolites as germinants, the mechanisms of germinant recognition remain to be elucidated. Unfortunately, the multimeric interactions of Ger receptor complexes and the hydrophobic nature of the Ger receptor subunits have hindered our understanding of the mechanism of germinant recognition.
To understand the molecular determinants of germinant recognition, we recently applied kinetic methods to study bacterial spore germination (1, 2, 18). Spore germination can be analyzed quantitatively by fitting optical density (OD) decreases to the Michaelis-Menten equation (2). The kinetic parameters obtained allow the determination of the apparent binding affinity (Km) of spores for the different cogerminants and the maximum rate of spore germination (Vmax). In these instances, Km refers to the concentration of substrate required to reach half of the maximal germination rate. These parameters can, in turn, be used to determine the mechanism of germination and potential interactions between germination receptors. Furthermore, by comparing apparent Km values to germinant concentrations in vivo, models for spore-germinant complex distribution can be proposed, and rate-limiting steps for the germination process can be derived. Thus, kinetic analysis can yield information on spore activation even if the identities of the germination receptors are not known.
Using this procedure, we were able to determine the mechanism for Bacillus anthracis germination with inosine and l-alanine. In turn, this information was used to design nucleoside analogs that inhibit B. anthracis spore germination in vitro and protect macrophages from anthrax cytotoxicity (2).
Since C. sordellii germination receptors have not been identified, we used chemical probes and kinetic methods to investigate the conditions necessary for spore germination. We found that C. sordellii spores germinate better at slightly acidic pH. Furthermore, germination rates varied slightly from 25 to 40°C. We also found that C. sordellii spores have an absolute requirement for a small amino acid, a basic amino acid, an aromatic amino acid, and bicarbonate (NaHCO3) for efficient germination. Kinetic analysis showed allosteric interaction for the putative l-phenylalanine and l-alanine germination receptors. In contrast, l-arginine or bicarbonate recognition followed typical Michaelis-Menten kinetics. The implication of germinant recognition and host environment is discussed.
MATERIALS AND METHODS
Bacterial strains and spore preparation.
Clostridium sordellii ATCC 9714 was obtained from the American Type Culture Collection (ATCC). C. sordellii cells were plated in brain heart infusion agar supplemented with sodium thioglycolate (0.5 g/liter) to yield single-cell clones (49). Single C. sordellii colonies were grown in liquid media and replated to obtain bacterial lawns. Plates were incubated for 7 days at 30°C in an anaerobic environment (10% CO2, 10% H2, 80% N2). The resulting bacterial lawns were collected by being flooded with ice-cold deionized water. Spores were pelleted by centrifugation and resuspended in fresh deionized water. After two washing steps, spores were separated from vegetative and partially sporulated forms by centrifugation through a 20 to 50% HistoDenz gradient. The spore pellet was washed five times with water, resuspended in sodium thioglycolate (0.5 g/liter), and stored at 4°C.
Preparation of germinant solution.
The AGFK mixture (100 mM l-asparagine, 10 mM d-glucose, 10 mM d-fructose, 50 mM KCl) was prepared as previously described (60). The defined medium employed was a modification of that described previously (33, 50). To prepare the defined medium, a buffer solution was made with 6.6 mM KH2PO4, 15 mM NaCl, 59.5 mM NaHCO3, and 35.2 mM Na2HPO4. Three solutions were prepared using this buffer as diluent. The first solution contained all salts at 1,000× concentrations (final concentrations were 10 mg/liter MgSO4·7H2O, 5 mg/liter FeSO4·7H2O, and 5 mg/liter MnCl2·4H2O). The second solution contained all amino acids, except cysteine, at 10× (concentrations were 100 to 500 mg/liter depending on solubility). Cysteine was prepared separately as a 10× solution in 0.2 N HCl. The third solution contained vitamins at 10× concentrations (final concentrations were 0.05 mg/liter d-biotin, 0.1 mg/liter p-amino benzoic acid, 0.05 mg/liter thiamine hydrochloride, 0.05 mg/liter pyridoxine, and 1.0 mg/liter nicotinic acid). The different solutions were added to buffer solution at the final concentration indicated. Stock (10×) solutions of each l-amino acid, sodium bicarbonate (NaHCO3), inosine, taurocholate, and l-lactate were prepared individually in deionized sterile water. Combinations of these solutions were tested to determine the germinants necessary for C. sordellii spore germination.
Determination of germinants for C. sordellii spores.
Changes in light diffraction during spore germination were monitored at an optical density of 580 nm (OD580) on a Biomate 5 spectrophotometer (ThermoElectron Corporation, Waltham, MA). C. sordellii spores were heat activated at 70°C for 30 min (17). After heat activation, spores were cooled to room temperature and resuspended in germination buffer (100 mM sodium phosphate buffer, pH 6.0) to an OD580 of 1, as done previously (2). The spore suspension was monitored for autogermination for 30 min. Germination experiments were carried out with spores that did not autogerminate. Experiments were performed in triplicate with at least two different spore preparations. Putative germinants were added individually or in combinations to a final concentration of 5 (l-Phe, l-Trp, and l-Tyr), 10 (l-Arg, l-His, l-Lys, l-Ser, d-glucose, and d-fructose), 25 (l-Ala and l-Gly), 50 (l-lactate, KCl, and NaHCO3), or 100 mM (d-Ala, l-Asp, and l-Glu). After the addition of germinants, the OD580 of the spore suspension was measured at 2-min intervals for 4 h. Spore germination rates were evaluated based on the decrease in OD580 at room temperature. Relative OD580 values were derived by dividing each OD580 reading obtained at different times by the OD580 obtained at the beginning of germination. All measurements showed standard deviations of less than 10%. Germination rates were calculated from the initial linear region of the germination curves (2). Germination rates were set to 100% for C. sordellii spores germinated in defined medium. The percentage of germination for other germinant combinations was calculated as a fraction of the rate of germination in defined medium.
Effect of d-amino acids on C. sordellii spore germination.
To test for d-alanine as an antagonist of spore germination, C. sordellii spores were treated with 25 mM l-alanine, 5 mM l-phenylalanine, 10 mM l-arginine, and 50 mM NaHCO3 (AFR*) supplemented with 0 or 100 mM d-alanine. The inhibition potential of d-phenylalanine and d-arginine on C. sordellii spore germination was tested similarly. To test for d-alanine as an agonist of spore germination, C. sordellii spores were treated with 5 mM l-phenylalanine, 10 mM l-arginine, and 50 mM NaHCO3 (FR*) supplemented with 100 mM d-alanine. Similarly, the agonist effect of d-phenylalanine and d-arginine was tested by substituting l-phenylalanine and l-arginine, respectively. Germination rates were determined as described above. Germination rates were set to 100% for C. sordellii spores germinated in AFR*. The percentage of germination for other conditions was calculated as a fraction of the rate of germination in AFR*.
Temperature dependence of C. sordellii spore germination.
C. sordellii spores were germinated with AFR*. The germination rate was determined as described above, except that the germination temperature was varied between 25 and 40°C. Germination rates were set to 100% for C. sordellii spores germinated at 37°C. The percentage of germination for other conditions was calculated as a fraction of the rate of germination at 37°C.
pH dependence of C. sordellii spore germination.
Individual C. sordellii spore aliquots were resuspended in 50 mM NaHCO3, and the pH was adjusted between 4.0 and 9.0. Germination was initiated by the addition of 25 mM l-alanine, 5 mM l-phenylalanine, and 10 mM l-arginine. Germination rates were determined as described above. Germination rates were set to 100% for C. sordellii spores germinated at pH 6. The percentage of germination for other conditions was calculated as a fraction of the rate of germination at pH 6.
Staining of spores and vegetative cells.
To confirm spore germination, treated C. sordellii spores were smeared across a glass slide, air dried, and heat fixed over a flame. Cells were stained using the Wirtz-Conklin staining technique as described previously (25). Briefly, heat-fixed spore/bacterial smears were immersed in boiling malachite green stain (5 g/100 ml water) for 1 min. Following destaining in distilled water, smears were counterstained with safranin-O (0.5 g/100 ml water) for 1 min. Smears subsequently were destained in distilled water and mounted. Cells were visualized using a Zeiss Axiophot microscope. The percentage of germination was calculated by comparing the number of red germinated C. sordellii cells to that of green ungerminated spores in three random fields. Results were confirmed by phase-contrast microscopy.
Kinetics of C. sordellii spore germination.
To determine l-alanine kinetic parameters for C. sordellii spore germination, spores were resuspended individually in solutions containing various concentrations of l-alanine and were supplemented with l-arginine (10 mM), l-phenylalanine (5 mM), and NaHCO3 (50 mM). Germination rates (v) were calculated as the slope of the initial linear portion of relative OD580 values over time. The resulting data were plotted as double reciprocal plots of 1/v versus 1/[l-alanine]. All plots were fitted using linear regression analysis to determine apparent Km and Vmax values. This setup allows the determination of the effect of saturating l-arginine, l-phenylalanine, and NaHCO3 on the apparent binding of l-alanine for C. sordellii spores (Km) and on the maximum germination rate (Vmax) of C. sordellii spores. Similarly, the apparent affinities (Km) and maximum germination rates (Vmax) of C. sordellii spores for l-arginine, l-phenylalanine, and NaHCO3 were tested individually in the presence of constant concentrations of all other germinants.
RESULTS
Germination in complex media.
Like other Clostridium species, spores of C. sordellii germinated efficiently in LB medium. In contrast, C. sordellii spores were unable to germinate in AGFK (l-asparagine, d-glucose, d-fructose, and KCl) solutions, a strong germinant combination for B. subtilis (15). Similarly, C. sordellii spores did not germinate in the presence of inosine or inosine-l-alanine. These compounds are strong germinants for B. anthracis and B. cereus (20, 30). C. sordellii spores also failed to germinate with taurocholate or taurocholate-glycine, compounds that induce the germination of C. difficile spores (57). Contrary to findings for C. bifermentans (59) and C. botulinum (3, 45), l-lactate had no effect on the germination efficiency of C. sordellii spores.
Germination in defined medium.
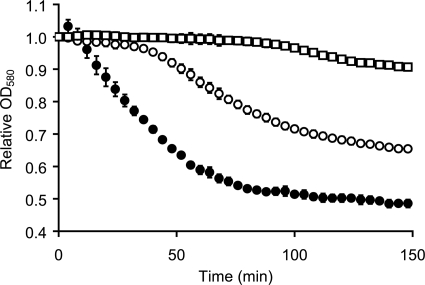
To determine whether combinations of amino acids can induce the germination of C. sordellii, we used a modification of defined medium used to germinate C. difficile and C. perfringens (33, 50). This medium contains all 20 l-amino acids plus salts (MgSO4·7H2O, FeSO4·7H2O, and MnCl2·4H2O), vitamins (d-biotin, p-amino benzoic acid, pyridoxine, nicotinic acid, and thiamine hydrochloride), and d-glucose. When the spores were suspended in this medium, the optical density of the suspension rapidly decreased, indicating that the spores were germinating (Fig. 1). Untreated spores showed no optical density change (data not shown). Phase-contrast microscopy and the staining of bacterial suspensions before and after treatment confirmed that all spores had germinated. C. sordellii spores germinated at the same rate in medium prepared without salts, vitamins, or d-glucose, suggesting that one or more amino acids are required for germination (data not shown).
FIG. 1.
C. sordellii spores germinate with a mixture of amino acids and NaHCO3. C. sordellii ATCC 9714 spores were germinated in defined medium (○), defined medium without NaHCO3 (□), or in a solution (AFR*) containing 25 mM l-alanine, 5 mM l-phenylalanine, 10 mM l-arginine, and 50 mM NaHCO3 (•). Germination was followed by a decrease of the OD580.
Bicarbonate effect on C. sordellii spore germination.
In contrast to the unimportance of salts, vitamins, and sugars, defined medium lacking NaHCO3 did not induce significant C. sordellii spore germination (Fig. 1). The small decrease in optical density shown in Fig. 1 was determined to be due to the settling of the spore suspension. Indeed, gentle agitation resulted in an optical density increase to starting levels. Furthermore, no germination was detected by the staining of the bacterial suspension even 3 h after treatment.
C. sordellii spores also were unable to germinate in NaHCO3 alone (data not shown). Furthermore, the substitution of bicarbonate for sulfate, chloride, or phosphate anions did not induce spore germination (data not shown). In contrast, C. sordellii spores germinated normally when NaHCO3 was replaced with KHCO3.
Amino acid requirement for C. sordellii spore germination.
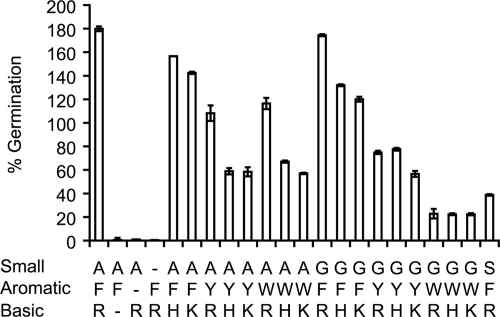
To identify which amino acids induce the germination of C. sordellii spores, solutions were prepared by supplementing NaHCO3 solutions with l-amino acids with similar side chains. Thus, C. sordellii spores were germinated in the presence of small (Ala, Ser, Thr, Gly, and Cys), hydrophobic (Leu, Ile, Met, and Val), aromatic (Phe, Tyr, and Trp), basic (Arg, Lys, and His), acidic (Asp and Glu), amide (Asn and Gln), and constrained (Pro) l-amino acid mixtures. None of these solutions alone was sufficient to trigger spore germination. C. sordellii spores were resuspended in solutions containing pairs and trios of amino acid groups. C. sordellii spore germination occurred only in solutions containing mixtures of small amino acids, aromatic amino acids, and basic amino acids.
To narrow further the best l-amino acid germinants, all possible combinations of small amino acids, basic amino acids, and aromatic amino acids were tested individually for their effect on C. sordellii spore germination.
Solutions that contained l-alanine and glycine induced stronger germination than solutions containing l-serine, l-threonine, or l-cysteine. In fact, solutions containing l-threonine or l-cysteine failed to trigger germination (data not shown). l-serine was a weak cogerminant only in the presence of l-phenylalanine and l-arginine (Fig. 2). Even with this optimal combination, spores treated with l-serine showed an 80% decrease in germination rate compared to that of l-alanine. Other amino acid combinations containing l-serine failed to activate spore germination (data not shown).
FIG. 2.
Amino acid combinations that trigger C. sordellii spore germination. C. sordellii ATCC 9714 spores were germinated in 50 mM NaHCO3 solutions containing combinations of small (A, G, S), aromatic (F, Y, W), and basic (R, H, K) amino acids. The percent germination was calculated in reference to spores germinated in defined medium. Amino acid combinations that did not activate spore germination are omitted for clarity. Amino acids are designated by their one-letter codes.
From the aromatic amino acid series, l-phenylalanine was the most active cogerminant. However, in the presence of l-alanine and l-arginine, both l-tryptophan and l-tyrosine were able to trigger germination rates that were only 40% slower than those of spores treated with l-alanine, l-arginine, and l-phenylalanine. Similarly, spores treated with l-tyrosine, glycine, and l-arginine showed germination rates that were 40% slower than those of spores treated with l-phenylalanine, glycine, and l-arginine. In contrast, substituting l-tryptophan for l-tyrosine in the presence of glycine and l-arginine resulted in a 90% reduction in the germination rate.
l-Arginine was shown to be the strongest cogerminant of the basic amino acids. Nevertheless, both l-lysine and l-histidine were able to substitute for l-arginine as the cogerminant. Spores treated with l-alanine, l-phenylalanine, and l-lysine (or l-histidine) showed only a 20% decrease in germination rate compared to that of spores treated with l-alanine, l-phenylalanine, and l-arginine.
Even though mixtures of l-alanine-glycine, l-phenylalanine, and l-arginine result in the highest germination rates, each one of these amino acids can be replaced partially with amino acids that have similar physical and chemical properties. As expected, binary combinations of l-alanine-l-phenylalanine, l-alanine-l-arginine, and l-phenylalanine-l-arginine failed to induce spore germination (Fig. 2).
Interestingly, C. sordellii spores treated with l-alanine, l-phenylalanine, and l-arginine show a faster germination rate than spores germinated with defined medium (Fig. 1). This is to be expected, since the defined medium contains amino acids that are weak germinants and will compete with l-alanine, l-phenylalanine, and l-arginine for binding. The binding of these alternative substrates will cause a fraction of the spore population to germinate at a lower rate.
Effect of d-amino acids on C. sordellii spore germination.
Bacterial spores contain two alanine isomers: l-alanine and d-alanine. l-Alanine has been shown to promote the germination of multiple bacterial spores (3, 7, 20, 30, 45), while d-alanine has been described to block germination (63). Since l-alanine is required for C. sordellii spore germination, we tested whether d-alanine could affect germination kinetics. As before, a mixture of l-alanine, l-phenylalanine, l-arginine, and NaHCO3 (AFR*) resulted in rapid initial rates of germination (Fig. 3). Supplementation with d-alanine did not reduce the rate or extent of germination under the conditions tested. Furthermore, d-alanine could not replace l-alanine as a cogerminant with l-phenylalanine, l-arginine, and NaHCO3 (FR*). Similar results were observed with d-phenylalanine and d-arginine.
FIG. 3.
Effect of d-alanine and acidic amino acids on C. sordellii spore germination. C. sordellii ATCC 9714 spores were germinated with 25 mM l-alanine; 5 mM l-phenylalanine; 10 mM l-arginine; 50 mM NaHCO3 (AFR*); AFR* supplemented with 100 mM d-alanine (d-Ala); or 5 mM l-phenylalanine, 10 mM l-arginine, and 50 mM NaHCO3 (FR*) supplemented with 100 mM d-Ala. C. sordellii spores also were germinated in the presence of l-aspartic acid alone (Asp) or Asp supplemented with 25 mM l-alanine, 5 mM l-phenylalanine, and 10 mM l-arginine (AFR). Results for l-glutamic acid are identical to those for l-aspartic acid and are not shown for clarity. The percent germination was calculated in reference to spores germinated with AFR*.
Effect of l-aspartic and l-glutamic acids on C. sordellii spore germination.
When spores were treated with NaHCO3 supplemented with acidic amino acids, a 20% decrease in optical density was observed (data not shown). Interestingly, this process was independent of other cogerminants, including bicarbonate (Fig. 3). However, spores treated with l-aspartic acid or l-glutamic acid failed to show germination by staining. These two techniques test different stages of spore germination. While an optical density decrease is related to the swelling of the spore core, staining with safranin occurs only after spore structures are degraded. Thus, it is possible that acidic amino acids are able to trigger germination, but the process then is aborted before completion. These results were confirmed by phase-contrast microscopy. While normally germinating spores change from phase bright to phase dark, aspartic acid-treated spores showed only a dimming of spore brightness. This is consistent with an aborted germination process (31). Neither l-aspartic nor l-glutamic acid is able to substitute for bicarbonate as a cogerminant with l-alanine, l-phenylalanine, and l-arginine (AFR) mixtures.
Optimal temperature for C. sordellii spore germination.
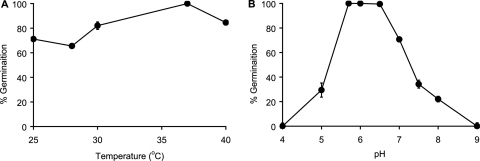
To define the optimal conditions for C. sordellii spore germination, germination assays were carried out at different temperatures. Remarkably, C. sordellii spores germinated well at all temperatures tested. Indeed, even though the maximum germination rate was obtained at 37°C, germination rates obtained between 25 and 40°C were within 30% of the maximum germination rate (Fig. 4A). Thus, C. sordellii spore germination is quite insensitive to physiological temperature changes.
FIG. 4.
Effect of temperature and pH on C. sordellii spore germination. C. sordellii spores were resuspended in ARF* (25 mM l-alanine, 5 mM l-phenylalanine, 10 mM l-arginine, and 50 mM NaHCO3). (A) Germination rates were determined at different temperatures. The percent germination was calculated relative to the germination rate at 37°C. (B) Germination rates were determined at different pH values. The percent germination was calculated relative to the germination rate at pH 6.
Optimal pH for C. sordellii spore germination.
To define the optimal conditions for C. sordellii spore germination, the pH of the germination buffer was varied. The rate and extent of C. sordellii spore germination was significantly diminished above pH 7.0 and below pH 5.7. The maximum rate and extent of spore germination occurred between pH 5.7 and 6.5 (Fig. 4B).
Kinetics of C. sordellii spore germination.
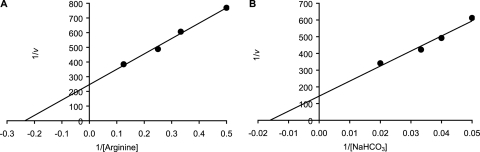
The kinetic parameters for l-alanine, l-arginine, l-phenylalanine, and NaHCO3 were determined individually with constant concentrations for the other three cogerminants. Spore germination with various l-arginine concentrations showed normal Michaelis-Menten kinetics, as demonstrated by linear double reciprocal plots (Fig. 5A). C. sordellii spores recognized l-arginine with an apparent binding constant (Km) of 4.3 mM and a maximum germination velocity (Vmax) of 0.24 OD/h (Table 1). Similarly, varying the NaHCO3 concentration allowed us to determine an apparent Km for bicarbonate of 68.9 mM and a Vmax of 0.42 OD/h (Fig. 5B).
FIG. 5.
Double reciprocal plots to determine kinetic parameters of l-arginine and NaHCO3. Germination rates were calculated from the linear segment of optical density changes over time. (A) Double reciprocal plot of C. sordellii spore germination at various l-arginine (2.0, 3.0, 4.0, and 8.0 mM) concentrations and constant l-alanine, l-phenylalanine, and NaHCO3 concentrations. (B) Double reciprocal plot of C. sordellii spore germination at various NaHCO3 (20, 25, 30, and 50 mM) concentrations and constant l-alanine, l-phenylalanine, and l-arginine concentrations.
TABLE 1.
Kinetic parameters for C. sordellii spore germination
| Germinant | Hill no. (n) | Km (mM) | Vmax (OD/h) |
|---|---|---|---|
| l-Alanine | 2.3 | 3.7 | 0.33 |
| l-Arginine | 1.0 | 4.3 | 0.24 |
| l-Phenylalanine | 2.5 | 0.008 | 0.21 |
| Sodium bicarbonate | 1.0 | 68.9 | 0.41 |
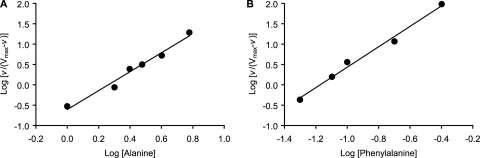
In contrast to the simple kinetics shown by l-arginine and NaHCO3, varying the concentration of l-alanine resulted in parabolic double reciprocal plots. These plots normally are seen for germinants that show allosteric behavior (1). Indeed, the Hill plot for l-alanine-mediated germination showed a slope of 2.3 (Fig. 6A). This is consistent with at least two interacting l-alanine-binding sites (Table 1). The apparent Km and Vmax for l-alanine was calculated to be 3.7 mM and 0.33 OD/h, respectively.
FIG. 6.
Hill plots for l-alanine and l-phenylalanine binding. Germination rates were calculated from the linear segment of optical density changes over time. (A) Hill plot of spore germination at various l-alanine (1.0, 2.0, 2.5, 3.0, 4.0, and 6.0 mM) concentrations shows a slope of 2.3. (B) Hill plot of spore germination at various l-phenylalanine (0.05, 0.08, 0.10, 0.20, and 0.40 mM) concentrations shows a slope of 2.5.
l-Phenylalanine-dependent spore germination also showed allosteric behavior. Indeed, Hill plots for l-phenylalanine-mediated germination yielded a slope of 2.5 (Fig. 6B). This is characteristic of a system with at least two interacting l-phenylalanine binding sites. The apparent Km for l-phenylalanine was calculated to be 0.088 mM. Thus, the binding of l-phenylalanine is approximately 50- and 800-fold stronger than those of l-arginine and NaHCO3, respectively. In contrast, the Vmax for l-phenylalanine-mediated germination was 0.21 OD/h, which is similar to those of the three other cogerminants.
DISCUSSION
It has been reported previously that spores of C. bifermentans, a close relative of C. sordellii, germinate in the presence of l-alanine, l-arginine, l-lactate, and l-phenylalanine (59). In this study, we showed that C. sordellii needs at least four different compounds to germinate: a small amino acid (l-alanine or glycine), a basic amino acid, an aromatic amino acid, and bicarbonate. Thus, C. sordellii spores seem to be quite promiscuous in the recognition of germinants.
Even though l-alanine is an essential germinant, d-alanine does not affect C. sordellii germination. This is in contrast to the inhibitory effect that d-alanine has on the germination of Bacilli spores (21, 47, 63). Similarly, neither d-phenylalanine nor d-arginine affected C. sordellii spore germination. This suggests that each amino acid receptor in C. sordellii has evolved to recognize the correct amino acid stereoisomer.
Bicarbonate is present at concentrations ranging between 35 and 90 mM in the female reproductive tract. These concentrations are much higher than those in other human tissues (58). The absolute requirement for bicarbonate suggests that C. sordellii spore germination has adapted to the environment of the female human host.
The fact that C. sordellii spores prefer slightly acidic pH for germination is intriguing. Maximal germination is observed at a pH range where both carbonic acid and bicarbonate are present in equilibrium. On the other hand, at more basic pH (where the concentration of carbonic acid is insignificant) or more acidic pH (where the concentration of bicarbonate is insignificant), no germination is detected. Thus, it seems that both the carbonic acid and bicarbonate forms are used as germinants. Since healthy females have a vaginal pH below 4.5 (22, 41), all bicarbonate forms would be protonated to the carbonic acid form, and C. sordellii spores would not be able to germinate under these conditions.
Bacteria vaginosis is the most common vaginal infection during the reproductive years. During bacterial vaginosis, the normal lactobacillus-dominated vaginal flora shifts to a population dominated by other organisms, including Neisseria gonorrhoeae, Chlamydia trachomatis, or anaerobic organisms (e.g., Clostridia species) (36). Bacterial vaginosis is characterized by vaginal pH values above 4.5 and is correlated with preterm birth in asymptomatic women and pelvic infections after induced abortions (27, 44). Thus, vaginal pH that correlates with pathogenicity results in the formation of bicarbonate-carbonic acid mixtures that are required for C. sordellii spore germination. Hence, altered vaginal pH conditions could result in a better environment for C. sordellii spores to germinate and establish infections (9).
Vaginal fluids contain high concentrations of alanine and arginine but lack phenylalanine under normal conditions (23). Thus, nonpregnant human females lack a key germinant for germination triggering. On the other hand, fetal growth and development is closely dependent on the availability of a constant supply of nutrients. This generates high concentrations of amino acids in the gestational sac. Phenylalanine and arginine are present at close to 0.1 mM, while alanine can reach 1 mM in coelomic and amniotic fluids (12, 32, 51). The amino acid pool available after abortion or delivery will contain all three amino acids and could serve as a signal for spore germination. Due to the allosteric behavior of l-phenylalanine, micromolar amounts of this amino acid will be sufficient to activate the germination program.
Even though the germination receptors that detect l-alanine, l-arginine, l-phenylalanine, and NaHCO3 in C. sordellii spores have not been characterized, titrations of the germination rate with NaHCO3 and l-arginine resulted in hyperbolas that could be analyzed using Michaelis-Menten approaches. This indicates the saturation of germinant binding to specific receptor sites. Furthermore, the allosteric behavior of l-phenylalanine and l-alanine recognition suggest that their putative receptors engage in protein-protein interactions that affect the corresponding binding sites.
The apparent binding constants (Km) that were obtained from the Michaelis-Menten analysis of germination kinetics represent the concentration of a compound required to obtain half the maximal germination rate. For a spore to germinate, however, germination receptors must be activated by their corresponding germinants. Thus, Km also represented the concentration of a germinant required for half of the spore population to be complexed. As a result, environmental concentrations below the apparent Km will result in a larger fraction of the spore population remaining in an uncomplexed, dormant form. Correspondingly, environmental concentrations above the apparent Km will result in mostly complexed, germinating spores. Consequently, the kinetic analysis of C. sordellii spore germination allows postulating a mechanism for spore activation by comparing the germinants' apparent binding constants (Km) to their available concentrations in the pregnant female reproductive tract (32).
Using estimated germinant concentrations (23), we can predict that in the normal female reproductive tract, the lack of l-phenylalanine, a suboptimal concentration of l-arginine, and low pH will cause spores to be bound to l-alanine only and will not germinate (Fig. 7A).
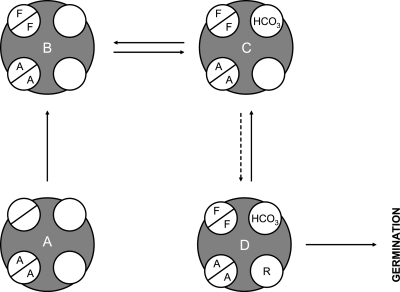
FIG. 7.
Model for C. sordellii spore activation in a postpregnancy host. C. sordellii spores are represented by gray circles. Germination receptors are represented by white circles. Allosteric receptors are represented by split circles. Solid arrows represent fast processes. Dashed arrows represent slow processes. Amino acids are represented by their single letter code. (A) Under normal conditions, the alanine-bound dormant spore would be the default state, since the human female genital track is acidic and has a low concentration of l-phenylalanine and l-arginine. Thus, only l-alanine will be allosterically bound to C. sordellii spores. The activation of germination can occur only when vaginal conditions change. For example, after a pregnancy is terminated, a new pool of amino acids containing l-phenylalanine may become available. (B) In this case, the allosteric behavior of l-alanine and l-phenylalanine will saturate C. sordellii spores. (C) The alanine-phenylalanine-spore complex will equilibrate with NaHCO3 to form the quaternary complex. (D) The activated C. sordellii spore will slowly bind l-arginine to complete the germination process.
We speculate that in postpregnancy females, the concentrations of l-alanine and l-phenylalanine increase. Due to their allosteric behavior, both l-alanine and l-phenylalanine will be saturating with respect to their apparent Km (Table 1). Hence, we expect that C. sordellii spores present in a postpregnancy reproductive tract always will be bound with l-alanine and l-phenylalanine (Fig. 7B).
In contrast, the apparent Km for bicarbonate-carbonic acid is close to their physiological concentrations. Thus, the doubly complexed spore population will be in equilibrium between bicarbonate-free (Fig. 7B) and bicarbonate-bound (Fig. 7C) forms.
Finally, even though l-arginine and l-phenylalanine have similar concentrations in the gestational sac (32), l-arginine binding does not show allostericity. Thus, the concentration of l-arginine is suboptimal with respect to its apparent Km. The binding of l-arginine will be the slowest part of the germination process (Fig. 7D).
Although we have used the available published literature to correlate germinant concentrations with mechanistic predictions, more research is necessary to establish the feasibility of our model.
In conclusion, C. sordellii spores use three structurally different amino acids as well as bicarbonate/carbonic acid as germination signals. The normal vaginal environment lacks the necessary signals and is too acidic to allow C. sordellii spore germination. We hypothesize that the human female reproductive tract after abortion and/or delivery will contain all signals needed for C. sordellii spores to germinate efficiently. In this case, l-arginine binding probably will serve as the rate-limiting step in the germination process. Integrating these different germination signals could allow C. sordellii spores to postpone germination until an appropriate environment (e.g., a postpregnancy reproductive tract) is encountered.
Acknowledgments
We thank Helen J. Wing, Jürgen Brojatsch, and Ronald Yasbin for their thoughtful discussions.
This work was supported by the RING-TRUE III 0447416 award from NSF-EPSCoR.
Footnotes
Published ahead of print on 13 November 2009.
REFERENCES
- 1.Abel-Santos, E., and T. Dodatko. 2007. Differential nucleoside recognition during Bacillus cereus 569 (ATCC 10876) spore germination. New J. Chem. 31:748-755. [Google Scholar]
- 2.Akoachere, M., R. C. Squires, A. M. Nour, L. Angelov, J. Brojatsch, and E. V. Abel-Santos. 2007. Identification of an in vivo inhibitor of Bacillus anthracis Sterne spore germination. J. Biol. Chem. 282:12112-12118. [DOI] [PubMed] [Google Scholar]
- 3.Alberto, F., V. Mason, D. R. Mason, F. Carlin, and M. W. Peck. 2003. Variability in spore germination response by strains of proteolytic Clostridium botulinum types A, B and F. Lett. Appl. Microbiol. 36:41-45. [DOI] [PubMed] [Google Scholar]
- 4.Aldape, M. J., A. E. Bryant, and D. L. Stevens. 2006. Clostridium sordellii infection: epidemiology, clinical findings, and current perspectives on diagnosis and treatment. Clin. Infect. Dis. 43:1436-1446. [DOI] [PubMed] [Google Scholar]
- 5.Al-Mashat, R. R., and D. J. Taylor. 1983. Production of diarrhoea and enteric lesions in calves by the oral inoculation of pure cultures of Clostridium sordellii. Vet. Res. 112:141-146. [DOI] [PubMed] [Google Scholar]
- 6.Amimoto, K., E. Oishi, H. Yasuhara, O. Sasaki, S. Katayama, T. Kitajima, A. Izumida, and T. Hirahara. 2001. Protective effects of Clostridium sordellii LT and HT toxoids against challenge with spores in guinea pigs. J. Vet. Med. Sci. 63:879-883. [DOI] [PubMed] [Google Scholar]
- 7.Atluri, S., K. Ragkousi, D. E. Cortezzo, and P. Setlow. 2006. Cooperativity between different nutrient receptors in germination of spores of Bacillus subtilis and reduction of this cooperativity by alterations in the GerB receptor. J. Bacteriol. 188:28-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beal, M. W. 2007. Update on medication abortion. J. Midwife. Women's Health 52:23-30. [DOI] [PubMed] [Google Scholar]
- 9.Brabin, L., S. A. Roberts, E. Fairbrother, D. Mandal, S. P. Higgins, S. Chandiok, P. Wood, G. Barnard, and H. C. Kitchener. 2005. Factors affecting vaginal pH levels among female adolescents attending genitourinary medicine clinics. Sex. Transm. Infect. 81:483-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark, S. 2003. Sudden death in periparturient sheep associated with Clostridium sordellii. Vet. Res. 153:340. [PubMed] [Google Scholar]
- 11.Cloud, J., and C. P. Kelly. 2007. Update on Clostridium difficile associated disease. Curr. Opin. Gastroenterol. 23:4-9. [DOI] [PubMed] [Google Scholar]
- 12.Cockburn, F., S. P. Robins, and J. O. Forfar. 1970. Free amino-acid concentrations in fetal fluids. Br. Med. J. iii:747-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen, A. L., J. Bhatnagar, S. Reagan, S. B. Zane, M. A. D'Angeli, M. Fischer, G. Killgore, T. S. Kwan-Gett, D. B. Blossom, W. J. Shieh, J. Guarner, J. Jernigan, J. S. Duchin, S. R. Zaki, and L. C. McDonald. 2007. Toxic shock associated with Clostridium sordellii and Clostridium perfringens after medical and spontaneous abortion. Obstet. Gynecol. 110:1027-1033. [DOI] [PubMed] [Google Scholar]
- 14.Collins, M. D., P. A. Lawson, A. Willems, J. J. Cordoba, J. Fernandez-Garayzabal, P. Garcia, J. Cai, H. Hippe, J. A. E. Farrow, P. A. Lawson, A. Willems, J. J. Cordoba, J. Fernandez-Garayzabal, P. Garcia, J. Cai, H. Hippe, and J. A. E. Farrow. 1994. The phylogeny of the genus Clostridium: proposal of five new genera and eleven new species combinations. Int. J. Syst. Bacteriol. 44:812-826. [DOI] [PubMed] [Google Scholar]
- 15.Corfe, B. M., A. Moir, D. Popham, and P. Setlow. 1994. Analysis of the expression and regulation of the gerB spore germination operon of Bacillus subtilis 168. Microbiology 140:3079-3083. [DOI] [PubMed] [Google Scholar]
- 16.De Groot, B., C. E. Dewey, D. D. Griffin, L. J. Perino, R. A. Moxley, and G. L. Hahn. 1997. Effect of booster vaccination with a multivalent clostridial bacterin-toxoid on sudden death syndrome mortality rate among feedlot cattle. J. Am. Vet. Med. Assoc. 211:749-753. [PubMed] [Google Scholar]
- 17.Desrosier, N. W., and F. Heiligman. 1956. Heat activation of bacterial spores. Food Res. 21:54-62. [Google Scholar]
- 18.Dodatko, T., M. Akoachere, N. Jimenez, Z. Alvarez, and E. Abel-Santos. Dissecting interactions between nucleosides and germination receptors in Bacillus cereus 569 spores. Microbiology, in press. [DOI] [PMC free article] [PubMed]
- 19.Fischer, M., J. Bhatnagar, J. Guarner, S. Reagan, J. K. Hacker, S. H. Van Meter, V. Poukens, D. B. Whiteman, A. Iton, M. Cheung, D. E. Dassey, W.-J. Shieh, and S. R. Zaki. 2005. Fatal toxic shock syndrome associated with Clostridium sordellii after medical abortion. N. Engl. J. Med. 353:2352-2360. [DOI] [PubMed] [Google Scholar]
- 20.Fisher, N., and P. Hanna. 2005. Characterization of Bacillus anthracis germinant receptors in vitro. J. Bacteriol. 187:8055-8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foster, S. J., and K. Johnstone. 1986. The use of inhibitors to identify early events during Bacillus megaterium KM spore germination. Biochem. J. 237:865-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gleeson, R. P., A. M. Elder, M. J. Turner, A. J. Rutherford, and M. G. Elder. 1989. Vaginal pH in pregnancy in women delivered at and before term. Br. J. Obstet. Gynaecol. 96:183-187. [DOI] [PubMed] [Google Scholar]
- 23.Gregoire, A. T., W. R. Lang, and K. Ward. 1959. The qualitative identification of free amino acids in human vaginal fluid. Ann. N. Y. Acad. Sci. 83:185-188. [DOI] [PubMed] [Google Scholar]
- 24.Grimes, D. A. 2003. Unsafe abortion: the silent scourge. Br. Med. Bull. 67:99-113. [DOI] [PubMed] [Google Scholar]
- 25.Hamouda, T., A. Y. Shih, and J. R. Baker. 2002. A rapid staining technique for the detection of the initiation of germination of bacterial spores. Lett. Appl. Microbiol. 34:86-90. [DOI] [PubMed] [Google Scholar]
- 26.Hao, Y., T. Senn, J. S. Opp, V. B. Young, T. Thiele, G. Srinivas, S. K. Huang, and D. M. Aronoff. Lethal toxin is a critical determinant of rapid mortality in rodent models of Clostridium sordellii endometritis. Anaerobe, in press. [DOI] [PMC free article] [PubMed]
- 27.Hauth, J. C., C. MacPherson, J. C. Carey, M. A. Klebanoff, S. L. Hillier, J. M. Ernest, K. J. Leveno, R. Wapner, M. Varner, W. Trout, A. Moawad, and B. Sibai. 2003. Early pregnancy threshold vaginal pH and Gram stain scores predictive of subsequent preterm birth in asymptomatic women. Am. J. Obstet. Gynecol. 188:831-835. [DOI] [PubMed] [Google Scholar]
- 28.Hibbs, C. M., D. R. Johnson, K. Reynolds, and R. Harrington. 1977. Clostridium sordellii isolated from foals. Vet. Med. Small. Anim. Clin. 72:256-258. [PubMed] [Google Scholar]
- 29.Ho, C. S., J. Bhatnagar, A. L. Cohen, J. K. Hacker, S. B. Zane, S. Reagan, M. Fischer, W.-J. Shieh, J. Guarner, S. Ahmad, S. R. Zaki, and L. C. McDonald. 2009. Undiagnosed cases of fatal Clostridium-associated toxic shock in Californian women of childbearing age. Am. J. Obstet. Gynecol. 201:459e1-459e7. [DOI] [PubMed] [Google Scholar]
- 30.Hornstra, L. M., Y. P. de Vries, M. H. Wells-Bennik, W. M. de Vos, and T. Abee. 2006. Characterization of germination receptors of Bacillus cereus ATCC 14579. Appl. Environ. Microbiol. 72:44-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishikawa, S., K. Yamane, and J. Sekiguchi. 1998. Regulation and characterization of a newly deduced cell wall hydrolase gene (cwlJ) which affects germination of Bacillus subtilis spores. J. Bacteriol. 180:1375-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jauniaux, E., B. Gulbis, E. Gerlo, and C. Rodeck. 1998. Free amino acid distribution inside the first trimester human gestational sac. Early Hum. Dev. 51:159-169. [DOI] [PubMed] [Google Scholar]
- 33.Karlsson, S., L. G. Burman, and T. Akerlund. 1999. Suppression of toxin production in Clostridium difficile VPI 10463 by amino acids. Microbiology 145:1683-1693. [DOI] [PubMed] [Google Scholar]
- 34.Khan, K. S., D. Wojdyla, L. Say, A. M. Gülmezoglu, and P. F. A. Van Look. 2006. WHO analysis of causes of maternal death: a systematic review. Lancet 367:1066-1074. [DOI] [PubMed] [Google Scholar]
- 35.Kimura, A. C., J. I. Higa, R. M. Levin, G. Simpson, Y. Vargas, and D. J. Vugia. 2004. Outbreak of necrotizing fasciitis due to Clostridium sordellii among black-tar heroin users. Clin. Infect. Dis. 38:e87-e91. [DOI] [PubMed] [Google Scholar]
- 36.Mania-Pramanik, J., S. C. Kerkar, P. B. Mehta, S. Potdar, and V. S. Salvi. 2008. Use of vaginal pH in diagnosis of infections and its association with reproductive manifestations. J. Clin. Lab. Anal. 22:375-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martinez, R. D., and T. D. Wilkins. 1988. Purification and characterization of Clostridium sordellii hemorrhagic toxin and cross-reactivity with Clostridium difficile toxin A (enterotoxin). Infect. Immun. 56:1215-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miech, R. P. 2005. Pathophysiology of mifepristone-induced septic shock due to Clostridium sordellii. Ann. Pharmacother. 39:1483-1488. [DOI] [PubMed] [Google Scholar]
- 39.Moir, A., B. M. Corfe, and J. Behravan. 2002. Spore germination. Cell. Mol. Life Sci. 59:403-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murray, S., and E. Wooltorton. 2005. Septic shock after medical abortions with mifepristone (Mifeprex, RU 486) and misoprostol. CMAJ 173:485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murta, E., P. Perfeito, T. Oliveira, M. Michelin, and P. Maluf. 2008. Relation between vaginal and endocervical pH in patients undergoing cold-knife conization and hysterectomy. Arch. Gynecol. Obstet. 277:43-46. [DOI] [PubMed] [Google Scholar]
- 42.Nicholson, W. L., N. Munakata, G. Horneck, H. J. Melosh, and P. Setlow. 2000. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol. Mol. Biol. Rev. 64:548-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ortega, J., B. Daft, R. A. Assis, H. Kinde, L. Anthenill, J. Odani, and F. A. Uzal. 2007. Infection of internal umbilical remnant in foals by Clostridium sordellii. Vet. Pathol. 44:269-275. [DOI] [PubMed] [Google Scholar]
- 44.Penney, G. 1997. Preventing infective sequelae of abortion. Hum. Reprod. 12:107-112. [PubMed] [Google Scholar]
- 45.Plowman, J., and M. W. Peck. 2002. Use of a novel method to characterize the response of spores of non-proteolytic Clostridium botulinum types B, E and F to a wide range of germinants and conditions. J. Appl. Microbiol. 92:681-694. [DOI] [PubMed] [Google Scholar]
- 46.Popoff, M. R. 1987. Purification and characterization of Clostridium sordellii lethal toxin and cross-reactivity with Clostridium difficile cytotoxin. Infect. Immun. 55:35-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Preston, R. A., and H. A. Douthit. 1988. Functional relationships between l- and d-alanine, inosine and NH4Cl during germination of spores of Bacillus cereus T. J. Gen. Microbiol. 134:3001-3010. [DOI] [PubMed] [Google Scholar]
- 48.Qa'Dan, M., L. M. Spyres, and J. D. Ballard. 2001. pH-enhanced cytopathic effects of Clostridium sordellii lethal toxin. Infect. Immun. 69:5487-5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rode, L. J., L. Pope, C. Filip, and L. D. Smith. 1971. Spore appendages and taxonomy of Clostridium sordellii. J. Bacteriol. 108:1384-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sacks, L. E., and P. A. Thompson. 1978. Clear, defined medium for the sporulation of Clostridium perfringens. Appl. Environ. Microbiol. 35:405-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saifer, A., E. A'Zary, C. Valenti, and L. Schneck. 1970. Quantitative cation-exchange chromatographic analysis of free amino acids in human amniotic fluid collected during early pregnancy. Clin. Chem. 16:891-895. [PubMed] [Google Scholar]
- 52.Schirmer, J., and K. Aktories. 2004. Large clostridial cytotoxins: cellular biology of Rho/Ras-glucosylating toxins. Biochim. Biophys. Acta 1673:66-74. [DOI] [PubMed] [Google Scholar]
- 53.Sebaihia, M., B. W. Wren, P. Mullany, N. F. Fairweather, N. Minton, R. Stabler, N. R. Thomson, A. P. Roberts, A. M. Cerdeno-Tarraga, H. Wang, M. T. Holden, A. Wright, C. Churcher, M. A. Quail, S. Baker, N. Bason, K. Brooks, T. Chillingworth, A. Cronin, P. Davis, L. Dowd, A. Fraser, T. Feltwell, Z. Hance, S. Holroyd, K. Jagels, S. Moule, K. Mungall, C. Price, E. Rabbinowitsch, S. Sharp, M. Simmonds, K. Stevens, L. Unwin, S. Whithead, B. Dupuy, G. Dougan, B. Barrell, and J. Parkhill. 2006. The multidrug-resistant human pathogen Clostridium difficile has a highly mobile, mosaic genome. Nat. Genet. 38:779-786. [DOI] [PubMed] [Google Scholar]
- 54.Setlow, P. 2003. Spore germination. Curr. Opin. Microbiol. 6:550-556. [DOI] [PubMed] [Google Scholar]
- 55.Setlow, P. 2006. Spores of Bacillus subtilis: their resistance to and killing by radiation, heat and chemicals. J. Appl. Microbiol. 101:514-525. [DOI] [PubMed] [Google Scholar]
- 56.Sinave, C., G. Le Templier, D. Blouin, F. Léveillé, and É. Deland. 2002. Toxic shock syndrome due to Clostridium sordellii: a dramatic postpartum and postabortion disease. Clin. Infect. Dis. 35:1441-1443. [DOI] [PubMed] [Google Scholar]
- 57.Sorg, J. A., and A. L. Sonenshein. 2008. Bile salts and glycine as cogerminants for Clostridium difficile spores. J. Bacteriol. 190:2505-2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vishwakarma, P. 1962. The pH and bicarbonate-ion content of the oviduct and uterine fluids. Fertil. Steril. 13:481-485. [DOI] [PubMed] [Google Scholar]
- 59.Waites, W. M., and L. R. Wyatt. 1971. Germination of spores of Clostridium bifermentans by certain amino acids, lactate and pyruvate in the presence of sodium or potassium ions. J. Gen. Microbiol. 67:215-222. [DOI] [PubMed] [Google Scholar]
- 60.Wax, R., and E. Freese. 1968. Initiation of the germination of Bacillus subtilis spores by a combination of compounds in place of l-alanine. J. Bacteriol. 95:433-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wheeldon, L. J., T. Worthington, A. C. Hilton, T. S. J. Elliott, and P. A. Lambert. 2008. Physical and chemical factors influencing the germination of Clostridium difficile spores. J. Appl. Microbiol. 105:2223-2230. [DOI] [PubMed] [Google Scholar]
- 62.Wilson, K. H. 1983. Efficiency of various bile salt preparations for stimulation of Clostridium difficile spore germination. J. Clin. Microbiol. 18:1017-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yasuda, Y., and K. Tochikubo. 1984. Relation between d-glucose and l- and d-alanine in the initiation of germination of Bacillus subtilis spores. Microbiol. Immunol. 28:197-207. [DOI] [PubMed] [Google Scholar]